Abstract
Background
Critically ill patients suffer from acute muscle wasting, which is associated with significant physical functional impairment. We describe data from nested muscle biopsy studies from two trials of functional electrical stimulation (FES) that did not shown improvements in physical function.
Methods
Primary cohort: single-centre randomized controlled trial. Additional healthy volunteer data from patients undergoing elective hip arthroplasty. Validation cohort: Four-centre randomized controlled trial. Intervention: FES cycling for 60-90min/day. Analyses: Skeletal muscle mRNA expression of 223 genes underwent hierarchal clustering for targeted analysis and validation.
Results
Positively enriched pathways between healthy volunteers and ICU participants were “stress response”, “response to stimuli” and “protein metabolism”, in keeping with published data. Positively enriched pathways between admission and day 7 ICU participants were “FOXO-mediated transcription” (admission = 0.48 ± 0.94, day 7 = − 0.47 ± 1.04 mean log2 fold change; P = 0.042), “Fatty acid metabolism” (admission = 0.50 ± 0.67, day 7 = 0.07 ± 1.65 mean log2 fold change; P = 0.042) and “Interleukin-1 processing” (admission = 0.88 ± 0.50, day 7 = 0.97 ± 0.76 mean log2 fold change; P = 0.054). Muscle mRNA expression of UCP3 (P = 0.030) and DGKD (P = 0.040) decreased in both cohorts with no between group differences. Changes in IL-18 were not observed in the validation cohort (P = 0.268). Targeted analyses related to intramuscular mitochondrial substrate oxidation, fatty acid oxidation and intramuscular inflammation showed PPARγ-C1α; (P < 0.001), SLC25A20 (P = 0.017) and UCP3 (P < 0.001) decreased between admission and day 7 in both arms. LPIN-1 (P < 0.001) and SPT1 (P = 0.044) decreased between admission and day 7. IL-18 (P = 0.011) and TNFRSF12A (P = 0.009) increased in both arms between admission and day 7. IL-1β (P = 0.007), its receptor IL-1R1 (P = 0.005) and IL-6R (P = 0.001) decreased in both arms between admission and day 7. No between group differences were seen in any of these (all p > 0.05).
Conclusions
Intramuscular inflammation and altered substrate utilization are persistent in skeletal muscle during first week of critical illness and are not improved by the application of Functional Electrical Stimulation-assisted exercise. Future trials of exercise to prevent muscle wasting and physical impairment are unlikely to be successful unless these processes are addressed by other means than exercise alone.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13054-023-04664-7.
Keywords: Muscle wasting, Critical illness, Exercise, Rehabilitation, Gene expression
Background
Critically ill patients suffer from acute muscle wasting, resulting in significant physical functional impairment [1, 2]. After discharge, patients struggle to regain muscle mass, strength and functional capacity [3]. This results in substantial functional limitations that persist in 70% of patients at 6–12 months, and 30% remain carer-dependent [2]. At 12 months, only 56% of previously employed patients return to work, with physical disability and fatigue as associative factors [4]. The scale and characteristics of this muscle wasting have now been described: rates of 2–3% loss per day are seen consistently, with preferential loss of Type II muscle fibres and evidence of non-excitable muscle membrane [1, 5]. Loss of muscle mass is the result of altered protein homeostasis from depressed muscle protein synthesis and increased protein degradation [1, 6].
Two parallel storylines have appeared in the literature in regard to acute muscle wasting and subsequent physical impairment: firstly, that neither physical nor nutritional interventions have been successful at preventing or treating this physical impairment [7, 8] and secondly, that the underpinning biology of altered protein homeostasis described in observational studies increasingly converges on common physiological and metabolic alterations [9].
Resistance exercise is a major stimulant of muscle protein synthesis, and exercise rehabilitation has grade I level evidence for improving physical function in other populations [10]. However, exercise interventions have not proven to be efficacious in the critically ill population. A systematic review of 43 trials demonstrated that physical rehabilitation in isolation cannot prevent or restore the loss of function associated with critical illness [11]. A narrative review of nine trials of increased substrate delivery did not demonstrate improvements in physical function [12]. Recently, the TARGET trial demonstrated no difference in quality of life or return to work rates with increased calorie delivery [13]. Increasing nutrition delivery in the EPANIC trial resulted in decreased muscle quality [14].
Persistent inflammation in critically ill patients is well described and contributes to both short- and long-term morbidity [15]. Intramuscular inflammation and cellular infiltration were first described in 1991 [16]. Tumour necrosis alpha (TNFα), hypoxia-inducible factor-1 (HIF1) and interleukin-6 (IL-6) and interleukin-10 (IL-10) have all been reported to increase [17–19]. The cellular infiltrates have been characterized to be CD68-positive macrophages, though not seen in all studies [17, 20, 21]. Mitochondrial dysfunction was first described in 2002 and confirmed in subsequent studies [22–24]. Inflammation and hypoxia signalling lead to altered carbohydrate and lipid substrate utilization, and insulin resistance [18]. The combination of these processes results in a range of alterations of cellular bioenergetics [18, 24]. Muscle protein synthesis, the facilitative mechanism of maintaining and increasing muscle mass in humans, is highly energy dependent [25]. Unless the bioenergetic impairment and intramuscular inflammation are addressed, it would seem unlikely that the usual stimuli of muscle protein synthesis (resistance exercise and amino acids) will be successful and therefore address the physical impairment of critical illness survivors.
In this study, we describe data from nested muscle biopsy studies within two trials of functional electrical stimulation (FES) that did not shown improvements in physical function [26, 27]. We hypothesized that the application of FES in the very early stages of critical illness may not alleviate the inflammation and alteration of cellular bioenergetics, which would explain the lack of efficacy of the intervention. If so, functional electrical stimulation may be an appropriate co-intervention to trial alongside interventions to address the underpinning biology.
Methods
Primary cohort
Single-centre prospective randomized controlled trial. Inclusion criteria were: mechanical ventilation and predicted ICU length of stay ≥ 7 days. FES cycling was performed 90min/day for 7 days/week. Full details including ethical approval are available in the study protocol and the original publication [26, 28].
Validation cohort
Nested sub-study of a four-centre (Australian and USA) randomized controlled trial. Inclusion criteria were: mechanical ventilation and sepsis, severe sepsis or systemic inflammatory response syndrome and ICU stay ≥ 4 days post-randomization. FES cycling was performed 60min/day, ≥ 5 days/week unilaterally with the unstimulated leg acting as a control. Full details including ethical approval are available in the original publication [27].
Healthy volunteers
In addition to the critically ill participants, metabolically healthy participants undergoing elective hip arthroplasty in spinal or epidural anaesthesia were recruited. After obtaining a prospective written informed consent, vastus lateralis muscle sample was taken by open technique as soon as the muscle was exposed during surgery.
