This cohort study investigates the timing of neuroaxonal pathology in individuals with multiple sclerosis who experience disability worsening.
Key Points
Question
When does neuroaxonal pathology occur in people with multiple sclerosis (MS) who experience disability worsening?
Findings
In this cohort study including 1899 individuals, serum neurofilament light chain elevation, as a sign of accelerated neuroaxonal injury, was detected approximately 1 year preceding disability worsening events associated with relapses and 1 to 2 years before worsening events independent of clinical relapses.
Meaning
Pronounced neuroaxonal damage precedes disability worsening events with or without preceding clinical relapses in people with MS, providing novel insights into the mechanisms contributing to accumulated worsening, their timing, and defining a potential window of dynamic central nervous system pathology that can be targeted with abortive therapies.
Abstract
Importance
Mechanisms contributing to disability accumulation in multiple sclerosis (MS) are poorly understood. Blood neurofilament light chain (NfL) level, a marker of neuroaxonal injury, correlates robustly with disease activity in people with MS (MS); however, data on the association between NfL level and disability accumulation have been conflicting.
Objective
To determine whether and when NfL levels are elevated in the context of confirmed disability worsening (CDW).
Design, Setting, and Participants
This study included 2 observational cohorts: results from the Expression, Proteomics, Imaging, Clinical (EPIC) study at the University of California San Francisco (since 2004) were confirmed in the Swiss Multiple Sclerosis Cohort (SMSC), a multicenter study in 8 centers since 2012. Data were extracted from EPIC in April 2022 (sampling July 1, 2004, to December 20, 2016) and SMSC in December 2022 (sampling June 6, 2012, to September 2, 2021). The study included 2 observational cohorts in tertiary MS centers. All participants of both cohorts with available NfL results were included in the study, and no eligible participants were excluded or declined to participate.
Exposure
Association between NfL z scores and CDW.
Main Outcome Measures
CDW was defined as Expanded Disability Status Scale (EDSS) worsening that was confirmed after 6 or more months and classified into CDW associated with clinical relapses (CDW-R) or independent of clinical relapses (CDW-NR). Visits were classified in relation to the disability worsening events into CDW(−2) for 2 visits preceding event, CDW(−1) for directly preceding event, CDW(event) for first diagnosis of EDSS increase, and the confirmation visit. Mixed linear and Cox regression models were used to evaluate NfL dynamics and to assess the association of NfL with future CDW, respectively.
Results
A total of 3906 EPIC visits (609 participants; median [IQR] age, 42.0 [35.0-50.0] years; 424 female [69.6%]) and 8901 SMSC visits (1290 participants; median [IQR] age, 41.2 [32.5-49.9] years; 850 female [65.9%]) were included. In CDW-R (EPIC, 36 events; SMSC, 93 events), NfL z scores were 0.71 (95% CI, 0.35-1.07; P < .001) units higher at CDW-R(−1) in EPIC and 0.32 (95% CI, 0.14-0.49; P < .001) in SMSC compared with stable MS samples. NfL elevation could be detected preceding CDW-NR (EPIC, 191 events; SMSC, 342 events) at CDW-NR(−2) (EPIC: 0.23; 95% CI, 0.01-0.45; P = .04; SMSC: 0.28; 95% CI, 0.18-0.37; P < .001) and at CDW-NR(−1) (EPIC: 0.27; 95% CI, 0.11-0.44; P < .001; SMSC: 0.09; 95% CI, 0-0.18; P = .06). Those findings were replicated in the subgroup with relapsing-remitting MS. Time-to-event analysis confirmed the association between NfL levels and future CDW-R within approximately 1 year and CDW-NR (in approximately 1-2 years).
Conclusions and Relevance
This cohort study documents the occurrence of NfL elevation in advance of clinical worsening and may hint to a potential window of ongoing dynamic central nervous system pathology that precedes the diagnosis of CDW.
Introduction
Diagnosing and treating disease progression is a critical unmet need in the care of people with multiple sclerosis (MS). Body fluid biomarkers hold the potential to assist in disentangling the complex interplay between neuronal, glial, and immune cells that ultimately cause irreversible disease progression. Neurofilament light chain (NfL) is a neuronal protein whose concentration in the blood of people with MS rises with neuroaxonal injury.1 NfL levels correlate with disease activity and treatment response: blood levels are higher during recent relapse and magnetic resonance imaging (MRI) activity and are lowered by treatment with disease-modifying treatments (DMTs)2,3 and remyelinating agents, such as clemastine.4 The monoclonal antibodies currently used in many assays (eg, Simoa) specifically detect NfL fragments related to neuroaxonal damage, thereby supporting the use of NfL as a surrogate marker of neurodegeneration.5
The link between NfL levels and confirmed disability worsening (CDW) in MS is poorly understood and partly contradictory.6,7,8,9,10 Evaluated populations differed regarding disease course (relapsing vs progressive), setting (clinical trials vs real-world population), definition of disability progression (single or combined outcome parameters), and most importantly, timing of NfL assessment (at baseline or longitudinally). Limited sample size and lack of sufficient quantitation of disease activity may be additional critical factors leading to discordant findings in the context of CDW.
In the present study, the association between longitudinal NfL measurements and different clinical types of CDW was evaluated in 2 deeply curated, long-term, real-world prospective MS cohorts: the Expression, Proteomics, Imaging, Clinical (EPIC) study at the University of California San Francisco (UCSF)11,12,13 and the Swiss Multiple Sclerosis Cohort (SMSC).2,14 Longitudinal dynamics of serum NfL levels were evaluated in association with CDW, with and without clinical relapses. In addition, we investigated the temporal association between NfL measurements and the risk of future disability worsening.
Methods
Cohort Description
The UCSF MS EPIC study prospectively conducted yearly evaluations (years 1-5 and 8-10, with optional visits at years 6-7) of people with MS. The SMSC was initiated in 2012 and is a prospective multicenter cohort study performed across 8 Swiss MS centers.2,14 Both studies included people with MS with all disease courses (clinically isolated syndrome, relapsing-remitting, primary- or secondary-progressive MS) regardless of age, Expanded Disability Status Scale (EDSS) score, or treatment status. The exclusion criteria were limited to patients who were not able to complete the standard study procedures. In both studies, visits covered basic demographics (included age, biological sex, and the following self-identified races and ethnicities: African American, Asian, Hispanic, White, and other), relapse history, disease course, DMT status, EDSS score evaluation by neurostatus-certified raters, blood sample collection, and MRI scans.11,12 The detailed study protocols are reported elsewhere.13,14 Visit scheduling was based on the corresponding study protocols. Unscheduled visits, eg, due to acute disease activity, are not considered study-specific and were not part of this analysis. Data were extracted from EPIC in April 2022 and SMSC in December 2022. The analysis included only visits with available NfL values. Both studies were approved by corresponding ethics committees. All participants signed written informed consent. Results were reported according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines.
