This randomized clinical trial examines data for patients with Alzheimer dementia to determine whether brexpiprazole is an efficacious, safe, and well-tolerated treatment for agitation.
Key Points
Question
Is brexpiprazole an efficacious, safe, and well-tolerated treatment for agitation in patients with Alzheimer dementia?
Findings
In this randomized clinical trial with 345 patients, brexpiprazole, 2 mg/d or 3 mg/d, demonstrated a statistically significant reduction in agitation (Cohen-Mansfield Agitation Inventory score) vs placebo over 12 weeks. No treatment-emergent adverse events had an incidence of 5% or greater with brexpiprazole and greater than placebo, and the discontinuation rates due to adverse events were similar across the groups.
Meaning
Brexpiprazole, 2 or 3 mg, reduced agitation in Alzheimer dementia and was generally well tolerated over 12 weeks.
Abstract
Importance
Agitation is a prevalent, distressing, and burdensome manifestation of Alzheimer dementia in need of an efficacious, safe, and well-tolerated treatment.
Objective
To confirm the efficacy, safety, and tolerability of brexpiprazole in patients with agitation in Alzheimer dementia.
Design, Setting, and Participants
This randomized clinical trial was a 12-week, double-blind, placebo-controlled, fixed-dose, parallel-arm trial that ran from May 2018 to June 2022 at 123 clinical trial sites in Europe and the United States. Participants included patients with agitation in Alzheimer dementia in a care facility or community-based setting. Stable Alzheimer disease medications were permitted.
Interventions
In this 2-arm trial, patients were randomized to receive oral brexpiprazole or placebo (2:1 ratio) for 12 weeks. Within the brexpiprazole arm, patients were further randomized to receive fixed doses of 2 mg/d or 3 mg/d in a 1:2 ratio.
Main Outcomes and Measures
The primary end point was change in Cohen-Mansfield Agitation Inventory total score (which measures the frequency of 29 agitated behaviors) from baseline to week 12 for brexpiprazole, 2 or 3 mg, vs placebo. Safety was assessed by standard measures, including treatment-emergent adverse events.
Results
A total of 345 patients were randomized to receive brexpiprazole (n = 228) or placebo (n = 117); completion rates were 198 (86.8%) for brexpiprazole and 104 (88.9%) for placebo. Mean (SD) age was 74.0 (7.5) years, and 195 of 345 patients were female (56.5%). Patients receiving brexpiprazole, 2 or 3 mg (n = 225), demonstrated statistically significantly greater improvement than those taking placebo (n = 116) in Cohen-Mansfield Agitation Inventory total score from baseline to week 12 (brexpiprazole baseline, 80.6, mean change, −22.6; placebo baseline, 79.2, mean change, −17.3; least-squares mean difference, −5.32; 95% CI, −8.77 to −1.87; P = .003; Cohen d effect size, 0.35). No treatment-emergent adverse events had an incidence of 5% or more with brexpiprazole and greater incidence than placebo. The proportion of patients who discontinued because of adverse events was 12 of 226 (5.3%) for brexpiprazole and 5 of 116 (4.3%) for placebo.
Conclusions and Relevance
In this study, patients with Alzheimer dementia who took brexpiprazole, 2 or 3 mg, showed a statistically significant improvement vs placebo in agitation over 12 weeks. Brexpiprazole was generally well tolerated over 12 weeks in this vulnerable patient population.
Trial Registration
ClinicalTrials.gov Identifier: NCT03548584
Introduction
Agitation associated with dementia is defined as excessive motor activity (eg, pacing, rocking, restlessness), verbal aggression (eg, speaking excessively loudly, screaming), or physical aggression (eg, grabbing, pushing, throwing objects), which causes excess distress or disability and cannot be solely attributed to a suboptimal care environment or another disorder (International Psychogeriatric Association criteria).1 Agitation in dementia is common2,3; has a negative effect on patient functioning, health outcomes, and quality of life3,4,5; increases caregiver distress and time spent caring5,6; and may contribute to the patient being institutionalized.7 Due to the lack of health-authority–approved pharmacological treatment options for agitation in dementia, physicians may prescribe off-label medications,8,9 despite having insufficient information about dosing, efficacy, and safety. Certain atypical antipsychotics have demonstrated efficacy on agitation in dementia but have an unfavorable benefit/risk profile that must be taken into consideration by patients and prescribing clinicians.10,11
Brexpiprazole is an atypical antipsychotic that acts on noradrenergic, serotonergic, and dopaminergic neurotransmitter systems,12 which are implicated in the neurochemistry of agitation in Alzheimer disease.13 Two prior randomized clinical trials suggested that brexpiprazole, 2 mg, may be efficacious, safe, and well tolerated in patients with agitation in Alzheimer dementia,14 indicating its potential as a new treatment for agitation, provided that results could be replicated. The aim of the present clinical trial was to confirm the efficacy, safety, and tolerability of brexpiprazole in patients with agitation in Alzheimer dementia.
Methods
This was a phase 3, multicenter, 12-week, randomized, double-blind, placebo-controlled, parallel-arm trial of brexpiprazole in patients with agitation in Alzheimer dementia (ClinicalTrials.gov identifier: NCT03548584). The trial protocol and statistical analysis plan are available in Supplement 1.
Patients and Study Design
The trial was conducted in accordance with the International Council for Harmonisation Good Clinical Practice guidelines and local regulatory requirements. The study protocol was approved by the governing institutional review board or independent ethics committee for each investigational site or country. All patients and/or their legal representatives provided electronic informed consent prior to the start of the study, after the intervention and possible adverse effects had been fully explained. Patients in the United States and their caregivers received a stipend to cover travel and meal costs.
Patients (a volunteer sample) were enrolled by investigators at 123 Alzheimer disease clinical trial sites in Europe (Bulgaria, Hungary, Serbia, Slovakia, Spain, Ukraine) and the United States. Key inclusion criteria were age 55 to 90 years; diagnosis of probable Alzheimer disease, defined by the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA) criteria15; Mini-Mental State Examination (MMSE)16 score of 5 to 22 at screening and baseline; previous computed tomography or magnetic resonance imaging scan of the brain with findings consistent with a diagnosis of Alzheimer disease; a diagnosis of agitation that meets the International Psychogeriatric Association definition (which was a provisional definition at the time)1; onset of agitation at least 2 weeks prior to screening; Neuropsychiatric Inventory (NPI) or NPI–Nursing Home version (NPI-NH) Agitation/Aggression domain score (frequency × severity) of 4 or greater at screening and baseline17,18; requiring pharmacotherapy for the treatment of agitation in the investigator’s judgment after an evaluation for reversible factors (eg, pain, infection, polypharmacy) and a trial of nonpharmacological interventions (eg, redirecting behavior, group activities, music therapy); living in a care facility or community-based setting (not living alone); and having an identified caregiver who has sufficient contact to describe the patient’s symptoms and behavior. An additional required inclusion criterion based on positivity for Cohen-Mansfield Agitation Inventory (CMAI) factor 1 (aggressive behavior [12 items: hitting, kicking, scratching, grabbing, pushing, hurting self or others, throwing things, cursing or verbal aggression, spitting, tearing things or destroying property, screaming, and biting]) was blinded to patients, caregivers, and investigators.19,20 To meet the CMAI factor 1 positivity criterion, 1 of the following must have been established at screening and baseline: 1 or more aggressive behaviors occurring several times per week, 2 or more aggressive behaviors occurring once or twice per week, or 3 or more aggressive behaviors occurring less than once per week.19,20,21 Key exclusion criteria were dementia or memory impairment due to a reason other than Alzheimer disease and any clinically significant neurological, psychiatric (except as specified), or unstable medical condition. Stable diabetes and asymptomatic major depressive disorder were permitted.