Functional electrical stimulation
Supine bikes with FES module RT300 System (© Restorative Therapies, Baltimore USA) Inc. 2005–2016. LB100108 Version 37 were used in both cohorts. Electrical stimulation was provided to the following muscles: rectus femoris; hamstrings; and gluteals and gastrocnemius using large size (20cm) electrodes. Electrodes were placed on both legs although muscle stimulation was only provided to the leg randomized to receive FES. The cycling only leg received sham electrical stimulation. The intervention was provided in addition to usual care rehabilitation by registered physiotherapists, experienced in critical care rehabilitation, who were not blinded to the randomization group.
Primary cohort
The intervention had a goal of being delivered 90 min per day, seven days a week. After warm-up phase (5 min of passive cycling), participants received therapy consisting of functional electrical stimulation or active cycling with duration adjusted per protocol and participants’ tolerance followed by relaxation phase (5 min of passive cycling). FES impulses had a pulse width of 250 μs, pulse frequency of 40 Hz and the lowest output per channel (in a range 0–60 mA) that allowed locomotive movement of lower extremities. To increase the intervention workload, both resistance (3–10 Nm) and cycling cadence were increased incrementally. When appropriate, FES was supplemented with protocolized goal-directed mobilization. Standard rehabilitation group received the usual care (SC), which was monitored but not protocolized. It consisted of passive muscle rehabilitation, in-bed exercises and standard mobilization, as appropriate.
Validation cohort
The intervention had a goal of being delivered for at least five out of seven day per week. Stimulation was set at 20–30 mA for all stimulated muscles and was titrated to achieve muscle contraction and participant comfort. The intervention was delivered to a single leg. Pulse width (microseconds) was set to 250 for average sized legs and was increased to 300 if the leg was oedematous. Frequency (Hertz) was set at a mean of 43.5 and increased as appropriate to 50 to induce a strong muscle contraction.
Sample collection
In all patients, using an aseptic technique under local anaesthetic injected into skin and fascia (i.e. 2 ml of 1% lignocaine) muscle biopsies were obtained from the vastus lateralis muscle 10 cm above the patella. Biopsies were performed by ICU medical staff using the percutaneous needle technique originally described by Bergstrom [29]. Biopsies were frozen in liquid nitrogen and stored at -80°C until biochemical analysis was performed.
Laboratory analyses: primary cohort and healthy volunteers
Total RNA was extracted from ∼20 mg frozen muscle tissue using TRI Reagent® (Thermo Fisher Scientific, MA, USA) according to the manufacturer’s protocol. Total RNA was quantified spectrophotometrically at 260 nm (NanoDrop ND-2000 Spectrophotometer; Thermo Fisher Scientific, MA, USA), and RNA purity was determined as the ratio of readings at 260/280 nm. Reverse transcription of RNA was carried out using a commercially available kit (SuperScript™ VILO™ cDNA Synthesis Kit, Thermo Fischer Scientific, MA, USA). Muscle mRNA expression of 224 genes from pathways associated with inflammation, insulin signalling, myogenesis, protein turnover, stress response, substrate metabolism, extracellular matrix remodelling, cellular amino acid transport and associated nuclear transcription factors were determined using custom designed RT-PCR OpenArray™ plates (Thermo Fischer Scientific, MA, USA) in combination with a QuantStudio 12K Flex Real-Time PCR system (Thermo Fischer Scientific, MA, USA) as we have previously described [30, 31]. A total of 223 genes were included in the final analysis. Relative muscle gene expression was calculated using the 2−ΔΔCT method. Gene expression values are presented as the log2 fold change in mRNA abundance calculated relative to healthy volunteers with the median cohort age, and the geometric mean of housekeeping genes β-actin and β2 microglobulin.
Laboratory analyses-validation cohort
Total RNA was extracted from 10 TO 20 mg muscle tissue using a commercially available kit, according to the manufacturer’s instructions (RNeasy Fibrous Tissue Mini Kit, Qiagen, VIC, Australia). RNA quality and concentration were determined using the NanoDrop 2000 spectrophotometer (Thermo Scientific, Waltham, MA, USA). RNA was transcribed into cDNA using iScript cDNA Synthesis Kit (Bio-Rad Laboratories, NSW, Australia). qPCR was performed using the Bio-Rad CFX384 PCR system (Bio- Rad Laboratories), in triplicate with reaction volumes of 10 ul, containing SsoAdvanced™ Universal SYBR® Green Supermix (Bio-Rad Laboratories), forward and reverse primers and cDNA template. The efficacy of B2M (beta2-microglobulin) as an endogenous control was examined using the equation 2−ΔCq. B2M mRNA was not influenced by the intervention and therefore was deemed an appropriate endogenous control. Data were analysed using a comparative quantification cycle (Cq) method where the amount of target relative to B2M is given by 2−ΔΔCq. Primers were designed using NCBI Primer BLAST from gene sequences obtained from GenBank and have bene described elsewhere [32] or are listed in Additional file 1: Table S1.
Data analysis
Untargeted functional enrichment analysis
Comparing the group difference in every gene would result in a considerable loss in statistical power due to the correction required for 223 individual statistical tests (to avoid family-wise error), leading to missing meaningful effects, or reporting type I errors. To overcome this, we used all 223 genes in the pathway approach to determine what pathways were perturbed by critical illness per se (corrected for family-wise error prior to pathway analysis). After applying a false discovery rate (FDR) of < 5% to adjust for family-wise error on these gene lists, in line with our previous work [30, 33] functional enrichment analysis was performed against the Reactome database using the PANTHER overrepresentation test (binomial test with no correction; released 2022–02-02; PANTHER version 17.0). The original gene list of 213 genes, as opposed to the whole genome, was used as the background. Network diagrams were generated using ShinyGo (version 0.76.2) using the Reactome pathway database and the whole genome as the background.
Targeted analysis and validation
The pathway analysis demonstrated key alterations in inflammatory and substrate utilization pathways. Forty genes specifically associated with substrate metabolism and inflammation were then selected a priori for a targeted investigation, by a researcher (ZP) not involved in data analysis. This gene list underwent an initial screening to identify genes which were differently expressed in ICU participants at admission compared with control participants using separate unpaired t tests, yielding 27 genes (substrate metabolism = 17, inflammation = 15). The effect of FES versus standard care on muscle gene expression was investigated using two-way repeated measure mixed effect models with time (admission vs day 7) and group (control vs FES) as factors. Furthermore, the expression of these 27 a priori selected genes underwent hierarchical clustering using Multiple Experiment Viewer (MeV, version 4.9.0) to generate a cluster of genes for validation in muscle samples obtained from the second FES study. The effect of FES versus standard care on muscle gene expression in the second FES study was investigated using two-way repeated measure mixed effect models with time (admission vs discharge) and leg condition (standard care vs FES) as factors.