NfL Measurement and Generation of NfL z Scores
In the EPIC study, serum NfL values were measured using a Simoa assay described previously.11,15 Age-adjusted NfL z scores were calculated using an assay-specific reference database of 485 healthy control samples from the Genome-Wide Association Study of Multiple Sclerosis (GeneMSA) study.15,16 In the SMSC, NfL concentrations were measured by NF-light Advantage kit (Quanterix) and age- and body mass index (BMI)–adjusted z scores were generated.2 NfL z scores are a measure of deviation from values observed in healthy controls: eg, an NfL z score of 1 means that the NfL concentration deviates by 1 SD from values in the reference database (84.1th percentile) adjusted for relevant physiological factors.
MRI
In the EPIC study, brain MRI scans were acquired on the 3-T GE scanner (GE Medical Systems) with standardized head positioning and pulse sequences that included high-resolution T1-weighted volume, with and without gadolinium-labeled diethylenetriamine pentaacetic acid, and T2-weighted images. The T2- and T1-weighted images were used to determine MS lesion borders using semiautomated lesion segmentation software (Amira [FEI] and Lesion Segmentation Toolbox [Structural Brain Mapping Group]). Brain MRI scans were performed annually in the SMSC. A standardized imaging protocol was applied across centers, including a 3-dimensional (3-D) magnetization-prepared rapid gradient echo (MPRAGE), a 3-D fluid-attenuated inversion recovery (FLAIR) sequence, and a postcontrast T1 sequence acquired at a spatial resolution of 1 mm3. T2-weighted lesion volume was automatically assessed using a deep learning–based approach17 and a longitudinal evaluation method,18 respectively, followed by manual quality assessment and correction. The number of contrast-enhancing lesions (CELs) was assessed manually. In this work, MRI data were included only when data regarding new or enlarged T2 lesions and CELs were available.
Definition of CDW and Related Visits
CDW was defined by applying EDSS step changes of at least 1.5, 1, and 0.5 points from reference EDSS scores of 0, 1 to 5.5, and greater than 5.5, respectively,12 confirmed in the subsequent visit (6/12 months in SMSC or 12 months in EPIC). In EPIC, EDSS worsening events at year 5 were confirmed at the next available visit (years 6-10). Recurrent events were identified using the roving reference EDSS approach.19 Relapses were defined as new, worsening, or recurrent neurologic symptoms that lasted for at least 24 hours in the absence of an infection, fever, or adverse reaction to a prescribed medication.20 CDW events were classified based on the presence of relapse into the following groups: CDW-NR, ie, no relapses between the EDSS reference visit and EDSS worsening event visit (CDW[event]) and CDW-R, ie, the presence of a relapse between reference visit and CDW(event). Visits related to the CDW(event) were classified into the following: a visit 2 encounters preceding the event, or CDW(−2), a visit directly preceding the event, or CDW(−1), and the consecutive confirmation visit, or CDW(+1). Samples collected outside the period between CDW(−2) and CDW(+1) or from participants without events over the entire follow-up and without relapses in the last year were assigned to the control group (stable MS[stMS]). CDW(event) visits had the highest classification priority in case of 2 consecutive events of the same pattern (ie, when they overlapped with CDW[−1] or CDW[−2] visits). The timing of visits in relation to the CDW(event) in the 2 cohorts is detailed in eFigure 1 in Supplement 1. Although the mean duration between CDW(−1) and CDW(−2) to the event was similar in both cohorts, the distribution of these intervals revealed a bimodal form for SMSC visits because in approximately one-third of the participants, visits were every 6 months.
Statistical Analysis
Summary statistics were applied to describe the different variables using median with IQR for continuous variables and frequencies (percentages) for categorical variables. Linear mixed-effects models, with a random intercept per participant, were used to evaluate the association between NfL z score (dependent variable) and CDW status of the visit (CDW[−2] to CDW[+1] vs stMS) adjusting for the following factors at sampling: sex, age at sampling, relapse within the last 90 days, treatment category (monoclonal antibodies [alemtuzumab, natalizumab, ocrelizumab, ofatumumab, rituximab], oral DMTs [cladribine, dimethyl fumarate, fingolimod, ozanimod, siponimod, teriflunomide], platform DMT [interferon beta, glatiramer acetate]), untreated disease course (progressive vs relapsing), and EDSS at sampling. BMI was added as an additional covariable in the EPIC cohort models, as the applied NfL z scores were only adjusted for age.
Next, the risk for future CDW based on NfL z score (recurrent CDW events and CDW-R and CDW-NR as competing events) was investigated using Cox regression models. In these analyses, NfL z score was used as a time-varying covariate (continuous or dichotomized based on defined z score cutoff 1 (ie, 84th percentile) estimating the risk of occurrence of CDW events at the next visit adjusting for age, BMI (in the EPIC cohort only), sex, EDSS at baseline, treatment category, disease subtype (relapsing vs progressive), and recent relapses (≤90 days) at sampling. NfL z score was thereby used as a time-varying covariate along the follow-up either using NfL z score at the current visit (ie, CDW[−1]) or at the previous visit (ie, CDW[−2]) resulting in estimates that reflect the hazard of CDW at the next visit, or 2 visits after, respectively. All analyses were performed in the EPIC cohort and the SMSC using SPSS, version 29 (IBM Corp) and R, version 4.1.2 (R Project for Statistical Computing). The analyses were performed separately in the EPIC study and the SMSC (authors A.A. and P.B./S.S., respectively), mutually confirming the results.
Results
Cohort Description
Baseline demographic and clinical characteristics are described in Table 1. NfL measurements were available for 3906 EPIC visits (609 participants; median [IQR] age, 42.0 [35.0-50.0] years; 424 female [69.6%]; 185 male [30.4%]), and 8901 SMSC visits (1290 participants; median [IQR] age, 41.2 [32.5-49.9] years; 850 female [65.9%]; 440 male [34.1%]). Participants self-identified with the following race and ethnicity categories in the EPIC cohort: 2 African American (0.3%), 2 Asian (0.3%), 22 Hispanic (3.6%) 582 White (95.6%), and 1 other race and ethnicity (0.2%). Participants self-identified with the following race and ethnicity categories in the SMSC: 4 African American (0.3%), 4 Asian (0.3%), 10 Hispanic (0.8%), 1265 White (98.1%), and 7 other race and ethnicity (0.5%). A total of 227 CDW events were seen in the EPIC cohort (CDW-R: 36 events; CDW-NR: 191 events) and 435 in the SMSC (CDW-R: 93 events; CDW-NR: 342 events). The majority of CDW events were sustained (ie, longitudinal EDSS scores remained higher than reference EDSS scores); 80.6% (29 of 36) and 90.3% (84 of 93) of CDW-R as well as 75.4% (144 of 191) and 88.0% (301 of 342) of CDW-NR events in the EPIC cohort and the SMSC, respectively. Most events were driven by worsening in more than 1 EDSS functional score (77.8% [28 of 36] for CDW-R and 89.0% [170 of 191] for CDW-NR in the EPIC cohort and 86.0% [80 of 93] and 81.9% [280 of 342] in the SMSC).