After screening, patients entered a 12-week double-blind treatment period in which they were randomized 2:1 to receive oral brexpiprazole or placebo. Although this was a 2-arm trial, patients in the brexpiprazole arm were further randomized 1:2 to receive brexpiprazole fixed doses of 2 mg/d or 3 mg/d. The 3-mg dose was included as recommended by the US Food and Drug Administration to explore the efficacy, safety, and tolerability of a higher dose of brexpiprazole than had previously been studied in Alzheimer disease. The trial design, including titration, is illustrated in eFigure 1 in Supplement 2.
Study drugs were taken orally once daily at the same time each day, preferably in the morning, without regard to meals. Brexpiprazole tablets and matching placebo tablets were provided by the sponsor, packaged in numbered, weekly blister cards. Treatments were assigned to patients using an eSource method via a fixed-block (block size 3) computer-generated randomization code provided by the sponsor and stratified by site. Treatment assignments were blinded to patients, caregivers, investigators, and sponsor personnel, including those involved in data analysis. Visits occurred every 2 weeks. Stable background medications for the treatment of Alzheimer disease were permitted, whereas antipsychotics, mood stabilizers, and anticonvulsants were prohibited. Limited use of benzodiazepines was permitted during the first 4 weeks of the double-blind treatment period and prohibited thereafter.
Assessments
Demographic information and medical history were recorded at the screening visit. Sex, race, and ethnicity were reported using US Census Bureau classifications; the protocol did not specify a method of collection.
Efficacy was assessed using the CMAI, a validated measure of the frequency of occurrence of 29 agitated behaviors in care facilities and community-based settings.19,22,23 Each item is scored from 1 (never occurs) to 7 (occurs a few times an hour), giving a total score range from 29 to 203 points.19 The CMAI was completed at each visit by the clinician based on an interview with the patient’s caregiver. Efficacy was also assessed using the clinician-rated Clinical Global Impression Severity of illness (CGI-S) and Improvement (CGI-I) scales,24 specifically applied to agitation, and the NPI-NH.18
Safety was assessed via treatment-emergent adverse events (TEAEs), body weight, laboratory tests, vital signs, electrocardiograms, the Sheehan Suicidality Tracking Scale,25 the MMSE (to assess cognitive dysfunction),16 and 3 extrapyramidal symptom rating scales: Simpson-Angus Scale,26 Abnormal Involuntary Movement Scale,24 and Barnes Akathisia Rating Scale.27
Statistical Analysis
The primary estimand was defined by the following components:
Population: patients with agitation in Alzheimer dementia.
Treatments: brexpiprazole, 2 mg/d or 3 mg/d (a single arm), or placebo.
Primary end point: change from baseline to week 12 in CMAI total score.
Measure of intervention effect: mean difference between the brexpiprazole and placebo arms.
Intercurrent events: premature treatment discontinuation (ie, early dropout) before week 12 attributable to adverse events, lack of efficacy, withdrawal of consent/assent, or any other cause.
Treatment effect was estimated under the hypothetical situation that no patients discontinued early from treatment. However, in clinical trial practice, some patients are likely to discontinue, and a mixed model for repeated measures was used to account for such patients, including all observed data regardless of completion status.
To control for experiment-wise type I error, a testing hierarchy was used in which the key secondary end point (change from baseline to week 12 in CGI-S score) was tested only if the primary end point was significant at the .035 level (2-sided). Other secondary end points (CMAI factor scores, CGI-I score, CMAI/CGI-I response rates) and prespecified exploratory end points (NPI-NH total score, CMAI total score by dose, CGI-S score by dose) were tested at a nominal .05 level (2-sided), with no adjustment for multiplicity.
Subgroup analyses of the primary efficacy end point were performed by region, sex, race, age, dementia severity, and psychosis status. Details of the sample size calculation, interim analysis, and statistical analysis methods are provided in the eMethods in Supplement 2.
Results
Patients
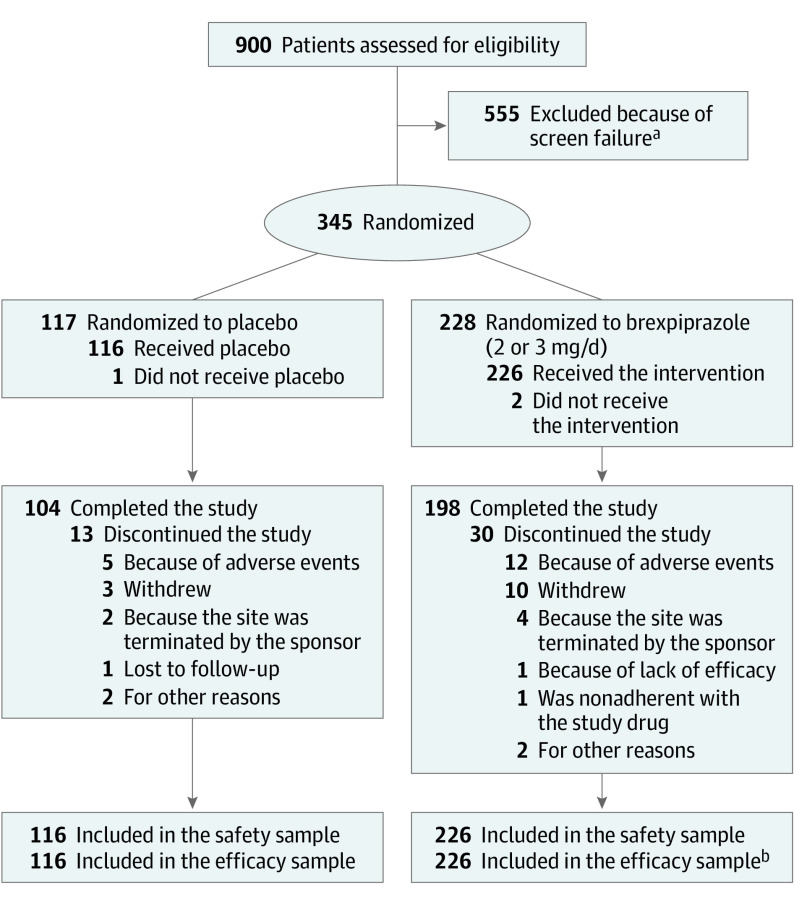
The trial was conducted between May 16, 2018, and June 1, 2022. Of 123 included sites (listed in eTable 1 in Supplement 2), 68 sites enrolled patients, of which 16 were classified as small sites. A total of 345 patients were randomized to receive brexpiprazole (n = 228) or placebo (n = 117) across the United States (152, 44.1%), Ukraine (107, 31.0%), Bulgaria (37, 10.7%), Serbia (19, 5.5%), Slovakia (13, 3.8%), Spain (11, 3.2%), and Hungary (6, 1.7%). Completion rates were 198 (86.8%) for brexpiprazole and 104 (88.9%) for placebo (Figure 1 and by dose in eFigure 2 in Supplement 2).
