Abstract
Background
This post hoc analysis of a large, phase 3 program evaluated the effects of upadacitinib on fatigue, bowel urgency, and abdominal pain in patients with moderately to severely active ulcerative colitis.
Methods
Induction data were pooled from 2 identical studies, the U-ACHIEVE induction and U-ACCOMPLISH studies. Patients in these studies received upadacitinib 45 mg once daily or placebo as induction treatment. Responders to induction treatment were rerandomized in the U-ACHIEVE maintenance study to upadacitinib 15 mg once daily, upadacitinib 30 mg, or placebo. The percentage of patients reporting no abdominal pain and no bowel urgency daily via an electronic diary and a meaningful within-person change (≥5 points) in the Functional Assessment of Chronic Illness Therapy–Fatigue score were evaluated.
Results
The results demonstrated a statistically significantly greater percentage of patients reporting no abdominal pain and absence of bowel urgency observed from week 2 (P < .001), with upadacitinib induction treatment and clinically meaningful improvements in Functional Assessment of Chronic Illness Therapy–Fatigue score observed at week 8 (P < .001), when compared with placebo. The maintenance study showed that significant and meaningful improvements in abdominal pain, bowel urgency, and Functional Assessment of Chronic Illness Therapy–Fatigue score achieved during induction were sustained through 52 weeks of maintenance treatment in upadacitinib- vs placebo-treated patients.
Conclusions
The findings of this study support the additional benefit of upadacitinib in treating moderately to severely active ulcerative colitis by demonstrating a statistically significant impact on clinically meaningful symptoms of fatigue, bowel urgency, and abdominal pain.
(U-ACHIEVE induction and maintenance studies; NCT02819635; U-ACCOMPLISH induction study; NCT03653026).
Keywords: quality of life, biologic therapies, ulcerative colitis
Key messages.
What is already known?
Patients with moderately to severely active ulcerative colitis (UC) showed significant improvements in fatigue, bowel urgency, and abdominal pain following induction treatment with upadacitinib (UPA); however, the effects of 52-week UPA maintenance treatment are unknown.
What is new here?
Patients with moderately to severely active UC who responded to UPA 45 mg induction treatment maintained improvements in fatigue, bowel urgency, and abdominal pain following 52-week UPA maintenance treatment compared with placebo.
How can this study help patient care?
The results of this study show that UPA, compared with placebo, has a long-term, positive impact in reducing the burden of fatigue, bowel urgency, and abdominal pain, which is meaningful for patients with moderately to severely active UC.
Introduction
Ulcerative colitis (UC) is a chronic, relapsing disease that typically involves inflammation of the mucosa and submucosa of the colon.1-3 The highest reported prevalence rates of UC were in Norway at 505 per 100 000, followed by 286 per 100 000 in the United States.4 Clinical remission is a treatment goal for patients with UC, and it has been proposed that this goal include symptomatic remission based on patient-reported outcomes (PROs) as well as on mucosal healing.5,6
In patients with UC, abdominal pain, bowel urgency, and fatigue are impairing symptoms that decrease quality of life and psychological well-being.7-12 In particular, symptoms of bowel urgency are associated with increased odds of depression, anxiety, fatigue, pain, and social impairment when compared with patients with no bowel urgency.13,14 After initiating biologic treatment, a significant percentage of patients with inflammatory bowel disease (IBD) continue to experience fatigue at 12 months and in remission, suggesting that fatigue is a long-term difficulty.15,16
Fatigue, bowel urgency, and abdominal pain are associated with disability in UC.17 Both abdominal pain and bowel urgency contribute to sleep disturbances, which are associated with IBD-related fatigue.13 Patients with UC have reported that frequent and sudden bowel urgency during the night resulted in less hours of sleep, which affected patients’ performance of daily activities the next day and their ability to work.11 As a result of fatigue, bowel urgency, and abdominal pain, patients with UC have reported a high burden of symptoms that reduce quality of life and work ability.17-19 Therefore, these symptoms are important to address in the management of UC.
Another unmet need in UC is that frequently utilized assessments of disease activity do not incorporate abdominal pain and bowel urgency. For example, the Mayo score evaluates stool frequency but not urgency and the Simple Clinical Colitis Activity Index does not include abdominal pain.1 As such, the American College of Gastroenterology recommends that abdominal pain and bowel urgency be considered during diagnosis and initial treatment of UC.1 As the burden and psychological impact of these symptoms are often underestimated by nurses and physicians, this leads to a gap in communication with patients.20,21
Assessing PROs provides insight into patients’ perspectives of treatment efficacy, which is lacking in IBD, and promotes patient-centered care.22,23 Therefore, the assessment and monitoring of symptoms of UC need to be improved. Additionally, further research of fatigue, bowel urgency, and abdominal pain in UC and the investigation of novel therapies and interventions that could ameliorate these effects is also needed.8,24
This post hoc analysis evaluated the effects of 8-week upadacitinib (UPA) (45 mg once daily [QD]) induction treatment and 52-week UPA (30 mg QD and 15 mg QD) maintenance treatment in induction responders on fatigue, bowel urgency, and abdominal pain in patients with moderately to severely active UC.