Table 1. Baseline and Follow-Up Characteristics of the Expression, Proteomics, Imaging, Clinical (EPIC) Study and Swiss Multiple Sclerosis Cohort (SMSC)a.
| Characteristic | EPIC | SMSC | P valueb |
|---|---|---|---|
| No. of participants | 609 | 1290 | NA |
| Age | 42.0 (35.0-50.0) | 41.2 (32.5-49.9) | .04 |
| Sex, No. (%) | |||
| Female | 424 (69.6) | 850 (65.9) | .11 |
| Male | 185 (30.4) | 440 (34.1) | |
| Race and ethnicity, No. (%) | |||
| African American | 2 (0.3) | 4 (0.3) | <.001 |
| Asian | 2 (0.3) | 4 (0.3) | |
| Hispanic | 22 (3.6) | 10 (0.8) | |
| White | 582 (95.6) | 1265 (98.1) | |
| Other | 1 (0.2) | 7 (0.5) | |
| EDSS | 1.5 (1.0-3.0) | 2.0 (1.5-3.5) | <.001 |
| RMS, No. (%) | 530 (87.0) | 1160 (89.9) | .06 |
| Disease duration, y | 6.0 (2.0-13.0) | 7.0 (2.1-13.8) | .03 |
| Disease modifying treatment, No. (%) | |||
| Monoclonal abs | 13 (2.1) | 321 (24.9) | <.001 |
| Orals | 3 (0.5) | 437 (33.9) | |
| Platform | 347 (57.0) | 156 (12.1) | |
| Untreated | 246 (40.4) | 376 (29.1) | |
| Follow-up, y | 10.2 (7.1-11.2) | 7.0 (4.4-8.7) | <.001 |
| Disease modifying treatment at last follow-up, No. (%) | |||
| Monoclonal absc | 66, (10.8) | 542 (42.0) | <.001 |
| Oralsd | 103 (16.9) | 498 (38.6) | |
| Platforme | 199 (32.7) | 56 (3.2) | |
| Untreated | 241 (39.6) | 194 (15.0) | |
| CDW events during follow-up, No. (%) | |||
| None | 419 (68.8) | 910 (70.5) | .29 |
| 1 Event | 158 (25.9) | 332 (25.7) | |
| 2 Events | 27 (4.4) | 41 (3.2) | |
| 3 Events or more | 5 (0.8) | 7 (0.5) | |
| CDW-R events during follow-up, No. (%) | |||
| None | 575 (94.4) | 1202 (93.2) | .59 |
| 1 Event | 32 (5.3) | 83 (6.4) | |
| 2 Events | 2 (0.3) | 5 (0.4) | |
| 3 Events or more | 0 (0) | 0 (0) | |
| CDW-NR events during follow-up, No. (%) | |||
| None | 446 (73.2) | 986 (76.4) | .15 |
| 1 Event | 139 (22.8) | 268 (20.8) | |
| 2 Events | 20 (3.3) | 34 (2.6) | |
| 3 Events or more | 4 (0.7) | 2 (0.2) |
Abbreviations: abs, antibodies; CDW, confirmed disability worsening; CDW-NR, confirmed disability worsening independent of clinical relapses; CDW-R, confirmed disability worsening with clinical relapses; EDSS, Expanded Disability Status Scale; NA, not applicable; orals, oral disease-modifying treatments; RMS, relapsing multiple sclerosis.
Values shown are median and interquartile range, unless mentioned otherwise.
P values calculated with Mann-Whitney test and χ2 test for median and for categorical variables, respectively.
Monoclonal abs include alemtuzumab, natalizumab, ocrelizumab, ofatumumab, and rituximab.
Orals include cladribine, dimethyl fumarate, fingolimod, ozanimod, siponimod, and teriflunomide.
Platform treatments include interferon beta, glatiramer acetate, and others (eg, immunosuppressive drugs).
Dynamics of NfL in Relation to CDW Events
CDW-R
NfL levels at CDW-R(−1) in 34 visits were 0.71 (95% CI, 0.35-1.07; P < .001) z score units higher compared with 2562 stMS visits in the EPIC cohort, whereas no such differences were found for the remaining time points (Figure 1 and eTables 1 and 2 in Supplement 1).
Figure 1. Neurofilament Light Chain (NfL) Dynamics in Relation to Future Disability Worsening.
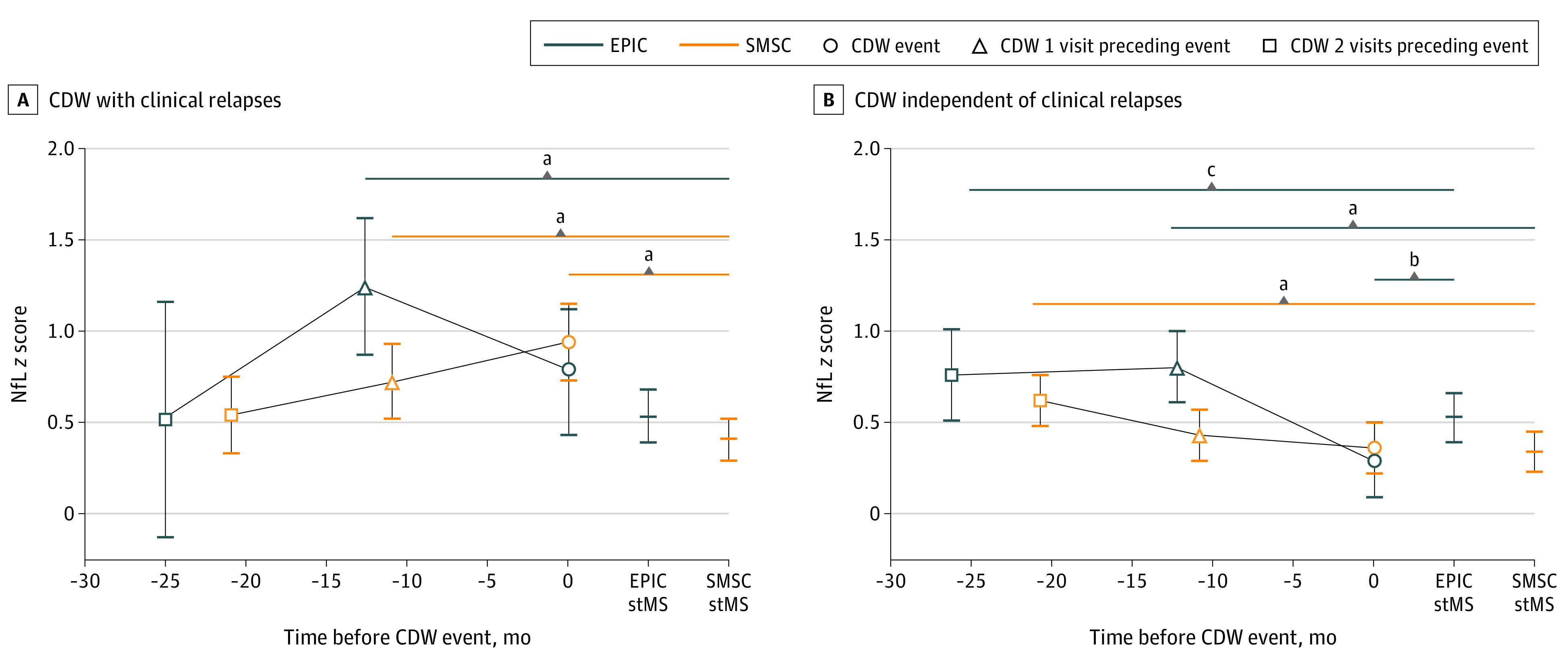
The y-axis showing the marginal means of NfL z scores and P values from mixed linear models correcting for age, sex, disease course (relapsing vs progressive multiple sclerosis [MS]), disease-modifying treatment (DMT) categories (high-efficacy monoclonal antibodies, oral DMT, platform DMT, and untreated), and recent relapse (within 90 days) for confirmed disability worsening associated with clinical relapses (CDW-R) and CDW independent of clinical relapses (CDW-NR), respectively. EPIC indicates Expression, Proteomics, Imaging, Clinical study; SMSC, Swiss Multiple Sclerosis Cohort; stMS, participants with stable MS without evidence of disease activity or CDW.
aP < .001.
bP < .01.
cP < .05.
