Abstract
Background
Fractures of the calcaneus (heel bone) comprise up to 2% of all fractures. These fractures are mostly caused by a fall from a height, and are common in younger adults. Treatment can be surgical or non‐surgical; however, there is clinical uncertainty over optimal management. This is an update of a Cochrane Review first published in 2013.
Objectives
To assess the effects (benefits and harms) of surgical versus conservative treatment of displaced intra‐articular calcaneal fractures.
Search methods
We searched the Cochrane Bone, Joint and Muscle Trauma Group Specialised Register, CENTRAL, MEDLINE, Embase, and clinical trials registers in November 2022.
Selection criteria
We included randomised controlled trials (RCTs) and quasi‐RCTs comparing surgical versus non‐surgical management of displaced intra‐articular calcaneal fractures in skeletally mature adults (older than 14 years of age). For surgical treatment, we included closed manipulation with percutaneous wire fixation, open reduction with internal fixation (ORIF) with or without bone graft, or primary arthrodesis. For non‐surgical treatment, we included ice, elevation and rest, or plaster cast or splint immobilisation.
Data collection and analysis
We used standard Cochrane methodological procedures. We collected data for the following outcomes: function in the short term (within three months of injury) or long term (more than three months after injury), chronic pain, health‐related quality of life (HRQoL) and ability to return to normal activities, as well as complications which may or may not have led to an unplanned return to theatre.
Main results
We included 10 RCTs and two quasi‐RCTs with 1097 participants. Sample sizes in studies ranged from 29 to 424 participants. Most participants were male (86%), and the mean age in studies ranged from 28 to 52 years. In the surgical groups, participants were mostly managed with ORIF with plates, screws, or wires; one study used only minimally invasive techniques. Participants in the non‐surgical groups were managed with a plaster cast, removable splint or a bandage, or with rest, elevation, and sometimes ice.
Risk of performance bias was unavoidably high in all studies as it was not possible to blind participants and personnel to treatment; in addition, some studies were at high or unclear risk of other types of bias (including high risk of selection bias for quasi‐RCTs, high risk of attrition bias, and unclear risk of selective reporting bias). We downgraded the certainty of all the evidence for serious risk of bias. We also downgraded the certainty of the evidence for imprecision for all outcomes (except for complications requiring return to theatre for subtalar arthrodesis) because the evidence was derived from few participants. We downgraded the evidence for subtalar arthrodesis for inconsistency because the pooled data included high levels of statistical heterogeneity.
We found that surgical management may improve function at six to 24 months after injury when measured using the American Orthopaedic Foot and Ankle Society (AOFAS) score (mean difference (MD) 6.58, 95% confidence interval (CI) 1.04 to 12.12; 5 studies, 319 participants; low‐certainty evidence). We are not aware of a published minimal clinically important difference (MCID) for the AOFAS score for this type of fracture. Previously published MCIDs for other foot conditions range from 2.0 to 7.9. No studies reported short‐term function within three months of injury. Surgical management may reduce the number of people with chronic pain up to 24 months after injury (risk ratio (RR) 0.56, 95% CI 0.37 to 0.84; 4 studies, 175 participants; low‐certainty evidence); this equates to 295 per 1000 fewer people with pain after surgical management (95% CI 107 to 422 per 1000). Surgical management may also lead to improved physical HRQoL (MD 6.49, 95% CI 2.49 to 10.48; 2 studies, 192 participants; low‐certainty evidence). This outcome was measured using the physical component score of the 36‐Item Short Form Health Survey. We used a change in effect of 5% to indicate a clinically important difference for this scoring system and thus judged that the difference in HRQoL between people treated surgically or non‐surgically includes both clinically relevant and not relevant changes for those treated surgically. There may be little or no difference in the number of people who returned to work within 24 months (RR 1.26, 95% CI 0.94 to 1.68; 5 studies, 250 participants; low‐certainty evidence) or who require secondary surgery for subtalar arthrodesis (RR 0.38, 95% CI 0.09 to 1.53; 3 studies, 657 participants; low‐certainty evidence). For other complications requiring return to theatre in people treated surgically, we found low‐certainty evidence for amputation (2.4%; 1 study, 42 participants), implant removal (3.4%; 3 studies, 321 participants), deep infection (5.3%; 1 study, 206 participants), and wound debridement (2.7%; 1 study, 73 participants). We found low‐certainty evidence that 14% of participants who were treated surgically (7 studies, 847 participants) had superficial site infection.
Authors' conclusions
Our confidence in the evidence is limited. Although pooled evidence indicated that surgical treatment may lead to improved functional outcome but with an increased risk of unplanned second operations, we judged the evidence to be of low certainty as it was often derived from few participants in studies that were not sufficiently robust in design. We found no evidence of a difference between treatment options in the number of people who needed late reconstruction surgery for subtalar arthritis, although the estimate included the possibility of important harms and benefits. Large, well‐conducted studies that attempt to minimise detection bias and that measure functional outcomes using calcaneal‐specific measurement tools would increase the confidence in these findings. Given that minimally invasive surgical procedures are already becoming more prevalent in practice, research is urgently needed to determine whether these newer surgical techniques offer better outcomes with regard to function, pain, quality of life, and postoperative complications for intra‐articular displaced calcaneal fractures.
Keywords: Adult; Female; Humans; Male; Middle Aged; Bandages; Chronic Pain; Chronic Pain/etiology; Fracture Fixation; Fracture Fixation/adverse effects; Fractures, Bone; Ice
Plain language summary
What are the benefits and risks of treating broken heel bones with or without an operation?
Key messages
• In people who have broken their heel bone, surgery may improve how people use their foot and ankle up to two years after their injury compared to non‐surgical treatments.
• Surgery may also reduce the number of people experiencing pain up to two years after injury, and may slightly improve their quality of life.
• The evidence came from only a few small studies that were not always well conducted, meaning that we have little confidence in the findings.
• More well‐conducted studies are needed to increase our confidence in the evidence. Future studies should use tools to measure outcomes that are designed specifically for heel bone fractures. They could also test newer surgical approaches that are not included in this review. Sometimes called 'minimally invasive', these newer methods limit the number of cuts or incisions the surgeon is required to make. This could lead to fewer postoperative complications and better long‐term outcome for the patient than the other types of surgery included in this review.
Broken heel bones
The calcaneus is a bone in the heel of the foot that helps to support the foot in normal walking. A broken heel bone typically occurs after a fall from a height or a high‐impact event such as a car crash, and is more common in young adults. It is a painful injury, and people may not be able to weight‐bear (put all their weight on the injured leg) for many weeks after injury. This is likely to limit physical activity and may lead to delays in the person returning to normal activities (such as work).
Treatments for a broken heel bone include:
• surgery using metal plates, screws, or wires to hold the broken pieces of bone together whilst they heal;
• non‐surgical treatment in which people will be asked to rest, keep their leg raised, and sometimes use ice to manage any swelling from the injury. People may also wear a plaster cast, a removable cast or splint, or a tight bandage.
For both treatments, it is likely that people will be asked not to weight‐bear for at least six weeks.
What did we want to find out?
We wanted to find out whether surgery or non‐surgical treatment works best for broken heel bones.
We wanted to know whether these treatments improved function (e.g. how well the person can use their ankle and foot), pain, quality of life, and ability to return to their normal activities (such as work). We were particularly interested in the longer‐term impact on people's lives up to about two years after injury. We were also interested in function in the first three months of injury.
We also wanted to find out if the treatments affected the number of complications and what the side effects were of surgical treatment.
What did we do?
We searched for studies that looked at surgery compared with non‐surgical treatment in people who were at least 14 years of age. We compared and summarised the results of the studies and rated our confidence in the evidence, based on factors such as study methods and sizes.
What did we find?
We found 12 studies that involved 1097 people with broken heel bones. The average age of study participants was 28 to 52 years, and 86% of participants were men, which is fairly typical for broken heel bones.
We found that compared to non‐surgical treatments, surgery may improve function within the first two years, although we were unsure if this improvement was big enough to make an important difference to people. No studies reported function in the first three months after injury.
Surgery may also reduce the number of people with pain and may result in a small but meaningful improvement to people's quality of life up to two years after injury. There may be little or no difference between treatments in the number of people able to return to their normal activities.
In one small study, a single person treated with surgery needed to have an amputation (removing the leg from the knee downwards), with no amputations in the group that had non‐surgical treatment; no other studies reported this outcome. There may be no difference between treatment options in the number of people who needed further surgery to fuse the joint around the heel bone.
For those treated with surgery, 14% had a wound infection that could be treated with antibiotics.
What are the limitations of the evidence?
We have little confidence in the evidence because people in the studies were aware of which treatment they received, which could have introduced bias. Also, in some studies people were not randomly placed into the different treatment groups, meaning that differences between groups could be due to differences between people rather than treatments. Furthermore, most studies involved only small numbers of people.
How up‐to‐date is the evidence?
This is an update of a previous review. The evidence is current to November 2022.
Summary of findings
Summary of findings 1. Surgical versus non‐surgical management for displaced intra‐articular calcaneal fractures.
| Surgical versus non‐surgical management for displaced intra‐articular calcaneal fractures | ||||||
| Population: people aged over 14 years with displaced intra‐articular calcaneal fractures Setting: treated in hospital Intervention: surgical management (ORIF, or closed reduction and percutaneous fixation) Comparison: non‐surgical management (rest, elevation, ice; below‐knee plaster casts, removable splints) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with non‐surgical treatment | Risk with surgery | |||||
| Function in the short term | ‐ | ‐ | ‐ | ‐ | ‐ | No studies reported function in the first 3 months after injury. |
|
Function in the long term Measured using AOFAS score (0 to 100, higher scores indicate improved function) Follow‐up: 6 to 24 months |
Mean scores in the non‐surgical groups ranged from 55 to 76.8. | MD 6.58 higher (1.04 higher to 12.12 higher) | ‐ | 319 (5 studies) | ⊕⊕⊝⊝ Lowa |
The MCID for AOFAS in this fracture type is not established. MCIDs for other foot conditions range from 2.0 to 7.9. |
|
Chronic pain Measured as number of people experiencing pain Follow‐up: 6 to 24 months |
Study population | RR 0.56 (0.37 to 0.84) | 175 (4 studies) | ⊕⊕⊝⊝ Lowa |
295 fewer people (95% CI 107 to 422 fewer) had chronic pain up to 24 months after surgery. | |
| 670 per 1000b | 375 per 1000 (248 to 563) | |||||
|
Health‐related quality of life (physical) Measured using SF‐36 (PCS) (score 0 to 100, higher scores indicate better physical HRQoL) Follow‐up: 12 to 24 months |
Mean scores in the non‐surgical group ranged from 37 to 42.5. | MD 6.49 higher (2.49 higher to 10.48 higher) | ‐ | 192 (2 studies) | ⊕⊕⊝⊝ Lowa |
Based on a 5% threshold (or 5 points on a 100‐point SF‐36 scale), this difference includes the possibility of a clinically important difference. |
|
Return to work Follow‐up: 12 to 24 months |
Study population | RR 1.26 (0.94 to 1.68) | 250 (5 studies) | ⊕⊕⊝⊝ Lowa |
||
| 603 per 1000b | 760 per 1000 (567 to 1000) | |||||
|
Complications requiring unplanned return to theatre Subtalar arthrodesis Follow‐up: 2 to 12 years |
Study population | RR 0.38 (0.09 to 1.53) |
657 (3 studies) |
⊕⊕⊝⊝ Lowc |
Other complications requiring unplanned return to theatre:
|
|
| 131 per 1000b | 50 per 1000 (12 to 200) |
|||||
|
Complications not requiring unplanned return to theatre Superficial infection (affecting only participants who had surgical treatment) Follow‐up: postoperative period |
Study population | See comment | 847 (7 studies) |
⊕⊕⊝⊝ Lowa |
59/419 (14%) participants in the surgical groups had superficial infection. Other complications not requiring unplanned return to theatre: 4/45 (9%) participants in surgical groups had wound dehiscence. |
|
| ‐ | 140 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AOFAS: American Orthopaedic Foot and Ankle Society; CI: confidence interval; HRQoL: health‐related quality of life; MCID: minimum clinically important difference; MD: mean difference; ORIF: open reduction and internal fixation; RR: risk ratio; SMD: standardised mean difference; SF‐36 (PCS): 36‐Item Short Form Health Survey (physical component score) | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level owing to serious risk of bias in the included studies and one level owing to imprecision because the analysis included few participants. bDerived from the pooled estimate of the non‐surgical treatment group. cDowngraded one level owing to serious risk of bias in the included studies and one level for inconsistency due to unexplained substantial levels of statistical heterogeneity in the pooled effect.
Background
Description of the condition
Calcaneal or heel fractures are fractures of the calcaneus, also called the heel bone or os calcis. They comprise 1% to 2% of all fractures (Humphrey 2019; Ibrahim 2007; Koval 2006). Most calcaneal fractures occur in younger, working‐age men (Vosoughi 2022). The economic impact of this injury to both the patient and society is considerable as a consequence of extended hospital stay, cost of treatment, residual pain, time to mobilisation, and delayed return to work (BOFAS; Schepers 2007). Studies suggest that people with these injuries can be incapacitated for up to three years and partially impaired for several years subsequently (Clarke 2007).
Heel bone fractures can be broadly divided into intra‐articular (where the articular or joint surfaces of the calcaneus are disrupted) and extra‐articular fractures (where the articular surfaces remain intact). Approximately two‐thirds of calcaneal fractures are intra‐articular (Vosoughi 2022). The majority of displaced intra‐articular fractures involve the posterior facet, which is the major weight‐bearing surface of the sub‐talar (talo‐calcaneal) joint (Koval 2006).
Displaced intra‐articular calcaneal fractures (DIACFs) are typically the result of high‐energy trauma, such as a fall or jump from a height. Patients present with a painful, swollen, and deformed heel. Some patients may be unable to walk properly or at all. Bruising around the heel extending into the arch of the foot is suggestive of calcaneal fracture; skin blistering may also result as a consequence of significant swelling. Approximately 2% to 3% of all calcaneal fractures are open, where the fractured bone is exposed, and 8% are bilateral (Vosoughi 2022).
Description of the intervention
It is generally agreed that undisplaced extra‐articular fractures should be managed conservatively, with non‐weight‐bearing for four to six weeks (Razik 2018); this may involve a combination of rest, analgesia, compression, and splinting. However, the treatment of DIACFs is more problematic. Historically, DIACFs were also treated conservatively with a combination of rest with elevation, ice, and immobilisation with plaster cast splintage, followed by physiotherapy and gradual mobilisation. However, this often led to delayed reconstruction of the malunited fracture, leaving patients with a painful and stiff foot which delayed or permanently prevented return to work and previous activities. Until the 1970s, operative treatment was technically challenging, and often led to postoperative infection, malunion, non‐union, and amputation (McLaughlin 1963).
In the last 20 years, improvements in anaesthesia, antibiotic prophylaxis, the Arbeitsgemanschaft für Osteosynthesefragen/Association for the Study of Internal Fixation (AO/ASIF) principles of fixation, advances in materials and implants, and computed imaging have led to improvements in outcome after operative repair. This has popularised fixation of many fractures, including those of the calcaneus. Operative treatments for DIACF include open reduction and internal fixation (ORIF) or primary arthrodesis (joint fusion). ORIF entails a skin incision through which the fracture fragments are visualised, realigned, and then held in position by plate and screws. Subtalar arthrodesis (joint fusion) is predominantly used for post‐traumatic arthritis resulting from either primary cartilage damage or joint incongruity. Non‐union after calcaneal fracture is rare and requires additional procedures (mostly bone grafting). Postoperative complications, such as surgical site infection and delayed wound healing, can occur after surgical stabilisation of calcaneal fractures. One study, based upon a retrospective record review (179 participants), reported that 25% of patients developed wound complications, with an increased risk observed in those with open fractures, diabetes, and amongst smokers (Folk 1999). A systematic review reported that people who had open reduction had a higher risk of postoperative wound infection and longer hospital stay in comparison to minimally invasive surgery (Zeng 2018).
The use of minimally invasive surgical (MIS) techniques has been reported to have fewer complications than other surgical methods that require larger incisions, and is becoming increasingly popular. Techniques include closed reduction (repositioning of the displaced bone fragments) and fixation with percutaneous pins or wires or limited open reduction and a combination of smaller plates and screws and finally fine wire frames.
How the intervention might work
Surgical fixation aims to restore, or at least improve, the normal three‐dimensional architecture of the hind foot, and secondarily the congruity of the facets of the subtalar joint. Calcaneal malunion can cause broadening of the heel with shoe‐wear impingement, fibulocalcaneal abutment and peroneal tendon impingement, altered ankle and hindfoot biomechanics with the potential risk of ankle impingement and late arthritis.
In the past, some advocated subtalar arthrodesis as the initial fracture treatment for all DIACFs (Harris 1946), because the severity of joint disruption was not retrievable, and early fusion allowed more rapid return to function than a persistently painful and stiff malunited fracture. It has been more recently advocated by some surgeons for fractures with severe joint damage (mostly Sanders Type IV fractures) (Buckley 2014).
Although advances in surgical techniques may have improved functional outcome for many patients, surgical fixation of these fractures is still technically challenging, and the risks of surgical complications such as surgical site infection and of treatment failure remain.
Why it is important to do this review
The previous version of this Cochrane Review identified four clinical trials involving 602 participants, and concluded that there was insufficient high‐quality evidence relating to clinical practice to establish whether surgical or conservative management would result in better outcomes following a displaced intra‐articular calcaneal fracture (Bruce 2013). However, since publication of that review, several other clinical trials have been completed, thus it was important to update the review in the light of the new evidence. This is the first update of Bruce 2013, and aims to provide a current synthesis of available data on this topic.
Objectives
To assess the effects (benefits and harms) of surgical versus conservative treatment of displaced intra‐articular calcaneal fractures.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) and quasi‐RCTs (in which the method of allocating participants to a treatment is not strictly random, e.g. by date of birth, hospital record number, alternation) evaluating surgical versus non‐surgical management of displaced intra‐articular calcaneal fractures (DIACFs).
Types of participants
We included people aged over 14 years, considered as skeletally mature, with DIACFs. Participants with unilateral or bilateral fractures were eligible for inclusion. We included all severities of fracture, both open and closed.
If a study included only a subset of eligible participants, we would only include the study if data were available separately for the subset of interest to this review.
Types of interventions
We included studies comparing surgical treatment of DIACFs with non‐surgical treatment. For surgical treatment, we included closed manipulation with percutaneous wire fixation, open reduction with internal fixation (ORIF) with or without bone graft, or primary arthrodesis. For non‐surgical treatment, we included ice, elevation and rest, or plaster cast or splint immobilisation.
We expected that rehabilitation, such as physiotherapy, was likely to be provided after definitive treatment for DIACFs. If studies incorporated multiple interventions, such as mobilisation strategies and physiotherapy after surgical or non‐surgical treatment, we collected this information. For the purposes of this review, we planned to use the surgical or non‐surgical component of multicomponent treatments as the studied intervention or comparison group.
Types of outcome measures
Critical outcomes
Function in the short term (within three months of injury). We included site‐specific measurement scores using validated instruments, such as the American Orthopaedic Foot and Ankle Society (AOFAS) score, which measures function and pain (Kitaoka 1994).
Function in the long term (up to 24 months after injury), using measures as above.
Chronic pain. We defined this as pain which lasted beyond the expected healing time of three months, up to 24 months after injury. We included recognised measurement tools such as visual analogue scores (VAS) or numerical rating scales (NRS).
Health‐related quality of life (HRQoL) in the long term (up to 24 months after injury). We included recognised measurement tools such as 36‐Item Short‐Form Health Survey (SF‐36) and EQ‐5D.
Return to normal activity (including work and leisure activity) up to 12 months after injury.
Postoperative complications requiring unplanned return to theatre. These included return to theatre for management of surgical site infection or breakdown, amputation, or management of subtalar osteoarthritis at least two years after injury.
Postoperative complications not requiring return to theatre. These included superficial site infection and wound dehiscence.
Other important outcomes
HRQoL in the short term (within three months of injury), using measures as above.
Ability to wear usual footwear (up to 24 months after injury).
Radiological measurements. We included measures of Böhler angle and Gissane angle.
Studies sometimes reported data at multiple time points. For short‐term data, we selected the data reported closest to three months after injury. For long‐term data, we considered data at any time point after three months and up to 24 months; we prioritised data closest to 24 months for inclusion in the review. We aimed to give preference to validated, patient‐reported outcome measures of function and pain. For return to theatre, such as for subtalar arthrodesis, we collected the latest time point in studies.
