Abstract
Purpose:
Biological and experimental evidence support restoration of normal zinc levels in malignant prostate cells as a promising prostate cancer treatment, yet the influence of zinc supplementation after diagnosis on prostate cancer survival in a human population is unknown.
Materials and Methods:
We prospectively assessed post-diagnostic zinc supplementation in relation to prostate cancer survival among 5788 men with nonmetastatic prostate cancer in the Health Professionals Follow-up Study (1986–2019). We used Cox regression models to estimate the multivariable hazard ratios (HRs) and 95% confidence intervals (CIs) of lethal prostate cancer (distant metastases or prostate cancer-specific death) and all-cause mortality according to post-diagnostic zinc supplement use and dosage.
Results:
During a median follow-up of 11 years, we documented 527 lethal prostate cancer events and 3198 all-cause deaths. 15% of men reported zinc supplement use post-diagnosis. Compared to non-users, post-diagnostic zinc supplement use was associated suggestively with a lower risk of lethal prostate cancer (HR [95% CI], 0.82 [0.60–1.13]) and significantly with all-cause mortality (0.84 [0.74–0.96]). The inverse association was mostly observed among men who used post-diagnostic zinc supplements of 1–24 mg/day (lethal prostate cancer: 0.55 [0.32–0.96]; all-cause mortality: 0.77 [0.64–0.93]), while higher dosage did not show a lower risk.
Conclusions:
Post-diagnostic low-dose zinc supplement use among nonmetastatic prostate cancer patients was associated with lower risk of lethal prostate cancer and all-cause mortality. A potential benefit of low-dose post-diagnostic zinc supplement for prostate cancer survival merits further study.
Keywords: Prostate cancer survival, Zinc supplementation, Prospective cohort
Introduction
Normal prostate tissue contains one of the highest concentrations of zinc in the body, and mechanistic evidence indicates zinc is important for prostate health1. In prostate cancer patients, decreased zinc levels are consistently observed in malignant tissue samples compared to normal prostate tissue across different populations and at various stages of malignancy2,3. This observation has led to the hypothesis that zinc treatment could slow or inhibit prostate cancer growth and invasion4. Numerous in vitro and animal studies showed that treatment of malignant prostate cells with zinc can induce apoptosis, inhibit cell migration and invasion5–7. However, few studies have investigated post-diagnosis zinc supplementation and prostate cancer survival in a human population.
Currently, only one Swedish cohort study examined dietary zinc level at diagnosis and prostate cancer survival8, which found men with adequate dietary zinc intake (15.6–20.1 mg/day) had lower risk of prostate cancer-specific mortality (hazard ratio [HR], 0.64; 95% confidence interval [CI], 0.44–0.94) compared to lower dietary intake (9–12.8 mg/day). The study, however, did not assess zinc intake at higher dosages attained through supplementation. While the recommended dietary allowance (RDA) of zinc for men aged 19 or above in the United States is 11 mg/day, some zinc supplements have much higher dosage, exceeding the RDA ten-fold. Previously in the Health Professionals Follow-up Study (HPFS), we found that men with supplemental zinc ≥100 mg/day had an increased risk of advanced prostate cancer among initially cancer-free men9.
Thus, we prospectively examined post-diagnostic zinc supplement use in relation to lethal prostate cancer and all-cause mortality among men diagnosed with nonmetastatic prostate cancer in the HPFS. We hypothesized that low-dose post-diagnostic zinc supplementation may associated with better survival while high-dose may do harm.
Methods
Study Population
The HPFS is an ongoing prospective cohort study of 51,529 US male health professionals who enrolled in 1986 at age of 40 to 75. Participants were mailed questionnaires at baseline and biennially to collect updated information on demographic, lifestyle factors, medical history, and disease outcome, with dietary information collected using validated semi-quantitative food frequency questionnaire (FFQ) every 4 years. The follow up rates exceeded 90% for each questionnaire. For this study, we included participants in the cohort diagnosed with incident prostate cancer between 1986 and 2016 whose cancers were nonmetastatic (TNM stage: T1-T3a, N0, M0) at the time of diagnosis.