Methods
Study Design and Patients
A Study to Evaluate the Safety and Efficacy of Upadacitinib (ABT-494) for Induction and Maintenance Therapy in Participants With Moderately to Severely Active UC (U-ACHIEVE induction study, NCT02819635; substudy 2) and A Study of the Efficacy and Safety of Upadacitinib (ABT-494) in Participants With Moderately to Severely Active UC (U-ACCOMPLISH study, NCT03653026) were 2 identical, double-blind, multicenter, placebo (PBO)-controlled, phase 3 trials. In the induction phase of both studies, patients 16 to 75 years of age were randomized 2:1 to UPA 45 mg QD or PBO. Patients were included in the induction studies if they had moderately to severely active UC, defined by an adapted Mayo score of 5 to 9 points and endoscopic subscore of 2 to 3 (confirmed by central reader).
Patients from these induction studies who had a clinical response after 8 weeks of UPA 45 mg QD induction, as well as induction responders from U-ACHIEVE phase 2b (substudy 1), were enrolled in a 52-week U-ACHIEVE maintenance study (substudy 3). Full details of these trials have been published elsewhere.25
The definition of a clinical response was a reduction in the Adapted Mayo Score (composed of stool frequency, rectal bleeding score [RBS], and findings of endoscopy) of ≥2 points from baseline and ≥30% from baseline of induction (hereafter referred to as baseline), plus a decrease in RBS ≥1 or an absolute RBS ≤1. In the maintenance study, patients were rerandomized 1:1:1 QD to UPA 15 mg, UPA 30 mg, or PBO.
Clinical Outcomes
Patients reported abdominal pain and bowel urgency daily via an electronic diary using a handheld device. Patients rated their daily abdominal pain on a scale of 0 to 3, with higher values indicating more severe symptoms, and confirmed daily bowel urgency (yes/no) in the last 24 hours. Abdominal pain and bowel urgency scores were calculated as an average of 3 consecutive days prior to each study visit. Patients were divided into 2 groups based on their score: abdominal pain (1, 2, or 3 days) or no abdominal pain (0 days) and bowel urgency (1, 2, or 3 days) or no bowel urgency (0 days). For these ranked secondary endpoints, a score of 0 was used to measure no abdominal pain and absence of bowel urgency, as this was the most stringent assessment. Study visits were at weeks 2, 4, 6, and 8 of the induction treatment and at weeks 0, 4, 8, 12, 20, 28, 36, 44, and 52 of the maintenance treatment. Results of psychometric validation analyses demonstrating that the abdominal pain and bowel urgency diary items are construct-valid, capable of distinguishing between groups known to be clinically different, and sensitive to change over time are described in the psychometric evaluation report provided in Supplemental Data File 1.
The Functional Assessment of Chronic Illness Therapy–Fatigue (FACIT-F) is made up of 13 items that assess self-reported fatigue and the impact it has on daily activities.26 The FACIT-F was administered electronically, and the score was measured at weeks 2 and 8 of induction treatment and weeks 0 and 52 of maintenance treatment. A change ≥5 points in FACIT-F score from induction or maintenance baseline was considered a meaningful within-person change (MWPC), based on anchor- and distribution-based analyses to establish the MWPC threshold.
Statistical Analyses
Analyses of this phase 3 data were performed in the intention-to-treat population that consisted of all randomized patients who received ≥1 dose of the study drug. In the maintenance study, only patients who achieved a clinical response were included. This post hoc analysis was based on data from patients who responded to 8-week UPA 45 mg QD induction treatment.
The percentage of patients who reported no abdominal pain and no bowel urgency and those who achieved MWPC in the FACIT-F were determined. Comparisons between each of the UPA groups and the PBO group were made. Missing data were reported using nonresponder imputation incorporating multiple imputation to handle missing data due to coronavirus disease 2019. The 95% confidence interval for the adjusted difference and P value were calculated according to the Cochran-Mantel-Haenszel test adjusted for strata. In the induction studies, adjustments were made for baseline corticosteroid use (yes or no), baseline adapted Mayo score (≤7 or >7), and biologic-inadequate responder (bio-IR) status (bio-IR or non–bio-IR) for the comparison of 2 treatment groups. In the maintenance study, adjustments were made for corticosteroid use at week 0 (yes or no), clinical remission status at week 0 (yes or no), and bio-IR status (bio-IR or non–bio-IR) for the comparison of 2 treatment groups.