Figure 1. Patient Disposition.
aDefined as a patient who provided informed consent but was not randomized. The top 5 categories of screen failure were blinded Cohen-Mansfield Agitation Inventory (CMAI) factor 1 criterion, investigator or sponsor discretion, unstable diabetes, abnormal test results (laboratory tests, vital signs, electrocardiograms), and Mini-Mental State Examination score.
bIn the brexpiprazole group, the efficacy sample comprised 226 patients who took at least 1 dose of study drug and who had a baseline and postbaseline CMAI measurement. However, 1 patient was excluded from efficacy analyses because their only postbaseline CMAI measurement was outside of the visit window.
Baseline demographic and clinical characteristics were generally similar between treatment groups (Table 1). Mean (SD) age was 74.0 (7.5) years (range, 56-90 years), 195 patients (56.5%) were female, 150 (43.5%) were male, 4 (1.2%) were Asian, 12 (3.5%) were Black, 108 (31.3%) were Hispanic, 329 (95.4%) were White, and 193 (55.9%) had moderate cognitive impairment (MMSE score of 13-18). During the trial, standard medications for Alzheimer disease (mostly memantine or donepezil) were taken by 184 patients (81.4%) receiving brexpiprazole and 95 (81.9%) receiving placebo, and concomitant medications for agitation (mostly lorazepam) were received at least once by 44 patients (19.5%) receiving brexpiprazole and 17 (14.7%) receiving placebo.
Table 1. Baseline Demographic and Clinical Characteristics (Randomized Sample).
| Characteristic | Placebo (n = 117), No. (%)a | Brexpiprazole, No. (%)a | ||
|---|---|---|---|---|
| 2 or 3 mg (n = 228) | 2-mg Subgroup (n = 75) | 3-mg Subgroup (n = 153) | ||
| Demographic | ||||
| Age, mean (SD), y | 73.0 (7.0) | 74.5 (7.7) | 74.3 (7.3) | 74.6 (8.0) |
| Age group, y | ||||
| <65 | 13 (11.1) | 24 (10.5) | 8 (10.7) | 16 (10.5) |
| ≥65 to <75 | 54 (46.2) | 83 (36.4) | 29 (38.7) | 54 (35.3) |
| ≥75 | 50 (42.7) | 121 (53.1) | 38 (50.7) | 83 (54.2) |
| Weight, mean (SD), kg | 71.3 (13.8) | 70.5 (15.5) | 70.9 (15.5) | 70.2 (15.5) |
| BMI, mean (SD)b | 26.6 (4.8) | 26.3 (4.7) | 26.6 (4.7) | 26.1 (4.7) |
| Sex | ||||
| Female | 60 (51.3) | 135 (59.2) | 43 (57.3) | 92 (60.1) |
| Male | 57 (48.7) | 93 (40.8) | 32 (42.7) | 61 (39.9) |
| Race | ||||
| Asian | 1 (0.9) | 3 (1.3) | 0 | 3 (2.0) |
| Black or African American | 1 (0.9) | 11 (4.8) | 5 (6.7) | 6 (3.9) |
| White | 115 (98.3) | 214 (93.9) | 70 (93.3) | 144 (94.1) |
| Ethnicity | ||||
| Hispanic or Latino | 37 (31.6) | 71 (31.1) | 25 (33.3) | 46 (30.1) |
| Not Hispanic or Latino | 80 (68.4) | 157 (68.9) | 50 (66.7) | 107 (69.9) |
| Living situation | ||||
| Care facility | 54 (46.2) | 96 (42.1) | 32 (42.7) | 64 (41.8) |
| Community-based setting | 63 (53.8) | 132 (57.9) | 43 (57.3) | 89 (58.2) |
| Clinical | ||||
| Time since Alzheimer disease diagnosis, mean (SD), mo | 34.1 (31.4) | 36.7 (36.9) | 34.5 (38.9) | 37.8 (36.0) |
| Time since onset of current agitation episode requiring pharmacotherapy, mean (SD), mo | 8.9 (10.7) | 10.0 (14.8) | 9.0 (14.4) | 10.5 (15.0) |
| CMAI total score, mean (SD) | 79.4 (17.6) | 80.4 (16.7) | 78.6 (15.5) | 81.2 (17.2) |
| CGI-S score as related to agitation, mean (SD) | 4.7 (0.7) | 4.7 (0.7) | 4.6 (0.7) | 4.7 (0.6) |
| NPI-NH total score, mean (SD) | 36.5 (17.0)c | 37.5 (17.7)d | 37.9 (17.1)e | 37.3 (18.1)f |
| NPI-NH Agitation/Aggression domain score, mean (SD) | 7.5 (2.1) | 7.7 (2.2) | 7.9 (2.4) | 7.6 (2.0) |
| Psychosis (≥4 NPI delusion/hallucination symptoms) | 21 (17.9) | 44 (19.3) | 14 (18.7) | 30 (19.6) |
| MMSE score, mean (SD) | 15.5 (3.9) | 15.6 (3.7) | 15.8 (3.2) | 15.5 (3.9) |
| MMSE score category | ||||
| Mild (>18) | 28 (23.9) | 53 (23.2) | 16 (21.3) | 37 (24.2) |
| Moderate (13-18) | 66 (56.4) | 127 (55.7) | 48 (64.0) | 79 (51.6) |
| Severe (≤12) | 23 (19.7) | 48 (21.1) | 11 (14.7) | 37 (24.2) |
Abbreviations: BMI, body mass index; CGI-S, Clinical Global Impression–Severity of illness; CMAI, Cohen-Mansfield Agitation Inventory; NPI, Neuropsychiatric Inventory; NPI-NH, Neuropsychiatric Inventory–Nursing Home version; MMSE, Mini-Mental State Examination.
Values are No. (%) unless otherwise described as mean (SD).
Calculated as weight in kilograms divided by height in meters squared.
n = 116.
n = 226.
n = 74.
n = 152.
Efficacy
Primary End Point
Brexpiprazole, 2 or 3 mg, demonstrated statistically significant greater improvement vs placebo on the change in CMAI total score from baseline to week 12, with a Cohen d effect size of 0.35 (Figure 2A, Table 2, absolute scores in eFigure 3A in Supplement 2, changes by subgroup in eFigure 4 in Supplement 2, and missing-not-at-random sensitivity analysis in eTable 2 in Supplement 2).
Figure 2. Change From Baseline in Cohen-Mansfield Agitation Inventory (CMAI) Total Score (Primary End Point) and Clinical Global Impression–Severity of Illness (CGI-S) Score as Related to Agitation (Key Secondary End Point): Efficacy Sample.
