Abstract
Background
Ulcerative colitis (UC) is a progressive chronic inflammatory disorder. Neutrophils play a critical role in regulating intestinal mucosal homeostasis in UC. Spleen tyrosine kinase (Syk) is involved in several inflammatory diseases. Here, we evaluated the effects and underlying mechanisms of Syk on neutrophil immune-responses in UC.
Methods
Syk expression in the colonic tissues of patients with UC was determined using quantitative reverse transcription-polymerase chain reaction (qRT-PCR), western blotting, and immunohistochemistry. Colonic biopsies from patients with UC were obtained for single-cell RNA-sequencing. Neutrophils isolated from peripheral blood were pre-treated with R788 (a Syk inhibitor) and gene differences were determined using RNA sequencing. Neutrophil functions were analyzed using qRT-PCR, flow cytometry, and Transwell assay. R788 was administered daily to mice with dextran sulfate sodium (DSS)-induced colitis to verify the effects of Syk on intestinal inflammation.
Results
Syk expression was increased in inflamed mucosa and neutrophils of patients with UC and positively correlated with disease activity. Pharmacological inhibition of Syk in neutrophils decreased the production of pro-inflammatory cytokines, chemokines, neutrophil extracellular traps, reactive oxygen species, and myeloperoxidase. Apoptosis and migration of neutrophils were suppressed by Syk blockade. Syk blockade ameliorated mucosal inflammation in DSS-induced murine colitis by inhibiting neutrophil-associated immune responses. Mechanistically, Syk regulated neutrophil immune-responses via the mammalian target of rapamycin kinase/rubicon-like autophagy enhancer-dependent autophagy pathway.
Conclusions
Our findings indicate that Syk facilitates specific neutrophil functional responses to mucosal inflammation in UC, and its inhibition ameliorates mucosal inflammation in DSS-induced murine colitis, suggesting its potential as a novel therapeutic target for UC treatment.
Keywords: Syk, UC, neutrophils, autophagy
Introduction
Ulcerative colitis (UC), a chronic relapsing inflammatory disease with continuous mucosal inflammation, mainly affects the rectum and colon and exhibits increasing incidence with high morbidity and mortality rates worldwide.1,2 Although several biological and oral small-molecule agents, such as Janus kinase inhibitors, have been used for the treatment of patients with UC, approximately one-third of patients do not respond to initial treatment and half of the patients lose response over time.3 Therefore, novel precision medicine strategies are urgently needed for UC treatment.
Although its precise etiology and pathology remain ambiguous, aberrant immune responses in the inflamed mucosa of UC are mainly characterized by the abnormal activation and infiltration of neutrophils with other immune cells, such as T cells, macrophages, and dendritic cells.4,5 Neutrophils are generally the first immune cell responders that play a crucial role in mucosal healing in the early stages of UC.6,7 During intestinal mucosal injury, activated neutrophils migrate to the inflammatory site and eradicate pathogens via phagocytosis, degranulation, release of reactive oxygen species (ROS), myeloperoxidase (MPO), and antibacterial peptides, and formation of neutrophil extracellular traps (NETs).8–10 Neutrophils also promote angiogenesis and intestinal epithelial cell proliferation to promote the intestinal mucosal wound repair.11,12 However, neutrophils act as a double-edged sword in the inflammatory response of UC. Excessive activation and infiltration of neutrophils leads to persistent mucosal injury and increases the risk of thrombosis in patients with UC.13,14 Moreover, neutrophil dysfunction may lead to therapeutic failure in patients with UC.15–17 Therefore, regulating the beneficial and detrimental effects of neutrophils on intestinal tissues plays an indispensable role in alleviating mucosal inflammation in UC.
Spleen tyrosine kinase (Syk) is a cytoplasmic non-receptor protein tyrosine kinase found in both hematopoietic and non-hematopoietic cells, including B cells, macrophages, neutrophils, and dendritic cells.18 It is involved in the pathogenesis of multiple conditions, including immune diseases and various malignancies.19–21 To date, only a few studies have demonstrated the critical pathogenic role of Syk in UC. Syk inhibition mitigates intestinal inflammation by reducing the production of tumor necrosis factor (TNF)-α in experimental colitis.22 Down-regulation of Syk expression in dendritic cells prevents colitis in murine models.23 Syk inhibits colitis by maintaining the microbial ecology and promoting inflammasome activation.24 However, whether Syk regulates neutrophil immune-responses in UC and whether inhibition of Syk relieves UC by improving the effects on neutrophils remain unclear.
In this study, we measured Syk levels in patients with UC and determined the effects of Syk on neutrophils using the small-molecule Syk inhibitor, R788. We found that Syk expression was significantly increased in the intestinal mucosa of patients with UC and positively correlated with disease activity. Generally, Syk is highly expressed on neutrophils. Here, Syk inhibition decreased the production and release of pro-inflammatory cytokines, chemokines, ROS, and MPO, and NETs formation by neutrophils and impaired their apoptosis and migration in vitro. Moreover, Syk blockade markedly alleviated dextran sulfate sodium (DSS)-induced colitis in mice. Our results indicate that Syk blockade alleviates intestinal mucosal damage by directly modulating neutrophil immune-responses via the mammalian target of rapamycin kinase (mTOR)/rubicon-like autophagy enhancer (RUBCNL)-dependent autophagy pathway, which may be a promising strategy for UC treatment.
Materials and methods
Patients
For this study, patients with UC and healthy controls (HCs) were recruited from the Department of Gastroenterology, Affiliated Hospital of Jining Medical University (Jining, Shandong, China) between May 2019 and August 2022. UC was diagnosed using standardized clinical, radiological, endoscopic, and histological criteria. We used the Mayo score to assess the clinical activity of UC and the UC Endoscopic Index of Severity (UCEIS) to grade the intestinal mucosal samples. The Mayo score system was assessed as stool frequency: normal number of stools (0), 1–2 stools (1), 3–4 stools (2), 5 or more stools (3); rectal bleeding: no blood seen (0), streaks of blood with stool less than half the time (1), obvious blood with stool most of the time (2), blood alone passed (3); findings on endoscopy: normal or inactive disease (0), mild disease (1), moderate disease (2), severe disease (3); physician's global assessment: normal (0), mild disease (1), moderate disease (2), severe disease (3).25 UCEIS was evaluated as vascular pattern: normal (0), patchy obliteration (1), obliterated (2); bleeding: none (0), mucosal (1), luminal mild (2), luminal moderate or severe (3); erosions and ulcers: none (0), erosions (1), superficial ulcer (2), deep ulcer (3).25 The characteristics of the patients with UC and HCs included in this study are listed in supplementary Table S1, see online supplementary material. This study was approved by the Institutional Review Board for Clinical Research at the Affiliated Hospital of Jining Medical University. Written informed consent was obtained from all subjects prior to their participation in this study.
Isolation and incubation of peripheral blood neutrophils
Peripheral blood neutrophils from HCs and patients with UC were isolated using the Ficoll–Hypaque density gradient (GE Healthcare, Piscataway, NJ, USA). Briefly, peripheral blood was collected in ethylene diamine tetraacetie acid anti-coagulation tubes, slowly laid on the surface of ficoll, and subjected to gradient centrifugation at 2000 rpm for 20 min at 20°C. After discarding the supernatant, the lowest layer was collected and treated twice or thrice with the red blood cell lysis buffer (BD Biosciences, San Diego, CA, USA) to eliminate erythrocytes. Isolated neutrophils (2 × 106 cells/ml) were resuspended in Roswell Park Memorial Institute-1640 medium supplemented with 0.5% fetal bovine serum (FBS) at 37°C in the presence of 5% CO2.
Single-cell RNA-sequencing analysis
Colonic biopsies of patients with UC and HCs were obtained via colonoscopy for single-cell RNA-sequencing (scRNA-seq) analysis by OE Biotech Co., Ltd (Shanghai, China). Libraries for scRNA-seq were generated using the BD Rhapsody platform and sequenced using the Illumina NovaSeq 6000 platform. Unique molecular identifiers (UMI) tools were applied for single-cell transcriptome analysis to identify the cell barcode whitelist, extract the cell barcode UMIs, and calculate the cell expression counts based on the filtered clean fastq data. Seurat54 software (version 4.0.0) was used for cell normalization and filtering, considering the MT percentage and minimum and maximum gene numbers. Uniform Manifold Approximation and Projection analysis were used for single cell–cell relationship descriptions. The FindAllMarkers function in Seurat was used to identify the marker genes in each cluster. Differentially expressed genes were identified using the FindMarkers function in Seurat. Moreover, P value < 0.05 and |log2fold-change| > 0.58 were set as the thresholds for significant differential expression.