Ethical Statement
At all study sites, the protocol and all study-related recruitment materials were approved by institutional review board or independent ethics committee. Written informed consent was obtained from all participants before study enrollment. Ethical principles outlined in the current Declaration of Helsinki, Good Clinical Practice guidelines and local regulatory requirements were applied in the conduct of this study. All patient data were anonymized to maintain patient confidentiality.
Results
Study Population
At induction baseline, 988 patients were evaluated, with 660 randomized to UPA and 328 to PBO (Table 1). Of the total population of patients, 37.6% (n = 248 of 660) of the UPA group and 37.8% (n = 124 of 328) of the PBO group were female. The median age was 41.0 (range, 17-76) years in the UPA group and 42.0 (range, 17-76) years in the PBO group. The mean duration of UC was 7.9 ± 6.8 years and 8.2 ± 8.0 years in the UPA and PBO groups, respectively.
Table 1.
Baseline demographics and disease characteristics.
| UPA (n = 660) | PBO (n = 328) | |
|---|---|---|
| Female | 248 (37.6) | 124 (37.8) |
| Age, y | 41.0 (17-76) | 42.0 (17-76) |
| Caucasian | 440 (66.7) | 224 (68.3) |
| Disease duration, y | 7.9 ± 6.8 | 8.2 ± 8.0 |
| Inadequate response to biologics | 340 (51.5) | 167 (50.9) |
| No abdominal paina | 65 (10.0) | 35 (10.8) |
| No bowel urgencya | 49 (7.6) | 26 (8.0) |
| FACIT-F score | 30.1 ± 11.7 | 31.5 ± 11.8 |
Values are n (%), median (range), or mean ± SD.
Abbreviations: FACIT-F, Functional Assessment of Chronic Illness Therapy–Fatigue; PBO, placebo; UPA, upadacitinib.
aUPA: n = 647; PBO: n = 325.
Abdominal Pain
Induction Treatment
At baseline, only 10% of patients reported no abdominal pain in the PBO and UPA groups (Figure 1). At weeks 2, 4, 6, and 8, a larger percentage of UPA-treated patients reported no abdominal pain when compared with PBO-treated patients (30.5% vs 15.5%, 39.1% vs 22.3%, 47.5% vs 23.5%, and 50.2% vs 23.8%, respectively). The differences at each time point were statistically significant (P < .001).
Figure 1.
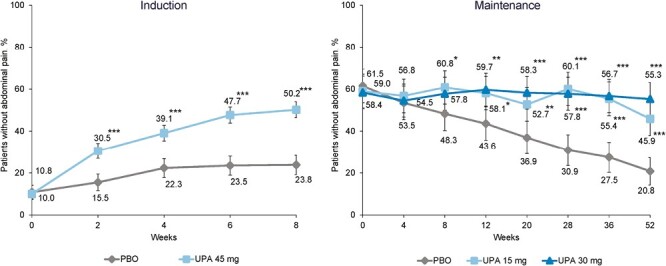
Percentage of patients reporting no abdominal pain. *P < .05, **P < .01, and ***P < .001 for upadacitinib (UPA) vs placebo (PBO). Maintenance results are based on induction responders. Nonresponder imputation incorporating multiple imputation to handle missing data due to coronavirus disease 2019.
Maintenance Treatment
At weeks 0 and 4 of maintenance treatment, values were similar across groups and differences were not significant (P > .05) (Figure 1), as all patients included had been treated with UPA 45 mg for induction and had recently experienced a clinical response. From week 8 of maintenance to week 52, the percentage of patients reporting no abdominal pain was maintained in UPA-treated patients and decreased in patients receiving PBO. Differences between PBO and both UPA treatment groups were statistically significant from week 12 (58.1% and 59.7% vs 43.6% for UPA 15 mg and UPA 30 mg vs PBO; P < .01) (Figure 1) to week 52 (55.3% and 45.9% vs 20.8% for UPA 15 mg and UPA 30 mg vs PBO; P < .001).
Bowel Urgency
Induction Treatment
At baseline, approximately 8% of patients reported no bowel urgency in the PBO and UPA groups. At weeks 2, 4, 6, and 8, a larger percentage of UPA-treated patients reported no bowel urgency when compared with PBO-treated patients (35.8% vs 13.7%, 44.3% vs 16.5%, 46.9% vs 19.0%, and 51.1% vs 23.8%, respectively). The differences at each time point were significant (P < .001).
