Abstract
Mental health is influenced by the gut-brain axis; for example, gut dysbiosis has been observed in patients with major depressive disorder (MDD). Gut microbial changes by fecal microbiota transplantation or probiotics treatment reportedly modulates depressive symptoms. However, it remains unclear how gut dysbiosis contributes to mental dysfunction, and how correction of the gut microbiota alleviates neuropsychiatric disorders. Our previous study showed that chronic consumption of Lactobacillus reuteri ATG-F4 (F4) induced neurometabolic alterations in healthy mice. Here, we investigated whether F4 exerted therapeutic effects on depressive-like behavior by influencing the central nervous system. Using chronic unpredictable stress (CUS) to induce anhedonia, a key symptom of MDD, we found that chronic F4 consumption alleviated CUS-induced anhedonic behaviors, accompanied by biochemical changes in the gut, serum, and brain. Serum and brain metabolite concentrations involved in tryptophan metabolism were regulated by CUS and F4. F4 consumption reduced the elevated levels of serotonin (5-HT) in the brain observed in the CUS group. Additionally, the increased expression of Htr1a, a subtype of the 5-HT receptor, in the medial prefrontal cortex (mPFC) of stressed mice was restored to levels observed in stress-naïve mice following F4 supplementation. We further demonstrated the role of Htr1a using AAV-shRNA to downregulate Htr1a in the mPFC of CUS mice, effectively reversing CUS-induced anhedonic behavior. Together, our findings suggest F4 as a potential therapeutic approach for relieving some depressive symptoms and highlight the involvement of the tryptophan metabolism in mitigating CUS-induced depressive-like behaviors through the action of this bacterium.
Keywords: Lactobacillus reuteri, Anhedonia, Prefrontal cortex, Serotonin
INTRODUCTION
Major depressive disorder (MDD), also known as depression, is a neuropsychiatric disease that negatively impacts an individual’s quality of life and burdens on society and the global economy [1-3]. It is characterized by various cognitive and psychophysical symptoms, including decreased motivation and persistent low mood [4]. Despite extensive research, the underlying mechanisms of MDD remains incompletely understood. However, accumulating evidence suggests that depression may be modulated by heterogeneous factors, from intrinsic to extrinsic [5]. Chronic stress originating from internal or external sources is a significant risk factor for depression. Prolonged exposure to stressors can disrupt the normal functioning of the neurotransmitter system, leading to psychophysical malfunction [6, 7]. Decades of animal studies have provided substantial evidence to support the chronic stress hypothesis, demonstrating similarities in symptomology between animal models and patients with depression [8, 9]. The chronic unpredictable stress (CUS) rodent model is widely recognized as a well-validated animal model for stress-induced depression. This model employs a variety of mild stressors to ensure high construct validity. Rodents who have experienced CUS consistently exhibit anhedonia, characterized by the inability to feel pleasure under normally pleasurable conditions, as a prominent feature alongside other depressive-like symptoms [10]. In addition, CUS-exposed mice often shown comprehensive physiological changes, including alterations in neurotransmitter systems [11-14].
Approximately one-third of patients with depression do not respond to conventional antidepressant drug therapies that primarily target the fine-tunning of neurotransmitters [15, 16]. As a result, there has been growing interest in novel therapeutic approaches, along with the pathophysiology of depression. Over the past decade, an increasing number of studies have investigated the relationship between depression and the gut-brain axis [17]. The gut-brain axis is a bidirectional signaling network that involves interactions among the intestinal microbiota, microbial metabolites, enteric nervous system, and neuroendocrine system [18]. Depending on the bacterial strain, the gut microbial environment generates neuroactive compounds, including neurotransmitters, amino acids, and microbial metabolites, that influence the nervous system. Bidirectional communication between bacterial strains and the central nervous system occurs through various biological pathways that modulate brain physiology. Alterations in the gut-brain axis can contribute to neuropsychiatric dysfunction. Conversely, restoring healthy gut microbial conditions may potentially improve mental disorders [19]. Probiotics are living microorganisms that have been suggested as a potential therapeutic strategy for treating depression by restoring the composition of gut microbiota [20, 21]. Preclinical studies have demonstrated the antidepressant-like effects of several probiotics in rodents, despite their underlying biological mechanisms [21]. In our previous study, we found that some Lactobacillus reuteri (L. reuteri) treatments under normal conditions showed gut microbial distribution differences compared to ampicillin-induced gut dysbiosis and neurotransmitter level changes in the serum, which might be indicative of the therapeutic potential for depression. Notably, mice receiving L. reuteri ATG-F4 (F4) exhibited increased serum levels of serotonin (or 5-hydroxytryptamine, 5-HT) compared to ampicillin-induced gut dysbiosis group and dopamine compared to both the normal condition and ampicillin-induced gut dysbiosis groups [22]. However, whether F4 exerts antidepressant-like effects remains unclear.
This study aimed to explore the potential of probiotic treatment in alleviating depressive-like behaviors, specifically anhedonia, induced by chronic stress. To investigate this, we administered F4 during CUS to examine its effects on CUS-induced anhedonic behaviors. We analyzed changes in the gut microbiota and metabolites to identify potential candidates involved in neurometabolic processes in the serum and brain. We identified changes in tryptophan metabolites in the serum and brain. Particularly, we observed increased 5-HT levels in the brains of chronically stressed mice, which were decreased by F4 treatment. Similarly, the enhancement of 5-HT1A receptor in the medial prefrontal cortex (mPFC) by CUS was restored by F4 supplementation. Finally, we confirmed that the prefrontal serotonergic system is associated with anhedonic behavior using adeno-associated virus (AAV)-mediated gene expression level adjustment. Overall, these findings suggest that the F4 strain alleviates chronic stress-induced anhedonic behaviors by modulating tryptophan metabolism and the prefrontal serotonergic system.
MATERIALS AND METHODS
Animals
Entire procedures using animals were approved by the Institutional Animal Care and Use Committee of Korea Brain Research Institute (IACUC-20-00023, IACUC-21-00006). 6-to-12-week-old C57BL6/N male mice (Orient-Bio Company, Busan, Republic of Korea) were employed in this study. Mice were maintained in a plastic cage under 24±2°C, 8:00~20:00 light/dark cycle in SPF grade animal facility. Also, mice were allowed to access chow and water ad libitum. Mice were group-housed during the chronic unpredictable stress (CUS) period. In the L. retueri ATG-F4 experiment, each group started with an initial animal count of 12. Dead mice during the CUS period were excluded from the results. In the Htr1a knockdown experiment, each group started with eight mice per group.
Chronic unpredictable stress (CUS)
CUS was performed as previously described [23, 24]. In brief, eight-week-old mice received 28 days of CUS. Various stressors were intentionally designed to maximize unpredictable effects. All CUS mice were exposed to two or three stressors per day for 28 days. After the 28th day of the CUS, the mice’s body weight was measured. And three days of sucrose preference test were followed. In the CUS schedule, stressors are randomly arranged to prevent subjects from predicting. Well-validated stressors were applied from the following list: cage tilt 45º for overnight, food deprivation for overnight, water deprivation for overnight, strobe light for overnight, bedding removal for overnight, wet bedding for overnight, housed with different inmates for overnight, cage rotation for an hour, swimming stress for 5 minutes, light off during the daytime for 3 hours, restraint stress for an hour.
Sucrose preference test (SPT)
On the last day of the CUS period, mice were single-housed and the water bottles were removed until 4 p.m. After the water restriction, mice were provided with a 1% sucrose-water solution (w/v) and a water bottle. Following three consecutive days, SPT was performed two-bottled choice of water and 1% sucrose-water solution (w/v) from 4 p.m. to the next day at 10 a.m. for three sessions. Each bottle was weighed at 4 p.m. and 10 a.m. to calculate preference for each session. The positions of the bottles were counterbalanced every session to prevent a place preference. Sucrose preference was calculated as a percentage using this formula: average of {1% sucrose solution intake/total (1% sucrose solution+water) intake}*100 (%). And sucrose preference was shown as an average value of three sessions.
Lactobacillus strains
The anonymous donor of Daejeon, South Korea, kindly provided fecal samples of newborn babies and L. reuteri ATG-F4 were isolated in June 2018 [25]. F4 was incubated in De Man Rogosa Sharp broth (Difco Laboratories Inc., NJ , USA) at 37°C for 16 h, then cell pellets were obtained by centrifugation (3000×g, 10 min, 4°C), and washed three times with phosphate-buffered saline (PBS; pH 7.4). For in vivo experiments, the cell pellets were resuspended in cryoprotectant solution and lyophilized using an FD8508 freeze-dryer (ilShinBioBase, Dongduchen, Korea). Based on the survival rate after freeze drying, the administration dose in vivo was calculated. To determine the survival rate, the freeze-dried F4 powder was resuspended in 0.9% sterilized saline and prepared daily for animal experimental periods. To analyze, the freeze-dried F4 powder was suspended in saline, and this was serially diluted to 10-to-8. After a serial dilution, diluted F4 powder was inoculated onto MRS agar and incubated at 37°C for 24 h. The colony number was counted, and the colony-forming unit (CFU) was calculated by colony number×dilution factor.
