ABSTRACT
Use of pneumococcal conjugate vaccines (PCVs) has led to substantial reductions in the global burden of pediatric pneumococcal disease. Expansion of serotype coverage has been achieved by increasing PCV valency, but this may carry the potential risk of antibody interference. A complementary 7-valent PCV (cPCV7) including polysaccharide conjugates from 7 non–13-valent (PCV13) serotypes was developed to potentially complement PCV13-mediated protection and expand serotype coverage. This study evaluated cPCV7 and PCV13 coadministered in separate limbs or separated in time in infants. This phase 2, multicenter, open-label study included 512 infants randomized 1:1:1 to receive cPCV7 coadministered with PCV13 at ages 2, 4, 6, and 12 months (cPCV7 Coadministered); cPCV7 given at ages 3, 5, 7, and 13 months, 3‒5 weeks after PCV13 (cPCV7 Separated); or PCV13 at ages 2, 4, 6, and 12 months followed by a single supplemental dose of cPCV7 at 13 months (PCV13 Control). Safety evaluations included local reactions, systemic events, and adverse events. Serotype-specific immunoglobulin G concentrations and opsonophagocytic activity titers were assessed. The safety profile of cPCV7 was similar to that of PCV13. cPCV7 was well-tolerated in infants when coadministered with or given separately from PCV13. Robust and functional immune responses for all cPCV7 serotypes were observed in both cPCV7 groups. No immunologic interference was observed for either the cPCV7 or PCV13 serotypes with coadministration. A single cPCV7 dose induced immune responses in toddlers. These findings support potential coadministration of a complementary PCV to supplement protection provided by existing PCVs.
Trial registration: ClinicalTrials.gov, NCT03550313.
KEYWORDS: Pneumococcal conjugate vaccine, pediatric, immunogenicity, safety, invasive pneumococcal disease, immunologic interference
Introduction
Streptococcus pneumoniae is a leading cause of meningitis, bacteremia, pneumonia, and acute otitis media and represents a significant global public health concern.1–3 The introduction of pneumococcal conjugate vaccines (PCVs) into national pediatric immunization programs has reduced the burden of pneumococcal disease (PD) in children.4 One such PCV is the 13-valent PCV (PCV13; Prevnar 13®, Pfizer Inc, Philadelphia, PA), which was licensed in the United States in 2010 and contains 13 polysaccharides (1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, and 23F), each conjugated to cross-reactive material 197 (CRM197).5 However, certain serotypes not contained in PCV13 continue to cause disease6–8; approximately 42% of invasive PD (IPD) in children is caused by non-PCV13 serotypes.9
A 20-valent PCV (PCV20; Prevnar 20®, Pfizer Inc, Philadelphia, PA) containing the PCV13 serotypes and 7 additional serotypes (8, 10A, 11A, 12F, 15B, 22F, and 33F) was developed to expand protection against non-PCV13 vaccine serotypes of public health importance.10 These 7 serotypes were selected based on several factors, such as their relative prevalence for causing IPD, generalized geographic distribution, and associations with antibiotic resistance and greater disease severity (eg, meningitis and mortality).9,11–14 PCV20 is currently licensed in the United States, Europe, Canada, Australia and other countries for adults ≥18 years of age, and in the United States in pediatric populations 6 weeks of age and older.10
Similar to licensure of PCV13, licensure of PCV20 was based on immunologic criteria demonstrating noninferior immune responses induced by PCV20 versus PCV13.5,10 For PCV13 licensure, immune responses were compared with those induced by the previously licensed 7-valent PCV (PCV7; Prevnar®, Pfizer Inc, Philadelphia, PA); for the serotypes shared by both vaccines, noninferiority was evaluated by direct comparison of immune responses, whereas for the additional serotypes in PCV13, responses were compared with the lowest response observed for PCV7 serotypes among PCV7 recipients.5 Data from a phase 2 safety and immunogenicity study in infants showed that immune responses elicited by PCV20 are similar to those elicited by PCV13 for the shared serotypes,15 and PCV20 was granted breakthrough therapy designation for pediatric use by the US Food and Drug Administration.16 Before data from the PCV20 program were available, a complementary 7-valent PCV (cPCV7) containing the 7 non-PCV13 serotypes in PCV20 was developed as an alternative strategy to PCV20 because of concerns regarding potential lower antibody responses in higher valency PCVs. There are multiple factors that may be involved in immune interference, including administration of the antigens simultaneously, administration of the antigens at the same local site, or interference at the vaccine formulation step.17 Potential use of cPCV7 might avoid possible drawbacks of increasing the number of polysaccharide conjugates contained within a single vaccine, and inclusion of fewer serotypes in the vaccine formulation might also address manufacturing complexity. cPCV7 uses the same platform and contains the same excipients as PCV13.
Here, we describe the safety and immunogenicity of cPCV7 coadministered with or given separately from PCV13 in healthy infants enrolled to receive a 4-dose series of both vaccines. This design enabled evaluation regarding immune interferences attributed to administration of PCV13 and cPCV7 at the same visit compared with separation by 3‒5 weeks. A control group of infants receiving a 4-dose series of PCV13 only was also included in the study. To further explore safety and immunogenicity of a single toddler dose, the control group received a single dose of cPCV7 at 13 months of age, after the 4-dose series of PCV13 was completed.
Methods
Study design and participants
This phase 2, multicenter, randomized, active-controlled, open-label, 3-arm parallel-design study was conducted at 39 sites in the United States comprising pediatric research centers, private practices, and academic institutions. The study was registered at ClinicalTrials.gov on June 8, 2018 (NCT03550313); a protocol and statistical plan are available at the clinicaltrials.gov website. Infants were randomized (1:1:1) using an interactive response technology system to receive cPCV7 coadministered with PCV13 at 2, 4, 6, and 12 months of age (cPCV7 Coadministered group); cPCV7 at 3, 5, 7, and 13 months of age, approximately 1 month after receiving PCV13 at 2, 4, 6, and 12 months of age (cPCV7 Separated group); or PCV13 at 2, 4, 6, and 12 months of age and cPCV7 at 13 months of age as a supplemental dose (PCV13 Control group; Figure 1). The interactive response system provided a randomization number and container number. PCV13 was administered according to the US-recommended schedule in all groups.5 In this report, Doses 1, 2, 3, and 4 refer to cPCV7 for the cPCV7 Coadministered and cPCV7 Separated groups and to PCV13 for the PCV13 Control group; the Supplemental Dose refers to cPCV7 in the PCV13 Control group. Routinely recommended infant immunizations were also permitted during the study. Laboratory personnel directly performing assays were blinded until all assays were complete.
Figure 1.
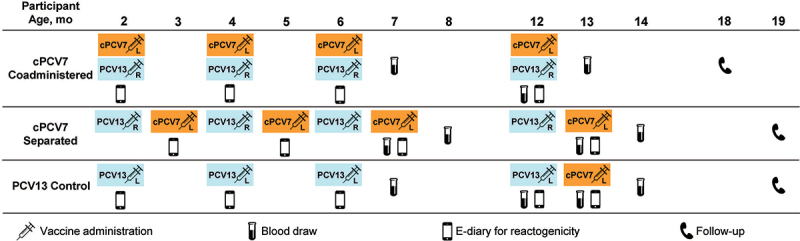
Study design. L denotes injection into the left leg, while R denotes injection into the right leg. cPCV7 = complementary 7-valent pneumococcal conjugate vaccine; e-diary = electronic diary; PCV13 = 13-valent pneumococcal conjugate vaccine.