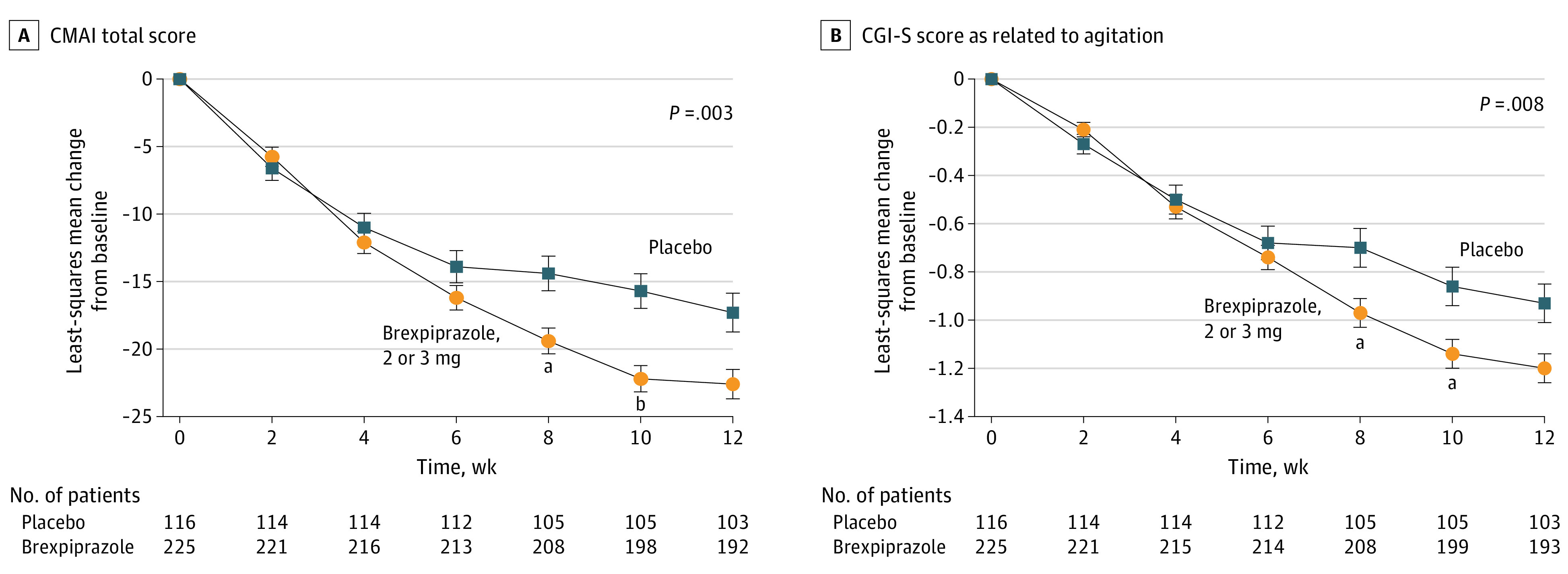
Baseline mean CMAI total scores: brexpiprazole, 80.6; placebo, 79.2. Baseline mean CGI-S scores: brexpiprazole, 4.7; placebo, 4.7. Footnotes indicate nominal P values with no adjustment for multiplicity.
aP < .01 vs placebo, mixed model for repeated measures.
bP < .001 vs placebo, mixed model for repeated measures.
Table 2. Summary of Efficacy End Points (Efficacy Sample).
| End point | Treatment group | No. of patients | Baseline, mean (SD) | At week 12, change from baseline, LS mean (SE), or No. (%) response rate | Treatment difference at week 12 vs placebo | ||
|---|---|---|---|---|---|---|---|
| LS mean difference or ratio of response rate (95% CI) | P value | Cohen d effect size | |||||
| Primary end point | |||||||
| CMAI total scorea | Brexpiprazole 2 or 3 mg | 225 | 80.6 (16.6) | −22.6 (1.1) | Difference, −5.32 (−8.77 to −1.87) | .003 | 0.35 |
| Placebo | 116 | 79.2 (17.5) | −17.3 (1.4) | ||||
| Key secondary end point | |||||||
| CGI-S score as related to agitationa | Brexpiprazole 2 or 3 mg | 225 | 4.7 (0.7) | −1.2 (0.1) | Difference, −0.27 (−0.47 to −0.07) | .008 | 0.31 |
| Placebo | 116 | 4.7 (0.7) | −0.9 (0.1) | ||||
| Secondary end points: change and difference b | |||||||
| CMAI factor 1: aggressive behavior scorea | Brexpiprazole 2 or 3 mg | 225 | 26.3 (7.3) | −9.1 (0.4) | Difference, −1.95 (−3.28 to −0.63) | .004 | 0.33 |
| Placebo | 116 | 26.5 (8.7) | −7.1 (0.6) | ||||
| CMAI factor 2: physically non-aggressive behavior scorea | Brexpiprazole 2 or 3 mg | 225 | 23.8 (7.3) | −6.5 (0.4) | Difference, −1.41 (−2.68 to −0.14) | .03 | 0.25 |
| Placebo | 116 | 23.2 (7.4) | −5.0 (0.5) | ||||
| CMAI factor 3: verbally agitated behavior scorea | Brexpiprazole 2 or 3 mg | 225 | 16.9 (4.7) | −4.4 (0.3) | Difference, −1.24 (−2.21 to −0.28) | .01 | 0.29 |
| Placebo | 116 | 16.3 (5.6) | −3.1 (0.4) | ||||
| CGI-I scorec | Brexpiprazole 2 or 3 mg | 225 | NA | 2.7 (1.1)d | Difference, −0.33 (−0.57 to −0.09)e | .007 | NA |
| Placebo | 116 | NA | 3.0 (1.1)d | ||||
| Secondary end points: response rate and ratio b | |||||||
| CMAI response ratec | |||||||
| ≥20% Improvement | Brexpiprazole 2 or 3 mg | 225 | NA | 154 (68.4) | Ratio, 1.41 (1.15 to 1.72)f | <.001 | NA |
| Placebo | 116 | NA | 55 (47.4) | ||||
| ≥30% Improvement | Brexpiprazole 2 or 3 mg | 225 | NA | 96 (42.7) | Ratio, 1.62 (1.18 to 2.23)f | .002 | NA |
| Placebo | 116 | NA | 30 (25.9) | ||||
| ≥40% Improvement | Brexpiprazole 2 or 3 mg | 225 | NA | 52 (23.1) | Ratio, 1.62 (1.00 to 2.61)f | .03 | NA |
| Placebo | 116 | NA | 17 (14.7) | ||||
| Improvement in agitation status | Brexpiprazole 2 or 3 mg | 225 | NA | 118 (52.4) | Ratio, 1.47 (1.14 to 1.89)f | .002 | NA |
| Placebo | 116 | NA | 43 (37.1) | ||||
| CGI-I response ratec | |||||||
| Score of very much improved or much improved | Brexpiprazole 2 or 3 mg | 225 | NA | 118 (52.4) | Ratio, 1.32 (1.03 to 1.69)f | .02 | NA |
| Placebo | 116 | NA | 47 (40.5) | ||||
| Exploratory end point b | |||||||
| NPI-NH total scorea | Brexpiprazole 2 or 3 mg | 215 | 37.7 (17.8) | −17.3 (0.9) | Difference, −4.60 (−7.33 to −1.88) | .001 | 0.39 |
| Placebo | 111 | 36.6 (17.2) | −12.7 (1.2) | ||||
Abbreviations: CGI-I, Clinical Global Impression–Improvement; CGI-S, Clinical Global Impression–Severity of illness; CMAI, Cohen-Mansfield Agitation Inventory; CMH, Cochran-Mantel-Haenszel; LS, least-squares; NA, not applicable; NPI-NH, Neuropsychiatric Inventory–Nursing Home version.
Mixed model for repeated measures.
P values for secondary and exploratory end points are nominal with no adjustment for multiplicity.
Last observation carried forward.
Mean (SD) score at week 12.