Results
Out of 150 participants enrolled into the primary study, samples were taken from 31 participants (16 intervention, 15 controls) to form primary study cohort at admission and day 7 (Additional file 1: Figure S1). In the secondary cohort, samples were taken from 11 participants (5 interventions, 6 controls) at admission and ICU discharge (Additional file 1: Figure S2). The characteristics of these participants are shown in Table 1. A total of 17 participants undergoing elective hip replacement surgery were recruited as healthy volunteers.
Table 1.
Baseline study subject characteristics
| Primary cohort (Czech Republic) | Validation cohort (Australia) | Czech Republic | |||
|---|---|---|---|---|---|
| Intervention n = 16 | Control n = 15 | Intervention n = 5 | Control n = 6 | Healthy Volunteers | |
| Age | 58 ± 17 | 64 ± 11 | 65 ± 8.5 | 60 ± 17.8 | 64 ± 14 |
| Sex (M/F) | 12/4 | 10/5 | 3/2 | 3/3 | 9/8 |
| BMI | 29.2 ± 5.9 | 33.3 ± 8.1 | 26 ± 3.5 | 22 ± 2.9 | 29.9 ± 3.4 |
| APACHE II | 22 ± 5 | 27 ± 7 | 21 ± 6.3 | 17 ± 4.3 | N/A |
| ICU LOS pre-recruitment | 1.4 ± 0.8 | 1.4 ± 0.7 | 2 ± 0.9 | 4 ± 2.2 | N/A |
| History of diabetes (%) |
6/10 (38%) |
7/8 (47%) |
0/0 (0%) |
2/6 (33%) |
0/17 (0%) |
| Pre-admission CCI | 2.9 ± 2.0 | 3.7 ± 2.8 | 2 ± 1.4 | 3 ± 2.9 | N/A |
| Diagnostic category | |||||
| Trauma | 8 (50%) | 4(25%) | 0 (0%) | 0 (0%) | |
| Surgical | 3 (19%) | 1 (7%) | 2 (40%) | 1 (16%) | |
| Medical | 5 (31%) | 10 (68%) | 3 (60%) | 5 (84%) | |
| Sepsis | 5 (31%) | 5 (33%) | 5 (100%) | 6 (100%) | |
Data are mean ± SD unless stated. BMI Body Mass Index, APACHE II Acute Physiology and Chronic Health Evaluation, ICU LOS Intensive Care Unit Length of Stay, CCI Charlson Comorbidity Index
Untargeted gene expression: pathway analysis
After applying a FDR < 5% correction, 137 genes were differentially expressed between healthy volunteers and ICU participants at ICU admission. The three most positively enriched pathways associated with this gene list were “Cellular response to stress” (healthy volunteers = -0.20 ± 0.50, ICU participants = 0.93 ± 0.74 mean log2 fold change from a nominal healthy volunteer; P = 0.047), “Cellular response to stimuli” (healthy volunteers = -0.20 ± 0.50, ICU participants = 0.93 ± 0.74 mean log2 fold change; P = 0.062) and “Metabolism of proteins” (healthy volunteers = − 0.12 ± 0.51, ICU participants = 0.93 ± 0.76 mean log2 fold change; P = 0.089) (Additional file 1: Figs. 1A, 2A, and Additional file 1: Table S2 for gene lists).
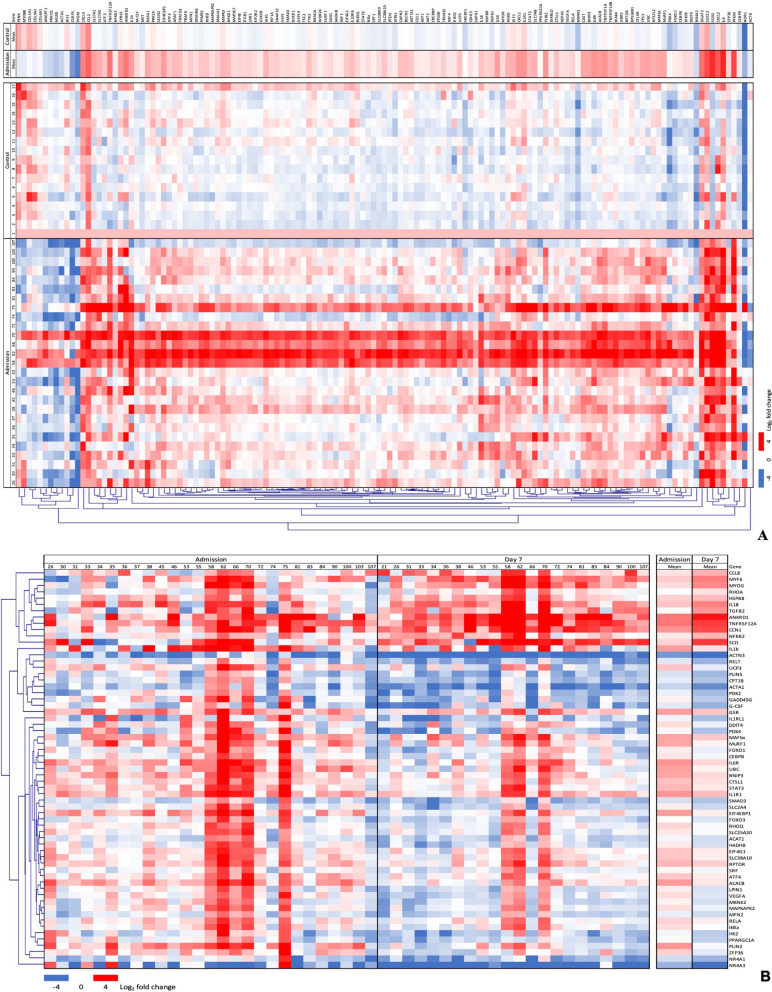
Fig. 1.
Heat maps of intramuscular log2 fold change differential gene expression between critically ill patients on day 1 (admission) versus healthy volunteers undergoing elective hip surgery (A) and critically ill patients on day 7 (B)
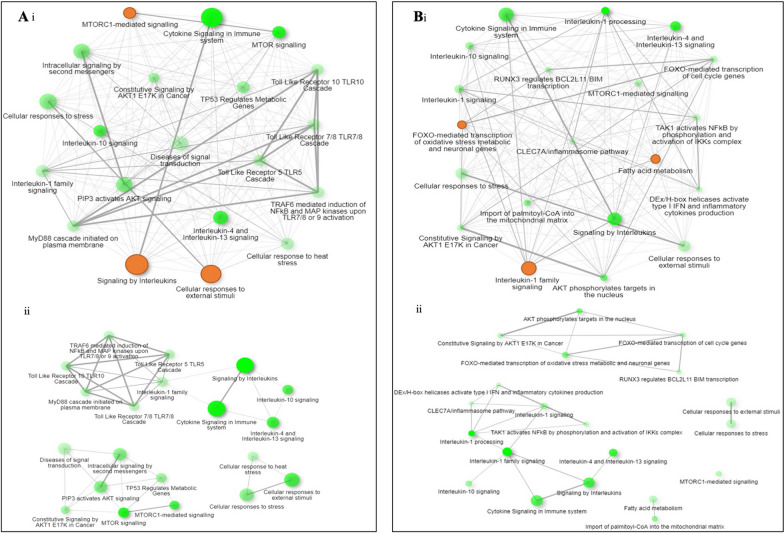
Fig. 2.
