Abstract
1,1-Dichloro-2,2-bis(4-chlorophenyl)ethylene (DDE), a toxic breakdown product of 1,1,1-trichloro-2,2-bis(4-chlorophenyl)ethane (DDT), has traditionally been viewed as a dead-end metabolite: there are no published reports detailing enzymatic ring fission of DDE by bacteria in either soil or pure culture. In this study, we investigated the ability of Pseudomonas acidovorans M3GY to transform DDE and its unchlorinated analog, 1,1-diphenylethylene (DPE). While strain M3GY could grow on DPE, cells grown on DPE as a sole carbon source could not degrade DDE. Cells grown on biphenyl, however, did degrade DDE. Mass balance analysis of [14C]DDE showed transformation of more than 40% of the recoverable radioactivity. Nine chlorinated metabolites produced from DDE were identified by gas chromatography-mass spectrometry–Fourier-transform infrared spectrometry (GC-MS-FTIR) from cultures grown on biphenyl. Recovery of these metabolites demonstrates that biphenyl-grown cells degrade DDE through a meta-fission pathway. This study provides a possible model for biodegradation of DDE in soil by biphenyl-utilizing bacteria.
1,1,1-Trichloro-2,2-bis(4-chlorophenyl)ethane (DDT) has been banned in the United States since 1972; however, more than 80% of the 4 billion pounds that were manufactured between 1940 and 1970 were used in U.S. agriculture (36). A large proportion of this chemical remains bound to the agricultural soils on which it was sprayed or dusted (38). These soils act as reservoirs from which DDT residues {DDT, DDE [1,1-dichloro-2,2-bis(4-chlorophenyl)ethylene], and DDD [1,1-dichloro-2,2-bis(4-chlorophenyl)ethane]} are slowly but continuously introduced into waterways as suspended sediment in agricultural runoff (9, 36).
Although DDT levels have dropped since 1972, 1990 levels in regional studies were still as much as 10 times higher than the chronic toxicity criterion established by the Environmental Protection Agency (36). While actual DDT levels may be decreasing, this decrease appears to be accompanied by a concomitant increase in DDE levels, as DDT is transformed mainly into DDE in aerobic environments (22, 38, 40). Thus, even though DDT had not been used since 1972, the body burden of DDE in some bird species was still increasing into the early 1980s (41). A direct negative correlation has been found between DDE levels in Alaskan hawks and eggshell thickness (6, 35). DDE has also been identified as a potent androgen receptor antagonist and has been linked to reports of abnormalities in male reproductive systems (27).
Despite being banned in the United States, DDT continues to be widely used in many developing nations. While DDE appears to dissipate more rapidly in tropical climates than in temperate climates (19), it continues to persist, being found to accumulate in food sources and human adipose tissue (19, 25, 26). The main mechanism by which DDE is dissipated from tropical soils is volatilization (19, 38). While limited mineralization of DDE from tropical soils has been reported (1), the extreme environmental conditions employed made it unclear what role microorganisms played in transforming DDE.
There are no published reports detailing bacterial mineralization of DDE in pure culture. However, You et al. have reported the “amicrobial” disappearance of DDE under reducing conditions (43). Massé et al. have also reported transformation of DDE via monohydroxylation of each ring and elimination of chloride; however, no further transformation was evident (31). In this study, we investigated the ability of Pseudomonas acidovorans M3GY to transform DDE and its unchlorinated analog, 1,1-diphenylethylene (DPE), via dioxygenation and subsequent meta cleavage.
MATERIALS AND METHODS
Chemicals.
Biphenyl, DPE, DDE, diphenylacetaldehyde, diphenylacetic acid, benzophenone, 2-hydroxybenzophenone, 3-hydroxybenzophenone, 4-chlorobenzaldehyde, 4-chlorobenzoate, 4-chloroacetophenone, and 4-chlorophenylacetic acid were purchased from Aldrich Chemical Co., Inc. (Milwaukee, Wis.) and, with the exception of 4-chloroacetophenone (97% pure), were determined to be greater than 99% pure by gas chromatography-mass spectrometry (GC-MS) analysis. [U-14C]DDE (>99.9% pure; specific activity, 13 μCi/mmol), N-nitrosyl-N-methylurea, and ethyl ether were purchased from Sigma Chemical Co. (St. Louis, Mo.). Radiochemical purity was confirmed by high-performance liquid chromatography using a FLO-ONE∖βeta radioactive-flow detector. N-Nitrosyl-N-methylurea was used to generate diazomethane (2). Scintisafe Plus 50 scintillation cocktail was obtained from Fisher Scientific (Pittsburgh, Pa.).
Bacterial culture and conditions.
P.acidovorans M3GY is a recombinant strain constructed by the continuous amalgamated culture method (30) and has been described elsewhere (32). M3GY was able to use DPE as a sole carbon source, though growth was poor without the addition of 0.005% yeast extract as a source of cofactors. M3GY was unable to grow on DDE as a sole carbon source. DPE metabolites were recovered from cells cultured in 2.8-liter flasks containing 1 liter of minimal salts medium (32) and either 200 mg of DPE or both 200 mg of DPE and 400 mg of biphenyl. Cells incubated with either 100 mg of DDE, 200 mg of DPE, and 50 mg of yeast extract or 100 mg of DDE and 500 mg of biphenyl were examined for transformation of DDE. Cultures of strain M3GY were incubated in the dark, on a rotary-platform shaker at 28°C and 200 rpm, for at least 25 days. Biphenyl-grown M3GY received 100 mg of additional biphenyl every 7 days. Abiotic controls consisted of minimal salts medium plus biphenyl and DDE, while biotic controls consisted of M3GY plus biphenyl but no DDE. Burkolderia cepacia LB400 was obtained from Donna Bedard (General Electric Co., Schenectady, N.Y.) and was cultured as described above.
