Abstract
Projected trends in population aging have forecasted a massive increase in the number of people with dementia, in particular in sub‐Saharan Africa and the Middle East and North Africa (MENA) region. Cognitive decline is a significant marker for dementia, typically assessed with standardized neuropsychological tools that have been validated in some well‐researched languages such as English. However, with the existing language diversity, current tools cannot cater to speakers of understudied languages, putting these populations at a disadvantage when it comes to access to early and accurate diagnosis of dementia. Here, we shed light on the detrimental impact of this language gap in the context of the MENA region, highlighting inadequate tools and an unacceptable lack of expertise for a MENA population of a half billion people. Our perspective calls for more research to unravel the exact impact of the language gap on the quality of cognitive decline assessment in speakers of understudied languages.
Highlights
Cognitive decline is a marker for dementia, assessed with neuropsychological tests.
There is a lack of culturally valid tests for speakers of understudied languages.
For example, suboptimal cognitive tests are used in the Middle East and North Africa region.
Linguistic diversity should be considered in the development of cognitive tests.
Keywords: behavioral assessment, cognitive abilities, cognitive decline, dementia, healthy aging, language, neuropsychology
1. INTRODUCTION
Here is a real challenge for a health‐care professional: assess the cognitive abilities of someone speaking Hausa or Fulani. It is unlikely that validated neuropsychological tests exist for speakers of those languages, so a trained health‐care professional might rely on some available or customized translated versions. For many speakers of similar understudied languages, the context can be much more challenging than that, starting in the first place with a lack of trained professionals who can accurately administer neuropsychological tests. This example is not limited to rare languages but applies to many languages spoken by hundreds of millions of people. 1 , 2 For instance, among the six official languages of the United Nations (Arabic, Chinese, English, French, Russian, and Spanish), robust psychometrically validated and culturally appropriate neuropsychological tests are lacking for some languages (e.g., Arabic), despite a growing number of speakers and internet users of these languages. This has far‐reaching consequences on the quality of health care offered to these populations, in particular in the current growing aging population and the forecasted high rates of people living with dementia. 3 The goal of this paper is to shed light on this language gap and its detrimental impact on the global fight against dementia, using the Middle East and North Africa (MENA) region as an illustrative case.
RESEARCH IN CONTEXT
Scoping review: The authors reviewed existing literature using traditional sources. All studies that adapted or validated neuropsychological tests to the Middle East and North Africa region are selected. Papers with similar aims in other contexts of testing cognitive decline in speakers of understudied languages are also selected and cited.
Interpretation: Our findings showed that speakers of understudied languages are at disadvantage when it comes to access to early and accurate diagnosis of dementia.
Future directions: The article calls for more research to gauge the exact detrimental impact of the language gap on access to clinically relevant cognitive tests in speakers of understudied languages. Future initiatives should help (a) develop culturally valid tests for speakers of different languages, (b) broaden the linguistic diversity of both participants and researchers in neuropsychology, and (c) collect comprehensive epidemiological data about dementia in linguistically diverse populations.
Cognitive decline assessment usually relies on a comprehensive evaluation process that incorporates self‐reported symptoms, physical and neurological examinations, and standardized cognitive tests. Common cognitive screening tools used in this process include the Mini‐Mental State Examination (MMSE), the Montreal Cognitive Assessment (MoCA), the Rowland Universal Dementia Assessment Scale (RUDAS), the Addenbrooke's Cognitive Assessment Version III (ACE‐III), and Mini‐Cog (see Table 1). 4 , 5 These tests can target various cognitive domains such as memory, attention, language, visuospatial abilities, and executive functions. These tests are important in particular when bedside cognitive status examination cannot provide a confident diagnosis. The assessment is typically administered one on one by a trained health‐care professional in a clinical setting. The examiner asks the patient to complete multiple tasks, including immediate and delayed recall of words or numbers, object naming, drawing geometric shapes, and answering questions related to orientation, using verbal stimuli primarily, and less dominantly visual and motor stimuli either in a paper‐based or computer‐based format. The examiner reads the instructions and questions to the patient as per the test protocol, and the patient is required to provide a verbal response to each part, except for the praxis subtests. Therefore, effective communication between the examiner and the patient is key to ensuring reliable and accurate scores. Moreover, some test items require patients to read, write, and perform mathematical calculations, thus assuming a minimum level of literacy of the patients; for example, MMSE has been reported in many studies to have an educational bias. 2 , 6 , 7 These neuropsychological tests have played an important role in cognitive assessment in the clinical setting, but they are still facing challenges regarding the lack of validation in non‐English speaking populations and their adaptation to other educational or cultural contexts. 6 , 8
TABLE 1.
Some widely used cognitive tests.
| Test abbreviation | Full test name | Cognitive domains | Cutoff score | Language of development and validation | Number of translated versions |
|---|---|---|---|---|---|
| MoCA | Montreal Cognitive Assessment | Short‐term memory, visuospatial abilities, executive function, attention, concentration, working memory, language, orientation to time and place | <26/30 | English, French | 100 (for the full version) |
| MMSE | Mini‐Mental State Examination | Attention and orientation, memory, registration, recall, calculation, language praxis |
Max. score 30 Cutoff: 19–27; the exact value is age and education dependent |
English | 73 |
| RUDAS | Rowland Universal Dementia Assessment Scale | Memory, orientation, praxis, recall, language | <22/30 | English | Not available |
| ACE‐III | Addenbrooke's Cognitive Examination‐III | Orientation and registration, attention and calculation, memory, language | <84 (lower bound)−88 (upper bound)/100 | English | 38 |
| Mini‐Cog | ‐ | Memory, executive functions | <3/5 | English | 23 |
Perhaps most importantly, testing patients should ideally be done in their primary native language. For instance, a recent work applied 11 neuropsychological tests spanning four different cognitive domains (attention, memory, language, visuo‐construction) to native and non‐native Swedish speakers 9 and showed consistently higher results in native speakers in all tests except for purely visuo‐constructive tests. Participants whose native languages are more distant to Swedish, in terms of vocabulary and alphabetical symbols, presented lower scores than participants with native languages closely related to the Swedish language, 9 suggesting that neuropsychological tests are not always reliable when administered in the non‐native language of the participant. In another study, native English speakers outperformed non‐native English speakers on several neuropsychological tests, 10 suggesting that delivering tasks in a non‐native language might negatively impact test performance. Similar observations have been reported for many other populations with diverse linguistic abilities, leading to the development of different language‐specific versions of these neuropsychological tests. 11 , 12 , 13 , 14 , 15 , 16 , 17 In this context, a plain translation of such tests might not be enough, that is, the transcripted word in another language might have a slightly different conceptual meaning and can bias test results. Thus, administering these translated versions to speakers of understudied languages might pose a real challenge to health‐care professionals to generate clinically useful scores, particularly when the diagnosis is based on comparing measured scores to prior cut‐off values.
Furthermore, current classic neuropsychological tests might not cater to the rapidly evolving cultural and linguistic diversity among the global older population. Indeed, the majority of the existing neuropsychological tests available for non‐English speakers are in most cases derived from existing English versions, leaving unanswered questions about the validity and reliability between different translated versions of the same tests. Moreover, in addition to language‐related factors, cultural factors could add another layer of complexity to existing assessment tools for dementia. 3 , 18 For instance, it has been shown recently that MMSE may have low specificity in minorities, with Black Americans, for example, tested at up to 42% false‐positive rate for cognitive impairment compared to a 6% false‐positive rate in White Americans. 19 Even in the updated MMSE‐2: Standard Version, in which problematic test items have been replaced to streamline translation and adaptation to other languages and cultures, some of the older subjects still faced difficulty in completing some items in an Arabic version of the test. 20 Similarly, two recent studies 21 , 22 evaluated the prevalence of dementia in a Saudi community using two different validated neuropsychological tests. Both studies recruited samples from the same city and yet they reported discrepant results; that is, a 16.6% prevalence rate with the 8‐item Alzheimer's Dementia test versus a 6.4% prevalence rate with a translated version of the MoCA test. This inconsistency raises concerns regarding the reliability of these translated tests. 21 , 22 In some cases, bias could be mitigated by modifying or replacing some test items. One example can be seen in MoCA, in which some items were modified to accommodate differences between Eastern versus Southern Asian populations. 2 Similar cultural factors were also reported in the Persian version of the test. 23 However, bias can also be observed at an instrument scale or task level rather than the individual test items or stimuli. For instance, a sample of Aruaco Indians from Colombia was unable to perform some tasks of a neuropsychological test that rely on a block design using a time limit due presumably to differences in time conception, 24 suggesting that cultural relevance, in addition to education level, might affect performance. Likewise, American participants outperformed Russian participants in timed cognitive tests due to potential differences in familiarity with timed testing procedures between the two cultures. 25
Overall, the limitations in existing adaptations and translations of English versions have led to many local initiatives to develop alternative tests tailored to non‐English speakers or cross‐cultural tests such as the Korean Dementia Screening Questionnaire, 26 the Neuropsychological Screening Battery for Hispanics, 27 and the Spanish and English Neuropsychological Assessment Scales. 28 Below, we appraise the utility of such adaptations and translations of English versions in the context of cognitive abilities assessment in Arabic speakers. To substantiate our conclusions, a scoping review has been conducted to gather evidence about current practices in cognitive assessment in the MENA region.
2. THE CASE OF THE MENA REGION
The MENA region offers an interesting case to gauge the impact of the language gap on the assessment of cognitive decline. The MENA region encompasses almost half a billion people, living in 22 countries with one of the highest forecasted rates of growth in the number of people living with dementia. Indeed, dementia is highly prevalent in the MENA region, ranging between 1.1% and 2.3% in people aged ≥ 50 years, and between 13.5% and 18.5% in people > 80 years. 29 This region has also a high Alzheimer's disease prevalence for people > 70 years. 30 The MENA region has a large socio‐economic diversity across many economic indicators, with people with very low income (e.g., Sudan, Yemen) to very high income (e.g., United Arab Emirates, Qatar), see Table 2. The cost of dementia is estimated at ≈ 0.25% to 1% of the total gross domestic product (GDP) in MENA countries. 31
TABLE 2.
