Summary
Gastroesophageal reflux disease (GERD) is associated with significant morbidity in patients with systemic sclerosis (SSc). Although the introduction of proton pump inhibitors (PPIs) into clinical care have represented a major achievement in the management of oesophago-gastric problems in SSc, PPIs are seldom fully effective in SSc patients, and the utilization of maximum PPI dosages is a very frequent clinical practice. However, currently there is little evidence currently to support the empiric use of PPIs in SSc which is especially relevant in regard to safety concerns of long-term exposure with have been raised in the general population. The purpose of this viewpoint is to highlight the significant beneficial impact of PPIs on GERD in SSc, while considering the potential adverse effects in this patient population. Furthermore, we highlight the unmet needs of SSc patients with GERD, and also propose an agenda for future research to optimise the safe and effective use of PPIs in SSc.
Introduction
Systemic sclerosis (SSc) is an autoimmune disease. Involvement of the upper and lower gastrointestinal tract (GIT) is one of the major characteristics of systemic sclerosis.1-3 In particular, upper GIT dysfunction is one of the main causes of complaints in patients with SSc, most commonly presenting as gastroesophageal reflux disease (GERD).2,4,5 Importantly, the integration of proton pump inhibitors (PPIs) into clinical care is a major achievement in the management of oesophago-gastric problems in SSc. PPIs are widely used to treat GERD in the general population, including in the long-term prevention and treatment of peptic ulcer disease. However, PPIs are seldom fully effective in SSc patients,6 and the utilization of maximum PPI dosages is a very frequent clinical practice. However, there is little evidence currently to support the empiric use of PPIs in SSc, especially in regard to the safety of long-term exposure. The purpose of this viewpoint is to highlight the significant beneficial impact of PPIs on GERD in SSc while considering the potential adverse effects that have been reported in the general population. Furthermore, we highlight the unmet needs of SSc patients with GERD and an agenda for future research to optimise the safe and effective use of PPIs in these patients.
Search strategy and selection criteria
We adopted a pragmatic approach to our review and therefore conducted a broad scoping review with critical expert opinion from relevant specialists. We searched the PubMed® database up to 1st January 2021 using the following keywords: ‘Systemic sclerosis’, ‘Scleroderma’, ‘Proton pump inhibitors’ and ‘PPIs’.
Viewpoint authorship
We adopted a pragmatic and inclusive approach and included rheumatologists with an interest in SSc, gastroenterology (CM), gastrointestinal surgery (PM-C), and with patient representation (IG).
High burden of upper gastrointestinal disease in SSc
Oesophageal involvement is very common in SSc and associated with significant morbidity,2,5 due to symptoms of GERD such as dyspepsia (e.g., heartburn) and/or dysmotility (e.g., dysphagia) or their sequelae. However, occasionally oesophageal dysfunction can be completely asymptomatic and only detected, for example, on endoscopic examination (i.e., performed for a particular clinical indication) or when evidence of oesophageal dilation is seen on cross-sectional chest imaging.7,8 The pathogenesis of GERD is complex and currently incompletely understood.9 In SSc, delayed gastrointestinal (GI) motility and incompetence of the lower oesophageal sphincter promotes gastric acid reflux and oesophageal inflammation. Oesophagitis might be particularly problematic leading to dysphagia and a risk of malnutrition. Oesophagitis can also induce anaemia which is challenging in a disease with a high cardiovascular burden. Furthermore, persistently uncontrolled GERD and oesophagitis can result in structural changes (i.e., stricture formation and Barrett’s oesophagus).10 In addition, oesophageal dysmotility may lead to aspiration which has been associated with low pulmonary function,11,12 and oropharyngeal disease manifestations (e.g., dysphonia and enamel decay).
Non-PPI treatment of GERD
Lifestyle modifications, although are these commonly implemented as adjunct management strategies (e.g., head of bed elevation, small but frequent meals, avoidance of triggering foods, smoking cessation), they are frequently ineffective in SSc. Therefore, as a result, pharmacological treatment is usually indicated in patients with SSc. Historically, histamine-2 receptor antagonists (H2RAs) were often ineffective in achieving sufficient control of reflux symptoms in patients with SSc and PPIs have become the first-line therapy.13 In patients with a partial response to PPIs, add-on-therapy can be considered including (but not limited to) H2RAs, alginates, sucralfate and promotility agents (e.g., metoclopramide, domperidone and prucalopride).14,15
Use of surgical approaches is rare and usually reserved for patients who have failed optimal medical therapy, and continue to experience persistent severe symptoms and evidence of oesophagitis or structural disease.16 However, major surgery (e.g., fundoplication and Roux-en-Y gastric bypass) should be considered as a last resort in severe cases only. This is because surgery is considered to provide little benefit, particularly if there is abnormal oesophageal manometry or gastroparesis or intestinal dysmotility, although early implementation prior to dysmotility has not been studied. Surgery can lead to serious complications including worsening of dysphagia and the need for recurrent endoscopic dilatation in patients with severe strictures.16,17
PPIs in patients with SSc
PPIs may be the class of drug that has had the greatest impact on the daily symptoms in the majority of patients with SSc. However, in SSc, no large, randomised, placebo-controlled trials of PPIs have been ever performed to clarify the dosing or duration of this therapy. The limited number of small studies that have shown short-term (e.g., 4 to 6 months) symptomatic benefit.18-20 Treatment efficacy in these studies have largely relied on patient reported GI symptoms, as well as different assessed objectives measures (e.g., of oesophageal inflammation or dysmotility).18-20 To date, the long-term benefit of treatment is yet to be confirmed.18-20 Furthermore, despite treatment with PPI, objective progression of oesophageal dysfunction has been reported in patients with SSc.6,20,21 There is a wide range of currently available PPIs; however, to date, there is no clear advantage with any particular agent. Therefore, the cheapest therapy is often prescribed, at least initially. Based on the known pharmacology of the medication, PPIs are prescribed, for example, typically 30-60 minutes before meals to optimise gastric acid suppression. However, the timing is less important with newer versions (e.g. Dexlansoprazole).22 Once- and twice-daily PPI regimens are utilised, the latter of which may confer greater gastric acid suppression.22,23
Despite treatment with PPIs, many patients with SSc still have evidence of continued GERD: in a prospective study of omeprazole 20mg twice daily for 4 weeks in 243 SSc patients, PPI-partial response (defined as <50% improvement in GERD visual analogue scale) was found in 53.9% of patients.6 Furthermore, despite high-dose PPI use, evidence of continued abnormal oesophageal acid reflux exposure is common in SSc patients.21 The mechanisms underlying PPI failure in SSc are believed to be largely driven by ineffective oesophageal clearance, with symptoms attributable to the reflux of gastric acid and/or other gastric contents.6,21
Risks of PPIs in the general population with relevance to patients with SSc
In the general population, a wide range of severe adverse effects/risks of PPIs, that may be especially relevant in SSc patients, has been reported, although no high-quality prospective studies have been performed. The major risks of PPIs, which are of practical concern to clinicians who care for patients with SSc are presented in Table 1. It is important to highlight that these data are based on administrative medical databases and large non-disease differentiated, non-SSc cohorts. It is not known if these findings apply to patients with SSc.