RNA-seq analysis
Neutrophils isolated from patients with UC and HCs were treated with lipopolysaccharide (LPS; 300 ng/ml, Sigma-Aldrich, St. Louis, MO, USA) in the absence or presence of Syk inhibitor R788 (2 μM; Selleck Chemicals, Houston, TX, USA) for 3 h, and total RNA was extracted for RNA-seq analysis (OE Biotech Co., Ltd). The purity and concentration of the total RNA preparations were examined using NanoDrop ND-2000. Transcriptome libraries were constructed using the VAHTS Universal V6 RNA-seq Library Prep kit and sequenced using Lumina NovaSeq 6000. HISAT2 was used for reference genome comparison and gene expression calculation, and the read count of each gene was obtained using HTSeq-count. Principal component analysis and gene count plotting were performed using R (v 3.2.0) to assess the sample biological duplications. Differentially expressed genes were identified using DESeq2, with Q value < 0.05 and fold-change > 2 or < 0.5. Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway and Gene Ontology (GO) enrichment analyses were performed based on hypergeometric distribution using R (v 3.2.0).
Determination of ROS levels in neutrophils
To determine the ROS levels produced by neutrophils, 2 × 106 neutrophils were stimulated with Syk inhibitor R788 (2 μM) in the presence of LPS (300 ng/ml) for 3 h. Cells (1 × 104) were collected and analyzed using an ROS assay kit (YEASEN, Shanghai, China). ROS probe [2',7’-dichlorodi-hydrofluorescein diacetate (DCFH-DA); 10 μM] was added into the medium and incubated with the neutrophils for 30 min. Then, cells were washed thrice with and resuspended in cold phosphate-buffered saline (PBS) for flow cytometric analysis (DxFLEX; Beckman Coulter, Inc., USA). The emission wavelength of DCFH-DA was set at 530 nm.
Analysis of NETs formation in neutrophils
Neutrophils were isolated from the peripheral blood of HCs and patients with UC and stimulated with LPS (300 ng/ml) for 3 h. Neutrophils (2 × 105) were seeded on glass slides, fixed with 4% paraformaldehyde, and stained with Hoechst 33342 (1:1000; Invitrogen, San Diego, CA, USA). NETs were visualized under a confocal microscope (LSM 710; ZEISS, Oberkochen, Germany).
Assessment of neutrophil apoptosis
To analyze neutrophil apoptosis after treatment with R788 or LPS, an annexin V-fluorescein isothiocyanate (FITC) Apoptosis Detection Kit (BD Bioscience, San Diego, CA, USA) was used following the manufacturer's instructions. Briefly, neutrophils isolated from peripheral blood were stimulated with LPS (300 ng/ml), R788 (2 μM), and LPS (300 ng/ml) combined with R788 (2 μM) for 3 h and resuspended in the binding buffer at a concentration of 1 × 106 cells/ml. Subsequently, the cells were stained with FITC-conjugated annexin V and propidium iodide (PI) for 15 min at room temperature in the dark. Flow cytometric analysis was performed using a Beckman DxFLEX flow cytometer and analyzed using the FlowJoVX Software (Tree Star, Inc., Ashland, OR, USA).
Transwell migration assay of neutrophils
Neutrophil migration was assessed using a Transwell chamber (8 μm; Corning, NY, USA). Briefly, neutrophils (5 × 105 cells/well) stimulated with or without R788 (2 μM) in the presence of LPS (300 ng/ml) were seeded in the upper well of the Transwell chamber, and tissue culture medium dissolved in formyl methionine leucine phenylalanine (fMLP; 50 nM; Sigma) or PBS was added to the bottom well. After incubation at 37°C for 2 h, migrated neutrophils were counted under a light microscope in at least five randomly selected high-power fields.
DSS-induced colitis model in mice
Specific pathogen-free C57BL/6 mice (age: 8–10-weeks-old and weight: 20–25 g), were obtained from Pengyue Experimental Animal Breeding Co., Ltd. (Jinan, Shandong, China). Animal studies were reviewed and approved by the Institutional Animal Care and Use Committee of Jining Medical University. Then, the DSS-induced colitis model was established. Briefly, two groups of mice (eight mice per group) were administered 2.5% DSS (molecular mass, 36 000–50 000; MP Biomedicals, Solon, OH, USA) in drinking water for 7 days, and in selected experiments, R788 (30 mg/kg) was gavaged daily. The characteristics of acute colitis, such as diarrhea, bloody stools, body weight, and survival rate, were recorded daily. All mice were sacrificed on the eighth day and colonic tissues were obtained. A small portion of the colon (0.5 cm) was fixed with 4% paraformaldehyde for hematoxylin and eosin staining and immunofluorescence. Another small part of the colon (0.5–1.0 cm) was used for RNA and protein extraction. Furthermore, peripheral blood cells were isolated from mice after red blood cell lysis for flow cytometric analysis.
Quantitative reverse transcription-polymerase chain reaction
Total RNA was extracted from the biopsies and cell samples using the TRIzol reagent (Invitrogen) and reverse-transcribed into cDNA using the 5x All-In-One RT Master Mix (abm, Richmond, BC, Canada), according to the manufacturers’ instructions. Reverse transcription-polymerase chain reaction (RT-PCR) was performed using the following conditions: 25°C for 10 min and 42°C for 15 min, followed by 85°C for 5 min. The synthesized cDNA was stored at −20°C. Quantative (q) RT-PCR was performed using SYBR green. All qRT-PCR analyses were performed in triplicate. All primers were synthesized by ShengGong BioTek (Shanghai, China), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an endogenous reference gene (supplementary Table S2, see online supplementary material).
Immunohistochemistry
Immunohistochemistry (IHC) was performed using 5-μm thick sections of paraffin-frozen biopsies from patients with UC and HCs. Sections were air dried overnight, fixed in acetone for 10 min, and washed with PBS for 5 min. After incubation with EnVision FLEX Peroxidase-Blocking Reagent for 10 min, these sections were incubated with the anti-t-Syk (1 : 500; ab40781; Abcam, Cambridge, MA, USA) and anti-p-Syk (1 : 300; ab62338; Abcam) antibodies at 4°C overnight. After washing with PBS, the sections were incubated for 60 min with horseradish peroxidase (HRP)-conjugated goat anti-rabbit antibody at room temperature. The color reaction was developed with 3,3’-diaminobenzidine and the sections were counterstained with hematoxylin. Finally, the slices were observed under a laser scanning confocal microscope (Zeiss LSM800; Oberkochen, Germany).
Western blotting
Tissue and cell samples were lysed using the radioimmunoprecipitation assay lysis buffer supplemented with 10% protease and phosphatase inhibitors (Beyotime, China). Equal amounts of proteins were loaded, separated on a 10% sodium dodecyl sulfate-polyacrylamide gel, transferred to polyvinylidene fluoride membranes, blocked with 5% non‐fat milk for 2 h at room temperature, and incubated with primary antibodies overnight at 4°C. Antibodies against t-Syk (1 : 1000; ab40781; Abcam), p-Syk (1 : 1000; ab62338; Abcam), RUBCNL (1 : 1000; 21183–1-AP; Proteintech), P62 (1 : 1000; PM045; MBL), and LC3 (1 : 1000, 43566; CST) were used as primary antibodies. Then, cells were incubated with HRP-conjugated secondary antibodies (1 : 3000; Beyotime, China) for 2 h at room temperature. The membranes were visualized in a dark room using an enhanced chemiluminescence reagent (Beyotime, China). Protein bands were quantified using ImageJ software. Results were normalized to those of the GAPDH internal control.