UV-visible light spectra.
Culture samples (1 ml each) were centrifuged at 10,000 × g for 5 min. The supernatant was then removed and analyzed on a Uvikon 860 spectrophotometer from Kontron Instruments (Zurich, Switzerland). Supernatants were scanned from 260 to 700 nm at a rate of 120 nm/min.
[14C]DDE disappearance assay.
Treatments were prepared by adding 100 ppm of DDE, 2.5 × 106 dpm of [14C]DDE, and 500 ppm of biphenyl in hexane to autoclaved 125-ml Wheaton bottles. The hexane was allowed to evaporate, and then 40 ml of an early-log-phase culture was added to each bottle. A foil-lined lid was used to seal each bottle. Glass test tubes (15 by 100 mm) containing 10 ml of 2 N NaOH were place inside the Wheaton bottle to collect any 14CO2 that had evolved. Abiotic controls consisted of minimal salts medium plus biphenyl and DDE. Biotic controls were obtained by adding 40 ml of B. cepacia LB400, in early log phase, to Wheaton bottles containing biphenyl and DDE. Strain LB400 was selected because of its ability to transform numerous chlorinated biphenyl congeners (3).
Analysis of [14C]DDE disappearance assay.
After 50 days, treatments were acidified with 1 ml of concentrated HCl. The treatments were then extracted four times with 20 ml of ethyl ether. The ether fractions were pooled and evaporated to dryness. The residue was extracted three times with 10 ml of 0.1 N NaOH. The NaOH fractions were then pooled to give the acid-extractable fraction. The residue was then further extracted three times with hexane. The hexane fractions were combined and dried over anhydrous Na2SO4 to give the neutral fraction. The treatments were then centrifuged at 25,900 × g for 15 min, the supernatant was decanted, and the pellet was washed twice with 10 ml of 50 mM NaHPO4 (pH 7). The supernatant and the two washes were combined to form the aqueous fraction. The pellet was then resuspended in 50 ml of 50 mM NaHPO4 (pH 7). Aliquots (1 ml) of each fraction were individually added to 19-ml volumes of scintillation cocktail and analyzed on a Beckman TD-4000 scintillation counter. The neutral hexane extract was also analyzed for the disappearance of DDE on a Hewlett-Packard (HP) 5890 series II gas chromatograph equipped with a flame ionization detector and an HP 5-ms capillary column (length, 25 m; inside diameter [i.d.], 0.25 mm; film thickness, 0.52 μm). The initial oven temperature was 155°C, but the temperature was immediately ramped to 250°C at 10°C/min and held at 250°C for 10 min. The injector and detector were at 200 and 260°C, respectively.
Metabolite recovery.
Metabolites were recovered from cells cultured in the dark for 25 days or more. Cultures were extracted with ethyl ether four times. The ether extracts were pooled and then back-extracted four times with 40 ml of cold 0.1 N NaOH. The NaOH fractions were pooled, acidified to pH 3 with concentrated HCl, and then reextracted four times with 30 ml of ether. The pooled ether extracts were then either dried over NaCl and concentrated by rotary evaporation at 4°C under reduced pressure or methylated with 10 to 15 ml of an ethereal solution of diazomethane, dried, and then concentrated by rotoevaporation.
GC-MS-FTIR analysis.
An HP model 5890 series II gas chromatograph coupled in parallel with an HP model 5965B Fourier-transform infrared (FTIR) detector and an HP model 5970B mass-selective detector (29) was used to identify the metabolites. The injector temperature was held at 200°C. The initial oven temperature was 37°C, but the temperature was immediately ramped to 250°C at 10°C/min and held at 250°C for 20 min. Helium, the carrier gas, was used at a flow rate of 0.25 ml/min. A 30-m-long by 0.32-mm-i.d. DB-5ms capillary column (film thickness, 0.25 μm) from J & W Scientific was used to separate the DPE metabolites. A 25-m-long by 0.25-mm-i.d. HP 5-ms capillary column (film thickness, 0.52 μm) was used to separate the DDE metabolites. The flow cell and transfer line were held at 260°C. The ion source and quadrupole were held at 100 and 250°C, respectively. The quadrupole was scanned from m/z 60 to m/z 450. For each spectrum obtained from the ether extracts, a library search was performed in the HP Pesticide Database, the National Bureau of Standards (NIST) 49K Database, the Environmental Protection Agency’s Infrared Vapor Phase Database, and the Robertet Flavor/Fragrance Infrared Database. Searches were also performed manually in the Wiley/NIST Registry of Mass Spectral Data (33) and the Eight Peak Index of Mass Spectra (37).
RESULTS
Unmethylated extracts from the supernatants of cultures grown on DPE yielded eight metabolites that were identifiable by GC-MS-FTIR analysis. Metabolites 1 to 4 had retention times, mass spectra, and infrared spectra identical to those of authentic samples of diphenylacetaldehyde (retention time, 17.5 min), diphenylacetic acid (retention time, 19.4 min), benzophenone (retention time, 16.21 min), 2-hydroxybenzophenone (retention time, 21.1 min), and 3-hydroxybenzophenone (retention time, 20.7 min). Benzophenone was present in large quantities and was by far the most abundant metabolite, although it could not support the growth of strain M3GY. Small amounts of metabolites 5 to 8 were recovered from cultures grown on DPE and were identified by both mass and infrared spectra. These spectra are consistent with those published for 2-phenylpropenoic acid (retention time, 13.5 min), 2-phenylpropanoic acid (retention time, 12.6 min), acetophenone (retention time, 10.9 min), and benzoic acid (retention time, 9.2 min), respectively. Metabolites 5 to 8 were present at much higher levels in cultures grown on both DPE and biphenyl than in cultures grown solely on DPE. No chlorinated metabolites were identified when DDE was added to cultures using DPE as a sole source of carbon and energy.