For scrutinizing the anti-anhedonic effects of F4 on chronic unpredictable stress, mice were separated into three groups. During the CUS timespan, mice received phosphate-buffered saline (PBS) containing freeze-dried F4 powder per os of each dilution, 0 CFU/ml and 5×108 CFU/ml. 250 ul of F4-suspended PBS solution was given using a feeding needle catheter at 10 AM daily. Control group mice have not received CUS, only administered 0 CFU/ml solution. CUS+Vehicle group and CUS+F4 group have undergone the CUS paradigm also with the p.o. administration of each dilution of F4-suspended PBS solution; 0 CFU/ml, 5×108 CFU/ml.
Sample extraction
One day after the last behavior test finished, the mice were anesthetized with the carbon dioxide (CO2) gas. Subsequently, the mice were laparotized and blood collection was performed using cardiac puncture. Cecal sample was collected by a cutoff using operation scissors. And the brain was removed from its head. All procedure was performed rapidly. Also, collected samples were placed immediately in -80°C deep freezer.
To perform real-time quantitative polymerase chain reaction (RT-qPCR) brain samples were sliced 1 mm coronal into the brain matrices. Target regions were micro-dissected from the applicable sections using tissue 14-gauge microdissection punches (BP-10F, Kai Medical). Regions of interest were the medial prefrontal cortex (mPFC), ventral hippocampus (vHIP), and basolateral amygdala (BLA).
Cecal microbiota analysis
Genomic DNA was extracted from fecal samples using the QIAamp PowerFecal Pro DNA Kit (QIAGEN). The quantity and quality of extracted DNA were measured using a Qubit 3.0 Fluorometer (Thermo Fisher Scientific) and agarose gel electrophoresis, respectively. The V4 hypervariable regions of the bacterial 16S rRNA were amplified with unique 8 bp barcodes and sequenced on the Illumina MiSeq PE300 platform according to the standard protocol [25]. Raw reads were analyzed using the QIIME pipeline [26] with the SILVA 132 database [27]. The non-parametric Kruskal-Wallis test was used to compare the differences in diversity indices and microbial taxa. The weighted UniFrac distances were previously obtained and used for PCoA [28].
LC-MS/MS instrumentation and analytical conditions
Tryptophan, kynurenine, kynurenic acid, tryptamine, 5-Hydroxytraptophan (5-HTP), serotonin (5-HT), and 5-Hydroxydindoleacetic acid (5-HIAA) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Ammonium formate (≥99.995% purity), formic acid (≥99.5% purity), high-performance liquid chromatography (HPLC) grade methanol (MeOH), HPLC grade acetonitrile (ACN) and HPLC grade water were purchased from Sigma-Aldrich. To prepare the samples, A 100 μl aliquot of serum was mixed with 500 μl of ice-cold MeOH with 1% formic acid. The brain tissue was homogenized in ice-cold MeOH with 1% formic acid (per gram of tissue by adding 4.0 ml of solution). The solution was mixed by vortex for 20 minutes and left for 2 h (4°C) to solidify the protein precipitate. After centrifugation at 14,000 rpm for 30 min at 4°C, the supernatant was filtrated by using a PVDF syringe filter (0.22 μm pore size) (Millipore, Billerica, MA). The filtered sample was then injected into the liquid chromatography-tandem mass spectrometry (LC-MS/MS) system. LC-MS/MS analyses were carried out using an Exion LC system connected to a QTRAP 4500 mass spectrometer (AB SCIEX, Framingham, MA, USA). For the analysis of Trp, 5-HT, MLT and 5-HIAA, the LC analyses were carried out using an Intrada Amino Acid column (150×2 mm, 3-μm particle size, Imtakt Kyoto, JPN). A 5 μl aliquot was used for the autosampler injection, and the flow rate was 0.6 ml/min at 40°C. A gradient mobile phase, consisting of (A) 100 mM Ammonium formate/ACN (80/20, v/v) solution and (B) 0.3% formic acid in ACN solution, was used. The gradient started at 80% B and was held at this value for 4 min. It then increased linearly to 0% B over 10 minutes, followed by a 2-minute hold at 0% B before returning to 80% B in 0.1 minute. Finally, the gradient was kept at 80% B for 8.9 min to re-equilibrate the column. The total analysis time was 25 min. For the analysis of KYN, KYNA, tryptamine and 5-HTP, the LC analyses were carried out using an ACQUITY UPLC HSS PFP VanGuard Pre-column (5×2.1 mm, 1.8-μm particle size, Waters, MA, USA) and ACQUITY UPLC HSS PFP Column, (100×2.1 mm, 1.8-μm particle size, Waters). A 2 μl aliquot was used for the autosampler injection, and the flow rate was 0.2 ml/min at 40°C. A gradient mobile phase was employed, consisting of (A) 0.2% formic acid in water solution and (B) 0.2% formic acid in MeOH/ACN (50/50, v/v) solution. The gradient started at 0% B and was held at this value for 0.5 min. The gradient increased linearly to 10% B in 2.5 min, 70% B in 1 min and 90% B in 4 min. Finally, the gradient was kept at 0% B for 7 min. The total analysis time was 15 min. The analysis was performed using an electrospray ionization source in positive mode. The operation conditions were as follows: ion spray voltage, 5500 V; curtain gas (CUR), 25 psi; collision gas (CAD), medium; ion source gas 1 (GS1) and ion source gas 2 (GS2), 50 and 50 psi; the turbo spray temperature (TEM), 450°C; entrance potential (EP), 10 V. Nitrogen was used in all cases. Analytes were quantificated by multiple reaction monitoring (MRM) employing the following precursor to product ion transitions and parameters: Trp, m/z 205.0→188.0 with declustering potential (DP) 61 V, collision energy (CE) 13 eV and collision cell exit potential (CXP), 10 V; KYN, m/z 209.0→94.0 with DP 6 V, CE 19 eV and CXP 8 V; KYNA, m/z 190.0→144.0 with DP 6 V, CE 27 eV and CXP 6 V; tryptamine, m/z 161.0→144.0 with DP 1 V, CE 19 eV and CXP 8 V; 5-HTP, m/z 221.1→204.1 with DP 61 V, CE 15 eV and CXP 8 V; 5-HT, m/z 177.1→160.0 with DP 46 V, CE 17 eV and CXP 8 V; MLT, m/z 233.1→174.1 with DP 61 V, CE 21 eV and CXP 12 V; 5-HIAA, m/z 192.0→146.1 with DP 1 V, CE 19 eV and CXP 10 V. SCIEX OS 2.0.0 software (AB SCIEX) was used for data acquisition and processing, and Analyst 3.3 software (AB SCIEX) was used for data analysis.
RNA extraction and RT-qPCR
The expression levels of Htr1a, Htr1b, and Htr2a were indicated by real-time quantitative polymerase chain reaction (RT-qPCR). The primer pair was designed using NCBI Primer-BLAST for the target genes. Gapdh, a housekeeping gene highly expressed in the brain, was chosen to perform relative quantification. Gapdh was also designed using NCBI Primer-BLAST.
Total RNA from brain tissues was extracted using the RNeasy Mini Kit (QIAgen®, USA). Tissue homogenization was performed by adding QIAzol Lysis Buffer RLT (350 μl with 1% β-mercaptoethanol). Further extraction steps followed the manufacturer’s instructions, and each RNA sample was supplementarily treated with DNase I buffer (QIAgen®, USA).
All RNA samples were evaluated for concentration and purity using a 4200 TapeStation (Agilent Technologies, Santa Clara, USA). Each RNA sample has normalized the concentration across the samples, and cDNA was synthesized using iScript reverse-transcriptase (1708891, Bio-Rad, Hercules, USA).
Specific quantification of target cDNA was conducted using the LightCycler® 480 SYBR Green I Master (Roche, Basal, Switzerland) on Light Cycler 480II (Roche, Basal, Switzerland). Each cDNA sample was run in triplicate. Gene expression analysis was performed using the ΔΔCt method [29], with Gapdh as the control gene for normalization. The primers used were as follows: Htr1a forward: 5’-CTTGGCTCATTGGCTTTCTC-3’, and reverse: 5’-CGCCGAAAGTGGAGTAGATG-3’ (Genbank: 15550). Htr1b forward: 5’-GCTTTGTGAACACCGACCAC-3’, and reverse: 5’-AGCGGGCTTCCACATAGATG-3’ (Genbank: 15551). Htr2a forward: 5’-AACCAACCTCTCCTGCGAAG-3’, and reverse: 5’-GGACACTGCCATGATGACCA-3’ (Genbank 15558). Gapdh forward: 5’-AACTTTGGCATTGTGGAAGG-3’, and reverse: 5’-ACACATTGGGGGTAGGAAC-3’ (Genbank 14433).