Immunofluorescence staining of NETs in colon tissues
Distal colon tissues of DSS-induced colitis mice were collected, fixed with 4% paraformaldehyde, embedded in a paraffin block, cut into 5-μm thick slices, and transferred to glass slides for subsequent use. After deparaffinization and rehydration, these slides were treated with 0.3% Triton X-100 for 10 min at room temperature and blocked with 10% FBS for 1 h. Subsequently, immunostaining was performed with rabbit anti-mouse citrullinated H3 (CitH3; 1 : 200; ab5103; Abcam) and rat anti-mouse Ly6G (1 : 200; RB6-8C5; Invitrogen) overnight at 4°C. After incubation with secondary antibodies coupled with Alexa Fluor Dyes (Invitrogen) for 1 h, the coverslips were mounted onto glass slides using the Slowfade Gold antifade mountant with 4',6-diamidino-2-phenylindole (Invitrogen) to counterstain the DNA. Images were observed via confocal microscopy.
Statistical analysis
Data are expressed as the mean ± standard error of the mean. Statistical analysis was conducted using SPSS V.20.0 (SPSS; Chicago, IL, USA). All experiments were conducted at least in triplicate. All data were normally distributed. Data were analyzed using the unpaired Student's t-test, one-way analysis of variance (ANOVA) with post hoc Tukey multiple comparison test, or two-way ANOVA with post hoc Bonferroni multiple comparison test. Spearman's correlation analysis was performed to analyze the correlation between Syk expression and the UCEIS, Mayo Score, erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP). Statistical significance was set at P < 0.05.
Results
Syk expression is elevated in the inflamed colonic mucosa of patients with UC
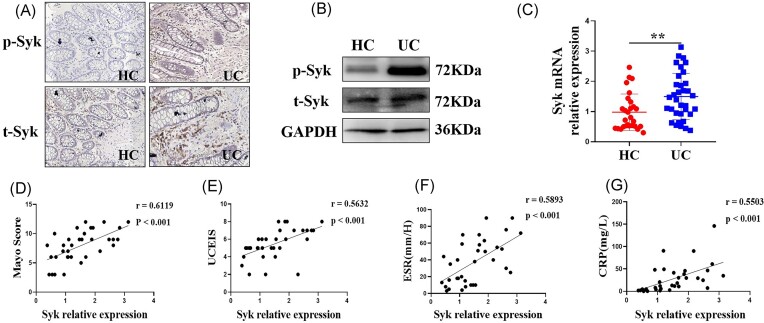
To explore the role of Syk in the pathogenesis of UC, we examined Syk expression in the colonic biopsies of patients with UC who underwent colonoscopy. Notably, increased protein expression of t-Syk and p-Syk were observed in the inflamed intestinal mucosa of patients with UC via IHC and western blotting (Fig. 1A and B). We also found higher Syk mRNA levels in intestinal mucosa of patients with UC compared to those in HCs (Fig. 1C). As the Mayo Score, UCEIS, ESR, and CRP are commonly used criteria to evaluate disease severity in patients with UC, we analyzed the correlation of Syk expression in the intestinal mucosa of patients with UC with the values of these parameters. As shown in Fig. 1D–G, Syk expression in the inflamed mucosa of patients with UC was positively correlated with the Mayo Score, UCEIS, ESR, and CRP. Taken together, these results indicate that Syk participates in the pathogenesis of UC and may serve as one potential therapeutic target in UC treatment.
Figure 1.
Expression of Syk is elevated in the inflamed colon mucosal biopsies of patients with UC. (A) Sections of colonic biopsies obtained via the endoscopy of HCs and patients with UC were analyzed for t-Syk and phosphorylated Syk (p-Syk) levels via immunohistochemistry. Original magnification × 200. (B) t-Syk and p-Syk protein expression in the colonic biopsies of HCs and patients with UC were determined via western blotting. (C) mRNA expression of Syk in the inflamed mucosa of patients with UC and HCs. RNA was isolated from the colonic biopsies of HC (n = 28) and patients with UC (n = 34). Syk mRNA levels were determined via qRT-PCR. Gene expression was normalized to GAPDH expression in each group. **P < 0.01. Correlation analysis between the Mayo Score (D), UCEIS (E), ESR (F), and CRP (G) and Syk mRNA expression levels in the inflamed mucosa of patients with UC.
Syk expression is markedly increased in neutrophils of patients with UC
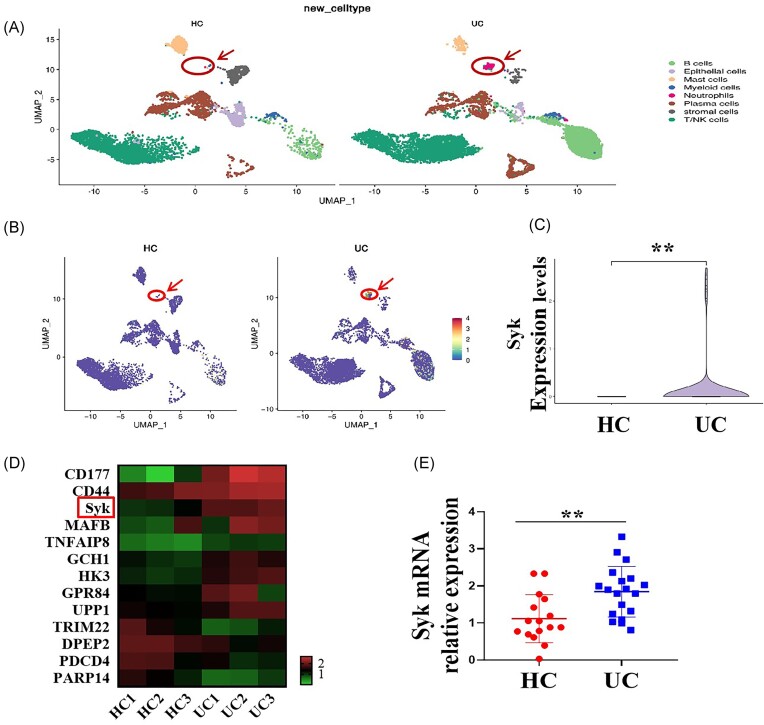
To determine Syk expression difference in neutrophils of the intestinal mucosa between patients with UC and HCs, ScRNA-seq was performed. By analyzing variably expressed genes across all immune cells, we identified eight major clusters: B cells, epithelial cells, mast cells, myeloid cells (monocytes/macrophages), neutrophils, plasma cells, stromal cells, and T/natural killer (NK) cells. Notably, the percentage of neutrophils in the inflamed intestinal mucosal tissues of patients with UC was 2.36%, which was much higher than the percentage of 0.02% observed in the normal intestinal mucosal tissues of HCs (Fig. 2A). In addition, Syk mRNA expression was significantly higher in the neutrophils of patients with UC than in those of HCs (Fig. 2B and C). Moreover, we isolated neutrophils from the peripheral blood cells of patients with UC and HCs for RNA-seq analysis and qRT-PCR and obtained a result consistent with that of scRNA-seq analysis (Fig. 2D and E). These data indicate that Syk signaling in neutrophils may contribute to mucosal inflammation in UC; however, its precise role requires further investigation.
Figure 2.
Syk expression is markedly elevated in the neutrophils of patients with UC. (A) Intestinal mucosal samples of patients with UC and HCs were used for scRNA-seq, and the percentages of neutrophils in the inflammatory intestinal mucosa of patients with UC and normal intestinal mucosa of HCs were determined. (B, C) Syk levels in the neutrophils of patients with UC and HCs were compared via scRNA-seq analysis. **P < 0.01. (D) Peripheral blood neutrophils isolated from patients with UC (n = 3) and HCs (n = 3) were used for RNA-seq and heat map analysis of some differentially expressed genes. (E) Peripheral blood neutrophils were collected from patients with UC (n = 19) and HCs (n = 16), and Syk mRNA expression levels were determined via qRT-PCR. **P < 0.01.