Supernatants from cultures containing biphenyl and DDE produced a yellow color (λmax, 399 nm) that was absent in biotic or abiotic controls. This color persisted under basic to neutral conditions but was abolished under acidic conditions (<pH 5). This color, which is characteristic of meta-fission products, continued to increase linearly in absorbance even after 25 days. No yellow color was observed in the supernatants of cultures containing DPE and DDE.
Sequential extractions of liquid cultures containing [14C]DDE demonstrated marked transformation of DDE by P. acidovorans M3GY (Table 1). Transformation was evidenced by a decrease in the percentage of radioactivity recoverable from the neutral extract and an increase in the percentage of radioactivity recoverable from the acid extractable fraction, when compared to the control. There was also a significant increase in the amount of radioactivity associated with the pellet. Despite transformation, the amount of radioactivity recovered from sample NaOH traps was not statistically significantly different from that recovered from control traps, indicating that no [14C]CO2 had evolved. The only peaks obtained from GC-flame ionization detection analysis of the neutral fraction had retention times of 14.52 and 19.34 min, identical to those of authentic samples of biphenyl and DDE, respectively.
TABLE 1.
Transformation of [14C-DDE]
| Straina | Percent recoverable radioactivity inb:
|
||||
|---|---|---|---|---|---|
| Neutral extract | Acid extract | Aqueous extract | Pellet | Total | |
| Control | 99.5 | 0.2 | 0.2 | 0.0 | 100.0 |
| M3GY | 57.0 | 21.2 | 3.1 | 17.7 | 99.0 |
| LB400 | 91.4 | 1.1 | 0.5 | 10.2 | 103.1 |
Control, uninoculated; M3GY, P.acidovorans M3GY grown on biphenyl; LB400, B. cepacia LB400 grown on biphenyl.
Values are averages of three replications, normalized for a DDE extraction efficiency of 91.1%.
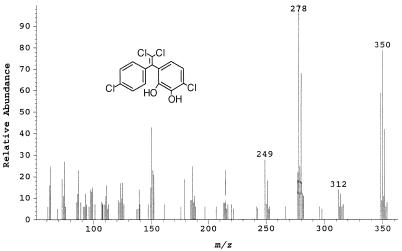
Extracts from supernatants of cells grown on biphenyl in the presence of DDE were analyzed both as methylated derivatives and without derivatization. Nine metabolites were identified either by their mass spectra or by both their mass and infrared spectra. Metabolite no. 1, which was recovered from the neutral ether extract, had a retention time of 24.9 min. The mass spectrum associated with this metabolite represents 1,1-di- chloro-2-(dihydroxy-4-chlorophenyl)-2-(4-chlorophenyl)ethyl- ene (C14H8O2Cl4) (Fig. 1). The molecular ion at m/z 348 displays a characteristic tetrachloro ratio (X:X+2:X+4:X+6:X+8) of 76.9:100:48.7:10.5:0.90, a result of the relative abundance of the most common chlorine isotopes, 35Cl and 37Cl. The loss of HCl gives rise to the fragment at m/z 312, which has the X:X+2:X+4:X+6 trichloro ratio of 100:97.5:31.7:3.4. The base peak at m/z 278, having the characteristic X:X+2:X+4 dichloro ratio, 100:65.0:10.6, is consistent with the loss of two chloride ions from the parent ion, a loss which is also common to DDE. The loss of CHO from the base peak yields the peak at m/z 249.
FIG. 1.
Mass spectrum of metabolite no. 1, 1,1-dichloro-2-(dihydroxy-4-chlorophenyl)-2-(4-chlorophenyl)ethylene.
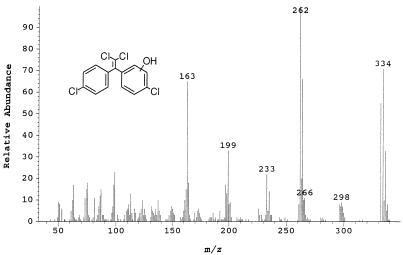
Metabolites 2 and 3, recovered from ethyl ether extracts of acidified culture supernatant, had retention times of 22.5 and 22.9 min, respectively. These mass spectra represent different isomers of 1,1-dichloro-2-(hydroxy-4-chlorophenyl)-2-(4-chlorophenyl)ethylene (C14H8OCl4). Both compounds had identical mass spectra (Fig. 2). The molecular ion at m/z 332 displays the characteristic tetrachloro ratio. The loss of a chlorine from the parent ion gives rise to the peak at m/z 297, which has a trichloro ratio. The base peak detected at m/z 262, which is ascribed to M-2Cl, has a dichloro ratio. The peak at m/z 233 represents the loss of CHO from the major m/z 262 fragment. Losses of CO and Cl give rise to the peak at m/z 199. The loss of HCl from the peak at m/z 199 yields the peak at m/z 163.
FIG. 2.
Mass spectrum of metabolites 2 and 3, meta and ortho isomers of 1,1-dichloro-2-(hydroxy-4-chlorophenyl)-2-(4-chlorophenyl)ethylene.
