Abstract
Background:
Low-field magnetic resonance imaging (LF-MRI) has become a valuable tool in the diagnosis of brain tumors due to its high spatial resolution and ability to acquire images in a short amount of time. However, the use of LF-MRI for intraoperative imaging during brain tumor surgeries has not been extensively studied. The aim of this systematic review is to investigate the impact of low-field intraoperative magnetic resonance imaging (LF-IMRI) on the duration of brain tumor surgery and the extent of tumor resection.
Methods:
A comprehensive literature search was conducted using PubMed, Scopus, and Google Scholar from February 2000 to December 2022. The studies were selected based on the inclusion criteria and reviewed independently by two reviewers. The gathered information was organized and analyzed using Excel.
Results:
Our review of 21 articles found that low-field intraoperative MRI (LF-IMRI) with a field below 0.3T was used in most of the studies, specifically 15 studies used 0.15T LF-IMRI. The T1-weighted sequence was the most frequently reported, and the average scanning time was 24.26 min. The majority of the studies reported a positive impact of LF-IMRI on the extent of tumor resection, with an increase ranging from 11% to 52.5%. Notably, there were no studies describing the use of ultra-low-field (ULF) intraoperative MRI.
Conclusion:
The results of this systematic review will aid neurosurgeons and neuroradiologists in making informed decisions about the use of LF-MRI in brain tumor surgeries. Further, research is needed to fully understand the impact of LF-MRI in brain tumor surgeries and to optimize its use in the clinical setting. There is an opportunity to study the utility of ULF-MRI in brain tumor surgeries.
Keywords: Brain tumors, Intraoperative, Low-field magnetic resonance imaging, Neuroimaging

INTRODUCTION
Magnetic resonance imaging (MRI) is a well-established noninvasive imaging technique that offers three-dimensional body images.[17] Its exceptional soft-tissue visualization and ability to distinguish tumors from surrounding normal tissue without radiation exposure have made it a cornerstone in oncology for diagnosis and treatment planning.[34] This reputation has been earned over years of effective utilization by oncologists.[34]
Technological advancements led to the development of the first MRI machine designed for intraoperative MRI (IMRI) in 1991.[2] In the realm of neuro-oncology, the use of IMRI represents a critical breakthrough. This advanced technology provides an unparalleled level of accuracy in assessing surgical performance and enables real-time monitoring of dynamic intraoperative changes. These include the complex shifts in brain anatomy that can occur during the procedure.[4,13,23] Such phenomena result from a complex interplay of variables, such as changes in intracranial pressure, gravity, head positioning, and brain edema. By capturing the intricate interplay between surgical maneuvers and the brain’s responses, IMRI provides the attending surgeon with invaluable insights into the intricate interaction between their actions and the brain’s response. Multiple studies in the medical literature have reported a better extent of resection (EOR) in brain tumors due to visualization of residual disease through IMRI, hence, improving survival.[10,11,25] Moreover, the use of IMRI can also reduce surgical complications and postoperative neurological deficits.[23]
Broadly, the MRI can be classified as low-field MRI (LFMRI) and high-field MRI (HF-MRI). At present, there is no consensus on the cutoff value of field strength to define the two varieties. However, generally, a field strength of 0.5T or less is considered LF-MRI, and a field strength of 1.5T or above is considered. HF-MRI.[24] In addition, ultra-low-field MRI (ULF-MRI) scanners have been defined with a field strength of <1 mT and typically use a standard AC power outlet. Furthermore, these are low-cost to build and operate.[15]
HF-MRI offers increased image resolution, field of view, and contrast visibility compared to low-field magnetic resonance imaging (LF-MRI). In addition, HF-MRI takes less time to produce the scans.[1] Nevertheless, LF-MRI offers less risk of device interactions, heating, and risk of metallic projectiles and noise, all at a lower cost and power consumption than HF-MRI.[1] These benefits are, further, enhanced with the use of ULF-MRI. LF-MRI and ULF-MRI having a lower cost of production, installation, and maintenance, in addition to lesser power need than HF-MRI, makes them a far more feasible option for low- and middle-income countries (LMICs) with an additional benefit of ultimately resulting in a smaller carbon footprint.[1]
Numerous studies have consistently demonstrated that incorporating LF-IMRI technology into surgeries for cerebral neoplasms yields remarkable benefits. LF-IMRI’s capacity to provide enhanced visualization of residual disease, guide more extensive resections, and ultimately lead to improved patient survival has been well-documented. By offering real-time insights into tumor extent and enabling surgeons to make informed decisions during surgery, LF-IMRI optimizes the precision and completeness of resection. This dynamic approach has been shown to directly correlate with improved long-term outcomes, as the technology empowers surgeons to address residual disease promptly. Consequently, LF-IMRI emerges as a transformative tool with the potential to reshape the landscape of cerebral neoplastic surgeries, offering enhanced patient care, and potentially changing the course of treatment and prognosis.[26]
Despite such compelling results for the use of LF-IMRI in cerebral tumors, there is little information on whether the use of LF-IMRI helps to increase EOR and achievement of gross total resection (GTR); in addition to the effect of LFIMRI on surgical time. Hence, this systematic review was conducted to evaluate and analyze the outcomes mentioned above for using LF-IMRI for brain tumors.