Zinc Supplement and Dietary zinc
Participants completed detailed information on use and dosage of dietary supplements biennially beginning in 198610. At baseline, participants were asked to identify themselves as “never-user”, “past only” or “current regular user” for each dietary supplement. Subsequent questionnaires collected updated information on current use status (yes, no) and dosage (pre-defined four levels) at each follow-up cycle. The pre-defined categories for zinc supplement dosage were: < 25, 25–74, 75–100 and ≥ 101 mg per day. Men who reported current zinc supplement use but missing dosage (<2.0% for each questionnaire cycle) were assigned the most common dosage level (25–74 mg/day).
Dietary zinc intake was calculated by multiplying the reported frequency of consumption of each item on FFQs by its zinc content, summing across from all foods, and adjusting for total caloric intake using the nutrient residual methods11. The validity of zinc intake was confirmed with validation studies using dietary records (Pearson correlation is 0.71)12. To avoid assessment during the period of active treatment and match dietary and supplement zinc information on the same questionnaire year, we defined post-diagnostic zinc supplement use as the exposure reported on the first questionnaire with FFQ collected at least 6 months after diagnosis. Pre-diagnostic zinc supplement use was based on the last questionnaire with FFQ reported before prostate cancer diagnosis.
Outcome Assessment and Follow-up
Our primary outcomes were lethal prostate cancer (distant metastases or prostate cancer specific death) and all-cause mortality. Self-reported prostate cancer diagnoses were confirmed through medical record and pathology report review. Stage, Gleason score, prostate-specific antigen (PSA) values at diagnosis, and distant metastases were collected by medical records, pathology reports, and study questionnaires sent to prostate cancer survivors and their attending physicians. Deaths in the cohorts were ascertained through reports by family members and searches of National Death Index. The underlying cause of death was determined by a physician endpoint review committee (blinded to any exposure information). The mortality ascertainment in the cohort is more than 98%13.
Statistical Analyses
We used Cox proportional hazards regression models with time since diagnosis as the time scale, accounting for left truncation due to differences between participants in the timing of post-diagnostic assessment14. For lethal prostate cancer, person-time was calculated from the return date of the questionnaire that was used for post-diagnostic assessment until date of diagnosis of distant metastases, death, or the end of follow-up (January 2019), whichever came first. For all-cause mortality, person time was calculated from the return date of the questionnaire used for post-diagnostic assessment until death or the end of follow-up (January 2019). (See Figure 1)
Figure 1.
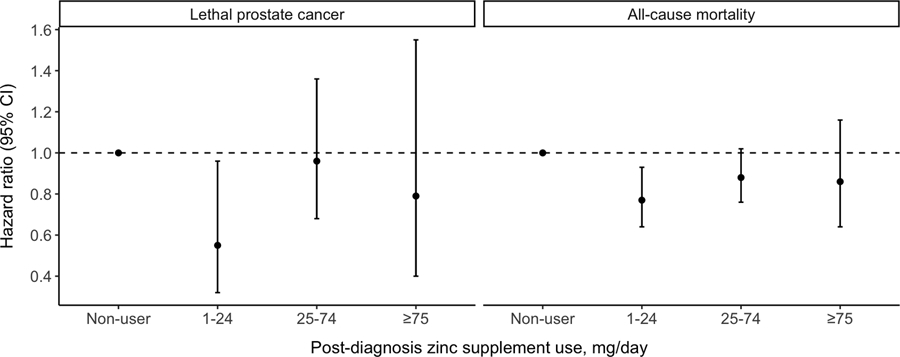
Multivariable hazard ratios (and 95% confidence intervals) of post-diagnosis zinc supplement use in relation to lethal prostate cancer and all-cause mortality among men with nonmetastatic prostate cancer. Multivariable model was adjusted for the same set of covariates as in Model3 in Table2.
We evaluated post-diagnostic zinc supplement by usage (non-user vs. user) and dosage (non-user, 1–24, 25–74, ≥75 mg/day). Basic model was adjusted for age at diagnosis, stage, Gleason score and primary treatment. The second model for lethal prostate cancer additionally included body mass index, vigorous physical activity, smoking, pre-diagnosis zinc supplement, selenium supplement15,16 and total number of different types of supplements (an indicator of behavioral tendency to use supplements). We included pre-diagnosis zinc supplement because we were interested in the association between post-diagnosis supplement use and prognosis, independent of pre-diagnostic use. We also considered models adjusted for PSA at diagnosis, multivitamin, vitamin A and E supplement use. The addition of these variables did not affect the main estimates and were excluded from the final models. For all-cause mortality, we additionally included history of diabetes mellitus, elevated cholesterol, high blood pressure, parental history of myocardial infarction and comorbid conditions. The proportional assumption was tested by plotting Schoenfeld residuals of the exposure against follow-up time and found to be satisfied. Other major causes of death including total cancer and cardiovascular disease mortality were evaluated as secondary outcomes.