Maintenance Treatment
At week 0 of maintenance treatment, values were similar across groups and differences were not significant (P > .05) (Figure 2), as patients included had recently experienced a clinical response. From week 4 of maintenance treatment to week 52, the percentage of patients reporting no bowel urgency was maintained in UPA-treated patients and decreased in patients receiving PBO. Differences between PBO and both UPA treatment groups were significant from week 8 (64.9% and 64.3% vs 49.7% for UPA 15 mg and UPA 30 mg vs PBO; P < .01) (Figure 2) to week 52 (56.1% and 63.6% vs 17.4% for UPA 15 mg and UPA 30 mg vs PBO; P < .001).
Figure 2.
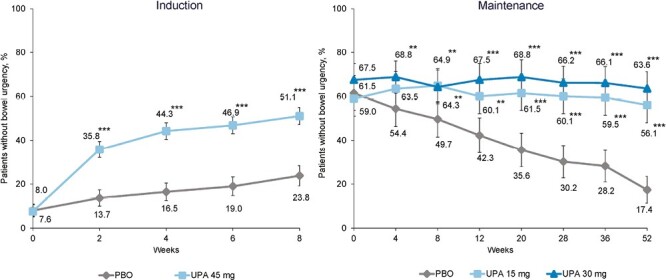
Percentage of patients reporting no bowel urgency. **P < .01 and ***P < .001 for upadacitinib (UPA) vs placebo (PBO). Maintenance results are based on induction responders. Nonresponder imputation incorporating multiple imputation to handle missing data due to coronavirus disease 2019.
FACIT-F score
Induction treatment
A greater percentage of patients administered UPA 45 mg in the induction treatment achieved MWPC (≥5 points) in the FACIT-F score at week 2 than patients treated with PBO (48.5% vs 26.3%; P < .001) (Figure 3). At week 8 of induction, a greater percentage of patients on UPA achieved MWPC in the FACIT-F score compared with PBO (59.1% vs 33.8%; P < .001) (Figure 3).
Figure 3.
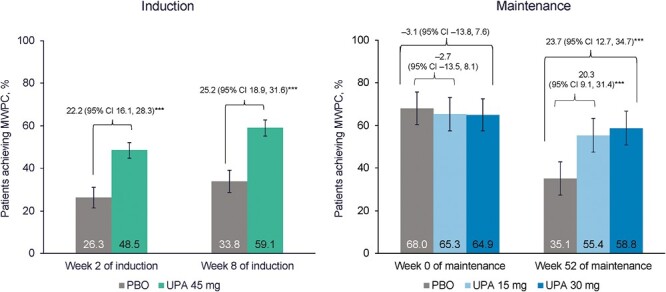
Percentage of patients reporting ≥5-point increase (meaningful within-person change [MWPC]) in Functional Assessment of Chronic Illness Therapy–Fatigue score at induction and at maintenance among induction responders. ***P < .001 for upadacitinib (UPA) vs placebo (PBO). Maintenance results are based on induction responders. Nonresponder imputation incorporating multiple imputation to handle missing data due to coronavirus disease 2019. CI, confidence interval.
Maintenance Treatment
At week 0 of the maintenance phase, the percentage of patients who achieved MWPC in the FACIT-F score was similar across the treatment groups (Figure 3). At week 52, the percentage of patients with a clinically meaningful change in FACIT-F score was maintained in the UPA groups and decreased in patients receiving PBO (55.4% and 58.8% vs 35.1% for UPA 15 mg and UPA 30 mg vs PBO; P < .001) (Figure 3).
Discussion
In this study, we demonstrated that a significantly higher percentage of UPA-treated patients with moderately to severely active UC reported no abdominal pain, no bowel urgency, and a meaningful change in FACIT-F score compared with PBO. Statistically significant improvements in the percentage of patients reporting no abdominal pain and no bowel urgency were observed as early as week 2 with UPA induction treatment. Clinically meaningful improvements in FACIT-F score were observed at week 8 when compared with PBO. The results of the maintenance study showed that significant and clinically meaningful improvements in abdominal pain, bowel urgency, and FACIT-F score achieved during the induction study were sustained through 52 weeks of maintenance treatment in UPA- vs PBO-treated patients.
Our results are consistent with a previous phase 2b study.27 A post hoc analysis of the phase 2b trial data showed that at week 8, a larger proportion of patients on UPA 45 mg QD (n = 56) vs PBO (n = 46) reported no abdominal pain (37.5% vs 13.0%) and no bowel urgency (46.4% vs 8.7%), with differences observed as significant for most but not all doses.28 These results are comparable to the findings presented in this phase 3 trial, in which a larger percentage of UPA- vs PBO-treated patients reported no abdominal pain (50.2% vs 23.8%) and no bowel urgency (51.1% vs 23.8%) at week 8 of induction. We expanded on these findings and demonstrated that treatment with UPA resulted in a sustained, beneficial effect with patients reporting no abdominal pain and no bowel urgency compared with PBO through week 52.