Network maps of muscle gene expression generated from the Reactome database using overrepresentation tests. Top maps (i) provide an overview of all enriched pathways, whereas bottom maps (ii) provide detail on the top three most enriched pathways shown in orange. A Differentially enriched pathways between critically ill patients on day 1 (admission) versus healthy volunteers undergoing elective hip surgery. B Differentially enriched pathways between critically ill patients on day 1 (admission) versus day 7
After applying a FDR < 5% correction, 63 genes were differentially expressed between ICU participants at admission compared with the same participants at day 7. The three most positively enriched pathways associated with this gene list were “FOXO-mediated transcription of oxidative stress, metabolic and neuronal genes” (admission = 0.48 ± 0.94, day 7 = − 0.47 ± 1.04 mean log2 fold change; P = 0.042), “Fatty acid metabolism” (admission = 0.50 ± 0.67, day 7 = 0.07 ± 1.65 mean log2 fold change; P = 0.042) and “Interleukin-1 processing” (admission = 0.88 ± 0.50, day 7 = 0.97 ± 0.76 mean log2 fold change; P = 0.054) (Figs. 1B and 2B). The small numerical difference between means at some time points is a result of pathways containing both genes that are up (positive expression value)- and downregulated (negative expression value).
Targeted gene expression (Mitochondrial substrate oxidation)
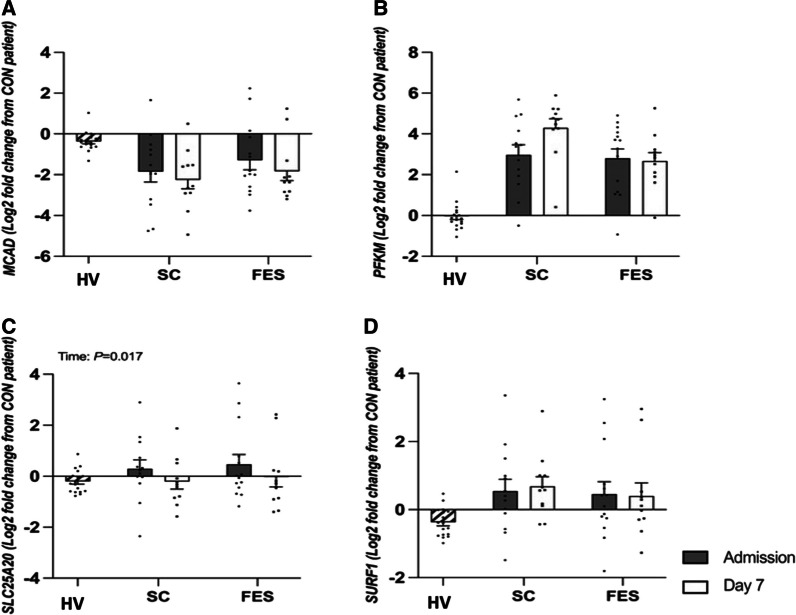
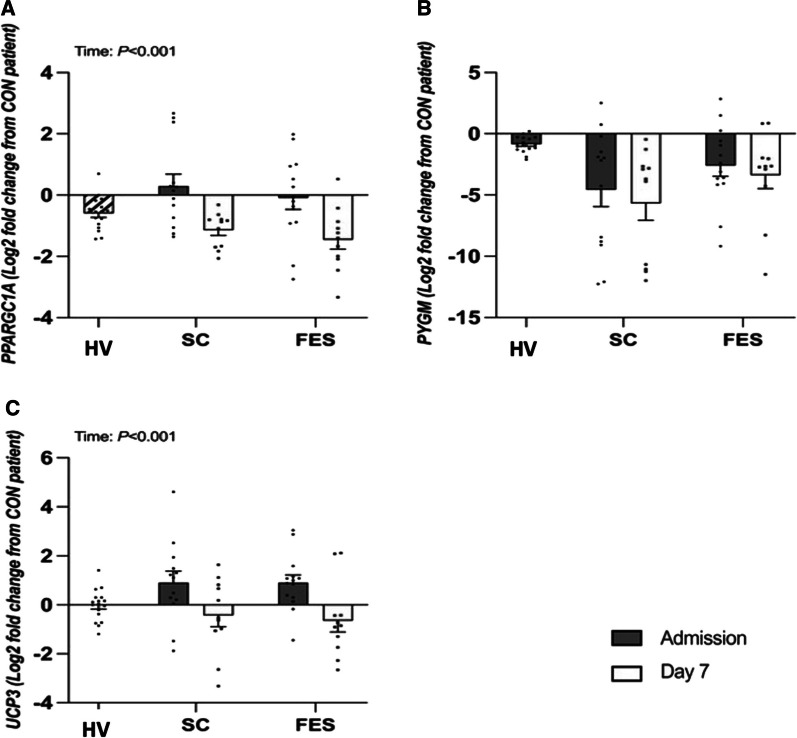
The log2 fold change in muscle expression of PPARγ-C1α; (P < 0.001), SLC25A20 (P = 0.017) and UCP3 (P < 0.001) decreased between admission and day 7 in both control and FES arms with no group difference (all interaction effect; P > 0.05, Figs. 3 and 4, Additional file 1: Table S3). No differences were seen between admission and day 7 in MCAD, PFKM, SURF-1 and PYGM gene expression (all p > 0.05).
Fig. 3.
Targeted fold change differences in skeletal muscle gene expression regulating mitochondrial substrate oxidation between healthy volunteers (HV) and critically ill patients either receiving routine standard of care (SC) or Functional Electrical Stimulation (FES) of genes; MCAD Acyl-CoA Dehydrogenase Medium Chain; PFKM Phosphofructokinase; SLC25a20 Solute Carrier Family 25 Member 20; and SURF-1 Surfeit locus protein 1. Data are expressed as mean ± SEM
Fig. 4.