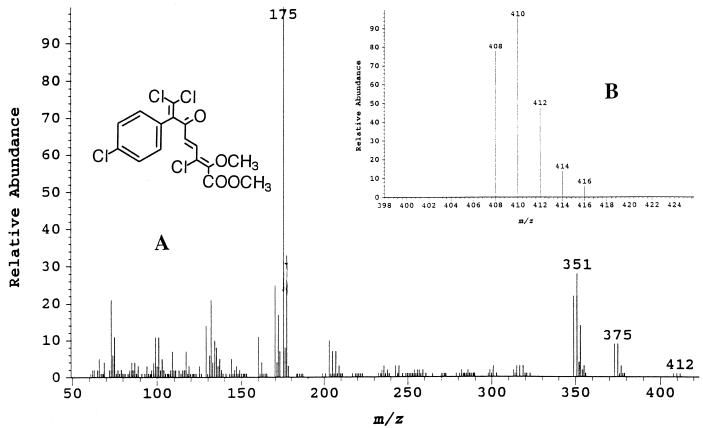
The spectrum of metabolite no. 4, with a retention time of 30.9 min, was obtained from ether extracts of acidified supernatant and was methylated with diazomethane. This spectrum is consistent with the structure of the dimethyl derivative of 6-oxo-2-hydroxy-7-(4-chlorophenyl)-4,8,8-trichloroocta-2,4- dienoic acid (C16H12O4Cl4) (Fig. 3a). The weak molecular ion at m/z 408 had a characteristic tetrachloro ratio but was fully resolved only by selected ion monitoring (Fig. 3b). The loss of a chloride ion results in the peak at m/z 373, which has a characteristic trichloro ratio. The peak at m/z 349, representing M-59, is consistent with the loss of COOCH3 from the molecular ion, a loss which is very characteristic of methyl esters (32). The base peak at m/z 175, which has a characteristic X:X+2 monochloro ratio of 100:32.5, represents the methylated aliphatic fragment cleaved at the carbonyl carbon (C-6).
FIG. 3.
(a) Mass spectrum of the dimethyl derivative of metabolite no. 4, 6-oxo-2-hydroxy-7-(4-chlorophenyl)-4,8,8-trichloroocta-2,4-dienoic acid. (b) Mass spectrum of the dimethyl derivative of metabolite no. 4 obtained by selected ion monitoring at m/z 408, 410, 412, 414, and 416.
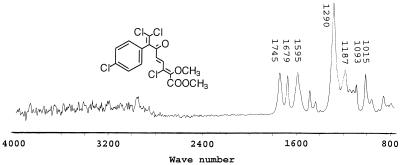
The infrared spectrum of metabolite no. 4 (retention time, 31.0 min.) shows two C=O stretches at 1,745 and 1,679 cm−1 (Fig. 4). At 1,595 cm−1 C=C stretching is evident. A strong C—O stretch, characteristic of an unsaturated ether, is observed at 1,290 cm−1, while a weaker C—O ether stretch is observed at 1,187 cm−1. Another set of weaker asymmetrical C—O stretches is observed at 1,093 and 1,015 cm−1.
FIG. 4.
Infrared spectrum of the dimethyl derivative of metabolite no. 4, 6-oxo-2-hydroxy-7-(4-chlorophenyl)-4,8,8-trichloroocta-2,4-dienoic acid.
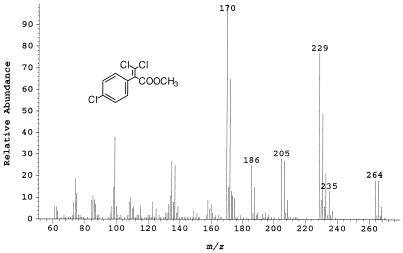
The mass spectrum of metabolite no. 5 (retention time, 18.3 min.) represents the methyl ester of 2-(4-chlorophenyl)-3,3-dichloropropenoic acid (C10H7O2Cl3) (Fig. 5). The weak molecular ion at m/z 264 has a characteristic trichloro ratio, although the X+8 peak is too low to be characterized. The peak at m/z 229 represents M-35, suggesting the loss of a chlorine. This is confirmed by the peak’s characteristic dichloro ratio. The peak at m/z 205 had a trichloro ratio similar to that of the parent ion and represents the loss of COOCH3 (M-59). The peak at m/z 170 is M-(59+35) and represents the loss of both the COOCH3 moiety (M-59) and a chlorine (M-35) from the parent ion.
FIG. 5.
Mass spectrum of the methyl ester of metabolite no. 5, 2-(4-chlorophenyl)-3,3-dichloropropenoic acid.
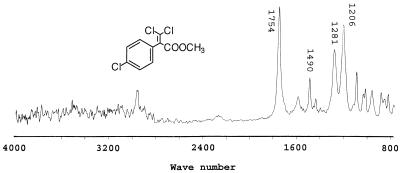
The infrared spectrum of metabolite no. 5 (retention time, 18.3 min.) displays a distinct C=O stretch at 1,754 cm−1 (Fig. 6). C=C stretching, characteristic of an aromatic compound, is found at 1,490 cm−1. Two C—O stretching bands are observed at 1,281 and 1,206 cm−1, indicating the presence of ether linkages.
FIG. 6.
Infrared spectrum of the methyl ester of metabolite no. 5, 2-(4-chlorophenyl)-3,3-dichloropropenoic acid.