Abdominal pain and bowel urgency are clinically important in UC; however, they are not frequently measured. The prevalence and impact of these symptoms on daily life in patients with UC was shown in a study in which abdominal pain and bowel urgency were reported by 58.1% and 62.5% of patients, respectively.29 Additionally, 40.9% and 43.5% of patients reported abdominal pain and bowel urgency to have a significant impact on their daily life; thus, there is a need for novel treatments.29,30 At baseline in the current study, only 10.0% and 7.6% of UPA-treated patients reported no abdominal pain and no bowel urgency, respectively. Following UPA induction treatment in this study, the percentage of patients reporting no abdominal pain and no bowel urgency increased to 50.2% and 51.1%, respectively, and this was maintained up to 52 weeks. The findings in this study illustrate that UPA can significantly alleviate the highly prevalent symptoms of abdominal pain and bowel urgency in patients with UC. Therefore, treatment with UPA had a positive effect on health-related quality of life and reduced the burden of these disabling symptoms, which is meaningful for patients with UC.
Regarding fatigue, a longitudinal prospective cohort study of patients with IBD presented findings that biologic therapy significantly improved the mean Short Inflammatory Bowel Disease Questionnaire fatigue score, which was strongly correlated with the FACIT-F score.15 Among patients with IBD reporting fatigue at baseline, 70% remained fatigued at week 14 and 61% at week 52.15 Thus, there is a need for novel treatments to improve symptoms of fatigue in patients with UC, as it is a long-lasting symptom experienced by a large proportion of patients.15 In the current study, significant and clinically meaningful improvements in fatigue were observed in UPA-treated patients at week 2, fatigue was further improved at week 8 of induction, and improvements were sustained through 52 weeks of maintenance treatment compared with PBO.
Managing the combination of bowel urgency, abdominal pain, and fatigue for patients with IBD is particularly difficult.31 Monitoring PROs is recognized by physicians as important in improving relationships with patients.21 Assessment and monitoring of abdominal pain, bowel urgency, and fatigue can facilitate conversation, and as such improve communication between patients and physicians. Additionally, effective treatments are needed in the management of UC to reduce these debilitating symptoms and their impact on psychological well-being and quality of life.8,9 Treatments that can reduce symptoms of bowel urgency may also decrease the impact this symptom has on fatigue and consequently the impact on ability to perform work and daily activities. The results of this study show that UPA has a long-term, positive impact in alleviating abdominal pain, bowel urgency, and fatigue among patients with moderately to severely active UC when compared with PBO.
Strengths of this study include the measurement of novel ranked secondary endpoints in UC and reporting on understudied PROs. Limitations of this study include the generalizability of the findings to patients with mild UC and the lack of granularity in the measurement of abdominal pain and bowel urgency. Therefore, it is difficult to assess patients who may further benefit from a reduction in pain or urgency. Abdominal pain and bowel urgency are significant symptoms to patients with UC, and so it is important for future research to focus on using these measures consistently across clinical research.
Conclusions
Treatment with UPA resulted in a significantly higher percentage of patients with moderately to severely active UC, reporting improvements in bowel urgency, abdominal pain, and fatigue in the UPA phase 3 program compared with PBO. Therefore, the results from this study support the use of UPA in the treatment of UC and demonstrate the importance of monitoring these symptoms given their relevance to patients with UC.
Supplementary Material
Acknowledgments
Medical writing support was provided by Natalie Mitchell, of Fishawack Facilitate Ltd, part of Fishawack Health, and funded by AbbVie. At all study sites, the protocol and all study-related recruitment materials were approved by institutional review board or independent ethics committee. Written informed consent was obtained from all participants before study enrolment. Ethical principles outlined in the current Declaration of Helsinki, Good Clinical Practice guidelines, and local regulatory requirements were applied in the conduct of this study. All patient data were anonymized to maintain patient confidentiality.
Contributor Information
Silvio Danese, Gastroenterology and Digestive Endoscopy, IRCCS Ospedale San Raffaele and Vita-Salute San Raffaele University, Milan, Italy.
Jacinda Tran, Comparative Health Outcomes, Policy, and Economics Institute, University of Washington, Seattle, WA, USA; AbbVie Inc, Chicago, IL, USA.
Geert D’Haens, Department of Gastroenterology and Hepatology, Amsterdam University Medical Center, Amsterdam, the Netherlands.
David T Rubin, Inflammatory Bowel Disease Center, University of Chicago Medicine, Chicago, IL, USA.
Nobuo Aoyama, Department of Gastroenterology, Gastrointestinal Endoscopy and IBD Center, Aoyama Medical Clinic, Kobe, Japan.