Syk promotes the production of pro-inflammatory cytokines and chemokines in neutrophils
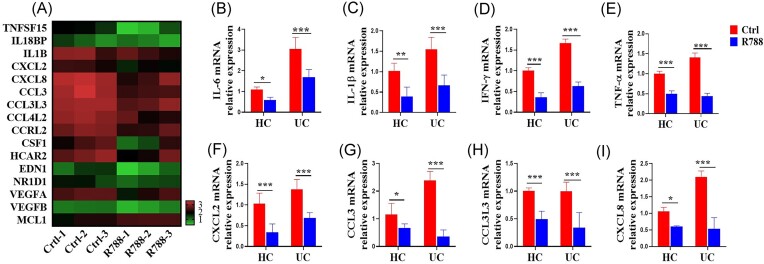
Activated neutrophils in the inflamed mucosa in patients with UC could release various pro-inflammatory cytokines and chemokines, which further activate and recruit other adaptive immune cells, such as monocytes and macrophages, to perpetuate and amplify intestinal inflammation. However, the role of Syk in the regulation of pro-inflammatory cytokines and chemokines by neutrophils in UC remains unknown. To assess whether Syk contributes to the expression of pro-inflammatory cytokines, chemokines, and other inflammatory mediators in neutrophils, we performed RNA-seq analysis of differential gene expression in neutrophils isolated from the peripheral blood of patients with UC after treatment with the Syk inhibitor, R788. We found marked downregulation in the levels of several cytokines, chemokines, and inflammation-related genes, such as TNF superfamily member 15 (TNFSF15), interleukin (IL)-1B, C-X-C motif chemokine ligand (CXCL)-2, CXCL8, C–C motif chemokine ligand (CCL)-3, CCL3L3, CCL4L2, colony-stimulating factor 1 (CSF1), vascular endothelial growth factor (VEGF)-A, and VEGFB, in the neutrophils of patients with UC after R788 treatment (Fig. 3A). In addition, we performed qRT-PCR to measure the levels of pro-inflammatory cytokines and chemokines in the neutrophils of patients with UC and HCs after R788 treatment. As shown in Fig. 3B–I, the mRNA levels of pro-inflammatory cytokines, such as IL-1β, IL-6, TNF-α, and interferon (IFN)-γ, and chemokines, such as CXCL2, CCL3, CCL3L3, and CXCL8, were markedly decreased by R788 treatment. Taken together, these results indicate that Syk markedly promotes pro-inflammatory cytokine and chemokine production by neutrophils in patients with UC.
Figure 3.
Blockade of Syk suppresses the production of pro-inflammatory cytokines and chemokines in neutrophils. (A) Neutrophils isolated from the peripheral blood of patients with UC (n = 3) were pre-treated in vitro with or without the Syk inhibitor, R788 (2 μM), for 3 h and used to detect the transcriptome differences via RNA-seq. Heat maps of some differential inflammation-related genes in neutrophils pre-treated with or without R788 were created. Neutrophils were isolated from patients with UC (n = 12) and HCs (n = 15) and cultured as described in (A). qRT-PCR was used to quantify the expression levels of some proinflammatory cytokines, such as interleukin (IL)-6 (B), IL-1β (C), interferon (IFN)-γ (D), and tumor necrosis factor (TNF)-α (E), and chemokines, such as the C-X-C motif chemokine ligand (CXCL)-2 (F), C–C motif chemokine ligand (CCL)-3 (G), CCL3L3 (H), and CXCL8 (I). Gene expression was normalized to GAPDH expression. *P < 0.05, **P < 0.01, and ***P < 0.001.
Syk promotes the release of ROS and MPO, and NETs formation by neutrophils
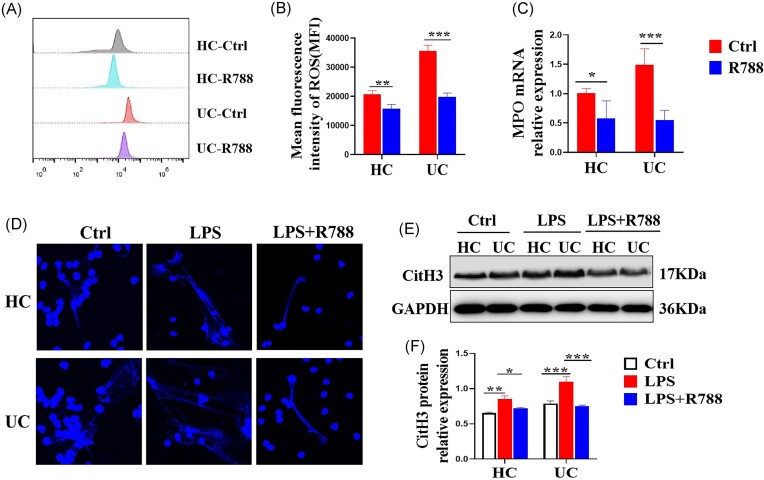
Aberrant and prolonged release of ROS and MPO, and NETs formation have been reported to have a direct effect on mucosal damage in UC;14 therefore, in this study, we investigated whether Syk affects these factors. Briefly, neutrophils were isolated from the peripheral blood of patients with UC and HCs and stimulated with the Syk inhibitor, R788. As shown in Fig. 4A–C, neutrophils isolated from HCs produced relatively low levels of ROS and MPO compared to those isolated from patients with UC. Moreover, we demonstrated that the levels of ROS and MPO released from neutrophils stimulated with R788 in vitro were significantly lower than those in the controls. Next, we found that blockade of Syk by R788 weakened the LPS-induced formation of NETs by neutrophils in both patients with UC and HCs (Fig. 4D). Moreover, we evaluated the protein expression of CitH3, an indicator of NETs formation, using western blotting. Consistently, expression levels of CitH3 protein in neutrophils of HCs and patients with UC were impaired after Syk inhibition (Fig. 4E and F). Collectively, these data indicate that Syk promotes the release of ROS and MPO, and NETs formation by neutrophils in patients with UC.
Figure 4.
Blockade of Syk inhibits the release of ROS and MPO, and NETs formation. (A) Neutrophils isolated from the peripheral blood of patients with UC (n = 10) and HCs (n = 10) were pre-treated in vitro with or without the Syk inhibitor, R788 (2 μM), for 3 h and collected for determination of ROS levels using flow cytometry. (B) Mean fluorescence intensity of ROS was statistically analyzed using the data from (A). **P < 0.01 and ***P < 0.001. (C) Neutrophils were isolated from patients with UC (n = 12) and HCs (n = 15) and cultured as described in (A). qRT-PCR was used to determine the mRNA levels of MPO. *P < 0.05 and ***P < 0.001. (D) Neutrophils were isolated from the peripheral blood of patients with UC (n = 10) and HCs (n = 10) and seeded on coverslips. Adherent neutrophils were stimulated with LPS (300 ng/ml) in the presence or absence of R788 (2 μM) for 3 h and stained with Hoechst 33342. NETs formation was examined via confocal microscopy (× 600). (E) Neutrophils were isolated from the peripheral blood of patients with UC (n = 8) and HCs (n = 8) and stimulated in vitro with LPS (300 ng/ml) in the presence or absence of R788 (2 μM) for 3 h. CitH3 protein expression was determined via western blotting. (F) Relative protein expression of CitH3 was statistically analyzed using the data from (E) with GAPDH as a reference. *P < 0.05, **P < 0.01, and ***P < 0.001.
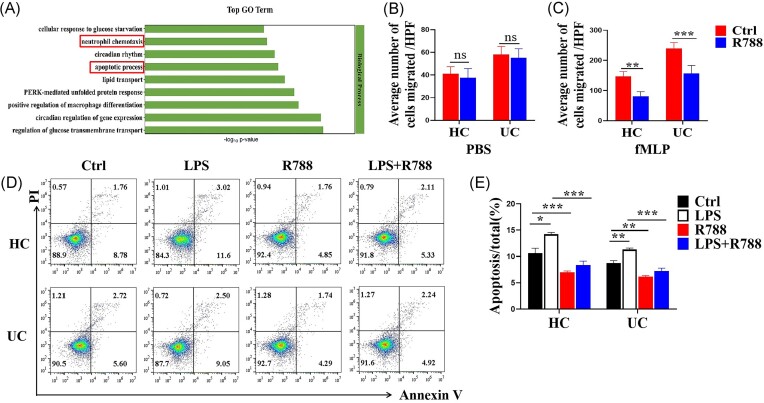
Syk augments the migration and apoptosis of neutrophils
GO analysis revealed that the biological functional differences between neutrophils of patients with UC treated with or without R788 were mainly reflected in neutrophil chemotaxis and the apoptotic process (Fig. 5A). Therefore, we hypothesized that Syk may affect neutrophil migration and apoptosis in the inflamed mucosa of patients with UC. To confirm our hypothesis, neutrophils were isolated from patients with UC and HCs and pre-incubated with or without R788 for 1 h. Transwell assays revealed that the migration of neutrophils was retarded in the presence of R788 (Fig. 5B and C).