Search methods for identification of studies
Electronic searches
For this update, we revised all our search strategies in line with current Cochrane Bone, Joint and Muscle Trauma Group practice. We searched the following databases:
Cochrane Bone, Joint and Muscle Trauma Group Specialised Register (8 November 2022);
Cochrane Central Register of Controlled Trials (CENTRAL) (8 November 2022, Issue 11);
MEDLINE (1 January 2011 to 7 November 2022); and
Embase (1 January 2011 to 7 November 2022).
In MEDLINE, we combined subject‐specific terms with the sensitivity‐maximising version of the Cochrane Highly Sensitive Search Strategy for identifying randomised trials (Lefebvre 2019). The search strategies for all databases are reported in Appendix 1. We did not apply any language restrictions. Details of the previous search strategies are available in Bruce 2013.
To identify ongoing trials, we searched the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (trialsearch.who.int) and ClinicalTrials.gov (clinicaltrials.gov) on 8 November 2022 (Appendix 1). Previously, we searched the ISRCTN registry (18 May 2015) and the Orthopaedic Trauma Association annual meeting archives from 1996 to May 2017.
Searching other resources
We checked the reference lists of relevant articles and contacted researchers involved with ongoing trials.
Data collection and analysis
We carried out collection and analysis according to the methods reported in the published protocol (Baliga 2010), with any important changes described in Differences between protocol and review.
Selection of studies
Two review authors (SL, MP, JS, or JB) independently examined the titles and abstracts of articles identified by the search. From this initial assessment, we obtained full versions of all potentially relevant articles. Thereafter, two review authors (SL, MP, JS, or JB) independently assessed study eligibility and reached consensus through discussion.
Data extraction and management
Two review authors (SL, MP, JS, or JB) independently extracted data using a piloted data extraction form. We extracted the following data.
Study methodology: publication type; sponsorship/funding/notable conflicts of interest of trial authors; study design; numbers of centres and locations; study inclusion and exclusion criteria; randomisation method; number of randomised participants, losses (and reasons for losses), and number analysed for each outcome
Population: baseline characteristics of the participants by group and overall (age, gender, type of fracture, number of bilateral fractures)
Intervention: details of each intervention; general surgical details; approaches used for early mobilisation, weight‐bearing and physiotherapy
Outcomes: outcomes relevant to the review (including measurement tools and time points of measures) and other outcomes reported in the studies; extraction of outcome data into Analysis tables or Additional tables in Review Manager 5 (Review Manager 2020)
Assessment of risk of bias in included studies
We assessed risk of bias in the included studies using the Cochrane risk of bias tool (Higgins 2011). We assessed the following domains.
Sequence generation (selection bias)
Allocation concealment (selection bias)
Blinding of participants and personnel (performance bias)
Blinding of outcome assessors (detection bias)
Incomplete outcome data (attrition bias)
Selective reporting (reporting bias)
Other risk of bias
For each domain, two review authors (SL, MP, JS, or JB) independently judged whether study authors made sufficient attempts to minimise bias in their design. We judged each domain as low, high, or unclear risk of bias and recorded these judgements in risk of bias tables. Consensus for risk of bias decisions was made through discussion.
Measures of treatment effect
For each outcome, we aimed to calculate summary estimates of treatment effect (with 95% confidence intervals (CI)). For dichotomous outcomes, we calculated risk ratios (RR). For continuous outcomes, we calculated mean differences (MD) between treatment groups. If studies used different measurement tools or scales, we would use standardised mean differences (SMDs).
Unit of analysis issues
We anticipated that the unit of randomisation and analysis in the included trials would be at the participant level. This was confirmed by an initial pilot screen of the published literature. However, bilateral calcaneal fractures are quite common, and trials including people with bilateral fractures may present results for fractures or limbs rather than individual participants. Where such unit of analysis issues arose, and appropriate corrections had not been made, we presented data for such trials where the disparity between the unit of analysis and randomisation was small. We used footnotes in the analyses tables to highlight this information.
Had we found eligible studies that were randomised clusters (e.g. of healthcare settings), we would have analysed data at the cluster rather than participant level. For multi‐arm studies, we would avoid double‐counting participants within the same analysis, either selecting only one pairwise group for comparison or, if appropriate, splitting the data from a shared comparison group to create more than one smaller comparison group.
Dealing with missing data
Where possible, we used intention‐to‐treat data in the analyses. Where data were missing or unsuitable for analysis (e.g. where intention‐to‐treat analysis was not presented), we contacted study authors for further information and data. Where data were missing to the extent that the study could not be included in the meta‐analysis and attempts to retrieve data had been exhausted, we presented and discussed results in the context of the findings. We used available‐case analysis, whereby data were only included for those for whom the result was known, thus denominators relate to those with data for the particular outcome in question. We did not impute missing values for the analyses.
Assessment of heterogeneity
When deciding whether meta‐analysis was appropriate, we assessed the clinical diversity across studies. This included assessment of the comparability of participant characteristics (such as age and type of fracture), interventions, co‐interventions, and outcomes. Where we deemed two or more studies to be clinically homogenous, we assessed the pooled data for statistical heterogeneity using Review Manager 5 (Review Manager 2020). We assessed heterogeneity by visual inspection of the forest plot along with the test for heterogeneity and the I² statistic (Higgins 2023). Our main quantitative assessment of heterogeneity was based on the I² statistic, using the following interpretation from the Cochrane Handbook for Systematic Reviews of Interventions:
0% to 40%: might not be important;
30% to 60%: may represent moderate heterogeneity;
50% to 90%: may represent substantial heterogeneity; and
75% to 100%: considerable heterogeneity (Deeks 2011).
Assessment of reporting biases
We planned to investigate the potential for publication bias and to explore possible small‐study biases using funnel plots; however, this was precluded by an insufficient number of studies (fewer than 10) for all outcomes (Sterne 2017).
To assess outcome reporting bias, we screened clinical trials registers for protocols and registration documents of included studies that were prospectively published, and we sourced all clinical trials register documents that were reported in the study reports of included studies. We used evidence of prospective registration to judge whether studies were at risk of selective reporting bias.
Data synthesis
We conducted meta‐analyses only where this was meaningful, that is where the treatments, participants, and the underlying clinical question were similar enough for pooling to make sense. We pooled results of comparable groups of trials using random‐effects models. We chose this model after careful consideration of the extent to which any underlying effect could truly be thought to be fixed, given the complexity of the interventions included in this review. We presented 95% CIs throughout.
In the event that studies used more than one measurement instrument for an outcome, we pooled data from the most commonly used tool and reported this as the primary data in the summary of findings table, Abstract, and Plain language summary. For completeness, we included study findings for all outcome measures in the review, including data that could not be used to calculate a summary statistic; we used tables in the appendix to present these data.
If effect sizes were statistically significant, we considered whether the point estimate (and its corresponding CI) was clinically important. Ideally, we aimed to base these decisions on established minimal clinically important differences (MCIDs) for the measurement tool.
Subgroup analysis and investigation of heterogeneity
Although we aimed to explore possible sources of heterogeneity between studies, key effect modifiers were insufficiently reported to allow for meaningful subgroup analysis. In this review update, we were interested in the following effect modifiers:
whether or not participants were smokers;
whether or not the injury included the presence of fibular impingement (fibulocalcaneal abutment);
type of fracture (Sanders classification types compared against one another);
type of surgery (open reduction and internal fixation versus minimally invasive procedures).
We planned to conduct formal tests for subgroup interactions in Review Manager 5 (Review Manager 2020), using between‐study or within‐study subgroups of these effect modifiers when the covariate distribution was sufficient (i.e. when sufficient participants or studies contributed to each subgroup).
We found that overall there were few studies and did not formally assess the interaction between these subgroups. However, we recognise the emerging trend in practice towards minimally invasive surgical (MIS) techniques in the management of this injury since the last version of this review. We therefore presented the studies in two groups within the analyses for measures of function – traditional extensile open approaches and minimally invasive procedures – to aid the reader in exploring clinical diversity between studies and plausible causes of heterogeneity.
Sensitivity analysis
We conducted sensitivity analyses by examining aspects of trial and review methodology. We excluded studies from the primary analysis that were at high risk of attrition bias, high or unclear risk of selection bias (arising from lack of allocation concealment), and those trials only reported in conference abstracts. We also re‐analysed pooled data using a fixed‐effect model in sensitivity analysis. We compared the results of sensitivity analyses with the primary analyses and reported effect estimates that differed in interpretation due to a notable change in size of effect or direction of effect.
Summary of findings and assessment of the certainty of the evidence
Two review authors used the GRADE system to assess the certainty of the body of evidence associated with the seven critical outcomes in the review (Schünemann 2019).
For outcomes that were reported using more than one measurement tool, and that could not be combined in analysis, we assessed the certainty of the evidence for the outcome that used a measurement tool with the most participants.
The GRADE approach assesses the certainty of a body of evidence based on the extent to which we can be confident that an estimate of effect or association reflects the item being assessed. Evaluation of the certainty of a body of evidence considers within‐study risks of bias, directness of the evidence (indirectness), heterogeneity of the data (inconsistency), precision of the effect estimates (imprecision), and risk of publication bias. The certainty of the evidence could be high, moderate, low or very low, downgraded by one or two levels depending on the presence and extent of concerns in each of the five GRADE domains. We used footnotes to describe reasons for downgrading the certainty of the evidence for each outcome and used these judgements when drawing conclusions in the review.
We used GRADEpro GDT software to prepare a summary of findings table (GRADEpro GDT).
Results
Description of studies
Results of the search
We updated the search to November 2022. We identified a total of 1235 records from the following databases: Cochrane Bone, Joint and Muscle Trauma Group Specialised Register (6); CENTRAL (287), MEDLINE (353), Embase (503), the WHO ICTRP (36), ClinicalTrials.gov (47), and the ISRCTN registry (3). After removal of duplicates, we screened 896 records which included backward citation searches of key references identified from the search results and studies that were ongoing in the previous version of this review (Bruce 2013). We reviewed the full text of 22 records and selected eight new studies (with 14 records); along with the four studies (with 22 records) previously included in Bruce 2013, we included a total of 12 studies (with 36 records) in the review. We excluded seven studies, and one study is awaiting classification. We found no ongoing studies. See Figure 1.
1.
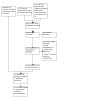
PRISMA flow chart. Searches conducted in November 2022.
Included studies
A summary table of study characteristics is presented in Appendix 2. For further details, see Characteristics of included studies.
One study was reported only as an abstract, and this included limited study information (Chrintz 1993). We contacted study authors from seven studies to request additional information (Buckley 2002; Chrintz 1993; Griffin 2014; Kulkarni 2015; Pandey 2018; Thordarson 1996). We received replies from the authors of only two studies (Buckley 2002; Griffin 2014), and only one of these study teams still had access to study information (Griffin 2014), thus information was limited to detail in published study reports.
Types of studies and setting
Whilst most studies were RCTs, two used methods to allocate participants to interventions that we assessed as quasi‐randomised (Kamath 2021; Parmar 1993).
Three studies were multicentre studies (Agren 2013; Buckley 2002; Griffin 2014), whilst the remaining studies were conducted at single centres. Studies were conducted in Sweden (Agren 2013), Canada (Buckley 2002), Denmark (Chrintz 1993), the UK (Griffin 2014; Parmar 1993), Pakistan (Hussain 2022), India (Kamath 2021; Kulkarni 2015; Sharma 2011), Iran (Nouraei 2011), Nepal (Pandey 2018), and the USA (Thordarson 1996).
Studies were published between 1993 and 2022. We noted that one study recruited participants at a much earlier time point (1977 to 1979) to the date of abstract publication (Chrintz 1993).
Types of participants
In total, 1097 participants with 1151 calcaneal fractures were recruited across the 12 studies. Randomisation in all studies was conducted at the participant level rather than the fracture level. Sample sizes ranged from 29, Kulkarni 2015, to 424, Buckley 2002.
All participants had DIACFs. Seven studies with 435 participants reported type of fracture as classified by Sanders (Agren 2013; Griffin 2014; Hussain 2022; Kamath 2021; Nouraei 2011; Sharma 2011; Thordarson 1996). Of these, 1% of participants had Type I fractures, 50% had Type II, 37% had Type III, and 12% had Type IV. Of those studies reporting sex distribution, 86% of participants were male. Mean ages of participants ranged from 28 years, Sharma 2011, to 52 years, Nouraei 2011. Only one study reported numbers of participants that smoked (Griffin 2014). In this study, 51% of participants in the surgical group smoked, and 56% of participants in the non‐surgical group smoked. There were no other notable differences between these studies in terms of participants.
No studies reported the number of included participants with fibula impingement. Griffin 2014 excluded 57 participants because they had fibula impingement. This was not described as exclusion criteria in any other studies, and it is possible that the remaining studies could have included some participants with fibula impingement.
Types of interventions
Most studies used ORIF using plates, screws, or wires (Agren 2013; Buckley 2002; Griffin 2014; Hussain 2022; Kulkarni 2015; Nouraei 2011; Parmar 1993; Sharma 2011; Thordarson 1996). In Kamath 2021 and Nouraei 2011, this was done using either an open or closed approach according to fixation types. Pandey 2018 used a minimally invasive approach with closed reduction and percutaneous screw fixation. Details were limited in Chrintz 1993, in which surgeons used a Steinmann pin technique.
Non‐surgical management included below‐knee casting (Hussain 2022; Kamath 2021; Kulkarni 2015; Nouraei 2011; Pandey 2018), patella tendon bandaging (Chrintz 1993), use of removable splints (Griffin 2014; Thordarson 1996), or rest and elevation with or without ice (Agren 2013; Buckley 2002; Parmar 1993; Sharma 2011).
Two studies did not report weight‐bearing protocols (Chrintz 1993; Pandey 2018). In all other studies, participants in both surgical and non‐surgical groups were non‐weight‐bearing for a minimum of six weeks.
Outcomes
Chrintz 1993 did not report any outcome data relevant to this review. However, all other studies reported data for at least one of our critical outcomes. Six studies also reported data for the other important review outcomes (Buckley 2002; Hussain 2022; Kulkarni 2015; Pandey 2018; Sharma 2011; Thordarson 1996).
Sources of funding and declarations of interest
One study reported funding from Arthritis Research UK (Griffin 2014), and one study reported that they received no funding (Hussain 2022). The remaining studies did not declare funding sources.
Five studies reported that their authors had no conflicts of interest (Griffin 2014; Hussain 2022; Kulkarni 2015; Nouraei 2011; Sharma 2011). The remaining studies did not report this information.
Excluded studies
We excluded seven studies (see Excluded studies). Two studies identified from clinical trials searches were not eligible because they compared two alternative surgical procedures (ACTRN12617001588381; IRCT2016051327872N1). We excluded the remaining five studies because they were not RCTs or quasi‐RCTs (Aslan 2019; Kashani 2013; Li 2016; Rajikumar 2017; Su 2017).
Studies excluded in earlier searches are described in the previous version of this review (Bruce 2013).
Studies awaiting classification
We found one study registered in an clinical trials register that was completed in Iran but for which we found no associated publication of the results (IRCT2017092720258N62). This study had a target sample size of 40 participants and compared ORIF with "routine treatment". Our attempts to contact the study authors for the results were unsuccessful; we were also unable to confirm whether "routine treatment" was non‐surgical. See Characteristics of studies awaiting classification.
Risk of bias in included studies
We did not conduct risk of bias assessments for Chrintz 1993 because this study contributed no outcome data to the review. For a summary of risk of bias judgements for all other studies, see Figure 2.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study. We did not conduct risk of bias assessment in Chrintz 1993 because this study contributed no outcome data to the review.
Allocation
Two studies were quasi‐randomised trials, and we judged these to be at high risk of selection bias for sequence generation (Kamath 2021; Parmar 1993). We judged a third study to be at high risk of selection bias (Nouraei 2011); although the method used for sequence generation was not described, we believe that manipulation of randomisation was possible owing to other information in the study report. Six studies used adequate methods for sequence generation, and we judged these to have a low risk of selection bias for sequence generation (Agren 2013; Buckley 2002; Griffin 2014; Hussain 2022; Kulkarni 2015; Pandey 2018). The remaining studies did not report information on sequence generation, and we judged risk of bias for this domain as unclear.
For allocation concealment, we judged the two quasi‐randomised trials to be at high risk of bias. Whilst Kulkarni 2015 used an appropriate method to allocate participants (drawing of chits), the number of participants was small in this study, and we expected that allocation concealment would not be possible when numbers of chits were small; we therefore judged this study to be at high risk of bias for this domain. Only three studies used adequate methods to conceal allocation, and we judged risk of bias for these to be low (Agren 2013; Buckley 2002; Griffin 2014). The remaining studies did not report information on allocation concealment, and we judged risk of bias for this domain as unclear.
Blinding
Given the intervention and comparison groups in this review, performance bias was inevitably high risk in all studies.
Similarly, for participant‐measured outcomes, detection bias was also high risk because participants were aware of their group allocation. Whilst some studies used independent personnel to assess clinical outcomes, we expected that blinding was unlikely in these studies because scars from surgical interventions would be visible. Unless clearly stated, we assumed that no attempts were made to blind outcome assessors to participants' treatment, and we judged these studies to be at high risk of detection bias. Only one study used methods to disguise scars during clinical outcome assessment, and we judged this study to be at low risk of detection bias for the relevant outcomes (Griffin 2014).
Incomplete outcome data
We judged four studies to be at high risk of attrition bias (Buckley 2002; Nouraei 2011; Parmar 1993; Thordarson 1996). In these studies, participant loss was large (more than 10%) or not balanced or explained by study authors. We judged the remaining studies to be at low risk of attrition bias because losses were few, reasonably justified, and balanced between groups.
Selective reporting
One study was prospectively registered with a clinical trials register and, because reported outcomes were consistent with planned outcomes, we judged this study to be at low risk of selective reporting bias (Griffin 2014). Another study was registered with a clinical trials register (Agren 2013), but this was done retrospectively, and we could not feasibly use these documents to assess this type of bias. We judged the risk of selective reporting bias in Agren 2013, and in all other studies that reported no protocol or clinical trials registration, to be unclear.
Other potential sources of bias
We could not be certain of the risk of other bias in Thordarson 1996 because we noted an unexplained change to the surgical procedure part‐way through the study report; we judged risk of other bias to be unclear in this study. We identified no other sources of bias in the remaining studies.
Effects of interventions
See: Table 1
Critical outcomes
See Table 1.
No studies reported outcome data for function in the short term (within three months of injury).
Function in the long term
Eight studies reported function up to 24 months after injury (Agren 2013; Buckley 2002; Griffin 2014; Hussain 2022; Kamath 2021; Kulkarni 2015; Pandey 2018; Sharma 2011). Studies used different instruments to report this outcome (American Orthopaedic Foot and Ankle Society score (AOFAS); Creighton‐Nebraska health foundation score; Kerr‐Atkins score; Modified Rowe's Score (MRS); Olerud‐Molander Ankle Score (OMAS); and a composite VAS score). Some studies measured function using more than one scale.
We pooled data for studies that reported function using the AOFAS score, as this was measured most frequently (Agren 2013; Griffin 2014; Kamath 2021; Pandey 2018; Thordarson 1996). We found a difference in function favouring surgical intervention (mean difference (MD) 6.58, 95% confidence interval (CI) 1.04 to 12.12, favours surgery; 5 studies, 319 participants; I2 = 80%; low‐certainty evidence; Analysis 1.1). In this analysis, function was measured at six months (Pandey 2018), 12 months (Agren 2013; Kamath 2021), and 24 months (Griffin 2014). We are not aware of any published thresholds for an MCID for the AOFAS score in calcaneal fractures. MCIDs for other foot conditions range from 2.0 to 7.9 (Chan 2017; Dawson 2007). We downgraded the certainty of the evidence by one level owing to serious risk of bias in the included studies, and one level owing to imprecision because the analysis included few participants. We noted substantial levels of statistical heterogeneity in this effect estimate but did not downgrade for inconsistency. The statistical heterogeneity was caused by the outlying result in Thordarson 1996 which included very few participants.
1.1. Analysis.