Key indicators of MENA region countries.
| Country | Population count (in millions), year of data collection | Literacy rate, adult total (% of people ages 15 and above), year | GDP per capita (US$ ×1000), year | Physicians (per 1,000 people), year | Spoken languages | Language(s) of instruction |
|---|---|---|---|---|---|---|
| Algeria | 44.2, 2021 | 81%, 2018 | 4.3, 2022 | 1.7, 2018 | Classic Arabic, Amazigh, Arabic dialect, French, Tacawit, Tamahaq | Arabic, French, Tamazight |
| Bahrain | 1.5, 2021 | 92%, 2011 | 30.2, 2022 | 0.9, 2015 | Classic Arabic, Arabic dialect, English, Persian, Urdu | Arabic |
| Comoros | 0.8, 2021 | 62%, 2021 | 1.5, 2022 | 0.3, 2018 | Comorian, French, classic Arabic | French, Arabic |
| Djibouti | 1.1, 2021 | Not available | 3.1, 2022 | 0.2, 2014 | French, classic Arabic, Arabic dialect, Somali, Afar | French, Arabic |
| Egypt. Arab Rep. | 109.3, 2021 | 73%, 2021 | 4.3, 2022 | 0.8, 2018 | Classic Arabic, Coptic, Arabic dialect, English, French | Arabic |
| Iraq | 43.5, 2021 | 86%, 2017 | 5.9, 2022 | 0.7, 2018 | Classic Arabic, Arabic dialect, Kurdish, Turkmen, Assyrian, Armenian, Aramaic, English | Arabic, Kurdish |
| Jordan | 11.1, 2021 | 98%, 2021 | 4.2, 2022 | 2.3, 2017 | Classic Arabic, Arabic dialect | Arabic, English |
| Kuwait | 4.3, 2021 | 96%, 2020 | 43.2, 2022 | 2.6, 2015 | Classic Arabic, Arabic dialect | Arabic, English |
| Lebanon | 5.6, 2021 | 95%, 2019 | 4.1, 2021 | 2.1, 2018 | Classic Arabic, Arabic dialect, French, English, Armenian | Arabic, English, French |
| Libya | 6.7, 2021 | 86%, 2004 | 6.7, 2022 | 21, 2017 | Classic Arabic, Arabic dialect, Amazigh, Tamahaq, Italian, English. | Arabic, English, French |
| Mauritania | 4.6, 2021 | 67%, 2021 | 2.2, 2022 | 0.2, 2018 | Classic Arabic, Hassaniya, French, Pular, Soninke, and Wolof | Arabic, French |
| Morocco | 37.1, 2021 | 76%, 2021 | 3.5, 2022 | 0.7, 2017 | Classic Arabic, Arabic dialect, Amazigh, Hassaniya, French, Spanish. | Arabic, Tamazight, French |
| Oman | 4.5, 2021 | 96%, 2018 | 25.1, 2022 | 2, 2018 | Classic Arabic, Arabic dialect | Arabic |
| Qatar | 2.7, 2021 | 93%, 2017 | 88.0, 2022 | 2.5, 2018 | Classic Arabic, Arabic dialect, English | Arabic, English |
| Saudi Arabia | 36.0, 2021 | 98%, 2020 | 30.4, 2022 | 2.6, 2018 | Classic Arabic, Arabic dialect | Arabic, English |
| Somalia | 17.1, 2021 | 5%, 1972 | 0.5, 2022 | 0.01, 2014 | Classic Arabic, Somali, English and Italian | Somali, Arabic, English |
| Sudan | 45.7, 2021 | 61%, 2018 | 1.1, 2022 | 0.3, 2017 | Classic Arabic, Arabic dialect, English, Nubian, Fur, Beja | English, Arabic |
| Syrian Arab Republic | 21.3, 2021 | 86%, 2014 | 0.5, 2020 | 1.3, 2016 | Classic Arabic, Arabic dialect, Kurdish, Armenian, Aramaic, Circassian | Arabic, English |
| Tunisia | 12.3, 2021 | 83%, 2021 | 3.87, 2022 | 1.3, 2017 | Classic Arabic, Arabic dialect, French, Berber language | Arabic, French |
| United Arab Emirates | 9.4, 2021 | 98%, 2021 | 53.8, 2022 | 2.5, 2018 | Classic Arabic, Arabic dialect | Arabic, English |
| West Bank and Gaza | 4.9, 2021 | 98%, 2020 | 3.8, 2022 | 0.8, 2001 | Classic Arabic, Arabic dialect | Arabic, English |
| Yemen, Rep. | 33.0, 2021 | 54%, 2004 | 0.7, 2022 | 0.5, 2014 | Classic Arabic, Arabic dialect | Arabic |
| Whole world | 8000, 2022 | 87%, 2020 | ‐ | 1.6, 2018 | 7,168 languages | ‐ |
Notes: Population count, Literacy rate, GDP per capita, and physicians data are sourced from the World Bank dataset. Classic Arabic is the formal MSA, and Arabic dialect is the spoken colloquial Arabic.
Abbreviations: GDP, gross domestic product; MSA, Modern Standard Arabic.
In terms of research investment and dissemination, MENA is a region with poor to moderate research productivity. 32 , 33 For instance, despite a large number of people suffering from diverse neurodegenerative diseases, 34 including dementia, 34 , 35 the contribution of the MENA region to research in dementia is relatively low compared to the global average research productivity. 36 Indeed, some countries had none‐to‐little information on dementia, indicating a considerable lack of awareness about dementia in these countries. 35 This might explain the scarcity of epidemiological studies about dementia in some MENA countries. Furthermore, dementia is sometimes associated with stigma, and so families have to take care of their members living with dementia in the absence of professional home care, in particular in small or rural communities. Likewise, many countries in the MENA region have a below‐world‐average number of physicians per 1000 inhabitants (Table 2), which is another factor negatively affecting the availability of quality cognitive assessment.
Perhaps most importantly, the MENA region is one of the classic examples of a linguistic situation with diglossia. 37 People in the MENA region typically use at least two languages, Modern Standard Arabic (MSA) as an instruction language in formal education and colloquial Arabic as a common or everyday language. MSA is relatively consistent across countries, but colloquial Arabic varies largely across countries with different varieties as local dialects. This language setting in the MENA raises questions on the impact of diglossia, on top of the widespread adoption of some languages as a “lingua franca” or as languages of instruction (e.g., English and French; Table 2), on dementia onset in the presence of high rates of diabetes, hypertension, and other comorbidities in the region. 38 This diglossic situation poses an additional challenge to the development of standardized tests for cognitive decline in the MENA region because diglossia can affect executive and cognitive functions. 39 , 40 , 41 , 42 This peculiar linguistic situation offers a good example for other similar situations in the world that are marked with diglossia such as speakers of Cantonese–Mandarin, Cypriot Greek, Swiss German, Serbo‐Croatian, and many other African and Asian dialects that are spoken by millions of people in the presence of a main national language (e.g., in Cameroon, speaking Fulani as a dialect in addition to French as an official language).
2.1. A scoping review about cognitive assessment in the MENA region
To identify and analyze gaps in cognitive assessment in the MENA region, a scoping review was performed following the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses extension for Scoping Reviews (PRISMA‐ScR) Checklist. 43 Articles were selected if they (1) were published between 2012 and February 2023, to cover the most recent decade since the Arab spring uprisings; (2) were published in English; (3) addressed questions related to the cognitive abilities of Arabic‐speaking adults; and (4) explicitly used neuropsychological tests or questionnaires for the assessment of cognitive abilities. Neuropsychology research published in Arabic is scarce. This literature mainly concerns paywalled papers in non‐indexed journals hosted by local universities that do not always offer a rigorous peer review process. Therefore, this review only considered research papers published in indexed English journals. Articles were excluded if they included Arab immigrants or non‐Arab subjects in their samples as these groups have unique demographics compared to indigenous Arabic populations that could exert an influence on their cognitive performance. 44 While it makes sense to assume that a validated test should work regardless of country of residence, Arab immigrants in Western countries have access to better health services and resources, including the chance of being examined by highly trained health‐care professionals, which is not the case for indigenous Arab people in many MENA countries (see Table 2). Moreover, Review papers were excluded as they do not provide empirical data. Search for relevant articles was conducted up until February 2023 using PubMed online database. The following keywords ((cognitive) AND ((decline) OR (impairment) OR (dysfunction)) AND ((assessment) OR (test)) AND (Arabic)) generated a total of 124 articles. These search terms are broad enough to encompass studies about dementia as well. These articles were compared against the selection criteria above, which resulted in a final list of 45 articles (see Figure 1 for more details). Relevant articles were reviewed and selected by authors OH and MLS. The final list of papers was exported into Mendeley referencing software.
FIGURE 1.
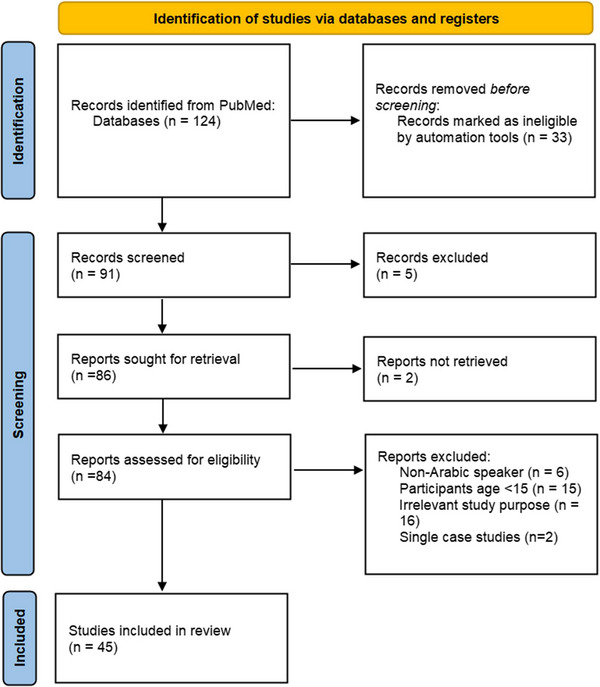
Preferred Reporting Items for for Systematic Reviews and Meta‐Analyses flow chart for study selection process. First the titles and abstracts were evaluated, then full‐text screening was done to exclude irrelevant articles. The final list included 45 articles.
Variables and information of interest were extracted from the selected articles and organized with Microsoft Excel 2019. They included the following details: title of the study, authors, year of publication, the assessment tool used, whether the tool is classic or self‐developed, the language of assessment, translation method (if applicable), targeted cognitive domains, cutoff scores used to evaluate cognitive status, information about participants’ number and age range, and any limitations and challenges reported by the original authors. Some neuropsychological tests were used to test cognitive abilities for diverse clinical questions not restricted to dementia (cf. last column of Table 3). Our rationale here was to comprehensively appraise the current use of neuropsychological tests for the overall assessment of cognitive abilities regardless of the original authors’ main clinical or research question of interest.
TABLE 3.
A detailed description of the 45 selected articles about cognitive abilities assessment in the MENA region.