MATERIALS AND METHODS
This systematic review was conducted according to the guidelines in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.[19,20] The study’s protocol was uploaded on the PROSPERO International Prospective Register of Systematic Reviews (registration number—CRD42023391879)
Search strategy
The medical literature was thoroughly searched from February 2000 to December 2022 by exploring the following electronic databases: PubMed, Scopus, and Google Scholar. The search strategy for all databases was developed using the keywords “magnetic resonance imaging” OR “MRI” OR “Low-field MRI,” AND “brain tumor,” OR “brain malignancy.” To guarantee that all pertinent research was included, reference lists of screened articles were also carefully examined. The complete search strategy utilized can be found in the supporting document.
Study selection
The study design selection process was comprehensive, involving the evaluation of various study designs for potential inclusion in the analysis. The literature search included studies that presented: (1) human subjects of all ages, (2) neurosurgical procedures for intracranial brain tumors, (3) the use of intraoperative LF-MRI, and (4) studies reporting outcomes regarding tumor assessment, the EOR, or survival of the patient in regard to the use of LF-IMRI. Studies were only included if the inclusion mentioned above criteria were met. Review articles, case reports, case series (n < 11), book chapters, guidelines, commentary, letters to the editors, and studies on animals were excluded from the study.
Data extraction
During the extraction process, the author’s names, date of publication, country of origin, sample size, histopathological diagnosis, radiological diagnosis, mean follow-up, additional imaging modalities, MRI field strength (Tesla), sequences utilized, scan frequency, Karnofsky performance status score, the EOR, that is GTR or subtotal resection (STR), operation time, and length of stay. A risk-of-bias assessment was performed using the Newcastle-Ottawa Scale to determine the bias associated with observational studies[29] and a Cochrane risk-of-bias tool for randomized trials (RoB 2.0) for randomized clinical trials.[30]
Data analysis
The data were extracted and organized in a tabular format on Microsoft Excel to facilitate clear representation and comprehension.
RESULTS
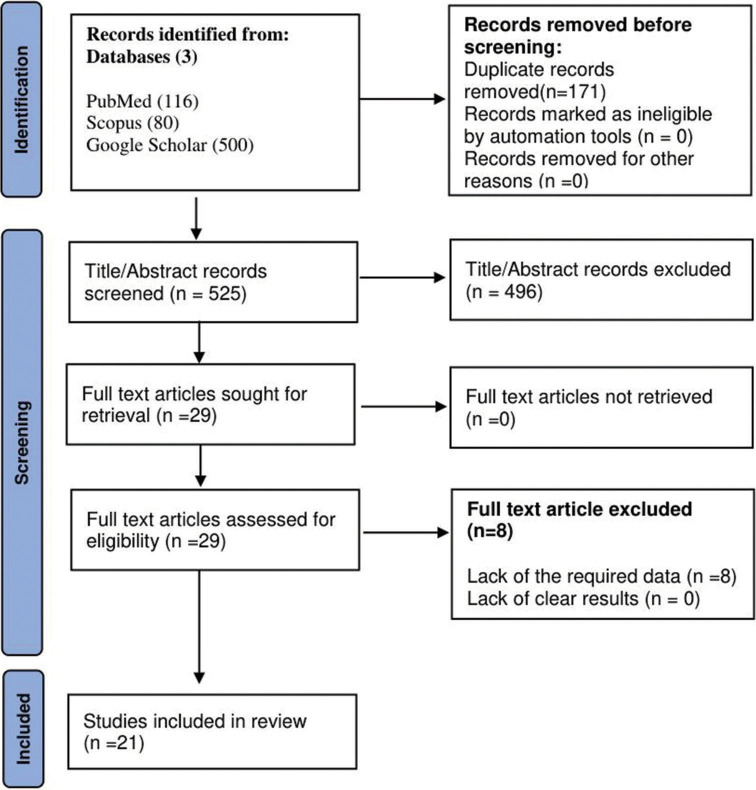
Our systematic review process screened 525 articles and, after evaluating the titles, abstracts, and full text, 21 of them were selected for inclusion in the final analysis.[3,6,7,12-14,16,18,21-23,25-28,32,33,35,36,38,39] The articles were screened and quality assessed independently by JM and AB, and any discrepancies were resolved by AA. The screening process and quality assessment procedure are thoroughly delineated in Figure 1 of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart, and Tables 1a and b, respectively.