The mass spectra of metabolites 6, 7, 8, and 9 were identical to and had the same retention times as authentic standards of 4-chlorophenylacetic acid (retention time, 13.2 min), 4-chloroacetophenone (retention time, 10.4 min), 4-chlorobenzaldehyde (retention time, 8.8 min), and 4-chlorobenzoic acid (retention time, 12.0 min), respectively. When extracts containing these metabolites were methylated, the mass spectra of metabolites 6 and 9 were no longer observed. New spectra, however, were obtained which were identical to the methyl ester derivatives of authentic 4-chlorophenylacetic acid and 4-chlorobenzoic acid (retention times, 10.7 min and 12.2 min, respectively).
DISCUSSION
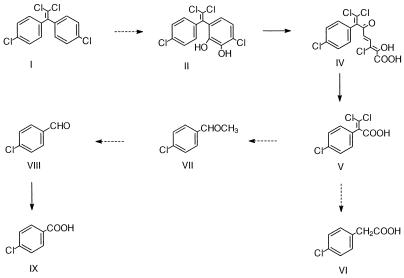
Although limited mineralization of DDE by fungi via dichlorobenzophenone has been demonstrated (5), this is the first published report detailing ring fission of DDE, long considered a dead-end metabolite and/or a conversion product of DDT (26, 34). Based on the information contained herein, we propose that biphenyl-grown P.acidovorans M3GY cometabolically transforms DDE in a manner analogous to the initial transformation of biphenyl (Fig. 7).
FIG. 7.
Proposed meta-fission pathway for the degradation of DDE by P.acidovorans M3GY. I, DDE; II, metabolite no. 1, 1,1-dichloro-2-(dihydroxy- 4-chlorophenyl)-2-(4-chlorophenyl)ethylene; IV, metabolite no. 4, 6-oxo-2-hydroxy-7-(4-chlorophenyl)-4,8,8-trichloroocta-2,4-dienoic acid; V, metabolite no. 5, 2-(4-chlorophenyl)-3,3-dichloropropenoic acid; VI, metabolite no. 6, 4-chlorophenylacetic acid; VII, metabolite no. 7, 4-chloroacetophenone; VIII, metabolite no. 8, 4-chlorobenzaldehyde; IX, metabolite no. 9, 4-chlorobenzoic acid.
Recovery of metabolite no. 1 from neutral extracts and of metabolites 2 and 3 from acid extracts suggests that DDE is attacked by a dioxygenase at the ortho and meta positions. Such an attack would give rise to a 2,3-dihydrodiol-DDE intermediate. Previous studies by Gibson et al. (16, 18) have shown that dihydrodiols are unstable, dehydrating rapidly under acidic conditions. Such a dehydration yields two different monohydroxy isomers of the parent diol, one substituted in the meta position and the other substituted in the ortho position. Similar results were obtained by Nadeau et al. during a study of DDT transformation by Alcaligenes eutrophus A5 (34). Based on the recovery of two hydroxy-DDT metabolites with identical mass spectra, Nadeau et al. suggested that DDT was subjected to initial ring oxidation by a dioxygenase (34). Metabolite no. 1 was not observed when supernatants were first acidified before extraction. Recovery of two monohydroxy-DDE metabolites (no. 2 and 3) under acidic conditions and a dihydroxy-DDE metabolite (no. 1) under neutral conditions is consistent with dioxygenase-mediated transformation of DDE.
Further evidence for the formation of dihydroxy-DDE arises from the accumulation of a ring cleavage product (metabolite no. 4). It is well established that meta cleavage of dihydroxybiphenyl and other aromatic diols leads to the production of unsaturated, α-hydroxy acids which absorb visible light (350 to 450 nm) strongly under basic conditions but not under acidic conditions (7, 10–12, 15, 24, 42). Nadeau et al. observed the accumulation of a metabolite with these spectral characteristics during the degradation of DDT, although no further analytical data were provided (34). Our observation of a yellow metabolite that absorbs light at 399 nm under basic conditions, but not under acidic conditions, is consistent with meta cleavage of dihydroxy-DDE.
Results from infrared and mass spectra indicate that this meta-cleavage product is 4,8,8-trichloro-7-(4-chlorophenyl)-2-hydroxy-6-oxo-octa-2,4-dienoic acid (metabolite no. 4). This DDE meta-cleavage metabolite was identified as its dimethyl derivative on the basis of both mass and infrared spectra. The loss of 59 mass units from the molecular ion (Fig. 3) is very characteristic of α-hydroxy esters due to the electron-withdrawing nature of the oxygen on the α carbon (2, 28). The lack of a visible O—H stretch in the infrared spectrum (Fig. 4) of metabolite no. 4 is consistent with methylation of both acid hydroxyl and α-hydroxyl groups. Methylation of nonacidic hydroxyl groups with diazomethane usually requires a Lewis acid as a catalyst (4); however, the keto/enol tautomerization that arises as a result of α,β unsaturation of C-1 in ring fission products makes the α-hydroxyl proton acidic enough to allow the oxygen to undergo methylation (2, 28).
The infrared spectrum of the DDE meta-fission metabolite displays nonsymmetrical C—O stretching bands at 1,290 and 1,188 cm−1 which are characteristic of unsaturated aliphatic esters. This ester results from methylation of the acid hydroxyl group. Nonsymmetrical C—O stretches were also observed at 1,093 and 1,015 cm−1 and arise from the ether linkage formed via methylation of the α-hydroxyl group. Similar ether stretches can be observed in infrared spectra of other methylated ring fission metabolites (2, 28).