Table 1.
The major risks of PPIs that have been highlighted in the general population which are of practical concern to clinicians who care for patients with SSc (note that this is not an exhaustive list).
| Risk of PPI | Relevance/explanation |
|---|---|
| Small intestinal bacterial overgrowth (SIBO) | SIBO is associated with significant disability in SSc. PPI use with reduced gastric acid production could cause/exacerbate SIBO. |
| Kidney disease | Increased risk of acute and chronic kidney disease from PPIs. Rarely interstitial nephritis from PPI. Patients with SSc have a spectrum of kidney disease including reduction in renal filtration. Scleroderma renal crisis is a medical emergency and can sometimes require permanent dialysis. |
| Pneumonia | Increased risk of community acquired pneumonia has been reported with PPIs. Patients with SSc often have interstitial lung disease and/or are receiving treatment with immune suppressive therapies. |
| Hypomagnesemia | Patients with SSc are at risk of hypomagnesemia from GI disease. |
| Osteoporosis and increased risk of fracture | Patients with SSc have an increased risk of low bone density/osteoporosis and fracture. |
Risk of Infection
An increased risk of infections (e.g., lung and urine) has been associated with PPI exposure, including SARS-CoV-2,24-27 and Clostridium difficile.24. The causes driving an increased risk of infection are likely multifactorial including from reduced acidity of the stomach contents and may not necessarily be causally related. For example, patients with severe oesophageal disease are more likely to be prescribed PPIs and have an increased risk of aspiration, therefore the relationship between PPI use and pulmonary infection may not necessarily be causal. Evidence of lung involvement [e.g., interstitial lung disease (ILD)] can be identified in the majority (~80%) of patients with SSc and often requires treatment with potent immunosuppressive therapy,28,29 increasing the risk of infection, even independent of the use of PPIs. For Covid-19, separation between progression of SSc-associated ILD and Covid-19 pneumonia can be very challenging, although certain radiographic parameters to facilitate the diagnosis have been identified.30 A systematic review, which included a meta-analysis of 8 observational studies in the general population, revealed that the overall risk (odds ratio) for pneumonia was very slightly elevated with both PPIs (1.27) and H2RAs (1.22).31 Almario et al,26 reported a dose-response relationship between PPIs and Covid-19. Individuals receiving treatment with (vs. without) PPIs once (2.15) or twice (3.67) daily were at moderately increased risk for reporting a positive Covid-19 test.26 However, individuals receiving treatment with H2RAs were not found to be at a higher risk of infection.26
Cardiovascular risk
An increased risk of cardiovascular disease is reported in SSc patients, including myocardial infarction, stroke, and peripheral vascular disease; however, this still remains a controversial issue.32,33 For example, it can be challenging to distinguish between primary heart disease (SSc targeting the heart) and secondary cardiac complications (common cardiovascular events related to atherosclerosis). Significant concerns have been raised about the cardiovascular risk of PPIs in the general population and adverse outcomes in patients with acute coronary syndromes or interventions. In fact, several authors have reported an increased risk of approximately 20 to 30% for myocardial infarction which is independent of clopidogrel use.34,35 The underlying mechanisms are incompletely understood and likely multifactorial. Chronic PPI use may increase plasma levels of dimethylarginine dimethylaminohydrolase resulting in reduced generation of vascular nitric oxide. Moreover, PPI-induced endothelial dysfunction has been also implicated in vascular remodelling.35 There are also significant drug interactions between certain PPIs (e.g., esomeprazole) and clopidogrel, resulting in reduced anti-platelet efficacy. In SSc, severe hypomagnesaemia (discussed later) could potentially induce fatal cardiac arrythmia.35
Risk of Small Intestinal Bowel Overgrowth
Small intestinal bowel overgrowth (SIBO) occurs in approximately 40% of SSc patients and is associated with significant GI-related morbidity and mortality.36,37 In the general population, PPIs have been reported to moderately increase the risk of SIBO.38,39 A meta-analysis of 11 studies (n=3134 patients), has identified that the risk (odds ratio) of SIBO in PPI users compared to non-users was 2.28. There is consistent evidence of an association between PPI use and SIBO in studies that use aspirate (e.g., duodenal or jejunal) cultures to diagnose SIBO, whereas conflicting results have been reported in studies employing glucose hydrogen breath tests.38,39 Taken together, these data suggest that PPIs could potentially cause and/or exacerbate SIBO in SSc patients although this has never been formally studied.