Eligible participants were healthy infants born at >36 weeks of gestation and approximately 2 months (≥42‒≤98 days) of age at the time of consent. Key exclusion criteria included previous receipt of a pneumococcal vaccine; contraindication to vaccination with PCV13 or diphtheria, tetanus, or pertussis vaccines; a history of IPD; significant neurologic disorder, history of seizure, or other medical or psychiatric condition that may increase the risk associated with study participation; and known or suspected immunodeficiency or receipt of immunosuppressive therapy.
This study was conducted in compliance with the ethical principles originating in or derived from the Declaration of Helsinki and with the International Council for Harmonisation Good Clinical Practice Guidelines. All local regulatory requirements were followed, particularly those affording greater protection to the safety of trial participants. A signed and dated informed consent form was obtained from each participant’s parent(s) or legal guardian(s) before performance of any study-related activity. The final protocol, amendments, and informed consent form were reviewed and approved by the institutional review boards and/or independent ethics committees for each of the participating investigational centers.
Interventions
In the cPCV7 Coadministered and cPCV7 Separated groups, participants received 0.5-mL injections of cPCV7 (Pfizer; lot number 17–004640) into the left anterolateral thigh and 0.5-mL injections of PCV13 (Pfizer; lot number 18–000291) into the right anterolateral thigh; in the PCV13 Control group, all injections were into the left anterolateral thigh. Each 0.5-mL cPCV7 dose included 2.2 µg of capsular saccharides from pneumococcal serotypes 8, 10A, 11A, 12F, 15B, 22F, and 33F individually conjugated to CRM197. Each 0.5-mL PCV13 dose was similarly formulated for serotypes 1, 3, 4, 5, 6A, 7F, 9V, 14, 18C, 19A, 19F, and 23F; for serotype 6B, the dose was 4.4 µg. Additional excipients for both cPCV7 and PCV13 include succinate buffer, sodium chloride, polysorbate 80, and aluminum as aluminum phosphate.
A diphtheria, tetanus, and acellular pertussis (DTaP)‒containing vaccine was administered concomitantly with Doses 1 to 3 in the cPCV7 Coadministered and PCV13 Control groups and with the first 3 PCV13 doses in the cPCV7 Separated group; a DTaP-containing vaccine (Pediarix; GSK Vaccines GmbH, Marburg, Germany), which also included polio and hepatitis B antigens, was provided to sites for this purpose. Other permitted vaccines included Haemophilus influenzae type b (all at 2, 4, and 6 months of age); measles, mumps, and rubella (12 months of age); rotavirus (anytime during the study), and inactivated influenza (during the influenza season); administration of other licensed vaccines was allowed within prespecified windows. Quadrivalent meningococcal conjugate vaccine was only permitted after blood collection 1 month following Dose 4/Supplemental Dose. The DTaP-containing vaccine and other permitted vaccines were administered into a limb other than the site of cPCV7 injection (all groups when cPCV7 was administered) or PCV13 injection (PCV13 Control group only).
Objectives and endpoints
The primary objective was to describe the safety profile of cPCV7 in healthy infants. Endpoints included the percentages of participants reporting prompted local reactions (ie, redness, swelling, and pain at the injection site) and systemic events (ie, fever, decreased appetite, drowsiness, and irritability) within 7 days of each dose (ie, cPCV7 for all doses in the cPCV7 Coadministered and cPCV7 Separated groups; PCV13 for Doses 1, 2, 3, and 4 and cPCV7 for the Supplemental Dose in the PCV13 Control group) as recorded in an electronic diary. Additional endpoints included adverse events (AEs) from Dose 1 to 1 month after Dose 3 and from Dose 4/Supplemental Dose to 1 month after Dose 4/Supplemental Dose, as well as serious AEs (SAEs) and newly diagnosed chronic medical conditions (NDCMCs) from Dose 1 to 6 months after Dose 4/Supplemental Dose.
The secondary objective was to describe the immunogenicity of cPCV7 administered with or separated by 1 month from PCV13 as measured by serotype-specific immunoglobulin G (IgG) concentrations for the 7 included serotypes. Exploratory objectives included further characterizing immunogenicity of cPCV7 in a subset of subjects, measured by serotype-specific opsonophagocytic activity (OPA) titers for the 7 cPCV7 serotypes, as well as describing immunogenicity of PCV13 when given with or separated by 1 month from cPCV7 and immunogenicity of cPCV7 following a 4-dose series of PCV13. Blood samples were collected for immunogenicity analyses at 1 month after Dose 3, before Dose 4, and 1 month after Dose 4 in all groups, as well as 1 month after the third dose of PCV13 in the cPCV7 Separated group and 1 month after the Supplemental Dose in the PCV13 Control group. Serotype-specific IgG concentrations were measured by the Pfizer Luminex assay. Serotype-specific OPA titers for cPCV7 serotypes were determined using serum from a randomly selected subset of participants at each blood draw and measured using the Pfizer OPA assay.
Statistical analysis
Sample size
The study planned to enroll approximately 690 participants. The study size in each cohort was not based on any formal statistical hypothesis testing for safety or immunogenicity endpoints; all statistical analyses were descriptive and no between-group comparisons were performed.
Safety
Safety results were descriptively summarized in the safety population, which included all participants who received ≥1 dose of cPCV7 (cPCV7 Coadministered and cPCV7 Separated groups) or PCV13 (PCV13 Control group) and had safety follow-up in the study.
Immunogenicity
The evaluable immunogenicity population was the primary population for immunogenicity results; this group included eligible participants who received the vaccines as randomized at the protocol-defined age ranges for the first vaccination and Dose 4 (Dose 4 population only), had a valid IgG concentration for ≥1 serotype from a blood sample collected within the prespecified windows for 1 month after Dose 3 or 1 month after Dose 4 (Dose 3 or Dose 4 populations, respectively), and had no major protocol deviations that would impact evaluable population eligibility. Immunogenicity results following the Supplemental Dose in the PCV13 Control group were summarized for the all-available Supplemental Dose immunogenicity population, which included participants who received the 4-dose series of PCV13 followed by cPCV7 (Supplemental Dose) and had ≥1 valid IgG concentration for the Supplemental Dose.
IgG geometric mean concentrations (GMCs), OPA geometric mean titers (GMTs; in a subset of participants), and geometric mean fold rises (GMFRs) in IgG concentrations and OPA titers and the corresponding 2-sided 95% CIs were calculated by exponentiating the mean logarithm of the concentrations, titers, or fold rises, respectively, and the corresponding CIs (based on the Student t distribution). The percentages of participants achieving predefined serotype-specific IgG concentration levels18 and the associated 2-sided 95% CIs (using the Clopper-Pearson method) are also reported.
Results
Participants
Due to significant challenges with enrollment and participant retention, the total number of randomized participants during June 1, 2018‒October 4, 2019 was 565 rather than the originally planned 690 (Figure 2); this smaller sample size was deemed adequate to provide the data necessary for endpoint evaluation with the knowledge gained from an infant PCV20 study.15 With continued feasibility challenges further compounded by the COVID-19 pandemic, study vaccinations and blood draws were halted in May 2020 and safety follow-up was continued through 6 months after the last dose of vaccine. Additionally, serious quality issues were identified for 2 study sites, resulting in early termination of 53 participants and the exclusion of their data from the analyses. Among the remaining 512 participants, 174, 170, and 168 were assigned to the cPCV7 Coadministered, cPCV7 Separated, and PCV13 Control groups, respectively, and 171, 147, and 166, respectively, received Dose 1 (ie, comprised the safety population). Of these, 137 (78.7%), 115 (67.6%), and 124 (73.8%) completed the 1-month visit after Dose 3 (ie, cPCV7 for the cPCV7 Coadministered and cPCV7 Separated groups, and PCV13 for the PCV13 Control group), and 90 (51.7%), 71 (41.8%), and 77 (45.8%) completed the 1-month visit after Dose 4. In the PCV13 Control group, 66 participants (39.3%) completed the 1-month visit after the Supplemental Dose.