| Tests used / battery | Country | Cognitive domain | Age and number | Language/dialect | Translation/adaptation | Norm data/cutoff | Limitations | Question of interest* | |
|---|---|---|---|---|---|---|---|---|---|
| Fakhry (2013) 100 | MMSE, MTS, CDT | UAE | Not mentioned by authors | Age = 20–40, N = 60 bipolar patients, 30 controls | Not mentioned | NA |
Cognitive impairment: MMSE < 25 MTS < 27 |
Psychotropic drugs’ effects on cognition could not be eliminated. | Q1 |
| Khedr (2015) 54 | MES, MMSE | Egypt | Memory, executive function, orientation, recall, attention, calculation, language processing, and constructional praxis | Age = 30–60, ≥ 60, n = 691 | Not mentioned | Not mentioned |
Normal: MES >75, MCI: MES= 62‐75, Dementia: MES<62 MMSE = 23/30 (Educated) MMSE =21/28 (illiterate) |
No limitations reported by authors. | Q1 |
| Darwish (2015) 46 | MoCA‐Arabic, RCFT, SDMT | Lebanon | Visual spatial memory, speed of processing | Age ≥30, n = 254 |
Arabic for instructions (unspecified dialect) Lebanese dialect for verbal fluency. |
MoCA‐Arabic: previously translated RCFT: translation of instructions, revision, pilot testing on 10 subjects SDMT: translation of instructions, revision. |
Cognitive impairment: MoCA ≤26 RCFT ≤5th percentile SDMT: not mentioned |
‐MoCA cutoff values and items were not appropriate for the tested population. ‐MoCA scores do not consider years of education. |
Q2 |
| Chaaya (2016) 74 | A‐RUDAS | Lebanon | Memory (registration and recall), body orientation, praxis, drawing, judgment, and language | Age ≥65, n = 232 elderly | Classical Arabic | Translation by native speakers, pilot testing on 10 individuals | Dementia: A‐RUDAS ≤22 | GMS (used for depression diagnosis) has not been separately validated in Arabic | Q4 |
| Nielsen (2016) 105 | RUDAS, IQCODE | Lebanon | Body orientation, praxis, drawing, judgment, memory, language. | Age ≥65, n = 225 elderly | RUDAS: classical Arabic, IQCODE: Arabic (unspecified dialect) | Previously translated and validated | Dementia: RUDAS <23/30, IQCODE >3.34/5 |
‐Diagnosis was not supported by in‐depth neuropsychological testing or ancillary investigations. ‐Inclusion of participants from long‐term care settings. ‐RUDAS might need clinical expertise to perform the task. |
Q4 |
| Ibrahim (2016) 101 | Arabic version of the Penn Computerized Neurocognitive Battery | Egypt | Sensorimotor integration speed, attention, face memory, abstraction, mental flexibility, manual dexterity, visual object learning and memory, nonverbal reasoning, spatial orientation, social cognition, emotion recognition, working memory. | Age = 21–62, n = 258 | Arabic (unspecified dialect) | Previously translated | NA, correlations between cognitive functions and antibody titers |
‐Hepatic functions were not estimated ‐Urine analysis for illicit drugs was not performed ‐HIV infection was not screened |
Q2 |
| Al‐Momani (2016) 56 | MMSE‐Arabic | Jordan | Not mentioned by authors | Age = 18–100, n = 221 nursing home residents | Arabic (unspecified dialect) | NA |
Impaired cognitive function: MMSE‐Arabic<25 |
‐Including a wide range of participants’ age. ‐Not all factors affecting gait and balance were considered ‐Cutoff scores of the tests were based on previous studies |
Q3 |
| Abou‐Mrad (2017) 45 | AD8, MoCA, MMSE, 3MS, BVMT‐R, LDS, CLNT, phonemic fluency, semantic fluency. | Lebanon | Memory, attention, language, construction, executive functioning, orientation, and visuospatial function, phonemic paraphasias, semantic paraphasias, circumlocutions, phonemic fluency, semantic fluency. | Age ≥60, n = 164 community dwelling older Lebanese adults without cognitive complaints. | Literary Arabic | Translation by linguistic experts, back‐translation. | Regression based norms. |
‐There was no comparison to a gold standard screening. ‐Medical imaging was not used to exclude significant intracranial findings. ‐Most participants were tested only once. |
Q4 |
| Albanna (2017) 20 | MMSE‐2:SV, Mini‐Cog | Qatar | Orientation, recall, attention, calculation, language processing and constructional praxis–cognitive function, memory, language comprehension, visual‐motor skills, and executive function | Age >60, n = 134 | Formal Arabic | Translation by experts, pilot testing on 20 subjects, back translation. |
Dementia: Combined score of MMSE‐2:SV and Mini‐Cog: 20/21. |
‐Some items of the batteries were not doable by the elderly. ‐The test sample was mostly from Qatar ‐Male subjects were more the females. ‐Arabic MMSE‐2 has low sensitivity to mild dementia. |
Q4 |
| Ben Jemaa (2017) 106 | A‐ADAS‐Cog | Tunisia | Memory, language, praxis | Age: 50–90, n = 124 NC, 33 N‐AD, 25 AD | Arabic, meeting cultural and linguistic need of the Arab populations. | Translation was based on equivalency |
AD ADAS‐Cog =10 |
No limitations reported by authors | Q4 |
| Bou‐Orm (2018) 102 | A‐IQCODE | Lebanon | Not mentioned by authors | Age ≥65, n = 502 | Arabic (unspecified dialect) | Previously translated and validated |
Cognitive decline: A‐IQCODE<3.34 |
No limitations reported by authors | Q2 |
| Alkhunizan (2018) 21 | MoCA | Saudi Arabia | Not mentioned by authors | Age ≥60, n = 171 patients (clinic) | MSA Arabic | Previously translated and validated |
MCI: A‐MoCA<26 Dementia: A‐MoCA<17 |
No limitations reported by authors |
Q1 |
| Farghaly (2018) 107 | Phonemic and Categorical verbal fluency (Using letter Haa only) | Egypt | Verbal fluency | Age >40, n = 79 NC, 32 CD | Arabic (dialect unspecified) | NA |
ROC analysis Dementia: animal <11 vegetables <11 Names <18 |
‐MMSE was used to classify cognitive status of the participants (cut‐offs not validated to Arabic populations) ‐Verbal fluency was used solely as an indicator for cognitive impairments |
Q4 |
| Saleh (2019) 47 | MoCA‐B‐Arabic | Egypt | Executive function, verbal fluency, calculation, abstraction, recall, naming, attention, orientation, visuospatial functions. | Age ≥ 60, n = 39 mild NCD,54 major NCD, 112 NC | MSA | Translated without cultural or linguistic modifications |
Mild NCD: A‐MoCA‐B: 21/22 Major NCD: A‐MoCA‐B:16/17 |
‐Small sample size. ‐Inability to measure inter‐rater or test–retest reliability. ‐Limited generalizability ‐Patients recruited from medical centers not a primary care facility |
Q4 |
| Almubark (2019) 69 | Arabic version of Cognistat | Saudi Arabia | Language construction, memory, calculations, reasoning, consciousness, orientation, attention. | Age = 18–60, n = 30 stroke and 32 TBI patients, 107 healthy adults | Standard Arabic | Translation by Native Arabic speakers, cultural adaptation, backward translation, pretest on 22 subjects. |
Percentile norms were reported. |
‐Sampling was restricted to 2 age groups, limiting the generalizability of the normative Cognistat profile. | Q4 |
| Al‐Joudi (2019) 70 | NTB used in the Division of Medical Psychology of the Johns Hopkins University School of Medicine | Saudi Arabia | Intelligence, confrontation naming ability, verbal comprehension and fluency, episodic memory, visuospatial learning and memory, frontal and executive functioning, psychomotor speed, fine motor control. | Age = 15–67, n = 56 | Formal Arabic language & common colloquial Arabic (not Saudi) | Translation by native Arabic speakers, cultural adaptation, backward translation. | NA, data analysis is done to investigate the battery test's ability in differentiating between controls and patients and between left and right temporal Epilepsy |
‐Many disease factors were not investigated for their relation to test performance. ‐Limited number of participants in the mesial temporal sclerosis group. ‐Not all battery tests were investigated for reliability ‐Degree of mesial temporal sclerosis was not accounted for in this study |
Q4 |
| El‐Hayeck (2019) 55 | A‐MMSE (GTD‐USJ) | Lebanon | Attention, registration, attention, calculation, language, visuospatial processing. | Age ≥55, n = 1010 literate community‐dwelling Lebanese residents | Arabic (unspecified dialect) | Translation and adaptation of the French version by the Working Group on Dementia at Saint Joseph University |
Cognitive impairment: A‐MMSE (GTD‐USJ) <23 |
‐Population may not be a random sample ‐Demographic information were not collected from subjects who declined participation ‐Insufficient number of subjects per categories ‐ Subjects with (A‐MMSE(GTD‐USJ) a score <16 was decided to need medical evaluation prior to validation of the tool ‐Including the data from participants who did not attend the medical consultation in the normative data ‐MMSE does not cover all cognitive domains |
Q4 |
| Ibrahim (2019) 57 | The Arabic version of Penn computerized neuropsychological battery, TMT‐ A&B, MMSE | Egypt | Sensorimotor integration speed, abstraction, mental flexibility, manual dexterity, visual object learning, memory, nonverbal reasoning, spatial orientation, social cognition, emotion recognition, attention, visual motor speed. | Age = 18–50, n = 94 |
Penn: Arabic (unspecified dialect) TMT and MMSE: not mentioned |
Previously translated | NA, significant difference between two groups |
‐Small sample size ‐All patients were stabilized on antipsychotic medications |
Q5 |
| Elsaid (2020) 111 | SF‐12v2 questionnaire | Egypt | Physical and mental component, eight health‐related domain scores | Age >56, n = 40 males, 20 females | Arabic (unspecified dialect) | Previously translated | Scores from excellent to poor: 5.0, 4.4, 3.4, 2.0, and 1.0 |
‐Small sample size ‐Immunological changes induced by Biobran/MGN‐3 were not assessed due to cost limitations. |
Q5 |
| Salama (2020) 48 | A‐MoCA, A‐BICAMS | Egypt | Associative learning, attention, executive function, inhibition, memory, processing speed | Mean age = 34.4, n = 20 NMOSD patients, 18 NC | Arabic (unspecified dialect) | Previously translated | significant difference between patients and controls | Small number of patients included from a single center | Q3 |
| Shalash (2020) 104 | NMSS, PDQ‐39‐Arabic | Egypt | NMSS measures 9 domains: (cardiovascular, sleep/fatigue, mood/cognition, perception/hallucinations, memory/attention, gastrointestinal, urinary, sexual, and miscellaneous symptoms) | Age = 35–77, n = 40 PD male patients, 25 NC |
NMSS: not mentioned. PDQ‐39: Arabic (unspecified dialect) |
Previously translated | NA, correlation between IIEF and the PDQ‐39 and NMSS |
‐Small sample size ‐Limited participants’ age range ‐Investigating the association of cognitive and autonomic factors with sexual dysfunction using more objective and specific tests is recommended. |
Q3 |
| Al‐Adawi (2020) 110 | A‐IQCODE, The modified Wisconsin Card‐Sorting Test, the tower of London, TMT, backward DS, verbal fluency. | Oman | Set‐shifting, cognitive flexibility, planning, the temporal organization of behavior, processing speed, initiation, speed of verbal responses. | Age = 18–35, n = 24 patients with executive dysfunction | Arabic (unspecified dialect) | Previously translated | IQCODE: “major improvement” = 1, “minor improvement” = 2, “did not change much” = 3, “minor deterioration” = 4, “major deterioration” = 5 |
‐ Open‐label study, without controls and with a relatively small sample size ‐Was not validated by a another catecholaminergic agonist ‐Limited generalizability as motor and functional metrics were not included ‐Include another cohort of TBI rather than those with executive functioning ‐Improvements could be a result of other factors |
Q5 |
| Qassem (2020) 65 | ACE‐III | Egypt | Attention and orientation, memory, verbal fluency, language, and visuospatial abilities | Age ≥60 patients, n = 37 dementia patients, 43 controls | Egyptian Arabic | Previously translated | Dementia: ACE‐III 72/100 |
Generalizability to rarer types of dementia was limited Cut‐offs were not validated in a second independent sample Specific cut‐offs could not be established for different age groups Subjects were recruited from one city Tested population did not include illiterates. |
Q4 |
| Zeinoun (2020) 60 | VMAT | Lebanon | Verbal memory |
Age ≥16, pilot study: 12 participants; study2: 199 NC, 16 MS population |
MSA and colloquial Arabic | The task was developed in Arabic | regression‐based norms |