Risk of Kidney disease
A higher risk of both acute kidney injury (AKI) and chronic kidney disease (CKD) due to PPI use has been reported in the general population.40-42 This is of potential concern because some SSc patients have evidence of kidney involvement, from life-threatening scleroderma renal crisis (SRC) to other forms of slowly progressing chronic renal dysfunction, the latter of which is frequently under-recognised.43 In recent decades, survival from SRC has improved significantly, in particular from the use of ACE inhibitors and tight blood pressure control.44-46 Although recovery of renal function can occur (even years) after SRC, a minority of patients require life-long renal replacement therapy (dialysis or renal transplant).46 Both AKI and acute interstitial nephritis have been attributed to PPI use.40,42 In a retrospective study using a large health maintenance organisation cohort (SSc not separated), PPI exposure (adjusted odds ratio) was associated with both AKI (4.35) and CKD (1.2).42 A dose-dependent relationship between PPI use (e.g., twice- vs once-daily dosing) and risk of CKD has been reported.41 Any relationship between renal function and use of PPI has not been studied in SSc. There is no evidence that use of PPI predisposes to SRC or influences the prognosis of SRC once it has occurred.
Hypomagnesemia
Profound hypomagnesemia has been reported in long-term PPI users,47 including SSc patients, resulting in symptomatic and life-threatening hypocalcaemia (e.g., cardiac arrythmia and tetany).47,48 In SSc, patients with existing nutritional or gastrointestinal (e.g., diarrhoea) problems could therefore potentially be at greater risk of hypomagnesemia when concomitant PPIs are prescribed.48
Other adverse effects of PPIs reported in the general population
An increased risk of dementia has been inconsistently reported with PPIs.49 In SSc patients, evidence of mild and severe (i.e., dementia) cognitive impairment has been reported from hypothesis-generating studies.50,51 Vitamin C and iron deficiency has also been reported with PPI.52 In SSc, iron deficiency is not uncommon,53 and is associated with a worse prognosis of pulmonary hypertension.54 A causative association between PPIs and gastric cancer remains controversial with current evidence based on observational studies with a high potential for unmeasured and confounding factors.55 Potential mechanisms include hypergastrinemia, gastric atrophy, chronic Helicobacter pylori infection and bacterial overgrowth.55
SSc-related PPI considerations
SSc-related calcinosis
Dystrophic deposition (subcutaneous or intracutaneous) of insoluble calcium salts is common in patients with SSc and responsible for significant morbidity.56,57 In SSc, long-term PPI exposure including high-dose PPI was significantly associated with calcinosis in a single center study which included both retrospective (n=199) and prospective (n=215) cohorts.58 It may well also relate to effects on magnesium as previously described. However, the association between high-dose PPIs and calcinosis should not reduce PPI use altogether, but rather encourage cautious PPI dosing in the presence of significant calcinosis. Further studies are indicated to confirm whether this association is specific to PPIs, as other anti-reflux medications, such as antacids, are associated with hypercalcemia/milk-alkali syndrome.
Interstitial lung disease
As previously described, ILD is a major cause of disease-related morbidity and mortality in SSc. There is strong evidence to strongly support the notion that GERD is associated with (and even may induce) ILD in SSc.59,60 Furthermore PPI-use has been reported to be correlated with the absence of radiological progression and improvement in survival of SSc-related ILD.61 However, a recent study found no relationship between PPI use and ILD progression in a multivariable analysis that adjusted for severity of reflux symptoms and severity of baseline ILD.62 It is important to highlight that the beneficial effect of PPIs on ILD is likely related to reduction of inhaled gastric contents resulting in chronic inflammation of the lung tissue.
Diarrhoea
Almost 50% of patients with SSc report having some degree of constipation and diarrhoea, affecting quality of life,63 and sometimes leading to weight loss and related fatigue. In addition, some key drugs used for treatment of SSc (e.g., nintedanib and mycophenolate mofetil) potentially cause diarrhoea, and sustained diarrhoea often leads to reduction of dosage or even discontinuation of these potentially disease-modifying drugs. It is often difficult to determine the underlying causes of diarrhoea, but discontinuation of PPIs sometimes results in improvement of diarrhoea since diarrhea is a common side effect of all PPIs.64
Osteoporosis and increased risk of fracture
In SSc patients, an increased risk of reduced bone density including osteoporosis and fracture has been reported.65,66 In the general population, an increased risk of fracture has been associated with PPI treatment.67-69 A meta-analysis (again without SSc separated), which included 24 observational studies (and over 2 million participants), concluded that PPI use was significantly associated with increased risk of hip fracture compared with no PPI use (odds ratio of 1.17).69 An increased risk of spinal and other-site fractures has also been reported.68 The risk was greater with higher doses of PPIs and was comparable with both short- and long-term PPI treatment,69 whereas, H2RAs have not been associated with a significant increased risk of hip fracture.67,69 All studies have been observational and thus a causative link between the use of PPI and osteoporosis remains to be established. Nevertheless, several hypothetical pathogeneses have been postulated. Hypochlorhydria is believed to decrease the absorption of micronutrients including calcium and vitamin B12, the latter of which can result in hyperhomocysteinemia which affects bone quality.52,70 Furthermore, chronic hypergastrinemia from PPI treatment may result in parathyroid hyperplasia and resultant bone resorption.71 Vitamin D levels should be monitored and to optimised, especially in patients at risk of osteoporosis.
Practical considerations: prescribing PPIs in SSc
In SSc patients, from the earlier stage of the disease, there is a need to review the non-pharmacological management of GERD including lifestyle measures (e.g., small frequent meals, elevation of the head of the bed, smoking cessation) as well as adherence. Reducing the use of other drug therapies that can cause GERD should also be considered. However, this can be challenging in SSc as other drugs, such as calcium channel blockers that can reduce the lower esophageal sphincter tone, thereby promoting reflux disease, are commonly prescribed for Raynaud’s phenomenon and/or digital ulcers.