Figure 2.
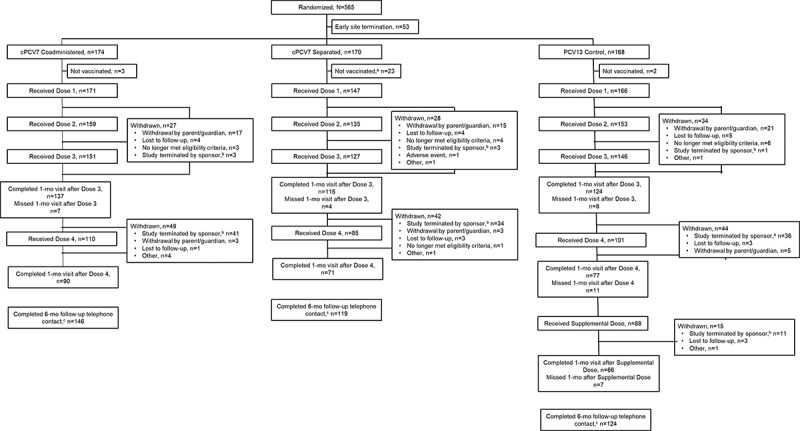
Participant disposition. Doses 1, 2, 3, and 4 refer to doses of cPCV7 administered in the cPCV7 Coadministered and cPCV7 Separated groups or doses of PCV13 administered in the PCV13 Control group. The Supplemental Dose refers to the cPCV7 dose given in the PCV13 Control group. aIncludes participants who received their first dose of PCV13 but did not receive Dose 1 of cPCV7. bVaccinations and blood draws were terminated; however, safety follow-up procedures were continued per protocol. cA telephone contact was attempted for all participants who were 6 months beyond their last vaccination at the time of the data cutoff, unless consent was withdrawn. cPCV7 = complementary 7-valent pneumococcal conjugate vaccine; PCV13 = 13-valent pneumococcal conjugate vaccine.
Demographic characteristics for sex, race, and ethnicity were generally similar among the vaccine groups (Table 1). Of note, the “not reported” category for race included participants who did not fall into categories specified by the electronic case report form; this applied to 48 participants (9.9%). The percentages of participants of Hispanic/Latinx ethnicity (47.6%‒52.0%) from all 3 vaccine groups were consistently higher than that of the general United States infant population.19 Mean age at Dose 1 was 65.9, 95.1, and 64.9 days in the cPCV7 Coadministered, cPCV7 Separated, and PCV13 Control groups, respectively, which was consistent with the protocol-defined vaccination schedules.
Table 1.
Demographic Characteristics (Overall Safety Population).
| Characteristic | Vaccine Group (as Administered) |
||
|---|---|---|---|
| cPCV7 Coadministered (N = 171) |
cPCV7 Separated (N = 147) |
PCV13 Control (N = 166) |
|
| Sex, n (%) | |||
| Male | 86 (50.3) | 67 (45.6) | 91 (54.8) |
| Female | 85 (49.7) | 80 (54.4) | 75 (45.2) |
| Race, na (%) | |||
| White | 102 (59.6) | 90 (61.2) | 105 (63.3) |
| Black or African American | 35 (20.5) | 26 (17.7) | 31 (18.7) |
| Asian | 7 (4.1) | 5 (3.4) | 3 (1.8) |
| American Indian or Alaskan Native | 4 (2.3) | 8 (5.4) | 3 (1.8) |
| Native Hawaiian or other Pacific Islander | 0 | 0 | 1 (0.6) |
| Multiracial | 6 (3.5) | 4 (2.7) | 6 (3.6) |
| Not reported | 17 (9.9) | 14 (9.5) | 17 (10.2) |
| Ethnicity, n (%) | |||
| Non-Hispanic/non-Latinx | 81 (47.4) | 77 (52.4) | 80 (48.2) |
| Hispanic/Latinx | 89 (52.0) | 70 (47.6) | 85 (51.2) |
| Not reported | 1 (0.6) | 0 | 1 (0.6) |
| Age at Dose 1b, d | |||
| Mean (SD) | 65.9 (9.53) | 95.1 (10.40) | 64.9 (8.22) |
| Median | 64.0 | 95.0 | 64.0 |
| Min, max | (43, 96) | (65, 126) | (43, 97) |
cPCV7 = complementary 7-valent pneumococcal conjugate vaccine; PCV13 = 13-valent pneumococcal conjugate vaccine.
aParticipants who were not part of the electronic case report form race options were included in the “not reported” category.
bRefers to the first vaccine dose (cPCV7 for the cPCV7 Coadministered and Separated groups and PCV13 for the PCV13 group). The older age of participants in the cPCV7 Separated group is consistent with cPCV7 receipt 3‒5 weeks after PCV13 administration at approximately 2 months of age, according to the study design.
Safety
Local reactions and systemic events
Across all vaccine groups, the percentage of participants who reported any local reaction within 7 days after administration of Dose 1, 2, 3, or 4 was lowest in the cPCV7 Separated group and highest in the cPCV7 Coadministered group across the 4 doses (Figure 3a). The most frequently reported reaction was pain at the injection site. Across all groups, the percentage of participants who reported any systemic events within 7 days after administration of Dose 1, 2, 3, or 4 was also lowest in the cPCV7 Separated group, with higher percentages in the cPCV7 Coadministered and PCV13 Control groups (Figure 3b). The most frequently reported systemic event was irritability. Most local reactions and systemic events were mild or moderate in severity and resolved within 1 to 3 days. The cPCV7 Supplemental Dose results are also provided in Figure 3.
Figure 3.