‐Sample was not representative of national age demographics ‐Participants were young and educated ‐MS group was assumed to have verbal learning and memory deficits without verification ‐VMAT scores were not correlated with other Arabic memory tests |
Q4 |
| Allataifeh (2020) 62 | BICAMS, Stroop test | Jordan | Learning, memory and mental processing speed, selective attention | Age >18, n = 110 individuals with MS | Arabic (unspecified dialect) | NA | regression‐based norms |
Participants were recruited from one geographical area Most participants had relapsing‐remitting MS (RM MS) |
Q3 |
| Qassem (2020) 65 | ACE‐III | Egypt | Attention, memory, fluency, language, visuospatial processing. | Age >60, n = 24 MCI patients, 54 controls | Egyptian Arabic | Previously translated |
Using ROC MCI: ACE‐III 81 /100 |
‐Lack of specific cut‐offs for different age groups ‐Cut‐offs were not validated in a second independent sample |
Q4 |
| Alshammari (2020) 58 | MMSE | Saudi Arabia | Orientation, registration, attention, calculation, recall, language. | Age = 60–93, n = 1299 | Arabic (unspecified dialect) | Previously translated |
Intact: 24‐30 Mild: 18‐23 severe: 0‐17 |
‐The cross‐sectional design of this study prevented us from establishing causality ‐Limited generalizability |
Q1 |
| Muayqil (2021) 49 | MoCA (A & B) | Saudi Arabia | Not mentioned by authors | Age = 18–80, n = 311 | MSA | Modifications to suit the culture and dialect, validation by a pilot study on 15 participants. |
Mean test value: MoCA‐A: 21.47 MoCA‐B: 24.37 |
‐Most participants had pervious concerns about their cognition (risk of bias) ‐ There was no screening to exclude any occult cognitive problems |
Q4 |
| Qassem (2021) 66 | m‐ACE‐III | Egypt | Attention, memory, fluency, language, visuospatial processing. | Age ≥, n = 24 MCI, 52 NC | Arabic (unspecified dialect) | Previously translated |
Using ROC MCI: ACE‐III = 21/30 |
Lack of specific cut‐offs for different age groups Cut‐offs were not validated in a second independent sample |
Q4 |
| Rababa (2021) 50 | MoCA | Jordan | Visuospatial/executive, naming, memory, attention, abstraction, detailed recall, language | Age = 55–103, n = 215 older adults | MSA | Previously translated | MCI: MoCA<30. |
PPI data was collected using medication cards which might have resulted in missing that could bias the output Limited generalizability due to the small sample size and inclusion of subjects from one geographical area |
Q3 |
| Assaf (2021) 51 | A‐MoCA, GDS, IADL | Lebanon | Not mentioned by authors | Age ≥60, n = 337 | Arabic (unspecified dialect) | Previously translated |
Objective cognitive impairment: MoCA≥26 MCI: MoCA <26, IADL (7/8). Dementia: MoCA <26, IADL <7. |
‐Limited generalizability as subjects had high socioeconomic and educational attainment ‐MoCA is subject to ceiling effects ‐Depression could be affecting MoCA results ‐Overlooking variables that could affect performance |
Q2 |
| Darwish (2022) 63 | BICAMS, SDMT, BVMT‐R, VMAT | Lebanon | Verbal learning, short‐term memory, long‐term memory, and recognition. | Age = 16–80, n = 180 healthy participants |
Lebanese Arabic dialect for administration MSA for tests |
Previously translated | Regression‐based norms |
‐Most of the MS sample was RRMS. ‐Unbalanced sex distribution. ‐More evidence is needed to support the validity of the BICAMS ‐Age groups were not matched in years of education. |
Q4 |
| Farahat (2022) 103 | WCST | Egypt | Executive function | Age = 25–52, n = 81 HCW (50 physicians, 31 nurses) | NA | NA | Compared cognitive performance in HCW before and after a 2‐week break. |
‐Executive dysfunction does not reflect a general cognitive decline. ‐HCW executive functioning baseline before their hospital stay was not measured ‐WCST results are impacted by IQ scores ‐limited study sample |
Q2 |
| Souissi (2022) 64 | T‐BICAM: SDMT, BVMT‐R, and TVLT | Tunisia | Processing speed, auditory/verbal learning, visuospatial memory | Age = 18–65, n = 104 MS patients and 104 NC | Tunisian Arabic | Instructions and stimuli were translated and standardized for Tunisian culture. |
MS: ROC analysis SDMT:39−40, BVMT‐R:26−27, TVLT:43−44 |
Most participants were highly educated. | Q4 |
| Alkeridy (2022) 109 | BADLS | Saudi Arabia | dependency in performing basic and instrumental activities of daily living. | N = 69, median age = 77 | Modern standard Arabic | Forward‐backward translation followed by pilot testing. | Non reported | Confirmatory factor analysis was not performed. The psychometric properties of the scale could change according to the change in literacy level in Saudi Arabia. | Q4 |
| Saguem (2022) 73 | BCIS | Tunisia | Self‐certainty and self‐reflectiveness | Age = 42±12.52, n = 150 patients | Literary Arabic | Repeated forward‐backward translation. | Non reported | No limitations reported by authors | Q4 |
| Haddad (2022) 53 | MoCA | Lebanon | Visuospatial abilities, short term memory, executive function, naming, attention, concentration, working memory, language, abstract reasoning, orientation | Age = 18–60, n = 120 patients | Arabic (unspecified dialect) | Previously translated |
MCI: A‐MoCA =21; Moderate cognitive impairments: A‐MoCA = 20.5; Severe cognitive impairments: A‐MoCA =19.5 |
‐Possibility of selection bias, participants cognitive function might be severely impaired ‐Limited sample size ‐Information bias might have occurred in the face‐face interview. ‐Some cognitive factors were not included ‐Missing some validity measures ‐MoCA is not compatible for illiterate participants. |
Q4 |
| El‐Hayeck (2022) 108 | A‐TNI93 (GTD‐USJ) | Lebanon | Episodic memory | Age ≥55, n = 332 | Assessment language was not mentioned | Pictures adapted to Lebanese culture |
Dementia: Free recall (FR) ≤6 Or total recall ≤8 |
‐Sample selection was not randomized ‐FR score was used to determine the participants who needed consultation before validation. ‐Possible selection bias if participation decline was due to cognitive dysfunction. −47% of individuals who needed a medical evaluation dropped out. ‐The CDR fails to detect frontotemporal dementia. ‐Target number of participants per group was not reached. ‐The low inter‐rater reproducibility suggests the need for more training before implementation. ‐Not all cognitive functions were evaluated by the tool |
Q4 |
| Kacem (2022) 71 | ECAS‐AR | Tunisia | Language, verbal fluency, executive functions, visuospatial domain, memory. | Age = 47–71, n = 85 ALS patients, 200 NC | MSA | Translated and double checked by an expert in Arabic language, back translated. | 2 SD below the mean control group score |
‐ECAS‐Ar efficiency might have been affected by the lack of a heterogeneous cohort from MENA. ‐Genetic mutations were not considered ‐Limited number of cases with a high level of education and with advanced ages. |
Q4 |
| Boujelbane (2022) 72 | The Neurotrack digital cognitive battery. | Tunisia | Processing speed, visual associative learning, attention, executive function, inhibition, associative and recognition memory. | Age = 62.24±7.52 years, n = 155 | Arabic (unspecified dialect) | Back translation and piloting using 15 subjects | Significant difference between groups |
‐Additional research is needed to compare the digital battery with traditional paper‐and‐pen assessments ‐Cultural adaptation was not performed ‐Groups were not matched by age or education. |
Q4 |
| Khatib (2022) 52 | MoCA 8.1 | Morocco | Visuospatial and executive functions, naming, memory, attention, language, abstraction, recall, orientation. | Age >50, n = 106 | Darija, Tamazight in its three variants (Tachelhit, Tarifit, Atlas Tamazight), and Arabic. | Translation by native speakers, back translation, pretest on 20 subjects |
Using ROC Dementia: MoCA <24.5, MMS<27.5 |
No limitations reported by authors | Q4 |
| Fray (2022) 59 | MMSE, ADAS‐Cog, FAB, GDS, IADLS, CDR | Tunisia | Attentional process, episodic memory, executive function, visuospatial function, praxis, gnosis, language. |
AD patients: 70.14±10.44, n = 144 NC: 69.13±14.56, n= 90 |
Arabic (unspecified dialect) | Previously translated | Differences in performance between the two groups on the domains of interest. |
‐Small sample size ‐The lack of correlation between APOE and other parameters involved in the pathophysiology of AD. |
Q2 |
| Alsebayel (2022) 22 | AD8 | Saudi Arabia | Not mentioned | Age > 60, n = 379 | Arabic (unspecified dialect) | Previously translated | AD8≥3 or AD8 ≥4 |
Causality is not confirmed. Probability of sampling bias The effect of risk factors control was not assessed |
Q1 |
| Soliman (2023) 67 | ECAS‐EG | Egypt | Executive function, verbal fluency, and language, memory, and visuospatial abilities | Age = 28–68, n = patient: 62, healthy controls:60. | Egyptian Arabic | translation‐backtranslation, adaptation |
ALS ≤104, ALS‐specific ≤72. |
Small sample size Participants were recruited from one center only (limited variability in demographic data). |
Q4 |
| Hassan (2023) 68 | FACT‐Cog version 3 | Lebanon | Not mentioned | Age = 52.05 ± 9.95, n = 134 patients. | Simple and acceptable language for the Lebanese population | The standard Functional Assessment of Chronic Illness Therapy (FACIT) translation methodology | FACT‐Cog mean population score of 83 |
Test–retest reliability and the construct validity of the scale were not assessed. The use of ‘QLQ‐30’ test for construct validity even though it is not the standard (it was the only available validated questionnaire for cognitive domains assessment in cancer patients) Difficulty in assessing reverse causality given that the study is cross‐sectional. The test has been used among female breast cancer patients only. |
Q3 |
Abbreviations: 25(OH)D, serum 25‐hydroxyvitamin D; 3MS, Modified Mini‐State test; A‐ADAS‐Cog, Arabic version Alzheimer's Disease Assessment Scale Cognitive subscale; ACE‐III, Addenbrooke's Cognitive Examination III; ADAS‐Cog, Alzheimer's Disease Assessment Scale Cognitive subscale; AD, Alzheimer's disease patients; AD8, Eight‐item Informant Interview to Differentiate Aging and Dementia; A‐IQCODE, Arabic version of Informant Questionnaire on Cognitive Decline in the Elderly; ALS, amyotrophic lateral sclerosis; A‐MMSE (GTD‐USJ), Arabic version of Mini‐Mental State Examination developed by the “Groupe de Travail sur les D ´emences de l'Université Saint Joseph”; APOE, apolipoprotein E; A‐RUDAS, Arabic Rowland Universal Dementia Assessment Scale; A‐TNI93 (GTD‐USJ): the Test of Nine Images developed by the “Groupe de Travail sur lesD ´emences de l'Université Saint Joseph”; BADLS, basic activities of daily living; BCIS, Beck Cognitive Insight Scale; BICAMS, Brief International Cognitive Assessment for Multiple Sclerosis; BVMT‐R, Brief Visuospatial Memory Test‐Revised; CD, clinically demented; CDR, Clinical Dementia Rating; CDT, Clock Drawing Test; DS, Digit Span; CLNT, Cross‐Linguistic Naming Test; ECAS‐AR, Arabic Edinburgh cognitive and behavioral Amyotrophic lateral sclerosis screen; ECAS‐EG, Edinburgh Cognitive and Behavioural Amyotrophic Lateral Sclerosis Screen; FAB, Frontal Assessment Battery; FACT‐Cog, The Functional Assessment of Cancer Therapy‐Cognitive Function; GDS, Geriatric Depression Scale; GMS, Geriatric Mental State; HCW, health‐care workers; IADL, Instrumental Activities of Daily Living Scale; IQCODE, Informant Questionnaire on Cognitive Decline in the Elderly; MCI, mild cognitive impairment; MES, Memory and Executive Screening test; MMSE, Mini‐Mental State Examination; MMSE‐2:SV, MMSE‐2 standard version; MoCA, Montreal Cognitive Assessment; MTS, Mental Test Score; NA, not applicable; N‐AD, non‐Alzheimer's disease dementia patients; NC, normal controls; NMOSD, Neuromyelitis optica spectrum disorder; NMSS, Non‐Motor Symptom Scale; NTB, Neuropsychological Test Battery; PDQ‐39, Parkinson's‐Disease Questionnaire; RCFT, Rey Complex Figure and Recognition Trial Test; ROC, receiver operating characteristic; RRMS, Relapsing remitting multiple sclerosis; SDMT, Symbol Digit Modalities; TMT, Trail Making Test; VMAT, Verbal Memory Arabic Test; WCST, Wisconsin Card Sorting Test.