Adjusted mean difference (95% CI) based on the CMH row mean scores differ test.
Brexpiprazole/placebo; CMH general association test.
Secondary End Points
Brexpiprazole, 2 or 3 mg, demonstrated statistically significant greater improvement vs placebo on the change in CGI-S score as related to agitation from baseline to week 12 (the key secondary end point), with a Cohen d effect size of 0.31 (Figure 2B, Table 2, and absolute scores in eFigure 3B in Supplement 2). Brexpiprazole, 2 or 3 mg, showed nominally significant greater improvement vs placebo on all other secondary end points (CMAI factor scores, CGI-I score, CMAI/CGI-I response rates) at week 12 (Table 2).
Exploratory End Points
Brexpiprazole, 2 or 3 mg, showed nominally significant greater improvement vs placebo on NPI-NH total score at week 12 (Table 2). By dose, both brexpiprazole dose subgroups, 2 and 3 mg, showed similar greater improvement than placebo on CMAI total score at week 12, with nominal significance (eFigure 5A in Supplement 2). On CGI-S score, brexpiprazole, 2 and 3 mg, showed similar improvements at week 12, with nominal significance vs placebo for brexpiprazole, 3 mg (eFigure 5B in Supplement 2).
Safety
Ninety-two patients (40.7%) reported TEAEs with brexpiprazole, 2 or 3 mg, and 36 (31.0%) with placebo, with no apparent effect of dose (Table 3). Headache was the only TEAE with an incidence 5% or greater in the brexpiprazole, 2 or 3 mg, group (15 [6.6%] vs 8 [6.9%] with placebo). The incidence of TEAEs in specific categories of interest for brexpiprazole and placebo, respectively, was as follows: any cardiovascular TEAE: 2 (0.9%), 1 (0.9%); any cerebrovascular TEAE: 0, 0; any extrapyramidal symptom–related TEAE: 8 (3.5%), 0; any somnolence/sedation TEAE: 9 (4.0%), 1 (0.9%); any accident or injury TEAE, including fall: 5 (2.2%), 4 (3.4%); any metabolism and nutrition disorder TEAE: 3 (1.3%), 2 (1.7%). The majority of TEAEs were mild or moderate in severity.
Table 3. Summary of Treatment-Emergent Adverse Events (Safety Sample).
| Event | Placebo (n = 116), No. (%) | Brexpiprazole, No. (%) | ||
|---|---|---|---|---|
| 2 or 3 mg (n = 226) | 2-mg Subgroup (n = 73) | 3-mg Subgroup (n = 153) | ||
| At least 1 TEAE | 36 (31.0) | 92 (40.7) | 28 (38.4) | 64 (41.8) |
| At least 1 serious TEAE | 3 (2.6)a | 6 (2.7)b | 0 | 6 (3.9) |
| Discontinuation due to adverse event | 5 (4.3)c | 12 (5.3)d | 1 (1.4) | 11 (7.2) |
| Death | 0 | 1 (0.4)e | 0 | 1 (0.7) |
| TEAEs with an incidence ≥2% in the brexpiprazole, 2 or 3 mg, group and an incidence greater than placebo | ||||
| Somnolence | 1 (0.9) | 8 (3.5) | 3 (4.1) | 5 (3.3) |
| Nasopharyngitis | 2 (1.7) | 7 (3.1) | 3 (4.1) | 4 (2.6) |
| Dizziness | 2 (1.7) | 6 (2.7) | 1 (1.4) | 5 (3.3) |
| Asthenia | 0 | 5 (2.2) | 0 | 5 (3.3) |
| Diarrhea | 1 (0.9) | 5 (2.2) | 3 (4.1) | 2 (1.3) |
| Urinary tract infection | 1 (0.9) | 5 (2.2) | 0 | 5 (3.3) |
| Other TEAEs of interest | ||||
| Fall | 2 (1.7) | 4 (1.8) | 2 (2.7) | 2 (1.3) |
| Akathisia | 0 | 2 (0.9) | 0 | 2 (1.3) |
| Extrapyramidal disorder | 0 | 2 (0.9) | 1 (1.4) | 1 (0.7) |
| Hip fracture | 1 (0.9) | 1 (0.4) | 0 | 1 (0.7) |
| Sedation | 0 | 1 (0.4) | 0 | 1 (0.7) |
Abbreviation: TEAE, treatment-emergent adverse event.
Hip fracture, positive SARS-CoV-2 test result, and psychotic disorder.
Urinary tract infection (n = 2), cardiac failure, COVID-19, pneumonia, fall, hip fracture, cachexia, dehydration, metabolic acidosis, mental status changes, acute kidney injury, and hypertension (some patients reported >1).
Positive SARS-CoV-2 test result (n = 2), agitation, psychotic disorder, and hypertensive crisis.
Asthenia (n = 2), anemia, COVID-19, pneumonia, urinary tract infection, viral infection, fall, hip fracture, alanine aminotransferase increased, aspartate aminotransferase increased, blood alkaline phosphatase increased, blood pressure increased, dehydration, metabolic acidosis, akathisia, dizziness, somnolence, hallucination, insomnia, mental status changes, acute kidney injury, and respiratory disorder (some patients reported >1).
Cardiac failure.
One patient died during the trial, in the brexpiprazole, 3 mg, subgroup, of cardiac failure (age, 78 years). The patient withdrew from the trial after 28 days because of the adverse event of hallucinations. The patient also had serious adverse events of pneumonia and cachexia. Death occurred 23 days after the last dose of brexpiprazole. An autopsy revealed coronary atherosclerosis, and the death was considered unrelated to brexpiprazole.
Mean (SD) increase in body weight from baseline to week 12 was 0.3 (2.8) kg in the brexpiprazole, 2 or 3 mg, group (n = 196) and 0.0 (2.0) kg in the placebo group (n = 104). At week 12, weight gain of 7% or more from baseline was experienced by 3 of 196 patients (1.5%) in the brexpiprazole, 2 or 3 mg, group and 0 of 104 in the placebo group; the corresponding values for weight loss of 7% or more were 2 of 196 (1.0%) and 1 of 104 (1.0%).
No patients reported TEAEs of suicidal ideation or behavior during the trial. Mean (SD) change in MMSE score from baseline to week 12 was 0.7 (2.8) in the brexpiprazole, 2 or 3 mg, group (n = 192) and 0.4 (2.1) in the placebo group (n = 103). There were no clinically meaningful between-group mean differences in laboratory test parameters, vital signs, or electrocardiograms, and extrapyramidal symptom rating scale score changes were minimal (eTable 3 in Supplement 2).
