We read with great interest the recent study by Gu et al.1 entitled SREBP-2 maintains glioblastoma stem cells through keeping the balance between cholesterol biosynthesis and uptake. Sterol regulatory element-binding proteins (SREBPs) are crucial lipogenesis-regulating transcription factors that include SREBP-1 (SREBP-1a, SREBP-1c) and SREBP-2. SREBP-1 mainly stimulates fatty acid synthesis, while SREBP-2 targets mostly genes involved in cellular cholesterol homeostasis. Targeting this family provides new insights into treatment of neoplasms. The authors performed a study aiming at investigating the crucial role of SREBP-2 in cholesterol homeostasis in glioblastoma stem cells (GSCs) as well as self-renewal and tumorigenesis.1 As blocking cholesterol metabolism has been recognized as a potential disincentive for malignant performance of glioblastoma (GBM), the detailed mechanism confirmed and novel targets revealed by this research work encourage exploration of new therapeutic strategies.
However, several aspects of the study deserve attention. First, the authors used Betulin as an inhibitor of SREBP-2 to attenuate GSC growth. However, Betulin showed low specificity and could function as an inhibitor for both SREBP-1 and SREBP-2.2 Based on a previous study,3 SREBP-1 was proven to contribute to GBM progression and tumorigenesis, and it owned higher expression level and prognostic relevance than SREBP-2. Thus, the authors did not assess effects of selective SREBP-2 blockade, and the observed effects could be influenced by inhibition of SREBP-1. In addition, it was reported that Betulin could not cross blood‒brain barrier, limiting its clinical translation potential.4 Recently, novel inhibitors (PCSK9-IN-9 and 5-O-methylembelin) have been reported to inhibit the mRNA level of SREBP-2 but not SREBP-15 (summarized in Table 1). Therefore, it is crucial to conduct further investigations on specific SREBP-2 inhibition, and the development of therapeutic targeting this druggable target. Furthermore, we believe that discussions surrounding the function of SREBP-1 and SREBP-2 in GBM progression are essential for a comprehensive understanding of targeting SREBPs therapy.
Table 1.
Summary of part of SREBPs inhibitors
| Agents | Formula | Structure | Action | Specificity |
|---|---|---|---|---|
| Fatostatin | C18H18N2S |
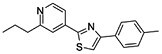
|
Inhibition of endoplasmic reticulum (ER)–Golgi translocation of SREBPs through binding to SCAP in Insulin-induced gene 1 protein (INSIG) independent manner | SREBP-1 and SREBP-2 |
| Betulin | C30H50O2 |
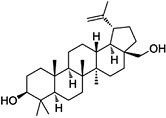
|
Inhibition of ER–Golgi translocation of SREBPs through binding to SCAP in INSIG-dependent manner | SREBP-1 and SREBP-2 |
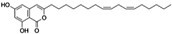
| 5-O-Methylembelin | C18H28O4 |
|
Inhibition of proprotein convertase subtilisin-kexin type 9 (PCSK9), inducible degrader of the low-density lipoprotein receptor (IDOL), and SREBP2 mRNA expression | SREBP2 |
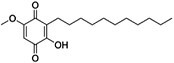
| PCSK9-IN-9 | C26H36O4 |
|
Inhibiting PCSK9, IDOL, and SREBP2 mRNA expression | SREBP-2 |
| PF-429242 | C25H37Cl2N3O2 |
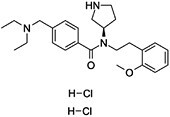
|
Inhibition of transcriptional activities of SREBPs | SREBP-1 and SREBP-2 |
| BioE-1115 | C19H18FN3O2 |
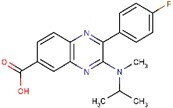
|
Inhibition of Pask resulting in inhibition of ER–Golgi translocation of SREBPs downstream or parallel to mTORC1, likely INSIG independent | Likely specific for SREBP-1a and -1c |
Second, we noticed that the authors confirmed the mRNA expression levels of cholesterol biosynthesis pathway genes in GSCs treated with low-density lipoprotein (LDL). Cholesterol, the major component of LDL, could be converted into oxysterols. When the cholesterol concentration reaches a certain level, oxysterols can prevent SREBP cleavage-activating protein (SCAP) from binding with SREBP-2, leading to the failed activation of SREBP-2, as revealed by Radhakrishnan et al.6 However, this study has not been cited. We convince that citing this article would provide enhanced evidence in support of the authors’ findings and would facilitate further research on the mechanism.
Third, impacts on downstream signaling induced by silencing SREBP-2 required further evaluation. In the authors’ view, cholesterol levels were essential for tumorigenesis. Since SREBP-2 inhibition could block cholesterol biosynthesis and uptake, variations in the concentration of cholesterol should be identified. Additionally, a previous study showed that silencing SREBP-2 modestly enhanced SREBP-1 cleavage, leading to abrogation of the antitumor effect.3 Therefore, changes in SREBP-1 levels should be recognized.