Comparison 1: Surgical versus non‐surgical management, Outcome 1: Function in the long term; measured using AOFAS
We also pooled data reported in the four of these studies that measured function using different measurement instruments: OMAS (Agren 2013), Kerr‐Atkins score (Griffin 2014), MRS (Kamath 2021), and a VAS score for pain and function (Pandey 2018). Agren 2013 and Kamath 2021 also assessed function using VAS scores that we did not include in this meta‐analysis; these data are reported in Appendix 3. We selected the formal functional instruments in preference to the unvalidated VAS scores rather than using data from both instruments in order to avoid double‐counting in the analyses. We found an imprecise effect estimate for function from this pooled analysis which included the possibility of both small harm and substantial benefit of surgery (standardised mean difference 0.60, 95% CI −0.10 to 1.30; 4 studies, 322 participants; Analysis 1.2).
1.2. Analysis.

Comparison 1: Surgical versus non‐surgical management, Outcome 2: Function in the long term; using measurement tools other than AOFAS score
Only two studies used a measurement tool (Kerr‐Atkins score) that was specific to calcaneal injuries (Griffin 2014; Sharma 2011). For Griffin 2014, we found little or no difference between treatment groups in function at 24 months (MD 4.10, 95% CI −3.36 to 11.56; 1 study, 143 participants; Analysis 2.1; Appendix 3). Sharma 2011 reported mean scores using this measurement tool, but without standard deviations or an effect estimate or P value (see Appendix 4).
2.1. Analysis.

Comparison 2: Surgical versus non‐surgical management: data reported using additional measurement tools, Outcome 1: Function in the long term
For completeness, we include other function data for Sharma 2011, as well as data for Buckley 2002, in Appendix 4, which are reported as mean scores without distribution values; we could not be certain of the time point in Buckley 2002 but expected that it fell outside of the time point specified in this review for long‐term follow‐up.
In three studies (86 participants), investigators translated scores into ordinal data, reporting function as excellent, good, satisfactory or fair, and poor (Hussain 2022; Kulkarni 2015; Thordarson 1996). We found little or no difference in the number of participants who judged function to be excellent (risk ratio (RR) 3.21, 95% CI 1.14 to 9.04), good (RR 1.13, 95% CI 0.75 to 1.71), or satisfactory or fair (RR 0.62, 95% CI 0.23 to 1.66) (Analysis 1.3).
1.3. Analysis.

Comparison 1: Surgical versus non‐surgical management, Outcome 3: Function in the long term; number of people with scores of excellent, good, or satisfactory/fair
Chronic pain
Five studies reported chronic pain up to 24 months after injury (Agren 2013; Hussain 2022; Nouraei 2011; Parmar 1993; Thordarson 1996).
When reported as the number of people experiencing pain at six months (Nouraei 2011), 12 months (Hussain 2022; Parmar 1993), and 24 months (Thordarson 1996), we found that people may be less likely to experience chronic pain after surgical treatment (RR 0.56, 95% CI 0.37 to 0.84; 4 studies, 175 participants; low‐certainty evidence; Analysis 1.4). We downgraded the certainty of this evidence by one level for serious risk of bias and one level for imprecision because the analysis included few participants.
1.4. Analysis.

Comparison 1: Surgical versus non‐surgical management, Outcome 4: Chronic pain; number of people with pain
When reported as pain scores in Agren 2013, we found no evidence of a difference between treatment groups at 12 months (MD 0.20, 95% CI −0.21 to 0.61; 1 study, 76 participants; Analysis 1.5). This was measured using a 10‐point VAS. Although we did not have an MCID threshold for VAS in these fracture types, we expected this effect estimate did not include a clinically important difference.
1.5. Analysis.

Comparison 1: Surgical versus non‐surgical management, Outcome 5: Chronic pain; measured using VAS
Agren 2013 also reported pain during weight‐bearing, and we reported the effect estimate for this (calculated using the calculator in Review Manager 5 (Review Manager 2020)) in Analysis 2.2 and Appendix 3; the finding was similar to pain at rest in this study.
2.2. Analysis.

Comparison 2: Surgical versus non‐surgical management: data reported using additional measurement tools, Outcome 2: Chronic pain
Health‐related quality of life (HRQoL) in the long term
Three studies reported HRQoL and measured this outcome using the physical and mental component scores (PCS and MCS) of the SF‐36 (Agren 2013; Buckley 2002; Griffin 2014).
When combining data from Agren 2013 and Griffin 2014 using the PCS of SF‐36, we found that people may have better physical HRQoL over two years when they have had surgical treatment (MD 6.49, 95% CI 2.49 to 10.48; 2 studies, 192 participants; low‐certainty evidence; Analysis 1.6); this was measured at 12 months in Agren 2013 and 24 months in Griffin 2014. Again, we did not have a threshold MCID for calcaneal fractures and therefore based our interpretation of the SF‐36 (PCS) using a 5% threshold (or 5 points on a 100‐point scale) as described in Ware 2005. We thus expected that this effect estimate included the possibility that some people treated surgically may have more clinically important improved HRQoL within 24 months than those treated without surgery. We downgraded the certainty of the evidence by one level for serious risk of bias and one level for imprecision because the analysis included few participants.
1.6. Analysis.

Comparison 1: Surgical versus non‐surgical management, Outcome 6: HRQoL in the long term; measured using SF‐36 (PCS)
Although we did not pool other data, we noted no evidence of a difference in mental health scores between treatment groups for the MCS of the SF‐36 or for HRQoL when measured using the EQ‐5D in Griffin 2014 (Analysis 2.3; Appendix 3).
2.3. Analysis.

Comparison 2: Surgical versus non‐surgical management: data reported using additional measurement tools, Outcome 3: HRQoL in the long term
We could not be certain of the time point at which data were reported in Buckley 2002, and expected that it may fall outside the time point specified in this review. However, for completeness we report data for Buckley 2002 in Appendix 4; these mean data were reported without distribution values.
Return to normal activity (including work and leisure activity)
Five studies reported this outcome (Griffin 2014; Hussain 2022; Kamath 2021; Parmar 1993; Thordarson 1996). The included studies used different definitions, and we pooled data that we judged to be most comparable; in all studies, this related to return to work rather than leisure activities. We found little or no difference between treatment groups in the number of people who returned to work 12 months after injury (RR 1.26, 95% CI 0.94 to 1.68; 5 studies, 250 participants; low‐certainty evidence; Analysis 1.7). In this analysis, we included data for two studies that fell outside the time point for this outcome; in Griffin 2014 and Thordarson 1996, this outcome was measured at 24 months. We downgraded the certainty of this evidence by one level for serious risk of bias and one level for imprecision because the analysis included few participants.
1.7. Analysis.

Comparison 1: Surgical versus non‐surgical management, Outcome 7: Return to normal activity (work)
Two studies also reported data for this outcome which they described as return to the same work, return to previous recreation activity, or having no limitations in daily or recreational activities (Parmar 1993; Thordarson 1996). We calculated effect estimates for these data using the calculator in Review Manager 5 (Review Manager 2020), and noted little or no difference between groups in these findings (Analysis 2.4; Appendix 3).
2.4. Analysis.

Comparison 2: Surgical versus non‐surgical management: data reported using additional measurement tools, Outcome 4: Return to normal activity
Postoperative complications requiring unplanned return to theatre
Data for unplanned return to theatre are reported in Analysis 1.8. Some of these events, for example implant removal, deep surgical site infection (SSI), and wound debridement, are necessarily only applicable to participants who had surgery. Others, such as amputation and subtalar arthrodesis, can affect both surgical and non‐surgical participants.
1.8. Analysis.

Comparison 1: Surgical versus non‐surgical management, Outcome 8: Postoperative complications requiring unplanned return to theatre
Amputation: one participant allocated to surgical treatment and no participants allocated to non‐surgical treatment required amputation (RR 2.86, 95% CI 0.12 to 68.23; 1 study, 82 participants; low‐certainty evidence).
Subtalar arthrodesis: 12 out of 321 participants allocated to surgical treatment (3.7%) and 44 out of 336 participants allocated to non‐surgical treatment (13%) had subtalar arthrodesis (RR 0.38, 95% CI 0.09 to 1.53; 3 studies, 657 participants; I2 = 67%; low‐certainty evidence). For this analysis, we used data at the last available time point at two years, Buckley 2002; Griffin 2014, and eight to 12 years, Agren 2013. Parmar 1993 also included later follow‐up data at 15 years, but we did not include these data (no events in either group) in this meta‐analysis because the rate of participant loss was substantial (32%) and therefore unreliable.
Implant removal: 11 out of 321 participants allocated to surgical treatment (3.4%) required implant removal (low‐certainty evidence).
Deep SSI: 11 out of 206 participants allocated to surgical treatment (5.3%) had deep SSI (low‐certainty evidence).
Wound debridement: 2 out of 73 participants allocated to surgical treatment (2.7%) required wound debridement in theatre (low‐certainty evidence).
We downgraded the certainty of the evidence for all complications by one level owing to serious risk of bias. For outcomes with few participants (amputation, implant removal, deep infection, and wound debridement), we also downgraded one level owing to imprecision. For subtalar arthrodesis, we downgraded another level for inconsistency because of unexplained substantial levels of statistical heterogeneity in the pooled effect.
Postoperative complications not requiring return to theatre
Seven studies reported superficial site infection (Agren 2013; Buckley 2002; Griffin 2014; Kamath 2021; Nouraei 2011; Pandey 2018; Thordarson 1996), with an incidence of 14% (59/419) in participants allocated to surgical treatment. Two studies reported wound dehiscence (Kamath 2021; Kulkarni 2015), with an incidence of 9% (4/45) in participants allocated to surgical treatment. Data in Analysis 1.9 are only applicable to participants who had surgery. We judged the certainty of the evidence for both of these types of complications to be low. We downgraded by one level owing to serious risk of bias and one level owing to imprecision because the evidence was from few participants.
1.9. Analysis.

Comparison 1: Surgical versus non‐surgical management, Outcome 9: Postoperative complications not requiring return to theatre
Other important outcomes
No studies reported data for HRQoL in the short term (within three months of injury).
Ability to wear usual footwear
Four studies reported this outcome. We pooled data, which were reported as ability to wear usual shoes, Agren 2013; Parmar 1993, and being able to wear all shoes comfortably, Nouraei 2011; Thordarson 1996. We found little or no difference between treatments for this outcome (RR 1.29, 95% CI 0.88 to 1.87; 4 studies, 219 participants; Analysis 1.10). This was reported at six months (Nouraei 2011), 12 months (Agren 2013; Parmar 1993), and 24 months (Thordarson 1996).
1.10. Analysis.

Comparison 1: Surgical versus non‐surgical management, Outcome 10: Ability to wear usual footwear
Radiological measurements
Böhler angle
Six studies measured this outcome (Buckley 2002; Hussain 2022; Kulkarni 2015; Pandey 2018; Sharma 2011; Thordarson 1996). We found that the Böhler angle was larger, and therefore showed more improvement, in participants who had surgery (MD 7.35°, 95% CI 3.81° to 10.89°, favours surgery; 2 studies, 72 participants; Analysis 1.11); this analysis included data at six months, Pandey 2018, and 12 months, Sharma 2011.
1.11. Analysis.

