Abstract
Purpose of review:
Apraxia of speech (AOS) is a motor speech disorder that has long been recognized to occur secondary to acute neurologic insults and, more recently, to neurodegenerative diseases as a harbinger for progressive supranuclear palsy and corticobasal syndrome. This article reviews recent findings regarding the clinic phenotypes of AOS, neuroimaging correlates, and the underlying disease processes.
Recent findings:
Two clinical subtypes of AOS map onto two underlying 4-Repeat tauopathies. New imaging techniques have recently been applied to the study of progressive AOS. There is no data on the impact of behavioral intervention, although studies of nonfluent/agrammatic primary progressive aphasia that include patients with AOS suggest some benefit in speech intelligibility and maintenance.
Summary:
While recent findings suggest subtypes of AOS exist that are linked to molecular pathology and have important implications for disease progression, further research is needed to assess outcome of behavioral and other types of intervention.
Keywords: apraxia of speech, progressive supranuclear palsy, corticobasal syndrome, phonetic, prosodic, tauopathy, degenerative disease
1.0. Introduction
Apraxia of speech (AOS) is a motor speech disorder that reflects disruptions in motor planning and programming. While AOS can occur as a developmental disorder (childhood apraxia of speech), and many of the same principles and descriptors apply to that clinical entity [1], this review will focus on acquired AOS that occurs secondary to acute neurological insult (e.g., stroke, tumor, traumatic brain injury), and, in particular, degenerative disease that involves the cortical regions that control speech production, such as the premotor cortex. Regardless of etiology, AOS can occur in isolation or with other communication difficulties, such as aphasia (language dysfunction) and/or dysarthria (neuromuscular execution impairments) and their distinction from one another has important implications for therapeutic targets, as well as disease prognosis in the case of progressive syndromes.
Primary progressive apraxia of speech (PPAOS) is the diagnosis given when apraxia of speech (AOS) is insidious, progressive, and the first or only clinical symptom associated with a neurodegenerative disease [2]. When later- and less prominent agrammatic aphasia is present, the term progressive apraxia of speech (PAOS) is applied [3]; this is in complement to the non-fluent agrammatic variant of Primary Progressive Aphasia (PPA), where aphasia occurs first and is more prominent [4]. Over the last decade, the clinical presentation [3, 5–9], underlying pathophysiology [2, 10–18], and evolving neurological picture that is heralded by PAOS [19–23] have been well detailed and recently summarized [24]. This review will focus on describing the clinical entity of AOS broadly followed by clarifying the clinical entity of PAOS, the terminology utilized, and the more recent discoveries regarding the related neurologic syndromes and underlying pathology.
1.1. What is apraxia of speech?
AOS is a motor speech disorder that affects a person’s ability to coordinate the movements of the muscles involved in speaking. AOS is often referred to as a planning and programming disorder, where the motor planning refers to the general strategies about what to do (i.e., the goal) and motor programming involve steps about how to accomplish plans (i.e., the procedure). When a step of the procedure is imprecisely or inaccurately executed, there may be an audible or visible attempt to correct it, referred to as groping. People with AOS describe knowing what they want to say but have difficulty coordinating the precise movements of the mouth, tongue and lips that are required to produce speech sounds, words, and sentences and the ability to do it quickly. Apraxic speech may be slow and effortful and is often characterized by distorted and/or substituted sounds, inconsistent errors, and excessive pausing between words or syllables of the same word.
1.2. What does apraxia of speech sound like?
The best way to recognize AOS is to listen to it. You can hear samples of progressive AOS in the following cited publications [3, 12, 23, 25–28]. After listening to the samples, it may be easier to understand the perceptual features used to describe AOS. Across etiologies, the clinical features of AOS are heterogeneous and not entirely agreed upon [1]; however, AOS consistently includes disruptions of articulation and prosody that become more prominent with increased word (or phrase) length and/or complexity. In neurodegenerative AOS, disruptions in either articulation or prosody can predominate the speech pattern [3, 12, 29, 30]. In most cases, both articulation and prosody disruptions are present but the perceptual predominance of one over the other informs a further diagnostic label: phonetic or prosodic predominant PAOS. Patients are referred to as having phonetic (articulatory) PAOS if sound distortions, distorted sound substitutions, or distorted additions clearly dominate the speech pattern, while prosodic PAOS is used to describe patients in whom slow rate and syllable or word segmentation clearly dominate the speech pattern [3, 12]. When neither phonetic nor prosodic features predominate, due to either mild or profound severity or a lack of clear perceptual predominance between those features, the term mixed PAOS is applied [3, 12]. In post-stroke AOS, the constellation of speech features are shared with those observed in progressive AOS, although the noted articulation disruptions classically predominate. There are limited investigations of subtypes of AOS in post-stroke AOS [30]. Resonance (e.g., hypernasality) and voice (e.g., strain or breathiness) disturbances are not typically seen in AOS, regardless of etiology.