The joint association of pre- and post-diagnostic zinc supplement use was assessed by classifying men according to their zinc supplement use status before and after diagnosis and treating non-users in both periods as the reference group. We conducted stratified analysis by primary treatment (radical prostatectomy vs. other), Gleason score (≤3+4, ≥4+3) and stage (T1, T2, T3a). We also examined whether the association differed by dietary zinc intake (<11, below RDA, vs. ≥11 mg/d) and multivitamin use (non-users vs. users). Finally, we examined total zinc intake post-diagnosis and simple updated post-diagnosis zinc supplement use with 2- or 4-year lag.
All analyses were performed using SAS version 9.4 (SAS Institute, Inc; Cary, NC) and results with a two-sided p-value <0.05 were considered statistically significant. The study protocol was approved by the institutional review boards of Harvard T.H. Chan School of Public Health, and those of participating registries as required.
Results
Among 5788 nonmetastatic prostate cancer patients, 851 reported zinc supplements use post-diagnosis. We observed 527 events of lethal prostate cancer and 3198 all-cause deaths during a median follow-up of 11 years. The primary causes of death were cardiovascular disease (26.3%), other cancers (15.5%), and prostate cancer (14.7%). The median time interval between prostate cancer diagnosis and the return of the post-diagnostic questionnaire was 2.4 years. Overall, post-diagnostic zinc supplement non-users and users had similar dietary zinc intake levels and clinical characteristics (all p-values > 0.1) except non-users were more likely to have radical prostatectomy as primary treatment (p<0.001, Table 1). Men who used post-diagnostic zinc supplement were more likely to use other dietary supplements compared to non-users (all p-values < 0.0001).
Table 1.
Age-standardized characteristics at diagnosis among 5788 men with nonmetastatic prostate cancer, by post-diagnosis zinc supplement use, 1986–2016*.
| Levels of supplement zinc dosage, mg/day |
||||
|---|---|---|---|---|
| Non-user | 1–24 mg/d | 25–74 mg/d | ≥75 mg/d | |
| (n=4937) | (n=290) | (n=470) | (n=91) | |
| Age at diagnosis, continuous, mean (SD) | 69.1 (7.0) | 69.9 (7.8) | 69.6 (7.0) | 70.1 (7.7) |
| Year of diagnosis, continuous, mean | 2000 | 2000 | 1999 | 1999 |
| White, % | 97 | 97 | 97 | 97 |
| Family history of prostate cancer†, % | 20 | 23 | 21 | 18 |
| BMI at diagnosis in kg/m2, mean (SD) | 26.0 (3.4) | 25.6 (3.0) | 25.8 (3.2) | 25.6 (3.1) |
| Clinical stage, % | ||||
| T1 | 62 | 56 | 61 | 62 |
| T2 | 35 | 43 | 36 | 36 |
| T3a | 3 | 1 | 3 | 3 |
| Gleason score, % | ||||
| 2–6 | 58 | 60 | 55 | 61 |
| 7 | 28 | 26 | 27 | 26 |
| 8–10 | 10 | 9 | 10 | 10 |
| Missing | 5 | 5 | 8 | 4 |
| Primary PCa treatment, % | ||||
| Radical prostatectomy | 48 | 41 | 44 | 39 |
| Radiation Therapy | 37 | 40 | 36 | 39 |
| Hormones (ADT) | 5 | 6 | 6 | 7 |
| Other | 10 | 13 | 15 | 16 |
| PSA levels at diagnosis, ng/mL % | ||||
| <4 | 12 | 16 | 12 | 11 |
| 4–9.9 | 57 | 59 | 53 | 56 |
| 10–19.9 | 17 | 11 | 19 | 16 |
| ≥20 | 7 | 7 | 8 | 7 |
| Missing | 7 | 7 | 8 | 11 |
| Current smoker, % | 3 | 3 | 4 | 2 |
| Current aspirin use, % | 44 | 49 | 44 | 45 |
| Diabetes, % | 10 | 7 | 11 | 8 |
| Vigorous physical activity in MET-h/week, mean (SD) | 9.1 (17.8) | 9.6 (19.1) | 10.9 (22.0) | 10.9 (20.8) |
| Dietary zinc intake in mg/day, mean (SD) ‡ | 12 (5) | 12 (4) | 12 (4) | 13 (8) |
| Multivitamin use, % | 62 | 78 | 78 | 76 |
| Selenium supplement use, % | 7 | 58 | 49 | 65 |
| Vitamin A supplement use, % | 3 | 42 | 30 | 42 |
| Vitamin E supplement use, % | 33 | 87 | 78 | 87 |
Abbreviations: BMI, body mass index; ADT, androgen-deprivation therapy; PSA, prostate-specific antigen; MET, metabolic equivalent.