Stereotaxic surgery
To identify Htr1a downregulation in medial prefrontal cortex (mPFC) effects on depressive symptoms, the adeno-associated virus vector (AAV) suppressing Htr1a expression was stereotactically injected two weeks before the CUS period. Viral gene knockdown was applied to validate the anti-depressive effect of Htr1a downregulation in the mPFC region. Adeno-associated virus (AAV) shRNA and negative control vectors were customized and constructed by VectorBuilder. AAV9 targeted sequence silencing 5’-CCCTGCTCAACCCAGTTATTTCTCGAGAAATAACTGGGTTGAGCAGGG-3’ were used in this study: pAAV [shRNA]-EGFP-U6>mHtr1a [shRNA#1] (VB220603-1002jcx, typical titer: >2×1013 GC/ml, minimum titer: >1013 GC/ml VectorBuilder, Chicago, USA), Ultra-purified scramble shRNA control AAV9 virus pAAV [shRNA]-EGFP-U6>Scramble_shRNA (VB010000-0023jze, minimum titer: >1013 GC/ml, VectorBuilder, Chicago, USA). AAV injections were performed in six weeks old mice with subsequent two weeks of recovery period for AAV expression. Before surgery, mice were anesthetized with an intraperitoneal injection of Ketamine (100 mg/kg)+Xylazine (10 mg/kg) mixture. After sufficient anesthesia, mice were placed on stereotaxic instruments (Stoelting, Illinois, USA). The coordinates of AAV injection were as follows: AP, +1.9 mm; ML, ±0.75 mm; DV, -2.6 mm; angle, 10° (relative to bregma). A total volume of 0.5 µl was bilaterally injected into mPFC a rate of 0.1 µl/min. After surgery, post-operative care was not provided to minimize side effect of pain medication to chronic stress exposure.
Histology
Mice were anesthetized with CO2 and transcardially perfused with 0.1 M phosphate-buffered saline (PBS) and 4% (w/v) paraformaldehyde (PFA) in PBS. Brains were removed, post-fixed overnight in 4% PFA, and then equilibrated in 30% sucrose in PBS at 4°C. 60 μm coronal sections containing mPFC were cut on a cryocut microtome (CM1860, Leica Biosystems) at -20°C. All sections were washed in PBS, mounted on a slide glass, and cover-slipped with VECTASIELD Hardset Antifade Mounting Medium with DAPI (H-1500, Vector Laboratories). Images were acquired using a fluorescence microscope (PANNORAMIC SCAN, 3DHISTEC Ltd.) with a 20× objective lens.
Statistical analysis
All statistical analyses were conducted using Prism 9 (GraphPad Software, USA). Normality (Shapiro-Wilk test) and equal variance (Bartlett’s test) were tested before parametric analysis. In experiment assessing Htr1a knockdown capacity with AAV-shRNA, Mann-Whitney U test was utilized as a post-hoc test. All dataset that met the assumptions of normality and homoscedasticity was analyzed using one-way ANOVA. As post-hoc tests, Fisher’s LSD and Tukey’s test were conducted. For sample comparison of means with unequal variances, Welch’s ANOVA was conducted; the analyses were followed by post hoc tests as unpaired Welch’s t-test. When the dataset did not adhere to the assumptions required for parametric statistics, Kruskal-Wallis test were employed, followed by post hoc tests using uncorrected Dunn’s test, and Dunn’s test. Detailed statistical analysis information was described in each figure legend. Main and interaction effects were considered significant at p<0.05. Post hoc testing was performed when the p-value of the initial test rounded to a value that was p<0.05. Data are presented as the mean±standard error mean (SEM). Significance levels were *p<0.05, **p<0.01, ***p<0.001, and ****p<0.0001.
RESULTS
Anti-anhedonic effect of Lactobacillus reuteri ATG-F4
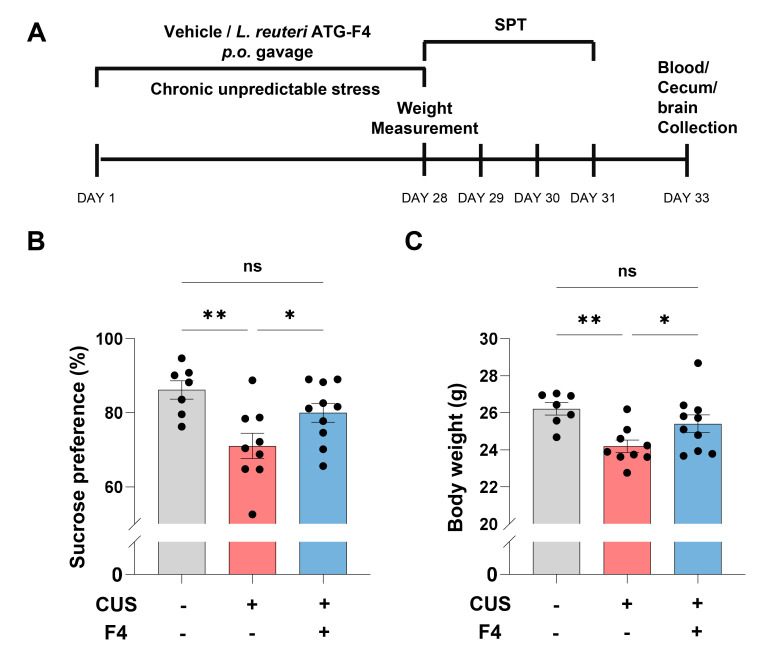
We conducted a sucrose preference test (SPT) to investigate whether F4 intake can alleviate anhedonia induced by CUS (Fig. 1A). Reduced interest in sweet solution indicates lower reward responsiveness, which is a decisive criterion for depression in human patients. Sucrose preference was significantly decreased in mice with CUS and vehicle (CUS+Veh) compared to control mice (Fig 1B). Mice treated with CUS and F4 (CUS+F4) showed rescue of sucrose preference compared to the CUS+Veh group (Fig. 1B). CUS also lowered the body weight, which was restored in the CUS+F4 group (Fig. 1C). These behavioral assays suggest that chronic F4 strain intake could recover CUS-induced anhedonia.
Fig. 1.
CUS-induced anhedonic behaviors were recovered by chronic Lactobacillus reuteri ATG-F4 consumption. (A) Experimental schedule. (B) Sucrose preference after CUS±F4. Uncorrected Fisher’s LSD; Control (n=7), CUS+Veh (n=9), CUS+F4 (n=10). (C) Body weight after CUS±F4. Uncorrected Fisher’s LSD; Control (n=7), CUS+Veh (n=9), CUS+F4 (n=10). *p<0.05, **p<0.01, ns (not significant).
Modulation of caecal microbiota by F4
We analyzed the bacterial community from cecum samples to verify whether behavioral changes were related to the restoration of the gut microbiota by F4 intake. The 16S rRNA sequencing data showed that CUS partially, but not significantly, induced gut dysbiosis (Supplementary Fig. 1). Bacterial richness was measured using the Chao1, which indicates bacterial richness, and Shannon index, which indicates species diversity. Both indices showed that all groups were not significantly different (Supplementary Fig. 1A, B). Unweighted UniFrac-based principal coordinate analysis was performed to compare group differences in the overall microbiota profile (Supplementary Fig. 1C~E). In PCo2, the CUS+F4 group showed an approaching tendency to the control group compared with the CUS+Veh group (Supplementary Fig. 1E). At the genus level, populations of Escherichia, Eisenbergiella, Ruminococcaceae NK4A214 and Lachnospiraceae UCG 009 were significantly increased in the CUS+F4 group compared to CUS+Veh group (Supplementary Fig. 1F~H). This suggests that F4 treatment ameliorates depressive symptoms with gut microbiome changes.
Effects of CUS and F4 on 5-HT and tryptophan metabolic pathways in the serum and brain
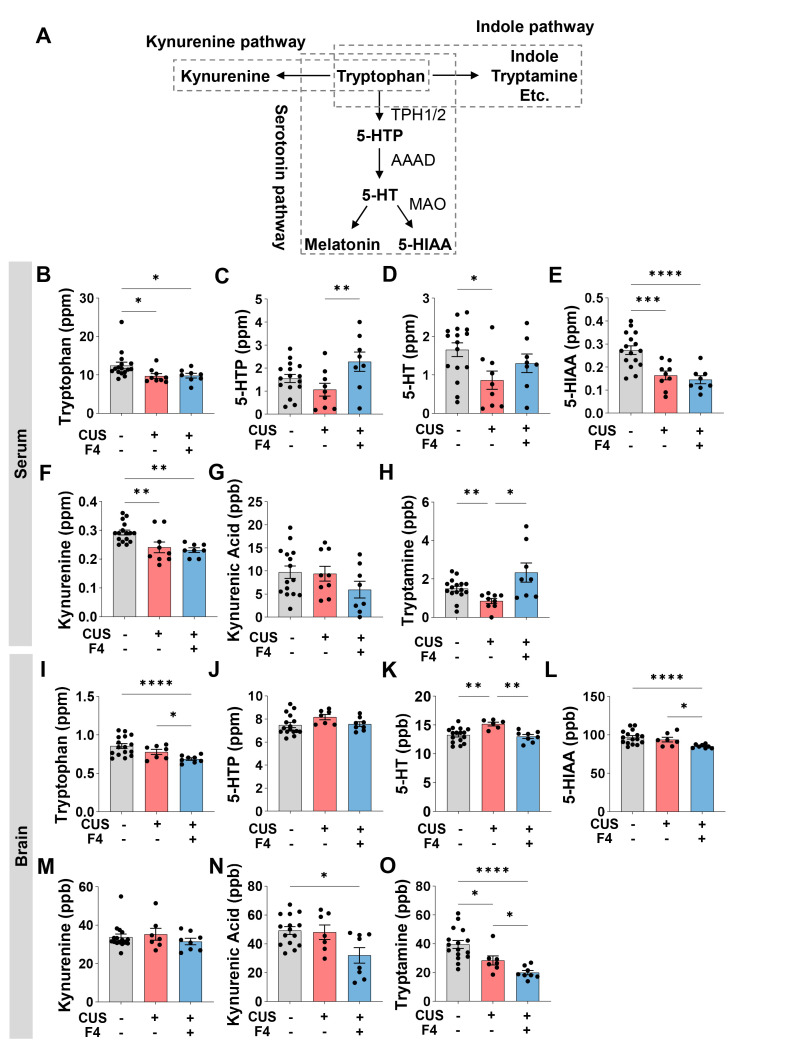
Tryptophan is involved in separate metabolic pathways, including 5-HT synthesis and the kynurenine and indole pathways (Fig. 2A). Therefore, we checked whether CUS and F4 treatment changed tryptophan and its metabolites because 5-HT, a metabolite of tryptophan, is an important neurotransmitter related to depression [30]. First, we found that serum tryptophan was decreased by CUS, but this reduction was not reversed by F4 treatment (Fig. 2B). Although 5-hydroxytrptophan (5-HTP) levels showed a decreasing trend following CUS, it was not significant. However, they were significantly increased by F4 treatment (Fig. 2C). Despite decreased 5-HT levels in the CUS group, which tended to increase after F4 treatment, this change was not significant (Fig. 2D). This was similar to our previous findings, in which serum 5-HT levels decreased as a result of ampicillin-induced gut dysbiosis [22]. Based on these findings, we infer that F4 may improve the decrease in serum 5-HT levels caused by gut dysbiosis, although the exact mechanism is unknown. Similar to serum tryptophan, serum 5-hydroxyindoleacetic acid (5-HIAA), a 5-HT metabolite, and kynurenine, a major metabolite in the kynurenine pathway, were decreased by CUS and F4 treatment (Fig. 2E, F). Serum tryptamine, a major metabolite of the indole pathway, was reduced by CUS and this effect was rescued by F4 treatment (Fig. 2H). However, since changes in tryptophan metabolites in the serum cannot directly explain the antidepressant effects of F4, we examined the changes in tryptophan metabolites in the brain.