1.3. What is apraxia of speech not?
1.3.1. Dysarthria.
The two primary dysarthria types that require careful differential diagnosis are ataxic and spastic, particularly for phonetic and prosodic predominant AOS, respectively. Generally speaking, automatic speech sequences (e.g. counting, saying the days of the week, singing a familiar song) may be produced without errors in AOS, while dysarthria is largely consistent across speech content [27]. The co-occurrence of non-verbal oral apraxia may also be considered confirmatory for AOS [31, 32]. More specific features to differentiate ataxic and spastic dysarthria from AOS are outlined next.
To differentiate between ataxic dysarthria and AOS, the speech AMRs (alternating motion rates; rapid repetitions of “puh,” “tuh,” and “kuh”) and sequential motion rates (SMRs; repetitions of the sequence “puhtuhkuh puhtuhkuh puhtuhkuh”) can be particularly helpful [27]. In AOS, SMRs are expected to be more challenging and error-filled than AMRs; the opposite may be observed in ataxic dysarthria. Audible and visible groping and increasing articulation errors with length and complexity also suggest AOS over ataxic dysarthria [27]. It is also unusual for ataxic dysarthria to occur in isolation, so the lack of coexisting limb, truncal and gait ataxia may be considered confirmatory.
Prosodic predominant AOS may be confused with a spastic dysarthria, given that slowed speech rate is a shared core feature. The primary diagnostic distinctions include 1) the absence of slowed physiologic movements (e.g., slowed tongue side to side or lip movements back to front) in AOS (and their presence in spastic dysarthria); 2) the relative preservation of the phonatory and resonance subsystems in AOS (that are commonly impaired in spastic dysarthria); 3) the sound-level errors in AOS are often inconsistent (and more so consistent in spastic dysarthria); 4) dysphagia and hyperreflexia (i.e., jaw jerk, palmomental, suck, snout reflexes) are absent in isolated AOS (and commonly co-occur with a spastic dysarthria).
1.3.2. Aphasia.
AOS must be differentiated from aphasia, which may be challenging given their frequency of co-occurrence [33]. Aphasia may cause difficulty with comprehension (understanding spoken language and reading) and expression (writing and speaking), while AOS only impacts speaking. When listening to a patient, they may have sound level speech errors and it may be difficult to determine if they reflect phonological or apraxic errors [34, 35] . There are some nuances in the quality of the speech sound level errors that may inform differential diagnosis (namely the presence of distorted substitutions in AOS, but not aphasia), but there are other clues as well. Additional prosodic abnormalities are consistent with AOS but not explained by aphasia alone. On the other hand, disruptions in the linguistic aspects of spoken or written language comprehension or writing would be supportive of aphasia rather than an isolated AOS. Interestingly, it is not uncommon for patients with isolated, progressive AOS to report “yes/no” or other binary reversals, saying or shaking their head no when they mean yes or thank you when they mean to say you’re welcome, even in the absence of other paraphasic errors (or language disturbances). A thorough speech-language evaluation may help describe the areas of communication that are relatively preserved (or impacted).
1.3.3. Impairment of general intellect.
AOS is a motor speech disorder and, as such, does not reflect an impairment of general intellect. Patients with PPAOS may perform in the abnormal range on timed verbal fluency tests [36], but this is largely attributable to motoric speed of the response rather than other neuropsychological impairment [37]. Many patients with AOS continue to work independently and without error, although the verbal demands of a given occupation may inform the decision to appropriately pursue disability services.
1.4. What causes apraxia of speech?
The supplementary motor area and the lateral premotor cortex are commonly involved in degenerative AOS [2, 3, 12], with structural and functional connectivity disruptions demonstrated on diffusion tensor imaging [38] and functional magnetic resonance imaging [11]. While these regions have also been implicated in stroke-related AOS, AOS in that context may result from broader areas of involvement, spanning the left hemisphere and, in particular, those regions associated with speech production (i.e., areas in the frontal lobe).