Figure 1:
Preferred reporting items for systematic reviews and meta-analyses flow diagram. Number (n).
Table 1a:
Newcastle-Ottawa scale quality assessment.
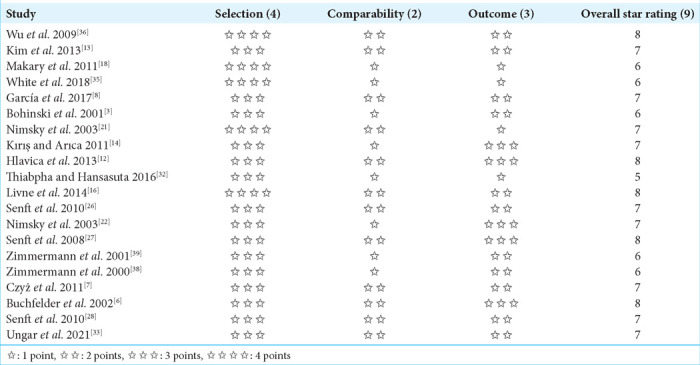
Table 1b:
Cochrane risk-of-bias tool for randomized trials (RoB 2.0).

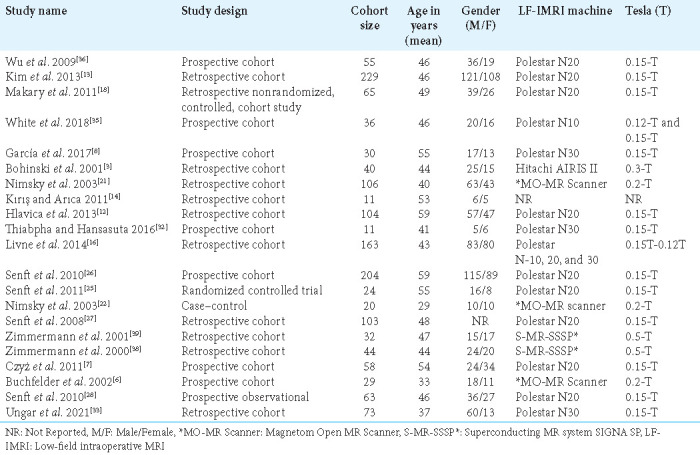
The majority of the studies were retrospective cohort studies (50%), followed by prospective cohort studies (33.3%) and one randomized controlled trial. The average number of participants in all of the studies was 54, with sample sizes ranging from 11 to 229. In addition, the mean age of the participants was 46.7 years, with a range between 29 and 59 years. A significant proportion of the included studies used the Polestar N20 with 0.15 tesla, while two of the studies utilized the superconducting MR system SIGNA SP 0.5T IMRI, as shown in Table 2.
Table 2:
General characteristics of the included studies.