This review has highlighted the possible trade-off associated with long-term PPI use, although it must be emphasized that the data come from observational and cohort studies that are considered hypothesis-generating rather than hypothesis-testing. We propose that clinicians should review PPI use as part of the ‘annual assessment’ process of SSc patients, including behavioral educations as well as actively considering to temporary withdrawal or substitution with other anti-acid therapies, or applying other management strategies (e.g., PPI dosage de-escalation, change to H2RA or pro-kinetic medications) when there is an issue or concern about the individual patient risk/benefit ratio of PPIs. Clinicians also need to be aware of the wide range of drug interactions with PPIs which could be particularly relevant in patients with SSc. For example, PPI use can impair mycophenolate mofetil absorption, which is increasingly used in SSc (e.g., for ILD).72,73
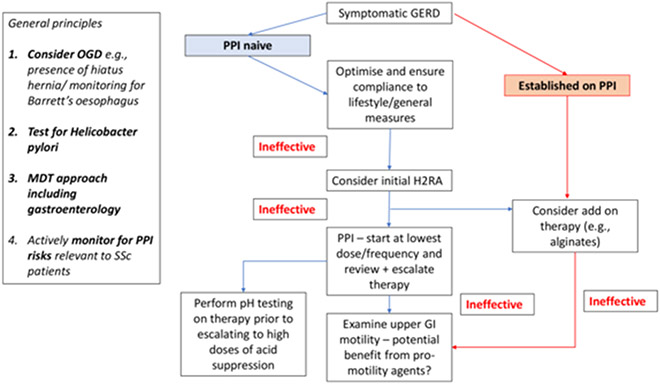
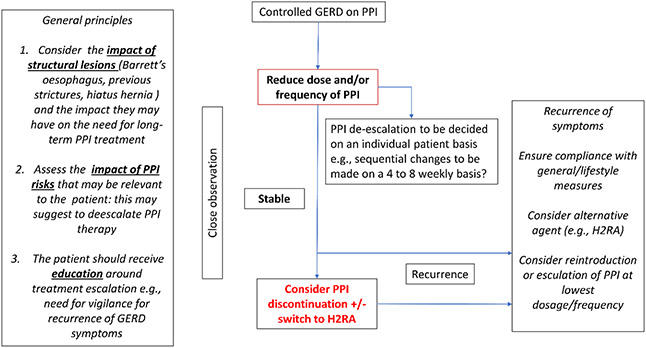
Based on the paucity of evidence and upon expert opinion, we present two potential pragmatic approaches to PPI management of SSc patients with symptomatic (Figure 1) and controlled GERD (Figure 2). General principles include considering the impact of structural disease (e.g., Barrett’s oesophagus and the presence of a hiatus hernia) and actively monitoring for PPI risks that are relevant to SSc patients. Adherence to general measures including lifestyle changes are mandatory and should be reviewed in all patients with symptomatic GERD, irrespective of prescribed drug therapy. An initial trial of H2RAs can be considered in treatment-naïve patients to try and avoid unnecessary exposure to PPIs, where possible. Consideration should be made to performing pH oesophageal testing on therapy prior to escalating to high doses of acid suppression. In patients with refractory GERD despite optimised PPI, upper GI motility should be examined and the role of standard (e.g., metoclopramide and domperidone) and non-standard pro-motility agents (e.g., prucalopride, pyridostigmine, buspirone) pro-motility agents should also be considered. In patients with controlled GERD on PPI, reduction in the dose and/or frequency of administration of PPI can be considered. Treatment de-escalation and on-demand use should be decided on an individual patient basis with a potential view toward PPI discontinuation/dose reduction or consideration of switching to an alternative therapy (including in combination) when possible (e.g., H2RA or pro-kinetics). However, it must be emphasised that alternative therapies are unlikely to be sufficient, as PPIs are much more effective for GERD than H2RAs. For patients with recurrent symptoms after PPI discontinuation, options could include the above alternative therapies prior to increasing the PPI dose.
Figure 1.
Management of symptomatic gastroesophageal reflux disease (GERD) in proton pump inhibitor (PPI) naive SSc patients and those established on PPI. Other causes odynophagia separate from GERD should also be potentially considered including presence of candida oesophagitis. In general, we do not advocate that gastroscopy should be performed in all SSc patients at baseline, but on the basis of a specific clinical indication (e.g., refractory GERD). Patients who initially respond to one type of PPI (e.g., omeprazole) may benefit from a trial of a different type of PPI (e.g., dexlansoprazole) e.g., due to better adherence from reduced frequency of dosing or differing drug treatment efficacy. Surgical intervention should be as a last resort in exceptional cases only and has a high probability of failure in the context of aperistalsis in SSc. H2RA: histamine-2 receptor antagonists; MDT: multi-disciplinary team; OGD: oesophago-gastro-duodenoscopy.
Figure 2.
Management of SSc patients with controlled gastroesophageal reflux disease (GERD) on proton pump inhibitor (PPI). H2RA: histamine-2 receptor antagonists.
Unmet needs and research agenda
The data presented in the general population clearly show that there is a need to examine PPIs in SSc patients using well-controlled prospective clinical trials because there have been little or no data to date. However, given that most patients with SSc are on a PPI (prescribed or over the counter) the prospects of an RCT in symptomatic patients seems low. What might be of interest would a randomised study of dose reduction in patients on a stable dose of a PPI, with one of the main outcome measures being the need to resume pre-study treatment
PPIs are widely prescribed in the general population and are now available to patients over-the-counter without a prescription. The strong advice is that PPIs should not be continued long-term without clinical review, including confirming the rationale for ongoing treatment. In patients with known structural disease, gastroscopy is likely to be needed to establish treatment success and suitability for PPI de-escalation.