Comparison 1: Surgical versus non‐surgical management, Outcome 11: Radiological measurements: Böhler angle
Other studies reported these data without distribution values, and we could not combine this information in analyses. We noted, however, that P values reported by study authors indicated that the Böhler angle was better for participants treated with surgery in Hussain 2022 (P = 0.001), Kulkarni 2015 (P < 0.001), and Thordarson 1996 (P = 0.001) (Appendix 4).
Gissane angle
Two studies measured this outcome (Hussain 2022; Kulkarni 2015). We could not combine these data in meta‐analysis because they were reported without distribution values. The P values reported by study authors indicated that the Gissane angle was improved after surgery in both studies (P < 0.001 for both studies) (Appendix 4).
Sensitivity analysis
We found no evidence that studies at high risk of attrition bias impacted our findings in all our primary analyses. For 'function in the long term', we noted that the effect estimate was less precise when we removed Kamath 2021 and Pandey 2018 from the primary analysis because these studies were at high and unclear risk of selection bias, respectively (MD 2.75, 95% CI −1.90 to 7.40; 2 studies, 190 participants); no other effect estimates in the remaining outcomes were impacted by risk of selection bias. No studies in our analyses were reported only as conference abstracts. Finally, we found that analyses of some outcomes differed when we used a fixed‐effect rather than a random‐effects model. In particular, the effect estimate was no longer imprecise for subtalar arthrodesis (RR 0.29, 95% CI 0.16 to 0.54, favours surgery; 3 studies, 657 participants) or for ability to wear usual footwear (RR 1.23, 95% CI 1.04 to 1.47; favours surgery; 4 studies, 219 participants).
Subgroup analysis
We did not conduct subgroup analysis because most studies did not report data according to our specified subgroups. Only Griffin 2014 reported outcome data for function (measured using the Kerr‐Atkins score) according to fracture type (Sanders Type II and Sanders Type III and IV), and we include these data in Appendix 5.
Discussion
Summary of main results
We included 12 studies (10 RCTs, two quasi‐RCTs) with 1097 participants who had DIACFs.
We found low‐certainty evidence from five studies (319 participants) that there may be an improvement in long‐term function (measured using the AOFAS score up to 24 months after surgery) when participants are treated surgically rather than non‐surgically. It is challenging to interpret the clinical importance of this difference because MCIDs for this instrument are not published for calcaneal fractures. However, we note that MCIDs for other foot injuries range from 2.0 to 7.9 (Chan 2017; Dawson 2007). Function was measured using a variety of instruments, and we found an imprecise effect estimate when pooling data from these other instruments; however, the point estimate was similar.
We also found low‐certainty evidence that people may be less likely to have chronic pain up to 24 months after surgery. We note that when the presence of pain was measured using a VAS in two small studies (118 participants), there was little or no difference between groups in long‐term pain. There may be a small but meaningful improvement in physical HRQoL for those treated surgically, but little or no difference in the number of people returning to normal activities (low‐certainty evidence).
Only one small study reported amputation, with the only event occurring in the surgical group, and there was no evidence of a difference in the number of people requiring subtalar arthrodesis, although this estimate includes the possibility of both important harms and benefits of surgery. For complications in those treated surgically, incidence rates were 3.4% (implant removal), 5.3% (deep SSI), 2.7% (wound debridement), 14% (superficial SSI), and 9% (wound dehiscence). We judged the evidence for all complications to be of low certainty.
There were insufficent data to perform our planned subgroup analyses. We did not formally assess any differences in treatment effect between open extensile approaches and minimally invasive surgical techniques, but noted no substantial differences on visual inspection of the forest plots. This inference should be treated with caution since only one study used only minimally invasive surgical approaches.
Overall completeness and applicability of evidence
Participants in the included studies were representative of people with DIACFs; most were men (86%), and the mean age of study participants was generally younger than 50 years. However, only four studies recruited participants after 2010 (Hussain 2022; Kamath 2021; Kulkarni 2015; Pandey 2018); these studies were small (including only 160 participants) and were all conducted in the Indian subcontinent. It is unclear whether changes in orthopaedic management, including rehabilitation approaches such as physiotherapy, could have impacted the findings of our review. In addition, most evidence came from small, single‐centre studies and may not account for the range of differences in surgical decision‐making within and between healthcare jurisdictions. Surgical interventions may vary widely, and there is also likely to be variability in the execution of each type of intervention. The studies included in this review involved participants with different fracture types. The limited evidence meant that we were unable to explore differences between study participants in subgroup analyses, such as the impact of different surgical techniques or fracture severity. Given the skills and experience required for calcaneal fracture surgery, outcome may also vary according to the learning curve of the individual surgeon (Fischer 2021). We did not explore this in the review, and acknowledge the challenges in capturing this information in RCTs and analysing the impact of surgeon experience within a meta‐analysis.
In this review, we included data for most outcomes within two years of injury; generally, later time points are exposed to a greater risk of attrition. Longer‐term follow‐up data were available in Agren 2013 (eight to 12 years) and Parmar 1993 (up to 15 years). For subtalar arthrodesis, data in Agren 2013 were only available at eight to 12 years' follow‐up, and we chose to pool these with earlier data from other studies rather than report them separately. The 15‐year data reported in Parmar 1993 had substantial rates of attrition, and we judged these data to be unreliable and did not include them in our analysis.
There is often a poor correlation between radiological and clinical outcomes, therefore we judged patient‐reported outcomes to be more important than radiological results. In this review, we focused on these critical outcomes. However, studies reported these measures using a variety of different instruments, particularly for the domain of function, which may capture different components of function and may have different statistical and measurement characteristics and performance. Whilst we were able to pool studies reporting the AOFAS score, we could not separately pool studies reporting the calcaneal‐specific Kerr‐Atkins score because this instrument was rarely used. In addition, some studies reported data without distribution values or denominators, and this limited our ability to include all available data in meta‐analyses.
Certainty of the evidence
We used the GRADE approach to formally assess the certainty of the evidence for the critical outcomes.
The review included quasi‐randomised trials, which were at high risk of selection bias, as well as some studies for which we could not determine whether adequate steps were taken to avoid selection bias. Whilst it was not possible to blind participants and personnel to treatment, and risk of performance bias was therefore inevitably high, we believe that steps could have been taken to reduce the risk of detection bias. However, only one study took measures to reduce the risk of detection bias that we judged to be sufficient (Griffin 2014). We also had concerns regarding levels of attrition in some studies, and could not rule out the possibility of selection bias in most studies because they neither had published protocols nor were registered with clinical trials registers. We downgraded the certainty of the evidence for all outcomes owing to serious risk of bias.
Most studies were small and, even when data were pooled, sample sizes were unlikely to be sufficient. We downgraded most of the evidence for imprecision because sample sizes were small. We also downgraded the certainty of the evidence for inconsistency, particularly for subtalar arthrodesis, because levels of statistical heterogeneity were substantial. The number of studies was insufficient to explore this inconsistency meaningfully, therefore the inconsistency between study results for this outcome remains unexplained.
All participants and types of interventions were consistent with our intended criteria, and we did not downgrade any of the evidence for indirectness. We could not rule out the possibility of publication bias, and insufficient studies in the meta‐analyses precluded an exploration of this risk.
Potential biases in the review process
We conducted a thorough search, and two review authors independently assessed study eligibility, extracted data, and assessed risk of bias in the included studies before reaching consensus between the two review authors. Whilst this update included some changes using the current methodological expectations for Cochrane Reviews (such as the inclusion of a summary of findings table), we do not expect that these changes would have introduced bias to the review process.
The included studies used more than one measurement tool to report similar outcome effects. We selected the tool that was most frequently used by study authors when pooling data, and presented these pooled analyses in the summary of findings table, Abstract, and Plain language summary. For function, the most frequently reported measure used the AOFAS score. We could not be certain whether this was the most reliable tool to measure function in calcaneal fractures. For completeness, we reported all other function scores in tables in the appendix to this review. Although our specified long‐term follow‐up was 24 months, we acknowledge that it is feasible that some outcomes such as pain or subtalar arthrodesis may change after 24 months and may change differentially between groups.
Agreements and disagreements with other studies or reviews
In the previous version of this review (Bruce 2013), we concluded that there was insufficient high‐quality evidence to establish the better treatment for calcaneal fractures. Whilst this updated review includes eight more studies (with an additional 495 participants), sample sizes in pooled analyses were still small and more likely to lead to imprecise findings. As a result, concerns remain about the robustness of the review findings.
When comparing our findings with those of other meta‐analyses, we noted that effect estimates for functional scores in these other reports included the possibility of no improvement in function (Meena 2017; Selim 2022). These analyses, however, included longer‐term data from Parmar 1993 (15 years after injury), which we did not include in our analysis. Whilst the longer‐term data for Parmar 1993 were not statistically significant, the point estimate favoured non‐surgical treatment; point estimates in the studies measuring function within 24 months of injury favoured surgery. Whilst the data in Selim 2022 were also imprecise for chronic pain, their analysis included non‐randomised studies that were not eligible for this review. Other reviews noted that combined complication rates were higher after surgery, and we also found evidence of people needing to return to theatre for implant removal, deep infection, and wound debridement. Luo 2016 concluded that subtalar arthrodesis may be less frequent after surgical treatment of calcaneal fractures, but their findings included substantial statistical heterogeneity, and they did not pool these data.
Authors' conclusions
Implications for practice.
Our confidence in the evidence in this review is limited. We judged the evidence to be of low certainty, as it was often derived from few participants in studies that were not sufficiently robust in design.
The pooled current evidence indicates that surgical treatment for people with displaced intra‐articular calcaneal fractures may lead to improved outcomes, particularly for function, pain, and physical quality of life. Balanced against this, people who have surgery may have a higher risk of unplanned further surgery for postoperative complications, although there may be no difference between treatment options in the risk of further surgery for subtalar arthritis.
Based on the existing evidence from randomised controlled trials, we are unable to determine if any one surgical technique (for those treated surgically) yields improved outcomes compared with others.
Implications for research.
Given that the certainty of the evidence in this review is low, and the impact of calcaneal fracture for individuals is so substantial, further good‐quality research is justified.
In general, future studies should be carefully designed and consider approaches to minimising detection and attrition bias in any assessment of function by healthcare personnel. Larger studies, with data that are also reported according to Sanders classification, smoking status, and presence of fibular impingement, would allow for analysis of potential effect modifiers in treatment options. We encourage researchers to consider the use of recognised measurements of function measured at two years following injury, in particular the use of instruments that are specifically designed for calcaneal fractures. The existing evidence from randomised trials would support, albeit with the caveat of low certainty, consideration of surgery for people with this type of injury due to at least a small benefit in patient‐reported outcomes at the cost of an increased risk of complications. It is unlikely in our view that further trials comparing traditional surgical and non‐surgical treatments could be delivered in the context of this and other authors' findings. Clinical practice has moved away from open reduction through extensile approaches in response to the potentially catastrophic complications and likelihood of only small to moderate benefits.
Minimally invasive surgical procedures, however, are becoming more prevalent, and these offer the possibility of improved outcomes observed with surgery with regard to function, pain, and quality of life at a potentially reduced risk of postoperative complications compared with older surgical techniques. Future studies should thus investigate the effectiveness of these new surgical techniques versus non‐surgical management. We anticipate that studies using the American Orthopaedic Foot and Ankle Society or Kerr‐Atkins instruments as a primary outcome tool would need to recruit samples in the order of 150 participants per treatment group to demonstrate small to moderate between‐group differences assuming P = 0.05 and with a power of 90%.
We recommend that researchers come together to perform larger, definitive trials at speed and scale. The evidence from this review and the included studies would suggest that trials in this field are time‐consuming and have extended recruitment periods. We would advocate for international collaboration in this field to accelerate the delivery of trial inferences to the benefit of clinicians and future patients. We also recognise the proliferation of different surgical techniques within the minimally invasive surgical spectrum and advise that any research group agree through consensus on an appropriate intervention description for proper evaluation and reporting in future meta‐analyses.
What's new
| Date | Event | Description |
|---|---|---|
| 7 November 2023 | New search has been performed | Title: we edited the title to describe that conservative interventions were non‐surgical. Review authors: we added three new review authors (SL, MP, XG) and removed two review authors (HR, BM). Searches and data extraction: we updated and re‐ran the searches for studies, extracted data on new studies, conducted risk of bias assessments on all included studies, and incorporated new data into the review. Results: this review update includes an additional eight studies. Conclusions: we made changes to the conclusions to reflect review findings for function. |
| 7 November 2023 | New citation required and conclusions have changed | We made changes to the conclusions to reflect review findings for function. |
History
Protocol first published: Issue 8, 2010 Review first published: Issue 1, 2013
Acknowledgements
We would like to thank Helen Richmond and Dr Bruno Mazuquin, who supported review work during earlier drafts of this publication.
See Bruce 2013 for acknowledgements of support in previous versions of this review.
Editorial and peer‐reviewer contributions
Cochrane Bone, Joint and Muscle Trauma Group supported the review authors in the development of this review.
The following people conducted the editorial process for this article:
Sign‐off Editor (final editorial decision): Tari Turner, Cochrane Australia;
Managing Editor (selected peer reviewers, collated peer‐reviewer comments, provided editorial guidance to authors, edited the article): Sam Hinsley, Cochrane Central Editorial Service;
Editorial Assistant (conducted editorial policy checks, collated peer‐reviewer comments, and supported editorial team): Sara Hales‐Brittain, Cochrane Central Editorial Service;
Copy Editor (copy‐editing and production): Lisa Winer, Cochrane Central Production Service;
Peer reviewers (provided comments and recommended an editorial decision): Stefan Rammelt, University Hospital Carl Gustav Carus, Dresden, Germany (clinical/content review); Nuala Livingstone, Cochrane Evidence Production and Methods Directorate (methods review); Steve McDonald, Cochrane Australia (search review). One additional peer reviewer provided clinical/content peer review but chose not to be publicly acknowledged.
Appendices
Appendix 1. Search strategies for this update (2011 to November 2022)
CENTRAL (via CRS Web)
The CENTRAL search was run in two stages: the first search was run in April 2017 and a top‐up search was run in November 2022.
Search 1
#1 MESH DESCRIPTOR Calcaneus EXPLODE ALL TREES (129) #2 MESH DESCRIPTOR Subtalar Joint (21) #3 #1 OR #2 (147) #4 MESH DESCRIPTOR Fractures, Bone EXPLODE ALL TREES (3844) #5 #3 AND #4 (56) #6 (calcan* adj2 fracture*):TI,AB,KY (88) #7 (calcis adj2 fracture*):TI,AB,KY (1) #8 #6 OR #7 (88) #9 #5 OR #8 (102)
Search 2 (top‐up search)
#1 MESH DESCRIPTOR Calcaneus EXPLODE ALL AND CENTRAL:TARGET (149) #2 MESH DESCRIPTOR Subtalar Joint EXPLODE ALL AND CENTRAL:TARGET (25) #3 #1 OR #2 (170) #4 MESH DESCRIPTOR Fractures, Bone EXPLODE ALL AND CENTRAL:TARGET (6947) #5 #3 AND #4 (70) #6 (calcan* and fracture*):AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET (330) #7 (calcis and fracture*):AB,EH,KW,KY,MC,MH,TI,TO AND CENTRAL:TARGET (18) #8 #6 OR #7 (337) #9 #8 OR #5 (338) #10 01/01/2017_TO_08/11/2022:CRSCREATED AND CENTRAL:TARGET (1012451) #11 #10 AND #9 (185)
MEDLINE (Ovid interface)
The MEDLINE search was run in two stages: the first search was run in April 2017 and a top‐up search was run in November 2022.
Search 1