(*) Questions of interest:
Q1: Assessing the cognitive status and screening for cognitive impairments in specific populations.
Q2: Risk factors for cognitive impairments.
Q3: Cognitive functions impact on other disorders.
Q4: Translation/adaptation/validation of classic tasks to be used in Arabic populations.
Q5: Therapy/intervention.
2.2. The unreliable testing of cognitive abilities
All retrieved details of interest are summarized in Table 3. Cognitive abilities were evaluated for different clinical or research purposes and applications (Table 3), in particular for the translation and validation of classic English tests to Arabic. Cognitive assessment research was conducted in 9 out of 22 MENA countries, with dominant contributions from Egypt, Lebanon, and Saudi Arabia. A total of 80 classic neuropsychological tests, batteries, scales, and questionnaires were used in this literature (see Table 3 for a full list), with the most frequent five tests being MoCA, MMSE, Brief Visuospatial Memory Test‐Revised, Brief International Cognitive Assessment for Multiple Sclerosis, and ACE‐III. By far, Arabic versions of MoCA and MMSE are the most used tests in the MENA region (n = 10 21 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 ), (n = 8 20 , 45 , 54 , 55 , 56 , 57 , 58 , 59 ), respectively. Interestingly, one study introduced the first Arabic memory test, The Verbal Memory Arabic Test. 60 There was a discrepancy in terms of the used or generated cutoff values even for the same test. For instance, Khatib et al. 52 applied a score of ≤ 24.5 on MoCA for dementia detection based on receiver operating characteristic curve derived from the study sample, while Assaf et al. 51 used a combined score of ≤ 26 on MoCA and a score of < 7 on the Instrumental Activities of Daily Living Scale (Table 3) to diagnose dementia based on the published cutoff score from Nasreddine et al. 61 Some studies reported data‐driven (e.g., regression‐based) cutoffs. 45 , 60 , 62 , 63
The participants’ age varied considerably across studies (Table 3, total of subjects = 10,018, age range: 15–103 years). To accommodate the different language abilities of the participants, 6 studies out of 45 reported the use of colloquial Arabic when translating the tasks from English (see Khatib et al., 52 Souissi et al., 64 Qassem et al., 65 , 66 Soliman et al., 67 and Hassan et al. 68 ), while one study 63 used it for task instructions only. To test the accuracy and validity of the translated versions, most articles adopted the forward‐backward translation method. 45 , 52 , 67 , 68 , 69 , 70 , 71 , 72 , 73 Likewise, some studies relied on piloting on an independent sample to evaluate the quality of the translation. 20 , 46 , 49 , 52 , 68 , 69 , 72 , 74 Last but not least, the main limitations acknowledged by the original authors concerned the small and insufficient sample sizes, lack of rigorous clinical validation, absence of cultural validation of the tools, potential bias in the results due to several factors related to sample selection and data collection, and disregarding possible confounding comorbidities.
What emerges from this literature is the use of suboptimal procedures for the assessment of cognitive abilities, reliance on unvalidated translated versions, significant differences in the use of colloquial Arabic in the communication between the clinicians and the participants, a lack of rigorous definitions of assessment criteria, and tests sometimes administered by very junior or less experienced clinical staff. It is thus not clear what added value these tests have brought to the diagnosis of dementia or to the quality of care offered to the tested people.
2.3. Recommendations for the MENA region
The recommendations below concern two main dimensions: the choice of language for test development and validation, and the presence of stigma about dementia and its damaging impact on research participation. But before laying down our recommendations, it is important to shed light on the level of research productivity in the MENA region. Fundamentally, the quality and usefulness of any developed neuropsychological tests in a given language depend on the volume and quality of research that involves speakers of that language. Thus, the development of specific tailored neuropsychological tests for cognitive decline assessment in Arabic speakers is contingent on a genuine promotion and support of research in the MENA region in the fields of cognitive and clinical neuroscience. However, current research metrics offer a poor portrait of research productivity in this region. For instance, according to SCImago country ranking based on many scientific indicators from the Scopus database, 75 the highest ranked MENA country in terms of total number of publications is Saudi Arabia at position 39 and 42 in cognitive neuroscience and neuropsychology, respectively. This ranking is much lower if one considers the citations to that published work. Overall, research in those fields is limited in terms of both volume and impact. This situation has been highlighted in many studies that looked at the contribution and significance of research about dementia produced in the MENA region. 31 , 36 , 76 This limited research productivity is a significant explanatory factor for the lack of both trained clinical experts and reliable standardized neuropsychological tests. 1
It is important to stress here that reliance on translated versions of research findings and clinical tests is not the solution to the problem. The lack of standardized neuropsychometric batteries for the MENA region is in itself a barrier that limits the development of research projects and collaborations. 31 To elevate the standards of neuropsychology in the MENA region toward serving the needs of its older population, significant investments are urgently needed to support the training of the next generation of clinical neuroscientists and neuropsychologists. An exchange program across countries would help to share expertise and promote the development of tests that can be validated across different populations and health‐care systems. This is key to the development of standardized tests about cognitive decline given the diversity in colloquial Arabic forms in the MENA region.
The use of colloquial Arabic might pose a challenge to how to validate the tests across groups and countries. A unified use of MSA would alleviate this issue to some extent, though this warrants future investigations given the context of diglossia and the high percentage of illiterate older adults in some MENA countries. While MSA is not frequently used by today's older generations, many existing tests include familiar items and high‐frequency words that are not particularly challenging for today's older population in the MENA region. We expect the use of MSA to become less of an issue for future generations given the widespread use of MSA in the education system and the media (see literacy rates and instruction languages in Table 2). 77 , 78 , 79 Still, we acknowledge the fact that MSA is facing a soaring competition from other initiatives, which we believe are counterproductive, to expand the use of spoken dialects to the written form. 80 We believe the best way to improve cognitive testing in the region is to promote the use of MSA, which can be done gradually by increasing the lexical similarity between colloquial Arabic and MSA 81 through the gradual inclusion of more MSA vocabulary into the current spoken dialects. Last but not least, the availability of large lexical databases 82 would facilitate the development of cognitive tests in MSA. Likewise, cultural norms and values should be considered in the development and validation stages to avoid cultural bias. Including populations from diverse backgrounds and using culturally relevant examples and materials can help develop and validate tests free from any cultural biases. Such endeavors will also benefit from fostering fruitful international collaborations with well‐established research centers, in particular with neuropsychologists from the Arab diaspora who can advise on the optimal procedures in the development and validation of tests.
Similarly, the MENA region needs to streamline the collection processes of large‐scale high‐quality data about aging and dementia. Indeed, one of the challenges frequently reported by researchers in the region, and by the studies reviewed here, concerns the lack of comprehensive epidemiological data about dementia. Collecting high‐quality data about dementia is extremely difficult due to a lack of resources, inadequate health‐care infrastructure in some countries, a deplorable stigma about dementia, and disinterest from the large public to participate in research. A multifaceted approach is required to address these limitations. Actively engaging the community through initiatives that promote dementia awareness, by sharing accurate information about its symptoms and causes, will play a crucial role in dismissing any misconceptions. Moreover, using media platforms to share personal stories can challenge dementia stereotypes and foster a more inclusive society. It is also very important in this context to raise awareness about the different types of dementia, the personalized nature of living with dementia, and its huge inter‐individual variability in its severity and progression. This has to go hand in hand with the adoption of a more positive language about dementia in the MENA region, which would ensure that people living with dementia are treated with respect. 83 For instance, some myths are still present in the general public about the idea that dementia is a normal part of aging, that memory loss is the only symptom of dementia, or that people at early stages of dementia cannot live independently.
To increase research participation, advocating online participation through the integration of mobile and telemedicine‐based services enhances the efficiency and accessibility of cognitive assessment, which in turn can support the collection of large‐scale data in naturalistic conditions. 84 , 85 Research studies have to adopt more inclusive criteria for the recruitment of older subjects. Indeed, restrictive exclusion criteria, often poorly justified from a scientific point of view (age limit, comorbidity, or disability type) represent one of the factors for the low recruitment rates of older adults in research. 86 , 87 Other (monetary) incentives can also be considered, 88 with an emphasis on the very positive impact of research participation as a “civic duty” for building healthier communities. In the same way, policy makers need to put in place safe data‐sharing protocols between MENA countries to accelerate the development of neuropsychological tests that can be validated on a large number of people. The creation of open‐access databases would also support open science in the MENA region in this domain (e.g., Albawardi et al. 89 ).
Furthermore, funding research should be at the level of the gravity of dementia as a regional health burden. For example, MENA countries can agree to dedicate at least 0.5% of their GDP to meeting the needs of their aging populations for better care and research outcomes. Last but not least, it is of paramount importance that the MENA region has its own neuropsychological society that can oversee the development of neuropsychology within the region, including neuropsychology for aging. This society could work closely with the current Middle East Psychological Association, which has a focus on mental health. Overall, we call for strong collaborations on an institutional but also regulatory body and government level to be competitive on the global stage in health‐care research and innovation, and also to be prepared for the implications and challenges that come with dementia within the next generation.
3. IMPLICATIONS FOR THE GLOBAL FIGHT AGAINST DEMENTIA
Many classic tests have translated versions suitable for speakers of diverse languages. We would argue that transcription is not enough to accommodate differences due to other cultural and socioeconomic features across different populations. Developing culturally valid tests for speakers of different languages, derived from a well‐established and research‐informed English version, can help provide accurate testing of cognitive abilities. This might mean using different items in a way that would make the tests relevant to the population of interest, at least with respect to feasibility, familiarity, and suitability. The use of different tests or test versions across different populations should not in principle pose a problem for global epidemiological studies as differences can still be considered statistically at the level of models. Put another way, the collection of valid data for different populations using tailored tests is a more desirable outcome than the requirement of consistency in data based on a single standardized test. This perspective is in line with the growing interest in precision neuropsychology by making sure that neuropsychological tests are tailored to the tested group.