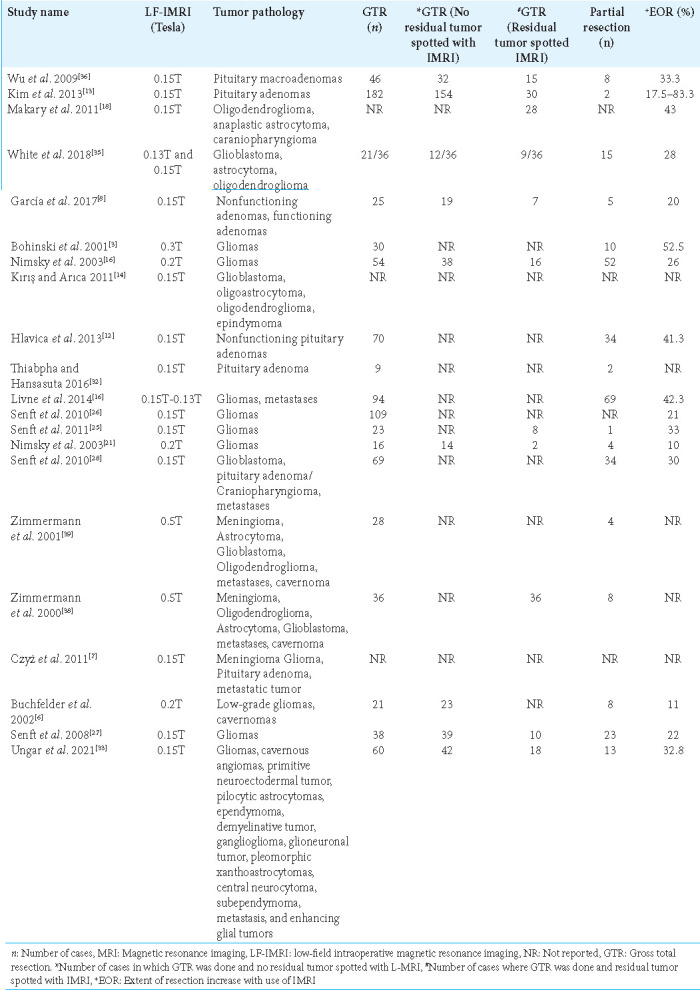
Gadolinium-based contrast was used in 12 of the studies to enhance image quality, while one study used a ferromagnetic contrast agent, and the others did not specify any contrast agent used. Neuronavigation was used as an adjunct imaging technology in 13 of the studies to assist the procedure in which LF-IMRI was used, while awake mapping was used in one study, and motor and sensory-evoked potential was used in two of the studies as adjunct technologies. In 20 studies that provided information on the sequences used in the analysis, T1-weighted sequences were commonly utilized. In addition, T2 and fluid-attenuated inversion recovery sequences were used in eight and five studies, respectively, alongside the T1 sequence. Thirteen studies mentioned the average scanning time for the LF-IMRI in the operating room. The average time for all the studies was 24.26 ± 18.6 standard deviation (SD) min, as shown in Table 3. In our review, it was observed that 17 of the studies investigated the effect of LF-IMRI on tumor resection. All of these 17 studies reported a favorable impact, with the percentage increase in tumor resection ranging from 11% to 52.5%. Table 4 shows, LF-IMRI resulted in increased resection in all of the studies that assessed this outcome.
Table 3:
Impact of LF-IoMRI on surgery duration.
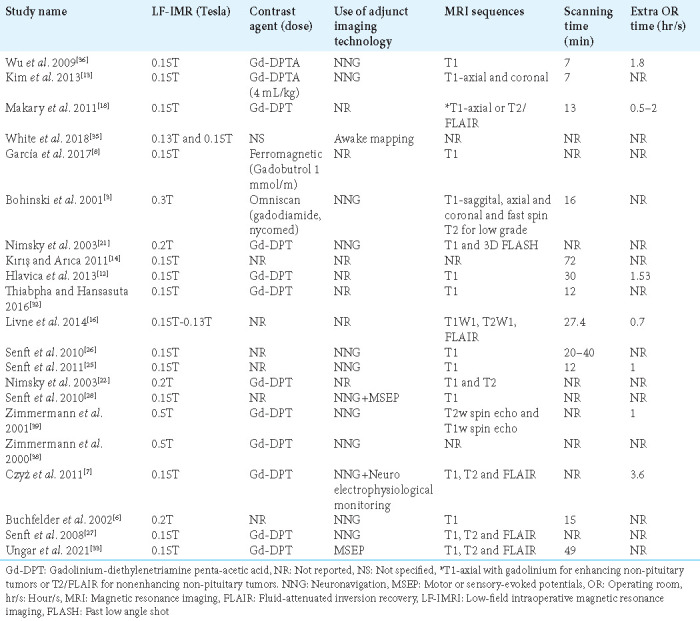
Table 4:
Impact of LF-IMRI on EOR.
DISCUSSION
This systematic review was conducted to enhance the understanding of the role and extent of aid LF-IMRI can offer neurosurgeons operating on brain tumors. The prevalent pattern noted in the studies under our review indicates that patients who underwent brain tumor surgery with the support of a low-field IMRI device, which is an economical imaging technology, exhibited increased rates of complete tumor resection, reduced complications, and improved progression-free survival. However, it is important to note that this came at the expense of a longer surgical duration.