Targeted fold change differences in skeletal muscle gene expression regulating mitochondrial substrate oxidation between healthy volunteers (HV) and critically ill patients either receiving routine standard of care (SC) or Functional Electrical Stimulation (FES) of genes; PPARGC1A = Peroxisome proliferator-activated receptor gamma coactivator 1-alpha; PYGM = Glycogen phosphorylase; UCP3 = Uncoupling Protein 3. Data are expressed as mean ± SEM
Targeted gene expression (Lipid synthesis)
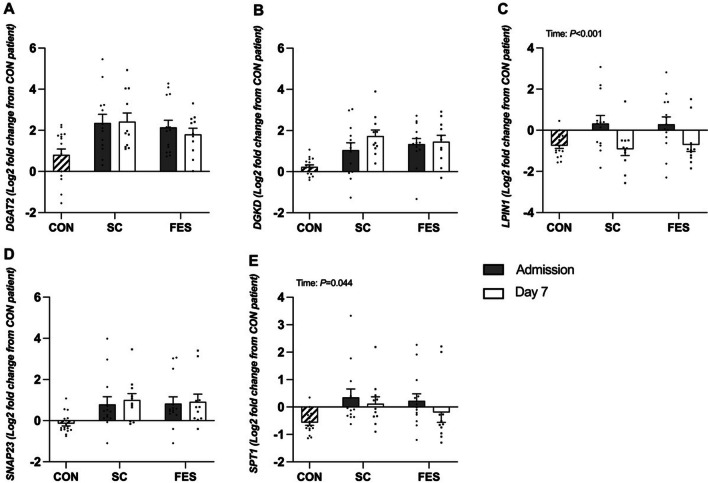
The log2fold change in muscle expression of LPIN-1 (P < 0.001) and SPT1 (P = 0.044) decreased between admission and day 7 in both control and FES arms with no group differences (both interactions effects; P > 0.05, Fig. 5, Additional file 1: Table S3). No differences were seen between admission and day 7 in DGKD, DGAT2 and SNAP 23 gene expression (all p > 0.05).
Fig. 5.
Targeted fold change differences in skeletal muscle genes regulating lipid synthesis between healthy volunteers (HV) and critically ill patients either receiving routine standard of care (SC) or Functional Electrical Stimulation (FES). DGAT2 = Diacylglycerol O-Acyltransferase 2; DGKD = Diacylglycerol Kinase Delta; LPN1 = Lipin-1; SNAP23 = Synaptosome Associated Protein 23; and SPT1 = Serine palmitoyltransferase 1. Data are expressed as mean ± SEM
Targeted gene expression (Intramuscular Inflammation)
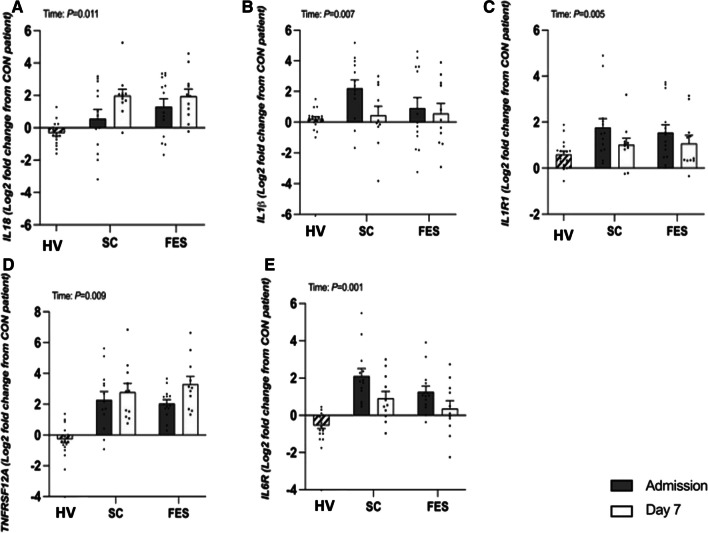
The log2fold change in muscle mRNA expression of Il-18 (P = 0.011) and TNFRSF12A (P = 0.009) increased in control and FES arms between admission and day 7, with no group differences (both interaction effects; P > 0.05). The mRNA expression of Il-1β (P = 0.007), its receptor Il-1R1 (P = 0.005) and Il-6R (P = 0.001) decreased in control and FES arms between admission and day 7 with no group differences (all interaction effects; P > 0.05, Fig. 6, Additional file 1: Table S3). No differences were seen in other inflammatory gene expression, (Additional file 1: Figure S3).
Fig. 6.
Targeted fold change differences in skeletal muscle genes regulating intramuscular inflammation between healthy volunteers (HV) and critically ill patients either receiving routine standard of care (SC) or Functional Electrical Stimulation (FES). IL-1b = Interleukin 1B; IL-1R1 = Interleukin 1 receptor 1; IL-18 = Interleukin 18; Il6R = Interleukin 6 receptor; and TNFRSF12A = Tumour necrosis factor receptor superfamily member 12A; Data are expressed as mean ± SEM
Validation of gene expression
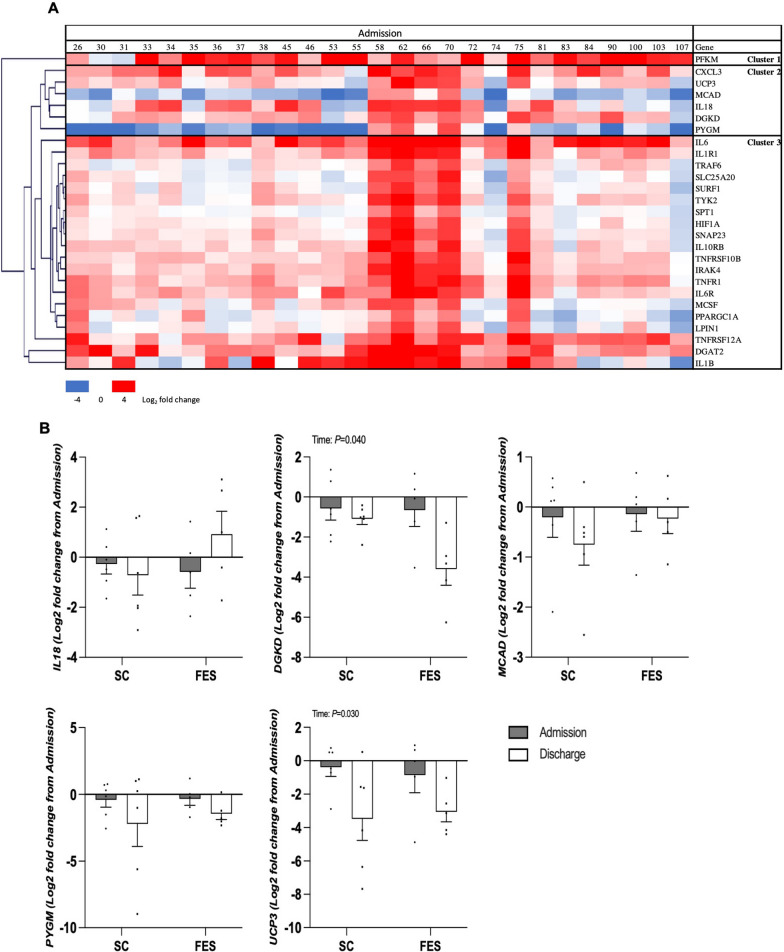
Hierarchal clustering was performed on 27 a priori selected genes which were differentially expressed between healthy volunteers and ICU participants at admission to identify a cluster in an unbiased manner that could be validated in a separate cohort (Fig. 7A). Clustering identified 3 distinct clusters and “cluster 2” was selected as it contained genes associated with both inflammation and substrate utilization, and however, CXCL3 could not be measured in validation samples due to methodological issue.