In current clinical practice most patients require long term PPI use. However, the judicious use of intermittent PPI therapy should be explored in which short treatment breaks are advocated in the minority of patients where symptom control with intermittent PPI use is potentially good practice. Another potential strategy could include rotating individual PPI drug therapies on a regular basis. Research could investigate alternative or novel drug therapies for GERD, thereby reducing the heavy reliance on PPIs in SSc patients. For example, vonoprazan, a novel potassium-competitive acid blocker, was found to be effective in SSc patients with refractory disease after PPI failure, both symptomatically and objectively (i.e., improving reflux oesophagitis on endoscopy).74,75
In SSc, a key area for research is to elucidate the aetiopathogenesis of GERD, which is still poorly understood, and thereby developing a targeted intervention strategy. Linking pathophysiology to symptoms may be helpful to define the potential benefit of prokinetics and potential for surgery which is recommended currently in exceptional cases only. Well-conducted studies are required to carefully delineate the role of surgical intervention for GERD in SSc, including to define the patients who are optimal candidates for good outcomes, the optimal surgical technique and long-term safety and outcomes. Furthermore, examining the efficacy of combination therapies targeting both acid reflux and physiologic dysfunction in the upper GI tract (e.g. oesophageal aperistalsis, ineffective oesophageal motility, hypotensive lower oesophageal sphincter, gastroparesis, and impaired gastric accommodation) and acid reflux in subsets of symptomatic SSc patients will also be important. Moreover, it will be important to determine the contribution of non-acid reflux to patient symptoms, particularly in patients with refractory GERD. It is also not clear whether acid suppression should be initiated in SSc patients and asymptomatic acid reflux/GERD. Another important aspect is that many clinicians strongly consider that oesophageal strictures have become much less frequent in SSc since the availability of PPIs. Indeed, in a recent international study which evaluated agreement on updated treatment recommendations for SSc treatment, experts indicated a very high level agreement (9.2 out of 10) that PPIs should be used for the treatment of SSc-related GERD including the and prevention of oesophageal ulcers and strictures’.76
Finally, the role of surgical intervention should also be critically appraised including (but not limited to) different techniques relating to fundoplication (Nissens, Toupets and Dor) and Roux-en-Y gastric bypasses. Some evidence recently published in the literature, has shown the feasibility of surgery (e.g., fundoplication) in SSc patients, reaching a very low rate of morbidity and mortality (not significantly different from the normal population) and very good outcome concerning GERD management.77-80 It should be highlighted that, in the context of lung transplantation in SSc patients, fundoplication has be shown to be feasible and some authors have reported better outcomes (e.g., prevention of organ rejection and improved survival in SSc).79 In general, for late-stage disease with an aperistaltic oesophagus fundoplication is typically considered as contraindicated as it usually leads to bolus obstruction by impairing swallowing. Similarly, the potential role of PPIs in the prevention of the progression of irreversible ILD (and potentially prevention of lung transplantation) in SSc should be definitively evaluated. Optimisation of GERD, including consideration of surgical options (e.g., for severe oesophageal dysfunction) can potentially improve patients’ eligibility for life-saving lung transplantation. Future research should also explore the opinions of other relevant stakeholders e.g., respiratory physicians and transplant surgeons.
Conclusion
Despite understandable concerns with PPIs, they are still the most effective drug for GERD in SSc. Therefore, PPIs are widely prescribed in SSc patient but frequently the ceiling dosage is rapidly reached. Although there are cohort and observational studies that raise legitimate concerns, the published data thus far published are not in well-controlled, hypothesis-testing trials in SSc patients, and combination therapies for GERD require further study. Therefore, SSc patients require a comprehensive approach to assessment including evaluation of GI involvement with support from gastroenterologists. Although the risks of PPIs in the general population may sometimes outweigh the benefits, this may be different in SSc where there is a lack of alternative treatment options and limited evidence to guide further management (e.g., switching drug therapies). Future research is required to delineate the safe and effective use of PPIs in patients with SSc to modify their reflux disease.
Footnotes
Declaration of interest:
MK - Grants or contracts from Boehringer Ingelheim and Ono Pharmaceuticals; Consulting fees from Boehringer Ingelheim and Mochida; Payment or honoria from Boehringer Ingelheim, Ono Pharmaceuticals, Abbvie, Janssen, Astellas and Bayer; Participation on a data monitoring or advisory board – Corbus and Horizon. The other authors declared no conflicts of interest.
References
- 1.Forbes A, Marie I. Gastrointestinal complications: the most frequent internal complications of systemic sclerosis. Rheumatology 2009; 48: iii36–9. [DOI] [PubMed] [Google Scholar]
- 2.Shreiner AB, Murray C, Denton C, Khanna D. Gastrointestinal Manifestations of Systemic Sclerosis. J scleroderma Relat Disord 2016; 1: 247–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hughes M. Significant weight loss in systemic sclerosis: a study from the EULAR Scleroderma Trials and Research (EUSTAR) database. Ann Rheum Dis 2020. DOI: 10.1136/annrheumdis-2020-217035. [DOI] [PubMed] [Google Scholar]