1 Calcaneus/ (6683) 2 Subtalar Joint/ (1339) 3 or/1‐2 (7678) 4 exp Fractures, Bone/ (165856) 5 and/3‐4 (2509) 6 ((calcan$ or calcis) adj5 fracture$).tw. (2283) 7 or/5‐6 (3073) 8 Randomized controlled trial.pt. (460602) 9 Controlled clinical trial.pt. (93977) 10 randomized.ab. (401705) 11 placebo.ab. (188357) 12 Drug therapy.fs. (1987944) 13 randomly.ab. (279258) 14 trial.ab. (420688) 15 groups.ab. (1721708) 16 or/8‐15 (4090002) 17 exp Animals/ not Humans/ (4389696) 18 16 not 17 (3536841) 19 7 and 18 (330) 20 (2011* or 2012* or 2013* or 2014* or 2015* or 2016* or 2017*).dc,dp,ed,ep,yr. (7552781) 21 19 and 20 (127)
Search 2 (top‐up search)
1 Calcaneus/ (7681) 2 Subtalar Joint/ (1575) 3 1 or 2 (8861) 4 exp Fractures, Bone/ (203736) 5 3 and 4 (2975) 6 ((calcan* or calcis) adj5 fracture*).tw. (2877) 7 5 or 6 (3726) 8 Randomized controlled trial.pt. (580212) 9 Controlled clinical trial.pt. (95085) 10 randomized.ab. (581518) 11 placebo.ab. (233070) 12 Drug therapy.fs. (2544435) 13 randomly.ab. (394911) 14 trial.ab. (622643) 15 groups.ab. (2430491) 16 or/8‐15 (5503069) 17 exp Animals/ not Humans/ (5061598) 18 16 not 17 (4796347) 19 7 and 18 (520) 20 (2017* or 2018* or 2019* or 2020* or 2021* or 2022*).ed,dt. (8968697) 21 19 and 20 (226)
EMBASE (Ovid interface)
The Embase search was run in two stages: the first search was run in April 2017 and a top‐up search was run in November 2022.
Search 1
1 Calcaneus Fracture/ (1985) 2 Subtalar Joint/ (1951) 3 Intraarticular Fracture/ or Joint Fracture/ (1898) 4 and/2‐3 (66) 5 ((calcan* or calcis) adj5 fracture*).tw. (2500) 6 or/1,4‐5 (3045) 7 Randomized controlled trial/ (440857) 8 Clinical trial/ (915971) 9 Controlled clinical trial/ (427735) 10 Randomization/ (72886) 11 Single blind procedure/ (26111) 12 Double blind procedure/ (136522) 13 Crossover procedure/ (50358) 14 Placebo/ (302707) 15 Prospective study/ (364324) 16 randomi#ed.tw. (664388) 17 ((clinical or controlled or comparative or placebo or prospective*) adj3 (trial or study)).tw. (901363) 18 (random* adj7 (allocat* or allot* or assign* or basis* or divid* or order*)).tw. (243963) 19 ((singl* or doubl* or trebl* or tripl*) adj7 (blind* or mask*)).tw. (201330) 20 (cross?over* or (cross adj1 over*)).tw. (86814) 21 ((allocat* or allot* or assign* or divid*) adj3 (condition* or experiment* or intervention* or treatment* or therap* or control* or group*)).tw. (330847) 22 RCT.tw. (23322) 23 or/7‐22 (2533990) 24 6 and 23 (344) 25 (2011* or 2012* or 2013* or 2014* or 2015* or 2016* or 2017*).dc,yr. (9479933) 26 24 and 25 (161)
Search 2 (top‐up search)
1 Calcaneus Fracture/ (2619) 2 Subtalar Joint/ (2777) 3 Intraarticular Fracture/ or Joint Fracture/ (2856) 4 2 and 3 (92) 5 ((calcan* or calcis) adj5 fracture*).tw. (2916) 6 1 or 4 or 5 (3687) 7 Randomized controlled trial/ (730432) 8 Controlled clinical study/ (467368) 9 Random*.ti,ab. (1836420) 10 randomization/ (95193) 11 intermethod comparison/ (289025) 12 placebo.ti,ab. (342671) 13 (compare or compared or comparison).ti. (557110) 14 ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab. (2582549) 15 (open adj label).ti,ab. (101366) 16 ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab. (254749) 17 double blind procedure/ (197211) 18 parallel group*1.ti,ab. (30256) 19 (crossover or cross over).ti,ab. (116153) 20 ((assign* or match or matched or allocation) adj5 (alternate or group*1 or intervention*1 or patient*1 or subject*1 or participant*1)).ti,ab. (388226) 21 (assigned or allocated).ti,ab. (457853) 22 (controlled adj7 (study or design or trial)).ti,ab. (418387) 23 (volunteer or volunteers).ti,ab. (265533) 24 trial.ti. (367123) 25 or/7‐24 (5471211) 26 (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) (6512858) 27 25 not 26 (4760126) 28 6 and 27 (758) 29 (2017* or 2018* or 2019* or 2020* or 2021* or 2022*).dc,yr. (10731930) 30 28 and 29 (342)
WHO ICTRP
calcan* AND fracture (36)
ClinicalTrials.gov
(calcaneal OR calcaneus) AND fracture from 2011 to 2017 (20) (calcaneal OR calcaneus) AND fracture from 2017 to 2022 (27)
ISRCTN Registry
calcaneal or calcaneus (3)
Appendix 2. Summary of study characteristics
| Study ID | Country | Number randomiseda | Data collection period | Participants | Surgical details | Comparison (non‐surgical management) | Outcome |
| Agren 2013 | Sweden | 82 | 1994 to 2012 | Mean age: 48.5 years Sex (M/F): 59/23 |
ORIF within 2 weeks. Involved extensile lateral approach, use of neutralisation plate, interfragmentary screws to achieve anatomic reduction | Rest, elevation, and non‐weight‐bearing | Follow‐up: 1 year, 8 to 12 years Primary outcome: composite pain and function score (VAS 0 to 100), quality of life (SF‐36) |
| Buckley 2002 | Canada | 424 | 1991 to 1997 | Mean age: 40 years Sex (M/F): 381/43 |
ORIF. Involving extended lateral approach, use of plate, screw or wire fixation. Autografting left to surgeon discretion. | Ice, elevation, rest Physiotherapy started after 6 weeks for all participants. |
Follow‐up: 2 to 4 weeks, 6, 12, 26, 52 weeks and 2 (minimum) to 8 years (maximum) Primary outcome: quality of life (SF‐36), disease‐specific scale (VAS) |
| Chrintz 1993 | Denmark | 68 | 1977 to 1979 | Age: not reported Sex: not reported |
Reduction and fixation with a Steinmann pin through calcaneus and talus followed by a PTB bandage for 12 weeks | PTB bandage for 12 weeks | Follow‐up: 6, 12 and a median of 80 weeks Pain, working capacity, activity in leisure time, working ability, tenderness on palpation or movement of the joints, duration of hospitalisation |
| Griffin 2014 | UK | 151 | 2007 to 2011 | Mean age: 46.5 years Sex (M/F): 127/24 |
ORIF within 3 weeks, involving extensile lateral approach, use of neutralisation plate, interfragmentary screws | Early gentle mobilisation with the fitting of a removable splint | Follow‐up: 6, 12, 18, 24 months Primary outcome: Kerr‐Atkin's score |
| Hussain 2022 | Pakistan | 32 | 2021 to 2022 | Mean age: surgical group 40 years; non‐surgical group 42 years Sex (M/F): 21/11 |
Surgery within 2 weeks of injury. Spinal, general, or epidural anaesthetic. Extensile lateral approach with single lateral plate. Stayed in hospital with limb in short leg non‐weight‐bearing splint. Weight‐bearing not authorised until 10 weeks postoperatively. Early subtalar RoM activities out of splint | Closed reduction was tried; short leg casts applied for 6 weeks and RoM exercises performed. In the case of swelling, temporary splint applied with a short leg POP back slab, suggested elevation, and anti‐inflammatory and analgesic medications | Follow‐up: 12 months Primary outcome: Modified Rowe’s score |
| Kamath 2021 | India | 55 participants (61 fractures) | Not reported | Mean age: surgical group 34.9 years; non‐surgical group 35 Sex (M/F): 55/0 |
Different surgical options: percutaneous reduction and fixation with Essex‐Lopresti manoeuvre/cannulated cancellous screws/K‐wires; or ORIF with plates/cannulated cancellous screws/K‐wires; splints with non‐weight‐bearing mobilisation for up to 4 weeks | Below‐knee cast and non‐weight‐bearing crutch walking for 6 weeks. Cast removed after 6 weeks. | Follow‐up: 12 months Primary outcome: Modified Rowe's score |
| Kulkarni 2015 | India | 29 (30 participants) | 2012 to 2014 | Mean age: 32.5 years Sex (M/F): 20/10 |
ORIF with calcaneal plates, and K‐wires 7 to 10 days after injury to allow local swelling to subside. The limb was then immobilised with plaster back slab for 4 to 5 weeks. | Below‐knee plaster cast once swelling had subsided, with partial weight‐bearing 7 to 8 weeks | Follow‐up: 12 months Primary outcome: Creighton‐Nebraska Score |
| Nouraei 2011 | Iran | 72 | 1998 to 2009 | Mean age: 49 years Sex: not reported |
ORIF with reconstruction plate and screw fixation Period of non‐weight‐bearing for 6 to 10 weeks after surgery. States that 2 different surgical procedures were used: 17 participants had Essex‐Lopresti technique (uses fluoroscopy x‐ray, closed reduction, internal fixation with pins and cast immobilisation); 14 participants had open reduction with lateral calcaneal approach and fixation with a reconstruction plate |
Splinting, ice pack, elevation, closed reduction and cast immobilisation. Ambulation after 3 days with crutches. Cast removed after 6 weeks. | Follow‐up: clinical examination at 2 weeks, 1, 3, 6 months, and 1 year. Questionnaire follow‐up at 6 months Outcomes: pain, swelling, limitation of activity, shoe‐wearing difficulty, range of motion, osteoarthritis, patient satisfaction |
| Pandey 2018 | Nepal | 44 | 2014 to 2015 | Mean age: 40.6 years Sex: not reported |
Closed reduction and percutaneous screw fixation | Below‐knee casting. No information provided on rehabilitation. | Follow‐up: 2 weeks (operative group only), 8 weeks, 3 months and 6 months Outcomes: function, (AOFAS), pain (VAS), Böhler's angle, heel height/width, complications and bone union |
| Parmar 1993 | UK | 80 (but only reported 56) | 1985 to 1992 | Mean age: 48.6 years Sex (M/F): surgical group 21/4; non‐surgical group 27/4 |
Open lateral reduction and K‐wire fixation of the posterior subtalar joint. Postoperative immobilisation in a plaster cast for 6 weeks All participants remained non‐weight‐bearing for 6 to 8 weeks, then gradual weight‐bearing started. |
Closed mobilisation of the hindfoot. Elevation and ice for 5 to 7 days, with movement encouraged as pain allowed, non‐weight‐bearing for 6 to 8 weeks All participants remained non‐weight‐bearing for 6 to 8 weeks, then gradual weight‐bearing started. |
Follow‐up: 12 and 24 months Outcomes: pain, use of analgesia, site/pattern of pain, sural nerve symptoms, walking difficulty, employment, recreation level, shoe wear, heel width, recovery plateau reached, compensation pending, no or mild problems |
| Sharma 2011 | India | 30 | 2003 to 2006 | Mean age (range): surgical group 28.1 (18 to 46) years; non‐surgical group 29.2 (25 to 60) years Sex (M/F): 21/9 |
ORIF after swelling had settled, involving extensile lateral approach, use of 3.5‐millimetre reconstruction plate, interfragmentary screws. Non‐weight‐bearing for 8 weeks | Limb elevated for 7 to 10 days with ice to control swelling. Non‐weight‐bearing for 8 weeks | Follow‐up: 12 weeks, 1 and 2 years Primary outcome: Kerr‐Atkin's score, function (AOFAS) |
| Thordarson 1996 | USA | 30 | Not reported | Mean age (range): surgical group 35 (23 to 57) years; non‐surgical group 36 (24 to 47) years Sex (M/F): surgical 12/3; non‐surgical: 9/2 |
Open reduction through an L‐shaped lateral approach, rigid fixation with contoured reconstruction plate and screws (first half of study) or calcaneal Y plate (second half of study). Early RoM exercises from day 3 postoperatively. Non‐weight‐bearing for 10 weeks, then partial weight‐bearing, then full weight‐bearing at 12 weeks postoperatively | Ice, elevation, and bulky Jones bandage dressing until oedema had improved, then fitted with a removable posterior splint. Followed by early range of motion exercises. Non‐weight‐bearing for 8 weeks | Follow‐up: surgical group: mean 17 months, range 11 to 25 months; conservative group: mean 14 months, range 9 to 23 months Outcome: composite outcome index (AOFAS) |
aTotal number of randomised participants was 1151. However, of these, Kulkarni 2015 randomised by fracture; Nouraei 2011 included 72 but reported on 61 participants; and Parmar 1993 randomised 80 but reported on 56 participants.
AOFAS: American Orthopaedic Foot and Ankle Society; K‐wires: Kirschner wires; M/F: male/female; ORIF: open reduction and internal fixation; POP: plaster of paris; PTB: patella tendon bearing; RoM: range of movement; SF‐36: 36‐Item Short Form Health Survey; VAS: visual analogue scale.
Appendix 3. Data reported using additional measurement tools
| Outcome | Study ID | Measurement tool and time point | Surgical; n | Conservative; n | Effect estimatea |
| Function in the long term | Agren 2013 | Composite VAS score for pain and function (0 to 100; higher scores indicate better outcome); participant‐measured scores At 12 months |
Mean (SD): 56.9 (± 26.4); 39 | Mean (SD): 54.8 (± 23.7); 37 | MD 2.10, 95% CI −9.17 to 13.37 Favours surgical treatment Analysis 2.1 |
| Function in the long term | Agren 2013 | OMAS score (0 to 100; higher scores indicate better outcome) At 12 months |
Mean (SD): 62.2 (± 24.2); 39 | Mean (SD): 60.9 (± 22.8); 37 | MD 1.30, 95% CI −9.27 to 11.87 Favours surgical treatment Analysis 2.1 |
| Function in the long term | Griffin 2014 | Kerr‐Atkins score (0 to 100; higher scores indicate better outcome) At 24 months |
Mean (SD): 69.8 (± 21.8); 69 | Mean (SD): 65.7 (± 23.7); 74 | MD 4.10, 95% CI −3.36 to 11.56 Favours surgical treatment Analysis 2.1 |
| Function in the long term | Kamath 2021 | MRS (0 to 100; higher scores indicate better outcome) At 1 year |
Mean (SD): 74.783 (± 11.229); 30 | Mean (SD): 57.368 (± 7.335); 31 | MD 17.41, 95% CI 12.64 to 22.19; 61 fractures (6 bilateral) Favours surgical treatment Analysis 2.1 |
| Function in the long term | Kamath 2021 | VASb (lower scores indicate better outcome; inverted in analysis) At 1 year |
Mean (SD): 3.348 (± 1.369); 30 | Mean (SD): 4.944 (± 0.802); 31 | MD 1.60, 95% CI 1.03 to 2.16; 61 fractures (6 bilateral) Favours surgical treatment Analysis 2.1 |
| Function in the long term | Pandey 2018 | VAS: combined pain and function measure (0 to 100; higher scores indicate better outcome) At 6 months |
Mean (SD): 56.73 (± 6.61); 22 | Mean (SD): 54 (± 5.19); 20 | MD 2.73, 95% CI −0.85 to 6.31 Favours surgical treatment Analysis 2.1 |
| Chronic pain | Agren 2013 | VAS during weight‐bearing At 1 year |
Mean (SD): 2.3 (± 2.1); 39 | Mean (SD): 2.6 (± 1.6); 37 | MD −0.30, 95% CI −1.14 to 0.54 Favours surgical treatment Analysis 2.2 |
| HRQoL | Agren 2013 | SF‐36 mental health score At 1 year |
Mean (SD): 52.5 (± 23.3); 39 | Mean (SD): 50.5 (± 21.9); 37 | MD 2.00, 95% CI −8.16 to 12.16 Favours surgical treatment Analysis 2.3 |
| HRQoL | Griffin 2014 | EQ‐5D At 2 years |
Mean (SD): 0.72 (± 0.22); 59 | Mean (SD): 0.66 (± 0.27); 62 | MD 0.06, 95% CI −0.03 to 0.15 Favour surgical treatment Analysis 2.3 |
| HRQoL | Griffin 2014 | SF‐36 mental health score At 2 years |
Mean (SD): 53.4 (± 11.4); 54 | Mean (SD): 53.6 (± 12.3); 62 | MD −0.20, 95% CI −4.51 to 4.11 Favours non‐surgical treatment Analysis 2.3 |
| Return to work | Parmar 1993 | Return to same work At 1 year |
Events: 19; 25 | Events: 21; 31 | RR 1.12, 95% CI 0.81 to 1.56 Favours surgical treatment Analysis 2.4 |
| Return to work | Thordarson 1996 | Return to same work Within 2 years |
Events: 12; 15 | Events: 4; 11 | RR 2.20, 95% CI 0.97 to 5.00 Favours surgical treatment Analysis 2.4 |
| Return to leisure activity | Parmar 1993 | Return to previous recreation level At 1 year |
Events: 16; 25 | Events: 23; 31 | RR 0.86, 95% CI 0.60 to 1.24 Favours non‐surgical treatment Analysis 2.4 |
| Return to leisure activity | Thordarson 1996 | No limitations on daily or recreational activities Within 2 years |
Events: 13; 15 | Events: 5; 11 | RR 1.91, 95% CI 0.97 to 3.75 Favours surgical treatment Analysis 2.4 |
aCalculated with the calculator in Review Manager 5 (Review Manager 2020). bThe outcome associated with VAS was ambiguous in the study report; we judged that it was used in this study as a measure of function. Range of scores was also not reported.
CI: confidence interval; HRQoL: health‐related quality of life; MD: mean difference; MRS: modified Rowe's score; n: number of participants; OMAS: Olerud‐Molander Ankle Score; RR: risk ratio; SD: standard deviation; SF‐36: 36‐Item Short Form Health Survey; VAS: visual analogue score
Appendix 4. Not included in analysis because data were insufficient to calculate effect estimates
| Outcome | Study ID | Measurement tool and time point | Surgical; n | Conservative; n | Between‐group difference |
| Function in the long term | Buckley 2002 | Composite VAS score (0 to 100; higher scores indicate better outcome); participant‐measured scores At average of 3 years (range 2 to 8 years) |
Mean: 68.6; 206a | Mean: 64.3; 218a | P = 0.12 |
| Function in the long term | Sharma 2011 | Kerr‐Atkins score (0 to 100; higher scores indicate better outcome) At 24 months |
Mean: 66.12; 15 | Mean: 60.22; 15 | NR |
| Function in the long term | Sharma 2011 | AOFAS score (0 to 100; higher scores indicate better outcome) At 24 months |
Mean: 72.13; 15 | Mean: 71.16; 15 | NR |
| Function in the long term | Sharma 2011 | VAS (range of scores and direction of scale not reported) At 24 months |
Mean: 1.83; 15 | Mean: 2.12; 15 | NR |
| HRQoL | Buckley 2002 | SF‐36 total score (0 to 100; higher scores indicate better outcome) At 2 to 8 years |
Mean: 68.7; 206a | Mean: 64.7; 218a | P = 0.13 |
| Böhler’s angle | Hussain 2022 | At 12 months | Postoperative: 29.22° Opposite normal side: 31.01°; 16 |
Postoperative: 11.21° Opposite normal side: 25°; 16 |
P = 0.001 |
| Böhler's angle | Kulkarni 2015 | At 12 months | Mean: 23.66°; 15 | Mean: 15.2º; 15 | P < 0.001 |
| Böhler's angle | Thordarson 1996 | Within 2 years | Mean: 26°; 15 | Mean: 8°; 11 | P = 0.001 |
| Gissane's angle | Hussain 2022 | At 12 months | Postoperative: 104.27° Opposite normal side: 102.08°; 16 |
Postoperative: 123.73° Opposite normal side: 106.58°; 16 |
P < 0.001 |
| Gissane's angle | Kulkarni 2015 | At 12 months | Mean: 137.6°; 15 | Mean: 160°; 15 | P < 0.001 |
aThe number of participants is not clearly reported in this study. Whilst these data are described as "complete case" data for all randomised participants, 115 participants were lost to follow‐up, and these data may be imputed for lost participants.
AOFAS: American Orthopaedic Foot and Ankle Society; HRQoL: health‐related quality of life; n: number of participants; NR: not reported; VAS: visual analogue scale
Appendix 5. Within‐study subgroup data: function measured using Kerr‐Atkins score
| Kerr‐Atkins scores inGriffin 2014(0 to 100; higher scores indicate better function) | ||
| Subgroup data | Surgical group; n | Non‐surgical group |
| Sanders Type II | Mean (SD): 74.3 (± 20.4); 33 | Mean (SD): 70.3 (± 24.9); 34 |
| Sanders Type III or IV | Mean (SD): 66.1 (± 21.9); 34 | Mean (SD): 63.0 (± 22.2); 38 |
n: number of participants per group; SD: standard deviation
Data and analyses
Comparison 1. Surgical versus non‐surgical management.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Function in the long term; measured using AOFAS | 5 | 319 | Mean Difference (IV, Random, 95% CI) | 6.58 [1.04, 12.12] |
| 1.1.1 Open reduction and internal fixation | 4 | 277 | Mean Difference (IV, Random, 95% CI) | 8.85 [1.03, 16.68] |
| 1.1.2 Minimally‐invasive surgery | 1 | 42 | Mean Difference (IV, Random, 95% CI) | 1.95 [‐0.71, 4.61] |
| 1.2 Function in the long term; using measurement tools other than AOFAS score | 4 | 322 | Std. Mean Difference (IV, Random, 95% CI) | 0.60 [‐0.10, 1.30] |
| 1.2.1 Open reduction and internal fixation | 3 | 280 | Std. Mean Difference (IV, Random, 95% CI) | 0.65 [‐0.27, 1.58] |
| 1.2.2 Minimally invasive surgery | 1 | 42 | Std. Mean Difference (IV, Random, 95% CI) | 0.45 [‐0.17, 1.06] |
| 1.3 Function in the long term; number of people with scores of excellent, good, or satisfactory/fair | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.3.1 Excellent | 3 | 88 | Risk Ratio (M‐H, Random, 95% CI) | 3.21 [1.14, 9.04] |
| 1.3.2 Good | 3 | 88 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.75, 1.71] |
| 1.3.3 Satisfactory/Fair | 3 | 88 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.23, 1.66] |
| 1.4 Chronic pain; number of people with pain | 4 | 175 | Risk Ratio (IV, Random, 95% CI) | 0.56 [0.37, 0.84] |
| 1.5 Chronic pain; measured using VAS | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.6 HRQoL in the long term; measured using SF‐36 (PCS) | 2 | 192 | Mean Difference (IV, Random, 95% CI) | 6.49 [2.49, 10.48] |
| 1.7 Return to normal activity (work) | 5 | 250 | Risk Ratio (IV, Random, 95% CI) | 1.26 [0.94, 1.68] |
| 1.8 Postoperative complications requiring unplanned return to theatre | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.8.1 Amputation | 1 | 82 | Risk Ratio (M‐H, Random, 95% CI) | 2.86 [0.12, 68.23] |