Fig. 7.
A Hierarchal clustering on 27 a priori selected genes which were differentially expressed between healthy volunteers (HV) and ICU patients at admission, identifying cluster for validation. B Differential gene expression in the validation cohort between ICU admission and discharge in ICU patients undergoing unilateral Functional Electrical Stimulation (FES) with the contralateral leg receiving standard care (SC). IL-18 = Interleukin-18; DGKD = Diacylglycerol Kinase Delta; MCAD = Acyl-CoA Dehydrogenase Medium Chain; PYGM = Glycogen phosphorylase; and UCP3 = Uncoupling Protein 3. Data are presented as fold change with gene expression normalized to patient’s admission values for each group, mean ± SEM
In a similar fashion to patterns of mRNA expression observed in the primary cohort, muscle mRNA expression of UCP3 (P = 0.030) decreased in both arms between admission and day 7 in the validation cohort with no group differences (interaction effect; P = 0.672). Further, a decrease in DGKD mRNA expression (P = 0.040) was observed in the validation cohort with no group differences (interaction effect; P = 0.128). Changes in IL-18 (intramuscular inflammation) were not observed in the validation cohort (P = 0.268, Fig. 7B, Additional file 1: Table S4).
Discussion
We performed an analysis of muscle biopsy samples collected prospectively as nested physiological sub-studies from two randomized controlled trials of Functional Electrical Stimulation (FES) in critically ill patients. The primary aim was to understand the underpinning mechanism of the failure of the intervention to improve patients’ functional outcomes. Gene expression data demonstrated an increase in intramuscular inflammation and altered substrate utilization, which was seen in both trials (primary and validation cohorts). Pathway analyses demonstrate these alterations to be consistent along congruent biological processes, deepening our understanding of these phenomena.
These data are consistent with previous data on the metabolic phenotype of skeletal muscle in critical illness, and importantly, we were not able to detect any significant or consistent changes of this pattern by the application of FES. The presence of critical illness seems to strongly dominate the possible influence of FES on muscle biology. These findings offer an insight into the mechanism of the lack of efficacy of exercise interventions to improve functional outcomes in critically ill patients.
Altered substrate utilization impedes both muscle mass and function
Decreases in pyruvate dehydrogenase kinase gene expression reflect the effects of hypoxia and inflammation on carbohydrate metabolism preventing pyruvate from entering the Krebs cycle, and diverting to lactate production (i.e. the Pasteur effect [34]). Decreases in lipid metabolism are seen at various points of the fatty acid oxidization process, with decrease in carnitine-acylcarnitine translocase and Acetyl-CoA acetyltransferase-1 gene expression seen. Decreases in Lipin-1 and increases in Stearoyl-CoA gene expression may be the causal pathways for accumulation of intramyocellular lipid seen in critically ill patients [20]. These metabolic derangements lead to the bioenergetic failure seen in critical illness, which will impede both muscle protein synthesis and muscle contractile force [18, 35]. These derangements would need to be addressed if FES protocols are to be effective in maintaining muscle mass and improving muscle function in critically ill patients.
Intramuscular inflammation may be the driver of anabolic resistance
Increases in pro-inflammatory cytokine gene expression within the skeletal muscle of critically ill patients have been well described, though there is significant heterogeneity in patients affected and concentrations seen, which is reflected in this analysis. The pathway analyses suggest that inflammation precedes metabolic alterations in substrate utilization. These metabolic alterations contribute to muscle dysfunction and generate an environment not conducive to favourable adaptations with normal anabolic signalling, i.e. anabolic resistance [36]. Anabolic resistance is prevalent in critical care settings decreasing nutritional protein incorporation into skeletal muscles [37]. These data add to the growing body of evidence that, in the same vein as exercise interventions, nutritional interventions are unlikely to be successful in the presence of intramuscular inflammation. Importantly, these data offer a caveat to the lack of efficacy of the FES intervention: there may still be a role for FES in patients without inflammation, or once inflammation has resolved.
Implications for future trials and clinical practice
FES represents the current state of the art in delivering resistance exercise (as a stimulus for muscle protein synthesis) to those unable to volitionally participate. These data represent a call for a cessation for trials of resistance exercise as standalone interventions, and a rationale to couple resistance exercise with co-interventions to address altered substrate utilization and intramuscular inflammation. The role of early resistance exercise in critically ill patients remains unclear, though a clear distinction must be made with early mobilization, for which there is sufficient data to show that this improves outcomes. The benefits of early mobilization have its causal roots across many processes (e.g. related to sedation-holds, delirium management, patient engagement, lung aeration) that are unrelated to maintenance of skeletal muscle mass and function [38]. The presence of altered substrate utilization and inflammation is likely to be causal in regard to muscle wasting, and future research may be better applied to exercise interventions once these processes have resolved.
Implications for future mechanistic research
The relationship between intramuscular inflammation, altered substrate utilization and loss of muscle mass has been described [18]. A similar relationship with muscle function has been less so. The lack of efficacy of FES interventions in critically ill patients could be attributed to compromised muscle adaptive responses and altered contractile activity. Exploring these potential mechanisms was outside the scope of this study, though in future such studies might identify what must be addressed to enhance therapeutic efficacy. These mechanisms are likely those that might similarly interfere with the expected adaptations to exercise, nutrition and/or pharmacological interventions in older adults or cancer cachexia where inflammation is a common mechanism [39, 40]. Loss of neuromuscular junction (NMJ) integrity has been reported in settings of acute and chronically elevated inflammation, such as in cancer cachexia and sarcopenia [41]. The temporary (or long-term) loss of a functioning connection between pre-synaptic and post-synaptic structures at the NMJ could account for some of the muscle wasting and weakness in critical care settings and compromise the efficacy of FES protocols. The dystrophin–glycoprotein complex (DGC) has well-described roles in normal force transmission, especially in the context of muscle diseases such as Duchenne muscular dystrophy, with evidence accumulating for other roles in anabolic and vascular signalling [42].
Strengths and limitations
This analysis has several strengths, based around the a priori decision to embed muscle biology analysis in clinical trials, the blinding of the laboratory analyses from clinical data, and the use of two independent cohorts from two groups in different continents using the same intervention. Limitations include the small number of samples in the validation cohort, and the limited amount of tissue available for analysis, requiring focused validation of biological processes and the minor differences in trial protocols between cohorts. Nevertheless, these data represent, to our knowledge, both the first embedded muscle biology analyses within a critical care exercise or rehabilitation trial, and the first paper to use primary and validation cohorts to demonstrate findings within skeletal muscle of critically ill patients.