In the SMSC, elevated NfL levels were found at CDW-R(−1) in 97 visits (estimate = 0.32; 95% CI, 0.14-0.49; P < .001) and at the event visit (CDW-R[event] in 93 visits; estimate = 0.53; 95% CI, 0.36-0.71; P < .001) compared with 6019 stMS visits (Figure 1 and eTables 1 and 2 in Supplement 1). Relapse onset was closer to the CDW-R(event) in the SMSC (median [IQR], −137 [−56 to −275] days) compared with the EPIC cohort (−166 [−70 to −270] days).
CDW-NR
In EPIC, NfL elevation was seen at both visits preceding the event (CDW-NR[−1]: 0.27; 95% CI, 0.11-0.44; P < .001; CDW-NR[−2]: 0.23; 95% CI, 0.01-0.45; P = .04). In SMSC, higher NfL z scores were seen at CDW-NR(−2) (0.28; 95% CI, 0.18- 0.37; P < .001) with a trend for higher NfL at CDW-NR(−1) (0.09; 95% CI, 0-0.18; P = .06) (Figure 1 and eTables 1 and 3 in Supplement 1).
All the previously mentioned analyses were adjusted for covariates relevant in MS, and the full models are shown in eTables 2 and 3 in Supplement 1. Besides visit status, the following significant associations with NfL z scores were found in CDW-R and CDW-NR analyses: NfL z scores were higher in younger patients, increased with longer disease duration, were strongly elevated after a recent relapse, tended to be lower under more effective DMT, and were associated with higher EDSS scores. Interestingly, the NfL elevation before CDW-NR was found over the entire EDSS range as indicated graphically in eFigure 2 in Supplement 1.
Moreover, restricting the analysis to relapsing remitting MS (83.5% [3262 of 3906] and 83.9% [7470 of 8901] of EPIC and SMSC samples, respectively), NfL elevation was still detected at CDW-NR(−1) in the EPIC cohort and CDW-NR(−2) in the EPIC cohort and the SMSC (eTables 4 and 5 and eFigure 3 in Supplement 1).
Acknowledging the different visit plan in both studies, we conducted a sensitivity analysis limiting the CDW definition and evaluation of NfL dynamics to the yearly visits in SMSC, which confirmed the NfL elevation seen when analyzing the whole cohort (eTable 6 in Supplement 1). An additional sensitivity analysis with adjustment for relapse and MRI activity was conducted in the subset of participants with a supplementary complete MRI data set (2145 time points in the EPIC study and 1735 in the SMSC). Clinical and/or imaging activity (new or enlarging T2-weighted lesions, CEL) was detected in 26 of 60 visits (43.3%) at CDW-NR(−2) and 38 of 94 visits (40.4%) at CDW-NR(−1) in the EPIC cohort and 23 of 56 visits (41.1%) and 35 of 91 visits (38.5%) in the SMSC, respectively. Following correction for any activity in the last year, higher NfL z score levels at CDW-NR(−2) (estimated difference = 0.22; 95% CI, 0.02-0.42; P = .03) but not at CDW-NR(−1) (0.12; 95% CI, −0.08 to 0.32; P = .25) were found in SMSC. In the EPIC study, the z scores were numerically higher with similar estimate difference as the SMSC but did not show a statistically significant difference compared with stMS (0.23; 95% CI, −0.06 to 0.52; P = .12; and 0.13; 95% CI, −0.11 to 0.38; P = .28 for CDW-NR[−2] and CDW-NR[−1], respectively) (eTable 7 and eFigure 4 in Supplement 1).
NfL Value and Future Risk of CDW
CDW-R
In both the EPIC cohort and the SMSC, higher NfL z scores were associated with the future occurrence of CDW-R (Table 2, Figure 2, and eTables 8, 9, 10, and 11 in Supplement 1). Indeed, an NfL z score greater than 1.0 was associated with a 70% (hazard ratio [HR], 1.70; 95% CI, 1.10-2.61; P = .02) higher risk for diagnosing CDW-R at the subsequent visit approximately 11.0 months later (SMSC) and a trend for a 91% (HR, 1.91; 95% CI, 0.94-3.87; P = .07) higher risk at 12.6 months later in the EPIC study.
Table 2. Association Between Neurofilament Light Chain (NfL) z Score and Future Risk of Confirmed Disability Worsening (CDW) in the Expression, Proteomics, Imaging, Clinical (EPIC) study and Swiss Multiple Sclerosis Cohort (SMSC)a.
| Variable | EPIC CDW-R(−2) | SMSC CDW-R(−2) | EPIC CDW-R(−1) | SMSC CDW-R(−1) | ||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| CDW-R | ||||||||
| Time preceding event (SD), mo | −25.1 (1.2) | −20.9 (5.3) | −12.6 (1.4) | −10.9 (3.1) | ||||
| NfL z score | 0.88 (0.63 to 1.22) | .44 | 1.01 (0.82 to 1.23) | .96 | 1.19 (0.97 to 1.47) | .10 | 1.20 (0.99 to 1.46) | .06 |
| NfL z score >1 vs ≤1 | 0.65 (0.19 to 2.25) | .49 | 1.09 (0.67 to 1.77) | .73 | 1.91 (0.94 to 3.87) | .07 | 1.70 (1.10 to 2.61) | .02 |
| EPIC CDW-NR(−2) | SMSC CDW-NR(−2) | EPIC CDW-NR(−1) | SMSC CDW-NR(−1) | |||||
| CDW-NR | ||||||||
| Time preceding event (SD), mo | −26.2 (6.4) | −20.7 (5.6) | −12.2 (1.3) | −10.8 (3.5) | ||||
| NfL z score | 1.16 (1.01 to 1.33) | .03 | 1.14 (1.04 to 1.24) | .004 | 1.19 (1.07 to 1.33) | .001 | 1.02 (0.94 to 1.10) | .72 |
| NfL z score >1 vs ≤1 | 1.25 (0.84 to 1.87) | .27 | 1.49 (1.20 to 1.84) | <.001 | 1.40 (1.06 to 1.85) | .02 | 0.94 (0.76 to 1.17) | .60 |
Abbreviations: CDW-NR, confirmed disability worsening independent of clinical relapses; CDW-R, confirmed disability worsening with clinical relapses; CDW-R/CDW-NR(−1), 1 visit preceding the event; CDW-R/CDW-NR(−2), 2 visits preceding the event; HR, hazard ratio.