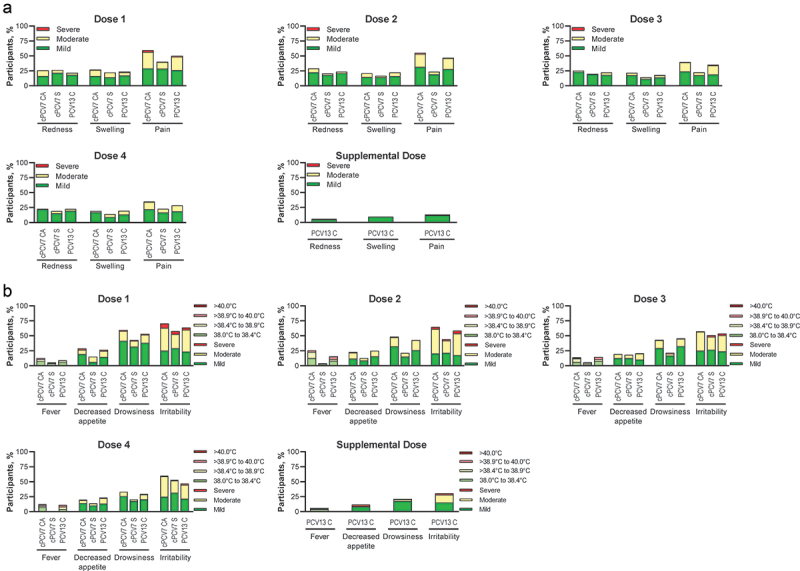
Participants reporting (a) local reactions and (b) systemic events within 7 days after Dose 1, Dose 2, Dose 3, Dose 4, and Supplemental Dose (safety population). Doses 1, 2, 3, and 4 refer to doses of cPCV7 administered in the cPCV7 Coadministered and cPCV7 Separated groups or doses of PCV13 administered in the PCV13 Control group. The Supplemental Dose refers to the cPCV7 dose given in the PCV13 Control group. For redness and swelling, mild was > 0.0 to 2.0 cm, moderate was > 2.0 to 7.0 cm, and severe was >7.0 cm. Pain at the injection site was mild if it hurt when gently touched (eg, participant whimpered, winced, protested, or withdrew), moderate if it hurt when gently touched with crying, and severe if it caused limitation of limb movement. For decreased appetite, mild was decreased interest in eating, moderate was decreased oral intake, and severe was refusal to feed. For drowsiness, mild was increased or prolonged sleeping bouts; moderate was slightly subdued, interfering with daily activity; and severe was disabling, not interested in usual daily activity. For irritability, mild was easily consolable; moderate was requiring increased attention; and severe was inconsolable, crying cannot be comforted. The numbers of subjects included in the analyses were as follows: Dose 1, n = 144‒166 per group; Dose 2, n = 126‒152 per group; Dose 3, n = 120‒143 per group; Dose 4, n = 79‒105 per group; Supplemental Dose, n = 85 for the PCV13 Control group. C = control; CA = coadministered; cPCV7 = complementary 7-valent pneumococcal conjugate vaccine; PCV13 = 13-valent pneumococcal conjugate vaccine; S = separated.
Adverse events
The percentages of participants reporting any AEs from Dose 1 to 1 month after Dose 3 and from Dose 4/Supplemental Dose to 1 month after Dose 4/Supplemental Dose were generally similar across vaccination groups (Table 2). Upper respiratory tract infection was the most frequently reported AE in all groups from Dose 1 to 1 month after Dose 3 (18.7%‒27.9%) and from Dose 4/Supplemental Dose to 1 month after Dose 4/Supplemental Dose (3.5%‒6.4%). One AE of erythema was reported in the cPCV7 Separated group that was considered related to vaccination; this AE began on the day of Dose 3 administration and lasted for 2 days. Severe AEs were reported by 2 participants (1.2%) in the cPCV7 Coadministered group, 2 participants (1.4%) in the cPCV7 Separated group, and 4 participants (2.4%) in the PCV13 Control group from Dose 1 to 1 month after Dose 3; none occurred in more than 1 individual. Similarly, the 2 severe AEs reported in the month following the Supplemental Dose did not occur in the same individual. Overall during the study period, SAEs were reported by 7 participants (4.1%) in the cPCV7 Coadministered group, 4 participants (2.7%) in the cPCV7 Separated group, and 9 participants (5.4%) in the PCV13 Control group. No SAEs reported were considered related to vaccine or led to a participant being withdrawn from the study. There were no deaths during the study. Few participants reported NDCMCs; none were considered related to the study vaccines.
Table 2.
Summary of AEs.
| Vaccine Group (as Administered) |
|||
|---|---|---|---|
| cPCV7 Coadministered | cPCV7 Separated | PCV13 Control | |
| Dose 1 to 1 mo after Dose 3a (overall safety population), nb (%) | |||
| Nc | 171 | 147 | 166 |
| Any AE | 99 (57.9) | 96 (65.3) | 94 (56.6) |
| Related AE | 0 | 1 (0.7) | 0 |
| Serious AE | 1 (0.6) | 2 (1.4) | 5 (3.0) |
| Severe AE | 2 (1.2) | 2 (1.4) | 4 (2.4) |
| Dose 4 to 1 mo after Dose 4a (Dose 4 safety population), nb (%) | |||
| Nd | 110 | 85 | 101 |
| Any AE | 26 (23.6) | 13 (15.3) | 26 (25.7) |
| Related AE | 0 | 0 | 0 |
| Serious AE | 0 | 0 | 0 |
| Severe AE | 0 | 0 | 0 |
| Supplemental Dose to 1 mo after Supplemental Dosea (Supplemental Dose safety population), nb (%) | |||
| Ne | – | – | 88 |
| Any AE | – | – | 16 (18.2) |
| Related AE | – | – | 0 |
| Serious AE | – | – | 2 (2.3) |
| Severe AE | – | – | 2 (2.3) |
| Dose 1a to end of study (overall safety population), nb (%) | |||
| Nc | 171 | 147 | 166 |
| Serious AE | 7 (4.1) | 4 (2.7) | 9 (5.4) |
| NDCMC | 13 (7.6) | 5 (3.4) | 12 (7.2) |
AE = adverse event; cPCV7 = complementary 7-valent pneumococcal conjugate vaccine; NDCMC = newly diagnosed chronic medical condition; PCV13 = 13-valent pneumococcal conjugate vaccine.
aDoses 1, 3, and 4 refer to doses of cPCV7 administered in the cPCV7 Coadministered and cPCV7 Separated groups or doses of PCV13 administered in the PCV13 Control group. The Supplemental Dose refers to the cPCV7 dose given in the PCV13 Control group.
bn = number of participants reporting ≥1 occurrence of the specified event.
cN = number of participants who received Dose 1 in the specified group.
dN = number of participants who received Dose 4 in the specified group.
eN = number of participants who received the Supplemental Dose in the specified group.
Immunogenicity
Responses at 1 month after dose 3
At 1 month after the third dose of cPCV7, strong immune responses to cPCV7 serotypes were observed in the cPCV7 Coadministered and cPCV7 Separated groups, with respective IgG GMC ranges of 1.92 µg/mL (serotype 12F) to 9.25 µg/mL (serotype 22F) and 3.35 µg/mL (serotype 12F) to 23.94 µg/mL (serotype 22F; Figure 4a). For all cPCV7 serotypes, the observed IgG GMCs were higher in the cPCV7 Separated group than in the cPCV7 Coadministered group. As expected, responses to the cPCV7 serotypes in the PCV13 Control group were not observed. The percentages of participants achieving predefined IgG concentrations for the cPCV7 serotypes were high in the cPCV7 Coadministered group (range, 89.8% [serotype 10A] to 98.4% [serotype 8]) and the cPCV7 Separated group (range, 98.1% [serotypes 10A and 12F] to 100% [serotypes 8, 15B, and 22F]) and much lower in the PCV13 Control group (≤6.4%; Figure 4b). Observed trends for OPA GMTs were similar to those for IgG GMCs, although the sample sizes were small (n = 38–46 across doses and groups; Figure 5a).
Figure 4.