Discussion
In patients with agitation in Alzheimer dementia, treatment with brexpiprazole (fixed doses of 2 or 3 mg) resulted in statistically significant greater improvements vs placebo in CMAI total score (primary end point) and CGI-S score as related to agitation (key secondary end point), supported by all other secondary efficacy end points. CMAI total score changes indicated an overall reduction in the frequency of agitated behaviors, and CMAI factor score changes indicated improvement of 3 distinct types of agitated behavior: aggressive, physically nonaggressive (excessive motor activity), and verbally agitated.19,20,22,28 At the individual patient level, responder analyses (secondary end points) suggested that brexpiprazole treatment may be clinically meaningful: first, a greater proportion of patients receiving brexpiprazole than placebo achieved a CGI-I score of 1, very much improved, or 2, much improved (52.4% vs 40.5%), which are widely regarded as clinically meaningful outcomes. Second, a greater proportion of patients receiving brexpiprazole than placebo achieved a meaningful within-patient change in CMAI total score of −20 points (57.2% vs 36.9% in a post hoc analysis); this meaningful within-patient change was determined by anchor- and distribution-based methods and approximately corresponds to a 2-point improvement in CGI-S score and a score of 2 on the CGI-I.29
This study was powered for analyses of the modified intention-to-treat (efficacy) sample and not for analyses of individual brexpiprazole doses or subgroups, the results from which should be interpreted with caution. Exploratory analyses by dose found no clinically relevant differences in efficacy between brexpiprazole, 2 and 3 mg. Results of subgroup analyses were generally consistent with the primary analysis. In the analysis by region, brexpiprazole response was consistent in the United States and Europe; however, there was an unexpectedly high placebo response in the United States with no discernible reason, which was not observed in a previous phase 3 trial of fixed-dose brexpiprazole.14,30
Two previous randomized phase 3 trials have investigated brexpiprazole for the treatment of agitation in Alzheimer dementia.14 In a fixed-dose trial, brexpiprazole, 2 mg (but not 1 mg), showed significant improvement vs placebo on CMAI total score (least-squares mean difference [LSMD], −3.77; P = .04; effect size, 0.25). In a flexible-dose trial, brexpiprazole, 0.5 mg to 2 mg, was not significantly different from placebo at week 12 (LSMD, −2.34; P = .15; effect size, 0.18); a post hoc analysis of patients titrated to 2 mg showed nominally significant separation vs placebo (LSMD, −5.06; P = .01; effect size, 0.41),14 with a comparable drug-placebo difference to the current study (LSMD, −5.32; P = .003; effect size, 0.35). Thus, observations from prior trials suggested that brexpiprazole, 2 mg, was an efficacious dose in patients with agitation in Alzheimer dementia. The current trial provides further evidence for efficacy of the 2-mg dose, as well as support for the 3-mg dose; the trial design does not allow for clinical recommendations on whether patients not responding to 2 mg might respond to 3 mg. An evaluation for reversible factors (such as pain) and a trial of nonpharmacological therapy should be performed before administration of brexpiprazole for agitation.
The trial reported here differed from the previous 2 trials in that it required patients to meet the International Psychogeriatric Association definition of agitation at entry, which was not available at the time of the previous trials.1,14 Furthermore, a post hoc analysis of the first 2 trials indicated that patients who did not meet the CMAI factor 1 (aggressive behavior) positivity criterion at baseline had insufficient baseline agitation severity to show measurable change over time.31 Hence, the present trial recruited an enriched sample who met the CMAI factor 1 positivity criterion at baseline, resulting in higher baseline CMAI total scores (79-81, depending on treatment group) than the previous trials (64-72),14 and a broad range of agitation symptoms across the CMAI factors. This enrichment may have enhanced signal detection (as shown by a higher LSMD in the current trial) and is likely to reflect the population of patients with Alzheimer dementia who require treatment for agitation in clinical practice.
Based on safety and tolerability data from the first 2 trials, the current trial used a faster titration schedule than the previous trials, reaching the 2-mg dose after 2 weeks as opposed to 4 weeks.14 Faster titration did not appear to affect the safety or tolerability of brexpiprazole in the current trial (with similar rates of discontinuation due to adverse events between trials) or the time point at which brexpiprazole, 2 mg, first separated from placebo on CMAI total score (week 8 in the present trial compared with week 6 or week 12 in the previous trials).
Brexpiprazole acts as an antagonist at noradrenergic α1B and α2C and serotonergic 5-HT2A receptors, as well as a partial agonist at 5-HT1A and dopaminergic D2 receptors, all with subnanomolar affinity.12 Thus, brexpiprazole acts on multiple receptors in the brain related to agitation, aggression, impulsiveness, arousal, and psychosis.13,32,33,34 Brexpiprazole has a moderate affinity for histamine H1 receptors, meaning that its effects on agitation are unlikely to be due to sedation.12,35
Comparing the efficacy of different antipsychotics for the treatment of agitation in Alzheimer dementia is challenging because of differences in trial design (including duration), study population (different settings, forms of dementia, and target symptom: psychosis or agitation), and outcome measure (BEHAVE-AD, NPI, CMAI, etc).36 A Cochrane review of these diverse trials concluded that atypical antipsychotics as a group (including olanzapine, quetiapine, and risperidone) have a small effect on agitation in dementia (standardized mean difference, −0.21).36
Since 2005, US prescribing information for atypical antipsychotics contains a boxed warning for increased risk of cerebrovascular events and mortality in elderly patients with dementia-related psychosis, supported by meta-analyses of data from randomized placebo-controlled trials.37,38,39,40 There were no cerebrovascular TEAEs in the current trial; however, patients and clinicians should be aware of this possible risk. Seven patients died during the 3 randomized phase 3 trials: 6 (0.9%) in the brexpiprazole groups and 1 (0.3%) in the placebo groups; these deaths were considered unrelated to brexpiprazole treatment by the investigators.31 Other warnings in the US prescribing information include neuroleptic malignant syndrome, tardive dyskinesia, orthostatic hypotension, syncope, and seizures (none of which were observed in this trial, although 2.7% of patients receiving brexpiprazole reported dizziness), and falls/fractures40 (a general concern with atypical antipsychotics in elderly patients,41 but which had placebo-level incidence in this trial). Somnolence, sedation, extrapyramidal symptoms, and urinary tract infection, which are associated with atypical antipsychotic treatment in elderly patients with dementia,10,42 had a low incidence with brexpiprazole (all <5%), albeit higher than placebo. Finally, whereas certain atypical antipsychotics have been associated with an increased risk of cognitive decline,43 mean MMSE score changes on brexpiprazole suggested that there was no worsening of cognition over 12 weeks. Overall, while the total incidence of TEAEs was higher with brexpiprazole than placebo, the rate of discontinuation due to adverse events was low and comparable between groups, and no new safety concerns were raised by observations in this trial.
Limitations
Standard of care for Alzheimer disease may differ between countries, which is difficult to account for in a multinational trial.44 The CMAI is completed based on information from caregivers, who may observe different behaviors according to their level of caregiving experience and the amount of time spent with the patient per day. A larger number of study sites was needed than originally anticipated (some sites did not recruit any patients and other sites were classified as small) due to the difficulty in recruiting patients with agitation in Alzheimer disease and due to the COVID-19 pandemic. The exclusion of patients with certain comorbidities and restrictions on concomitant therapy could limit the generalizability of the findings. The sample was predominantly White, and care should be taken when extrapolating tolerability data to other races. The trial did not include a measure of patient functioning. Finally, this study had limited duration of treatment, and longer-term efficacy and safety data are needed, particularly with regard to cognition and mortality; an extension trial providing data on longer-term brexpiprazole treatment has recently been completed (ClinicalTrials.gov identifier: NCT03594123).