Our review revealed that the use of low-field IMRI has been associated with a significant increase in the number of patients who achieved GTR of brain tumors. Attaining GTR is of paramount importance, as it has been shown to be associated with prolonged survival in various brain tumor types. A meta-analysis conducted by Brown et al. reported that patients who underwent GTR had better 1-year and 2-year survival rates compared to those who had STR. In addition, patients who achieved GTR had a lower likelihood of disease progression within 6 months.[5] Another meta-analysis by Xia et al. demonstrated that patients who underwent GTR had better 5-year and 10-year survival rates compared to those who had STR.[37]
While aiming for GTR, preservation of neurological function postsurgery is crucial. Maximal preservation of neurological function is not only essential to preserve a good quality of life but is also associated with better survival.[9] There were multiple patients in our patient pool where GTR was planned, and the LF-IMRI showed residual tumors; however, the surgeons did not choose to resect further due to the tumor being in very close proximity to the brain’s eloquent areas to avoid neurological deficit postsurgery. Nevertheless, multiple studies that employed the use of IMRI and resected additional tissue with the help of IMRI did not report a higher percentage of neurological deficit compared to patients in which IMRI was not utilized.[25,31]
One setback of performing surgeries with LF-IMRI is the additional surgical time taken. The time taken for the scan ranged from 7 min to 72 min, with a mean and standard deviation of 24.26 ± 18.6 SD min. Furthermore, the additional time taken to complete the surgery ranged from 30 min to 250 min in our study. This leads to prolonged surgeries and additional time in surgical suits, leading to a higher operative cost.[31] Better EOR coming at a higher cost puts emphasis on the need for further prospective research with long-term follow-ups, which can help clarify if the increased EOR, specifically through the use of LF-IMRI, improves survival and other outcomes including but not limited to disease progression and de novo postsurgical neurological deficits. This can significantly help us understand how cost-effective mass installation of LF-MRIs will be.
Limitations
There are some limitations to this review, which should be considered when interpreting the results. The included studies may not have controlled for all variables that could have influenced the results, contributing to confounding factors. The studies varied in design, lacking consistent control groups for direct resection comparison. This heterogeneity might affect conclusion generalization. Surgeons’ inconsistent preIMRI resection perceptions introduced potential bias, possibly inflating LF-IMRI’s attributed resection improvement. Due to the limited amount of literature available on the topic, case series with sample sizes of >10 were included in the study.
Future direction
Going forward, prospective and randomized controlled trials with large sample sizes are necessary to evaluate the utility of LF-MRI in routine neurosurgical care and to determine how LF-MRI can be effectively implemented in areas with limited resources, it is crucial to carry out prospective studies in LMICs. It is also important to study the feasibility and utility of the emerging field of ULF-MRI in the neurosurgery operating room.
CONCLUSION
Our review highlighted that LF-MRI has the potential to be an important tool for intraoperative brain tumor imaging, which is known to increase the EOR and subsequently improve outcomes of brain tumors. To fully realize the potential of LFMRI in LMICs, where access to high-field MRI is often limited, efforts should be made to increase the accessibility of LF-MRI machines, develop specialized protocols, provide training and education for healthcare professionals, conduct research and evaluations, develop teleradiology linkages with experts in LFMRI interpretation, and partner with equipment manufacturers to create low-cost and low-maintenance machines. With these efforts, LF-MRI has the potential to improve diagnosis and treatment outcomes of brain tumors in LMICs.
Footnotes
How to cite this article: Altaf A, Shakir M, Malik M, Arif A, Islam O, Mubarak F, et al. Intraoperative use of low-field magnetic resonance imaging for brain tumors: A systematic review. Surg Neurol Int 2023;14:357.
Contributor Information
Ahmed Altaf, Email: ahmedaltafgagan@gmail.com.
Muhammad Shakir, Email: muhammad.shakir@alumni.aku.edu.
Muhammad Jawad Amin Malik, Email: muhammad.malik3@scholar.aku.edu.
Aabiya Arif, Email: aabiya.arif@gmail.com.
Omar Islam, Email: omarislam9902@gmail.com.
Fatima Mubarak, Email: fatima.mubarak@aku.edu.
Eddie Knopp, Email: Eknopp@hyperfine.io.
Khan Siddiqui, Email: Ksiddiqui@hyperfine.io.