The DDE meta-fission metabolite also displays a strong C=C stretch, observed at 1,595 cm−1. This may result from conjugation of the double bonds in the unsaturated aliphatic moiety with the methylene carbons on the aromatic ring. Finally, two C=O stretches are observed at 1,679 and 1,745 cm−1. The low wave number of the first C=O stretch (1,679 cm−1) is characteristic of α,β-unsaturated ketones and corresponds to the C-8 carbonyl group. The second C=O stretch, at 1,745 cm−1, is characteristic of aliphatic esters and corresponds to the C-1 carbonyl group.
Hydrolase activity against 2-hydroxy-6-oxo-6-phenylhexa-2,4-dienoate, the meta-cleavage product of biphenyl, at the C-6 carbonyl group results in the formation of benzoate (14). In like manner, the DDE meta-cleavage product appears to undergo direct attack at the C-6 carbonyl group, yielding 2-(4-chlorophenyl)-3,3-propenoic acid (metabolite no. 6). This is also analogous to the production of 2-phenylpropenoic acid from DPE by strain M3GY when grown on DPE plus biphenyl. Focht and Alexander found evidence of a similar hydrolase activity, recovering 3,3,3-trichloro-2-phenylpropanoic acid from Hydrogenomonas sp. cultures incubated with 1,1-trichloro-2,2-bis-[phenyl]ethane, a DDT analog (11).
Further work is required to elucidate the steps involved in the transformation of 3,3-dichloro-2-(4-chlorophenyl)propenoic acid; however, reduction of C-3 could yield 3,3-dichloro-2-(4-chlorophenyl)ethane, a hypothetical intermediate. This is analogous to the reduction of 2-phenylpropenoic acid to 2-phenylpropanoic acid in the metabolism of DPE by biphenyl-grown cells. Such a reductive step is also consistent with the findings of Francis et al. (13), who recovered 2-(4-chlorophenyl)propanoic acid from Pseudomonas sp. cultures incubated with 1-(4-chlorophenyl)-1-phenylethylene.
Degradation of this hypothetical intermediate may proceed via decarboxylation to yield 1,1-dichloro-(4-chlorophenyl)ethane, a polychlorinated ethylbenzene. Ethylbenzene has been shown by others to undergo oxidation of the aliphatic side chain to yield 1-phenylethanol, which is further oxidized to yield acetophenone (17, 39). Utkin et al. (39) also showed that the terminal methyl group of ethylbenzene undergoes oxidation to yield phenylacetic acid. Recovery of both 4-chloroacetophenone and 4-chlorophenylacetic acid from culture supernatants suggests the existence of a common chloroethylbenzene precursor.
In contrast to previous studies which showed that chloroacetophenones were transformed to chlorophenols via chlorophenyl acetate (20, 21), strain M3GY appears to oxidizes 4-chloroacetophenone to 4-chlorobenzoate. This may appear similar to the transformation of 4-hydroxyacetophenone to 4-hydroxybenzoic acid (23), but the production of 4-chlorobenzaldehyde by M3GY suggests that there exists another mechanism for removal of the methyl group from the aliphatic moiety (23). Darby et al. have speculated on an alternative pathway for the transformation of 4-hydroxyacetophenone to 4-hydroxybenzaldehyde via complete oxidation and subsequent decarboxylation of the terminal methyl group (8). We suggest that a similar mechanism may be responsible for transformation of 4-chloroacetophenone to 4-chlorobenzaldehyde. Resting cells rapidly transformed 4-chlorobenzaldehyde to 4-chlorobenzoate (data not shown).
There was no apparent cometabolism of DDE by DPE-grown cultures, which is not surprising in as much as the predominant pathway for DPE metabolism by DPE-grown cultures is via oxidation of the aliphatic moiety to benzophenone. Thus, even though DPE-grown cultures yielded trace amounts of metabolites consistent with a meta-fission pathway (DPE metabolites 4 to 9), the enzymes involved in this meta-fission pathway did not appear to be induced at levels high enough to afford detectable DDE transformation. However, when biphenyl was added as a cosubstrate to cultures containing only DPE, much lower levels of benzophenone were found and much higher levels of a yellow ring fission metabolite and of DPE metabolites 5 to 9 were produced than in cultures containing only DPE. This ring fission product (λmax = 423 nm) was not produced from biphenyl (λmax = 435 nm) (7).
The metabolites of DPE identified from cultures grown on either DPE alone or DPE plus biphenyl are consistent with earlier studies showing two distinct catabolic pathways for DPE (12). DPE metabolites 4 to 9, which are consistent with a DPE ring fission pathway, were analogous to the chlorinated metabolites recovered after ring fission of DDE by cultures grown on biphenyl.
Our study also suggests a possible mechanism for the production of 4-chlorobenzoate from DDT as observed by Nadeau et al. (34). Hydrolase activity on the DDT ring fission product could produce 3,3,3-trichloro-2-(4-chlorophenyl)propionic acid. Decarboxylation and subsequent oxidation could yield α,α,α-4-tetrachloroacetophenone, which could be metabolized in a similar manner to 4-chloroacetophenone, an intermediate in the DDE pathway, which is degraded to 4-chlorobenzoate.
In conclusion, these results demonstrate that DDE can be degraded by bacteria in pure culture when the correct pathway is induced. Biphenyl was capable of effecting this induction, while the more structurally similar compound DPE was not. When grown on biphenyl in the presence of [14C]DDE, strain M3GY was able to effect greater than 40% transformation of recoverable radioactivity from liquid culture. These results suggest a possible model for biodegradation of DDE in soil by biphenyl-utilizing bacteria.