| 1.8.2 Subtalar arthrodesis | 3 | 657 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.09, 1.53] |
| 1.8.3 Implant removal | 3 | 657 | Risk Ratio (M‐H, Random, 95% CI) | 6.98 [1.23, 39.52] |
| 1.8.4 Deep infection | 1 | 424 | Risk Ratio (M‐H, Random, 95% CI) | 24.33 [1.44, 410.30] |
| 1.8.5 Wound debridement | 1 | 151 | Risk Ratio (M‐H, Random, 95% CI) | 5.34 [0.26, 109.35] |
| 1.9 Postoperative complications not requiring return to theatre | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.9.1 Superficial infection | 7 | 847 | Risk Ratio (M‐H, Random, 95% CI) | 12.04 [3.68, 39.33] |
| 1.9.2 Wound breakdown/dehiscence | 2 | 91 | Risk Ratio (M‐H, Random, 95% CI) | 4.84 [0.57, 40.68] |
| 1.10 Ability to wear usual footwear | 4 | 219 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.88, 1.87] |
| 1.11 Radiological measurements: Böhler angle | 2 | 72 | Mean Difference (IV, Random, 95% CI) | 7.35 [3.81, 10.89] |
Comparison 2. Surgical versus non‐surgical management: data reported using additional measurement tools.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Function in the long term | 4 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.2 Chronic pain | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.3 HRQoL in the long term | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.4 Return to normal activity | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Agren 2013.
| Study characteristics | ||
| Methods | RCT, parallel design, 2‐armed trial | |
| Participants |
Total number of randomised participants: 82 Source population: multicentre, 5 trauma centres in Stockholm, Sweden Inclusion criteria: intra‐articular fracture, displaced > 2 mm, > 18 years of age Exclusion criteria: peripheral neurovascular disease, an open fracture, uncontrolled diabetes mellitus and medical contraindications to surgery Baseline characteristics for surgical group
Baseline characteristics for non‐surgical group
|
|
| Interventions |
Surgical group
Non‐surgical group
|
|
| Outcomes |
Timing of assessments: 12 months, and 8 to 12 years Outcomes reported in this review: composite pain and function score (VAS 0 to 100), quality of life (SF‐36). Secondary outcomes: pain, function, and alignment (AOFAS, 0‐to‐100 scale), pain and function (Olerud‐Molander score 0 to 100), pain at rest (VAS 0 to 10), pain during weight‐bearing (VAS 0 to 10), ability to wear shoes comfortably, postoperative complications (subtalar arthritis, subtalar arthrodesis (fusion)). Full data on radiological measurements (Böhler's angle) by treatment group not reported. |
|
| Notes |
Study dates: 1994 to 1998 Funding sources: not reported Declarations of interest: not reported Notes: contact with study authors was not undertaken because key data were extracted from publications |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation was used with an equal number of participants in each group. Randomisation took place after verifying the fracture by CT scan but before classification of the fracture. |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes contained assignment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to nature of intervention, treating surgeons and participants were not blinded to allocation |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The treating surgeon performed clinical follow‐up within the first 6 months. At 1, 8 and 12 years, follow‐up was undertaken by 1 of 2 "unbiased" surgeons who had not been involved in the treatment. However, because study authors did not describe methods to disguise scars, we judged that detection bias was still possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 76/82 (92%) follow up at 1 year |
| Selective reporting (reporting bias) | Unclear risk | No protocol paper available, and the study was registered retrospectively (NCT01615744; first received 11 June 2012). It is not feasible to use the clinical trials documents to effectively assess risk of selective outcome reporting bias. |
| Other bias | Low risk | We identified no other sources of bias |
Buckley 2002.
| Study characteristics | ||
| Methods | RCT, parallel design, 2‐armed trial | |
| Participants |
Total number of randomised participants: 424 (471 fractures) Source population: initially 7 centres (14 surgeons) in Canada recruited, but data from only 4 (6 surgeons) were included as they complied with the requirement of a minimum of 20 participants followed up for at least 2 years from each participating surgeon Inclusion criteria: intra‐articular fracture, displaced > 2 mm on CT scan Exclusion criteria: medical contraindication to surgery; previous calcaneal injury; coexistent foot injury; head injury; injury that had occurred more than 14 days before presentation Overall baseline characteristics
Baseline characteristics for surgical group
Baseline characteristics for non‐surgical group
|
|
| Interventions |
Surgical group
Non‐surgical group
*Flow of participant information is not clearly reported in the text. Although 64 participants were lost to follow‐up, complication data for these participants were tracked by nurses. Study authors report "complete case" data for 242 participants for function and HRQoL, but they do not describe how they accounted for lost participants in these data. |
|
| Outcomes |
Timing of assessments: 2 to 4 weeks, 6, 12, 26, 52 weeks, and 2 (minimum) to 8 years (maximum) Outcomes reported in review: quality of life (SF‐36), disease‐specific scale (VAS) (primary outcomes); Böhler's angle, postoperative complications (treatment failure/subtalar arthrodesis) Note: we did not report data for Böhler angle because we could not determine whether the data were between time points (before and after surgery) or between intervention groups (surgical and non‐surgical) or were mean postoperative data. |
|
| Notes |
Study dates: April 1991 to December 1997 Funding sources: not reported Declarations of interest: not reported Notes:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number tables used after eligible for entry into study. Random numbers generated at the site of the Principal Investigator. |
| Allocation concealment (selection bias) | Low risk | Central administrative site sent random number assignments to study centres. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to nature of intervention, treating surgeons and participants were not blinded to the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessment by postal questionnaire sent to study participants (primary outcome of quality of life). Assessment of CT scans to assess quality of reduction was conducted by lead study author who was not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 309/424 (73%) followed up for minimum of 2 years, maximum of 8 years (mean 3 years). Response rate to questionnaire is not clearly reported. A later publication by O'Brien 2004 stated that 319 participants completed the RCT at 2 to 8 years but reported different SF‐36 values from the main paper (Buckley 2002). We also noted exclusion of data from 3 centres. The derivation of the data for the primary outcomes so that it is from the 'complete study group' is not shown. Study report states that intention‐to‐treat analysis was conducted. However, 44 cases (44/424 (10%)) who went on to have subtalar fusion were excluded from analyses. |
| Selective reporting (reporting bias) | Unclear risk | No protocol or clinical trials registration. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias |
Chrintz 1993.
| Study characteristics | ||
| Methods | RCT, parallel design, 2‐armed trial | |
| Participants |
Total number of randomised participants: 68 Source population: single centre; Copenhagen area, Denmark Inclusion criteria: only unilateral fractures included Exclusion criteria: no previous calcaneus fracture No baseline characteristics reported. |
|
| Interventions |
Surgical group
Non‐surgical group
|
|
| Outcomes |
Timing of assessments: 6, 12, and a median of 80 weeks Outcomes reported in study: pain, working capacity, activity in leisure time, working ability, tenderness on palpation or movement of the joints, duration of hospitalisation. Radiographic assessment of subtalar arthrosis. However, data for only one of these outcomes were reported: radiography outcome at a median of 80 weeks after treatment. |
|
| Notes |
Study dates: 1977 to 1979 Funding sources: not reported Declarations of interest: not reported Notes:
|
|
Griffin 2014.
| Study characteristics | ||
| Methods | RCT, parallel design, pragmatic, 2‐armed trial | |
| Participants |
Total number of randomised participants: 151 Source population: multicentre; participants recruited from 22 UK hospitals; all centres were regional referral centres for calcaneal fractures. Surgeons were "recognised as specialists in the treatment of these injuries". Inclusion criteria: intra‐articular fracture, displaced > 2 mm, > 18 years of age Exclusion criteria: gross deformity of the hindfoot, other serious leg injuries sufficient to affect the outcome at 2 years, not fit for surgery, peripheral vascular disease Overall baseline characteristics
Baseline characteristics for surgical group
Baseline characteristics for non‐surgical group
|
|
| Interventions |
Surgical group
Non‐surgical group
|
|
| Outcomes |
Timing of assessments: 6, 12, 18, 24 months Outcomes reported in review: Kerr‐Atkin's score, function (AOFAS), quality of life (SF‐36 and EQ‐5D), postoperative complications, reoperation (removal of metal implants and subtalar arthrodesis), return to work Other outcomes: heel width, walking speed, movement (dorsiflexion, plantar, eversion, inversion) and gait symmetry indices Note: study conducted subgroup analysis according to Sanders classification and sex |
|
| Notes |
Study dates: 2007 to 2009 Funding sources: Arthritis Research UK Declarations of interest: study authors declared no conflicts of interest Notes: Dr Nicholas Parsons and Mr Damian Griffin were contacted (JB) by email 23 October 2015 for clarification regarding allocation concealment. The lead senior statistician on the study (NP) clarified that they had used a central randomisation service by telephone and confirmed allocation concealment. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Participants were randomised 1:1 to receive operative and non‐operative treatment using a minimisation algorithm". Comment: randomisation was by participant and individuals with bilateral fractures received the same treatment on both sides. Although sequence generation was adequate for this study, we note that a large number of eligible participants did not consent to study participation because of a preference for one treatment over another. We could not rule out the possibility that participants were provided with information from surgeons about their fracture type that influenced their decision to consent. |
| Allocation concealment (selection bias) | Low risk | Secure telephone randomisation service used managed by an independent clinical trials unit (York, UK). |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to nature of intervention, treating surgeons and participants were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participant‐reported outcomes were captured by postal questionnaire and because participants were aware of group allocation, detection bias for these outcomes was high risk. The assessment of clinical outcomes at 2 years was performed by a physiotherapist who was unaware of treatment allocation. During clinical examination, thin socks were worn to obscure surgical scars and maintain blinding of the physiotherapist. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 143/151 (95%) patients completed follow‐up at 2 years. Reasons for no follow‐up included death (n = 3), participant withdrawal (n = 2) and lost to follow up (n = 3); these losses were balanced between intervention groups. |
| Selective reporting (reporting bias) | Low risk | Study was prospectively registered in a clinical trials register (ISRCTN37188541). Reported primary and secondary outcomes were consistent with those in the clinical trials register documents. |
| Other bias | Low risk | We identified no other sources of bias |
Hussain 2022.
| Study characteristics | ||
| Methods | RCT, parallel design, 2‐armed trial | |
| Participants |
Total number of randomised participants: 32 Source population: single centre, Pakistan Inclusion criteria: 19 to 67 years of age, Sanders Type II and III closed fractures < 3 weeks old, had normal bipedal gait before injury Exclusion criteria: calcaneal injuries connected with spinal injuries, pathological fractures, peripheral vasculopathy, or any medical contraindication to surgery, no informed consent Overall baseline characteristics
Baseline characteristics for surgical group
Baseline characteristics for non‐surgical group
|
|
| Interventions |
Surgical group
Non‐surgical group
|
|
| Outcomes |
Timing of assessments: 1 year Outcomes reported in review: pain from exercise (n,%), pain from daily activities (n,%), pain from weight‐bearing (n,%); RoM; restriction from work; Böhler's angle, Gissane angle; function (using MRS: excellent/good/satisfactory/poor); adverse events (delayed wound healing, subtalar arthritis, calcaneal malunion, peroneal tendonitis, heel exostosis) Other outcomes: gait; radiological union time |
|
| Notes |
Study dates: January 2021 to January 2022 Funding sources: none Declarations of interest: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number tables |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to nature of intervention, treating surgeons and participants were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No details of blinding of outcome assessors, and we assume there was no blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | No protocol or clinical trials registration reported. It is not feasible to effectively assess selective outcome reporting without these documents |
| Other bias | Low risk | We identified no other sources of bias |
Kamath 2021.
| Study characteristics | ||
| Methods | Q‐RCT, 2‐armed trial | |
| Participants |
Total number of randomised participants: 55 participants (61 fractures) Source population: single centre, India Inclusion criteria: closed displaced intra‐articular calcaneal fractures, aged 18 to 65 years of age Exclusion criteria: undisplaced fracture (Sanders Type I), extra‐articular fractures, comorbidity such as diabetes, associated spine fractures with neurological deficits, open fractures Overall baseline characteristics
Baseline characteristics for surgical group
Baseline characteristics for non‐surgical group
|
|
| Interventions |
Surgical group
Non‐surgical group
|
|
| Outcomes |
Timing of assessments: 1 year Outcomes reported in review: MRS, VAS, AOFAS, participant work outcome/restriction, complications (gait abnormality, stiffness, heel pain, plaster sores, wound infection, wound dehiscence), Böhler's angle |
|
| Notes |
Study dates: not reported Funding sources: not reported Declarations of interest: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomisation by alternate allocation |
| Allocation concealment (selection bias) | High risk | Alternate allocation and therefore concealment is not possible |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to nature of intervention, treating surgeons and participants were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | It is not possible to blind participants (for any PROMs). Study authors do not report if investigators were blinded to treatment allocation and we therefore assumed that they were not |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses reported |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report whether there is a protocol or clinical trials registration. It is not feasible to effectively assess risk of selective outcome reporting bias without these documents |
| Other bias | Low risk | We identified no other sources of bias |
Kulkarni 2015.
| Study characteristics | ||
| Methods | RCT, parallel design, 2‐armed trial | |
| Participants |
Total number of randomised participants: 29 (30 fractures) Source population: single centre, India Inclusion criteria: adults 18 to 50 years of age Exclusion criteria: open fractures, extra‐articular fractures, and fractures presenting after 2 weeks Overall baseline characteristics
Baseline characteristics for surgical group
Baseline characteristics for non‐surgical group
|
|
| Interventions |
Surgical group
Non‐surgical group
|
|
| Outcomes |
Timing of assessments: 12 months Outcomes reported in review: Creighton‐Nebraska Score, Böhler's angle, Gissane's angle, wound dehiscence Other outcomes: heel varus angle, sural nerve injury |
|
| Notes |
Study dates: June 2012 to September 2014 Funding sources: not reported Declarations of interest: "all authors have none to declare" Notes: study authors were contacted for further information, but no response was received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "One chit was picked whenever the treatment modality of a new patient was to be was decided" Comment: as this was comparable to the drawing of lots, we judged this method of randomisation to be adequate |
| Allocation concealment (selection bias) | High risk | High risk of bias due to allocation method and potential to predict allocation from number of remaining chits |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to nature of intervention, treating surgeons and participants were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No information given on outcome data collection or who conducted clinical assessments or undertook interpretation of x‐rays, and we therefore assumed that no blinding took place |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | No protocol or clinical trials registration. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | Data reported by fracture rather than by participant. However because there was only 1 bilateral fracture, we did not expect this to impact the findings. |
Nouraei 2011.
| Study characteristics | ||
| Methods | RCT, parallel design, 2‐armed trial | |
| Participants |
Total number of randomised participants: 72 Source population: single centre, Iran Inclusion criteria: displaced intra‐articular fracture. States in text that 11 participants had bilateral fracture, but no description about allocation to intervention Exclusion criteria: states that "reasons that patients were not selected for surgery were as follows: participant disagreement with surgery, open fractures (not suitable for open reduction internal fixation), combined injuries (head trauma, cardiovascular disorders, severe osteoporosis...), also severe comminution" Overall baseline characteristics
Baseline characteristics for surgical group (for participants included in analysis)
Baseline characteristics for non‐surgical group (for participants included in analysis)
|
|
| Interventions |
Surgical group
Non‐surgical group
*Total number randomised is 72. However, 11 participants were lost to follow‐up, and the groups to which they originally had been allocated are unknown. |
|
| Outcomes |
Timing of assessments: clinical examination at 2 weeks, 1, 3, 6 months, and 1 year. Questionnaire follow‐up at 6 months Outcomes reported in this review: pain on walking (n,%), ability to wear footwear, complications. Study authors state that function was recorded using Kerr‐Atkins score, but results for this outcome were not reported. Other outcomes: swelling, limitation of activity, RoM, osteoarthritis, patient satisfaction |
|
| Notes |