Recent relapse (within 90 days) at measurement visit, and expanded disability status scale (EDSS) at baseline.
Figure 2. Future Risk of Confirmed Disability Worsening With Clinical Relapses (CDW-R) Based on Neurofilament Light Chain (NfL) z Score.
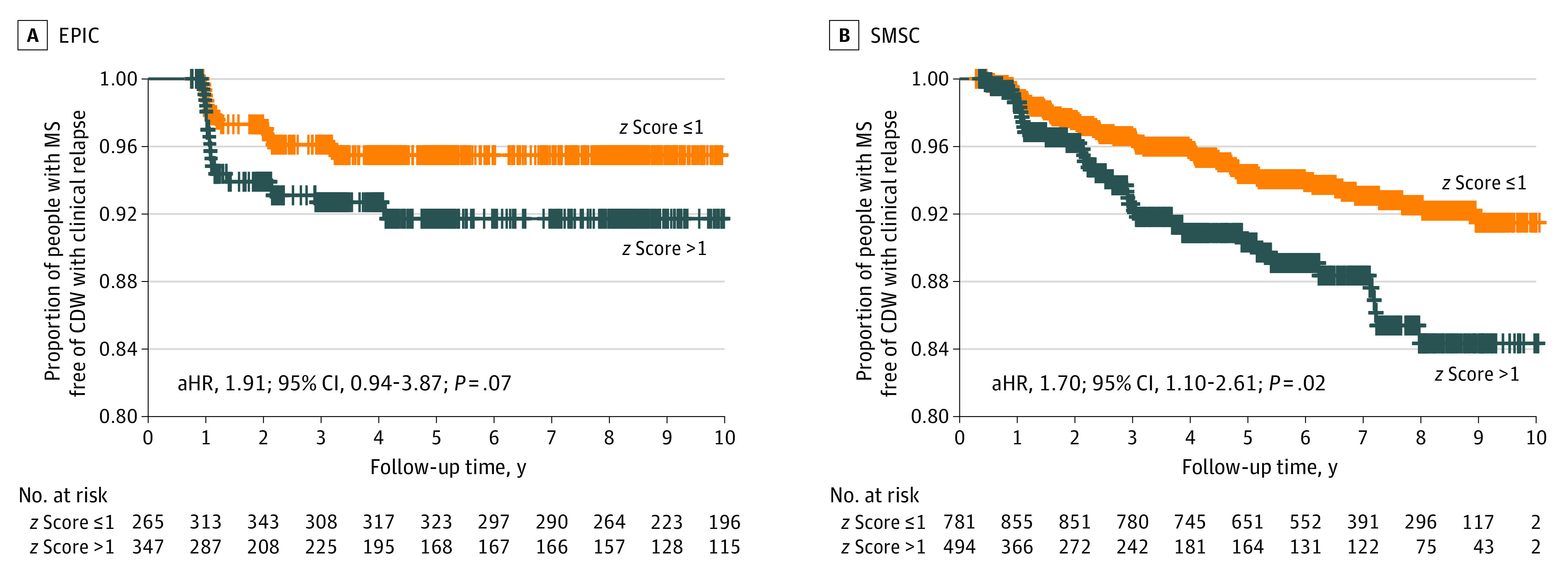
In the Expression, Proteomics, Imaging, Clinical (EPIC) study (A) and Swiss Multiple Sclerosis Cohort (SMSC) (B), NfL z score less than or equal to 1 or greater than 1 was used as a time-varying covariate, correcting for age, sex, disease course (relapsing vs progressive multiple sclerosis [MS]), disease-modifying treatment (DMT) category (monoclonal antibodies, oral DMT, platform DMT, and untreated), recent relapse (within 90 days) at measurement visit, and Expanded Disability Status Scale at baseline. aHR indicates adjusted hazard ratio.
CDW-NR
Considering visits from both the EPIC study and the SMSC in a time series related to CDW-NR, NfL levels close to CDW-NR, ie, at CDW-NR(−1) in the EPIC cohort and CDW-NR(−2) in the SMSC, were associated with higher risk for CDW-NR events (Table 2, Figure 3, and eTables 12, 13, 14, and 15 in Supplement 1). For CDW-NR, an NfL z score greater than 1.0 was associated with a 40% (HR, 1.40; 95% CI, 1.06-1.85; P = .02) and 49% (HR, 1.49; 95% CI, 1.20-1.84; P < .001) higher risk of diagnosing CDW-NR in approximately 12 months in the EPIC cohort and approximately 21 months in the SMSC, respectively. In comparison, increased NfL levels at visits closest to the event (SMSC CDW-NR[−1]: −10.8 months) or visits further away from the event (EPIC CDW-NR[−2]: −26.2 months) were not consistently associated with higher CDW-NR risk, a finding in line with the results of the mixed models.
Figure 3. Future Risk of Confirmed Disability Worsening Independent of Clinical Relapses (CDW-NR) based on Neurofilament Light Chain (NfL) z Score.