Last, the authors used in vivo experiments to confirm SREBP-2-mediated tumorigenesis, which provided solid evidence. However, the alteration of key molecules, such as SREBP-2 and Squalene monooxygenase (SQLE), was not confirmed in xenograft tissues. We believe that further confirmation by immunochemistry and immunofluorescence is required and could provide more favorable proof for their conclusions.
We recognize the importance of the knowledge gap Gu et al. sought to fill by producing available data on targeting cholesterol metabolism for GBM treatment, and we believe it is important to inform your readers on the limitations in this study. The refinement of these limitations is more conducive to the further enhancement of the conclusions of the aforementioned article and thus contributes to the in-depth study of the corresponding mechanisms.
Contributor Information
Yanfei Sun, Department of Neurosurgery, Qilu Hospital, Cheeloo College of Medicine and Institute of Brain and Brain-Inspired Science, Shandong University, Jinan, China; Jinan Microecological Biomedicine Shandong Laboratory and Shandong Key Laboratory of Brain Function Remodeling, Jinan, China.
Guangjing Mu, Department of Neurosurgery, Qilu Hospital, Cheeloo College of Medicine and Institute of Brain and Brain-Inspired Science, Shandong University, Jinan, China; Jinan Microecological Biomedicine Shandong Laboratory and Shandong Key Laboratory of Brain Function Remodeling, Jinan, China.
Zhiwei Xue, Department of Neurosurgery, Qilu Hospital, Cheeloo College of Medicine and Institute of Brain and Brain-Inspired Science, Shandong University, Jinan, China; Jinan Microecological Biomedicine Shandong Laboratory and Shandong Key Laboratory of Brain Function Remodeling, Jinan, China.
Shuai Wang, Department of Neurosurgery, NYU Grossman School of Medicine, New York, NY 10016, USA.
Xingang Li, Department of Neurosurgery, Qilu Hospital, Cheeloo College of Medicine and Institute of Brain and Brain-Inspired Science, Shandong University, Jinan, China.
Mingzhi Han, Department of Neurosurgery, Qilu Hospital, Cheeloo College of Medicine and Institute of Brain and Brain-Inspired Science, Shandong University, Jinan, China; Jinan Microecological Biomedicine Shandong Laboratory and Shandong Key Laboratory of Brain Function Remodeling, Jinan, China.
Conflict of interest statement
The authors declare that there are no potential conflicts of interest.
Funding
This work was supported by the Natural Science Foundation of China (82103277), the Research Project of Jinan Microecological Biomedicine Shandong Laboratory (JNL-2022003A, JNL-2022041C), Shandong Excellent Young Scientists Fund Program (2022HWYQ-035), the Department of Science & Technology of Shandong Province (SYS202202), Shandong Provincial Natural Science Foundation (ZR2021QH030), the Special Foundation for Taishan Young Scholars (tsqn202211041), and the Qilu Young Scholar Program of Shandong University, China.
Data availability
All data generated or analyzed during this study are included in this article.
Consent for publication
All authors have agreed to publish this manuscript.
Author contributions
YS, SW, and MH designed the project. YS, GM, and ZX wrote the manuscript. MH and XL supervised the project. All authors contributed to the article and approved the submitted version.
Ethical statement
All experimental procedures were approved by the institutional research ethics committee of Shandong University.
References
- 1. Gu D, Zhou F, You H, et al. SREBP2 maintains glioblastoma stem cells through keeping the balance between cholesterol biosynthesis and uptake. Neuro Oncol. 2023:noad060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tang JJ, Li JG, Qi W, et al. Inhibition of SREBP by a small molecule, betulin, improves hyperlipidemia and insulin resistance and reduces atherosclerotic plaques. Cell Metab. 2011;13(1):44–56. [DOI] [PubMed] [Google Scholar]
- 3. Geng F, Cheng X, Wu X, et al. Inhibition of SOAT1 suppresses glioblastoma growth via blocking SREBP-1-mediated lipogenesis. Clin Cancer Res. 2016;22(21):5337–5348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pozharitskaya ON, Karlina MV, Shikov AN, et al. Pharmacokinetics and tissue disposition of nanosystem-entrapped betulin after endotracheal administration to rats. Eur J Drug Metab Pharmacokinet. 2017;42(2):327–332. [DOI] [PubMed] [Google Scholar]
- 5. Pel P, Kim YM, Kim HJ, et al. Isocoumarins and benzoquinones with their proprotein convertase subtilisin/kexin type 9 expression inhibitory activities from dried roots of Lysimachia vulgaris. ACS Omega. 2022;7(50):47296–47305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Radhakrishnan A, Ikeda Y, Kwon HJ, Brown MS, Goldstein JL.. Sterol-regulated transport of SREBPs from endoplasmic reticulum to Golgi: oxysterols block transport by binding to Insig. Proc Natl Acad Sci USA. 2007;104(16):6511–6518. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article.