Wen Zhou, AbbVie Inc, Chicago, IL, USA.
Dapo Ilo, AbbVie Inc, Chicago, IL, USA.
Xuan Yao, AbbVie Inc, Chicago, IL, USA.
Yuri Sanchez Gonzalez, AbbVie Inc, Chicago, IL, USA.
Remo Panaccione, Inflammatory Bowel Disease Unit, Division of Gastroenterology and Hepatology, University of Calgary, Calgary, AB, Canada.
Author Contribution
J.T.: substantial contributions to conception and design; acquisition of data; data analysis; interpretation of data; involved in drafting or revising critically for important intellectual content. Y.S.G.: substantial contributions to conception and design; interpretation of data; involved in drafting or revising critically for important intellectual content. X.Y.: acquisition of data; data analysis; interpretation of data; involved in drafting or revising critically for important intellectual content. S.D., G.D., D.T.R., N.A., W.Z., D.I., R.P.: interpretation of data; been involved in drafting or revising critically for important intellectual content. All authors had access to the data results, and participated in the development, review, and approval of this manuscript.
Funding
This work was supported by AbbVie Inc. AbbVie participated in the study design, research, data collection, analysis and interpretation of data, writing, reviewing, and approving the publication. No honoraria or payments were made for authorship.
Conflict of Interest
S.D. has received consultancy fees from AbbVie, Allergan, Amgen, AstraZeneca, Biogen, Boehringer Ingelheim, Celgene, Celltrion, Ferring, Gilead, Hospira, Janssen, Johnson & Johnson, MSD, Mundipharma, Pfizer, Roche, Sandoz, Takeda, TiGenix, UCB, and Vifor Pharma. J.T. was a summer intern at AbbVie at the time this study was conducted; is currently an AbbVie contractor and PhD student at University of Washington; and has received financial support from the Agency for Healthcare Research and Quality, National Institutes of Health, ARCS Foundation, and Washington Research Foundation.
G.D. has served as an adviser for AbbVie, Ablynx, Allergan, Alphabiomics, Amakem, Amgen, AM Pharma, Arena Pharmaceuticals, AstraZeneca, Avaxia, Biogen, Bristol-Myers Squibb, Boehringer Ingelheim, Celgene/Receptos, Celltrion, Cosmo, Echo Pharmaceuticals, Eli Lilly, Engene, Ferring, Dr. Falk Pharma, Galapagos, Genentech/Roche, Gilead, Glaxo Smith Kline, Gossamerbio, Hospira/Pfizer, Immunic, Johnson and Johnson, Kintai Therapeutics, Lycera, Medimetrics, Millennium/Takeda, Medtronics, Mitsubishi Pharma, Merck Sharp Dome, Mundipharma, Nextbiotics, Novo Nordisk, Otsuka, Pfizer/Hospira, Photopill, Prodigest, Prometheus Laboratories/Nestlé, Progenity, Protagonist, RedHill, Robarts Clinical Trials, Salix, Samsung Bioepis, Sandoz, Seres/Nestle, Setpoint, Shire, Teva, Tigenix, Tillotts, Topivert, Versant, and Vifor; and has received speaker fees from AbbVie, Biogen, Ferring, Johnson and Johnson, Merck Sharp Dome, Mundipharma, Norgine, Pfizer, Samsung Bioepis, Shire, Millennium/Takeda, Tillotts, and Vifor. D.T.R. has received grant support from Takeda; and served as a consultant for AbbVie, Altrubio, Arena Pharmaceuticals, Bellatrix Pharmaceuticals, Boehringer Ingelheim Ltd, Bristol-Myers Squibb, Celgene Corp/Syneos, Connect BioPharma, GalenPharma/Atlantica, Genentech/Roche, Gilead Sciences, InDex Pharmaceuticals, Ironwood Pharmaceuticals, Iterative Scopes, Janssen Pharmaceuticals, Lilly, Materia Prima, Pfizer, Prometheus Biosciences, Reistone, Takeda, and Techlab Inc. N.A. has no conflict of interest to report.