- 4.Frantz C, Avouac J, Distler O, et al. Impaired quality of life in systemic sclerosis and patient perception of the disease: A large international survey. Semin Arthritis Rheum 2016; 46: 115–23. [DOI] [PubMed] [Google Scholar]
- 5.Denaxas K, Ladas SD, Karamanolis GP. Evaluation and management of esophageal manifestations in systemic sclerosis. Ann Gastroenterol 2018; 31: 165–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foocharoen C, Chunlertrith K, Mairiang P, et al. Prevalence and predictors of proton pump inhibitor partial response in gastroesophageal reflux disease in systemic sclerosis: a prospective study. Sci Rep 2020; 10: 769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhalla M, Silver RM, Shepard JA, McLoud TC. Chest CT in patients with scleroderma: prevalence of asymptomatic esophageal dilatation and mediastinal lymphadenopathy. AJR Am J Roentgenol 1993; 161: 269–72. [DOI] [PubMed] [Google Scholar]
- 8.Lahcene M, Oumnia N, Matougui N, Boudjella M, Tebaibia A, Touchene B. Esophageal involvement in scleroderma: clinical, endoscopic, and manometric features. ISRN Rheumatol 2011; 2011: 325826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Emmanuel A. Current management of the gastrointestinal complications of systemic sclerosis. Nat Rev Gastroenterol Hepatol 2016; 13: 461–72. [DOI] [PubMed] [Google Scholar]
- 10.Wipff J, Coriat R, Masciocchi M, et al. Outcomes of Barrett’s oesophagus related to systemic sclerosis: a 3-year EULAR Scleroderma Trials and Research prospective follow-up study. Rheumatology (Oxford) 2011; 50: 1440–4. [DOI] [PubMed] [Google Scholar]
- 11.Marie I, Dominique S, Levesque H, et al. Esophageal involvement and pulmonary manifestations in systemic sclerosis. Arthritis Rheum 2001; 45: 346–54. [DOI] [PubMed] [Google Scholar]
- 12.Ma L, Zhu Q, Zhang Y, et al. Esophagus involvement in systemic sclerosis: ultrasound parameters and association with clinical manifestations. Arthritis Res Ther 2021; 23: 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zell S, Carmichael JM, Reddy AN. Rational approach to long-term use of H2-antagonists. Am J Med 1987; 82: 796–802. [DOI] [PubMed] [Google Scholar]
- 14.Janiak P, Thumshirn M, Menne D, et al. Clinical trial: the effects of adding ranitidine at night to twice daily omeprazole therapy on nocturnal acid breakthrough and acid reflux in patients with systemic sclerosis--a randomized controlled, cross-over trial. Aliment Pharmacol Ther 2007; 26: 1259–65. [DOI] [PubMed] [Google Scholar]
- 15.Foocharoen C, Chunlertrith K, Mairiang P, et al. Effectiveness of add-on therapy with domperidone vs alginic acid in proton pump inhibitor partial response gastro-oesophageal reflux disease in systemic sclerosis: randomized placebo-controlled trial. Rheumatology 2017; 56: 214–22. [DOI] [PubMed] [Google Scholar]
- 16.Hansi N, Thoua N, Carulli M, et al. Consensus best practice pathway of the UK scleroderma study group: gastrointestinal manifestations of systemic sclerosis. Clin Exp Rheumatol 2014; 32: S-214–21. [PubMed] [Google Scholar]
- 17.Kent MS, Luketich JD, Irshad K, et al. Comparison of surgical approaches to recalcitrant gastroesophageal reflux disease in the patient with scleroderma. Ann Thorac Surg 2007; 84: 1710–6. [DOI] [PubMed] [Google Scholar]
- 18.Hendel L, Hage E, Hendel J, Stentoft P. Omeprazole in the long-term treatment of severe gastro-oesophageal reflux disease in patients with systemic sclerosis. Aliment Pharmacol Ther 1992; 6: 565–77. [DOI] [PubMed] [Google Scholar]
- 19.Muro Y, Sugiura K, Nitta Y, et al. Scoring of reflux symptoms associated with scleroderma and the usefulness of rabeprazole. Clin Exp Rheumatol 2009; 27: 15–21. [PubMed] [Google Scholar]
- 20.Pakozdi A, Wilson H, Black CM, Denton CP. Does long term therapy with lansoprazole slow progression of oesophageal involvement in systemic sclerosis? Clin Exp Rheumatol 2009; 27: 5–8. [PubMed] [Google Scholar]
- 21.Stern EK, Carlson DA, Falmagne S, et al. Abnormal esophageal acid exposure on high-dose proton pump inhibitor therapy is common in systemic sclerosis patients. Neurogastroenterol Motil 2018; 30: 10.1111/nmo.13247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carlson DA, Hinchcliff M, Pandolfino JE. Advances in the evaluation and management of esophageal disease of systemic sclerosis. Curr Rheumatol Rep 2015; 17: 475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang H, Yang Z, Ni Z, Shi Y. A Meta-Analysis and Systematic Review of the Efficacy of Twice Daily PPIs versus Once Daily for Treatment of Gastroesophageal Reflux Disease. Gastroenterol Res Pract 2017; 2017: 9865963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kwok CS, Arthur AK, Anibueze CI, Singh S, Cavallazzi R, Loke YK. Risk of Clostridium difficile infection with acid suppressing drugs and antibiotics: meta-analysis. Am J Gastroenterol 2012; 107: 1011–9. [DOI] [PubMed] [Google Scholar]
- 25.Lanas-Gimeno A, Hijos G, Lanas Á. Proton pump inhibitors, adverse events and increased risk of mortality. Expert Opin Drug Saf 2019; 18: 1043–53. [DOI] [PubMed] [Google Scholar]
- 26.Almario CV, Chey WD, Spiegel BMR. Increased Risk of COVID-19 Among Users of Proton Pump Inhibitors. Am J Gastroenterol 2020; 115: 1707–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koyyada A. Long-term use of proton pump inhibitors as a risk factor for various adverse manifestations. Therapie 2021; 76: 13–21. [DOI] [PubMed] [Google Scholar]
- 28.Denton CP, Khanna DK. Systemic sclerosis. Lancet 2017; 390: 1685–99. [DOI] [PubMed] [Google Scholar]