Figure 5.
Blockade of Syk inhibits the migration and apoptosis of neutrophils. (A) Neutrophils were isolated from the peripheral blood of patients with UC (n = 3) and stimulated with or without R788 (2 μM) for 3 h. RNA-seq analysis was performed. Differentially expressed genes between the two groups were enriched for GO functional analysis. (B, C) Neutrophils were isolated from the peripheral blood of patients with UC (n = 10) and HCs (n = 10) and pre-treated with or without R788 (2 μM). Neutrophil migration was analyzed in a Transwell plate (8 μm) after stimulation with PBS (B) or fMLP (C) for 1 h. **P < 0.01 and ***P < 0.001. (D) Neutrophils were isolated from the peripheral blood of patients with UC (n = 10) and HCs (n = 10), pre-treated with or without R788 (2 μM) or LPS (300 ng/ml) for 3 h, and stained with annexin V to determine the number of apoptotic neutrophils via flow cytometry. (E) Percentages of apoptotic neutrophils were statistically analyzed using the data from (D). *P < 0.05, **P < 0.01, and ***P < 0.001.
Peripheral neutrophils were isolated from patients with UC and HCs and stimulated with LPS with or without R788 treatment in vitro for 3 h to determine the changes in neutrophil apoptosis. Flow cytometric analysis revealed that R788 directly suppressed neutrophil apoptosis in vitro in the majority of patients with UC and HCs (Fig. 5D and E). Taken together, these data indicate that Syk enhances the migration and apoptosis of neutrophils in patients with UC.
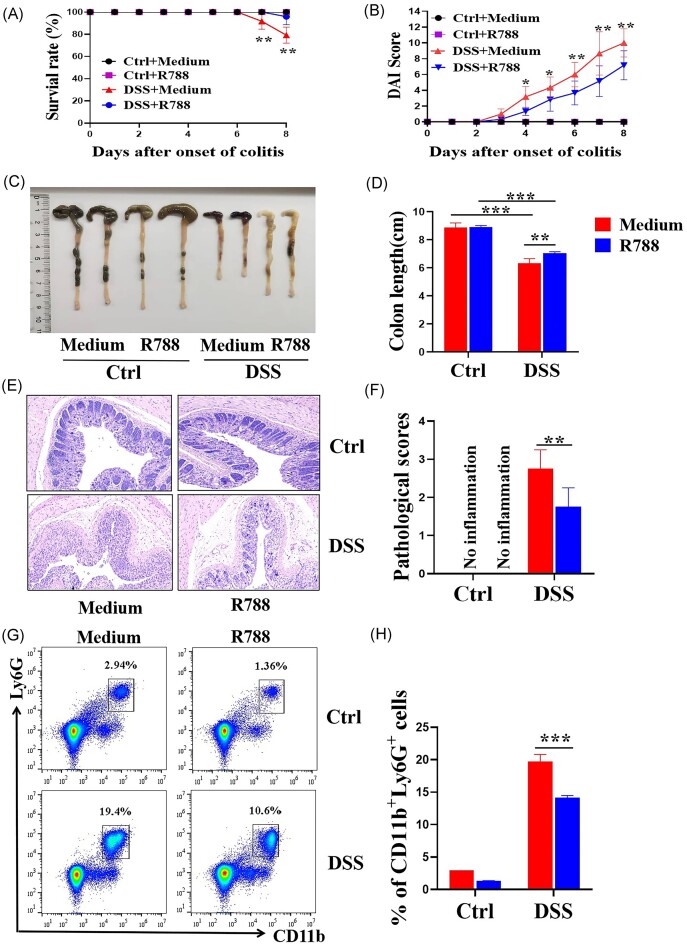
Blockade of Syk alleviates DSS-induced colitis in mice
To determine the role of Syk in colonic inflammation, we established a mouse model of DSS-induced colitis. Syk-selective inhibitor R788 (30 mg/kg) was intragastric administered to mice daily. As shown in Fig. 6A–F, mice treated with R788 exhibited milder colitis, characterized by higher survival rate, lower disease activity index, less-shortened colon length, and lower pathological scores than those in mice without R788 treatment.
Figure 6.
Blockade of Syk alleviates DSS-induced colitis in mice. Wild-type mice (n = 8 in each group) were administered 2.5% DSS in drinking water for 7 days. Mice from the DSS-treated group were gavaged with R788 (30 mg/kg) daily. (A) Survival rates of mice over 8 days observation. **P < 0.01. (B) Disease activity index (DAI) was calculated daily during the 8 days observation. *P < 0.05 and **P < 0.01. (C) Gross morphology of the colon on day 8 when mice were sacrificed. (D) Statistical length of the colon in different groups. **P < 0.01 and ***P < 0.001. (E) Representative hematoxylin and eosin staining images of distal colonic sections (original magnification × 100). (F) Changes in the pathological scores of colonic sections were calculated as indicated. **P < 0.01. (G) Immune cells were isolated from the peripheral blood of DSS-induced mice on day 8, and expression levels of Ly6G and CD11b were determined via flow cytometry. (H) Percentage of Ly6G+CD11b+ cells was calculated using the data from (G). ***P < 0.001.
A high percentage of neutrophils in peripheral blood is a marker of acute inflammatory response. We observed that the percentage of neutrophils in the peripheral blood of Syk-blocked mice was markedly decreased compared to that in the peripheral blood of wild-type mice after DSS exposure (Fig. 6G and H). Taken together, these data indicate that blocking Syk alleviates DSS-induced colitis in mice.
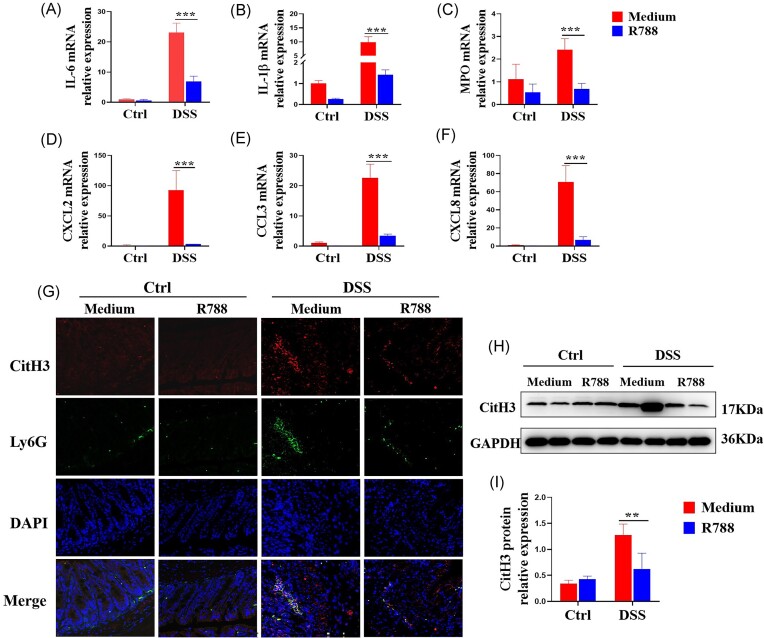
Blockade of Syk inhibits neutrophil-associated inflammatory mediator production and NETs formation in DSS-induced murine colitis
To further investigate whether Syk modulates neutrophil immune-responses during intestinal colitis, fresh colonic samples were obtained from a murine colitis model and total RNA was extracted to determine the inflammatory cytokine expression via qRT-PCR. As shown in Fig. 7A–F, expression levels of pro-inflammatory mediators, such as IL-1β, IL-6, MPO, CXCL2, CCL3, and CXCL8, were significantly decreased in the colonic tissues of Syk-blocked mice.
Figure 7.