S. Ather Enam, Email: ather.enam@aku.edu.
Declaration of patient consent
Patients’ consent not required as patients’ identities were not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The author(s) confirms that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
SEARCH STRATEGY
PubMed: (No of articles retrieved, 116)
((“magnetic resonance imaging” AND (“low-field” OR “low field” OR “low field”) AND (“Brain Neoplasms”[Mesh] OR “brain tumor” OR “brain tumor” OR “cns tumor” OR “cns tumor” OR “central nervous system tumor” OR “central nervous system tumor” OR “brain malignancy” OR “cns malignancy” OR “central nervous system malignancy” OR “glioma” OR “medulloblastoma” OR “ependymoma” OR “meningioma”) AND (“surgery, computer-assisted” OR “neuronavigation” OR “therapy, computer-assisted”))
Scopus (No of articles retrieved, 80)
(“intraoperative” OR “neuronavigation”) AND (“magnetic resonance imaging”) AND “low-field” AND (“Brain Neoplasm” OR “brain tumor” OR “cns tumor” OR “cns tumor” OR “central nervous system tumor” OR “central nervous system tumor” OR “brain malignancy” OR “cns malignancy” OR “central nervous system malignancy” OR “glioma” OR “medulloblastoma” OR “ependymoma” OR “meningioma”)
Google Scholar: (No of articles retrieved, 500)
((“magnetic resonance imaging”|“Neuroimaging”) (“lowfield”|“lowfield”|“low field”) (“Brain Neoplasms”|“brain tumor” |“brain tumor”|“cns tumor”|“cns tumor”| “central nervous system tumor”|“central nervous system tumor” |“brain malignancy”|“cns malignancy”| “central nervous system malignancy”|“glioma” |“medulloblastoma” |“ependymoma”|“meningioma”) (“surgery, computer-assisted ”|“neuronavigation”|“therapy, computer-assisted”))
REFERENCES
- 1.Arnold TC, Freeman CW, Litt B, Stein JM. Low-field MRI: Clinical promise and challenges. J Magn Reson Imaging. 2023;57:25–44. doi: 10.1002/jmri.28408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Black PM, Moriarty T, Alexander E, 3rd, Stieg P, Woodard EJ, Gleason PL, et al. Development and implementation of intraoperative magnetic resonance imaging and its neurosurgical applications. Neurosurgery. 1997;41:831–42. doi: 10.1097/00006123-199710000-00013. discussion 842-5. [DOI] [PubMed] [Google Scholar]
- 3.Bohinski RJ, Kokkino AK, Warnick RE, Gaskill-Shipley MF, Kormos DW, Lukin RR, et al. Glioma resection in a shared-resource magnetic resonance operating room after optimal image-guided frameless stereotactic resection. Neurosurgery. 2001;48:731–42. doi: 10.1097/00006123-200104000-00007. discussion 742-4. [DOI] [PubMed] [Google Scholar]
- 4.BRAIN SHIFT - Golby lab. Available from: https://golbylab.bwh.harvard.edu/brain-shift [Last accessed on 2023 Jun 14]
- 5.Brown TJ, Brennan MC, Li M, Church EW, Brandmeir NJ, Rakszawski KL, et al. Association of the extent of resection with survival in glioblastoma: A systematic review and meta-analysis. JAMA Oncol. 2016;2:1460–9. doi: 10.1001/jamaoncol.2016.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buchfelder M, Fahlbusch R, Ganslandt O, Stefan H, Nimsky C. Use of intraoperative magnetic resonance imaging in tailored temporal lobe surgeries for epilepsy. Epilepsia. 2002;43:864–73. doi: 10.1046/j.1528-1157.2002.46201.x. [DOI] [PubMed] [Google Scholar]
- 7.Czyż M, Tabakow P, Lechowicz-Głogowska B, Jarmundowicz W. Prospective study on the efficacy of low-field intraoperative magnetic resonance imaging in neurosurgical operations. Neurol Neurochir Pol. 2011;45:226–34. doi: 10.1016/s0028-3843(14)60075-x. [DOI] [PubMed] [Google Scholar]
- 8.García S, Reyes L, Roldán P, Torales J, Halperin I, Hanzu F, et al. Does low-field intraoperative magnetic resonance improve the results of endoscopic pituitary surgery? Experience of the implementation of a new device in a referral center. World Neurosurg. 2017;102:102–10. doi: 10.1016/j.wneu.2017.02.094. [DOI] [PubMed] [Google Scholar]