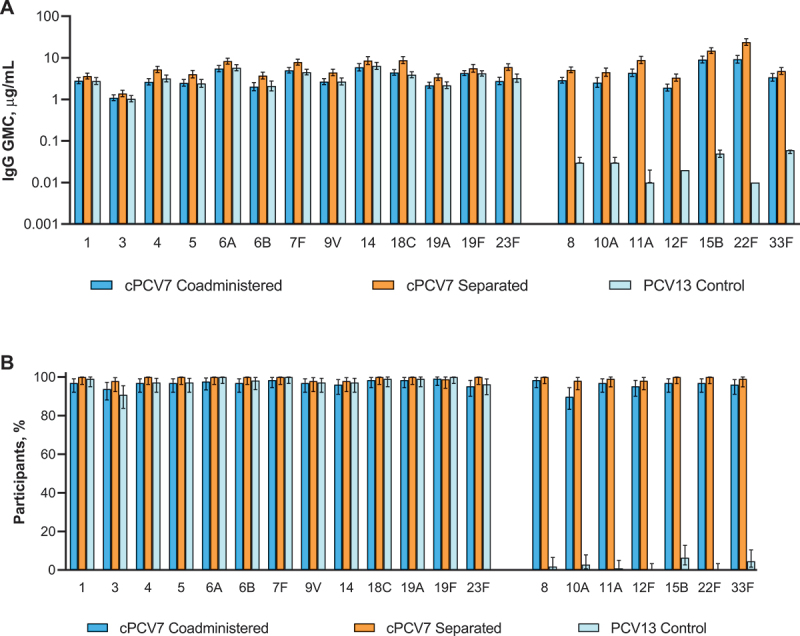
Serotype-specific (a) IgG GMCs and (b) percentages of participants achieving predefined IgG concentrations at 1 month after Dose 3 (cPCV7 serotypes) or 1 month after the third PCV13 dose (PCV13 serotypes; Dose 3 evaluable immunogenicity population). Dose 3 refers to the third dose of cPCV7 administered in the cPCV7 Coadministered and cPCV7 Separated groups or the third dose of PCV13 administered in the PCV13 Control group. For the PCV13 serotypes, data are shown from 1 month after the third dose of PCV13 for all groups. The predefined IgG concentration was ≥0.35 μg/mL for serotypes 1, 3, 4, 6A, 7F, 8, 9V, 10A, 11A, 12F, 14, 15B, 18C, 19F, 22F, 23F, and 33F; ≥0.23 μg/mL for serotype 5; ≥0.10 μg/mL for serotype 6B; and ≥0.12 μg/mL for serotype 19A.18 Error bars display the upper and lower bounds of the 2-sided 95% CIs for (a) GMCs and (b) percentages of participants achieving predefined IgG concentrations, calculated based on the Student t distribution and the Clopper-Pearson method, respectively. Assay results below the LLOQ were set to 0.5 × LLOQ in the GMC calculations. The numbers of subjects included in the analyses were as follows: n = 128 for the cPCV7 Coadministered group, n = 94‒107 for the cPCV7 Separated group, and n = 109 for the PCV13 Control group. cPCV7 = complementary 7-valent pneumococcal conjugate vaccine; GMC = geometric mean concentration; IgG = immunoglobulin G; LLOQ = lower limit of quantitation; PCV13 = 13-valent pneumococcal conjugate vaccine.
Figure 5.
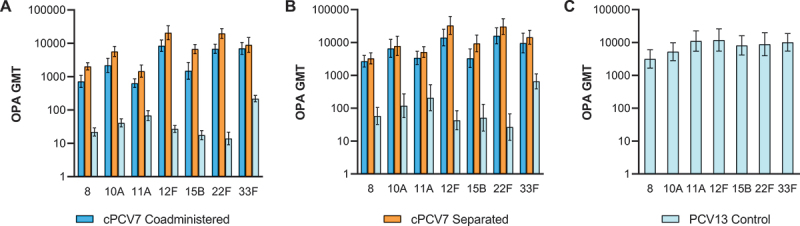
Serotype-specific OPA GMTs for the cPCV7 serotypes at (a) 1 month after Dose 3 (Dose 3 evaluable immunogenicity population), (b) 1 month after Dose 4 (Dose 4 evaluable immunogenicity population), and (c) 1 month after the Supplemental Dose (Supplemental Dose all-available immunogenicity population). Doses 3 and 4 refer to doses of cPCV7 administered in the cPCV7 Coadministered and cPCV7 Separated groups or doses of PCV13 administered in the PCV13 Control group. The Supplemental Dose refers to the cPCV7 dose given in the PCV13 Control group. OPA titers were determined based on serum from a randomly selected subset of participants. Error bars display the upper and lower bounds of the 2-sided 95% CIs for GMTs calculated based on the Student t distribution. Assay results below the LLOQ were set to 0.5 × LLOQ in the GMT calculations. At 1 month after Dose 3, n = 38‒46 for the cPCV7 Coadministered group, n = 38‒45 for the cPCV7 Separated group, and n = 40‒44 for the PCV13 Control group. At 1 month after Dose 4, n = 24‒28 for the cPCV7 Coadministered group, n = 25‒28 for the cPCV7 Separated group, and n = 24‒29 for the PCV13 Control group. At 1 month after the Supplemental Dose, n = 21‒26 for the PCV13 Control group. cPCV7 = complementary 7-valent pneumococcal conjugate vaccine; GMT = geometric mean titer; LLOQ = lower limit of quantitation; OPA = opsonophagocytic activity; PCV13 = 13-valent pneumococcal conjugate vaccine.
One month after the third dose of PCV13, robust responses to PCV13 serotypes were observed across all vaccine groups (Figure 4a). IgG GMCs for the PCV13 serotypes were generally numerically highest in the cPCV7 Separated group (range, 1.39 µg/mL [serotype 3] to 8.77 µg/mL [serotype 18C]) and similar between the cPCV7 Coadministered group (range, 1.10 µg/mL [serotype 3] to 5.93 µg/mL [serotype 14]) and PCV13 Control group (range, 1.04 µg/mL [serotype 3] to 6.39 µg/mL [serotype 14]). The percentages of participants achieving predefined serotype-specific IgG concentrations were high in all groups (cPCV7 Coadministered, 93.8% [serotype 3] to 99.2% [serotype 19F]; cPCV7 Separated, 97.9% [serotypes 3, 9V, and 14] to 100% [serotypes 1, 4, 5, 6A, 6B, 7F, 18C, 19A, and 23F]; PCV13 Control, 90.8% [serotype 3] to 100% [serotypes 6A, 7F, and 19F]; Figure 4b).
Responses at 1 month after dose 4
After the fourth dose of cPCV7, IgG GMCs of the cPCV7 serotypes ranged from 3.15 μg/mL (serotype 12F) to 25.68 μg/mL (serotype 22F) in the cPCV7 Coadministered group and 2.57 μg/mL (serotype 12F) to 29.92 μg/mL (serotype 22F) in the cPCV7 Separated group (Figure 6). Unlike at 1 month after Dose 3, the observed IgG GMCs were slightly higher for most serotypes in the cPCV7 Coadministered group than in the cPCV7 Separated group; IgG GMFRs from before Dose 4 to 1 month after Dose 4 ranged from 6.7 (serotype 33F) to 10.6 (serotype 10A) in the cPCV7 Coadministered group and from 2.5 (serotype 8) to 4.0 (serotype 22F) in the cPCV7 Separated group (Table 3). The observed OPA GMTs at 1 month after Dose 4 generally remained higher in the cPCV7 Separated Group compared with the cPCV7 Coadministered group (n = 24‒29 across doses and groups; Figure 5b). Ranges of OPA GMFRs from 1 month after Dose 3 to 1 month after Dose 4 were 1.3 (serotype 33F) to 6.2 (serotype 11A) in the cPCV7 Coadministered group and 1.4 (serotypes 10A, 12F, 15B, and 33F) to 2.5 (serotype 11A) in the cPCV7 Separated group (Table 4), demonstrating boosting for all 7 serotypes.
Figure 6.