Blockade of Syk inhibits neutrophil-associated inflammatory mediator production and NETs formation in DSS-induced murine colitis. Colon tissues were obtained from mice on day 8, and total RNA was extracted to examine the mRNA levels of IL-6 (A), IL-1β (B), MPO (C), CXCL2 (D), CCL3 (E), and CXCL8 (F) via qRT-PCR. Gene expression was normalized to GAPDH expression. ***P < 0.001. (G) Representative immunofluorescent images of CitH3 in the mice colon tissues. (H) Protein levels of CitH3 in mice colon tissues were determined via western blotting. (I) Relative protein expression of CitH3 was statistically analyzed using the data from (H) with GAPDH as a reference. **P < 0.01.
We also observed a marked decrease in CitH3 expression levels in the colon tissue sections of Syk-blocked mice compared to those of the controls via immunofluorescence staining (Fig. 7G) and western blotting (Fig. 7H and I). Collectively, these data suggest that Syk blockade ameliorates DSS-induced colitis, at least partially, by impairing neutrophil-associated inflammatory mediator production and reducing NETs formation by neutrophils.
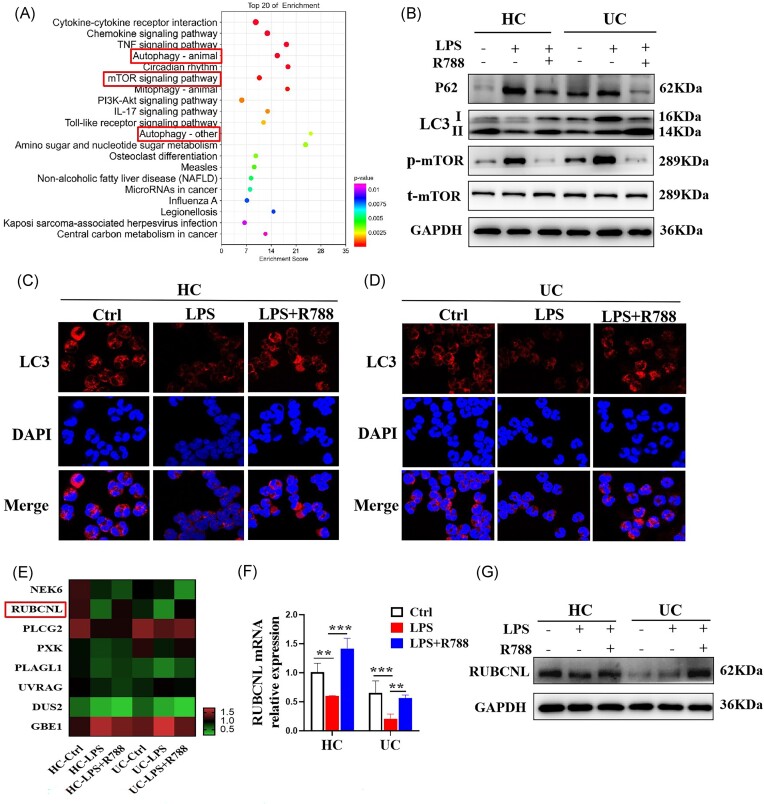
Syk modulates neutrophil immune-responses via the mTOR/RUBCNL-dependent autophagy pathway
To investigate the underlying mechanism by which Syk regulates neutrophil immune-responses, we isolated neutrophils from the peripheral blood of patients with UC and HCs and performed RNA-seq analysis to investigate the differential gene profiles in neutrophils with or without R788 treatment. KEGG enrichment and pathway analyses revealed that the differentially expressed genes were mainly associated with cytokine–cytokine receptor interaction, chemokine signaling pathway, TNF signaling pathway, mTOR signaling pathway, and autophagy (Fig. 8A). Autophagy is an important regulator of neutrophil function and neutrophil-mediated inflammation.26 To determine whether Syk modulates neutrophil immune-responses via autophagy, we isolated peripheral blood neutrophils from patients with UC and HCs and stimulated them with LPS in the absence or presence of R788. Western blotting analysis revealed that Syk inhibition enhanced the expression levels of autophagy-specific protein light chain 3 (LC3)-II, while reducing the expression levels of LC3-I, P62, and p-mTOR (Fig. 8B). Consistently, immunofluorescence staining revealed the increased expression of LC3 after R788 treatment in neutrophils from UC patients and HCs (Fig. 8C and D), indicating an augmentation in autophagy.
Figure 8.
Syk modulates neutrophil immune-responses via the mechanistic mTOR/RUBCNL-dependent autophagy pathway. Neutrophils were isolated from the peripheral blood of patients with UC and HCs and stimulated with LPS (300 ng/ml) in the absence or presence of R788 (2 μM) for 3 h. (A) RNA-seq analysis was performed. KEGG pathway analysis of differential gene expression in patients with UC (n = 3). (B) p62, LC3, and p-mTOR levels were determined via western blotting. GAPDH was used as a loading control. (C, D) Autophagic flux was detected via immunofluorescence staining for LC3. (E) Heat maps of some differential genes may interact with Syk via RNA-seq. RUBCNL mRNA and protein levels were determined via qRT-PCR (F) and western blotting (G), respectively. Gene expression was normalized to GAPDH expression. **P < 0.01 and ***P < 0.001.
Next, we focused on the genes related to the autophagy of neutrophils based on RNA-seq analysis and found that R788 reversed the LPS-induced decrease in the mRNA expression of RUBCNL (also known as Pacer), which is an autophagy enhancer and mediator of mTORC1 signaling, in the regulation of autophagosome maturation (Fig. 8E).27 Thus, we hypothesized that Syk affects autophagy in neutrophils by regulating RUBCNL expression. To verify this, we isolated neutrophils from patients with UC and HCs and stimulated them with LPS in the presence or absence of R788. As shown in Fig. 8F and G, LPS reduced the mRNA and protein levels of RUBCNL in the neutrophils of patients with UC and HCs, but after blockade of Syk by R788, the reduced expression of RUBCNL induced by LPS was improved. These results suggest that Syk may regulate neutrophil functions via the mTOR/RUBCNL autophagy pathway.
Discussion
Being a subtype of inflammatory bowel disease, the typical pathological feature of UC is the formation of neutrophilic cryptitis and crypt abscesses.28 Neutrophils are involved in the pathogenesis and progression of UC. Neutrophil dysfunction results in aberrant immune responses in UC, and balancing their beneficial and detrimental roles may be a potential therapeutic strategy for UC management.29 Syk coordinates the complicated intracellular signaling pathways downstream of immunoreceptors in neutrophils to enable appropriate cellular responses to the extracellular cues at infection sites.30,31 However, the specific role of Syk in mediating neutrophil activity in patients with UC remains ambiguous. In the present study, we investigated the role and underlying mechanisms of Syk in the regulation of neutrophil functions in UC. We found that Syk expression was increased in inflamed mucosa and neutrophils of patients with UC, and positively correlated with disease activity. Syk significantly promoted the production of pro-inflammatory mediators (e.g. cytokines, chemokines, ROS, MPO, and NETs) in neutrophils, and enhanced the apoptosis and migration of neutrophils via the mTOR/RUBCNL-dependent autophagy pathway.
Neutrophil-derived pro-inflammatory cytokines and chemokines can mediate the cross-talk between themselves and other immune cells and profoundly shape the local immune responses in the intestinal mucosa, playing important roles in the initiation and progression of mucosal inflammation.32 Here, we found that the production of neutrophil-derived pro-inflammatory cytokines, such as IL-6, IL-1β, TNF-α, and IFN-γ, and chemokines, such as CXCL2, CCL3, CCL3L3, and CXCL8, was significantly inhibited in Syk-blocked neutrophils. CXCL2 and CXCL8 trigger increased neutrophil recruitment to the intestinal lamina propria. CCL3 is an important contributor in the recruitment of other immune cells, including monocytes, macrophages, and NK cells, to boost local mucosal immune responses.33,34 RNA-seq analysis revealed that Syk-blocked neutrophils had low CSF1 transcript levels. CSF1, also known as the macrophage colony-stimulating factor, is a prominent chemokine involved in the proliferation and polarization of macrophages, with detrimental effects on UC cells.35 Therefore, the blockade of Syk in neutrophils may impair subsequent inflammation in UC.