Fig. 2.
CUS and F4 consumption changed 5-HT and tryptophan metabolic pathways in the serum and brain. (A) A simplified schematic diagram of tryptophan metabolic pathways. (B~H) Concentrations of tryptophan metabolites in serum. (B) Tryptophan. Unpaired Welch’s t-test; Control (n=16), CUS+Veh (n=9), CUS+F4 (n=8). (C) 5-HTP. Uncorrected Fisher’s LSD; Control (n=16), CUS+Veh (n=9), CUS+F4 (n=8). (D) 5-HT. Uncorrected Fisher’s LSD; Control (n=16), CUS+Veh (n=9), CUS+F4 (n=8). (E) 5-HIAA. Uncorrected Fisher’s LSD; Control (n=16), CUS+Veh (n=9), CUS+F4 (n=8). (F) Kynurenine. Uncorrected Fisher’s LSD; Control (n=16), CUS+Veh (n=9), CUS+F4 (n=8). (G) Kynurenic acid. Uncorrected Fisher’s LSD; Control (n=16), CUS+Veh (n=9), CUS+F4 (n=8). (H) Tryptamine. Unpaired Welch’s t-test; Control (n=16), CUS+Veh (n=9), CUS+F4 (n=8). (I~O) Concentrations of tryptophan metabolites in brain. (I) Tryptophan. Unpaired Welch’s t-test; Control (n=16), CUS+Veh (n=7), CUS+F4 (n=8). (J) 5-HTP. Uncorrected Fisher’s LSD; Control (n=16), CUS+Veh (n=7), CUS+F4 (n=8). (K) 5-HT. Uncorrected Fisher’s LSD; Control (n=16), CUS+Veh (n=7), CUS+F4 (n=8). (L) 5-HIAA. Unpaired Welch’s t-test; Control (n=16), CUS+Veh (n=7), CUS+F4 (n=8). (M) Kynurenine. Uncorrected Dunn’s test. Control (n=16), CUS+Veh (n=7), CUS+F4 (n=8). (N) Kynurenic acid. Uncorrected Dunn’s test; Control (n=16), CUS+Veh (n=7), CUS+F4 (n=8). (O) Tryptamine. Unpaired Welch’s t-test; Control (n=16), CUS+Veh (n=7), CUS+F4 (n=8); 5-HTP, 5-hydroxytryptophan; 5-HT, Serotonin; 5-HIAA, 5-Hydroxydindoleacetic acid. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
Brain tryptophan levels tended to decrease in response to CUS (Fig. 2I). We found that brain tryptophan levels were significantly decreased in the CUS+F4 group (Fig. 2I). These reductions were believed to be due to reduced tryptophan intake and serum tryptophan levels (Fig. 2B). Brain 5-HTP showed no differences among the three groups, but tended to be elevated in the CUS+Veh group (Fig. 2J). Next, brain 5-HT levels were increased by CUS and recovered by F4 treatment (Fig. 2K). While it remains unclear how F4 treatment during CUS lowered brain tryptophan levels, this is likely to be a major contributor to the reduction in brain 5-HT caused by F4. We considered that the increase in brain 5-HT was due to CUS and its decrease by F4 could be a key mechanism underlying the antidepressant effects of F4. CUS did not change the 5-HIAA levels in the brain, but it was significantly reduced in the CUS+F4 group (Fig. 2L). The similarity in the differences in brain 5-HT and 5-HIAA levels between the CUS+Veh and CUS+F4 groups suggests that the conversion of 5-HT to 5-HIAA by monoamine oxidase (MAO) may not be influenced by F4 treatment. Brain tryptamine levels were significantly altered; CUS-induced lower brain tryptamine levels, which were also significantly reduced in the F4 treatment group compared to those in the CUS+Veh group (Fig. 2O). This result implies that the indole pathway is prominently affected by CUS and F4 treatment. Contrastingly, brain kynurenine levels were similar among the control, CUS+Veh, and CUS+F4 groups (Fig. 2M). Additionally, kynurenic acid levels were significantly reduced in the CUS+F4 group (Fig. 2N). This indicates that CUS regulates the kynurenine pathway, and it is possible that F4 further modulated this pathway because there was a decrease in tryptophan in the CUS+F4 group without a corresponding change in kynurenine, but with kynurenic acid changes.
Effects of CUS and F4 on 5-HT receptor mRNA expression in the mPFC
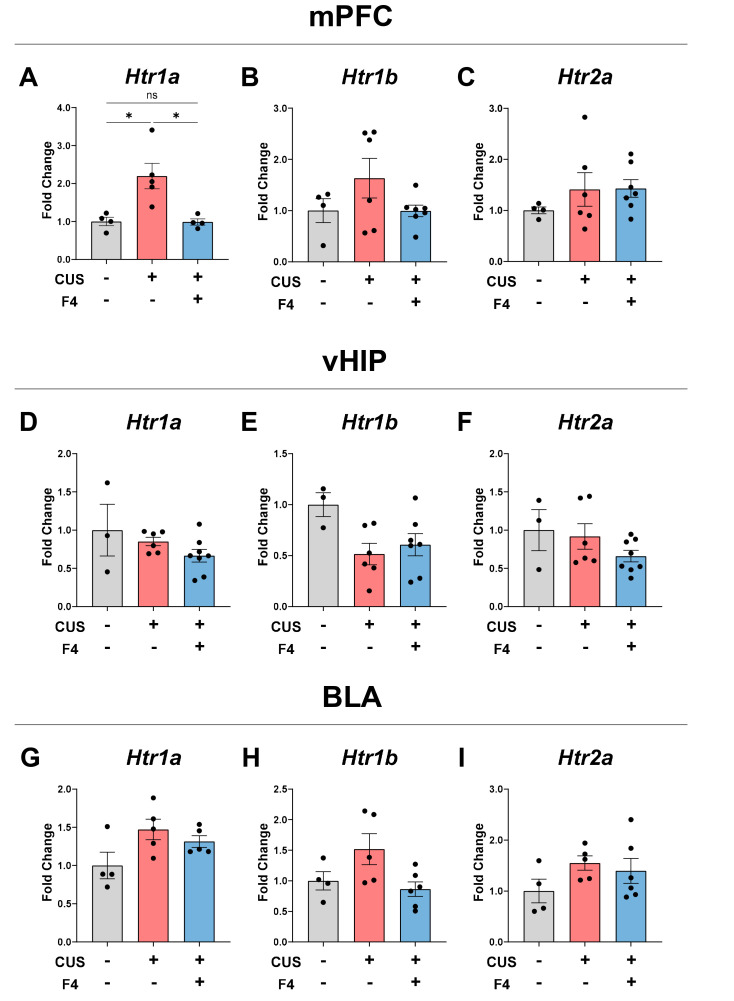
To identify the biological correlates of behavioral and neurochemical changes in the brain, we measured the expression levels of the 5-HT receptors Htr1a, Htr1b, and Htr2a using real-time quantitative polymerase chain reaction (RT-qPCR). We evaluated the medial prefrontal cortex (mPFC), ventral hippocampus (vHIP), and basolateral amygdala (BLA), which are known to be involved in the serotonergic pathway and may also affect depression [30, 31]. Notably, the mRNA level of Htr1a in the mPFC was markedly increased in the CUS+Veh group. Moreover, the increase in mRNA fold change in stressed mice was restored by F4 intake to a level similar to that of the control group (Fig. 3A). In contrast, Htr1b and Htr2a in the mPFC showed no significant differences between all groups (Fig. 3B, C). Regarding vHIP, there was no effect of CUS or F4 on all the three 5-HT receptors (Fig. 3D~F). In the BLA, there were no significant differences in Htr1a mRNA levels between all groups (Fig. 3G~I). These results indicate that the alteration of 5-HT and 5-HT1a receptor in the mPFC is a major pathophysiological mechanism and therapeutic target in CUS-induced anhedonia.