Study dates: December 1998 and January 2009 Funding sources: not reported Declarations of interest: "authors have no conflict of interests" Notes: study authors were contacted for further information, but no reply was received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | No details provided. States that "consecutive participants were randomly allocated to surgical and non surgical groups". We noted that participants were excluded from the surgical group if they had comminuted fractures, but these fracture types were not excluded from the non‐surgical group (4 of 30 participants in the non‐surgical group had comminuted fractures). We judged this to indicate possible manipulation of the randomisation method. |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to nature of intervention, treating surgeons and participants were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Participant‐reported outcomes were collected by questionnaire at 6 months, and participants were aware of their treatment allocation. No information given on clinical outcome data collection or interpretation of x‐rays, and we assumed that no blinding took place |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 11 (15%) participants dropped after hospital discharge but these are reported as exclusions not as losses to follow‐up. Actual number randomised to each intervention arm is not reported. |
| Selective reporting (reporting bias) | Unclear risk | No protocol or clinical trials registration. It is not feasible to effectively assess risk of selective reporting bias without these documents. Patient satisfaction is included as an outcome in the Methods section but is not reported in the Results section of the study report. |
| Other bias | Low risk | We identified no other sources of bias |
Pandey 2018.
| Study characteristics | ||
| Methods | RCT, parallel design, 2‐armed trial | |
| Participants |
Total number of randomised participants: 44 Source population: single centre, Nepal Inclusion criteria: not reported. The authors state that "Patients presenting with calcaneal fracture were screened for eligibility by clinico radiological evaluation". Exclusion criteria: not reported Baseline characteristics for surgical group
Baseline characteristics for non‐surgical group
|
|
| Interventions |
Surgical group
Non‐surgical group
|
|
| Outcomes |
Timing of assessments: 2 weeks (operative group only), 8 weeks, 3 months and 6 months Outcomes reported in review: AOFAS, VAS pain and function score, Böhler's angle, complications Other outcomes: calcaneal height and width, bone union |
|
| Notes |
Study dates: March 2014 to May 2015 Funding sources: not reported Declarations of interest: not reported Notes: no data presented for functional scores at baseline. No information about ethical approval, and journal is not indexed in the main biomedical databases. Study authors were contacted for more information, but no reply was received. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomized into two groups [...] using computer generated random number selection." |
| Allocation concealment (selection bias) | Unclear risk | No details provided on who generated numbers or who had access. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to nature of intervention, treating surgeons and participants were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No information given on outcome data collection, or who conducted clinical assessments or undertook interpretation of x‐rays. We therefore judged that no blinding took place |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 42/44 (95%) followed up for 6 months. |
| Selective reporting (reporting bias) | Unclear risk | No protocol or clinical trials registration. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias |
Parmar 1993.
| Study characteristics | ||
| Methods | Q‐RCT, parallel design, 2‐armed trial | |
| Participants |
Total number of randomised participants: 80. No explanation about discrepancy in 24 missing participants, as sample drops from 80 to 56 at follow‐up Source population: single centre, UK Inclusion criteria: all participants had x‐ray and CT scans preoperatively; displaced intra‐articular fractures entered into trial Exclusion criteria: undisplaced and extra‐articular fractures were treated conservatively, bilateral fractures except when extra‐articular fracture on 1 side was of no clinical significance and allowed full weight‐bearing after initial bed rest, and those "who could not be randomised" (no reasons given) Overall baseline characteristics
Baseline characteristics for surgical group
Baseline characteristics for non‐surgical group
|
|
| Interventions |
Surgical group
Non‐surgical group
*80 participants randomised overall, but this is not described by group. No explanation about discrepancy in 24 missing participants, as sample drops from 80 to 56 at follow‐up |
|
| Outcomes |
Timing of assessments: 12 and 24 months (main 1993 paper); mean follow‐up 23 months (surgical follow‐up: mean 25.3 months; conservative: 21.6 months); later publication of 15‐year outcomes on subset of 26/56 responding survivors (46%) (Ibrahim 2007) Outcomes reported in review: pain, employment, shoe wear, recreation level Other outcomes: use of analgesia, site/pattern of pain, walking difficulty, heel width, recovery plateau reached, compensation pending, no or mild problems, sural nerve symptoms. At 15 years: multiple outcomes including AOFAS Hindfoot scale, Calcaneal Fracture Scoring system and Foot Function Index and subtalar arthrodesis Note: we did not include 15‐year data for subtalar arthrodesis, as this information was available for only 26 of the original sample, and we judged that attrition was too high to provide reliable data |
|
| Notes |
Study dates: participants recruited from 1985 and 1992 Funding sources: not reported Declarations of interest: not reported Notes: data in Parmar 1993 also reported for undisplaced fractures. Data difficult to interpret for some outcomes at 1 year, partly because data were presented as percentages. We attempted to contact the lead author on a later paper for more information (Ibrahim 2007), but the email address was no longer valid. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quasi‐randomised by year of birth, with odd years entering the operative group and even years the conservative group |
| Allocation concealment (selection bias) | High risk | Allocation predictable if year of birth is known |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to nature of intervention, treating surgeons and participants were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No details provided about blinding of outcome assessors at 1 to 2 years follow‐up and we therefore assumed no blinding took place. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Lack of detail about numbers randomised (n = 80) and data reported at 2 years (n = 56). Previous review contacted authors and were informed that 24 participants were excluded because follow‐up was less than 1 year (30%). Results presented as whole percentages ‐ these often do not correspond to whole numbers, indicating either errors in the calculation of percentages or different denominators. |
| Selective reporting (reporting bias) | Unclear risk | No protocol or clinical trials registration. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias |
Sharma 2011.
| Study characteristics | ||
| Methods | RCT, parallel design, 2‐armed trial | |
| Participants |
Total number of randomised participants: 30 Source population: single centre, India Inclusion criteria: intra‐articular fracture, closed, Sanders Type II only, age between 18 and 60 years Exclusion criteria: any associated injuries or open injury Baseline characteristics for surgical group
Baseline characteristics for non‐surgical group
|
|
| Interventions |
Surgical group
Non‐surgical group
|
|
| Outcomes |
Timing of assessments: 12 weeks, 1 and 2 years Outcomes reported in review: Kerr‐Atkin's score, function (AOFAS), VAS score (combined pain and function score), postoperative Böhler's angle |
|
| Notes |
Study dates: participants recruited between 2003 and 2006 Funding sources: not reported Declarations of interest: study authors report no conflicts of interest Notes:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Authors stated "randomization was based on sealed envelope technique" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to nature of intervention, treating surgeons and participants were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Methods of outcome data collection not clearly described. No information given on clinical outcome data collection or interpretation of x‐rays and CT scans and we therefore assumed that no blinding took place. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No report of numbers lost to follow‐up. We assumed that 100% follow‐up was achieved |
| Selective reporting (reporting bias) | Unclear risk | No protocol or clinical trials registration. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Low risk | We identified no other sources of bias |
Thordarson 1996.
| Study characteristics | ||
| Methods | RCT, parallel design, 2‐armed trial | |
| Participants |
Total number of randomised participants: 30 Source population: single centre, 1 participating surgeon, Los Angeles, CA, USA Inclusion criteria: displaced unilateral intra‐articular fracture (Sanders Type II and III). All had preoperative CT scan. Exclusion criteria: displaced Sanders Type IV, peripheral vascular disease, diabetes, systemic illness, drug or alcohol abuse, psychotics or bilateral injuries, unwilling to participate in study Baseline characteristics for surgical group
Baseline characteristics for non‐surgical group
|
|
| Interventions |
Surgical group
Non‐surgical group
|
|
| Outcomes |
Timing of assessments: overall 9 to 25 months; surgical group: mean 17 months, range 11 to 25 months; conservative group: 14 months, range 9 to 23 months Outcomes reported in this review: composite outcome index (AOFAS), scores ranged from 0 to 100 (pain, daily activity, shoe wear, walking, exercise, and work), Böhler's angle Other outcomes: RoM of subtalar joint, residual displacement of posterior facet |
|
| Notes |
Study dates: not reported Funding sources: not reported Declarations of interest: not reported Notes: although 26/30 participants completed the functional questionnaire, only 11/30 had a physical examination conducted by an independent examiner (8 surgical, 3 conservative) in outpatient clinic at follow‐up. Clinical data are thus based on 11/30 (37%) of the original sample. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated how sequence generated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Drawing a sealed unmarked envelope with type of treatment enclosed within." Comment: insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to nature of intervention, treating surgeons and participants were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Participant‐reported outcomes were collected by questionnaire, and participants were aware of their treatment allocation. Of the 26 participants, 11 had a clinical assessment by an independent examiner but methods used to blind these assessors is not reported. The remainder were assessed by non‐independent clinical examiners. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Total 4/30 (13%) lost to follow‐up by 15 months: 1 surgical and 3 conservative |
| Selective reporting (reporting bias) | Unclear risk | No protocol or clinical trials registration. It is not feasible to effectively assess risk of selective reporting bias without these documents. |
| Other bias | Unclear risk | We noted use of an non‐validated scoring system although questionnaire was found to correlate highly with the validated AOFAS. Change in surgical procedure halfway through the trial, the reason for this is not explained and we could not be certain whether this influenced the findings in the surgical group. |
AOFAS: American Orthopaedic Foot and Ankle Society; BMI: body mass index; CD: compact disc; CT scan: computed tomography scan; K‐wires: Kirschner wires; M/F: male/female; MIS: minimally invasive surgery; MRS: Modified Rowe's Score; n: number of participants; ORIF: open reduction and internal fixation; POP: plaster of paris; PTB: patella tendon bearing; Q‐RCT: quasi‐randomised controlled trial; RCT: randomised controlled trial; RoM: range of motion; SD: standard deviation; SF‐36: 36‐Item Short Form Health Survey; VAS: visual analogue score
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| ACTRN12617001588381 | RCT, target sample size of 110 participants with calcaneal fractures. We excluded this ongoing study because it compared 2 surgical interventions (ORIF and transarticular tibio‐talo‐calcaneal nailing). |
| Aslan 2019 | Plate and screw osteosynthesis versus circular leg cast in 54 participants with intra‐articular calcaneal fractures. Wrong study design (participants were not randomised to groups) |
| IRCT2016051327872N1 | RCT, target sample size of 36 participants with calcaneal fractures. We excluded this study because it compared 2 surgical interventions (conventional and minimally invasive techniques). |
| Kashani 2013 | Participants were recruited from 2 hospitals in Iran and treated surgically or non‐surgically for Sanders Type II fractures. We contacted the study authors, who confirmed that participants were not randomised, therefore we excluded this study from the review. |
| Li 2016 | Study comparing surgical with non‐surgical treatment of the medial process of the calcaneal tuberosity in 18 people. Wrong study design (participants were not randomised to groups) |
| Rajikumar 2017 | Open reduction and locking plate fixation versus conservative management in 20 people with calcaneal fractures. Wrong study design (participants were not randomised to groups) |
| Su 2017 | Surgical versus non‐surgical treatment of Sanders II‐III calcaneal fractures in 60 elderly people. Wrong study design (participants were not randomised to groups) |
ORIF: open reduction and internal fixation; RCT: randomised controlled trial
Characteristics of studies awaiting classification [ordered by study ID]
IRCT2017092720258N62.
| Methods |
Design: RCT, parallel design Setting: Valiasr Hospital, Iran |
| Participants |
Inclusion criteria: people with calcaneal fractures Sanders II and III Exclusion criteria: presence of other fractures, especially at the foot of the same side; bilateral calcaneous fracture; multiple fractures; open fracture; diabetes; rheumatoid arthritis; < 15 years of age; fractures > 3 weeks old Targeted sample size: 40 |
| Interventions |
Intervention arm: open reduction and internal fixation Control group: routine treatment |
| Outcomes | After 1 year: Gissane and Böhler's angles, function (AOFAS), pain (VAS), "conduct previous activities (observation)", complications (wound healing) |
| Notes | Registration was done retrospectively, and the current status states that recruitment is complete. We contacted the study authors (14 February 2019) to clarify what their control group treatment consisted of. However, from the 3 emails sent to the available email addresses, only 1 email was delivered (f.farokhi@arakmu.ac.ir). We received no response. We contacted study authors again (24 Novemeber 2022) to clarify the status of the study. Again, the emails failed to deliver. We await publication of this study to assess its eligibility for this review. |
AOFAS: American Orthopaedic Foot and Ankle Society; RCT: randomised controlled trial; VAS: visual analogue scale
Differences between protocol and review
Changes made to review since last publication
Author team: Sharon Lewis (SL), Michael Pritchard (MP), Josh Solomon (JS), and Xavier Griffin (XG) joined the review author team. Helen Richmond and Bruno Mazuquin left the team.
Title: we edited the title to more clearly state that conservative interventions referred to non‐surgical management.
Types of interventions: we removed the comparison type of 'early or delayed mobilisation', as this was not meaningful for this review.
Types of outcome measures: we reorganised the outcomes into 'critical outcomes' and 'other important outcomes' so that they were more closely aligned with our choice of outcomes for the summary of findings table. In this review update, we did not include objective measures of impairment (e.g. range of movement) or time to union (bone healing), as we consider these to be less important than patient‐measured outcomes such as quality of life. We added time points for measurement of outcomes, with a preference for long‐term assessment up to 24 months.
Data extraction and management: we added additional information to this section of the Methods to include a summary of the information extracted from the included studies.
Data synthesis: we used the random‐effects model (rather than a fixed‐effect model) for all primary meta‐analyses to account for the likely differences between surgery techniques and populations. We evaluated this decision in sensitivity analyses. Some studies used more than one measurement tool to report data. In the current version of the review, we prioritised the pooling of data for the most commonly used tool for each outcome and reported this as our primary analysis in the summary of findings table, Abstract, and Plain language summary.
Subgroup analysis: insufficient information in the included studies precluded subgroup analysis in this review update. We used this update to re‐evaluate which effect modifiers were most likely to influence the review findings. In the current update, we considered only four key subgroups, which are listed in the Methods section.
Sensitivity analysis: we updated the Methods section to provide a more specific list of sensitivity analyses, including the use of effects models in the analyses.
Summary of findings and assessment of certainty of the evidence: we used the GRADE approach to assess the certainty of the evidence in the current update. This approach is consistent with current methodological expectations for Cochrane Reviews.
Contributions of authors
SL (systematic reviewer) screened and identified included studies, extracted study data, interpreted the findings, and drafted the review.
MP (systematic reviewer) screened and identified included studies, extracted study data, interpreted the findings, and drafted the review.
JS (medical student at the time of contribution) screened and identified included studies, extracted study data.
XG (content expert, trauma and orthopaedics) interpreted the findings, reviewed and approved the final review.
JB (guarantor, content expert, trauma and orthopaedics) screened and identified included studies, extracted study data, interpreted the findings, reviewed and approved the final review, and is guarantor of the content.
The original review was conceived by Alisdair Sutherland, who is no longer an author on this review. For all other previous contributions, see Bruce 2013.
Sources of support
Internal sources
-
University of Warwick, UK
Warwick Clinical Trials Unit
-
Queen Mary University of London, UK
Support to Cochrane Bone, Joint and Muscle Trauma Group up to 31 March 2023
External sources
-
NIHR Cochrane Infrastructure funding, UK
Awarded to the Cochrane Bone, Joint and Muscle Trauma Group up to 31 March 2023
-
NIHR, UK
This work acknowledges the support of the National Institute for Health Research Barts Biomedical Research Centre (NIHR203330)
Declarations of interest
SL (review author and Deputy Co‐ordinating Editor of the Bone, Joint and Muscle Trauma Group) has no known conflicts of interest. SL was not involved in the editorial process.
MP has no known conflicts of interest.
JS has no known conflicts of interest.