Some examples of culturally valid tests include the Seoul Neuropsychological Screening Battery, a comprehensive battery developed for Korean‐speaking individuals. 61 Similarly, NEUROPSI 90 and the Spanish National Institutes of Health (NIH) Toolbox Cognition Battery 91 have been developed to standardize both the administration and the scoring of cognitive abilities in Spanish‐speaking populations. These batteries consist of a set of adapted classical tests combined with culturally relevant items. They are the result of a multi‐center collaborative effort that allowed for extensive normative data collection and accounted for the cultural and socio‐economic characteristics of their targeted populations. These batteries have underscored the need to develop and validate population‐specific neuropsychological tests. For example, it has been shown that demographic factors had significant effects on cognitive scores in the Spanish version of the NIH Toolbox Cognition Battery following a pattern that completely differed from that observed in the English version. 91
However, it might not be realistically feasible to develop tests for every group and in every spoken language. Thousands of languages are unlikely to motivate significant research and hence speakers of such languages might need to be tested in other widely spoken languages. Therefore, translated versions might be the only option for some populations. In this context, translation efforts can be supported by new advancements in artificial intelligence (AI) tools. For instance, recent sophisticated AI architectures are able to offer expert‐level translations for many languages. 92 There is also a surge in interest in developing alternative AI‐based translators that can learn from a small amount of data given that many languages have a low presence on the internet. In this context, the recent initiative by Meta “no language left behind” is an exciting opportunity that would have many ramifications in the near future for the health‐care sector. 93 For example, in typical clinical scenarios in which the health‐care provider does not speak the primary language of the patient, the use of professional interpreters is usually considered. Now, with the advancement in AI‐based translators, such tools can offer a cost‐effective solution to any language barrier when delivering care to populations with high language diversity (see example in Panayiotou et al. 94 ). There is, for instance, an interest in the development of AI‐based cognitive tests, 95 including chatbots powered by the like of ChatGPT to conduct cognitive tests, but these chatbots based on large language models do not perform well in all languages (e.g., Seghier 96 ). We reckon that the use of AI in neuropsychology is still in its infancy, facing many limitations with respect to generalizability of AI algorithms and availability of high‐quality and bias‐free data. Other challenges for AI concern accountability, transparency, data security, non‐equal representation of different groups in the data used to train and test AI models, mistrust due to a lack of understanding of how AI‐based diagnostics are generated, risk of decisions being based on irrelevant features, and difficulty in establishing clinical validity of AI tools. 97 , 98
In summary, the availability of reliable and valid tests for English speakers is the result of a very active research community in cognitive neuroscience, too often relying too closely on English‐speaking people. Indeed, research in cognitive neuroscience, as in many domains, speaks English, and this has many ramifications on our understanding of cognition at large, 99 but also on measuring cognitive abilities for clinical purposes. The research community in cognitive science needs to uphold inclusion by broadening the linguistic diversity of both its participants and researchers. Journals, funding bodies, and scientific societies should put in place guidelines on how to ensure language diversity during the selection of participants so that the sampling process is fair and inclusive. Furthermore, initiatives can be created to offer mentoring opportunities for researchers of understudied languages; for instance, researchers of understudied languages can be invited to spend time at clinical institutions that have expertise in administering neuropsychological tests about cognitive abilities in patients. By working on both sides of the challenge, namely the development of neuropsychological tests in understudied languages and upskilling health‐care staff in neuropsychology for diverse populations, we can offer equal opportunities when it comes to access to culturally valid and clinically useful diagnostic tools for the study of healthy and pathological aging.
CONFLICTS OF INTEREST STATEMENT
The authors declare no conflicts of interest. Author disclosures are available in the supporting information.
CONSENT STATEMENT
This article does not contain any data from human participants or animals, hence informed consent was not required.
Supporting information
Supplementary Information
ACKNOWLEDGMENTS
The authors thank BME colleagues for their support. This work was funded by Khalifa University (grant numbers FSU‐2022‐006 and RC2‐2018‐022).
Hatahet O, Roser F, Seghier ML. Cognitive decline assessment in speakers of understudied languages. Alzheimer's Dement. 2023;9:e12432. 10.1002/trc2.12432
REFERENCES
- 1. Fasfous AF, Al‐Joudi HF, Puente AE, Pérez‐García M. Neuropsychological measures in the Arab world: a systematic review. Neuropsychol Rev. 2017;27(2):158‐173. doi: 10.1007/S11065-017-9347-3 [DOI] [PubMed] [Google Scholar]
- 2. Rosli R, Tan MP, Gray WK, Subramanian P, Chin AV. Cognitive assessment tools in Asia: a systematic review. Int Psychogeriatr. 2016;28(2):189‐210. doi: 10.1017/S1041610215001635 [DOI] [PubMed] [Google Scholar]
- 3. Jessen F, Amariglio RE, Buckley RF, et al. The characterisation of subjective cognitive decline. Lancet Neurol. 2020;19(3):271‐278. Lancet Publishing Group. doi: 10.1016/S1474-4422(19)30368-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cognitive Testing—PsychDB. https://www.psychdb.com/cognitive‐testing (accessed Jul. 11, 2023)
- 5. Mini‐Cog© – Quick Screening for Early Dementia Detection. https://mini‐cog.com/ (accessed Jul. 11, 2023)
- 6. Carnero‐Pardo C. Should the mini‐mental state examination be retired? Neurología (English Edition). 2014;29(8):473‐481. doi: 10.1016/J.NRLENG.2013.07.005 [DOI] [PubMed] [Google Scholar]
- 7. Devenney E, Hodges JR. The mini‐mental state examination: pitfalls and limitations. Pract Neurol. 2017;17(1):79‐80. doi: 10.1136/PRACTNEUROL-2016-001520 [DOI] [PubMed] [Google Scholar]
- 8. O'Driscoll C, Shaikh M. Cross‐cultural applicability of the montreal cognitive assessment (MoCA): a systematic review. J Alzheimer Dis. 2017;58(3):789‐801. IOS Press, doi: 10.3233/JAD-161042 [DOI] [PubMed] [Google Scholar]
- 9. Stålhammar J, Hellström P, Eckerström C, Wallin A. Neuropsychological test performance among native and non‐native swedes: second language effects. Arch Clin Neuropsychol. 2022;37(4):826‐838. doi: 10.1093/ARCLIN/ACAA043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kisser JE, Wendell CR, Spencer RJ, Waldstein SR. Neuropsychological performance of native versus non‐native English speakers. Arch Clin Neuropsychol. 2012;27(7):749‐755. doi: 10.1093/ARCLIN/ACS082 [DOI] [PubMed] [Google Scholar]
- 11. Kaczmarek B, Ilkowska Z, Kropinska S, et al. Applying ACE‐III, M‐ACE and MMSE to diagnostic screening assessment of cognitive functions within the polish population. Int J Environ Res Public Health. 2022;19(19):doi: 10.3390/IJERPH191912257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Peixoto B, Machado M, Rocha P, et al. Validation of the Portuguese version of Addenbrooke's Cognitive Examination III in mild cognitive impairment and dementia. Adv Clin Exp Med. 2018;27(6):781‐786. doi: 10.17219/ACEM/68975 [DOI] [PubMed] [Google Scholar]
- 13. Manjavong M, Limpawattana P, Sawanyawisuth K. Can RUDAS be an alternate test for detecting mild cognitive impairment in older adults, Thailand? Geriatrics (Basel). 2021;6(4):doi: 10.3390/GERIATRICS6040117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Daniel B, Agenagnew L, Workicho A, Abera M. Validation of the Rowlands Universal Dementia Assessment Scale (RUDAS) to detect major neurocognitive disorder among elderly people in Ethiopia, 2020. PLoS One. 2022;17(1):doi: 10.1371/JOURNAL.PONE.0262483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Broche‐Pérez Y, López‐Pujol HA. Validation of the cuban version of addenbrooke's cognitive examination‐revised for screening mild cognitive impairment. Dement Geriatr Cogn Disord. 2017;44(5‐6):320‐327. doi: 10.1159/000481345 [DOI] [PubMed] [Google Scholar]
- 16. Pan FF, Cui L, Li QJ, Guo QH. Validation of a modified Chinese version of Mini‐Addenbrooke's Cognitive Examination for detecting mild cognitive impairment. Brain Behav. 2022;12(1):doi: 10.1002/BRB3.2418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Qassem T, Khater MS, Emara T, et al. Validation of the Mini‐Addenbrooke's cognitive examination in mild cognitive impairment in Arabic speakers. Dement Geriatr Cogn Disord. 2021;50(2):178‐182. doi: 10.1159/000517580 [DOI] [PubMed] [Google Scholar]
- 18. Verghese J, Noone ML, Johnson B, et al. Picture‐based memory impairment screen for dementia. J Am Geriatr Soc. 2012;60(11):2116‐2120. doi: 10.1111/J.1532-5415.2012.04191.X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stephenson J. Racial barriers may hamper diagnosis, care of patients with Alzheimer disease. JAMA. 2001;286(7):779‐780. doi: 10.1001/JAMA.286.7.779 [DOI] [PubMed] [Google Scholar]
- 20. Albanna M, Yehya A, Khairi A, et al. Validation and cultural adaptation of the arabic versions of the mini–mental status examination – 2 and mini‐cog test. Neuropsychiatr Dis Treat. 2017;13:793. doi: 10.2147/NDT.S126825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Alkhunizan M, Alkhenizan A, Basudan L. Prevalence of mild cognitive impairment and dementia in Saudi Arabia: a community‐based study. Dement Geriatr Cogn Dis Extra. 2018;8(1):98‐103. doi: 10.1159/000487231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Alsebayel FM, Alangari AM, Almubarak FH, Alhamwy R. Prevalence of dementia and its associated risk factors among geriatric patients visiting primary healthcare centers in Riyadh, Saudi Arabia: a cross‐sectional study. Cureus. 2022;14(4):doi: 10.7759/CUREUS.24394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Badrkhahan SZ, Sikaroodi H, Sharifi F, Kouti L, Noroozian M. Validity and reliability of the Persian version of the Montreal Cognitive Assessment (MoCA‐P) scale among subjects with Parkinson's disease. Applied Neuropsychology:Adult. 2020;27(5):431‐439. doi: 10.1080/23279095.2019.1565762 [DOI] [PubMed] [Google Scholar]