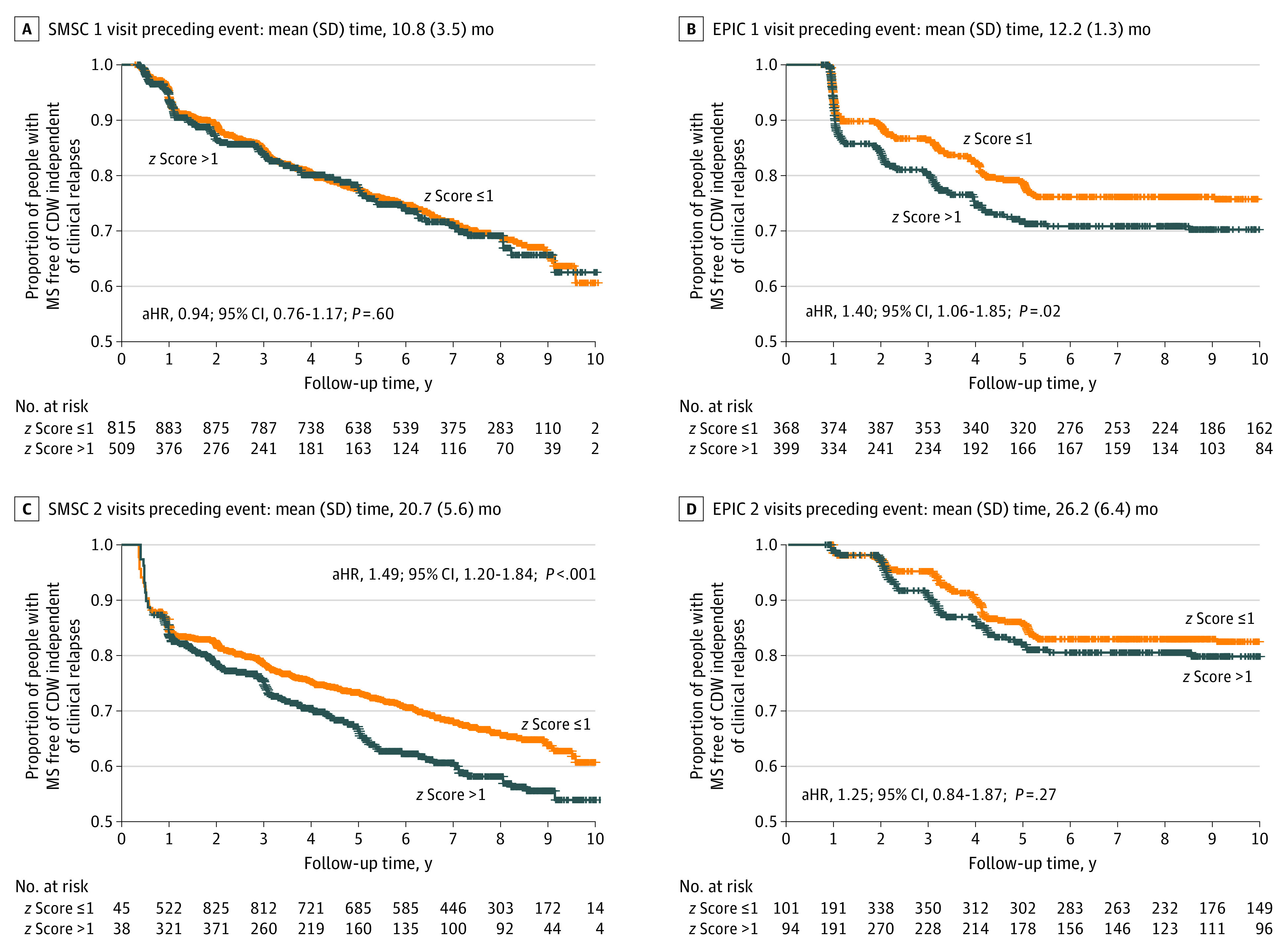
NfL z score less than or equal to 1 or greater than 1 was used as a time-varying covariate, either at CDW-NR(−1) or CDW-NR(−2), correcting for age, sex, disease course (relapsing vs progressive multiple sclerosis [MS]), disease-modifying treatment (DMT) category (high-efficacy monoclonal antibodies, oral DMT, platform DMT, and untreated), recent relapse (within 90 days) at measurement visit, and Expanded Disability Status Scale (EDSS) at baseline. NfL z score was associated with higher risk for future CDW-NR at visits CDW-NR(−1) in the Expression, Proteomics, Imaging, Clinical (EPIC) study (B) and CDW-NR(−2) in Swiss Multiple Sclerosis Cohort (SMSC) (C). NfL z scores at visits closest to the event, CDW-NR(−1) in SMSC (A), or too distant from the event, CDW-NR(−2) in EPIC (D), were not significantly associated with CDW-NR. aHR indicates adjusted hazard ratio.
Discussion
This work evaluated the longitudinal dynamics of NfL levels in 2 large, prospective, longitudinal cohorts. Increased NfL levels were found preceding relapse-associated worsening (CDW-R; approximately 1 year) and worsening independent of clinical relapses (CDW-NR; approximately 12-24 months). High NfL levels were associated with CDW-R events in the following 13 months and CDW-NR events occurring between approximately 1 to 2 years in the future.
The more descriptive term, CDW, in combination with or without relapses to define the groups (CDW-R and CDW-NR), was used instead of relapse-associated worsening (RAW) and progression independent of relapse activity (PIRA).21 RAW could imply the association with incomplete relapse remission, which can be evaluated through clinical visits at and following the time of relapse. This requirement could limit the application of this term in longitudinal population cohorts with the wide interval between visits. On the other hand, PIRA could imply the lack of disease activity beyond clinically diagnosed relapses. In fact, PIRA is a considerably heterogeneous group regarding MRI activity, which was recently reported in more than 50% of PIRA cases in early MS.22
The reported NfL elevation preceding CDW events highlights the value of NfL as an early biomarker of disability worsening and points to the existence of different windows of dynamic central nervous system pathology preceding CDW-R and CDW-NR. Further studies of those distinct periods, which we here propose to call “pre–CDW-R and CDW-NR,” could help define the chronicle of events preceding CDW that can potentially be therapeutically influenced. Furthermore, our findings hint to the necessity for optimized terms to describe CDW in MS depending on its temporal association with any detectable focal inflammatory disease activity. That includes relapses (regardless of the outcome), MRI activity, and/or potentially significant NfL surge beyond a yet-to-define cutoff.
Although in the EPIC cohort, NfL elevation was detectable only 1 visit per year preceding CDW-R, NfL z scores at CDW-R event visits were still significantly higher compared with control samples in the SMSC. In the SMSC, relapses were closer to CDW-R(event) than in the EPIC cohort due to a higher visit frequency, permitting capturing of time points closer to the relapse. Furthermore, relapse dates should be considered cautiously due to possible recall bias. Regardless, such NfL elevation could potentially demonstrate ongoing neuroaxonal pathology at the time of diagnosing CDW-R. Otherwise, it could merely reflect a spill-over effect with NfL levels regressing to the mean after the main burden of axonal pathology took place before and during the clinical diagnosis of relapse.
In addition, our study describes a novel NfL elevation related to CDW-NR that is more temporally distant (between 1-2 years) preceding the event compared to CDW-R. The sequence of events leading to CDW-NR is not entirely understood and involves complex pathophysiology, including demyelination, glial activation, and neuroaxonal damage. Finding elevated NfL z scores at both CDW-NR(−1) and CDW-NR(−2) with lower estimates compared with CDW-R suggest a more prolonged, slow neuroaxonal pathology preceding CDW-NR that decreases in intensity in advance of the clinically identifiable event. Such slowly ongoing neuroaxonal involvement aligns with the finding of slowly expanding/smoldering lesions associated with progressive MS.23 Another potential explanation is the association between elevated NfL levels and temporally distant, focal subclinical activity preceding CDW-NR with more delayed clinical manifestation. Moreover, substantial heterogeneity could exist in the CDW-NR population based on genetic risk profile, disease course, duration, and treatments.22,24
Our findings help understand the complex landscape of conflicting evidence for association between NfL and CDW. NfL assessment at the time of EDSS-based CDW diagnosis could be less informative, as the components of the pathophysiology associated with NfL increase already took place and were potentially concluded before the event visit. Moreover, evaluating treatment success in preventing CDW-R/CDW-NR should allow the investigated treatment to intervene during pre–CDW-R and CDW-NR. For example, in a clinical trial aiming to prevent CDW-NR (ie, instead of or PIRA), events diagnosed in the first few months may be beyond the intervention’s potential influence. On the other hand, events diagnosed after more than 1 year are potentially salvageable events as the intervention may have had sufficient time to exert its effect.