Fig. 3.
CUS-induced upregulation of Htr1a in the mPFC was recovered by F4 treatment. (A~C) mRNA expression levels of 5-HT receptor types in the mPFC. (A) Htr1a. Tukey’s test; Control (n=4), CUS+Veh (n=5), CUS+F4 (n=4). (B) Htr1b. Tukey’s test; Control (n=4), CUS+Veh (n=5), CUS+F4 (n=7). (C) Htr2a. Tukey’s test; Control (n=4), CUS+Veh (n=5), CUS+F4 (n=7). (D~F) mRNA expression levels of 5-HT receptor types in the vHIP. (D) Htr1a. Tukey’s test; Control (n=3), CUS+Veh (n=6), CUS+F4 (n=8). (E) Htr1b. Tukey’s test; Control (n=3), CUS+Veh (n=6), CUS+F4 (n=8). (F) Htr2a. Dunn’s test; Control (n=3), CUS+Veh (n=6), CUS+F4 (n=8). (G~I) mRNA expression levels of 5-HT receptor types in the BLA. (G) Htr1a. Tukey’s test; Control (n=4), CUS+Veh (n=5), CUS+F4 (n=6). (H) Htr1b. Tukey’s test; Control (n=4), CUS+Veh (n=5), CUS+F4 (n=6). (I) Htr2a. Tukey’s test; Control (n=4), CUS+Veh (n=5), CUS+F4 (n=6). *p<0.05, ns (not significant). mPFC, medial prefrontal cortex; vHIP, ventral hippocampus; BLA, basolateral amygdala.
Recapitulation of the anti-anhedonic effect by Htr1a knockdown in the mPFC
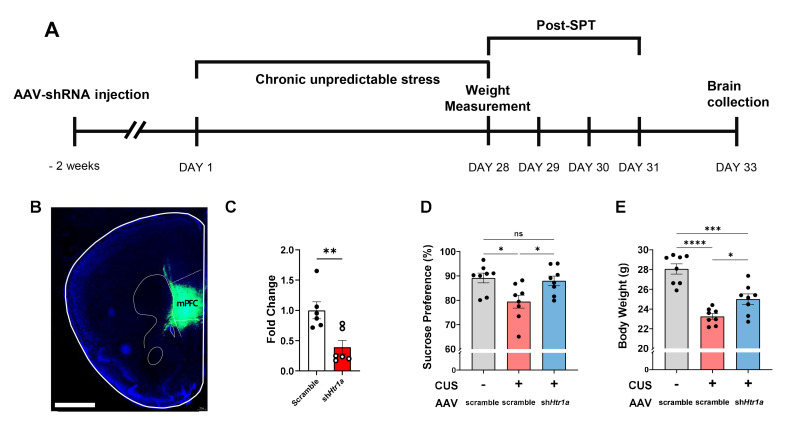
We tested whether the balance of the serotonergic system in the mPFC mimicked the anti-anhedonic effects of the F4 treatment (Fig. 4A). The short hairpin RNA (shRNA) used to knockdown Htr1a was delivered by an adeno-associated virus (AAV) into the mPFC (Fig. 4B), and its efficiency was validated by RT-qPCR (Fig. 4C). Two weeks before CUS, the mice received Htr1a shRNA vector bilaterally in the mPFC (Fig. 4A). Notably, the diminished sucrose preference in the CUS group was rescued by the knockdown of Htr1a in the mPFC (Fig. 4D). In addition, body weight was significantly decreased in the CUS group compared to that in the control group and was restored by Htr1a downregulation (Fig. 4E). These results indicate that the modulation of the serotonergic system in the mPFC controls CUS-induced anhedonia.
Fig. 4.
CUS-induced anhedonic behaviors were alleviated by Htr1a knockdown in the mPFC. (A) Experimental schedule. (B) Representative images of AAV injection site in the mPFC. Scale bar=1,000 μm (C) Efficiency of Htr1a knockdown (KD) by AAV expression in the mPFC. Mann-Whitney U test; Scramble (n=6), Htr1a KD (n=6). (D) Sucrose preference after CUS±Htr1a KD. Tukey’s test; No CUS+AAV-scramble (n=8), CUS+AAV-scramble (n=8), CUS+AAV-shHtr1a (n=8). (E) Body weight after CUS±Htr1a KD. Tukey’s test; No CUS+AAV-scramble (n=8), CUS+AAV-scramble (n=8), CUS+AAV-shHtr1a (n=8). *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001, ns (not significant).
DISCUSSION
This study investigated the effects of the F4 strain on CUS-induced anhedonia and explored its underlying mechanisms. Our results showed that F4 intake improved CUS-induced anhedonic behavior, as measured by the SPT (Fig. 1B). These improvements are likely attributable to the modulation of tryptophan metabolism and the serotonergic system in the mPFC (Fig. 3, 4).
Anhedonia, which is characterized by a diminished ability to experience pleasure, is a prominent behavioral feature of depression [10]. Lower reward responsiveness is a behavioral characteristic of CUS. Thus, the CUS mouse model is a valuable tool for investigating novel treatments for depression owing to its high validity. In the current study, F4 treatment of CUS-exposed animals resulted in the recovery of body weight reduction and elevated responsiveness to reward in the SPT (Fig. 1B, C). These results are consistent with the existing literature, suggesting that the administration of specific bacterial strains can alleviate depressive-like behaviors [31-33]. Thus, our results support the hypothesis that F4 exerts therapeutic effects on depressive symptoms.
In this study, 16S rRNA sequencing data showed no apparent alterations in the distribution of the gut microbial community (Supplementary Fig. 1). Overall, bacterial richness and diversity were not significantly affected by stress or F4 treatment (Supplementary Fig. 1A, B). Unlike previous studies [33, 34], our results indicate that neither stress nor F4 treatment combined with stress can modify gut microbial conditions. However, a few species of bacteria, such as Escherichia, Shigella, Eisenbergiella, Ruminococcaceae NK4A214, and Lachnospiraceae UCG 009, were significantly increased in the CUS+F4 group compared to the CUS+Veh group (Supplementary Fig. 1F~H). Interestingly, a human study revealed that Escherichia and Ruminococcus were the dominant phylotypes in healthy controls compared to patients with MDD [35]. This suggests that F4 intake partially influences the intestinal bacterial community. A previous study on chronic stress showed that probiotics did not change the bacterial community distribution based on 16S rRNA sequencing data [31]. Nevertheless, similar to our findings, some studies have identified differences in the bacterial composition at the genus level [33].
We quantified the primary metabolites in the 5-HT synthesis and tryptophan metabolism pathways in both the serum and brain to analyze how they were related to changes in anhedonia and body weight (Fig. 2). The relative abundance of metabolites between the groups varied in the serum and brain. Diverse biochemical compounds, including 5-HT, undergo independent metabolic pathways within the body that are distinct from those within the brain owing to the presence of the blood-brain barrier [36, 37]. However, changes in tryptophan-related metabolism have been observed in both the brain and serum under stressful conditions [38-40]. To begin with, examining the changes in 5-HT, the serum 5-HT level decreased due to CUS (Fig. 2D), which is consistent with the results of previous studies [33, 41-43]. The synthesis of 5-HT occurs in both the central nervous system and gastrointestinal tract [36, 44]. Regardless of the location in the gut-brain axis, the synthetic cascade is similar [36, 45]. 5-HT is synthesized from the amino acid tryptophan via a short metabolic pathway. Both 5-HTP and 5-HIAA are metabolites of the serotonin synthesis pathway [45]. Approximately 90% of 5-HT is produced in the gastrointestinal tract [36, 44], and 5-HT-related transmission is suggested to be one of the critical signaling pathways through the microbiota-gut-brain axis [46-48]. Several studies have suggested that the antidepressant-like effects of probiotics are associated with 5-HT synthesis and tryptophan metabolism [49-51]. Another animal model of depression utilizing chronic social defeat stress with different L. reuteri strains showed changes in 5-HT levels [33]. Although our results showed that F4 intake did not fully block serum 5-HT reduction (Fig. 2D), Xie et al. [33] demonstrated that 5-HT levels were restored by L. reuteri 3 consumption. However, behavioral changes were restored in both cases [33]. This suggests that serum 5-HT levels may not be directly correlated with behavioral changes. The correlation between the peripheral and central 5-HT systems is controversial because 5-HT cannot cross the blood-brain barrier, resulting in the functional isolation of both 5-HT systems [44]. In the brain, unlike the alterations observed in the serum, 5-HT levels increased in the stress-naïve group and decreased in the ATG-F4-treated group under stressful conditions (Fig. 2K). Polymorphisms related to 5-HT signaling have been implicated in the pathophysiology of depression and in responses to antidepressant treatment [52-54]. In our study, 5-HT levels increased in the CUS+Veh group, which was attenuated by F4 treatment (Fig. 2K). Similar increases in brain 5-HT levels have been reported in patients with depression and animal studies involving chronic stress [55, 56]. Additionally, this is believed to be attributable to an increase in tryptophan hydroxylase 2 (TPH2)-expressing neurons that produce and release serotonin in the dorsal raphe nucleus induced by stress [57]. Examination of the variations in serum 5-HTP, a precursor of 5-HT, revealed an increase in 5-HTP levels in the F4-treated group compared to those in the stress-only group (Fig. 2C). Unlike 5-HT, which cannot cross the blood-brain barrier, 5-HTP can [37]. In a previous study, serum 5-HTP levels significantly increased after dietary intervention in malnutrition in weaning rats, the rats also showed shift in gut microbiota composition. Additionally, the relative abundance of the Lachnospiraceae family increased, which is similar to our finding that the relative abundance of Lachnospiraceae UCG 009 was higher in the F4-treated group than in stressed animals [58]. Lactococcus lactis WHH2078, a lactic acid bacteria like Lactobacillus, increased serum 5-HTP levels through oral administration and reduced depressive symptoms under chronic stress [59]. The final step in the synthesis pathway of 5-HT is its conversion into 5-HIAA. In the brain, 5-HIAA levels significantly decreased in the F4-treated group (Fig. 2L). A study reported that bifidobacterium-treated Sprague-Dawley rats showed attenuated 5-HIAA levels in the frontal cortex [60]. Another study showed that 5-HIAA levels were decreased in the frontal cortex of a group treated with the antidepressant imipramine [61]. However, both 5-HIAA and 5-HT were decreased, so it cannot be directly concluded whether there was a decrease in the conversion of 5-HT to 5-HIAA.