- 24. Ardila A, Moreno S. Neuropsychological test performance in Aruaco Indians: an exploratory study. J Int Neuropsychol Soc. 2001;7(4):510‐515. doi: 10.1017/S1355617701004076 [DOI] [PubMed] [Google Scholar]
- 25. Agranovich AV, Puente AE. Do Russian and American normal adults perform similarly on neuropsychological tests? Preliminary findings on the relationship between culture and test performance. Arch Clin Neuropsychol. 2007;22:273‐282. doi: 10.1016/j.acn.2007.01.003 [DOI] [PubMed] [Google Scholar]
- 26. Yang DW, Chey JY, Kim SY, Kim S. The development and validation of Korean dementia screening questionnaire (KDSQ). J Korean Neurol Assoc. 2002;20(2):0–000. [Google Scholar]
- 27. Pontón MO, Satz P, Herrera L, et al. Normative data stratified by age and education for the Neuropsychological Screening Battery for Hispanics (NeSBHIS): Initial report. J Int Neuropsychol Soc. 1996;2(2):96‐104. doi: 10.1017/S1355617700000941 [DOI] [PubMed] [Google Scholar]
- 28. Mungas D, Reed BR, Crane PK, Haan MN, González H. Spanish and English Neuropsychological Assessment Scales (SENAS): further development and psychometric characteristics. Psychol Assess. 2004;16(4):347‐359. doi: 10.1037/1040-3590.16.4.347 [DOI] [PubMed] [Google Scholar]
- 29. El‐Metwally A, Toivola P, Al‐Rashidi M, et al. Epidemiology of Alzheimer's disease and dementia in Arab countries: a systematic review. Behav Neurol. 2019;2019 doi: 10.1155/2019/3935943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gustavsson A, Norton N, Fast T, et al. Global estimates on the number of persons across the Alzheimer's disease continuum. Alzheimer's & Dementia. 2023;19(2):658‐670. doi: 10.1002/ALZ.12694 [DOI] [PubMed] [Google Scholar]
- 31. Al Sinawi H, Qassem T, Al Harrasi A. Dementia in the Arab World. Handbook of Healthcare in the Arab World. 2021;2953‐2966. doi: 10.1007/978-3-030-36811-1_132 [DOI] [Google Scholar]
- 32. el Rassi R, Meho LI, Nahlawi A, Salameh JS, Bazarbachi A, Akl EA. Medical research productivity in the Arab countries: 2007‐2016 bibliometric analysis. J Glob Health. 2018;8(2):20411. doi: 10.7189/JOGH.08.020411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gul S, Nisa NT, Shah TA, Gupta S, Jan A, Ahmad S. Middle East: research productivity and performance across nations. Scientometrics. 2015;105(2):1157‐1166. doi: 10.1007/S11192-015-1722-3/TABLES/5 [DOI] [Google Scholar]
- 34. Benamer HTS. Neurological disorders in the Arab world. Neurological Disorders in the Arab World. 2014;9783319072579:1‐195. doi: 10.1007/978-3-319-07257-9/COVER [DOI] [Google Scholar]
- 35. Bhalla D, Lotfalinezhad E, Amini F, et al. Incidence and risk profile of dementia in the regions of Middle East and North Africa. Neuroepidemiology. 2018;50(3‐4):144‐152. doi: 10.1159/000487761 [DOI] [PubMed] [Google Scholar]
- 36. el Masri J, Dankar R, el Masri D, Chanbour H, el Hage S, Salameh P. The Arab countries’ contribution to the research of neurodegenerative disorders. Cureus. 2021;13(8):doi: 10.7759/CUREUS.17589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fernández MA, Diglossia, 1993;23:doi: 10.1075/LISL23 [DOI] [Google Scholar]
- 38. Rizzi L, Rosset I, Roriz‐Cruz M. Global epidemiology of dementia: Alzheimer's and vascular types. Biomed Res Int. 2014;2014:doi: 10.1155/2014/908915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Abu‐Rabia S, Kedan ES, Wattad H, Abu‐Rabia S, Kedan ES, Wattad H. The influence of diglossia on syntactic proficiency in modern standard Arabic among regular and struggling readers. Creat Educ. 2022;13(1):252‐282. doi: 10.4236/CE.2022.131016 [DOI] [Google Scholar]
- 40. Antoniou K, Grohmann KK, Kambanaros M, Katsos N. The effect of childhood bilectalism and multilingualism on executive control. Cognition. 2016;149:18‐30. doi: 10.1016/J.COGNITION.2015.12.002 [DOI] [PubMed] [Google Scholar]
- 41. Schwieter JW, Liceras JM, Fernández Fuertes R, et al. Is there an effect of diglossia on executive functions? An investigation among adult diglossic speakers of Arabic. Languages 2022. 2022;7(4):312. doi: 10.3390/LANGUAGES7040312 [DOI] [Google Scholar]
- 42. Liu K, Zhu L. The Effects of Diglossia on Cognitive Ability. Proceedings of the 2022 8th International Conference on Humanities and Social Science Research (ICHSSR 2022) . 2022;664:214‐218. doi: 10.2991/ASSEHR.K.220504.039 [DOI] [Google Scholar]
- 43. Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA‐ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467‐473. doi: 10.7326/M18-0850/SUPPL_FILE/M18-0850_SUPPLEMENT.PDF [DOI] [PubMed] [Google Scholar]
- 44. Llorente AM, Pontón MO, Taussig IM, Satz P, Clark J, Green V. Patterns of American immigration and their influence on the acquisition of neuropsychological norms for hispanics. Arch Clin Neuropsychol. 1999;14(7):603‐614. [Online]. Available: https://academic.oup.com/acn/article/14/7/603/1876 [PubMed] [Google Scholar]
- 45. Abou‐Mrad F, Chelune G, Zamrini E, Tarabey L, Hayek M, Fadel P. Screening for dementia in Arabic: normative data from an elderly Lebanese sample. Clin Neuropsychol. 2017;31(sup1):1‐19. doi: 10.1080/13854046.2017.1288270 [DOI] [PubMed] [Google Scholar]
- 46. Darwish H, Zeinoun P, Ghusn H, Khoury B, Tamim H, Khoury SJ. Serum 25‐hydroxyvitamin D predicts cognitive performance in adults. Neuropsychiatr Dis Treat. 2015;11:2217. doi: 10.2147/NDT.S87014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Saleh AA, Abd El Hamid Ali Alkholy RS, Khalaf OO, et al. Validation of Montreal Cognitive Assessment‐Basic in a sample of elderly Egyptians with neurocognitive disorders. Aging Ment Health. 2019;23(5):551‐557. doi: 10.1080/13607863.2018.1428936 [DOI] [PubMed] [Google Scholar]
- 48. Salama S, Marouf H, Reda MI, Mansour AR, ELKholy O, Levy M. Cognitive functions in Egyptian neuromyelitis optica spectrum disorder. Clin Neurol Neurosurg. 2020;189:105621. doi: 10.1016/J.CLINEURO.2019.105621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Muayqil TA, Alamri NK, Alqahtani AM, et al. Normative and equated data of the original and basic versions of the montreal cognitive assessment among community dwelling Saudi Arabians. Behav Neurol. 2021;2021:doi: 10.1155/2021/5395627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rababa M Rababa'h A. The inappropriate use of proton pump inhibitors and its associated factors among community‐dwelling older adults. Heliyon. 2021;7(7):e07595. doi: 10.1016/J.HELIYON.2021.E07595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Assaf G, el Khoury J, Jawhar S, Rahme D. Mild Cognitive Impairment and modifiable risk factors among Lebanese older adults in primary care. Asian J Psychiatr. 2021;65:doi: 10.1016/j.ajp.2021.102828 [DOI] [PubMed] [Google Scholar]
- 52. Khatib N, Harch IE, Lamkaddem A, et al. The Moroccan MoCA test: translation, cultural adaptation, and validation. Applied Neuropsychology:Adult. 2022;doi: 10.1080/23279095.2022.2119143 [DOI] [PubMed] [Google Scholar]
- 53. Haddad C, Salameh P, Sacre H, Clément J‐P, Calvet B. The use of the Montreal Cognitive Assessment (MoCA) screening tool to evaluate cognitive deficits in Lebanese in‐patients with schizophrenia. Asian J Psychiatr. 2022;70:103029. doi: 10.1016/j.ajp.2022.103029 [DOI] [PubMed] [Google Scholar]
- 54. Khedr E, Fawi G, Abbas MAA, et al. Prevalence of mild cognitive impairment and dementia among the elderly population of qena governorate, upper Egypt: A community‐based study. J Alzheimer Dis. 2015;45(1):117‐126. doi: 10.3233/JAD-142655 [DOI] [PubMed] [Google Scholar]
- 55. El‐Hayeck R, Baddoura R, Wehbé A, et al. An Arabic version of the mini‐mental state examination for the lebanese population: reliability, validity, and normative data. J Alzheimer Dis. 2019;71(2):525‐540. doi: 10.3233/JAD-181232 [DOI] [PubMed] [Google Scholar]
- 56. Al‐Momani M, Al‐Momani F, Alghadir AH, Alharethy S, Gabr SA. Factors related to gait and balance deficits in older adults. Clin Interv Aging. 2016;11:1043. doi: 10.2147/CIA.S112282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ibrahim I, Tobar S, Fathi W, et al. Randomized controlled trial of adjunctive Valproate for cognitive remediation in early course schizophrenia. J Psychiatr Res. 2019;118:doi: 10.1016/j.jpsychires.2019.08.011 [DOI] [PubMed] [Google Scholar]
- 58. Alshammari SA, Alhamdan AA, Bindawas SM, et al. Assessing the cognitive status of older adults attending primary healthcare centers in Saudi Arabia using the Mini‐Mental State Examination. Saudi Med J. 2020;41(12):1315‐1323. doi: 10.15537/smj.2020.12.25576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Fray S, Achouri‐Rassas A, Belal S, Messaoud T. Missing apolipoprotein E e4 allele associated with nonamnestic Alzheimer's disease in a Tunisian population. J Genet. 2022;101:doi: 10.1007/s12041-022-01384-9 [DOI] [PubMed] [Google Scholar]
- 60. Zeinoun P, Farran N, Khoury SJ, Darwish H. Development, psychometric properties, and pilot norms of the first Arabic indigenous memory test: the Verbal Memory Arabic Test (VMAT). J Clin Exp Neuropsychol. 2020;42(5):505‐515. doi: 10.1080/13803395.2020.1773408 [DOI] [PubMed] [Google Scholar]
- 61. Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: A Brief Screening Tool For Mild Cognitive Impairment [Online]. Available: www.mocatest. [DOI] [PubMed]
- 62. Allataifeh E, Khalil H, Almhdawi K, et al. The clinical correlates of participation levels in people with multiple sclerosis. NeuroRehabilitation. 2020;47(2):153‐160. doi: 10.3233/NRE-203131 [DOI] [PubMed] [Google Scholar]
- 63. Darwish H, Zeinoun P, Farran N, Ghusn H, Yamout B, Khoury SJ. The Brief International Cognitive Assessment in Multiple Sclerosis (BICAMS): Validation in Arabic and Lebanese Normative Values. doi: 10.1017/S1355617721000102 [DOI] [PubMed]
- 64. Souissi A, Mrabet S, Ferchichi W, et al. Tunisian version of the brief international cognitive assessment for multiple sclerosis: Validation and normative values. 2022;doi: 10.1016/j.msard.2021.103444 [DOI] [PubMed]
- 65. Qassem T, Khater MS, Emara T, et al. Validation of the Egyptian‐Arabic Version of the Addenbrooke's Cognitive Examination III (ACE‐III) in Diagnosing Dementia. Dement Geriatr Cogn Disord. 2020;49(2):179‐184. doi: 10.1159/000507758 [DOI] [PubMed] [Google Scholar]
- 66. Qassem T, Khater MS, Emara T, et al. Validation of the addenbrooke's cognitive examination‐III in mild cognitive impairment in arabic speakers in Egypt. Dement Geriatr Cogn Disord. 2021;49(4):418‐422. doi: 10.1159/000510952 [DOI] [PubMed] [Google Scholar]