Estimates are age-standardized to the age distribution of the study population at prostate cancer diagnosis, except age itself.
Family history of prostate cancer in first-degree relatives.
Nutrients are adjusted for total energy intake using the residual method.
Overall, we observed that post-diagnosis zinc supplements users had better prostate cancer survival (Table 2). Compared to non-users, post-diagnosis zinc supplement use was associated with a suggestively lower risk of lethal prostate cancer (HR [95% CI], 0.82 [0.60–1.13]) and significantly lower all-cause mortality (0.84 [0.74–0.96]). The inverse association was mostly observed at 1–24 mg/day (lethal prostate cancer: 0.55 [0.32–0.96]; all-cause mortality: 0.77 [0.64–0.93]), while higher dosage did not show any association.
Table 2.
Multivariable hazard ratios (and 95% confidence intervals, CI) of post-diagnosis zinc supplement use in relation to lethal prostate cancer and all-cause mortality among men with nonmetastatic prostate cancer.
| Hazard Ratio (95% CI) | |||||
|---|---|---|---|---|---|
| Non-user | User | By dosage |
|||
| Outcome | 1–24 mg/d | 25–74 mg/d | ≥75 mg/d | ||
| Lethal Prostate cancer | |||||
| Person-year | 54390 | 9892 | 3410 | 5479 | 1003 |
| No. of events: | 443 | 84 | 15 | 57 | 12 |
| Crude rate (per 1000 person-years) | 8.1 | 8.5 | 4.4 | 10.4 | 12.0 |
| Model 1* | 1.0 | 0.93 (0.74, 1.18) | 0.51 (0.31, 0.86) | 1.13 (0.85, 1.49) | 1.21 (0.68, 2.15) |
| Model 2† | 1.0 | 0.82 (0.60, 1.13) | 0.55 (0.32, 0.96) | 0.96 (0.68, 1.36) | 0.79 (0.40, 1.55) |
| All-cause mortality | |||||
| Person-year | 55631 | 10023 | 3426 | 5573 | 1024 |
| No. of events: | 2701 | 497 | 151 | 290 | 56 |
| Crude rate (per 1000 person-years) | 48.6 | 49.6 | 44.1 | 52.0 | 54.7 |
| Model 1* | 1.0 | 0.91 (0.83, 1.00) | 0.79 (0.67, 0.93) | 0.97 (0.86, 1.10) | 0.99 (0.76, 1.29) |
| Model 3‡ | 1.0 | 0.84 (0.74, 0.96) | 0.77 (0.64, 0.93) | 0.88 (0.76, 1.02) | 0.86 (0.64, 1.16) |
Model1: adjusted for age at diagnosis, stage (T1, T2, T3a), Gleason score (<7, 7, ≥8, missing) and treatment (radical prostatectomy, radiation, hormones, other).
Model2: additional adjusted for body mass index (<25, 25–30, ≥30 kg/m2), vigorous physical activity (<1, 1 to <3, ≥3 MET-h/week), smoking (never, former/quit > 10 years ago, former/quit ≤ 10 years ago, current), pre-diagnosis zinc supplement use (non-user, 1–24 mg/d, 25–74 mg/d, ≥75 mg/d), selenium supplement use (non-user, current) and total number of supplements use (0, 2–3, ≥4).
Model3: adjusted for same factors as model2 and additionally adjusted for diabetes mellitus (yes or no), elevated cholesterol (yes or no), high blood pressure (yes or no), parental history of myocardial infarction before age 60 years, and comorbid condition (yes or no; conditions included myocardial infarction, coronary artery bypass or angioplasty, stroke, emphysema or chronic obstructive pulmonary disorder, and Parkinson disease).