2.0. Broader Neurologic Entity: What company does PPAOS keep?
2.1. Clinical
A diagnosis of PAOS requires that other neurological signs and symptoms are no more than equivocal. If AOS occurs in the context of a more widespread neurodegenerative syndrome, such as corticobasal syndrome (CBS), the more appropriate diagnosis would be CBS (with AOS), not PAOS. PAOS is a neurodegenerative disorder that progresses over time with other neurological signs and symptoms becoming present as the syndrome evolves [19]. Additional neurological signs and symptoms typically occur five years from time of onset of speech problems [20, 39]. The most common signs and symptoms that occur later in time in patients with PAOS include aphasia, as well as those linked to the extrapyramidal system. Aphasia occurs quite commonly as mentioned above, and, in fact, when present, worsens survival [40]. Bradykinesia and rigidity, but not tremor (of any kind) is very common later in the disease course [20]. It is also very common to observe changes in balance and gait ultimately resulting in patients being wheelchair bound and being in an akinetic rigid state close to the time of death. Ideomotor limb apraxia also commonly develops, where it tends to be asymmetric. Hence, patients with PAOS often will later meet criteria for probable CBS [41]. Less common, is the presence of a vertical supranuclear gaze palsy [39, 42]. It seems to be the case that those patients who present with the phonetic subtype of PAOS are more likely to evolve into the CBS, or CBS/PSP hybrid syndrome while those with the prosodic subtype tends to show features of the Richardson syndrome variant of progressive supranuclear palsy [17]. Gait freezing is rarely seen in patients who started off with PAOS [39]. Additional features such as sleep disturbances, dysphagia and urinary incontinence frequently occur, with sleep disturbances occurring early in the disease course [43] while dysphagia and urinary incontinence occur later in the disease course [19]. Mild behavioral abnormalities and executive dysfunction may also develop [44]. In summary, in the latter stages of the disease, most patients who started off as PAOS, ends up having a classic extra-pyramidal syndrome [19, 20, 39, 45]. While often developing features of CBS and Richardson syndrome variant of PSP, features of MSA including stridor, autonomic dysfunction, ataxia and rapid eye movement sleep disorder are rare in PAOS. Hallucinations and other psychotic symptoms that are common in dementia with Lewy bodies are also extremely rare in patients with PAOS.
2.1. Imaging
Different types of imaging modalities have been reported in patients with PAOS and recently summarized [24]. As mentioned earlier, anatomical imaging with brain MRI scans have found atrophy of the lateral premotor and supplementary motor area (SMA) in patients with PAOS [2, 3, 19]. Hypometabolism is also observed in these regions on [18F] fluorodeoxyglucose (FDG) PET [2, 3, 19, 45], and often FDG-PET is more sensitive than MRI to detect early and subtle changes in patients with PAOS. Unlike in patients with PPA where the left hemisphere is linked to aphasia, in PAOS both right and left hemisphere are involved [45]. With progression of the disease, atrophy and hypometabolism become more striking, with spread into inferior frontal regions, primary motor cortex and brainstem [19–21, 46].
In addition to MRI and FDG-PET, there are more recent imaging studies in PAOS using PET ligands to detect beta-amyloid and tau proteins, and other imaging techniques including diffusion tensor imaging (DTI), diffusion tractography, resting state functional MRI, Ioflupane (I-123) dopamine transporter (DAT) scanning, and quantitative susceptibility mapping (QSM) to assess different aspects of brain structure, function and biology. Amyloid PET scans in PAOS have been found to be abnormal, showing evidence of beta-amyloid deposition, in about 20% of patients [47]. However, unlike in Alzheimer’s disease (AD) where both beta-amyloid and tau PET scans show global/widespread increased uptake, tau PET uptake in PAOS is limited and focally affects the lateral premotor and SMA regions [18]. Hence, in the vast majority of PAOS patients, a positive beta-amyloid scan does not suggest a primary underlying pathology of AD. Focal tau uptake on PET scans is unlike to all be due to AD type paired helical filament tau and may represent a combination of off target binding to some neurodegenerative [48] as well as some binding to non-AD tau. Diffusion tensor imaging studies have shown involvement of the white matter underlying the premotor cortex, although changes tend to be more striking in those with phonetic AOS [12, 19]. Patients with phonetic AOS also show degeneration of the superior frontal white matter and body of the corpus callosum, while those with prosodic AOS show degeneration of the superior cerebellar peduncle [12], a structure that is often affected in patients with the Richardson syndrome variant of PSP [49]. Diffusion tractography demonstrates abnormal diffusion in PAOS patients in the SMA commissural fibers, as well as white matter tracts from the SMA to the basal ganglia and cortical regions, such as inferior frontal cortex via the frontal aslant tract and U-fibers to motor cortex [38]. Functional connectivity disruptions have also been identified in patients with PAOS using resting state functional MRI, with reduced connectivity from the SMA and premotor cortex to other regions of the brain, including the rest of the speech and language network [11, 50]. DAT scan results have been underwhelming, with abnormal striatal uptake observed in some PAOS patients, although DAT scan abnormalities do not match with the presence of parkinsonism in these patients [16]. Most recently, QSM studies have shown abnormal magnetic susceptibility in PAOS patients in the putamen, red nucleus and cerebellar dentate regions when compared with controls [51], suggesting the presence of iron deposition and concurring with findings in PSP [52]. No differences were identified between the phonetic and prosodic PAOS subtypes although relatively small sample sizes may have limited the findings.