Strengths and Limitations
A strength of this study is that almost all findings could be replicated in both cohorts despite their different designs, which lends credence to the results. This study has some limitations. The different study designs and timing of visits used in the 2 cohorts posed a challenge for harmonizing the analysis. More frequent NfL assessment (eg, every 3 months) using minimally invasive methods such as dried blood spots might enable a better definition of the time windows of NfL elevation. Moreover, CDW was diagnosed based on EDSS, which is a less sensitive outcome metric when combined with the timed 25-foot walk test, and 9-hole peg test. In addition, detailed MRI data were available only in a subset of the participants. Finally, the differences we detected were on group levels, which might underestimate the heterogeneity in causes leading to progression within each CDW-R and CDW-NR.
Conclusions
In summary, this cohort study documented the temporal dynamics of NfL levels in association with different patterns of CDW in MS, defined a potential window for future CDW-abortive treatments, and provided insights for designing clinical trials.
eFigure 1. Distribution of Time Points of Visits in Relation to Diagnosis of Confirmed Disability Worsening With and Independent of Clinical Relapses
eFigure 2. NfL Dynamics in Relation to Disability Worsening Independent of Clinical Relapses in Relation to Expanded Disability Status Scale at Sampling
eFigure 3. Subgroup Analysis in Relapsing Remitting MS: NfL Dynamics in Relation to Disability Worsening Independent of Clinical Relapses
eFigure 4. Sensitivity Analysis in Patients With Complete MRI Information at Each Visit: NfL Dynamics in Relation to Confirmed Disability Worsening Independent of Clinical Relapses Adjusted for Clinical and Radiological Disease Activity
eTable 1. NfL z Score (Marginal Means) in Relation to Confirmed Disability Worsening With and Independent of Clinical Relapses Compared to Stable Multiple Sclerosis
eTable 2. Mixed Effect Models Estimating Longitudinal NfL z Scores in Context of Confirmed Disability Worsening With Clinical Relapses
eTable 3. Mixed Effect Models Estimating Longitudinal NfL z Scores in Context of Confirmed Disability Worsening Independent of Clinical Relapses
eTable 4. Subgroup Analysis in People With Relapsing-Remitting Multiple Sclerosis: NfL z Score (Marginal Means) in Relation to Confirmed Disability Worsening Independent of Clinical Relapses Compared to Stable Multiple Sclerosis
eTable 5. Subgroup Analysis in People With Relapsing-Remitting Multiple Sclerosis: Mixed-Effects Models Evaluating Longitudinal NfL z Scores in Cases With Confirmed Disability Worsening Independent of Clinical Relapses in Participants With Relapsing-Remitting Multiple Sclerosis
eTable 6. Sensitivity Analyses in SMSC Restricted to Yearly Visits: NfL z Score (Marginal Means) in Relation to Confirmed Disability Worsening
eTable 7. Sensitivity Analysis in Participants With MRI Information Available at Each Visit: Mixed-Effects Models Estimating Longitudinal NfL z Scores in Context of Confirmed Disability Worsening Independent of Relapses, Accounting for Clinical and Magnetic Resonance Imaging Activity
eTable 8. Association Between NfL z Score and Future Risk of Confirmed Disability Worsening With Clinical Relapses at Visit CDW-R(−1)
eTable 9. Association Between NfL z Score > 1.0 and Future Risk of Confirmed Disability Worsening With Clinical Relapses at Visit CDW-R(−1)
eTable 10. Association Between NfL z Score and Future Risk of Confirmed Disability Worsening With Clinical Relapses at Visit CDW-R(−2)
eTable 11. Association Between NfL z Score > 1.0 and Future Risk of Confirmed Disability Worsening With Clinical Relapses at Visit CDW-R(−2)
eTable 12. Association Between NfL z Score and Future Risk of Confirmed Disability Worsening Independent of Clinical Relapses at Visit CDW-NR(−1)
eTable 13. Association Between NfL z Score > 1.0 and Future Risk of Confirmed Disability Worsening Independent of Clinical Relapses at Visit CDW-NR(−1)
eTable 14. Association Between NfL z Score and Future Risk of Confirmed Disability Worsening Independent of Clinical Relapses at Visit CDW-NR(−2)
eTable 15. Association Between NfL z Score > 1.0 and Future Risk of Confirmed Disability Worsening Independent of Clinical Relapses at Visit CDW-NR(−2)
Nonauthor Collaborators
Data Sharing Statement
References
- 1.Khalil M, Pirpamer L, Hofer E, et al. Serum neurofilament light levels in normal aging and their association with morphologic brain changes. Nat Commun. 2020;11(1):812. doi: 10.1038/s41467-020-14612-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benkert P, Meier S, Schaedelin S, et al. ; NfL Reference Database in the Swiss Multiple Sclerosis Cohort Study Group . Serum neurofilament light chain for individual prognostication of disease activity in people with multiple sclerosis: a retrospective modelling and validation study. Lancet Neurol. 2022;21(3):246-257. doi: 10.1016/S1474-4422(22)00009-6 [DOI] [PubMed] [Google Scholar]
- 3.Kuhle J, Kropshofer H, Haering DA, et al. Blood neurofilament light chain as a biomarker of MS disease activity and treatment response. Neurology. 2019;92(10):e1007-e1015. doi: 10.1212/WNL.0000000000007032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abdelhak A, Cordano C, Boscardin WJ, et al. Plasma neurofilament light chain levels suggest neuroaxonal stability following therapeutic remyelination in people with multiple sclerosis. J Neurol Neurosurg Psychiatry. 2022;jnnp-2022-329221. doi: 10.1136/jnnp-2022-329221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shaw G, Madorsky I, Li Y, et al. Uman-type neurofilament light antibodies are effective reagents for the imaging of neurodegeneration. Brain Commun. 2023;5(2):fcad067. doi: 10.1093/braincomms/fcad067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gafson AR, Jiang X, Shen C, et al. Serum neurofilament light and multiple sclerosis progression independent of acute inflammation. JAMA Netw Open. 2022;5(2):e2147588. doi: 10.1001/jamanetworkopen.2021.47588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bridel C, Leurs CE, van Lierop ZYGJ, et al. Serum neurofilament light association with progression in natalizumab-treated patients with relapsing-remitting multiple sclerosis. Neurology. 2021;97(19):e1898-e1905. doi: 10.1212/WNL.0000000000012752 [DOI] [PubMed] [Google Scholar]