Compared to non-users both before and after diagnosis, continued-users at 1–24 mg/day had lower risk of all-cause mortality (0.79 [0.63–0.99]), while discontinued users had worse survival (1.24 [1.07–1.44], Table 3). When stratified by dietary zinc intake, patients with 25–74 mg/day zinc supplement intake had significant lower risk of all-cause mortality (0.74 [0.75, 0.96]) in the low diet zinc group (<11 mg/day), while among high diet zinc group (≥11 mg/day), the inverse association was observed for patients with zinc supplement use of 1–25 mg/day (0.70 [0.55, 0.90], Table 4). Similar associations were observed for lethal prostate cancer (eTable1 and 2), although with limited sample size across dosage groups. As for total zinc intake, compared to men with post-diagnosis total zinc intake of <10 mg/day, those with 30–40 mg/day had lower risk of all-cause mortality (eFigure 1).
Table 3.
Multivariable hazard ratios (and 95% confidence intervals, CI) of joint association of pre- and post-diagnosis zinc supplement use in relation to all-cause mortality among men with nonmetastatic prostate cancer.
| Post-diagnosis use | |||||
|---|---|---|---|---|---|
| Non-user | 1–25 mg/d | 25–74 mg/d | >75 mg/d | ||
| All-cause mortality | |||||
| Pre-diagnosis non-user | No. of events | 2506 | 63 | 99 | 12 |
| HR (95% CI)* | 1.0 | 0.93 (0.71, 1.20) | 0.89 (0.72, 1.09) | 1.07 (0.60, 1.89) | |
| Pre-diagnosis user | No. of events | 195 | 88 | 191 | 44 |
| HR (95% CI)* | 1.24 (1.07, 1.44) | 0.79 (0.63, 0.99) | 1.05 (0.90, 1.24) | 1.02 (0.75, 1.40) | |
Adjusted age at diagnosis, stage (T1, T2, T3a), Gleason score (<7, 7, ≥8, missing), treatment (radical prostatectomy, radiation, hormones, other), body mass index (<25, 25–30, ≥30 kg/m2), vigorous physical activity (<1, 1 to <3, ≥3 MET-h/week), smoking (never, former/quit > 10 years ago, former/quit ≤ 10 years ago, current), selenium supplement use (non-user, current), total number of supplements use (0, 2–3, ≥4), diabetes mellitus (yes or no), elevated cholesterol (yes or no), high blood pressure (yes or no), parental history of myocardial infarction before age 60 years, and comorbid condition (yes or no; conditions included myocardial infarction, coronary artery bypass or angioplasty, stroke, emphysema or chronic obstructive pulmonary disorder, and Parkinson disease).
Table 4.
Multivariable hazard ratios (and 95% confidence intervals, CI) of post diagnosis zinc supplement use in relation all-cause mortality among men with nonmetastatic prostate cancer, stratified by dietary zinc intake.
| Post-diagnosis use | ||||||
|---|---|---|---|---|---|---|
| Non-user | User | By dosage |
||||
| Dietary zinc intake | 1–25 mg/d | 25–74 mg/d | >75 mg/d | |||
| All-cause mortality | ||||||
| Low diet zinc | No. of events | 985 | 175 | 59 | 97 | 19 |
| HR (95% CI)* | 1.0 | 0.79 (0.63, 0.98) | 0.90 (0.66, 1.22) | 0.74 (0.57, 0.96) | 0.69 (0.41, 1.15) | |
| High diet zinc | No. of events | 1716 | 322 | 92 | 193 | 37 |
| HR (95% CI)* | 1.0 | 0.89 (0.76, 1.05) | 0.70 (0.55, 0.90) | 0.97 (0.81, 1.17) | 1.10 (0.76, 1.60) | |
Note: Low diet zinc: dietary zinc intake <11 mg/day, below the recommended dietary allowance for zinc; high diet zinc: dietary zinc intake ≥11 mg/day.
Adjusted for age at diagnosis, stage (T1, T2, T3a), Gleason score (<7, 7, ≥8, missing), treatment (radical prostatectomy, radiation, hormones, other), body mass index (<25, 25–30, ≥30 kg/m2), vigorous physical activity (<1, 1 to <3, ≥3 MET-h/week), smoking (never, former/quit > 10 years ago, former/quit ≤ 10 years ago, current), selenium supplement use (non-user, current), total number of supplements use (0, 2–3, ≥4), diabetes mellitus (yes or no), elevated cholesterol (yes or no), high blood pressure (yes or no), parental history of myocardial infarction before age 60 years, and comorbid condition (yes or no; conditions included myocardial infarction, coronary artery bypass or angioplasty, stroke, emphysema or chronic obstructive pulmonary disorder, and Parkinson disease).