Wen Zhou, Dapo Ilo, Xuan Yao, and Yuri Sanchez Gonzalez are full-time employees of AbbVie and may hold AbbVie stock and/or stock options. R.P. has received consulting fees from AbbVie, Abbott, Alimentiv (formerly Robarts), Amgen, Arena Pharmaceuticals, AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Celgene, Celltrion, Cosmos Pharmaceuticals, Eisai, Elan, Eli Lilly, Ferring, Galapagos, Genentech, Gilead Sciences, GlaxoSmithKline, Janssen, Merck, Mylan, Oppilan, Pandion, Pharma, Pandion Pharma, Pfizer, Progenity, Protagonist Therapeutics, Roche, Satisfai Health, Sandoz, Schering-Plough, Shire, Sublimity Therapeutics, Theravance Biopharma, UCB, and Takeda Pharmaceuticals; received speaker fees from AbbVie, Arena Pharmaceuticals, Celgene, Eli Lilly, Ferring, Gilead Sciences, Janssen, Merck, Pfizer, Roche, Sandoz, Shire, and Takeda Pharmaceuticals; served on the advisory board for AbbVie, Amgen, Arena Pharmaceuticals, Bristol-Myers Squibb, Celgene, Celltrion, Eli Lilly, Ferring, Galapagos, Genentech, Gilead Sciences, GlaxoSmithKline, Janssen, Merck, Mylan, Oppilan Pharma, Pandion Pharma, Pfizer, Sandoz, Shire, Sublimity Therapeutics, Theravance Biopharma, and Takeda Pharmaceuticals; and received research/educational support from AbbVie, Ferring, Janssen, Pfizer, and Takeda.
Data Availability
AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymized, individual and trial-level data (analysis data sets), as well as other information (eg, protocols and Clinical Study Reports), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications.
This clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal and Statistical Analysis Plan and execution of a Data Sharing Agreement. Data requests can be submitted at any time and the data will be accessible for 12 months, with possible extensions considered. For more information on the process, or to submit a request, visit the following link: https://www.abbvieclinicaltrials.com/hcp/data-sharing/.
REFERENCES
- 1. Rubin DT, Ananthakrishnan AN, Siegel CA, Sauer BG, Long MD.. ACG clinical guideline: ulcerative colitis in adults. Am J Gastroenterol. 2019;114(3):384-413. [DOI] [PubMed] [Google Scholar]
- 2. Ungaro R, Colombel J-F, Lissoos T, Peyrin-Biroulet L.. A treat-to-target update in ulcerative colitis: a systematic review. Am J Gastroenterol. 2019;114(6):874-883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lynch WD, Hsu R.. Ulcerative Colitis. Treasure Island, FL: StatPearls; 2022. Available at: https://www.ncbi.nlm.nih.gov/books/NBK459282/ [Google Scholar]
- 4. Ng SC, Shi HY, Hamidi N, et al. . Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2017;390(10114):2769-2778. [DOI] [PubMed] [Google Scholar]
- 5. Danese S, Roda G, Peyrin-Biroulet L.. Evolving therapeutic goals in ulcerative colitis: towards disease clearance. Nat Rev Gastroenterol Hepatol. 2020;17(1):1-2. [DOI] [PubMed] [Google Scholar]
- 6. Turner D, Ricciuto A, Lewis A, et al. . STRIDE-II: an update on the selecting therapeutic targets in inflammatory bowel disease (STRIDE) initiative of the international organization for the study of IBD (IOIBD): determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology. 2021;160(5):1570-1583. [DOI] [PubMed] [Google Scholar]
- 7. Armuzzi A, Liguori G.. Quality of life in patients with moderate to severe ulcerative colitis and the impact of treatment: A narrative review. Dig Liver Dis. 2021;53(7):803-808. [DOI] [PubMed] [Google Scholar]
- 8. Danese S, Allez M, van Bodegraven AA, et al. . Unmet medical needs in ulcerative colitis: an expert group consensus. Dig Dis. 2019;37(4):266-283. [DOI] [PubMed] [Google Scholar]
- 9. Jones JL, Nguyen GC, Benchimol EI, et al. . The impact of inflammatory bowel disease in Canada 2018: quality of life. J Can Assoc Gastroenterol. 2019;2(Suppl 1):S42-S48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Borren NZ, van der Woude CJ, Ananthakrishnan AN.. Fatigue in IBD: epidemiology, pathophysiology and management. Nat Rev Gastroenterol Hepatol. 2019;16(4):247-259. [DOI] [PubMed] [Google Scholar]
- 11. Dubinsky MC, Irving PM, Panaccione R, et al. . Incorporating patient experience into drug development for ulcerative colitis: development of the urgency numeric rating scale, a patient-reported outcome measure to assess bowel urgency in adults. J Patient Rep Outcomes. 2022;6(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Villoria A, García V, Dosal A, et al. . Fatigue in out-patients with inflammatory bowel disease: prevalence and predictive factors. PLoS One. 2017;12(7):e0181435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McGing JJ, Radford SJ, Francis ST, Serres S, Greenhaff PL, Moran GW.. The aetiology of fatigue in inflammatory bowel disease and potential therapeutic management strategies. Aliment Pharmacol Ther. 2021;54(4):368-387. [DOI] [PubMed] [Google Scholar]