2.2. Pathology
An association between tauopathy, especially a 4-repeat tauopathy such as PSP and CBD and PAOS has been known now for over a decade [14, 53]. However, recently it was demonstrated that patients with phonetic PAOS were more likely to show underlying CBD pathology while patients with prosodic PAOS were more likely to show underlying PSP pathology [17]. It has also been shown that if a patient with phonetic PAOS has PSP pathology, that the PSP pathology tends to be atypical with greater burden of tau deposition in cortical regions compared to what is seen in typical PSP pathology [17]. Some patients with PAOS have also been found to have a rare 4R tauopathy known as globular tauopathy (GGT) [54]. This 4R tauopathy tends to show tau pathology in the motor cortex and corticospinal tract. It is unknown, however, whether patients with PAOS and GGT have more clinical, or imaging involvement of the motor cortex compared to PAOS patients with CBD or PSP pathology. Pick’s disease, a 3R tauopathy has also been found in patients with AOS when AOS is not the primary presenting syndrome [17]. That is, when AOS occurs in the context of a progressive agrammatic aphasia (i.e., non-fluent/agrammatic variant of PPA). Lastly, non-tau pathology has been found in a handful patients with AOS occurring in the context of a dysexecutive or behavioral syndrome [17].
No genetic abnormalities have been found to date to be associated with PAOS including abnormalities in the microtubule associated protein, tau gene [55].
3.0. Available Treatment
At this time, there are no available curative or disease modifying treatments for PAOS or evolving neurologic syndromes. However, symptomatic behavioral treatment is recommended, as research has demonstrated its potential to maintain speech function or slow the progression of deterioration through working with a speech-language pathologist to improve speech production skills [56]. Recent studies have looked at transcranial direct current stimulation (tDCS) [57] and transcranial magnetic stimulation (TMS) [58] and shown some promise in improving speed and accuracy of speech, although more work is needed. Referrals to speech-language pathologists may facilitate identifying augmentative and alternative means of communication, and referrals to occupational therapy may support alternative means of access in the cases where limb rigidity, spasticity, or apraxia preclude typing on speech generating devices. Regularly meeting with a speech-language pathologist also allows for identification and management of later emerging speech, language, cognitive, and/or swallowing difficulties
4.0. Case Study
A 72-year-old woman presented with a two to three-year history of progressive speech changes. She first noted a slight hesitancy in her speech, at first only noticeable to her. She noted that her speech was slow and that she had to concentrate on enunciating carefully. She also described being short of breath when talking, but not during physical exertion. She could not keep up with recitation of poems or the pledge of allegiance. She denied any chewing or swallowing difficulties. She denied difficulty with auditory or reading comprehension, or any difficulties with respect to word finding.
On examination, she demonstrated a markedly slow overall speech rate, moderately reduced words per breath group, moderate segmentation between words, and mild segmentation within words, with additional mild false starts, sound prolongations, and inconsistent articulation errors. Her overall score on the Apraxia of Speech Rating Scale-3 was 13 (2 phonetic subscore, 6 prosodic subscore) [26, 59]. The examination of the structure and function of her oral mechanism was unremarkable. There was good symmetry, speed, strength, and range of motion of the speech articulators, although she initially said “blow” when asked to execute that command. The examination demonstrated adequate confrontation naming abilities, repetition, verbal and written comprehension, and writing abilities. She had no cognitive deficits, behavioral change, parkinsonism, limb ideomotor apraxia or any other neurological findings. Her overall examination was therefore consistent with the prosodic subtype of primary progressive apraxia of speech (PPAOS) with non-verbal oral apraxia; there was no evidence of dysarthria or aphasia on examination. FDG-PET demonstrated hypometabolism in the left greater than right SMA and left lateral premotor cortex extending into the middle and inferior frontal gyri (Figure).