Neutrophils exert powerful bactericidal effects via phagocytosis, degranulation, release of ROS and MPO, and formation of NETs. Despite the immune-protective roles of ROS, MPO, and NETs, abnormally high levels of ROS and NETs in the inflamed intestinal mucosa are associated with persistent mucosal damage in UC. Excessive production of ROS and a concomitant imbalance between oxidant and antioxidant levels lead to oxidative DNA damage in intestinal epithelial cells in UC.36 NETs accumulate in the inflamed intestinal mucosa of patients with active UC and sustain the inflammatory signals.37 Recent studies also proved that NETs impair the intestinal barrier function and induce intestinal damage and thrombosis in the inflamed intestinal mucosa.38,14 Here, we found that Syk-blocked neutrophils produced lower levels of ROS, MPO, and NETs, which may contribute to the compensatory regulation of detrimental neutrophil functions. In addition, we established a DSS-induced murine colitis model to verify the role of Syk in modulating neutrophil functions in vivo. Consistent with the in vitro findings, the levels of MPO and NETs in the inflamed intestinal mucosa were decreased by Syk inhibitor treatment, possibly due to the impact of Syk on neutrophils.
Neutrophils have the shortest lifespan among circulating leukocytes, and strengthening their lifespan is important for effective host defense at the sites of inflammation or tissue injury.39 RNA sequencing revealed increased mRNA levels of myeloid cell leukemia-1, which is involved in the negative regulation of neutrophil apoptosis,40 after Syk inhibition in neutrophils. Moreover, the apoptosis rates of neutrophils in patients with UC and HCs decreased after treatment with Syk inhibitors compared with those of the untreated neutrophils. These data indicate that the blockade of Syk inhibits neutrophil apoptosis. Massive infiltration of neutrophils into the intestinal mucosa is involved in the pathogenesis of UC, and neutrophil migration to the intestinal crypts is associated with mucosal injury.13 In the present study, we found that blocking Syk significantly suppressed the migration of neutrophils. Taken together, our data indicate that blocking Syk restricts neutrophil apoptosis and migration. This regulation not only prolongs neutrophil lifespan and strengthens their retention at sites of inflammation but also prevents excessive infiltration of neutrophils in the inflamed mucosa, thereby preventing tissue damage and maintaining intestinal homeostasis.
Autophagy, an intracellular degradation and energy recycling mechanism, is an important regulator of the immunological activity of neutrophils.41 Autophagy-deficient neutrophils exhibit reduced degranulation and NADPH oxidase-mediated ROS generation.26 Syk is involved in the regulation of autophagy in the pathological processes of several diseases. Syk-mediated autophagy is essential for epithelial–mesenchymal plasticity and breast cancer metastasis.42 Syk regulates major histocompatibility complex-II expression via autophagy in macrophages and may contribute to the regulation of adaptive immune responses in atherosclerosis.43 In our study, KEGG enrichment and pathway analyses revealed that the differentially expressed genes after blockade of Syk in neutrophils were associated with autophagy, indicating that Syk suppresses autophagy in the neutrophils of both patients with UC and HCs, as evidenced by the increased LC3II/I ratio and decreased P62 expression following Syk inhibition. Moreover, we found that Syk acts upstream of the mTOR pathway, and its inhibition decreases mTOR pathway activation, which is consistent with the findings of a previous study.44 Downregulation of p-mTOR expression further increases the expression of RUBCNL, an autophagy enhancer gene that interacts with mTOR and regulates autophagosome maturation.27 Thus, we concluded that Syk may partially modulate neutrophil immune-responses via the mTOR/RUBCNL-dependent autophagy pathway. To the best of our knowledge, this is the first study to identify the role of Syk in the regulation of neutrophil autophagy.
Collectively, our results demonstrated that Syk is a critical regulator of neutrophil immune-responses in patients with UC. Syk promoted the production of pro-inflammatory mediators and augmented the apoptosis and migration of neutrophils partially via the mTOR/RUBCNL-dependent autophagy pathway. Moreover, blockade of Syk restricted neutrophil hyperactivation and dysfunction, thereby controlling the inflammatory responses during intestinal mucosal inflammation. Therefore, targeting Syk may provide new insights into the modulation of neutrophil immune-responses to counterbalance intestinal inflammation in UC.
Supplementary Material
Acknowledgement
This work was supported by grants from the National Natural Science Foundation of China (Grants No. 82270562, 82200591 and 81901655), Tai Shan Young Scholar Foundation of Shandong Province (Grant No. tsqn202103190), TCM Science and Technology Development Plan of Shandong Province (Grants No. Q-2022133, Q-2022134, M-2023173), Research Fund for Academician Lin He New Medicine (Grant No. JYHL2022FZD05), and Postdoctoral Fund of the Affiliated Hospital of Jining Medical University (Grant No. JYFY303574).
Contributor Information
Fengqin Zhu, Department of Gastroenterology, Affiliated Hospital of Jining Medical University, Jining Medical University, Jining 272000, China.
Dehuai Jing, Department of Gastroenterology, Affiliated Hospital of Jining Medical University, Jining Medical University, Jining 272000, China.
Huihui Zhou, Department of Gastroenterology, Affiliated Hospital of Jining Medical University, Jining Medical University, Jining 272000, China.
Zongjing Hu, Department of Gastroenterology, Affiliated Hospital of Jining Medical University, Jining Medical University, Jining 272000, China.
Yan Wang, Department of Gastroenterology, Affiliated Hospital of Jining Medical University, Jining Medical University, Jining 272000, China.
Guiyuan Jin, Medical Research Center, Affiliated Hospital of Jining Medical University, Jining Medical University, Jining 272000, China.
Yonghong Yang, Medical Research Center, Affiliated Hospital of Jining Medical University, Jining Medical University, Jining 272000, China.
Guangxi Zhou, Department of Gastroenterology, Affiliated Hospital of Jining Medical University, Jining Medical University, Jining 272000, China.
Author Contributions
F.Z. and G.Z. conceived and designed the experiments. F.Z. performed the experiments. F.Z., G.J., and Y.Y. analyzed the data. D.J., H.Z., Z.H., and Y.W contributed to the clinical data and specimens. F.Z. and G.Z. wrote the manuscript. All authors discussed and revised the manuscript.
Conflict of Interest
The authors declare that they have no competing interests.