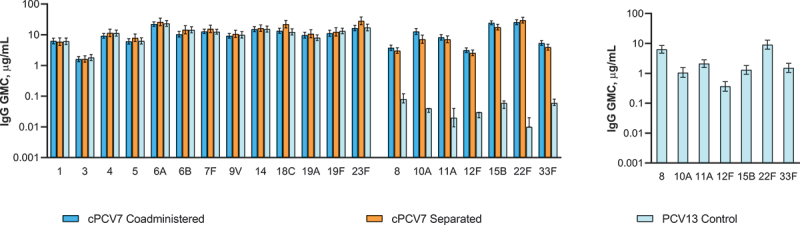
Serotype-specific IgG GMCs 1 month after Dose 4 (cPCV7 serotypes) or 1 month after the fourth PCV13 dose (PCV13 serotypes; Dose 4 evaluable immunogenicity population) and 1 month after the Supplemental Dose (Supplemental Dose all-available immunogenicity population). Dose 4 refers to the fourth dose of cPCV7 administered in the cPCV7 Coadministered and cPCV7 Separated groups or the fourth dose of PCV13 administered in the PCV13 Control group. For the PCV13 serotypes, data are shown from 1 month after the fourth dose of PCV13 for all groups. The Supplemental Dose refers to the cPCV7 dose given in the PCV13 Control group. Error bars display the upper and lower bounds of the 2-sided 95% CIs for GMCs calculated based on the Student t distribution. Assay results below the LLOQ were set to 0.5 × LLOQ in the GMC calculations. At 1 month after Dose 4, n = 76 for the cPCV7 Coadministered group, n = 52‒57 for the cPCV7 Separated group, and n = 68 for the PCV13 Control group. At 1 month after the Supplemental Dose, n = 66 for the PCV13 Control group. cPCV7 = complementary 7-valent pneumococcal conjugate vaccine; GMC = geometric mean concentration; IgG = immunoglobulin G; LLOQ = lower limit of quantitation; PCV13 = 13-valent pneumococcal conjugate vaccine.
Table 3.
IgG GMFRs for the cPCV7 Serotypes from Before Dose 4 to 1 Month After Dose 4a (Evaluable Immunogenicity Population) and from Before Supplemental Dose to 1 Month After Supplemental Dosea (PCV13 Control Group Only; Supplemental Dose All-Available Immunogenicity Population).
| Before Dose 4 to 1 Month After Dose 4a |
Before Supplemental Dose to 1 Month After Supplemental Dosea |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vaccine Group (as Administered) |
||||||||||||
| cPCV7 Coadministered Group |
cPCV7 Separated Group |
PCV13 Control Group |
PCV13 Control Group |
|||||||||
| Serotype | nb | GMFRc | 95% CI | nb | GMFRc | 95% CI | nb | GMFRc | 95% CI | nb | GMFRc | 95% CI |
| 8 | 69 | 8.4 | (6.6, 10.7) | 52 | 2.5 | (2.0, 3.3) | 63 | 1.3 | (1.1, 1.5) | 60 | 83.2 | (53.2, 130.1) |
| 10A | 69 | 10.6 | (8.0, 14.1) | 52 | 3.7 | (2.8, 4.8) | 63 | 1.1 | (0.9, 1.3) | 60 | 28.8 | (18.5, 44.7) |
| 11A | 69 | 9.0 | (6.9, 11.7) | 52 | 3.3 | (2.5, 4.4) | 63 | 1.3 | (0.9, 1.7) | 60 | 104.0 | (61.9, 174.8) |
| 12F | 69 | 9.1 | (7.4, 11.2) | 52 | 3.6 | (2.9, 4.5) | 63 | 1.1 | (0.9, 1.4) | 60 | 16.9 | (11.5, 24.7) |
| 15B | 69 | 8.5 | (6.2, 11.7) | 52 | 3.7 | (2.9, 4.7) | 63 | 1.3 | (1.0, 1.7) | 60 | 27.0 | (19.2, 38.0) |
| 22F | 69 | 8.7 | (6.5, 11.7) | 52 | 4.0 | (2.9, 5.5) | 63 | 1.4 | (0.9, 2.2) | 60 | 848.1 | (485.7, 1481.1) |
| 33F | 69 | 6.7 | (5.3, 8.6) | 52 | 3.2 | (2.5, 4.1) | 63 | 1.2 | (1.0, 1.5) | 60 | 31.0 | (22.0, 43.8) |
cPCV7 = complementary 7-valent pneumococcal conjugate vaccine; GMFR = geometric mean fold rise; IgG = immunoglobulin G; PCV13 = 13-valent pneumococcal conjugate vaccine.
aDose 4 refers to cPCV7 administered in the cPCV7 Coadministered and cPCV7 Separated groups or PCV13 administered in the PCV13 Control group. The Supplemental Dose refers to the cPCV7 dose given in the PCV13 Control group.
bn = number of participants with valid and determinate assay results at both of the specified visits.
cGMFRs and the corresponding 2-sided 95% CIs were calculated by exponentiating the mean logarithm of IgG fold rises and the corresponding CIs (based on the Student t distribution).
Table 4.
OPA GMFRs for the cPCV7 Serotypes from 1 Month After Dose 3a to 1 Month After Dose 4a (Evaluable Immunogenicity Population) and from Before Supplemental Dose to 1 Month After Supplemental Dosea (PCV13 Control Group Only; Supplemental Dose All-Available Immunogenicity Population)b.
| 1 Month After Dose 3a to 1 Month After Dose 4a |
Before Supplemental Dose to 1 Month After Supplemental Dosea |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vaccine Group (as Administered) |
||||||||||||
| cPCV7 Coadministered Group |
cPCV7 Separated Group |
PCV13 Control Group |
PCV13 Control Group |
|||||||||
| Serotype | nc | GMFRd | 95% CI | nc | GMFRd | 95% CI | nc | GMFRd | 95% CI | nc | GMFRd | 95% CI |
| 8 | 26 | 3.8 | (2.2, 6.6) | 23 | 1.9 | (1.3, 2.9) | 23 | 2.1 | (1.3, 3.5) | 24 | 75.7 | (30.2, 189.8) |
| 10A | 27 | 3.5 | (1.4, 8.7) | 19 | 1.4 | (0.7, 2.6) | 23 | 2.4 | (1.1, 5.2) | 19 | 42.7 | (13.8, 132.5) |
| 11A | 26 | 6.2 | (3.7, 10.4) | 22 | 2.5 | (1.4, 4.5) | 20 | 2.3 | (1.0, 5.3) | 18 | 53.1 | (16.2, 174.1) |
| 12F | 23 | 1.9 | (0.8, 4.6) | 18 | 1.4 | (0.6, 3.1) | 26 | 1.3 | (0.8, 2.1) | 23 | 304.5 | (127.7, 726.4) |
| 15B | 21 | 2.6 | (0.9, 7.3) | 24 | 1.4 | (0.7, 2.6) | 25 | 2.4 | (0.7, 7.9) | 25 | 205.7 | (58.0, 729.7) |
| 22F | 19 | 2.4 | (1.1, 5.1) | 19 | 1.6 | (0.9, 2.7) | 24 | 1.9 | (0.6, 6.1) | 22 | 360.7 | (113.7, 1144.5) |
| 33F | 20 | 1.3 | (0.5, 3.1) | 21 | 1.4 | (0.7, 2.9) | 22 | 2.6 | (1.5, 4.4) | 22 | 15.6 | (7.7, 31.6) |
cPCV7 = complementary 7-valent pneumococcal conjugate vaccine; GMFR = geometric mean fold rise; OPA = opsonophagocytic activity; PCV13 = 13-valent pneumococcal conjugate vaccine.
aDoses 3 and 4 refer to doses of cPCV7 administered in the cPCV7 Coadministered and cPCV7 Separated groups or doses of PCV13 administered in the PCV13 Control group. The Supplemental Dose refers to the cPCV7 dose given in the PCV13 Control group.
bOPA titers were determined based on serum from a randomly selected subset of participants.
cn = number of participants with valid and determinate assay results at both of the specified visits.
dGMFRs and the corresponding 2-sided 95% CIs were calculated by exponentiating the mean logarithm of OPA fold rises and the corresponding CIs (based on the Student t distribution).