Conclusions
Intramuscular inflammation and altered substrate utilization are persistent in skeletal muscle during first week of critical illness and are not improved by the application of Functional Electrical Stimulation-assisted exercise. Future trials of exercise to prevent muscle wasting and physical impairment are unlikely to be successful unless these processes are addressed by other means than exercise alone.
Supplementary Information
Author contributions
FD, LD, SB and ZP conceived, designed and implemented clinical study. TJ, FD, GL, MK, RK,ZP, AK and TU conceived and designed analyses. Analysis was performed by TJ and MK. Manuscript was drafted by TJ, MK and ZP. All authors reviewed and approved the final manuscript.
Funding
This study was supported by grants 16-28663A and NU21J-06-00078 of AZV (Agentura pro zdravotnicky vyzkum) of the Czech Ministry of Health and the National Health and Medical Research Council of Australia (App1079460), the Mrs. Sheila S. Pakula and Dr Lawrence C. Pakula Patient Recovery Fund in Pulmonary and Critical Care Medicine at Johns Hopkins University and The Intensive Care Foundation of Australia and Austin Health Medical Research Fund. Restorative Therapies provided the RT 300 supine cycle ergometer to the Austin Health site.
Availability of data and materials
Source data can be obtained from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
Applicable for both human and/or animal studies. Ethical committees, Internal Review Boards and guidelines followed must be named. When applicable, additional headings with statements on consent to participate and consent to publish are also required.
Competing of interests
Z. A. P. reports personal fees from Faraday Pharmaceuticals, Lyric Pharmaceuticals, Bioage Fresenius Kabi, Nestlé, Orion and GlaxoSmithKline, outside the submitted work.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
S. Berney, F. Duska and Z. Puthucheary joint senior authors.
References
- 1.Puthucheary ZA, Rawal J, McPhail M, et al. Acute skeletal muscle wasting in critical illness. JAMA. 2013;310(15):1591–1600. doi: 10.1001/jama.2013.278481. [DOI] [PubMed] [Google Scholar]
- 2.Herridge MS, Cheung AM, Tansey CM, et al. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med. 2003;348(8):683–693. doi: 10.1056/NEJMoa022450. [DOI] [PubMed] [Google Scholar]
- 3.Ohtake PJ, Lee AC, Scott JC, et al. Physical impairments associated with post-intensive care syndrome: systematic review based on the world health organization's international classification of functioning. Disability and Health Framework Physical therapy. 2018;98(8):631–645. doi: 10.1093/ptj/pzy059. [DOI] [PubMed] [Google Scholar]
- 4.McPeake J, Mikkelsen ME, Quasim T, et al. Return to employment after critical illness and its association with psychosocial outcomes. A systematic review and meta-analysis. Ann Am Thorac Soc. 2019;16(10):1304–1311. doi: 10.1513/AnnalsATS.201903-248OC. [DOI] [PubMed] [Google Scholar]
- 5.Latronico N, Bolton CF. Critical illness polyneuropathy and myopathy: a major cause of muscle weakness and paralysis. Lancet Neurol. 2011;10(10):931–941. doi: 10.1016/S1474-4422(11)70178-8. [DOI] [PubMed] [Google Scholar]
- 6.Chapple LS, Kouw IWK, Summers MJ, et al. Muscle protein synthesis after protein administration in critical illness. Am J Respir Crit Care Med. 2022;206(6):740–749. doi: 10.1164/rccm.202112-2780OC. [DOI] [PubMed] [Google Scholar]
- 7.Heyland DK, Patel J, Compher C, et al. The effect of higher protein dosing in critically ill patients with high nutritional risk (EFFORT Protein): an international, multicentre, pragmatic, registry-based randomised trial. Lancet. 2023. [DOI] [PubMed]
- 8.Investigators TS, the ACTG, Hodgson CL, et al. Early Active Mobilization during Mechanical Ventilation in the ICU. The New England Journal of Medicine 2022;387(19):1747–1758. [DOI] [PubMed]
- 9.Bear DE, Parry SM, Puthucheary ZA. Can the critically ill patient generate sufficient energy to facilitate exercise in the ICU? Curr Opin Clin Nutr Metab Care. 2018;21(2):110–115. doi: 10.1097/MCO.0000000000000446. [DOI] [PubMed] [Google Scholar]
- 10.Smidt N, de Vet HC, Bouter LM, et al. Effectiveness of exercise therapy: a best-evidence summary of systematic reviews. Aust J Physiother. 2005;51(2):71–85. doi: 10.1016/S0004-9514(05)70036-2. [DOI] [PubMed] [Google Scholar]
- 11.Waldauf P, Jiroutkova K, Krajcova A, Puthucheary Z, Duska F. Effects of rehabilitation interventions on clinical outcomes in critically ill patients: systematic review and meta-analysis of randomized controlled trials. Crit Care Med. 2020;48(7):1055–1065. doi: 10.1097/CCM.0000000000004382. [DOI] [PubMed] [Google Scholar]
- 12.Bear DE, Wandrag L, Merriweather JL, et al. The role of nutritional support in the physical and functional recovery of critically ill patients: a narrative review. Crit Care. 2017;21(1):226. doi: 10.1186/s13054-017-1810-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deane AM, Little L, Bellomo R, et al. Outcomes six months after delivering 100% or 70% of enteral calorie requirements during critical illness (TARGET). A randomized controlled trial. Am J Respirat Critical Care Med. 2020;201(7):814–822. doi: 10.1164/rccm.201909-1810OC. [DOI] [PubMed] [Google Scholar]
- 14.Casaer MP, Langouche L, Coudyzer W, et al. Impact of early parenteral nutrition on muscle and adipose tissue compartments during critical illness. Crit Care Med. 2013;41(10):2298–2309. doi: 10.1097/CCM.0b013e31828cef02. [DOI] [PubMed] [Google Scholar]
- 15.Mira JC, Brakenridge SC, Moldawer LL, Moore FA. Persistent inflammation, immunosuppression and catabolism syndrome. Crit Care Clin. 2017;33(2):245–258. doi: 10.1016/j.ccc.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Helliwell TR, Coakley JH, Wagenmakers AJ, et al. Necrotizing myopathy in critically-ill patients. J Pathol. 1991;164(4):307–314. doi: 10.1002/path.1711640406. [DOI] [PubMed] [Google Scholar]
- 17.De Letter MA, van Doorn PA, Savelkoul HF, et al. Critical illness polyneuropathy and myopathy (CIPNM): evidence for local immune activation by cytokine-expression in the muscle tissue. J Neuroimmunol. 2000;106(1–2):206–213. doi: 10.1016/S0165-5728(99)00252-0. [DOI] [PubMed] [Google Scholar]
- 18.Puthucheary ZA, Astin R, McPhail MJW, et al. Metabolic phenotype of skeletal muscle in early critical illness. Thorax. 2018;73(10):926–935. doi: 10.1136/thoraxjnl-2017-211073. [DOI] [PubMed] [Google Scholar]