In stratified analysis, the inverse associations between low-dose post-diagnosis zinc supplement and prostate cancer survival were observed in men who had radical prostatectomy, among men with low-risk cancer (Gleason score ≤3+4 or T1–2 stage, eTable 3), and in both multivitamin non-user and user group (eTable 4). For death of other major causes (eTable 5), post-diagnostic zinc supplement was associated with lower risks of respiratory diseases (0.43 [0.25, 0.72]) and other disease mortality (0.76 [0.59, 0.99]). When examining simple-update post-diagnosis zinc supplement use with 2- or 4- year lag, similar inverse association was observed for 1–24 mg/d and all-cause mortality (0.78 [0.63, 0.96], eTable 6).
Discussion
In this large prospective cohort study, we found that low-dose (1–24 mg/day) post-diagnostic zinc supplement use was associated with a lower risk of lethal prostate cancer and all-cause mortality among men with nonmetastatic prostate cancer. These associations persisted after adjusting for various confounding factors and in both low and high dietary zinc intake groups. Our findings provide novel evidence for a potential benefit of low-dose zinc supplementation among nonmetastatic prostate cancer patients.
To date, most epidemiologic studies had examined the association between zinc supplementation and prostate cancer incidence9,17–19, providing limited support for a protective role of zinc supplementation in prostate carcinogenesis as suggested by mechanistic studies. Only one Swedish study examined dietary zinc at diagnosis and prostate cancer survival8. It reported that compared to men whose dietary zinc intake <12.8 mg/day, men with adequate dietary zinc (15.6–20.1 mg/day) had lower risk of prostate cancer-specific mortality (0.64 [0.44–0.94]), especially localized tumors patients (0.24 [0.09–0.66]). In current study, we also observed that the inverse associations persisted with low-risk cancers (Gleason score ≤3+4 or T1–2 stage), but not in high-risk groups (Gleason score ≥4+3 or T3a stage). Possible reasons could be the small number of cases in high-risk groups but could also be that early-stage prostate cancer may still respond to modification by an adequate dosage of zinc supplementation, whereas advanced-stage disease have limited reaction to nutritional intervention. Laboratory studies have showed that ZIP1 transporter, which functions to uptake zinc from the circulation, is lost in poorly differentiated prostate tumors3,20.
Previously in the HPFS, we showed excessive zinc supplementation (>100 mg/day) may substantially increase the risk of aggressive prostate cancer among initially cancer-free men9. In current study, we were interested how post-diagnosis zinc supplementation could influence prognosis among nonmetastatic prostate cancer patients. Our restriction to nonmetastatic cancer patients also minimized potential reverse causation from advanced cancer, as men with more advanced stage might be more likely to use zinc supplement for self-care. In addition, zinc supplement use was not more common among men with advanced stage (Table 1). Our joint analysis of pre- and post-diagnosis zinc supplement use showed that compared to non-users in both periods, continued users at a low dosage had lower risk of all-cause mortality, whereas discontinued users had a significantly worse survival. It is possible systemic adaptations to a high zinc level before diagnosis may lead to a withdrawal effect (zinc transporters unable to pick up enough zinc from a low zinc environment) among discontinued users and negatively influence prognosis. Lastly, the reason we did not observe any association between excessive dosage of post-diagnostic zinc supplementation and prostate cancer survival could be the diminished expression of zinc uptake transporters in the tumors, which result in the inability to continually increase intratumorally zinc levels despite higher zinc supplement intake21.
In the stratified analysis by dietary zinc intake (<11, below RDA, vs. ≥11 mg/day), the potential beneficial dosage level of zinc supplement was higher in low diet zinc group compared to the one in high diet zinc group, suggesting there might be an optimal level of adequate zinc intake that is important for prostate cancer survival. Our analysis on total zinc intake suggests, compared to men with inadequate zinc intake (<10 mg/day), having 30–40 mg/day (from foods and low-dose supplemental zinc) may associated with lower risk of all-cause mortality (eFigure 1). Both laboratory22–24 and epidemiological9,19 studies had suggested that zinc at an optimal level is beneficial whereas at both deficient and high levels it may enhance tumor growth.