Tryptophan is the primary component in 5-HT biosynthesis [45, 62]. However, the tryptophan metabolic pathway is not limited to 5-HT synthesis [45, 62, 63]. More than 90% of tryptophan is metabolized to kynurenine, which also participates in various metabolic pathways [45, 62]. Serum tryptophan levels significantly decreased under stress conditions (Fig. 2B). Lower plasma tryptophan levels have been observed in patients with MDD [64]. This suggests that the abundance of serum tryptophan may be a potential indicator of a stress condition. There were no significant differences in kynurenine levels in the brain (Fig. 2M). Contrastingly, serum kynurenine levels were lower in the CUS+Veh and CUS+F4 groups than those in the control group (Fig. 2F). Lower kynurenine levels were identified in a meta-analysis of MDD [65]. However, brain kynurenine levels were no significant differences among the groups. Unlike alterations in kynurenine levels, changes in kynurenic acid levels were observed only in the brain. Kynurenic acid levels were significantly lower in the CUS+F4 group than in the control group. The kynurenine pathway is also associated with inflammation. Kynurenic acid is an N-methyl-D-aspartate receptor (NMDAR) antagonist known for its protective effects against excitotoxic and apoptotic damage [63, 66]. However, in schizophrenia, elevated levels of kynurenine acid in the brain can lead to an excessive blockade of NMDAR, a known trigger of psychiatric symptoms [66]. A previous study reported that kynurenic acid in the postmortem PFC of the brain was increased in patients with schizophrenia patients who had higher levels of proinflammatory cytokines. This indicates that dysregulation of the kynurenine pathway can lead to neuropsychiatric diseases [67].
Tryptamine is a biochemical involved in the indole pathway. Tryptamine levels in the brain were significantly altered (Fig. 2O). In addition to the decrease in tryptamine levels in the CUS+Veh group compared to the control group, the CUS+F4 group exhibited a significant reduction in tryptamine levels compared to the CUS+Veh and control groups. Additionally, the serum tryptamine levels were altered (Fig. 2H). In the serum, the level of tryptamine was significantly lower in the CUS+Veh group than in the control group. Further, a significant increase in tryptamine levels was observed in the CUS+F4 group compared to that in the CUS+Veh group. Only a few studies have recognized tryptamine as a subtle neuromodulator of trace amine-associated receptor 1 (TAAR1) with the ability to influence neuronal cell responses independent of binding to postsynaptic receptors [68, 69]. Additionally, activation of TAAR1 suggests potential treatments for neuropsychiatric diseases [70, 71]. Thus, changes in tryptamine levels under stress conditions and F4 treatment are a possible candidates for the antidepressant-like effects of F4. While changes in brain 5-HT alterations are expected to play a significant role in behavioral changes during chronic stress, F4 influences various aspects of the tryptophan-involved metabolic pathways, including 5-HT synthesis, the kynurenine pathway, and the indole pathway. Since tryptophan metabolites are known to affect neuropsychiatric symptoms, further research is required to confirm the direct influence of F4.
We also analyzed the expression of 5-HT receptors in the mPFC, vHIP, and BLA, which are involved in depression and the serotonin pathway [53]. Htr1a mRNA levels were significantly increased in the CUS group, while F4 treatment reduced Htr1a mRNA expression, specifically in the mPFC. A previous study of the region- and receptor-specific effects of chronic psychosocial stress showed similar increases in Htr1a expression in the mPFC of stressed animals [72]. Another study investigated the modulation of the monoaminergic system by antidepressants with different mechanisms of action reported increased Htr1a expression in CUS-treated rats, which was blocked by three antidepressants [73]. Furthermore, increased Htr1a receptor RNA and protein expression in the CUS model was associated with DNA methylation at the 681 CpG promoter site, and the antidepressant imipramine reversed these changes at the Htr1a promoter CpG site [74]. In addition, chronic restraint stress revealed differential regulation of the serotonin-related transcription factors Freud-1 and NUDR, with Htr1a mRNA upregulation in the mPFC [75]. Higher levels of Htr1a in the mPFC of chronically stressed animals are thought to mediate a feedback loop that alters the serotonergic raphe neurons [76]. Therefore, increased Htr1a levels may incidentally affect serotonergic modulation in the brain. Further functional investigations of the mPFC-projecting 5-HTergic pathway may provide a comprehensive understanding of the anti-anhedonic effects of F4.
In summary, our findings demonstrate that the consumption of L. reuteri ATG-F4 alleviates symptoms related to CUS-induced anhedonia through physiological changes in the gut, serum, and brain. Specifically, F4 treatment modulates tryptophan metabolism changes, which can cause the reversal of 5-HT levels in the brain and decrease Htr1a expression in the mPFC.
Supplemental Materials
ACKNOWLEDGEMENTS
This research was supported by the KBRI Basic Research Program through Korea Brain Research Institute funded by Ministry of Science and ICT (23-BR-02-02 and 23-BR-02-07) and by AtoGen Co., Ltd..
References
- 1.Patel V, Chisholm D, Parikh R, Charlson FJ, Degenhardt L, Dua T, Ferrari AJ, Hyman S, Laxminarayan R, Levin C, Lund C, Medina Mora ME, Petersen I, Scott J, Shidhaye R, Vijayakumar L, Thornicroft G, Whiteford H DCP MNS Author Group, author. Addressing the burden of mental, neurological, and substance use disorders: key messages from Disease Control Priorities, 3rd edition. Lancet. 2016;387:1672–1685. doi: 10.1016/S0140-6736(15)00390-6. [DOI] [PubMed] [Google Scholar]
- 2.Haroz EE, Ritchey M, Bass JK, Kohrt BA, Augustinavicius J, Michalopoulos L, Burkey MD, Bolton P. How is depression experienced around the world? A systematic review of qualitative literature. Soc Sci Med. 2017;183:151–162. doi: 10.1016/j.socscimed.2016.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lund C, Brooke-Sumner C, Baingana F, Baron EC, Breuer E, Chandra P, Haushofer J, Herrman H, Jordans M, Kieling C, Medina-Mora ME, Morgan E, Omigbodun O, Tol W, Patel V, Saxena S. Social determinants of mental disorders and the sustainable development goals: a systematic review of reviews. Lancet Psychiatry. 2018;5:357–369. doi: 10.1016/S2215-0366(18)30060-9. [DOI] [PubMed] [Google Scholar]
- 4.American Psychiatric Association, author. Diagnostic and statistical manual of mental disorders: DSM-5. 5th ed. American Psychiatric Publishing; Arlington, VA: 2013. [Google Scholar]
- 5.Otte C, Gold SM, Penninx BW, Pariante CM, Etkin A, Fava M, Mohr DC, Schatzberg AF. Major depressive disorder. Nat Rev Dis Primers. 2016;2:16065. doi: 10.1038/nrdp.2016.65. [DOI] [PubMed] [Google Scholar]
- 6.Tafet GE, Bernardini R. Psychoneuroendocrinological links between chronic stress and depression. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:893–903. doi: 10.1016/S0278-5846(03)00162-3. [DOI] [PubMed] [Google Scholar]
- 7.Tafet GE, Nemeroff CB. The links between stress and depression: psychoneuroendocrinological, genetic, and environmental interactions. J Neuropsychiatry Clin Neurosci. 2016;28:77–88. doi: 10.1176/appi.neuropsych.15030053. [DOI] [PubMed] [Google Scholar]
- 8.Gururajan A, Reif A, Cryan JF, Slattery DA. The future of rodent models in depression research. Nat Rev Neurosci. 2019;20:686–701. doi: 10.1038/s41583-019-0221-6. [DOI] [PubMed] [Google Scholar]
- 9.Krishnan V, Nestler EJ. Animal models of depression: molecular perspectives. Curr Top Behav Neurosci. 2011;7:121–147. doi: 10.1007/7854_2010_108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Willner P. Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology (Berl) 1997;134:319–329. doi: 10.1007/s002130050456. [DOI] [PubMed] [Google Scholar]
- 11.Matuszewich L, McFadden LM, Friedman RD, Frye CA. Neurochemical and behavioral effects of chronic unpredictable stress. Behav Pharmacol. 2014;25:557–566. doi: 10.1097/FBP.0000000000000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sequeira-Cordero A, Salas-Bastos A, Fornaguera J, Brenes JC. Behavioural characterisation of chronic unpredictable stress based on ethologically relevant paradigms in rats. Sci Rep. 2019;9:17403. doi: 10.1038/s41598-019-53624-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Riel E, Meijer OC, Steenbergen PJ, Joëls M. Chronic unpredictable stress causes attenuation of serotonin responses in cornu ammonis 1 pyramidal neurons. Neuroscience. 2003;120:649–658. doi: 10.1016/S0306-4522(03)00355-5. [DOI] [PubMed] [Google Scholar]