- 19.Langhans C, Weber-Carstens S, Schmidt F, et al. Inflammation-induced acute phase response in skeletal muscle and critical illness myopathy. PLoS ONE. 2014;9(3):e92048. doi: 10.1371/journal.pone.0092048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Puthucheary ZA, Phadke R, Rawal J, et al. Qualitative ultrasound in acute critical illness muscle wasting. Crit Care Med. 2015;43(8):1603–1611. doi: 10.1097/CCM.0000000000001016. [DOI] [PubMed] [Google Scholar]
- 21.Wollersheim T, Grunow JJ, Carbon NM, et al. Muscle wasting and function after muscle activation and early protocol-based physiotherapy: an explorative trial. J Cachexia Sarcopenia Muscle. 2019;10(4):734–747. doi: 10.1002/jcsm.12428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brealey D, Brand M, Hargreaves I, et al. Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet. 2002;360(9328):219–223. doi: 10.1016/S0140-6736(02)09459-X. [DOI] [PubMed] [Google Scholar]
- 23.Fredriksson K, Hammarqvist F, Strigard K, et al. Derangements in mitochondrial metabolism in intercostal and leg muscle of critically ill patients with sepsis-induced multiple organ failure. Am J Physiol Endocrinol Metab. 2006;291(5):E1044–1050. doi: 10.1152/ajpendo.00218.2006. [DOI] [PubMed] [Google Scholar]
- 24.Jiroutkova K, Krajcova A, Ziak J, et al. Mitochondrial function in skeletal muscle of patients with protracted critical illness and ICU-acquired weakness. Crit Care. 2015;19:448. doi: 10.1186/s13054-015-1160-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kafri M, Metzl-Raz E, Jona G, Barkai N. The Cost of Protein Production. Cell Rep. 2016;14(1):22–31. doi: 10.1016/j.celrep.2015.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Waldauf P, Hruskova N, Blahutova B, et al. Functional electrical stimulation-assisted cycle ergometry-based progressive mobility programme for mechanically ventilated patients: randomised controlled trial with 6 months follow-up. Thorax. 2021;76(7):664–671. doi: 10.1136/thoraxjnl-2020-215755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berney S, Hopkins RO, Rose JW, et al. Functional electrical stimulation in-bed cycle ergometry in mechanically ventilated patients: a multicentre randomised controlled trial. Thorax. 2021;76(7):656–663. doi: 10.1136/thoraxjnl-2020-215093. [DOI] [PubMed] [Google Scholar]
- 28.Waldauf P, Gojda J, Urban T, et al. Functional electrical stimulation-assisted cycle ergometry in the critically ill: protocol for a randomized controlled trial. Trials. 2019;20(1):724. doi: 10.1186/s13063-019-3745-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bergstrom J. Percutaneous needle biopsy of skeletal muscle in physiological and clinical research. Scand J Clin Lab Invest. 1975;35(7):609–616. doi: 10.3109/00365517509095787. [DOI] [PubMed] [Google Scholar]
- 30.Jameson TSO, Pavis GF, Dirks ML, et al. Reducing NF-kappaB signaling nutritionally is associated with expedited recovery of skeletal muscle function after damage. J Clin Endocrinol Metab. 2021;106(7):2057–2076. doi: 10.1210/clinem/dgab106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pavis GF, Jameson TSO, Dirks ML, et al. Improved recovery from skeletal muscle damage is largely unexplained by myofibrillar protein synthesis or inflammatory and regenerative gene expression pathways. Am J Physiol Endocrinol Metab. 2021;320(2):E291–E305. doi: 10.1152/ajpendo.00454.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Caldow MK, Cameron-Smith D, Levinger P, McKenna MJ, Levinger I. Inflammatory markers in skeletal muscle of older adults. Eur J Appl Physiol. 2013;113(2):509–517. doi: 10.1007/s00421-012-2458-x. [DOI] [PubMed] [Google Scholar]
- 33.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Stat Soc: Ser B (Methodol) 1995;57(1):289–300. [Google Scholar]
- 34.Seagroves TN, Ryan HE, Lu H, et al. Transcription factor HIF-1 is a necessary mediator of the pasteur effect in mammalian cells. Mol Cell Biol. 2001;21(10):3436–3444. doi: 10.1128/MCB.21.10.3436-3444.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Connolly B, Maddocks M, MacBean V, et al. Nonvolitional assessment of tibialis anterior force and architecture during critical illness. Muscle Nerve. 2018;57(6):964–972. doi: 10.1002/mus.26049. [DOI] [PubMed] [Google Scholar]
- 36.Crossland H, Skirrow S, Puthucheary ZA, Constantin-Teodosiu D, Greenhaff PL. The impact of immobilisation and inflammation on the regulation of muscle mass and insulin resistance: different routes to similar end-points. J Physiol. 2019;597(5):1259–1270. doi: 10.1113/JP275444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Puthucheary Z, Rooyackers O. Anabolic Resistance: An Uncomfortable Truth for Clinical Trials in Preventing Intensive Care Acquired Weakness and Physical Functional Impairment. American Journal of Respiratory and Critical Care Medicine. 2022. [DOI] [PMC free article] [PubMed]
- 38.Balas MC, Burke WJ, Gannon D, et al. Implementing the awakening and breathing coordination, delirium monitoring/management, and early exercise/mobility bundle into everyday care: opportunities, challenges, and lessons learned for implementing the ICU Pain, Agitation, and Delirium Guidelines. Crit Care Med. 2013;41(9 Suppl 1):S116–127. doi: 10.1097/CCM.0b013e3182a17064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lynch GS. Identifying the challenges for successful pharmacotherapeutic management of sarcopenia. Expert Opin Pharmacother. 2022;23(11):1233–1237. doi: 10.1080/14656566.2022.2076593. [DOI] [PubMed] [Google Scholar]
- 40.Webster JM, Kempen L, Hardy RS, Langen RCJ. Inflammation and skeletal muscle wasting during cachexia. Front Physiol. 2020;11:597675. doi: 10.3389/fphys.2020.597675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu H, Ranjit R, Richardson A, Van Remmen H. Muscle mitochondrial catalase expression prevents neuromuscular junction disruption, atrophy, and weakness in a mouse model of accelerated sarcopenia. J Cachexia Sarcopenia Muscle. 2021;12(6):1582–1596. doi: 10.1002/jcsm.12768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gumerson JD, Michele DE. The dystrophin-glycoprotein complex in the prevention of muscle damage. J Biomed Biotechnol. 2011;2011:210797. doi: 10.1155/2011/210797. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Source data can be obtained from the corresponding author upon reasonable request.