Relatively few studies have investigated zinc supplementation on all-cause mortality25–27. A randomized clinical trial of antioxidants and zinc supplement on eye diseases showed that participants randomized to zinc (80mg/d) had a reduction in all-cause mortality (0.83 [0.73–0.95], p=0.008), largely related to a decrease in circulatory system disease deaths26. Data from the National Health and Nutrition Examination Survey suggested adequate zinc intake (from foods not supplements) was associated with reduced all-cause or cardiovascular disease mortality27. Besides prostate cancer specific survival, our analysis showed post-diagnosis zinc supplement use seems also lower respiratory diseases and other disease mortality. Zinc has critical effects in immune function, oxidative stress and aging. Concurrent zinc deficiency may complicate many chronic diseases, affect adversely immunological status, increase oxidative stress, and lead to generation of inflammatory cytokines28.
Study strengths include prospective and repeated assessment of supplement use and dosage biennially, long follow-up, and verified disease and death endpoints. However, our study is not without limitations. First, zinc supplement dosage was pre-defined on questionnaires and we were unable to accurately calculate total zinc supplementation from multivitamin and individual zinc supplement. However, our stratified analysis showed similar inverse association in both multivitamin users and non-user groups. Second, as our population were mostly dietary zinc sufficient, we were unable to separate a truly zinc deficient group. Nonetheless, the stratified analysis showed the beneficial effect persisted in both low and high dietary zinc groups. Third, we did not have information on drugs and foods that can block zinc absorption or biological measures of zinc concentration in prostate cancer tissue. Forth, potential measurement errors in the self-reported questionnaires exist. However, given the prospective study design, any mismeasurement in the exposures would tend to be non-differential, typically attenuating associations. Finally, our results may not be generalizable to all populations as our cohort consists of health professionals who were mostly well-nourished. Future studies evaluating how baseline nutritional status modify the effect of post-diagnostic zinc supplementation on prostate cancer prognosis are needed.
In conclusion, we observed that low-dose post-diagnosis zinc supplement use was associated with lower risk of lethal prostate cancer and all-cause mortality among men with nonmetastatic prostate cancer.
Supplementary Material
Acknowledgments:
We would like to thank the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY. The authors assume full responsibility for analyses and interpretation of these data. We are grateful to the participants and research staff of the Health Professionals Follow-up Study.
Funding information:
The project was supported by funding from an administrative supplement from the National Cancer Institute grants. The Health Professionals Follow-up study is supported by grant number U01 CA167552. K.S. is Prostate Cancer Foundation Young Investigator. L.M. is supported by the Prostate Cancer Foundation.
Abbreviations:
- BMI
body mass index
- CI
confidence interval
- FFQ
food frequency questionnaire
- HPFS
Health Professionals Follow-up Study
- HR
Hazard ratio
- PSA
prostate-specific antigen
- RDA
recommended dietary allowance
Footnotes
Conflict of interest: None declared.
Data availability:
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
- 1.Li D, Stovall DB, Wang W, Sui G. Advances of Zinc Signaling Studies in Prostate Cancer. Int J Mol Sci 2020;21(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zaichick V, Sviridova TV, Zaichick SV. Zinc in the human prostate gland: normal, hyperplastic and cancerous. Int Urol Nephrol 1997;29(5):565–574. [DOI] [PubMed] [Google Scholar]
- 3.Kolenko V, Teper E, Kutikov A, Uzzo R. Zinc and zinc transporters in prostate carcinogenesis. Nat Rev Urol 2013;10(4):219–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang J, Zhao H, Xu Z, Cheng X. Zinc dysregulation in cancers and its potential as a therapeutic target. Cancer Biol Med 2020;17(3):612–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Costello LC, Franklin RB. Cytotoxic/tumor suppressor role of zinc for the treatment of cancer: an enigma and an opportunity. Expert Rev Anticancer Ther 2012;12(1):121–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.To PK, Do MH, Cho JH, Jung C. Growth Modulatory Role of Zinc in Prostate Cancer and Application to Cancer Therapeutics. Int J Mol Sci 2020;21(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sauer AK, Vela H, Vela G, Stark P, Barrera-Juarez E, Grabrucker AM. Zinc Deficiency in Men Over 50 and Its Implications in Prostate Disorders. Front Oncol 2020;10:1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Epstein MM, Kasperzyk JL, Andren O, et al. Dietary zinc and prostate cancer survival in a Swedish cohort. Am J Clin Nutr 2011;93(3):586–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leitzmann MF, Stampfer MJ, Wu K, Colditz GA, Willett WC, Giovannucci EL. Zinc supplement use and risk of prostate cancer. J Natl Cancer Inst 2003;95(13):1004–1007. [DOI] [PubMed] [Google Scholar]