- 29.Hoffmann-Vold A-M, Maher TM, Philpot EE, et al. The identification and management of interstitial lung disease in systemic sclerosis: evidence-based European consensus statements. Lancet Rheumatol 2020; 2: e71–83. [DOI] [PubMed] [Google Scholar]
- 30.Orlandi M, Landini N, Sambataro G, et al. The role of chest CT in deciphering interstitial lung involveemtn in systemic sclerosis versus COVID-19. Rheumatology (Oxford) 2022; 61: 1600–1609. [DOI] [PubMed] [Google Scholar]
- 31.Eom C-S, Jeon CY, Lim J-W, Cho E-G, Park SM, Lee K-S. Use of acid-suppressive drugs and risk of pneumonia: a systematic review and meta-analysis. C Can Med Assoc J = J l’Association medicale Can 2011; 183: 310–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Au K, Singh MK, Bodukam V, et al. Atherosclerosis in systemic sclerosis: a systematic review and meta-analysis. Arthritis Rheum 2011; 63: 2078–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Man A, Zhu Y, Zhang Y, et al. The risk of cardiovascular disease in systemic sclerosis: a population-based cohort study. Ann Rheum Dis 2013; 72: 1188–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shah NH, LePendu P, Bauer-Mehren A, et al. Proton Pump Inhibitor Usage and the Risk of Myocardial Infarction in the General Population. PLoS One 2015; 10: e0124653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ariel H, Cooke JP. Cardiovascular Risk of Proton Pump Inhibitors. Methodist Debakey Cardiovasc J 2019; 15: 214–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McFarlane IM, Bhamra MS, Kreps A, et al. Gastrointestinal Manifestations of Systemic Sclerosis. Rheumatology (Sunnyvale) 2018; 8: 235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morrisroe K, Baron M, Frech T, Nikpour M. Small intestinal bacterial overgrowth in systemic sclerosis. J Scleroderma Relat Disord 2019; 5: 33–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lo W-K, Chan WW. Proton pump inhibitor use and the risk of small intestinal bacterial overgrowth: a meta-analysis. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc 2013; 11: 483–90. [DOI] [PubMed] [Google Scholar]
- 39.Su T, Lai S, Lee A, He X, Chen S. Meta-analysis: proton pump inhibitors moderately increase the risk of small intestinal bacterial overgrowth. J Gastroenterol 2018; 53: 27–36. [DOI] [PubMed] [Google Scholar]
- 40.Antoniou T, Macdonald EM, Hollands S, et al. Proton pump inhibitors and the risk of acute kidney injury in older patients: a population-based cohort study. C open 2015; 3: E166–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lazarus B, Chen Y, Wilson FP, et al. Proton Pump Inhibitor Use and the Risk of Chronic Kidney Disease. JAMA Intern Med 2016; 176: 238–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hart E, Dunn TE, Feuerstein S, Jacobs DM. Proton Pump Inhibitors and Risk of Acute and Chronic Kidney Disease: A Retrospective Cohort Study. Pharmacotherapy 2019; 39: 443–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Livi R, Teghini L, Pignone A, Generini S, Matucci-Cerinic M, Cagnoni M. Renal functional reserve is impaired in patients with systemic sclerosis without clinical signs of kidney involvement. Ann Rheum Dis 2002; 61: 682–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Woodworth TG, Suliman YA, Li W, Furst DE, Clements P. Scleroderma renal crisis and renal involvement in systemic sclerosis. Nat Rev Nephrol 2016; 12: 678–91. [DOI] [PubMed] [Google Scholar]
- 45.Bruni C, Cuomo G, Rossi FW, Praino E, Bellando-Randone S. Kidney involvement in systemic sclerosis: From pathogenesis to treatment. J Scleroderma Relat Disord 2018; 3: 43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hughes M, Zanatta E, Sandler RD, Avouac J, Allanore Y. Improvement with time of vascular outcomes in systemic sclerosis: a systematic review and meta-analysis study. Rheumatology 2021; published online Nov 15. DOI: 10.1093/rheumatology/keab850. [DOI] [PubMed] [Google Scholar]
- 47.Cundy T, Dissanayake A. Severe hypomagnesaemia in long-term users of proton-pump inhibitors. Clin Endocrinol (Oxf) 2008; 69: 338–41. [DOI] [PubMed] [Google Scholar]
- 48.Low ASL, Lal S, Farrell AJ, Herrick AL. Profound hypomagnesaemia causing symptomatic hypocalcaemia--an underdiagnosed and potentially life-threatening problem in systemic sclerosis? Rheumatology (Oxford). 2014; 53: 767–9. [DOI] [PubMed] [Google Scholar]
- 49.Gomm W, von Holt K, Thomé F, et al. Association of Proton Pump Inhibitors With Risk of Dementia: A Pharmacoepidemiological Claims Data Analysis. JAMA Neurol 2016; 73: 410–6. [DOI] [PubMed] [Google Scholar]
- 50.Lin T-M, Chen W-S, Sheu J-J, Chen Y-H, Chen J-H, Chang C-C. Autoimmune rheumatic diseases increase dementia risk in middle-aged patients: A nationwide cohort study. PLoS One 2018; 13: e0186475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wuriliga, Xu D, He Y, et al. Mild cognitive impairment in patients with systemic sclerosis and features analysis. Rheumatology 2021; published online Oct 26. DOI: 10.1093/rheumatology/keab787. [DOI] [Google Scholar]
- 52.Ito T, Jensen RT. Association of long-term proton pump inhibitor therapy with bone fractures and effects on absorption of calcium, vitamin B12, iron, and magnesium. Curr Gastroenterol Rep 2010; 12: 448–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sari A, Gill A, Nihtyanova SI, Ong VH, Denton CP. THU0398 Unexplained iron deficiency is frequent in systemic sclerosis. Ann Rheum Dis 2018; 77: 413 LP – 414. [Google Scholar]
- 54.Ruiter G, Lanser IJ, de Man FS, et al. Iron deficiency in systemic sclerosis patients with and without pulmonary hypertension. Rheumatology 2014; 53: 285–92. [DOI] [PubMed] [Google Scholar]