- 8.Uphaus T, Steffen F, Muthuraman M, et al. NfL predicts relapse-free progression in a longitudinal multiple sclerosis cohort study. EBioMedicine. 2021;72:103590. doi: 10.1016/j.ebiom.2021.103590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ziemssen T, Arnold DL, Alvarez E, et al. Prognostic value of serum neurofilament light chain for disease activity and worsening in patients with relapsing multiple sclerosis: results from the phase 3 ASCLEPIOS I and II trials. Front Immunol. 2022;13:852563. doi: 10.3389/fimmu.2022.852563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leppert D, Kropshofer H, Häring DA, et al. Blood neurofilament light in progressive multiple sclerosis: post hoc analysis of 2 randomized controlled trials. Neurology. 2022;98(21):e2120-e2131. doi: 10.1212/WNL.0000000000200258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cantó E, Barro C, Zhao C, et al. Association between serum neurofilament light chain levels and long-term disease course among patients with multiple sclerosis followed up for 12 years. JAMA Neurol. 2019;76(11):1359-1366. doi: 10.1001/jamaneurol.2019.2137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cree BAC, Hollenbach JA, Bove R, et al. ; University of California, San Francisco MS-EPIC Team . Silent progression in disease activity-free relapsing multiple sclerosis. Ann Neurol. 2019;85(5):653-666. doi: 10.1002/ana.25463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cree BA, Gourraud PA, Oksenberg JR, et al. ; University of California, San Francisco MS-EPIC Team . Long-term evolution of multiple sclerosis disability in the treatment era. Ann Neurol. 2016;80(4):499-510. doi: 10.1002/ana.24747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Disanto G, Benkert P, Lorscheider J, et al. ; SMSC Scientific Board . The Swiss Multiple Sclerosis Cohort study (SMSC): a prospective Swiss wide investigation of key phases in disease evolution and new treatment options. PLoS One. 2016;11(3):e0152347. doi: 10.1371/journal.pone.0152347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Disanto G, Barro C, Benkert P, et al. ; Swiss Multiple Sclerosis Cohort Study Group . Serum neurofilament light: a biomarker of neuronal damage in multiple sclerosis. Ann Neurol. 2017;81(6):857-870. doi: 10.1002/ana.24954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barro C, Benkert P, Disanto G, et al. Serum neurofilament as a predictor of disease worsening and brain and spinal cord atrophy in multiple sclerosis. Brain. 2018;141(8):2382-2391. doi: 10.1093/brain/awy154 [DOI] [PubMed] [Google Scholar]
- 17.Andermatt S, Pezold S, Cattin PC. Automated segmentation of multiple sclerosis lesions using multidimensional gated recurrent units. In: Crimi A, Bakas S, Kuijf H, Menze B, Reyes M, eds. Brainlesion: Glioma, Multiple Sclerosis, Stroke, and Traumatic Brain Injuries. 2018. doi: 10.1007/978-3-319-75238-9_3 [DOI] [Google Scholar]
- 18.Fartaria MJ, Kober T, Granziera C, Bach Cuadra M. Longitudinal analysis of white matter and cortical lesions in multiple sclerosis. Neuroimage Clin. 2019;23:101938. doi: 10.1016/j.nicl.2019.101938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kappos L, Butzkueven H, Wiendl H, et al. ; Tysabri® Observational Program (TOP) Investigators . Greater sensitivity to multiple sclerosis disability worsening and progression events using a roving vs a fixed reference value in a prospective cohort study. Mult Scler. 2018;24(7):963-973. doi: 10.1177/1352458517709619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17(2):162-173. doi: 10.1016/S1474-4422(17)30470-2 [DOI] [PubMed] [Google Scholar]
- 21.Kappos L, Wolinsky JS, Giovannoni G, et al. Contribution of relapse-independent progression vs relapse-associated worsening to overall confirmed disability accumulation in typical relapsing multiple sclerosis in a pooled analysis of 2 randomized clinical trials. JAMA Neurol. 2020;77(9):1132-1140. doi: 10.1001/jamaneurol.2020.1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tur C, Carbonell-Mirabent P, Cobo-Calvo A, et al. Association of early progression independent of relapse activity with long-term disability after a first demyelinating event in multiple sclerosis. JAMA Neurol. 2023;80(2):151-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calvi A, Tur C, Chard D, et al. Slowly expanding lesions relate to persisting black-holes and clinical outcomes in relapse-onset multiple sclerosis. Neuroimage Clin. 2022;35:103048. doi: 10.1016/j.nicl.2022.103048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cagol A, Schaedelin S, Barakovic M, et al. Association of brain atrophy with disease progression independent of relapse activity in patients with relapsing multiple sclerosis. JAMA Neurol. 2022;79(7):682-692. doi: 10.1001/jamaneurol.2022.1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Distribution of Time Points of Visits in Relation to Diagnosis of Confirmed Disability Worsening With and Independent of Clinical Relapses
eFigure 2. NfL Dynamics in Relation to Disability Worsening Independent of Clinical Relapses in Relation to Expanded Disability Status Scale at Sampling
eFigure 3. Subgroup Analysis in Relapsing Remitting MS: NfL Dynamics in Relation to Disability Worsening Independent of Clinical Relapses
eFigure 4. Sensitivity Analysis in Patients With Complete MRI Information at Each Visit: NfL Dynamics in Relation to Confirmed Disability Worsening Independent of Clinical Relapses Adjusted for Clinical and Radiological Disease Activity
eTable 1. NfL z Score (Marginal Means) in Relation to Confirmed Disability Worsening With and Independent of Clinical Relapses Compared to Stable Multiple Sclerosis
eTable 2. Mixed Effect Models Estimating Longitudinal NfL z Scores in Context of Confirmed Disability Worsening With Clinical Relapses
eTable 3. Mixed Effect Models Estimating Longitudinal NfL z Scores in Context of Confirmed Disability Worsening Independent of Clinical Relapses
eTable 4. Subgroup Analysis in People With Relapsing-Remitting Multiple Sclerosis: NfL z Score (Marginal Means) in Relation to Confirmed Disability Worsening Independent of Clinical Relapses Compared to Stable Multiple Sclerosis
eTable 5. Subgroup Analysis in People With Relapsing-Remitting Multiple Sclerosis: Mixed-Effects Models Evaluating Longitudinal NfL z Scores in Cases With Confirmed Disability Worsening Independent of Clinical Relapses in Participants With Relapsing-Remitting Multiple Sclerosis
eTable 6. Sensitivity Analyses in SMSC Restricted to Yearly Visits: NfL z Score (Marginal Means) in Relation to Confirmed Disability Worsening
eTable 7. Sensitivity Analysis in Participants With MRI Information Available at Each Visit: Mixed-Effects Models Estimating Longitudinal NfL z Scores in Context of Confirmed Disability Worsening Independent of Relapses, Accounting for Clinical and Magnetic Resonance Imaging Activity
eTable 8. Association Between NfL z Score and Future Risk of Confirmed Disability Worsening With Clinical Relapses at Visit CDW-R(−1)
eTable 9. Association Between NfL z Score > 1.0 and Future Risk of Confirmed Disability Worsening With Clinical Relapses at Visit CDW-R(−1)
eTable 10. Association Between NfL z Score and Future Risk of Confirmed Disability Worsening With Clinical Relapses at Visit CDW-R(−2)
eTable 11. Association Between NfL z Score > 1.0 and Future Risk of Confirmed Disability Worsening With Clinical Relapses at Visit CDW-R(−2)
eTable 12. Association Between NfL z Score and Future Risk of Confirmed Disability Worsening Independent of Clinical Relapses at Visit CDW-NR(−1)
eTable 13. Association Between NfL z Score > 1.0 and Future Risk of Confirmed Disability Worsening Independent of Clinical Relapses at Visit CDW-NR(−1)
eTable 14. Association Between NfL z Score and Future Risk of Confirmed Disability Worsening Independent of Clinical Relapses at Visit CDW-NR(−2)
eTable 15. Association Between NfL z Score > 1.0 and Future Risk of Confirmed Disability Worsening Independent of Clinical Relapses at Visit CDW-NR(−2)
Nonauthor Collaborators
Data Sharing Statement