- 10.Kim HJ, Giovannucci E, Rosner B, Willett WC, Cho E. Longitudinal and secular trends in dietary supplement use: nurses’ health study and health professionals follow-up study, 1986–2006. Journal of the Academy of Nutrition and Dietetics 2014;114(3):436–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Willett W Nutritional epidemiology Oxford university press; 2012. [Google Scholar]
- 12.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol 1992;135(10):1114–1126; discussion 1127–1136. [DOI] [PubMed] [Google Scholar]
- 13.Rich-Edwards JW, Corsano KA, Stampfer MJ. Test of the National Death Index and Equifax Nationwide Death Search. Am J Epidemiol 1994;140(11):1016–1019. [DOI] [PubMed] [Google Scholar]
- 14.Cain KC, Harlow SD, Little RJ, et al. Bias due to left truncation and left censoring in longitudinal studies of developmental and disease processes. Am J Epidemiol 2011;173(9):1078–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giovannucci E, Liu Y, Platz EA, Stampfer MJ, Willett WC. Risk factors for prostate cancer incidence and progression in the health professionals follow-up study. Int J Cancer 2007;121(7):1571–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kenfield SA, Van Blarigan EL, DuPre N, Stampfer MJ, E LG, Chan JM. Selenium supplementation and prostate cancer mortality. J Natl Cancer Inst 2015;107(1):360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahmad A, Mahmoud AM, Al-Alem U, et al. Zinc Intake and Risk of Prostate Cancer: Case-Control Study and Meta-Analysis. Plos One 2016;11(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kristal AR, Arnold KB, Neuhouser ML, et al. Diet, supplement use, and prostate cancer risk: results from the prostate cancer prevention trial. Am J Epidemiol 2010;172(5):566–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Y, Coogan P, Palmer JR, Strom BL, Rosenberg L. Vitamin and mineral use and risk of prostate cancer: the case-control surveillance study. Cancer Causes Control 2009;20(5):691–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson LA, Kanak MA, Kajdacsy-Balla A, Pestaner JP, Bagasra O. Differential zinc accumulation and expression of human zinc transporter 1 (hZIP1) in prostate glands. Methods 2010;52(4):316–321. [DOI] [PubMed] [Google Scholar]
- 21.Golovine K, Makhov P, Uzzo RG, Shaw T, Kunkle D, Kolenko VM. Overexpression of the zinc uptake transporter hZIP1 inhibits nuclear factor-kappaB and reduces the malignant potential of prostate cancer cells in vitro and in vivo. Clin Cancer Res 2008;14(17):5376–5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong PF, Abubakar S. Comparative transcriptional study of the effects of high intracellular zinc on prostate carcinoma cells. Oncol Rep 2010;23(6):1501–1516. [DOI] [PubMed] [Google Scholar]
- 23.Prasad AS, Mukhtar H, Beck FW, et al. Dietary zinc and prostate cancer in the TRAMP mouse model. J Med Food 2010;13(1):70–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ko YH, Woo YJ, Kim JW, et al. High-dose dietary zinc promotes prostate intraepithelial neoplasia in a murine tumor induction model. Asian J Androl 2010;12(2):164–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwingshackl L, Boeing H, Stelmach-Mardas M, et al. Dietary Supplements and Risk of Cause-Specific Death, Cardiovascular Disease, and Cancer: A Systematic Review and Meta-Analysis of Primary Prevention Trials. Adv Nutr 2017;8(1):27–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chew EY, Clemons TE, Agron E, et al. Long-term effects of vitamins C and E, beta-carotene, and zinc on age-related macular degeneration: AREDS report no. 35. Ophthalmology 2013;120(8):1604–1611 e1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen F, Du M, Blumberg JB, et al. Association Among Dietary Supplement Use, Nutrient Intake, and Mortality Among U.S. Adults: A Cohort Study. Ann Intern Med 2019;170(9):604–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chasapis CT, Loutsidou AC, Spiliopoulou CA, Stefanidou ME. Zinc and human health: an update. Arch Toxicol 2012;86(4):521–534. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