Figure:
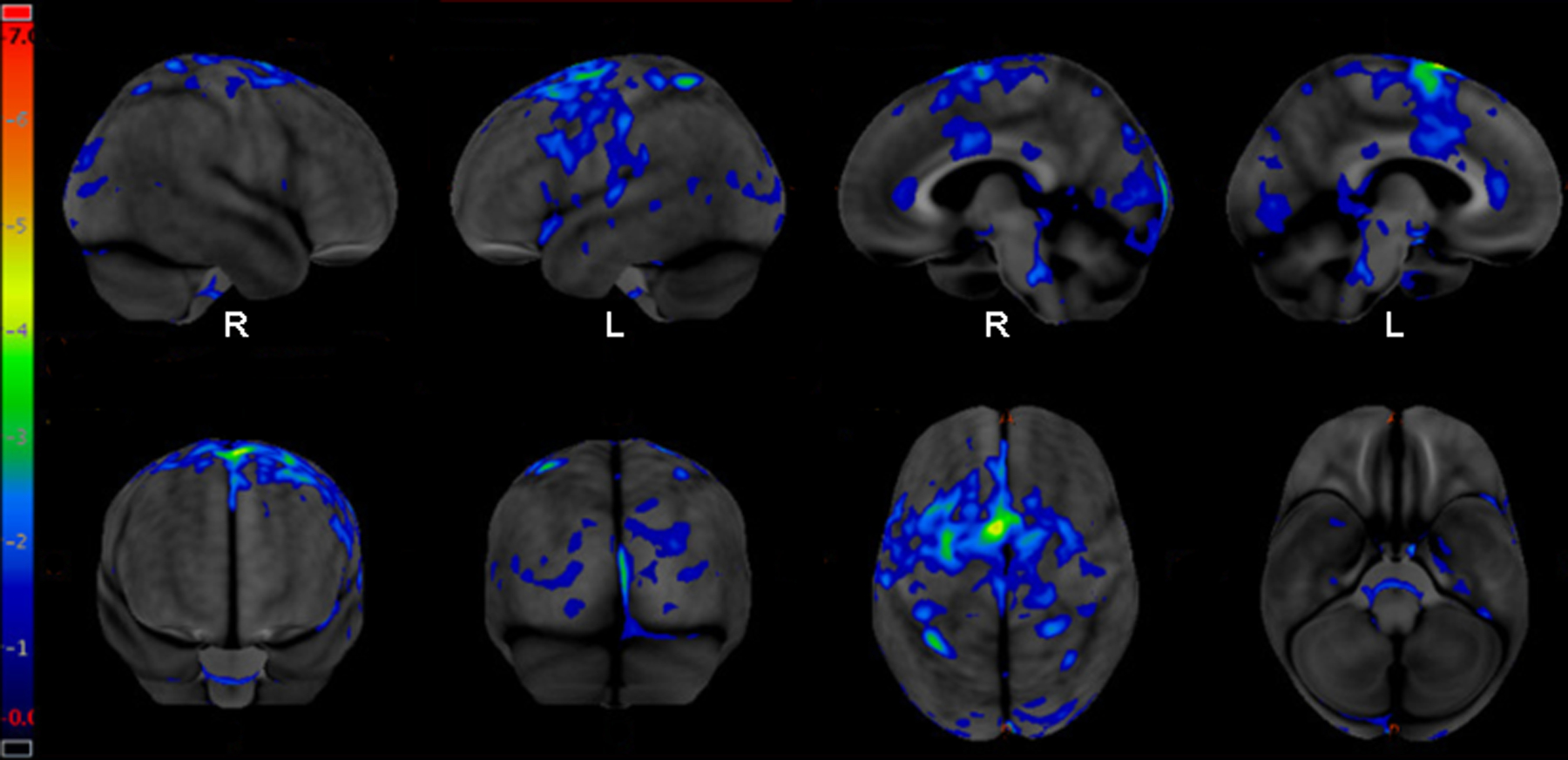
[18F] fluorodeoxyglucose PET (FDG-PET) demonstrating hypometabolism mainly in the right and left premotor and supplementary motor areas. The left hemisphere is slightly more affected than the right hemisphere and there is subtle extension of hypometabolism into left middle and inferior frontal cortex.
Over time, she developed difficulty with balance and experienced falls about once monthly. She started to shake her head “yes” when she meant to shake her head “no” and say “thank you” when she meant to say “you’re welcome.” Otherwise, she had no difficulty with word order or error. Her handwriting became larger and less precise. Neurologically, she developed slowed vertical saccades, and right greater than left ideomotor apraxia, as well as slowing of complex hand sequencing (e.g., Luria sequence). Overall, she was developing a PSP-CBS overlap syndrome.
5.0. Conclusions
Progressive AOS can occur in the absence of aphasia and as a harbinger of progressive supranuclear palsy and corticobasal syndrome. The two clinical subtypes of AOS, with phonetic and prosodic predominant speech disturbances, map onto underlying CBS or PSP, respectively. New imaging techniques have documented the cortical and subcortical areas of involvement and their progression along the disease course. FDG-PET continues to be the most sensitive diagnostic biomarker and exploration is ongoing to identify those that might better predict individual patterns of progression. There is no data on the impact of behavioral intervention, although studies of nonfluent/agrammatic variant of primary progressive aphasia that include patients with AOS suggest some benefit in speech intelligibility and maintenance; further research is needed to assess outcomes of behavioral and other types of intervention.