- 14.Lages YVM, Rossi AD, Krahe TE, Landeira-Fernandez J. Effect of chronic unpredictable mild stress on the expression profile of serotonin receptors in rats and mice: a meta-analysis. Neurosci Biobehav Rev. 2021;124:78–88. doi: 10.1016/j.neubiorev.2021.01.020. [DOI] [PubMed] [Google Scholar]
- 15.Al-Harbi KS. Treatment-resistant depression: therapeutic trends, challenges, and future directions. Patient Prefer Adherence. 2012;6:369–388. doi: 10.2147/PPA.S29716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, Niederehe G, Thase ME, Lavori PW, Lebowitz BD, McGrath PJ, Rosenbaum JF, Sackeim HA, Kupfer DJ, Luther J, Fava M. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163:1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- 17.Choi TY, Choi YP, Koo JW. Mental disorders linked to crosstalk between the gut microbiome and the brain. Exp Neurobiol. 2020;29:403–416. doi: 10.5607/en20047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Foster JA, McVey Neufeld KA. Gut-brain axis: how the microbiome influences anxiety and depression. Trends Neurosci. 2013;36:305–312. doi: 10.1016/j.tins.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 19.Cenit MC, Sanz Y, Codoñer-Franch P. Influence of gut microbiota on neuropsychiatric disorders. World J Gastroenterol. 2017;23:5486–5498. doi: 10.3748/wjg.v23.i30.5486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu RT, Walsh RFL, Sheehan AE. Prebiotics and probiotics for depression and anxiety: a systematic review and meta-analysis of controlled clinical trials. Neurosci Biobehav Rev. 2019;102:13–23. doi: 10.1016/j.neubiorev.2019.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yong SJ, Tong T, Chew J, Lim WL. Antidepressive mechanisms of probiotics and their therapeutic potential. Front Neurosci. 2020;13:1361. doi: 10.3389/fnins.2019.01361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beck BR, Park GS, Jeong DY, Lee YH, Im S, Song WH, Kang J. Multidisciplinary and comparative investigations of potential psychobiotic effects of Lactobacillus strains isolated from newborns and their impact on gut microbiota and ileal transcriptome in a healthy murine model. Front Cell Infect Microbiol. 2019;9:269. doi: 10.3389/fcimb.2019.00269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koo JW, Duman RS. IL-1beta is an essential mediator of the antineurogenic and anhedonic effects of stress. Proc Natl Acad Sci U S A. 2008;105:751–756. doi: 10.1073/pnas.0708092105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koo JW, Russo SJ, Ferguson D, Nestler EJ, Duman RS. Nuclear factor-κB is a critical mediator of stress-impaired neurogenesis and depressive behavior. Proc Natl Acad Sci U S A. 2010;107:2669–2674. doi: 10.1073/pnas.0910658107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Huntley J, Fierer N, Owens SM, Betley J, Fraser L, Bauer M, Gormley N, Gilbert JA, Smith G, Knight R. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012;6:1621–1624. doi: 10.1038/ismej.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Peña AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yilmaz P, Parfrey LW, Yarza P, Gerken J, Pruesse E, Quast C, Schweer T, Peplies J, Ludwig W, Glöckner FO. The SILVA and "All-Species Living Tree Project (LTP)" taxonomic frameworks. Nucleic Acids Res. 2014;42:D643–D648. doi: 10.1093/nar/gkt1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lozupone C, Lladser ME, Knights D, Stombaugh J, Knight R. UniFrac: an effective distance metric for microbial community comparison. ISME J. 2011;5:169–172. doi: 10.1038/ismej.2010.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 30.Moncrieff J, Cooper RE, Stockmann T, Amendola S, Hengartner MP, Horowitz MA. The serotonin theory of depression: a systematic umbrella review of the evidence. Mol Psychiatry. 2022 doi: 10.1038/s41380-022-01661-0. doi: 10.1038/s41380-022-01661-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bharwani A, Mian MF, Surette MG, Bienenstock J, Forsythe P. Oral treatment with Lactobacillus rhamnosus attenuates behavioural deficits and immune changes in chronic social stress. BMC Med. 2017;15:7. doi: 10.1186/s12916-016-0771-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bercik P, Park AJ, Sinclair D, Khoshdel A, Lu J, Huang X, Deng Y, Blennerhassett PA, Fahnestock M, Moine D, Berger B, Huizinga JD, Kunze W, McLean PG, Bergonzelli GE, Collins SM, Verdu EF. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication. Neurogastroenterol Motil. 2011;23:1132–1139. doi: 10.1111/j.1365-2982.2011.01796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xie R, Jiang P, Lin L, Jiang J, Yu B, Rao J, Liu H, Wei W, Qiao Y. Oral treatment with Lactobacillus reuteri attenuates depressive-like behaviors and serotonin metabolism alterations induced by chronic social defeat stress. J Psychiatr Res. 2020;122:70–78. doi: 10.1016/j.jpsychires.2019.12.013. [DOI] [PubMed] [Google Scholar]
- 34.Huang L, Lv X, Ze X, Ma Z, Zhang X, He R, Fan J, Zhang M, Sun B, Wang F, Liu H. Combined probiotics attenuate chronic unpredictable mild stress-induced depressive-like and anxiety-like behaviors in rats. Front Psychiatry. 2022;13:990465. doi: 10.3389/fpsyt.2022.990465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiang H, Ling Z, Zhang Y, Mao H, Ma Z, Yin Y, Wang W, Tang W, Tan Z, Shi J, Li L, Ruan B. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav Immun. 2015;48:186–194. doi: 10.1016/j.bbi.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 36.El-Merahbi R, Löffler M, Mayer A, Sumara G. The roles of peripheral serotonin in metabolic homeostasis. FEBS Lett. 2015;589:1728–1734. doi: 10.1016/j.febslet.2015.05.054. [DOI] [PubMed] [Google Scholar]
- 37.Nakatani Y, Sato-Suzuki I, Tsujino N, Nakasato A, Seki Y, Fumoto M, Arita H. Augmented brain 5-HT crosses the blood-brain barrier through the 5-HT transporter in rat. Eur J Neurosci. 2008;27:2466–2472. doi: 10.1111/j.1460-9568.2008.06201.x. [DOI] [PubMed] [Google Scholar]
- 38.Correia AS, Vale N. Tryptophan metabolism in depression: a narrative review with a focus on serotonin and kynurenine pathways. Int J Mol Sci. 2022;23:8493. doi: 10.3390/ijms23158493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miura H, Ozaki N, Sawada M, Isobe K, Ohta T, Nagatsu T. A link between stress and depression: shifts in the balance between the kynurenine and serotonin pathways of tryptophan metabolism and the etiology and pathophysiology of depression. Stress. 2008;11:198–209. doi: 10.1080/10253890701754068. [DOI] [PubMed] [Google Scholar]
- 40.Karu N, McKercher C, Nichols DS, Davies N, Shellie RA, Hilder EF, Jose MD. Tryptophan metabolism, its relation to inflammation and stress markers and association with psychological and cognitive functioning: Tasmanian Chronic Kidney Disease pilot study. BMC Nephrol. 2016;17:171. doi: 10.1186/s12882-016-0387-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou Y, Cong Y, Liu H. Folic acid ameliorates depression-like behaviour in a rat model of chronic unpredictable mild stress. BMC Neurosci. 2020;21:1. doi: 10.1186/s12868-020-0551-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao J, Niu C, Wang J, Yang H, Du Y, Wei L, Li C. The depressive-like behaviors of chronic unpredictable mild stress-treated mice, ameliorated by Tibetan medicine Zuotai: involvement in the hypothalamic-pituitary-adrenal (HPA) axis pathway. Neuropsychiatr Dis Treat. 2018;14:129–141. doi: 10.2147/NDT.S151107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang X, Li W, You B, Tang W, Gan T, Feng C, Li C, Yang R. Serum metabonomic study on the antidepressant-like effects of ellagic acid in a chronic unpredictable mild stress-induced mouse model. J Agric Food Chem. 2020;68:9546–9556. doi: 10.1021/acs.jafc.0c02895. [DOI] [PubMed] [Google Scholar]
- 44.Berger M, Gray JA, Roth BL. The expanded biology of serotonin. Annu Rev Med. 2009;60:355–366. doi: 10.1146/annurev.med.60.042307.110802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Höglund E, Øverli Ø, Winberg S. Tryptophan metabolic pathways and brain serotonergic activity: a comparative review. Front Endocrinol (Lausanne) 2019;10:158. doi: 10.3389/fendo.2019.00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Layunta E, Buey B, Mesonero JE, Latorre E. Crosstalk between intestinal serotonergic system and pattern recognition receptors on the microbiota-gut-brain axis. Front Endocrinol (Lausanne) 2021;12:748254. doi: 10.3389/fendo.2021.748254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carabotti M, Scirocco A, Maselli MA, Severi C. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol. 2015;28:203–209. [PMC free article] [PubMed] [Google Scholar]