- 9.Gerritsen JK, Zwarthoed RH, Kilgallon JL, Nawabi NL, Versyck G, Jessurun CA, et al. Impact of maximal extent of resection on postoperative deficits, patient functioning, and survival within clinically important glioblastoma subgroups. Neuro Oncol. 2023;25:958–72. doi: 10.1093/neuonc/noac255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Golub D, Hyde J, Dogra S, Nicholson J, Kirkwood KA, Gohel P, et al. Intraoperative MRI versus 5-ALA in high-grade glioma resection: A network meta-analysis. J Neurosurg. 2020;134:484–98. doi: 10.3171/2019.12.JNS191203. [DOI] [PubMed] [Google Scholar]
- 11.Hatiboglu MA, Weinberg JS, Suki D, Rao G, Prabhu SS, Shah K, et al. Impact of intraoperative high-field magnetic resonance imaging guidance on glioma surgery: A prospective volumetric analysis. Neurosurgery. 2009;64:1073–81. doi: 10.1227/01.NEU.0000345647.58219.07. discussion 1081. [DOI] [PubMed] [Google Scholar]
- 12.Hlavica M, Bellut D, Lemm D, Schmid C, Bernays RL. Impact of ultra-low-field intraoperative magnetic resonance imaging on extent of resection and frequency of tumor recurrence in 104 surgically treated nonfunctioning pituitary adenomas. World Neurosurg. 2013;79:99–109. doi: 10.1016/j.wneu.2012.05.032. [DOI] [PubMed] [Google Scholar]
- 13.Kim EH, Oh MC, Kim SH. Application of low-field intraoperative magnetic resonance imaging in transsphenoidal surgery for pituitary adenomas: Technical points to improve the visibility of the tumor resection margin. Acta Neurochir (Wien) 2013;155:485–93. doi: 10.1007/s00701-012-1608-6. [DOI] [PubMed] [Google Scholar]
- 14.Kırış T, Arıca O. Impact of a low-field intraoperative MRI on the surgical results for high-grade gliomas. Acta Neurochir Suppl. 2011;109:55–9. doi: 10.1007/978-3-211-99651-5_9. [DOI] [PubMed] [Google Scholar]
- 15.Liu Y, Leong AT, Zhao Y, Xiao L, Mak HK, Tsang AC, et al. A low-cost and shielding-free ultra-low-field brain MRI scanner. Nat Commun. 2021;12:7238. doi: 10.1038/s41467-021-27317-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Livne O, Harel R, Hadani M, Spiegelmann R, Feldman Z, Cohen ZR. Intraoperative magnetic resonance imaging for resection of intra-axial brain lesions: A decade of experience using low-field magnetic resonance imaging, Polestar N-10, 20, 30 systems. World Neurosurg. 2014;82:770–6. doi: 10.1016/j.wneu.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 17.Magnetic Resonance Imaging (MRI) National institute of biomedical imaging and bioengineering. Available from: https://www.nibib.nih.gov/science-education/science-topics/magnetic-resonance-imaging-mri [Last accessed on 2023 Jun 15]
- 18.Makary M, Chiocca EA, Erminy N, Antor M, Bergese SD, Abdel-Rasoul M, et al. Clinical and economic outcomes of low-field intraoperative MRI-guided tumor resection neurosurgery. J Magn Reson Imaging. 2011;34:1022–30. doi: 10.1002/jmri.22739. [DOI] [PubMed] [Google Scholar]
- 19.McInnes MD, Moher D, Thombs BD, McGrath TA, Bossuyt PM, the PRISMA-DTAGroup et al. Preferred reporting items for a systematic review and meta-analysis of diagnostic test accuracy studies: The PRISMA-DTA Statement. JAMA. 2018;319:388–96. doi: 10.1001/jama.2017.19163. [DOI] [PubMed] [Google Scholar]
- 20.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int J Surg. 2010;8:336–41. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 21.Nimsky C, Ganslandt O, Buchfelder M, Fahlbusch R. Glioma surgery evaluated by intraoperative low-field magnetic resonance imaging. Acta Neurochir Suppl. 2003;85:55–63. doi: 10.1007/978-3-7091-6043-5_8. [DOI] [PubMed] [Google Scholar]
- 22.Nimsky C, Ganslandt O, Hofmann B, Fahlbusch R. Limited benefit of intraoperative low-field magnetic resonance imaging in craniopharyngioma surgery. Neurosurgery. 2003;53:72–80. doi: 10.1227/01.neu.0000068728.08237.af. discussion 80-1. [DOI] [PubMed] [Google Scholar]
- 23.Rogers CM, Jones PS, Weinberg JS. Intraoperative MRI for brain tumors. J Neurooncol. 2021;151:479–90. doi: 10.1007/s11060-020-03667-6. [DOI] [PubMed] [Google Scholar]