References
Papers of particular interest, published recently, have been highlighted as:
* Of importance
** Of major importance
- 1.Allison KM, Cordella C, Iuzzini-Seigel J, Green JR (2020) Differential Diagnosis of Apraxia of Speech in Children and Adults: A Scoping Review. J Speech Lang Hear Res 63:2952–2994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Josephs KA, Duffy JR, Strand EA, Machulda MM, Senjem ML, Master AV, Lowe VJ, Jack CR Jr, Whitwell JL (2012) Characterizing a neurodegenerative syndrome: primary progressive apraxia of speech. Brain 135:1522–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Josephs KA, Duffy JR, Strand EA, Machulda MM, Senjem ML, Lowe VJ, Jack CR, Whitwell JL (2013) Syndromes dominated by apraxia of speech show distinct characteristics from agrammatic PPA. Neurology 81:337–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gorno-Tempini M, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SE, Manes F (2011) Classification of primary progressive aphasia and its variants. Neurology 76:1006–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bouvier L, Monetta L, Laforce R Jr, Vitali P, Bocti C, Martel‐Sauvageau V (2021) Progressive apraxia of speech in Quebec French speakers: A case series. Int J Lang Commun Disord 56:528–548 ** The authors report a relatively large series of patients with PAOS and PPAOS in French speakers from Quebec demonstrating that these syndromic diagnoses are not limited to English speakers.
- 6.Bouvier L, Monetta L, Vitali P, Laforce R Jr, Martel-Sauvageau V (2021) A Preliminary Look Into the Clinical Evolution of Motor Speech Characteristics in Primary Progressive Apraxia of Speech in Québec French. Am J Speech Lang Pathol 30:1459–1476 [DOI] [PubMed] [Google Scholar]
- 7.Duffy J (2006) Apraxia of speech in degenerative neurologic disease. Aphasiology 20(6):511–527 [Google Scholar]
- 8.Duffy JR, Strand EA, Clark H, Machulda M, Whitwell JL, Josephs KA (2015) Primary progressive apraxia of speech: clinical features and acoustic and neurologic correlates. Am J Speech Lang Pathol 24:88–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poole ML, Brodtmann A, Darby D, Vogel AP (2017) Motor Speech Phenotypes of Frontotemporal Dementia, Primary Progressive Aphasia, and Progressive Apraxia of Speech. J Speech Lang Hear Res 60:897–911 [DOI] [PubMed] [Google Scholar]
- 10.Botha H, Duffy JR, Whitwell JL, et al. (2015) Classification and clinicoradiologic features of primary progressive aphasia (PPA) and apraxia of speech. Cortex 69:220–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Botha H, Utianski RL, Whitwell JL, et al. (2018) Disrupted functional connectivity in primary progressive apraxia of speech. Neuroimage Clin 18:617–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Utianski RL, Duffy JR, Clark HM, et al. (2018) Prosodic and phonetic subtypes of primary progressive apraxia of speech. Brain Lang 184:54–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whitwell JL, Duffy JR, Strand EA, Machulda MM, Senjem ML, Gunter JL, Kantarci K, Eggers SD, Jack CR, Josephs KA (2013) Neuroimaging comparison of primary progressive apraxia of speech and progressive supranuclear palsy. Eur J Neurol 20:629–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Josephs KA, Duffy JR, Strand E, et al. (2006) Clinicopathological and imaging correlates of progressive aphasia and apraxia of speech. Brain 129:1385–1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Josephs KA, Duffy JR, Fossett TR, et al. (2010) Fluorodeoxyglucose f18 positron emission tomography in progressive apraxia of speech and primary progressive aphasia variants. Arch Neurol 67:596–605 [DOI] [PubMed] [Google Scholar]
- 16.Seckin ZI, Whitwell JL, Utianski RL, et al. (2020) Ioflupane 123I (DAT scan) SPECT identifies dopamine receptor dysfunction early in the disease course in progressive apraxia of speech. J Neurol 267:2603–2611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Josephs KA, Duffy JR, Clark HM, et al. (2021) A molecular pathology, neurobiology, biochemical, genetic and neuroimaging study of progressive apraxia of speech. Nat Commun 12:3452. ** The authors identified an association between phonetic AOS and corticobasal degeneration pathology, and between prosodic AOS and progressive supranuclear palsy pathology highlighting the importance of subtyping AOS.