Conclusions
Treatment of agitation is essential to increase the comfort, quality of life, and safety of patients with Alzheimer dementia; to ease the burden on their caregivers; and to allow patients to live at home longer. In this 12-week clinical trial, brexpiprazole, 2 or 3 mg, showed a statistically significant improvement vs placebo on agitation in patients with Alzheimer dementia. Brexpiprazole was generally well tolerated over 12 weeks in this vulnerable patient population. Overall, brexpiprazole, 2 or 3 mg, appears to have a favorable benefit/risk profile in the treatment of agitation in Alzheimer dementia. Based on the results of this trial, together with a previous trial, brexpiprazole was approved in the United States for the treatment of agitation associated with dementia due to Alzheimer disease.
Trial Protocol and Statistical Analysis Plan
eMethods. Statistical Analysis
eReferences
eFigure 1. Study Design
eFigure 2. Patient Disposition by Dose
eFigure 3. CMAI Total Score and CGI-S Score as Related to Agitation (Efficacy Sample)
eFigure 4. Change From Baseline in CMAI Total Score at Week 12 by Subgroup (Efficacy Sample)
eFigure 5. Change From Baseline in CMAI Total Score and CGI-S Score as Related to Agitation by Dose (Exploratory Analysis, Efficacy Sample)
eTable 1. List of Principal Investigators and Trial Sites
eTable 2. CMAI Total Score Sensitivity Analysis – MNAR Using Pattern Mixture Model With Multiple Imputation and Dropout Due to Adverse Event or Withdrawal By Patient as MNAR (Efficacy Sample)
eTable 3. Suicidality, EPS, Cognitive Dysfunction, Body Weight, Metabolic Parameters, and QT Interval (Safety Sample)
Data Sharing Statement
References
- 1.Sano M, Cummings J, Auer S, et al. Agitation in cognitive disorders: progress in the International Psychogeriatric Association consensus clinical and research definition. Int Psychogeriatr. 2023:1-13. doi: 10.1017/S1041610222001041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Halpern R, Seare J, Tong J, Hartry A, Olaoye A, Aigbogun MS. Using electronic health records to estimate the prevalence of agitation in Alzheimer disease/dementia. Int J Geriatr Psychiatry. 2019;34(3):420-431. doi: 10.1002/gps.5030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fillit H, Aigbogun MS, Gagnon-Sanschagrin P, et al. Impact of agitation in long-term care residents with dementia in the United States. Int J Geriatr Psychiatry. 2021;36(12):1959-1969. doi: 10.1002/gps.5604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Okura T, Plassman BL, Steffens DC, Llewellyn DJ, Potter GG, Langa KM. Prevalence of neuropsychiatric symptoms and their association with functional limitations in older adults in the United States: the Aging, Demographics, and Memory Study. J Am Geriatr Soc. 2010;58(2):330-337. doi: 10.1111/j.1532-5415.2009.02680.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khoo SA, Chen TY, Ang YH, Yap P. The impact of neuropsychiatric symptoms on caregiver distress and quality of life in persons with dementia in an Asian tertiary hospital memory clinic. Int Psychogeriatr. 2013;25(12):1991-1999. doi: 10.1017/S1041610213001518 [DOI] [PubMed] [Google Scholar]
- 6.Okura T, Langa KM. Caregiver burden and neuropsychiatric symptoms in older adults with cognitive impairment: the Aging, Demographics, and Memory Study (ADAMS). Alzheimer Dis Assoc Disord. 2011;25(2):116-121. doi: 10.1097/WAD.0b013e318203f208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cloutier M, Gauthier-Loiselle M, Gagnon-Sanschagrin P, et al. Institutionalization risk and costs associated with agitation in Alzheimer’s disease. Alzheimers Dement (N Y). 2019;5:851-861. doi: 10.1016/j.trci.2019.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Antonsdottir IM, Smith J, Keltz M, Porsteinsson AP. Advancements in the treatment of agitation in Alzheimer’s disease. Expert Opin Pharmacother. 2015;16(11):1649-1656. doi: 10.1517/14656566.2015.1059422 [DOI] [PubMed] [Google Scholar]
- 9.Aigbogun MS, Cloutier M, Gauthier-Loiselle M, et al. Real-world treatment patterns and characteristics among patients with agitation and dementia in the United States: findings from a large, observational, retrospective chart review. J Alzheimers Dis. 2020;77(3):1181-1194. doi: 10.3233/JAD-200127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yunusa I, Alsumali A, Garba AE, Regestein QR, Eguale T. Assessment of reported comparative effectiveness and safety of atypical antipsychotics in the treatment of behavioral and psychological symptoms of dementia: a network meta-analysis. JAMA Netw Open. 2019;2(3):e190828. doi: 10.1001/jamanetworkopen.2019.0828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reus VI, Fochtmann LJ, Eyler AE, et al. The American Psychiatric Association practice guideline on the use of antipsychotics to treat agitation or psychosis in patients with dementia. Am J Psychiatry. 2016;173(5):543-546. doi: 10.1176/appi.ajp.2015.173501 [DOI] [PubMed] [Google Scholar]
- 12.Maeda K, Sugino H, Akazawa H, et al. Brexpiprazole I: in vitro and in vivo characterization of a novel serotonin-dopamine activity modulator. J Pharmacol Exp Ther. 2014;350(3):589-604. doi: 10.1124/jpet.114.213793 [DOI] [PubMed] [Google Scholar]
- 13.Liu KY, Stringer AE, Reeves SJ, Howard RJ. The neurochemistry of agitation in Alzheimer’s disease: a systematic review. Ageing Res Rev. 2018;43:99-107. doi: 10.1016/j.arr.2018.03.003 [DOI] [PubMed] [Google Scholar]
- 14.Grossberg GT, Kohegyi E, Mergel V, et al. Efficacy and safety of brexpiprazole for the treatment of agitation in Alzheimer’s dementia: two 12-week, randomized, double-blind, placebo-controlled trials. Am J Geriatr Psychiatry. 2020;28(4):383-400. doi: 10.1016/j.jagp.2019.09.009 [DOI] [PubMed] [Google Scholar]
- 15.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939-944. doi: 10.1212/WNL.34.7.939 [DOI] [PubMed] [Google Scholar]
- 16.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198. doi: 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- 17.Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44(12):2308-2314. doi: 10.1212/WNL.44.12.2308 [DOI] [PubMed] [Google Scholar]
- 18.Wood S, Cummings JL, Hsu MA, et al. The use of the neuropsychiatric inventory in nursing home residents: characterization and measurement. Am J Geriatr Psychiatry. 2000;8(1):75-83. doi: 10.1097/00019442-200002000-00010 [DOI] [PubMed] [Google Scholar]
- 19.Cohen-Mansfield J. Instruction Manual for the Cohen-Mansfield Agitation Inventory (CMAI). Research Institute of the Hebrew Home of Greater Washington; 1991. [Google Scholar]