- 24.Seifert V, Gasser T, Senft C. Low field intraoperative MRI in glioma surgery. Acta Neurochir Suppl. 2011;109:35–41. doi: 10.1007/978-3-211-99651-5_6. [DOI] [PubMed] [Google Scholar]
- 25.Senft C, Bink A, Franz K, Vatter H, Gasser T, Seifert V. Intraoperative MRI guidance and extent of resection in glioma surgery: A randomised, controlled trial. Lancet Oncol. 2011;12:997–1003. doi: 10.1016/S1470-2045(11)70196-6. [DOI] [PubMed] [Google Scholar]
- 26.Senft C, Franz K, Ulrich CT, Bink A, Szelényi A, Gasser T, et al. Low field intraoperative MRI-guided surgery of gliomas: A single center experience. Clin Neurol Neurosurg. 2010;112:237–43. doi: 10.1016/j.clineuro.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 27.Senft C, Seifert V, Hermann E, Franz K, Gasser T. Usefulness of intraoperative ultra low-field magnetic resonance imaging in glioma surgery. Neurosurgery. 2008;63:257–66. doi: 10.1227/01.NEU.0000313624.77452.3C. discussion 266-7. [DOI] [PubMed] [Google Scholar]
- 28.Senft C, Ulrich CT, Seifert V, Gasser T. Intraoperative magnetic resonance imaging in the surgical treatment of cerebral metastases. J Surg Oncol. 2010;101:436–41. doi: 10.1002/jso.21508. [DOI] [PubMed] [Google Scholar]
- 29.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–5. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 30.Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 31.Taslimi S, Ye VC, Wen PY, Zadeh G. Lessons learned from contemporary glioblastoma randomized clinical trials through systematic review and network meta-analysis: Part 2 recurrent glioblastoma. Neurooncol Adv. 2021;3:vdab029. doi: 10.1093/noajnl/vdab029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thiabpha A, Hansasuta A. Initial experience with ultra-low-field intraoperative magnetic resonance imaging in endoscopic endonasal transsphenoidal surgery for pituitary adenoma at ramathibodi hospital. J Med Assoc Thai. 2016;99(Suppl 3):S30–8. [PubMed] [Google Scholar]
- 33.Ungar L, Zibly Z, Wohl A, Harel R, Hadani M, Attia M, et al. Utility of the polestar N30 low-field MRI system for resecting non-enhancing intra-axial brain lesions. Neurol Neurochir Pol. 2021;55:202–11. doi: 10.5603/PJNNS.a2021.0017. [DOI] [PubMed] [Google Scholar]
- 34.Vickers AJ, Thiruthaneeswaran N, Coyle C, Manoharan P, Wylie J, Kershaw L, et al. Does magnetic resonance imaging improve soft tissue sarcoma contouring for radiotherapy? BJR Open. 2019;1:20180022. doi: 10.1259/bjro.20180022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.White T, Zavarella S, Jarchin L, Nardi D, Schaffer S, Schulder M. Combined brain mapping and compact intraoperative MRI for brain tumor resection. Stereotact Funct Neurosurg. 2018;96:172–81. doi: 10.1159/000488991. [DOI] [PubMed] [Google Scholar]
- 36.Wu JS, Shou XF, Yao CJ, Wang YF, Zhuang DX, Mao Y, et al. Transsphenoidal pituitary macroadenomas resection guided by PoleStar N20 low-field intraoperative magnetic resonance imaging: Comparison with early postoperative high-field magnetic resonance imaging. Neurosurgery. 2009;65:63–70. doi: 10.1227/01.NEU.0000348549.26832.51. [DOI] [PubMed] [Google Scholar]
- 37.Xia L, Fang C, Chen G, Sun C. Relationship between the extent of resection and the survival of patients with low-grade gliomas: A systematic review and meta-analysis. BMC Cancer. 2018;18:48. doi: 10.1186/s12885-017-3909-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zimmermann M, Seifert V, Trantakis C, Kühnel K, Raabe A, Schneider JP, et al. Open MRI-guided microsurgery of intracranial tumours. Preliminary experience using a vertical open MRI-scanner. Acta Neurochir (Wien) 2000;142:177–86. doi: 10.1007/s007010050021. [DOI] [PubMed] [Google Scholar]
- 39.Zimmermann M, Seifert V, Trantakis C, Raabe A. Open MRI-guided microsurgery of intracranial tumours in or near eloquent brain areas. Acta Neurochir (Wien) 2001;143:327–37. doi: 10.1007/s007010170086. [DOI] [PubMed] [Google Scholar]