ACKNOWLEDGMENT
This work was supported in part by the Kearney Foundation of Soil Science.
REFERENCES
- 1.Agarwal H C, Singh D K, Sharma V B. Persistence, metabolism and binding of p,p′-DDT in soil in Delhi, India. J Environ Sci Health Part B. 1994;29:73–86. [Google Scholar]
- 2.Arensdorf J J, Focht D D. A meta cleavage pathway for 4-chlorobenzoate, an intermediate in the metabolism of 4-chlorobiphenyl by Pseudomonas cepacia P166. Appl Environ Microbiol. 1995;61:443–447. doi: 10.1128/aem.61.2.443-447.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bedard D L, Unterman R, Bopp L H, Brennan M J, Haberl M L, Johnson C. Rapid assay for screening and characterizing microorganisms for the ability to degrade polychlorinated biphenyls. Appl Environ Microbiol. 1986;51:761–768. doi: 10.1128/aem.51.4.761-768.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Black H T. The preparation and reactions of diazomethane. Aldrichim Acta. 1983;16:3–9. [Google Scholar]
- 5.Bumpus J A, Powers R H, Sun T A. Biodegradation of DDE (1,1-dichloro-2,2-bis(4-chlorophenyl)ethylene) Mycol Res. 1993;97:95–98. [Google Scholar]
- 6.Cade T J, Lincer J L, Clayton M W, Roseneau D G, Swartz L G. DDE residues and eggshell changes in Alaskan falcons and hawks. Science. 1971;172:955–957. doi: 10.1126/science.172.3986.955. [DOI] [PubMed] [Google Scholar]
- 7.Catelani D, Columbi A, Sorlini C, Treccani V. Metabolism of biphenyl. 2-Hydroxy-6-oxo-6-phenylhexa-2,4-dienoate: the meta-cleavage product from 2,3-dihydroxybiphenyl by Pseudomonas putida. Biochem J. 1973;134:1063–1066. doi: 10.1042/bj1341063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Darby J M, Taylor D G, Hopper D J. Hydroquinone as the ring-fission substrate in the catabolism of 4-ethylphenol and 4-hydroxyacetophenone by Pseudomonas putida JD1. J Gen Microbiol. 1987;133:2137–2146. [Google Scholar]
- 9.Edwards C A. Persistent pesticides in the environment. 2nd ed. Cleveland, Ohio: CRC Press; 1973. [Google Scholar]
- 10.Focht D D, Alexander M. Bacterial degradation of diphenylmethane, a DDT model substrate. Appl Microbiol. 1970;20:608–611. doi: 10.1128/am.20.4.608-611.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Focht D D, Alexander M. DDT metabolites and analogs: ring fission by Hydrogenomonas. Science. 1970;170:91–92. doi: 10.1126/science.170.3953.91. [DOI] [PubMed] [Google Scholar]
- 12.Focht D D, Joseph H. Degradation of 1,1-diphenylethylene by mixed cultures. Can J Microbiol. 1974;20:631–635. doi: 10.1139/m74-096. [DOI] [PubMed] [Google Scholar]
- 13.Francis A J, Spanggord R J, Ouchi G I, Bramhall R, Bohonos N. Metabolism of DDT analogues by a Pseudomonas sp. Appl Environ Microbiol. 1976;32:213–216. doi: 10.1128/aem.32.2.213-216.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furukawa K, Hirose J, Suyama A, Zaiki T, Hayashida S. Gene components responsible for discrete substrate specificity in the metabolism of biphenyl (bph operon) and toluene (tod operon) J Bacteriol. 1993;175:5224–5232. doi: 10.1128/jb.175.16.5224-5232.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Furukawa K, Tonomura K, Kamibayashi A. Effect of chlorine substitution on the biodegradability of polychlorinated biphenyls. Appl Environ Microbiol. 1978;35:223–227. doi: 10.1128/aem.35.2.223-227.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gibson D T, Hensley M, Yoshioka H, Mabry T J. Formation of (+)-cis-2,3-dihydroxy-1-methylcyclohexa-2,4-diene from toluene by Pseudomonas putida. Biochemistry. 1970;9:1626–1630. doi: 10.1021/bi00809a023. [DOI] [PubMed] [Google Scholar]
- 17.Gibson D T, Gschwendt B, Yeh W K, Kobal V M. Initial reactions in the oxidation of ethylbenzene by Pseudomonas putida. Biochemistry. 1973;12:1520–1528. doi: 10.1021/bi00732a008. [DOI] [PubMed] [Google Scholar]
- 18.Gibson D T, Roberts R L, Wells M C, Kobal V M. Oxidation of biphenyl by a Beijerinckia sp. Biochem Biophys Res Commun. 1973;50:211–219. doi: 10.1016/0006-291x(73)90828-0. [DOI] [PubMed] [Google Scholar]
- 19.Hassan A. Special issue on DDT in the tropics: appraisal of overall programme accomplishments and conclusions. J Environ Sci Health Part B. 1994;29:205–228. [Google Scholar]
- 20.Havel J, Reineke W. Microbial degradation of chlorinated acetophenones. Appl Environ Microbiol. 1993;59:2706–2712. doi: 10.1128/aem.59.8.2706-2712.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Higson F K, Focht D D. Bacterial degradation of ring-chlorinated acetophenones. Appl Environ Microbiol. 1990;56:3678–3685. doi: 10.1128/aem.56.12.3678-3685.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hitch R K, Day H R. Unusual persistence of DDT in some western USA soils. Bull Environ Contam Toxicol. 1992;48:259–264. doi: 10.1007/BF00194381. [DOI] [PubMed] [Google Scholar]