XG (Co‐ordinating Editor of the Cochrane Bone, Joint and Muscle Trauma Group) is funded by an National Institute for Health and Care Research (NIHR) Clinician Scientist Grant. Further funding from industry and charitable grants are and have been made available to his institution. All decisions relating to the design, conduct, analysis, write‐up, and publication of research are independent of these funders. He has ongoing expert consultancy with several companies; none involve the development of any implant for use in calcaneal fracture care. XG was not involved in the editorial process.
JB (guarantor) is Editor of the Cochrane Wounds Group. JB receives National Institute for Health and Care Research (NIHR) Health Technology Assessment and Programme Grants for Applied Research funding for non‐wound‐related rehabilitation research studies (2020 to present). JB is supported by NIHR Research Capability Funding via University Hospitals Coventry and Warwickshire NHS Trust. JB is a member of the NIHR Research for Patient Benefit Panel West Midland, UK.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Agren 2013 {published data only}
- NCT01615744. Surgical vs conservative treatment of displaced intra-articular calcaneal fractures: a prospective RCT [Surgical vs. conservative treatment of displaced intra-articular calcaneal fractures: a prospective, randomized, controlled multicenter trial]. clinicaltrials.gov/show/NCT01615744 (first received 11 June 2012).
- Younger A. A calcaneal fracture study illustrates a need for better statistical methods for orthopaedic outcomes. Journal of Bone and Joint Surgery - American Volume 2013;95(15):111. [DOI] [PubMed] [Google Scholar]
- Ågren P-H, Mukka S, Tullberg T, Wretenberg P, Sayed-Noor AS. Factors affecting long-term treatment results of displaced intra-articular calcaneal fractures: a post hoc analysis of a prospective, randomized, controlled multicenter trial. Journal of Orthopedic Trauma 2014;28(10):564-8. [DOI] [PubMed] [Google Scholar]
- Ågren P-H, Wretenberg P, Sayer-Noor AS. Operative versus nonoperative treatment of displaced intra-articular calcaneal fractures. Journal of Bone and Joint Surgery - American Volume 2013;95(15):1351-7. [DOI] [PubMed] [Google Scholar]
Buckley 2002 {published data only}
- Barla J, Buckley R, McCormack R, Pate G, Leighton R, Petrie D, et al. Displaced intraarticular calcaneal fractures: long-term outcome in women. Foot and Ankle International 2004;25(12):853-6. [DOI] [PubMed] [Google Scholar]
- Brauer CA, Manns BJ, Ko M, Donaldson C, Buckley R. An economic evaluation of operative compared with nonoperative management of displaced intra-articular calcaneal fractures. Journal of Bone and Joint Surgery - British Volume 2005;87(12):2741-9. [DOI] [PubMed] [Google Scholar]
- Buckley R, Tough S, McCormack R, Pate G, Leighton R, Petrie D et al. Operative compared with nonoperative treatment of displaced intra-articular calcaneal fractures: a prospective, randomized, controlled multicenter trial. Journal of Bone and Joint Surgery - American Volume 2002;84(10):1733-44. [DOI] [PubMed] [Google Scholar]
- Buckley RE, Loucks C. Bohler's angle-correlation with long-term outcome in displaced intra-articular calcaneal fractures. Orthopaedic Transactions 1997;21(4):1349-50. [DOI] [PubMed] [Google Scholar]
- Dooley P, Buckley R, Tough S, McCormack B, Pate G, Leighton R. Bilateral calcaneal fractures; operative versus nonoperative treatment. Foot and Ankle International 2004;25(2):47-52. [DOI] [PubMed] [Google Scholar]
- Hildebrand KA, Buckley RE, Mohtadi NG, Faris P. Functional outcome measures after displaced intra-articular calcaneal fractures. Journal of Bone and Joint Surgery - British Volume 1996;78(1):119-23. [PubMed] [Google Scholar]
- Howard JL, Buckley R, McCormack R, Pate G, Leighton R, Petrie D, et al. Complications following management of displaced intra-articular calcaneal fractures: a prospective randomized trial comparing open reduction internal fixation with nonoperative management. Journal of Orthopaedic Trauma 2003;17(4):241-9. [DOI] [PubMed] [Google Scholar]
- Kingwell S, Buckley R, Willis N. The association between subtalar joint motion and outcome satisfaction in patients with displaced intraarticular calcaneal fractures. Foot and Ankle International 2004;25(9):666-73. [DOI] [PubMed] [Google Scholar]
- Lawrence S. Displaced intra-articular calcaneal fracture treatment. Orthopedics 2010;33(11):836-7. [DOI] [PubMed] [Google Scholar]
- O'Brien J, Buckley R, McCormack R, Pate G, Leighton R, Petrie D, et al. Personal gait satisfaction after displaced intraarticular calcaneal fractures: a 2-8 year followup. Foot and Ankle International 2004;25:657-65. [DOI] [PubMed] [Google Scholar]
- Tufescu TV, Buckley R. Age, gender, work capability, and worker's compensation in patients with displaced intraarticular calcaneal fractures. Journal of Orthopaedic Trauma 2001;15(4):275-9. [DOI] [PubMed] [Google Scholar]
Chrintz 1993 {published data only}
- Chrintz H, Sonne-Holm S. Clinical results after conservative versus operative treatment of dislocated intraarticular fractures of calcaneus [Abstract]. Acta Orthopaedica Scandinavica - Supplementum 1993;251:64. [Google Scholar]
- Chrintz H, Sonne-Holm S. Radiograhic results after conservative versus operative treatment of dislocated intraarticular fractures of calcaneus [Abstract]. Acta Orthopaedica Scandinavica - Supplementum 1993;251:63-4. [Google Scholar]
Griffin 2014 {published data only}ISRCTN37188541
- Dickenson EJ, Parsons N, Griffin DR. Open reduction and internal fixation versus nonoperative treatment for closed, displaced, intra-articular fractures of the calcaneus: long-term follow-up from the HeFT randomized controlled trial. The Bone & Joint Journal 2021;103-B(6):1040-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Griffin D, Parsons N, Shaw E, Kulikov Y, Hutchinson C, Thorogood M, et al. Operative versus non-operative treatment for closed, displaced, intra-articular fractures of the calcaneus: randomised controlled trial. BMJ 2014;349:10.1136/bmj.g4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISRCTN37188541. UK heel fracture trial: surgical treatment versus non-operative care [Improved functional outcome in heel fracture with surgical treatment versus non-operative care: a randomised controlled trial]. www.controlled-trials.com/ISRCTN37188541 (first received 7 February 2006).
- Lui TH, Ling SK. Calcaneal fractures have universally poor outcomes regardless of management. Evidence Based Medicine 2015;20(1):13. [DOI] [PubMed] [Google Scholar]
Hussain 2022 {published data only}
- Hussain F, Bhutto AM, Ahmed W, Palh HB, Mahar SA, Laghari AR, et al. A comparison between functional results in intra-articular displaced calcaneus fractures managed with conservative and operative treatment: a randomized controlled trial. Pakistan Journal of Medical and Health Sciences 2022;16:633-6. [DOI: ] [Google Scholar]
Kamath 2021 {published data only}
- Kamath KR, Mallya S, Hegde A. A comparative study of operative and conservative treatment of intraarticular displaced calcaneal fractures. Scientific Reports 2021;11:3946. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kulkarni 2015 {published data only}
- Kulkarni HG, Mane V, Gaonkar K. Plating for intra-articular calcaneal fractures... Is it an overkill? Journal of Clinical Orthopaedics and Trauma 2015;6:153-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nouraei 2011 {published data only}
- Nouraei MH, Moosa FM. Operative compared to non-operative treatment of displaced intra-articular calcaneal fractures. Journal of Research in Medical Sciences 2011;16(8):1014-9. [PMC free article] [PubMed] [Google Scholar]
Pandey 2018 {published data only}
- Pandey AK, Shrestha BP, Khanal GP, Rijal R, Pokharel B. Comparing closed reduction and percutaneous screw fixation versus below knee cast for closed displaced intra-articular calcaneal fracture – functional and radiological outcome. International Journal of Scientific Research 2018;7(2):67-9. [Google Scholar]
Parmar 1993 {published data only}
- Ibrahim T, Rowsell M, Rennie W, Brown A, Taylor G, Gregg P. Displaced intra-articular calcaneal fractures: 15 year follow-up of a randomised controlled trial of conservative versus operative treatment. Journal of Bone and Joint Surgery - British Volume 2009;91(Suppl 1):80. [DOI] [PubMed] [Google Scholar]
- Ibrahim T, Rowsell M, Rennie W, Brown AR, Taylor GJ, Gregg PJ. Displaced intra-articular calcaneal fractures: 15-year follow-up of a randomised controlled trial of conservative versus operative treatment. Injury 2007;38(7):848-55. [DOI] [PubMed] [Google Scholar]
- Lowrie IG, Triffitt PD, Gregg PJ. A controlled, prospective, randomised trial of operative versus conservative treatment of displaced intra-articular fractures of the os calcis: a preliminary report. Journal of Bone and Joint Surgery - British Volume 1990;72(5):948. [Google Scholar]
- Parmar H, Triffitt P, Lowrie I, Gregg PJ. A prospective randomized trial of operative and conservative treatment of displaced intra-articular fractures of the os calcis [Abstract]. Journal of Bone and Joint Surgery - British Volume 1992;74(Suppl 3):269. [Google Scholar]
- Parmar HV, Triffitt PD, Gregg PJ. Intra-articular fractures of the calcaneum treated operatively or conservatively. A prospective study. Journal of Bone and Joint Surgery - British Volume 1993;75(6):932-7. [DOI] [PubMed] [Google Scholar]
- Pavic R. Displaced intra-articular calcaneal fractures: 15 year follow-up of a randomised controlled trial of conservative versus operative treatment (Letter). Injury 2008;39(3):380. [DOI] [PubMed]
Sharma 2011 {published data only}
- Sharma V, Dogra A. Sanders type II calcaneum fractures - surgical or conservative treatment? A prospective randomized trial. Journal of Clinical Orthopaedics and Trauma 2011;2(1):35-8. [Google Scholar]
Thordarson 1996 {published data only}
- Thordarson DB, Krieger L. ORIF versus non-operative treatment of intra-articular fractures of the calcaneus: a prospective randomized trial [Abstract]. Orthopaedic Transactions 1997;21(2):584-5. [DOI] [PubMed] [Google Scholar]
- Thordarson DB, Krieger L. ORIF versus non-operative treatment of intraarticular fractures of the calcaneus: a prospective randomised trial [Abstract]. Orthopaedic Transactions 1996;20(1):23. [Google Scholar]
- Thordarson DB, Krieger LE. Operative versus nonoperative treatment of intra-articular fractures of the calcaneus: a prospective randomized trial. Foot and Ankle International 1996;17(1):2-9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
ACTRN12617001588381 {published data only}
- ACTRN12617001588381. Transarticular tibio-talo-calcaneal nailing versus open reduction and internal fixation for treatment of the elderly ankle fracture: a multi-centre, prospective, randomised controlled trial. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=373765&isReview=true (first received 2017).
Aslan 2019 {published data only}
- Aslan A, Sargın S, Gülcü A, Konya MN. Clinical, radiological and patient-reported outcomes in intra-articular calcaneal fractures: comparison of conservative and surgical treatment. Eklem Hastalik Cerrahisi 2019;30(2):143-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
IRCT2016051327872N1 {published data only}
- IRCT2016051327872N1. Comparison between conventional and minimally invasive technique for treatment of calcaneal fracture. www.irct.ir/trial/22743 (first received 17 July 2017).
Kashani 2013 {published data only}
- Kashani MB, Kachooei AR, Ebrahimi H, Peivandi MT, Amelfarzad S, Bekhradianpoor N, et al. Comparative study of peroneal tenosynovitis as the complication of intraarticular calcaneal fracture in surgically and non-surgically treated patients. Indian Red Crescent Medical Journal 2013;15(10):e11378. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 2016 {published data only}
- Li B, Wu G, Yang Y. Conservative versus surgical treatment for displaced fracture of the medial process of the calcaneal tuberosity. Journal of Orthopaedic Surgery 2016;24(2):163-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Rajikumar 2017 {published data only}
- Rajikumar V, Bajuri MY, Ali-Noor I, Sridharan R. A 3D CT scan assessment of the outcome of displaced intra-articular calcaneal fractures; operative vs non operative treatment. Malaysian Orthopaedic Journal 2017;11 (Suppl A). [Google Scholar]
Su 2017 {published data only}
- Su J, Cao X. Can operations achieve good outcomes in elderly patients with Sanders II-III calcaneal fractures? Medicine (Baltimore) 2017;96(29):e7553. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
IRCT2017092720258N62 {published and unpublished data}
- IRCT2017092720258N62. Comparison of the minimally invasive surgical treatment outcomes of intra-extra articular calcaneal fracture’s and non operative treatment in Valiasr hospital. www.irct.ir/trial/18000 (first received 29 October 2017).
Additional references
BOFAS
- British Orthopaedic Foot & Ankle Society. Calcaneal fractures. www.bofas.org.uk/hyperbook/trauma/calcaneal-fracture#:~:text=The%20economic%20impact%20of%20calcaneal,and%20delayed%20return%20to%20work. (accessed 22 August 2023).
Buckley 2014
- Buckley R, Leighton R, Sanders D, Poon J, Coles CP, Stephen D, et al. Open reduction and internal fixation compared with ORIF and primary subtalar arthrodesis for treatment of Sanders type IV calcaneal fractures: a randomized multicenter trial. Journal of Orthopaedic Trauma 2014;28(10):577-83. [PMID: ] [DOI] [PubMed] [Google Scholar]
Chan 2017
- Chan HY, Chen JY, Zainul-Abidin S, Ying H, Koo K, Rikhraj IS. Minimal clinically important differences for American Orthopaedic Foot & Ankle Society Score in hallux valgus Surgery. Foot & Ankle International 2017;38(5):551-557. [DOI] [PubMed] [Google Scholar]
Clarke 2007
- Clarke M. Fractures of the Calcaneus: Trauma. In: Saunders R, editors(s). Trauma: Core Knowledge in Orthopaedics. 1st edition. Philadelphia: Elsevier Mosby, 2007:386-402. [Google Scholar]
Dawson 2007
- Dawson J, Doll H, Coffey J, Jenkinson C, Oxford and Birmingham Foot and Ankle Clinical Research Group. Responsiveness and minimally important change for the Manchester-Oxford foot questionnaire (MOXFQ) compared with AOFAS and SF-36 assessments following surgery for hallux valgus. Osteoarthritis and Cartilage 2007;15(8):918-31. [PMID: ] [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta-analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Fischer 2021
- Fischer S, Meinert M, Neun O, Colcuc C, Gramlich Y, Hoffmann R, et al. Surgical experience as a decisive factor for the outcome of calcaneal fractures using locking compression plate: results of 3 years. Archives of Orthopaedic and Trauma Surgery 2021;141(10):1691-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Folk 1999
- Folk JW, Starr AJ, Early JS. Early wound complications of operative calcaneus fractures: analysis of 190 fractures. Journal of Orthopaedic Trauma 1999;13(5):369-72. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Version accessed 14 February 2023. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Harris 1946
- Harris RI. Fractures of the os calcis: their treatment by tri-radiate traction and subastragalar fusion. Annals of Surgery 1946;124(6):1082-1100. [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2023
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated August 2023). Cochrane, 2023. Available from training.cochrane.org/handbook.
Humphrey 2019
- Humphrey JA, Woods A, Robinson AHN. The epidemiology and trends in the surgical management of calcaneal fractures in England between 2000 and 2017. The Bone & Joint Journal 2019;101-B(2):140-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ibrahim 2007
- Ibrahim T, Rowsell M, Rennie W, Brown AR, Taylor GJS, Gregg PJ. Displaced intra-articular calcaneal fractures: 15-year follow-up of a randomised controlled trial of conservative versus operative treatment. Injury 2007;38(7):848-55. [DOI] [PubMed] [Google Scholar]
Kitaoka 1994
- Kitaoka HB, Alexander IJ, Adelaar RS, Nunley JA, Myerson MS, Sanders M. Clinical rating systems for the ankle-hindfoot, midfoot, hallux, and lesser toes. Foot Ankle International 1994;15(7):349-53. [DOI] [PubMed] [Google Scholar]
Koval 2006
- Koval KJ, Zuckerman JD. Handbook of Fractures. 3rd edition. Philadelphia: Lippincott, Williams & Wilkins, 2006. [Google Scholar]
Lefebvre 2019
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf MI, et al. Chapter 4: Searching for and selecting studies. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from training.cochrane.org/handbook/archive/v6.
Luo 2016
- Luo X, Li Q, He S, He S. Operative versus nonoperative treatment for displaced intra-articular calcaneal fractures: a meta-analysis of randomized controlled trials. The Journal of Foot and Ankle Surgery 2016;55(4):821-8. [DOI] [PubMed] [Google Scholar]
McLaughlin 1963
- McLaughlin HL. Treatment of late complications after os calcis fractures. Clinical Orthopaedics & Related Research 1963;30:111-5. [MEDLINE: ] [PubMed] [Google Scholar]
Meena 2017
- Meena S, Hooda A, Sharma P, Mittal S, Sharma J, Chowdhury B. Operative versus non operative treatment of displaced intraarticular fracture of the calcaneum: a meta-analysis of randomized controlled trials. Acta Orthopaedica Belgica 2017;83(1):161-9. [PMID: ] [PubMed] [Google Scholar]
Razik 2018
- Razik A, Harris M, Trompeter A. Calcaneal fractures: where are we now? Strategies in Trauma and Limb Reconstruction 2018;13(1):1-11. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- Review Manager (RevMan). Version 5.4. Copenhagen: The Cochrane Collaboration, 2020.
Schepers 2007
- Schepers T, Lieshout EM, Ginhoven TM, Heetveld MJ, Patka P. Current concepts in the treatment of intra-articular calcaneal fractures: results of a nationwide survey. International Orthopaedics 2008;32(5):711-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2019
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editors(s). Cochrane Handbook for Systematic Reviews of Interventions. 2nd edition. Chichester (UK): John Wiley & Sons, 2019:375-402. [Google Scholar]
Selim 2022
- Selim A, Ponugoti N, Chandrashekar S. Systematic review of operative vs nonoperative treatment of displaced intraarticular calcaneal fractures. Foot & Ankle Orthopaedics 2022;7(2):24730114221101609. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sterne 2017
- Sterne JA, Egger M, Moher D, Boutron I (editors). Chapter 10: Addressing reporting biases. In: Higgins JP, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017), Cochrane, 2017. Available from training.cochrane.org/handbook/archive/v5.2.
Vosoughi 2022
- Vosoughi AR, Borazjani R, Ghasemi N, Fathi S, Mashhadiagha A, Hoveidaei AH. Different types and epidemiological patterns of calcaneal fractures based on reviewing CT images of 957 fractures. Foot and Ankle Surgery 2022;28(1):88-92. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ware 2005
- Ware JE, Kosinski MA, Gandek B. SF-36 Health Survey: Manual and Interpretation Guide. Lincoln: Quality Metric Inc, 2005. [Google Scholar]
Zeng 2018
- Zeng Z, Yuan L, Zeng S, Sun Y, Huang F. Minimally invasive versus extensile lateral approach for Sanders type II and III calcaneal fractures: a meta-analysis of randomized controlled trials. International Journal of Surgery 2018;50:143-56. [PMID: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Baliga 2010
- Baliga S, Sutherland A, Bruce J. Surgical versus conservative interventions for displaced intra-articular calcaneal fractures. Cochrane Database of Systematic Reviews 2010, Issue 8. Art. No: CD008628. [DOI: 10.1002/14651858.CD008628] [DOI] [PubMed] [Google Scholar]
Bruce 2013
- Bruce J, Sutherland A. Surgical versus conservative interventions for displaced intra-articular calcaneal fractures. Cochrane Database of Systematic Reviews 2013, Issue 1. Art. No: CD008628. [DOI: 10.1002/14651858.CD008628.pub2] [DOI] [PubMed] [Google Scholar]