- 18.Utianski RL, Whitwell JL, Schwarz CG, et al. (2018) Tau-PET imaging with [18F]AV-1451 in Primary Progressive Apraxia of Speech. Cortex 99:358–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Josephs KA, Duffy JR, Strand EA, et al. (2014) The evolution of primary progressive apraxia of speech. Brain 137:2783–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seckin ZI, Duffy JR, Strand EA, et al. (2020) The evolution of parkinsonism in primary progressive apraxia of speech: A 6-year longitudinal study. Parkinsonism Relat Disord 81:34–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whitwell JL, Duffy JR, Machulda MM, et al. (2017) Tracking the development of agrammatic aphasia: A tensor-based morphometry study. Cortex 90:138–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Whitwell JL, Weigand S, Duffy J, Clark H, Strand E, Machulda M, Spychalla A, Senjem M, Jack CR, Josephs KA (2017) Predicting clinical decline in progressive agrammatic aphasia and apraxia of speech. Neurology 89:2271–2279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Utianski RL, Duffy JR, Clark HM, Strand EA, Boland SM, Machulda MM, Whitwell JL, Josephs KA (2021) Clinical Progression in Four Cases of Primary Progressive Apraxia of Speech. Am J Speech Lang Pathol 27:1303–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duffy JR, Utianski RL, Josephs KA (2021) Primary progressive apraxia of speech: from recognition to diagnosis and care. Aphasiology 35:560–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Botha H, Josephs KA (2019) Primary progressive aphasias and apraxia of speech. Contin Lifelong Learn Neurol 25:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duffy JR, Martin PR, Clark HM, Utianski RL, Strand EA, Whitwell JL, Josephs KA (2023) The Apraxia of Speech Rating Scale: Reliability, Validity, and Utility. Am J Speech Lang Pathol 1–23 [DOI] [PMC free article] [PubMed]
- 27.Duffy JR (2020) Motor Speech Disorders: Substrates, Differential Diagnosis, and Management, 4e. Mosby, St. Louis [Google Scholar]
- 28.Utianski RL (2019) Primary progressive aphasia and other frontotemporal dementias: Diagnosis and treatment of associated communication disorders Plural Publishing [Google Scholar]
- 29.Mailend ML, Maas E (2020) To Lump or to Split? Possible Subtypes of Apraxia of Speech. Aphasiology 35:592–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takakura Y, Otsuki M, Sakai S, et al. (2019) Sub-classification of apraxia of speech in patients with cerebrovascular and neurodegenerative diseases. Brain Cogn 130:1–10 [DOI] [PubMed] [Google Scholar]
- 31.Botha H, Duffy JR, Strand EA, Machulda MM, Whitwell JL, Josephs KA (2014) Nonverbal oral apraxia in primary progressive aphasia and apraxia of speech. Neurology 82:1729–1735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morihara K, Ota S, Kakinuma K, Kawakami N, Higashiyama Y, Kanno S, Tanaka F, Suzuki K (2023) Buccofacial apraxia in primary progressive aphasia. Cortex 158:61–70 [DOI] [PubMed] [Google Scholar]
- 33.Duffy JR, Strand EA, Josephs KA (2014) Motor speech disorders associated with primary progressive aphasia. Aphasiology 28:1004–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ash S, McMillan C, Gunawardena D, Avants B, Morgan B, Khan A, Moore P, Gee J, Grossman M (2010) Speech errors in progressive non-fluent aphasia. Brain Lang 113:13–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Croot K, Ballard K, Leyton CE, Hodges JR (2012) Apraxia of speech and phonological errors in the diagnosis of nonfluent/agrammatic and logopenic variants of primary progressive aphasia. J Speech Lang Hear Res 55:S1562–72 [DOI] [PubMed] [Google Scholar]
- 36.Scheffel L, Duffy JR, Strand EA, Josephs KA (2021) Word Fluency Test Performance in Primary Progressive Aphasia and Primary Progressive Apraxia of Speech. Am J Speech Lang Pathol 30:2635–2642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Polsinelli AJ, Machulda MM, Martin PR, et al. (2021) Neuropsychological Profiles of Patients with Progressive Apraxia of Speech and Aphasia. J Int Neuropsychol Soc 1–11 [DOI] [PMC free article] [PubMed]
- 38.Valls Carbo A, Reid RI, Tosakulwong N, et al. (2022) Tractography of supplementary motor area projections in progressive speech apraxia and aphasia. NeuroImage Clin 34:102999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Whitwell JL, Stevens CA, Duffy JR, et al. (2019) An Evaluation of the Progressive Supranuclear Palsy Speech/Language Variant. Mov Disord Clin Pract 6:452–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Whitwell JL, Martin P, Duffy JR, Clark HM, Utianski RL, Botha H, Machulda MM, Strand EA, Josephs KA (2021) Survival analysis in primary progressive apraxia of speech and agrammatic aphasia. Neurol Clin Pract 11:249–255 ** The authors found evidence for patients with PPAOS to have better survival compared to patients with PAOS (i.e., having aphasia) highlighting the importance of distinguishing PPAOS from PPA.