- 14. Sninsky JA, Barnes EL, Zhang X, Long MD.. Urgency and its association with quality of life and clinical outcomes in ulcerative colitis patients. Am J Gastroenterol. 2022;117(5):769-776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Borren NZ, Tan W, Colizzo FP, et al. . Longitudinal trajectory of fatigue with initiation of biologic therapy in inflammatory bowel diseases: a prospective cohort study. J Crohns Colitis. 2020;14(3):309-315. [DOI] [PubMed] [Google Scholar]
- 16. Chavarría C, Casanova MJ, Chaparro M, et al. . Prevalence and factors associated with fatigue in patients with inflammatory bowel disease: A multicentre study. J Crohns Colitis. 2019;13(8):996-1002. [DOI] [PubMed] [Google Scholar]
- 17. Allen PB, Gower-Rousseau C, Danese S, Peyrin-Biroulet L.. Preventing disability in inflammatory bowel disease. Therap Adv Gastroenterol. 2017;10(11):865-876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Calvet X, Argüelles-Arias F, López-Sanromán A, et al. . Patients’ perceptions of the impact of ulcerative colitis on social and professional life: results from the UC-LIFE survey of outpatient clinics in Spain. Patient Prefer Adherence. 2018;12:1815-1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Carpio D, López-Sanromán A, Calvet X, et al. . Perception of disease burden and treatment satisfaction in patients with ulcerative colitis from outpatient clinics in Spain: UC-LIFE survey. Eur J Gastroenterol Hepatol. 2016;28(9):1056-1064. [DOI] [PubMed] [Google Scholar]
- 20. Schreiber S, Panés J, Louis E, et al. . Perception gaps between patients with ulcerative colitis and healthcare professionals: an online survey. BMC Gastroenterol. 2012;12:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rubin DT, Hart A, Panaccione R, et al. . Ulcerative colitis narrative global survey findings: communication gaps and agreements between patients and physicians. Inflamm Bowel Dis. 2021;27(7):1096-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. El-Matary W. Patient-reported outcome measures in inflammatory bowel disease. Can J Gastroenterol Hepatol. 2014;28(10):536-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Geldof J, LeBlanc J-F, Lucaciu L, et al. . Are we addressing the top 10 research priorities in IBD? Frontline Gastroenterol. 2021;12(7):564-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Borren NZ, Long MD, Sandler RS, et al. . Longitudinal trajectory of fatigue in patients with inflammatory bowel disease: a prospective study. Inflamm Bowel Dis. 2021;27(11):1740-1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Danese S, Vermeire S, Zhou W, et al. . Upadacitinib as induction and maintenance therapy for moderately to severely active ulcerative colitis: results from the phase 3, multicentre, double-blind, randomised trials. Lancet. 2022;399(10341):2113-2128. [DOI] [PubMed] [Google Scholar]
- 26. Tinsley A, Macklin E, Korzenik J, et al. . Validation of the functional assessment of chronic illness therapy-fatigue (FACIT-F) in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2011;34(11-12):1328-1336. [DOI] [PubMed] [Google Scholar]
- 27. Sandborn WJ, Ghosh S, Panes J, et al. . Efficacy of upadacitinib in a randomized trial of patients with active ulcerative colitis. Gastroenterology. 2020;158(8):2139-2149.e14. [DOI] [PubMed] [Google Scholar]
- 28. Ghosh S, Sanchez Gonzalez Y, Zhou W, et al. . Upadacitinib treatment improves symptoms of bowel urgency and abdominal pain, and correlates with quality of life improvements in patients with moderate to severe ulcerative colitis. J Crohns Colitis. 2021;15(12):2022-2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hibi T, Ishibashi T, Ikenoue Y, Yoshihara R, Nihei A, Kobayashi T.. Ulcerative colitis: disease burden, impact on daily life, and reluctance to consult medical professionals: results from a Japanese internet survey. Inflamm Intest Dis. 2020;5(1):27-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rapport F, Clement C, Seagrove AC, et al. . Patient views about the impact of ulcerative colitis and its management with drug treatment and surgery: a nested qualitative study within the CONSTRUCT trial. BMC Gastroenterol. 2019;19(1):166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dibley L, Khoshaba B, Artom M, et al. . Patient strategies for managing the vicious cycle of fatigue, pain and urgency in inflammatory bowel disease: impact, planning and support. Dig Dis Sci. 2021;66(10):3330-3342. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymized, individual and trial-level data (analysis data sets), as well as other information (eg, protocols and Clinical Study Reports), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications.
This clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal and Statistical Analysis Plan and execution of a Data Sharing Agreement. Data requests can be submitted at any time and the data will be accessible for 12 months, with possible extensions considered. For more information on the process, or to submit a request, visit the following link: https://www.abbvieclinicaltrials.com/hcp/data-sharing/.