At 1 month after the fourth dose of PCV13, robust immune responses to the PCV13 serotypes were observed across all groups (Figure 6). IgG GMCs ranged from 1.61 μg/mL (serotype 3) to 22.25 μg/mL (serotype 6A) in the cPCV7 Coadministered group, 1.63 μg/mL (serotype 3) to 28.36 μg/mL (serotype 23F) in the cPCV7 Separated group, and 1.82 μg/mL (serotype 3) to 23.42 μg/mL (serotype 6A) in the PCV13 Control group.
Responses at 1 month after the supplemental dose
At 1 month after the Supplemental Dose of cPCV7 in the PCV13 Control group, immune responses in IgG concentrations were observed for all cPCV7 serotypes; IgG GMCs ranged from 0.39 μg/mL (serotype 12F) to 9.87 μg/mL (serotype 22F; Figure 6). IgG GMFRs from before to 1 month after the Supplemental Dose ranged from 16.9 (serotype 12F) to 848.1 (serotype 22F; Table 3). OPA GMTs after the Supplemental Dose (PCV13 Control group, n = 21‒26) ranged from 3156.9 (serotype 8) to 11,888.1 (serotype 12F; Figure 5c), with OPA GMFRs from before to 1 month after the Supplemental Dose for the cPCV7 serotypes ranging from 15.6 (serotype 33F) to 360.7 (serotype 22F; Table 4).
Discussion
This study evaluated the safety and immunogenicity of cPCV7 coadministered with or given separately from PCV13. cPCV7 was well tolerated, and AEs reported in the study were consistent with medical events or conditions that are expected with vaccinations in this age group. The milder tolerability profile observed in the cPCV7 Separated group may be attributable at least in part to the lack of coadministration with other infant vaccines in that group, as opposed to the other groups, at Doses 1 to 4. Although this study was not powered to make formal comparisons between cPCV7 groups, there was no suggestion of interference with IgG responses to cPCV7 when coadministered with or given separately from PCV13, or responses to PCV13 when coadministered or given separately from cPCV7 compared with the PCV13 Control group.
cPCV7 induced robust and functional immune responses to all 7 serotypes 1 month after Doses 3 and 4 in both cPCV7 groups, suggesting that coadministration with PCV13 and other childhood vaccines may have been a feasible strategy for these serotypes in the event that PCV20 had shown major immunologic interferences. This is important because implementation of the schedule used in the cPCV7 Separated group would have been extraordinarily challenging given that no other vaccines are recommended at the proposed ages of cPCV7 administration.20 The slightly higher immune responses to the cPCV7 serotypes after Dose 3 in the cPCV7 Separated group compared with the cPCV7 Coadministered group may be partially explained by the slightly older age (approximately 1 month older), which is associated with improved immune responses due to a more mature immune system,21 at cPCV7 administration in the cPCV7 Separated group rather than by major interferences in the cPCV7 Coadministered group. cPCV7 also induced responses after a single dose (Supplemental Dose) in participants approximately 13 months of age after a 4-dose series of PCV13.
Similar to results observed for the cPCV7 serotypes, immune responses to PCV13 serotypes were robust across all three groups, further supporting potential use of a complementary PCV coadministered with an existing multivalent PCV and other childhood vaccines. Although immune responses to PCV13 after Dose 3 were robust and similar between groups, they were slightly higher (IgG GMCs, 1.39−8.77 µg/mL) in the cPCV7 Separated group compared with the cPCV7 Coadministered (1.10−5.93 µg/mL) and PCV13 Control (1.04−6.39 µg/mL) groups. The mechanisms underlying these higher immune responses are not known but theoretically may involve carrier priming enhancement from interval doses of diphtheria-containing vaccine (cPCV7 and Pediarix). This phenomenon has been postulated to explain responses observed with CRM197-, diphtheria toxoid‒, or tetanus toxoid‒containing polysaccharide conjugate vaccines, whereby immune responses were theoretically primed by prevaccination with vaccines containing antigens similar to the carrier protein.22 The higher responses were not present, or were less marked, after Dose 4.
Notable study strengths include the randomized design and active comparator control group. Limitations include that the study was not powered for formal quantitative comparisons between cPCV7 groups and results were descriptive only. The numbers of participants in each group after Dose 3 and particularly after Dose 4 were relatively small; while these data are informative, conclusions regarding immune responses are limited. Participants were also exclusively from the United States, potentially limiting generalizability to other countries. Additionally, coadministered cPCV7 was not given in the same limb as PCV13, and thus potential interference resulting from competition of the 2 vaccines within individual lymph nodes17 was not assessed.
Overall, a complementary vaccine platform has certain advantages, especially if future single-expanded-valency PCV formulations show immunologic interferences rendering suboptimal immune responses. Addition of a complementary vaccine to a schedule that includes an existing PCV or in a setting where there is herd protection could allow for expansion of serotype coverage without the need to redevelop the existing vaccine. Disadvantages of this platform include the need for additional doses and clinic visits, which may lead to feasibility issues and/or missed doses.23 The difficulties with enrollment and high discontinuation rates in this study suggest that this may be a challenging approach in infants; of note, however, in this particular study, this challenge was compounded by the COVID-19 pandemic.
Conclusions
The safety and tolerability of cPCV7 were similar when given separately or coadministered with PCV13, and the safety profile was similar to that of PCV13. cPCV7 elicited IgG and functional antibody responses to the 7 vaccine serotypes as a 4-dose series in infants and as a single supplemental dose in toddlers 13 months of age. Coadministration of cPCV7 with PCV13 did not elicit immunologic interference in the responses to serotypes contained in either vaccine. Separate administration of cPCV7 elicited slightly higher immune responses to both vaccines after 3 infant doses compared with coadministration; however, the clinical significance of the increased responses is unknown. The results of this study support the concept of using a complementary vaccine to expand pneumococcal serotype coverage.
Acknowledgments
Editorial/medical writing support was provided by Allison R. Gillies, PhD, and Judith Kandel, PhD (ICON, Blue Bell, PA), and was funded by Pfizer Inc
Funding Statement
This study was sponsored by Pfizer Inc. Pfizer was involved in the study design; data collection, analysis, and interpretation; writing the report; and the final decision to submit the article for publication.
Disclosure statement
Michael W. Simon and Regine Bataille have no conflicts to declare. Nicole S. Caldwell, Bradford Gessner, Luis Jodar, Erik Lamberth, Yahong Peng, Daniel A. Scott, Lanyu Lei, Peter C. Giardina, William C. Gruber, Kathrin U. Jansen, Allison Thompson, and Wendy Watson are employees of Pfizer and may hold stock or stock options.
Data availability statement
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions and exceptions, Pfizer may also provide access to the related individual de-identified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.