- 20.Rabinowitz J, Davidson M, De Deyn PP, Katz I, Brodaty H, Cohen-Mansfield J. Factor analysis of the Cohen-Mansfield Agitation Inventory in three large samples of nursing home patients with dementia and behavioral disturbance. Am J Geriatr Psychiatry. 2005;13(11):991-998. doi: 10.1097/00019442-200511000-00010 [DOI] [PubMed] [Google Scholar]
- 21.Brodaty H, Ames D, Snowdon J, et al. A randomized placebo-controlled trial of risperidone for the treatment of aggression, agitation, and psychosis of dementia. J Clin Psychiatry. 2003;64(2):134-143. doi: 10.4088/JCP.v64n0205 [DOI] [PubMed] [Google Scholar]
- 22.Cohen-Mansfield J, Marx MS, Rosenthal AS. A description of agitation in a nursing home. J Gerontol. 1989;44(3):M77-M84. doi: 10.1093/geronj/44.3.M77 [DOI] [PubMed] [Google Scholar]
- 23.Finkel SI, Lyons JS, Anderson RL. Reliability and validity of the Cohen-Mansfield Agitation Inventory in institutionalized elderly. Int J Geriatr Psychiatry. 1992;7(7):487-490. doi: 10.1002/gps.930070706 [DOI] [Google Scholar]
- 24.Guy W. ECDEU Assessment Manual for Psychopharmacology, Revised. National Institute of Mental Health; 1976. [Google Scholar]
- 25.Coric V, Stock EG, Pultz J, Marcus R, Sheehan DV. Sheehan Suicidality Tracking Scale (Sheehan-STS): preliminary results from a multicenter clinical trial in generalized anxiety disorder. Psychiatry (Edgmont). 2009;6(1):26-31. [PMC free article] [PubMed] [Google Scholar]
- 26.Simpson GM, Angus JWS. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand Suppl. 1970;212:11-19. doi: 10.1111/j.1600-0447.1970.tb02066.x [DOI] [PubMed] [Google Scholar]
- 27.Barnes TRE. A rating scale for drug-induced akathisia. Br J Psychiatry. 1989;154:672-676. doi: 10.1192/bjp.154.5.672 [DOI] [PubMed] [Google Scholar]
- 28.Cohen-Mansfield J, Marx MS, Werner P. Agitation in elderly persons: an integrative report of findings in a nursing home. Int Psychogeriatr. 1992;4(suppl 2):221-240. doi: 10.1017/S1041610292001285 [DOI] [PubMed] [Google Scholar]
- 29.Oberdhan D, Meunier J, Loubert A, Creel K, Larsen KG, Aggarwal J. Agitation associated with dementia due to Alzheimer’s disease: defining a meaningful within-patient change threshold for the Cohen-Mansfield Agitation Inventory (CMAI). Poster presented at 2023 ASCP Annual Meeting; May 30–June 2, 2023; Miami Beach, FL. Accessed July 3, 2023. https://ascpp.org/ascp-meetings/ascp-annual-meeting/
- 30.Otsuka Pharmaceutical Co . Brexpiprazole for the treatment of agitation associated with Alzheimer’s dementia. Sponsor briefing document; FDA Psychopharmacologic Drugs Advisory Committee and Peripheral and Central Nervous System Drugs Advisory Committee; April 14, 2023. Accessed May 11, 2023. https://www.fda.gov/media/167068/download
- 31.Grossberg GT, Lee D, Slomkowski M, et al. Tackling agitation in Alzheimer’s dementia: brexpiprazole Phase III trial results. J Prev Alzheimers Dis. 2022;9(suppl 1):S10-S11. doi: 10.14283/jpad.2022.96 [DOI] [Google Scholar]
- 32.Centenaro LA, Vieira K, Zimmermann N, Miczek KA, Lucion AB, de Almeida RMM. Social instigation and aggressive behavior in mice: role of 5-HT1A and 5-HT1B receptors in the prefrontal cortex. Psychopharmacology (Berl). 2008;201(2):237-248. doi: 10.1007/s00213-008-1269-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Puig MV, Gulledge AT. Serotonin and prefrontal cortex function: neurons, networks, and circuits. Mol Neurobiol. 2011;44(3):449-464. doi: 10.1007/s12035-011-8214-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gannon M, Wang Q. Complex noradrenergic dysfunction in Alzheimer’s disease: low norepinephrine input is not always to blame. Brain Res. 2019;1702:12-16. doi: 10.1016/j.brainres.2018.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miller DD. Atypical antipsychotics: sleep, sedation, and efficacy. Prim Care Companion J Clin Psychiatry. 2004;6(suppl 2):3-7. [PMC free article] [PubMed] [Google Scholar]
- 36.Mühlbauer V, Möhler R, Dichter MN, Zuidema SU, Köpke S, Luijendijk HJ. Antipsychotics for agitation and psychosis in people with Alzheimer’s disease and vascular dementia. Cochrane Database Syst Rev. 2021;12(12):CD013304. doi: 10.1002/14651858.CD013304.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jeste DV, Blazer D, Casey D, et al. ACNP White Paper: update on use of antipsychotic drugs in elderly persons with dementia. Neuropsychopharmacology. 2008;33(5):957-970. doi: 10.1038/sj.npp.1301492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. JAMA. 2005;294(15):1934-1943. doi: 10.1001/jama.294.15.1934 [DOI] [PubMed] [Google Scholar]
- 39.Schneider LS, Dagerman K, Insel PS. Efficacy and adverse effects of atypical antipsychotics for dementia: meta-analysis of randomized, placebo-controlled trials. Am J Geriatr Psychiatry. 2006;14(3):191-210. doi: 10.1097/01.JGP.0000200589.01396.6d [DOI] [PubMed] [Google Scholar]
- 40.Otsuka Pharmaceutical Co . Rexulti® (brexpiprazole) tablets, for oral use. Prescribing information [United States]. May 2023. Accessed July 3, 2023. https://www.otsuka-us.com/media/static/Rexulti-PI.pdf
- 41.Fraser LA, Liu K, Naylor KL, et al. Falls and fractures with atypical antipsychotic medication use: a population-based cohort study. JAMA Intern Med. 2015;175(3):450-452. doi: 10.1001/jamainternmed.2014.6930 [DOI] [PubMed] [Google Scholar]
- 42.Ma H, Huang Y, Cong Z, et al. The efficacy and safety of atypical antipsychotics for the treatment of dementia: a meta-analysis of randomized placebo-controlled trials. J Alzheimers Dis. 2014;42(3):915-937. doi: 10.3233/JAD-140579 [DOI] [PubMed] [Google Scholar]
- 43.Vigen CLP, Mack WJ, Keefe RS, et al. Cognitive effects of atypical antipsychotic medications in patients with Alzheimer’s disease: outcomes from CATIE-AD. Am J Psychiatry. 2011;168(8):831-839. doi: 10.1176/appi.ajp.2011.08121844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cummings J, Reynders R, Zhong K. Globalization of Alzheimer’s disease clinical trials. Alzheimers Res Ther. 2011;3(4):24. doi: 10.1186/alzrt86 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol and Statistical Analysis Plan
eMethods. Statistical Analysis
eReferences
eFigure 1. Study Design
eFigure 2. Patient Disposition by Dose
eFigure 3. CMAI Total Score and CGI-S Score as Related to Agitation (Efficacy Sample)
eFigure 4. Change From Baseline in CMAI Total Score at Week 12 by Subgroup (Efficacy Sample)
eFigure 5. Change From Baseline in CMAI Total Score and CGI-S Score as Related to Agitation by Dose (Exploratory Analysis, Efficacy Sample)
eTable 1. List of Principal Investigators and Trial Sites
eTable 2. CMAI Total Score Sensitivity Analysis – MNAR Using Pattern Mixture Model With Multiple Imputation and Dropout Due to Adverse Event or Withdrawal By Patient as MNAR (Efficacy Sample)
eTable 3. Suicidality, EPS, Cognitive Dysfunction, Body Weight, Metabolic Parameters, and QT Interval (Safety Sample)
Data Sharing Statement