- 48.Yano JM, Yu K, Donaldson GP, Shastri GG, Ann P, Ma L, Nagler CR, Ismagilov RF, Mazmanian SK, Hsiao EY. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161:264–276. doi: 10.1016/j.cell.2015.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wei CL, Wang S, Yen JT, Cheng YF, Liao CL, Hsu CC, Wu CC, Tsai YC. Antidepressant-like activities of live and heat-killed Lactobacillus paracasei PS23 in chronic corticosterone-treated mice and possible mechanisms. Brain Res. 2019;1711:202–213. doi: 10.1016/j.brainres.2019.01.025. [DOI] [PubMed] [Google Scholar]
- 50.Tian P, Wang G, Zhao J, Zhang H, Chen W. Bifidobacterium with the role of 5-hydroxytryptophan synthesis regulation alleviates the symptom of depression and related microbiota dysbiosis. J Nutr Biochem. 2019;66:43–51. doi: 10.1016/j.jnutbio.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 51.Liang S, Wang T, Hu X, Luo J, Li W, Wu X, Duan Y, Jin F. Administration of Lactobacillus helveticus NS8 improves behavioral, cognitive, and biochemical aberrations caused by chronic restraint stress. Neuroscience. 2015;310:561–577. doi: 10.1016/j.neuroscience.2015.09.033. [DOI] [PubMed] [Google Scholar]
- 52.Ślifirski G, Król M, Turło J. 5-HT receptors and the development of new antidepressants. Int J Mol Sci. 2021;22:9015. doi: 10.3390/ijms22169015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Andrews PW, Bharwani A, Lee KR, Fox M, Thomson JA., Jr Is serotonin an upper or a downer? The evolution of the serotonergic system and its role in depression and the antidepressant response. Neurosci Biobehav Rev. 2015;51:164–188. doi: 10.1016/j.neubiorev.2015.01.018. [DOI] [PubMed] [Google Scholar]
- 54.Yohn CN, Gergues MM, Samuels BA. The role of 5-HT receptors in depression. Mol Brain. 2017;10:28. doi: 10.1186/s13041-017-0306-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kennett GA, Chaouloff F, Marcou M, Curzon G. Female rats are more vulnerable than males in an animal model of depression: the possible role of serotonin. Brain Res. 1986;382:416–421. doi: 10.1016/0006-8993(86)91355-7. [DOI] [PubMed] [Google Scholar]
- 56.Adell A, Garcia-Marquez C, Armario A, Gelpi E. Chronic stress increases serotonin and noradrenaline in rat brain and sensitizes their responses to a further acute stress. J Neurochem. 1988;50:1678–1681. doi: 10.1111/j.1471-4159.1988.tb02462.x. [DOI] [PubMed] [Google Scholar]
- 57.Zhao H, Mohamed NE, Chan SJ, Tan CT, Tao R, Yu VC, Wong PT. Absence of stress response in dorsal raphe nucleus in modulator of apoptosis 1-deficient mice. Mol Neurobiol. 2019;56:2185–2201. doi: 10.1007/s12035-018-1205-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wei Z, Zhou N, Zou L, Shi Z, Dun B, Ren G, Yao Y. Soy protein alleviates malnutrition in weaning rats by regulating gut microbiota composition and serum metabolites. Front Nutr. 2021;8:774203. doi: 10.3389/fnut.2021.774203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gao K, Farzi A, Ke X, Yu Y, Chen C, Chen S, Yu T, Wang H, Li Y. Oral administration of Lactococcus lactis WHH2078 alleviates depressive and anxiety symptoms in mice with induced chronic stress. Food Funct. 2022;13:957–969. doi: 10.1039/D1FO03723D. [DOI] [PubMed] [Google Scholar]
- 60.Desbonnet L, Garrett L, Clarke G, Bienenstock J, Dinan TG. The probiotic bifidobacteria infantis: an assessment of potential antidepressant properties in the rat. J Psychiatr Res. 2008;43:164–174. doi: 10.1016/j.jpsychires.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 61.Xu Y, Ku BS, Yao HY, Lin YH, Ma X, Zhang YH, Li XJ. Antidepressant effects of curcumin in the forced swim test and olfactory bulbectomy models of depression in rats. Pharmacol Biochem Behav. 2005;82:200–206. doi: 10.1016/j.pbb.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 62.Roth W, Zadeh K, Vekariya R, Ge Y, Mohamadzadeh M. Tryptophan metabolism and gut-brain homeostasis. Int J Mol Sci. 2021;22:2973. doi: 10.3390/ijms22062973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Réus GZ, Jansen K, Titus S, Carvalho AF, Gabbay V, Quevedo J. Kynurenine pathway dysfunction in the pathophysiology and treatment of depression: evidences from animal and human studies. J Psychiatr Res. 2015;68:316–328. doi: 10.1016/j.jpsychires.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Samad N, Yasmin F, Manzoor N. Biomarkers in drug free subjects with depression: correlation with tryptophan. Psychiatry Investig. 2019;16:948–953. doi: 10.30773/pi.2019.0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ogyu K, Kubo K, Noda Y, Iwata Y, Tsugawa S, Omura Y, Wada M, Tarumi R, Plitman E, Moriguchi S, Miyazaki T, Uchida H, Graff-Guerrero A, Mimura M, Nakajima S. Kynurenine pathway in depression: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2018;90:16–25. doi: 10.1016/j.neubiorev.2018.03.023. [DOI] [PubMed] [Google Scholar]
- 66.Perkins MN, Stone TW. An iontophoretic investigation of the actions of convulsant kynurenines and their interaction with the endogenous excitant quinolinic acid. Brain Res. 1982;247:184–187. doi: 10.1016/0006-8993(82)91048-4. [DOI] [PubMed] [Google Scholar]
- 67.Kindler J, Lim CK, Weickert CS, Boerrigter D, Galletly C, Liu D, Jacobs KR, Balzan R, Bruggemann J, O'Donnell M, Lenroot R, Guillemin GJ, Weickert TW. Dysregulation of kynurenine metabolism is related to proinflammatory cytokines, attention, and prefrontal cortex volume in schizophrenia. Mol Psychiatry. 2020;25:2860–2872. doi: 10.1038/s41380-019-0401-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Miller GM. The emerging role of trace amine-associated receptor 1 in the functional regulation of monoamine transporters and dopaminergic activity. J Neurochem. 2011;116:164–176. doi: 10.1111/j.1471-4159.2010.07109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zucchi R, Chiellini G, Scanlan TS, Grandy DK. Trace amine-associated receptors and their ligands. Br J Pharmacol. 2006;149:967–978. doi: 10.1038/sj.bjp.0706948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Grandy DK, Miller GM, Li JX. "TAARgeting Addiction"--the Alamo bears witness to another revolution: an overview of the plenary symposium of the 2015 Behavior, Biology and Chemistry Conference. Drug Alcohol Depend. 2016;159:9–16. doi: 10.1016/j.drugalcdep.2015.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gainetdinov RR, Hoener MC, Berry MD. Trace amines and their receptors. Pharmacol Rev. 2018;70:549–620. doi: 10.1124/pr.117.015305. [DOI] [PubMed] [Google Scholar]
- 72.Carneiro-Nascimento S, Powell W, Uebel M, Buerge M, Sigrist H, Patterson M, Pryce CR, Opacka-Juffry J. Region- and receptor-specific effects of chronic social stress on the central serotonergic system in mice. IBRO Neurosci Rep. 2021;10:8–16. doi: 10.1016/j.ibneur.2020.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Martín-Hernández D, Pereira MP, Tendilla-Beltrán H, Madrigal JLM, García-Bueno B, Leza JC, Caso JR. Modulation of monoaminergic systems by antidepressants in the frontal cortex of rats after chronic mild stress exposure. Mol Neurobiol. 2019;56:7522–7533. doi: 10.1007/s12035-019-1619-x. [DOI] [PubMed] [Google Scholar]
- 74.Le François B, Soo J, Millar AM, Daigle M, Le Guisquet AM, Leman S, Minier F, Belzung C, Albert PR. Chronic mild stress and antidepressant treatment alter 5-HT1A receptor expression by modifying DNA methylation of a conserved Sp4 site. Neurobiol Dis. 2015;82:332–341. doi: 10.1016/j.nbd.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Iyo AH, Kieran N, Chandran A, Albert PR, Wicks I, Bissette G, Austin MC. Differential regulation of the serotonin 1A transcriptional modulators five prime repressor element under dual repression-1 and nuclear-deformed epidermal autoregulatory factor by chronic stress. Neuroscience. 2009;163:1119–1127. doi: 10.1016/j.neuroscience.2009.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Celada P, Puig MV, Casanovas JM, Guillazo G, Artigas F. Control of dorsal raphe serotonergic neurons by the medial prefrontal cortex: involvement of serotonin-1A, GABA(A), and glutamate receptors. J Neurosci. 2001;21:9917–9929. doi: 10.1523/JNEUROSCI.21-24-09917.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.