References
- 1. Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009; 361:2066–78. doi: 10.1056/NEJMra0804647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2018; 390:2769–78. doi: 10.1016/S0140-6736(17)32448-0. [DOI] [PubMed] [Google Scholar]
- 3. Mao R, Chen M. Precision medicine in IBD: genes, drugs, bugs and omics. Nat Rev Gastroenterol Hepatol. 2022; 19:81–2. doi: 10.1038/s41575-021-00555-w. [DOI] [PubMed] [Google Scholar]
- 4. Chang JT. Pathophysiology of inflammatory bowel diseases. N Engl J Med. 2020; 383:2652–64. doi: 10.1056/NEJMra2002697. [DOI] [PubMed] [Google Scholar]
- 5. Maloy KJ, Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature. 2011; 474:298–306. doi: 10.1038/nature10208. [DOI] [PubMed] [Google Scholar]
- 6. Parkos CA. Neutrophil-epithelial interactions: a double-edged sword. Am J Pathol. 2016; 186:1404–16. doi: 10.1016/j.ajpath.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fournier BM, Parkos CA. The role of neutrophils during intestinal inflammation. Mucosal Immunol. 2012; 5:354–66. doi: 10.1038/mi.2012.24. [DOI] [PubMed] [Google Scholar]
- 8. Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013; 13:159–75. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- 9. Nicolas-Avila JA, Adrover JM, Hidalgo A. Neutrophils in homeostasis, immunity, and cancer. Immunity. 2017; 46:15–28. doi: 10.1016/j.immuni.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 10. Wang W, Zhang J, Zheng N, et al. The role of neutrophil extracellular traps in cancer metastasis. Clin Transl Med. 2020; 10: e126. doi: 10.1002/ctm2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Christoffersson G, Vågesjö E, Vandooren J, et al. VEGF-A recruits a proangiogenic MMP-9-delivering neutrophil subset that induces angiogenesis in transplanted hypoxic tissue. Blood. 2012; 120:4653–62. doi: 10.1182/blood-2012-04-421040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sumagin R, Brazil JC, Nava P, et al. Neutrophil interactions with epithelial-expressed ICAM-1 enhances intestinal mucosal wound healing. Mucosal Immunol. 2016; 9:1151–62. doi: 10.1038/mi.2015.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brazil JC, Louis NA, Parkos CA. The role of polymorphonuclear leukocyte trafficking in the perpetuation of inflammation during inflammatory bowel disease. Inflamm Bowel Dis. 2013; 19:1556–65. doi: 10.1097/MIB.0b013e318281f54e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li T, Wang C, Liu Y, et al. Neutrophil extracellular traps induce intestinal damage and thrombotic tendency in inflammatory bowel disease. J Crohns Colitis. 2020; 14:240–53. doi: 10.1093/ecco-jcc/jjz132. [DOI] [PubMed] [Google Scholar]
- 15. Liu C, Mo LH, Feng BS, et al. Twist1 contributes to developing and sustaining corticosteroid resistance in ulcerative colitis. Theranostics. 2021; 11:7797–812. doi: 10.7150/thno.62256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lu H, Lin J, Xu C, et al. Cyclosporine modulates neutrophil functions via the SIRT6-HIF-1α-glycolysis axis to alleviate severe ulcerative colitis. Clin Transl Med. 2021; 11:e334. doi: 10.1002/ctm2.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Friedrich M, Pohin M, Jackson MA, et al. IL-1-driven stromal-neutrophil interactions define a subset of patients with inflammatory bowel disease that does not respond to therapies. Nat Med. 2021; 27:1970–81. doi: 10.1038/s41591-021-01520-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Geahlen RL. Getting Syk: spleen tyrosine kinase as a therapeutic target. Trends Pharmacol Sci. 2014; 35:414–22. doi: 10.1016/j.tips.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mócsai A, Ruland J, Tybulewicz VL. The SYK tyrosine kinase: a crucial player in diverse biological functions. Nat Rev Immunol. 2010; 10:387–402. doi: 10.1038/nri2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu S, Liao Y, Chen B, et al. Critical role of Syk-dependent STAT1 activation in innate antiviral immunity. Cell Rep. 2021; 34:108627. doi: 10.1016/j.celrep.2020.108627. [DOI] [PubMed] [Google Scholar]
- 21. Cooper N, Ghanima W, Hill QA, et al. Recent advances in understanding spleen tyrosine kinase (SYK) in human biology and disease, with a focus on fostamatinib. Platelets. 2023; 34:2131751. doi: 10.1080/09537104.2022.2131751. [DOI] [PubMed] [Google Scholar]
- 22. Can G, Ayvaz S, Can H, et al. The Syk inhibitor fostamatinib decreases the severity of colonic mucosal damage in a rodent model of colitis. J Crohns Colitis. 2015; 9:907–17. doi: 10.1093/ecco-jcc/jjv114. [DOI] [PubMed] [Google Scholar]
- 23. Hang L, Blum AM, Kumar S, et al. Downregulation of the syk signaling pathway in intestinal dendritic cells is sufficient to induce dendritic cells that inhibit colitis. J Immunol. 2016; 197:2948–57. doi: 10.4049/jimmunol.1600063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Malik A, Sharma D, Malireddi RKS, et al. SYK-CARD9 signaling axis promotes gut fungi-mediated inflammasome activation to restrict colitis and colon cancer. Immunity. 2018; 49:515–530.e5. doi: 10.1016/j.immuni.2018.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rubin DT, Ananthakrishnan AN, Siegel CA, et al. ACG Clinical guideline: ulcerative colitis in adults. Am J Gastroenterol. 2019;114:384–413. doi: 10.14309/ajg.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 26. Bhattacharya A, Wei Q, Shin JN, et al. Autophagy is required for neutrophil-mediated inflammation. Cell Rep. 2015; 12:1731–9. doi: 10.1016/j.celrep.2015.08.019. [DOI] [PubMed] [Google Scholar]
- 27. Cheng X, Ma X, Zhu Q, et al. Pacer is a mediator of mTORC1 and GSK3-TIP60 signaling in regulation of autophagosome maturation and lipid metabolism. Mol Cell. 2019; 73:788–802.e7. doi: 10.1016/j.molcel.2018.12.017. [DOI] [PubMed] [Google Scholar]
- 28. Ungaro R, Mehandru S, Allen PB, et al. Ulcerative colitis. Lancet. 2017; 389:1756–70. doi: 10.1016/S0140-6736(16)32126-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen H, Wu X, Xu C, et al. Dichotomous roles of neutrophils in modulating pathogenic and repair processes of inflammatory bowel diseases. Precis Clin Med. 2021; 4:246–57. doi: 10.1093/pcmedi/pbab025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Futosi K, Fodor S, Mócsai A. Reprint of neutrophil cell surface receptors and their intracellular signal transduction pathways. Int Immunopharmacol. 2013; 17:1185–97. doi: 10.1016/j.intimp.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 31. Lowell CA. Src-family and Syk kinases in activating and inhibitory pathways in innate immune cells: signaling cross talk. Cold Spring Harb Perspect Biol. 2011; 3:a002352. doi: 10.1101/cshperspect.a002352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mantovani A, Cassatella MA, Costantini C, et al. Neutrophils in the activation and regulation of innate andadaptive immunity. Nat Rev Immunol. 2011; 11:519–31. doi: 10.1038/nri3024. [DOI] [PubMed] [Google Scholar]
- 33. Tecchio C, Cassatella M. Neutrophil-derived chemokines on the road to immunity. Semin Immunol. 2016; 28:119–28. doi: 10.1016/j.smim.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kucharzik T, Hudson J, Lugering A, et al. Acute induction of human IL-8 production by intestinal epithelium triggers neutrophil infiltration without mucosal injury. Gut. 2005; 54:1565–72. doi: 10.1136/gut.2004.061168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Muñoz-Garcia J, Cochonneau D, Télétchéa S, et al. The twin cytokines interleukin-34 and CSF-1: masterful conductors of macrophage homeostasis. Theranostics. 2021; 11:1568–93. doi: 10.7150/thno.50683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pereira C, Grácio D, Teixeira JP, et al. Oxidative stress and DNA damage: implications in inflammatory bowel disease. Inflamm Bowel Dis. 2015; 21:2403–17. doi: 10.1097/MIB.0000000000000506. [DOI] [PubMed] [Google Scholar]
- 37. Dinallo V, Marafini I, Di Fusco D, et al. Neutrophil extracellular traps sustain inflammatory signals in ulcerative colitis. J Crohns Colitis. 2019; 13:772–84. doi: 10.1093/ecco-jcc/jjy215. [DOI] [PubMed] [Google Scholar]
- 38. Lin EY, Lai HJ, Cheng YK, et al. Neutrophil extracellular traps impair intestinal barrier function during experimental colitis. Biomedicines. 2020; 8:275. doi: 10.3390/biomedicines8080275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Geering B, Simon HU. Peculiarities of cell death mechanisms in neutrophils. Cell Death Differ. 2011; 18:1457–69. doi: 10.1038/cdd.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Milot E, Filep JG. Regulation of neutrophil survival/apoptosis by Mcl-1. Scientific World Journal. 2011; 11:1948–62. doi: 10.1100/2011/131539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yu Y, Sun B. Autophagy-mediated regulation of neutrophils and clinical applications. Burns Trauma. 2020; 8:tkz001. doi: 10.1093/burnst/tkz001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shinde A, Hardy SD, Kim D, et al. Spleen tyrosine kinase-mediated autophagy is required for epithelial-mesenchymal plasticity and metastasis in breast cancer. Cancer Res. 2019; 79:1831–43. doi: 10.1158/0008-5472.CAN-18-2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Choi SH, Gonen A, Diehl CJ, et al. SYK regulates macrophage MHC-II expression via activation of autophagy in response to oxidized LDL. Autophagy. 2015; 11:785–95. doi: 10.1080/15548627.2015.1037061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Schweig JE, Yao H, Coppola K, et al. Spleen tyrosine kinase (SYK) blocks autophagic Tau degradation in vitro and in vivo. J Biol Chem. 2019; 294:13378–95. doi: 10.1074/jbc.RA119.008033. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.