- 67. Soliman R, Rashed HR, Moustafa RR, et al. Egyptian adaptation and validation of the Edinburgh Cognitive and Behavioral Amyotrophic Lateral Sclerosis Screen (ECAS‐EG). Neurolog Sci. 2023;1:1‐10. doi: 10.1007/S10072-023-06639-6/FIGURES/4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hassan M, Barakat Z, Fares Y, Abou‐Abbas L. Cognitive functioning in women with breast cancer: psychometric properties of the Arabic version of the Functional Assessment of Cancer Therapy‐Cognitive Function Tool. Health Qual Life Outcomes. 2023;21(1)1‐10. doi: 10.1186/S12955-023-02095-0/TABLES/5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Almubark BM, Cattani A, Floccia C, Translation, cultural adaptation, and validation of Cognistat for its use in Arabic‐speaking population with acquired brain injury. Eur J Phys Rehabil Med. 2019;55(5):595‐604. doi: 10.23736/S1973-9087.18.05530-2 [DOI] [PubMed] [Google Scholar]
- 70. Al‐Joudi HF, Mincari L, Baz S, et al. Standardization of an Arabic‐language neuropsychological battery for epilepsy surgical evaluations. J Int Neuropsychol Soc. 2019;25(7):doi: 10.1017/S1355617719000432 [DOI] [PubMed] [Google Scholar]
- 71. Kacem I et al. Arabic adaptation of the Edinburgh cognitive and behavioural Amyotrophic lateral sclerosis screen (ECAS‐AR).Rev Neurol (Paris). 2022;178(8):817‐825. doi: 10.1016/j.neurol.2021.12.015 [DOI] [PubMed] [Google Scholar]
- 72. Boujelbane MA, Trabelsi K, Boukhris O, et al. The use of digital technology to assess cognitive function in tunisian adults. J Alzheimers Dis. 2022;88(4):1545‐1552. doi: 10.3233/JAD-220398 [DOI] [PubMed] [Google Scholar]
- 73. Saguem BN, Braham A, Ines MK, Mtiraoui A, Nakhli J, Nasr SB. Validation of Beck Cognitive Insight Scale – Arabic version in a Tunisian sample. Encephale. 2022;49(3):234‐240. doi: 10.1016/j.encep.2021.11.005 [DOI] [PubMed] [Google Scholar]
- 74. Chaaya M, Phung TKT, el Asmar K, Atweh S, Ghusn H, Khoury RM. Aging & Mental Health Validation of the Arabic Rowland Universal Dementia Assessment Scale (A‐RUDAS) in elderly with mild and moderate dementia. 2016;doi: 10.1080/13607863.2015.1043620 [DOI] [PubMed]
- 75. SCImago nd, SJR—ASCImago Journal & Country Rank. https://www.scimagojr.com (accessed Mar. 23, 2023)
- 76. Karam G, Itani L. Dementia : a review from the Arab Region=الخرف : ملخص من العالم العربي. المجلة العربية للطب النفسي 2013;44(473):1‐16. doi: 10.12816/0000102 [DOI] [Google Scholar]
- 77. Living Arabic Project. http://livingarabic.com/ (accessed Jul. 02, 2023)
- 78. Successful Arabic language Curriculum Reform in Morocco. https://allafrica.com/stories/202011041009.html (accessed Jul. 02, 2023)
- 79. Gregory L, Taha Thomure H, Kazem A, Boni A, Elsayed MAA, Taibah N. Advancing Arabic Language Teaching and Learning. World Bank; 2021;doi: 10.1596/35917 [DOI] [Google Scholar]
- 80. Schmitt GA. Relevance of Arabic Dialects: A Brief Discussion. 2019;doi: 10.1007/978-3-319-73400-2_79-1 [DOI]
- 81. Kwaik KA, Saad M, Chatzikyriakidis S, Dobnika S. A lexical distance study of Arabic Dialects. Procedia Comput Sci. 2018;142:2‐13. doi: 10.1016/J.PROCS.2018.10.456 [DOI] [Google Scholar]
- 82. Boudelaa S, Marslen‐Wilson WD. Aralex: a lexical database for modern standard Arabic. Behavior Research Methods. 2010;42:481‐487. doi: 10.3758/BRM.42.2.481 [DOI] [PubMed] [Google Scholar]
- 83. Swaffer K. Dementia: stigma, language, and dementia‐friendly. Dementia (London). 2014;13(6):709‐716. doi: 10.1177/1471301214548143 [DOI] [PubMed] [Google Scholar]
- 84. Öhman F, Hassenstab J, Berron D, Schöll M, Papp KV. Current advances in digital cognitive assessment for preclinical Alzheimer's disease. Alzheimer Dementng. 2021;13(1):John Wiley and Sons Inc, doi: 10.1002/dad2.12217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Commercial Deployment at Leading Psychiatry and Neurology Centre in UAE—Cognetivity. https://cognetivity.com/commercial‐deployment‐at‐leading‐psychiatry‐and‐neurology‐centre‐in‐uae/ (accessed Mar. 09, 2023)
- 86. Mody L, Miller DK, McGloin JM, et al. Recruitment and retention of older adults in aging research. J Am Geriatr Soc. 2008;56(12):2340‐2348. doi: 10.1111/J.1532-5415.2008.02015.X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Lacey RJ, Wilkie R, Wynne‐Jones G, Jordan JL, Wersocki E, Mcbeth J. Evidence for strategies that improve recruitment and retention of adults aged 65 years and over in randomised trials and observational studies: a systematic review. Age Ageing. 2017;46(6):895‐903. doi: 10.1093/AGEING/AFX057 [DOI] [PubMed] [Google Scholar]
- 88. Abdelazeem B, Abbas KS, Amin MA, et al. The effectiveness of incentives for research participation: a systematic review and meta‐analysis of randomized controlled trials. PLoS One. 2022;17(4):doi: 10.1371/JOURNAL.PONE.0267534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Albawardi NM, Shaikh Q, Alahaideb W. et al. Development of the Arabic Health Measures database: a bibliometric analysis of Arabic health‐related measures. Health Res Policy Syst. 2022;20(1):doi: 10.1186/S12961-022-00890-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Ostrosky‐Solís F, Ardila A, Rosselli M. NEUROPSI: a brief neuropsychological test battery in Spanish with norms by age and educational level. J Int Neuropsychol Soc. 1999;5(5):413‐433. doi: 10.1017/S1355617799555045 [DOI] [PubMed] [Google Scholar]
- 91. Casaletto KB, Umlauf A, Marquine M, et al. Demographically corrected normative standards for the Spanish language version of the NIH toolbox cognition battery. J Int Neuropsychol Soc. 2016;22(3):364‐374. doi: 10.1017/S135561771500137X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Popel M, Tomkova M, Tomek J, et al. Transforming machine translation: a deep learning system reaches news translation quality comparable to human professionals. Nat Commun. 2020;11(1):1‐15. doi: 10.1038/s41467-020-18073-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Team N, Costa‐jussà MR, Cross J, et al. No Language Left Behind: Scaling Human‐Centered Machine Translation. Accessed: Mar. 23, 2023. [Online]. Available: https://github.com/facebookresearch/fairseq/tree/nllb
- 94. Panayiotou A, Gardner A, Williams S, et al. Language translation apps in health care settings: expert opinion. JMIR Mhealth Uhealth. 2019;7(4):e11316. doi: 10.2196/11316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Sirilertmekasakul C, Rattanawong W, Gongvatana A, Srikiatkhachorn A. The current state of artificial intelligence‐augmented digitized neurocognitive screening test. Front Hum Neurosci. 2023;17:1133632. doi: 10.3389/FNHUM.2023.1133632/BIBTEX [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Seghier ML. ChatGPT: not all languages are equal. Nature. 2023;615(7951):216. doi: 10.1038/D41586-023-00680-3 [DOI] [PubMed] [Google Scholar]
- 97. Petersson L, Larsson I, Nygren JM, et al. Challenges to implementing artificial intelligence in healthcare: a qualitative interview study with healthcare leaders in Sweden. BMC Health Serv Res. 2022;22(1):1‐16. doi: 10.1186/S12913-022-08215-8/FIGURES/1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Tsopra R, Fernandez X, Luchinat C, et al. A framework for validating AI in precision medicine: considerations from the European ITFoC consortium. BMC Med Inform Decis Mak. 2021;21(1):doi: 10.1186/S12911-021-01634-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Blasi DE, Henrich J, Adamou E, Kemmerer D, Majid A. Over‐reliance on English hinders cognitive science. Trends Cogn Sci, 2022;26(12):1153‐1170. doi: 10.1016/J.TICS.2022.09.015 [DOI] [PubMed] [Google Scholar]
- 100. Fakhry H, el Ghonemy SH, Salem A. Cognitive functions and cognitive styles in young euthymic patients with bipolar i disorder. J Affect Disord. 2013;151(1):369‐377. doi: 10.1016/j.jad.2013.05.095 [DOI] [PubMed] [Google Scholar]
- 101. Ibrahim I, Salah H, El Sayed H, et al. Hepatitis C virus antibody titers associated with cognitive dysfunction in an asymptomatic community‐based sample. J Clin Exp Neuropsychol. 2016;38(8):861‐868. doi: 10.1080/13803395.2016.1168780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Bou‐Orm IR, Khamis AM, Chaaya M. Determinants of poor cognitive function using A‐IQCODE among Lebanese older adults: a cross‐sectional study. Aging Ment Health. 2017;22(6):844‐848. doi: 10.1080/13607863.2017.1301879 [DOI] [PubMed] [Google Scholar]
- 103. Farahat SA, Amin OR, Hamdy HS, Fouad MM. The impact of work‐related stress on the cognition domain of executive functioning of health care workers during the COVID‐19 pandemic. Int Arch Occup Environ Health. 2022;95(5):1079‐1090. doi: 10.1007/S00420-021-01814-8/FIGURES/2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Shalash A, Hamid E, Elrassas H, Abushouk AI, Salem HH. Sexual dysfunction in male patients with Parkinson's disease: related factors and impact on quality of life. Neurological Sciences. 2020;41(8):2201‐2206. doi: 10.1007/S10072-020-04328-2/TABLES/4 [DOI] [PubMed] [Google Scholar]
- 105. Nielsen TR, Kieu T, Phung T, Chaaya M, Mackinnon A, Waldemar G. Combining the Rowland Universal Dementia Assessment Scale and the Informant Questionnaire on Cognitive Decline in the Elderly to Improve Detection of Dementia in an Arabic‐Speaking Population. Original Research Article Dement Geriatr Cogn Disord. 2016;41:46‐54. doi: 10.1159/000441649 [DOI] [PubMed] [Google Scholar]
- 106. ben Jemaa S, Romdhane NA, Bahri‐Mrabet A, Jendli A, le Gall D, Bellaj T. An Arabic Version of the cognitive subscale of the Alzheimer's Disease Assessment Scale (ADAS‐Cog): Reliability, validity, and normative data. Journal of Alzheimer's Disease. 2017;60(1):11‐21. doi: 10.3233/JAD-170222 [DOI] [PubMed] [Google Scholar]
- 107. Farghaly M, Hussein M, Hassan A, Hegazy M, Sabbah A. Testing of Verbal Fluency in Egyptians: Cultural and Educational Challenges. Cogn Behav Neurol. 2018;31(3):133‐141. doi: 10.1097/WNN.0000000000000160 [DOI] [PubMed] [Google Scholar]
- 108. El‐Hayeck R, Baddoura R, Wehbé A, et al. An adapted Arabic version of the Test of Nine Images for the illiterate Lebanese population: Validation and preliminary normative data. J Int Neuropsychol Soc. 2022:1‐8. doi: 10.1017/s1355617722000236 [DOI] [PubMed] [Google Scholar]
- 109. Alkeridy WA, Muayqil TA, al Khalifah RA, Mohammedin AS, Khallaf RA, Bucks RS. Arabic translation and cross‐cultural adaptation of the Bristol Activities of Daily Living Scale (BADLS). Alzheimers Dement. 2022;17:e050856. doi: 10.1002/alz.050856 [DOI] [PubMed] [Google Scholar]
- 110. Al‐Adawi S, Al‐Naamani A, Jaju S, et al. Methylphenidate improves executive functions in patients with traumatic brain injuries: a feasibility trial via the idiographic approach. BMC Neurol. 2020;20(1):103. doi: 10.1186/S12883-020-01663-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Elsaid AF, Fahmi RM, Shaheen M, Ghoneum M. The enhancing effects of Biobran/MGN‐3, an arabinoxylan rice bran, on healthy old adults’ health‐related quality of life: a randomized, double‐blind, placebo‐controlled clinical trial. Quality of Life Research. 2020;29(2):357‐367. doi: 10.1007/S11136-019-02286-7/TABLES/4 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information