- 41.Armstrong MJ, Litvan I, Lang AE, Bak TH, Bhatia KP, Borroni B, et al. (2013) Criteria for the diagnosis of corticobasal degeneration. Neurology 80:496–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hokelekli FO, Duffy JR, Clark HM, et al. (2022) Autopsy Validation of Progressive Supranuclear Palsy-Predominant Speech/Language Disorder Criteria. Mov Disord Off J Mov Disord Soc 37:213–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hokelekli FO, Ali F, Carlos AF, et al. (2021) Sleep disturbances in the speech-language variant of progressive supranuclear palsy. Parkinsonism Relat Disord 91:9–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hokelekli FO, Duffy JR, Clark HM, Utianski RL, Botha H, Stierwalt JA, Strand EA, Machulda MM, Whitwell JL, Josephs KA (2022) Cross-Sectional and Longitudinal Assessment of Behavior in Primary Progressive Apraxia of Speech and Agrammatic Aphasia. Dement Geriatr Cogn Disord 51:193–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Robinson CG, Duffy JR, Clark HA, et al. (2023) Clinicopathological associations of hemispheric dominance in primary progressive apraxia of speech. Eur J Neurol 30:1209–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tetzloff KA, Duffy JR, Strand EA, et al. (2018) Clinical and imaging progression over 10 years in a patient with primary progressive apraxia of speech and autopsy-confirmed corticobasal degeneration. Neurocase 24:111–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Josephs KA, Duffy JR, Strand EA, Machulda MM, Senjem ML, Lowe VJ, Jack CR, Whitwell JL (2014) APOE ε4 influences β-amyloid deposition in primary progressive aphasia and speech apraxia. Alzheimers Dement 10:630–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lowe V, Curran G, Fang P, et al. (2016) An autoradiographic evaluation of AV-1451 Tau PET in dementia. Acta Neuropathol Commun 4:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Whitwell JL, Höglinger GU, Antonini A, et al. (2017) Radiological biomarkers for diagnosis in PSP: Where are we and where do we need to be? Mov Disord Off J Mov Disord Soc 32:955–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sintini I, Duffy JR, Clark HM, et al. (2022) Functional connectivity to the premotor cortex maps onto longitudinal brain neurodegeneration in progressive apraxia of speech. Neurobiol Aging 120:105–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Satoh R, Arani A, Senjem ML, et al. (2023) Spatial patterns of elevated magnetic susceptibility in progressive apraxia of speech. NeuroImage Clin 38:103394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sjöström H, Granberg T, Westman E, Svenningsson P (2017) Quantitative susceptibility mapping differentiates between parkinsonian disorders. Parkinsonism Relat Disord 44:51–57 [DOI] [PubMed] [Google Scholar]
- 53.Josephs KA, Boeve B, Duffy J, Smith G, Knopman D, Parisi J, Petersen R, Dickson D (2005) Atypical progressive supranuclear palsy underlying progressive apraxia of speech and nonfluent aphasia. Neurocase 283–296 [DOI] [PubMed]
- 54.Buciuc M, Koga S, Pham NTT, et al. (2023) The many faces of globular glial tauopathy: A clinical and imaging study. Eur J Neurol 30:321–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Flanagan EP, Baker MC, Perkerson RB, Duffy JR, Strand EA, Whitwell JL, Machulda MM, Rademakers R, Josephs KA (2015) Dominant frontotemporal dementia mutations in 140 cases of primary progressive aphasia and speech apraxia. Dement Geriatr Cogn Disord 39:281–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Henry ML, Hubbard HI, Grasso SM, et al. (2018) Retraining speech production and fluency in non-fluent/agrammatic primary progressive aphasia. Brain 141:1799–1814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Themistocleous C, Webster K, Tsapkini K (2021) Effects of tDCS on Sound Duration in Patients with Apraxia of Speech in Primary Progressive Aphasia. Brain Sci 11:335. * The authors provide some evidence for transcranial direct current stimulation to be beneficial to maximizing the efficacy of speech therapy in patients with PAOS.
- 58.Shpiner DS, McInerney KF, Miller M, Allen J, Rice J, Luca CC, Adams D, Gomes-Osman J (2019) High frequency repetitive transcranial magnetic stimulation for primary progressive apraxia of speech: A case series. Brain Stimulat 12:1581–1582 [DOI] [PubMed] [Google Scholar]
- 59.Strand EA, Duffy JR, Clark HM, Josephs KA (2014) The Apraxia of Speech Rating Scale: A new tool for diagnosis and description of AOS. J Commun Disord 51:43–50 [DOI] [PMC free article] [PubMed] [Google Scholar]