- 23.Hopper D J, Jones H G, Elmorsi E A, Rhodes-Roberts M E. The catabolism of 4-hydroxyacetophenone by an Alcaligenes sp. J Gen Microbiol. 1985;131:1807–1814. [Google Scholar]
- 24.Horvath R S. Co-metabolism of methyl- and chloro-substituted catechols by an Achromobacter sp. possessing a new meta-cleaving oxygenase. Biochem J. 1970;119:871–876. doi: 10.1042/bj1190871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kashyap R, Iyer L R, Singh M M. Evaluation of daily dietary intake of dichloro-diphenyl-trichloroethane (DDT) and benzene hexachloride (BHC) in India. Arch Environ Health. 1994;49:63–66. doi: 10.1080/00039896.1994.9934417. [DOI] [PubMed] [Google Scholar]
- 26.Kaushik C P. Persistence and metabolism of HCH and DDT in soil under subtropical conditions. Soil Biol Biochem. 1991;23:131–134. [Google Scholar]
- 27.Kelce W R, Stone C R, Laws S C, Gray L E, Kemppainen J A, Wilson E M. Persistent DDT metabolite p,p′-DDE is a potent androgen receptor antagonist. Nature. 1995;375:581–585. doi: 10.1038/375581a0. [DOI] [PubMed] [Google Scholar]
- 28.Koh S-C, McCullar M V, Focht D D. Biodegradation of 2,4-dichlorophenol through a distal meta-fission pathway. Appl Environ Microbiol. 1997;63:2054–2057. doi: 10.1128/aem.63.5.2054-2057.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krock K A, Wilkins C L. Qualitative analysis of contaminated environmental extracts by multidimensional gas chromatography with infrared and mass spectral detection (MDGC-IR-MS) J Chromatogr A. 1996;726:167–178. [Google Scholar]
- 30.Kröckel L, Focht D D. Construction of chlorobenzene-utilizing recombinants by progenitive manifestation of a rare event. Appl Environ Microbiol. 1987;53:2470–2475. doi: 10.1128/aem.53.10.2470-2475.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Massé R, Lalanne D, Messier F, Sylvestre M. Characterization of new bacterial transformation products of 1,1,1-trichloro-2,2-bis-(4-chlorophenyl)ethane (DDT) by gas chromatography/mass spectrometry. Biomed Environ Mass Spectrom. 1989;18:741–752. doi: 10.1002/bms.1200180917. [DOI] [PubMed] [Google Scholar]
- 32.McCullar M V, Brenner V, Adams R H, Focht D D. Construction of a novel polychlorinated biphenyl-degrading bacterium: utilization of 3,4′-dichlorobiphenyl by Pseudomonas acidovorans M3GY. Appl Environ Microbiol. 1994;60:3833–3839. doi: 10.1128/aem.60.10.3833-3839.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McLafferty F W, Stauffer D B, editors. The Wiley/NBS registry of mass spectral data. New York, N.Y: John Wiley and Sons; 1989. [Google Scholar]
- 34.Nadeau L J, Menn F-M, Breen A, Sayler G S. Aerobic degradation of 1,1,1,-trichloro-2,2-bis(4-chlorophenyl)ethane (DDT) by Alcaligenes eutrophus A5. Appl Environ Microbiol. 1994;60:51–55. doi: 10.1128/aem.60.1.51-55.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peakall D B. DDE: its presence in peregrine eggs in 1948. Science. 1974;183:673–674. doi: 10.1126/science.183.4125.673. [DOI] [PubMed] [Google Scholar]
- 36.Rinella J F, Hamilton PA, McKenzie S W. Persistence of the pesticide DDT in the Yakima River Basin, Washington. Washington, D.C: U.S. Geological Survey; 1993. [Google Scholar]
- 37.The Royal Chemical Society. Eight peak index of mass spectra. 4th ed. London, United Kingdom: The Mass Spectrometry Data Centre, The Royal Society of Chemistry; 1991. [Google Scholar]
- 38.Spencer W F, Singh G, Taylor C D, LeMert R A, Cliath M M, Farmer W J. DDT persistence and volatility as affected by management practices after 23 years. J Environ Qual. 1996;25:815–821. [Google Scholar]
- 39.Utkin I B, Yakimov M M, Matveeva L N, Kozlyak E I, Rogozhin I S, Solomon Z G, Bezborodov A M. Degradation of styrene and ethylbenzene by Pseudomonas species Y2. FEMS Microbiol Lett. 1991;77:237–242. [Google Scholar]
- 40.Wedemeyer G. Dechlorination of 1,1,1-trichloro-2,2-bis(p-chlorophenyl)ethane by Aerobacter aerogenes. Appl Environ Microbiol. 1967;15:569–574. doi: 10.1128/am.15.3.569-574.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.White D H, Krynitsky A J. Wildlife in some areas of New Mexico and Texas (USA) accumulate elevated DDE residues, 1983. Arch Environ Contam Toxicol. 1986;15:149–158. doi: 10.1007/BF01059964. [DOI] [PubMed] [Google Scholar]
- 42.Yagi O, Sudo R. Degradation of polychlorinated biphenyls by microorganisms. J Water Pollut Control Fed. 1980;52:1035–1043. [Google Scholar]
- 43.You G, Sayles G D, Kupferle J M, Kim I S, Bishop PL. Anaerobic DDT biotransformation: enhancement by application of surfactants and low oxidation reduction potential. Chemosphere. 1996;32:2269–2284. [Google Scholar]