- 55.Cheung KS, Leung WK. Long-term use of proton-pump inhibitors and risk of gastric cancer: a review of the current evidence. Therap Adv Gastroenterol 2019; 12: 1756284819834511–1756284819834511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Herrick A, Gallas A. Systemic-sclerosis-related calcinosis. J Scleroderma Relat Disord 2016; 1: 177–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hughes M, Hodgson R, Harris J, et al. Imaging calcinosis in patients with systemic sclerosis by radiography, computerised tomography and magnetic resonance imaging. Semin Arthritis Rheum 2019. DOI:doi.org/ 10.1016/j.semarthrit.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 58.Host LV, Campochiaro C, Afonso A, Nihtyanova SI, Denton CP, Ong VH. High proton pump inhibitor exposure increases risk of calcinosis in systemic sclerosis. Rheumatology 2021; 60: 849–54. [DOI] [PubMed] [Google Scholar]
- 59.Savarino E, Bazzica M, Zentilin P, et al. Gastroesophageal reflux and pulmonary fibrosis in scleroderma: a study using pH-impedance monitoring. Am J Respir Crit Care Med 2009; 179: 408–13. [DOI] [PubMed] [Google Scholar]
- 60.Christmann RB, Wells AU, Capelozzi VL, Silver RM. Gastroesophageal reflux incites interstitial lung disease in systemic sclerosis: clinical, radiologic, histopathologic, and treatment evidence. Semin Arthritis Rheum 2010; 40: 241–9. [DOI] [PubMed] [Google Scholar]
- 61.Kreuter M, Bonella F, Kathrin K, et al. Long-term outcome of SSc-associated ILD: improved survival in PPI treated patients. Ann Rheum Dis 2021; 80: 670–671 [Abstract]. [Google Scholar]
- 62.Volkman E, Tashkin D, Leng M, Kim G, Goldin J, Roth M. Severity of Gastroesophageal Reflux, but Not the Use of Proton Pump Inhibitors, Is Associated with Radiographic Progression of Interstitial Lung Disease in Systemic Sclerosis (abstract). 2021. [Google Scholar]
- 63.Bassel M, Hudson M, Taillefer SS, Schieir O, Baron M, Thombs BD. Frequency and impact of symptoms experienced by patients with systemic sclerosis: results from a Canadian National Survey. Rheumatology (Oxford) 2011; 50: 762–7. [DOI] [PubMed] [Google Scholar]
- 64.Kinoshita Y, Ishimura N, Ishihara S. Advantages and Disadvantages of Long-term Proton Pump Inhibitor Use. J Neurogastroenterol Motil 2018; 24: 182–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Omair MA, Pagnoux C, McDonald-Blumer H, Johnson SR. Low bone density in systemic sclerosis. A systematic review. J Rheumatol 2013; 40: 1881–90. [DOI] [PubMed] [Google Scholar]
- 66.Chuealee W, Foocharoen C, Mahakkanukrauh A, Suwannaroj S, Pongchaiyakul C, Nanagara R. Prevalence and predictive factors of osteoporosis in Thai systemic sclerosis. Sci Rep 2021; 11: 9424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Eom C-S, Lee S-S. Risk of fracture and pneumonia from acid suppressive drugs. World J Methodol 2011; 1: 15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhou B, Huang Y, Li H, Sun W, Liu J. Proton-pump inhibitors and risk of fractures: an update meta-analysis. Osteoporos Int a J Establ as result Coop between Eur Found Osteoporos Natl Osteoporos Found USA 2016; 27: 339–47. [DOI] [PubMed] [Google Scholar]
- 69.Poly TN, Islam MM, Yang H-C, Wu CC, Li Y-CJ. Proton pump inhibitors and risk of hip fracture: a meta-analysis of observational studies. Osteoporos Int a J Establ as result Coop between Eur Found Osteoporos Natl Osteoporos Found USA 2019; 30: 103–14. [DOI] [PubMed] [Google Scholar]
- 70.Tahir R, Patel PN. Role of Proton Pump Inhibitors in Calcium Absorption, Bone Resorption, and Risk of Hip Fracture. J Pharm Technol 2007; 23: 275–80. [Google Scholar]
- 71.Yang Y-X. Chronic proton pump inihibitor therapy and calcium metabolism. Curr Gastroenterol Rep 2012; 14: 473–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ogawa R, Echizen H. Drug-drug interaction profiles of proton pump inhibitors. Clin Pharmacokinet 2010; 49: 509–33. [DOI] [PubMed] [Google Scholar]
- 73.Andréasson K, Neringer K, Wuttge DW, et al. Mycophenolate mofetil for systemic sclerosis: drug exposure exhibits considerable inter-individual variation-a prospective, observational study. Arthritis Res Ther 2020; 22 : 230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tabuchi M, Minami H, Akazawa Y, et al. Use of vonoprazan for management of systemic sclerosis-related gastroesophageal reflux disease. Biomed reports 2021; 14: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shirai Y, Kawami N, Iwakiri K, Kuwana M. Use of vonoprazan, a novel potassium-competitive acid blocker, for the treatment of proton pump inhibitor-refractory reflux esophagitis in patients with systemic sclerosis. J Scleroderma Relat Disord 2021; 23971983211021748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.de Vries-Bouwstra JK, Allanore Y, Matucci-Cerinic M, Balbir-Gurman A. Worldwide Expert Agreement on Updated Recommendations for the Treatment of Systemic Sclerosis. J Rheumatol 2020; 47: 249–254. [DOI] [PubMed] [Google Scholar]
- 77.Yan J, Strong AT, Sharma G, et al. Surgical management of gastroesophageal reflux disease in patients with systemic sclerosis. Surg Endosc 2018; 32: 3855–3860. [DOI] [PubMed] [Google Scholar]
- 78.Aiolfi A, Nosotti M, Matsushima K, Perali C, Ogliari C, Del Papa N, Bonitta G, Bona D. Surgical treatment of recalcitrant gastroesophageal reflux disease in patients with systemic sclerosis: a systematic review. Langenbecks Arch Surg 2021; 406: 1353–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Loganathan P, Gajendran M, Davis B, McCallum R. Efficacy and Safety of Robotic Dor Fundoplication on Severe Gastroesophageal Reflux Disease in Patients With Scleroderma. J Investig Med High Impact Case Rep 2021; 406: 1353–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Leiva-Juárez MM, Urso A, Costa J, et al. Fundoplication after lung transplantation in patients with systemic sclerosis–related end-stage lung disease. J Scleroderma Relat Disord 2021; 23971983211016210. [DOI] [PMC free article] [PubMed] [Google Scholar]