References
- 1.Said MA, Johnson HL, Nonyane BA, Deloria-Knoll M, O’Brien KL, AGEDD Adult Pneumococcal Burden Study Team . Estimating the burden of pneumococcal pneumonia among adults: a systematic review and meta-analysis of diagnostic techniques. PLoS One. 2013;8(4):e60273. doi: 10.1371/journal.pone.0060273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang SS, Johnson KM, Ray GT, Wroe P, Lieu TA, Moore MR, Zell ER, Linder JA, Grijalva CG, Metlay JP, et al. Healthcare utilization and cost of pneumococcal disease in the United States. Vaccine. 2011;29(18):3398–10. doi: 10.1016/j.vaccine.2011.02.088. [DOI] [PubMed] [Google Scholar]
- 3.O’Brien KL, Wolfson LJ, Watt JP, Henkle E, Deloria-Knoll M, McCall N, Lee E, Mulholland K, Levine OS, Cherian T.. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet. 2009;374(9693):893–902. doi: 10.1016/S0140-6736(09)61204-6. [DOI] [PubMed] [Google Scholar]
- 4.Wahl B, O’Brien KL, Greenbaum A, Majumder A, Liu L, Chu Y, Luksic I, Nair H, McAllister DA, Campbell H, et al. Burden of Streptococcus pneumoniae and Haemophilus influenzae type b disease in children in the era of conjugate vaccines: global, regional, and national estimates for 2000-15. Lancet Glob Health. 2018;6(7):e744–57. doi: 10.1016/S2214-109X(18)30247-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prevnar 13 (pneumococcal 13-valent conjugate vaccine [diphtheria CRM197 protein]). Full Prescribing Information. Philadelphia, PA: Pfizer Inc; 2017. [Google Scholar]
- 6.Hausdorff WP, Hanage WP. Interim results of an ecological experiment - conjugate vaccination against the pneumococcus and serotype replacement. Hum Vaccin Immunother. 2016;12(2):358–74. doi: 10.1080/21645515.2015.1118593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Isturiz RE, Ramirez J, Self WH, Grijalva CG, Counselman FL, Volturo G, Ostrosky-Zeichner L, Peyrani P, Wunderink RG, Sherwin R, et al. Pneumococcal epidemiology among US adults hospitalized for community-acquired pneumonia. Vaccine. 2019;37(25):3352–61. doi: 10.1016/j.vaccine.2019.04.087. [DOI] [PubMed] [Google Scholar]
- 8.van der Linden M, Falkenhorst G, Perniciaro S, Imohl M. Effects of infant pneumococcal conjugate vaccination on serotype distribution in invasive pneumococcal disease among children and adults in Germany. PLoS One. 2015;10(7):e0131494. doi: 10.1371/journal.pone.0131494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balsells E, Guillot L, Nair H, Kyaw MH. Serotype distribution of Streptococcus pneumoniae causing invasive disease in children in the post-PCV era: a systematic review and meta-analysis. PLoS One. 2017;12(5):e0177113. doi: 10.1371/journal.pone.0177113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prevnar 20 (pneumococcal 20-valent conjugate vaccine). Full Prescribing Information. Philadelphia, PA: Pfizer Inc; 2023. [Google Scholar]
- 11.Wroe PC, Lee GM, Finkelstein JA, Pelton SI, Hanage WP, Lipsitch M, Stevenson AE, Rifas-Shiman SL, Kleinman K, Dutta-Linn MM, et al. Pneumococcal carriage and antibiotic resistance in young children before 13-valent conjugate vaccine. Pediatr Infect Dis J. 2012;31(3):249–54. doi: 10.1097/INF.0b013e31824214ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flasche S, Van Hoek AJ, Sheasby E, Waight PA, Andrews N, Sheppard CL, George R, Miller E. Effect of pneumococcal conjugate vaccination on serotype-specific carriage and invasive disease in England: a cross-sectional study. PLoS Med. 2011;8(4):e1001017. doi: 10.1371/journal.pmed.1001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harboe ZB, Thomsen RW, Riis A, Valentiner-Branth P, Christensen JJ, Lambertsen L, Krogfelt KA, Konradsen HB, Benfield TL. Pneumococcal serotypes and mortality following invasive pneumococcal disease: a population-based cohort study. PLoS Med. 2009;6(5):e1000081. doi: 10.1371/journal.pmed.1000081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lepoutre A, Varon E, Georges S, Gutmann L, Levy-Bruhl D, Microbiologists of Epibac, ORP Networks . Impact of infant pneumococcal vaccination on invasive pneumococcal diseases in France, 2001-2006. Euro Surveill. 2008;13(35):18962. doi: 10.2807/ese.13.35.18962-en. [DOI] [PubMed] [Google Scholar]
- 15.Senders S, Klein NP, Lamberth E, Thompson A, Drozd J, Trammel J, Peng Y, Giardina PC, Jansen KU, Gruber WC, et al. Safety and immunogenicity of a 20-valent pneumococcal conjugate vaccine in healthy infants in the United States. Pediatr Infect Dis J. 2021;40(10):944–51. doi: 10.1097/INF.0000000000003277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pfizer Inc . Data from Pfizer’s adult and pediatric clinical trial programs for 20-valent pneumococcal conjugate vaccine presented at IDWeek 2020. [accessed 2022 Mar 22]. https://www.pfizer.com/news/press-release/press-release-detail/data-pfizers-adult-and-pediatric-clinical-trial-programs-20.
- 17.Dagan R, Poolman J, Siegrist CA. Glycoconjugate vaccines and immune interference: a review. Vaccine. 2010;28(34):5513–23. doi: 10.1016/j.vaccine.2010.06.026. [DOI] [PubMed] [Google Scholar]
- 18.Tan CY, Immermann FW, Sebastian S, Pride MW, Pavliakova D, Belanger KA, Watson W, Scott DA, Sidhu M, Jansen KU, et al. Evaluation of a validated Luminex-based multiplex immunoassay for measuring immunoglobulin G antibodies in serum to pneumococcal capsular polysaccharides. mSphere. 2018;3(4):e00127–00118. doi: 10.1128/mSphere.00127-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.The Annie E. Casey Foundation . KIDS COUNT data center. Child population by race and age group in the United States. [accessed 2021 June 29]. https://datacenter.kidscount.org/data/tables/8446-child-population-by-race-and-age-group#detailed/1/any/false/1729,37,871,870,573,869,36,868,867,133/68,69,67,12,70,66,71,13|62,63,30/17077,17078.
- 20.Centers for Disease Control and Prevention . Recommended child and adolescent immunization schedule for ages 18 years or younger. [accessed 2021 Dec 21]. https://www.cdc.gov/vaccines/schedules/downloads/child/0-18yrs-child-combined-schedule.pdf.
- 21.Zimmermann P, Curtis N. Factors that influence the immune response to vaccination. Clin Microbiol Rev. 2019;32(2). doi: 10.1128/CMR.00084-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pobre K, Tashani M, Ridda I, Rashid H, Wong M, Booy R. Carrier priming or suppression: understanding carrier priming enhancement of anti-polysaccharide antibody response to conjugate vaccines. Vaccine. 2014;32(13):1423–30. doi: 10.1016/j.vaccine.2014.01.047. [DOI] [PubMed] [Google Scholar]
- 23.Ventola CL. Immunization in the United States: recommendations, barriers, and measures to improve compliance: part 1: childhood vaccinations. Pharmacy And Therapeutics. 2016;41(7):426–36. https://www.ncbi.nlm.nih.gov/pubmed/27408519. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions and exceptions, Pfizer may also provide access to the related individual de-identified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.


