Abstract
Background:
Food protein–induced enterocolitis syndrome (FPIES) is a rare, non–immunoglobulin E (IgE) mediated gastrointestinal food hypersensitivity. It is a clinical diagnosis commonly characterized by profuse vomiting 1 to 4 hours after ingestion of the triggering food(s).
Objective:
The objective was to increase awareness of FPIES and review the epidemiology, clinical presentation, pathogenesis, diagnosis, and management of FPIES. The lack of availability of a definite biomarker or diagnostic tool often leads to a delay in diagnosis.
Methods:
A literature search of salient articles that described case reports and case series of FPIES and their management were analyzed.
Results:
A case of FPIES with a literature review is presented with emphasis on clinical pearls and pitfalls. FPIES is a diagnosis of exclusion and the mainstay of treatment is avoidance of the trigger food(s) for at least 12–18 months from the last exposure.
Conclusion:
As FPIES is a non-IgE-mediated reaction, allergy testing via skin-prick test or blood tests to measure food IgE antibodies is not routinely recommended. Many children outgrow FPIES by 3–4 years of age. Supervised oral food challenge is recommended to assess acquisition of tolerance.
Keywords: Food protein induced enterocolitis syndrome, FPIES, Hypersensitivity reaction, IgE, Food allergies
Question
A 5-month-old boy presents with recurrent vomiting and watery diarrhea 2 hours after his second ingestion of cow’s milk-based formula. Results of a sepsis workup initiated at an emergency department visit during these episodes was negative. His pediatrician suspects food protein–induced enterocolitis syndrome (FPIES) and requests confirmatory testing. His parents inquired if he will be able to outgrow his “allergy” when he grows up. Which of the following statements is correct:
Skin-prick test to milk will determine his allergy status
He will have FPIES his whole life
He will need to have an epinephrine autoinjector prescription
He should undergo an oral food challenge in 12–18 months
He is at risk to have FPIES to other food
Food protein–induced enterocolitis syndrome (FPIES) is a non–immunoglobulin E (IgE) mediated gastrointestinal food hypersensitivity. It is characterized by profuse vomiting and lethargy that occurs 1 to 4 hours after ingestion of the triggering food. FPIES is predominantly a disease of the pediatric population, although it has been reported in adults.1 The prevalence is rising, with an incidence between 0.015 and 0.7% on population-based cohort studies from Israel, Spain, and Australia.2,3 It primarily affects the male population. However, female predilection is reported in adult FPIES.1 Despite being considered as a non–IgE-mediated entity, FPIES has been associated with atopic diseases in ∼30–60% of the population.4 The role of genetics in FPIES is unknown. There is no strong familial association in FPIES. Only 7% of infants with FPIES had siblings with a history of FPIES.3
FPIES presents a diagnostic challenge given its overlapping clinical presentation with multiple disease processes, such as gastroenteritis, sepsis, metabolic diseases, and IgE-mediated food allergies. This can lead to a misdiagnosis and/or a delay in diagnosis.5 Therefore, it is essential to consider a broad differential diagnosis and maintain a high clinical suspicion.
CLINICAL CHARACTERISTICS
The clinical presentation of FPIES is varied and nonspecific, ranging from mild symptoms to severe and life-threatening ones. FPIES may present in an acute or chronic form. The initial reaction typically occurs after the first or second exposure to the trigger food but has been reported to occur after several exposures. Acute FPIES is characterized by sudden profuse, repetitive vomiting that occurs 1 to 4 hours after ingestion of the triggering food. Patients often appear pale, lethargic, or limp. Diarrhea may occur 6 to 8 hours later. In severe conditions, it may progress to hypotension, acidemia, and shock. Hypothermia secondary to circulatory volume loss can also occur. These clinical scenarios can resemble sepsis, which warrants an extensive workup. Unlike IgE-mediated food allergy, skin or respiratory symptoms are absent in FPIES. These symptoms typically resolved within several hours, and patients are well until the triggering food is re-introduced.
Chronic FPIES is seen when the triggering food is regularly and/or repeatedly ingested. A common scenario involves young infants who are starting cow’s milk or soy formula. It usually presents as intermittent but progressive vomiting and diarrhea without a temporal relationship to the triggering food, which leads to hypoalbuminemia, poor weight gain, and failure to thrive. Given the broad differential diagnosis for failure to thrive, the diagnosis of chronic FPIES is often delayed.
Eliciting triggers such as cow’s milk, grains, soy, fruits, vegetables, legumes, seeds, eggs, meat, poultry, and seafood have been reported in FPIES. The most common food trigger in pediatrics FPIES is cow’s milk, grains, and soy, although this varies between regions and culture. In countries with early introduction of seafood, fish is also recognized as a more common trigger food. Recent studies have highlighted the emergence of egg, peanut, and tree nut as the cause of FPIES in infants, which might be linked to the current recommendation of early introduction of allergenic foods.6−8 The most common trigger foods in the adult population are grains and seafood.1 Similar to IgE-mediated allergy to fish and shellfish, seafood FPIES in pediatric patients seems to have a protracted course and often lasts to adulthood.6 Adult onset FPIES to seafood is also a long-lasting or permanent condition.
Most patients with FPIES reacted to a single food (65–80%, depending on the study population). However, 5% of patients in the U.S. case series reacted to as many as six foods.9 Patients with FPIES to multiple foods present at a younger age and were more likely to have FPIES to fruits and/or vegetables.3 Cross-reaction to various food types within the same food group (e.g., rice and other grains, soy, and legumes) have been noted.4 Children with egg and cow's milk FPIES have been shown to be able to tolerate baked or fermented forms of the trigger foods.10 The same study also showed that children with fish FPIES are able to tolerate different fish species, which suggests specific sensitivity.10
PATHOPHYSIOLOGY
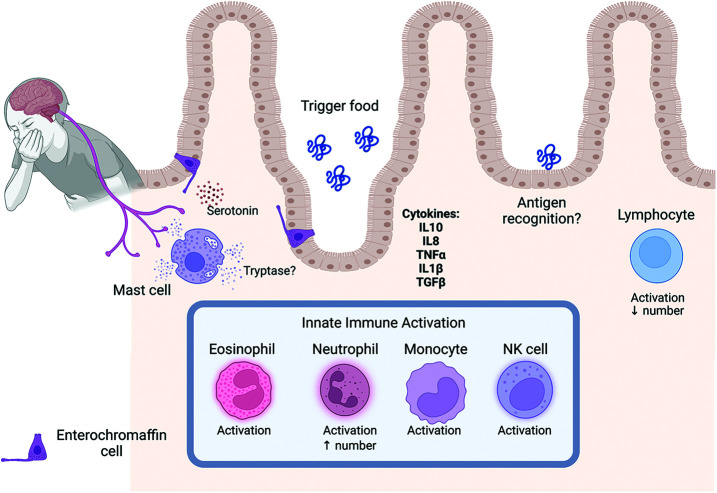
The pathophysiology of FPIES remains unclear. FPIES is considered a non–IgE-mediated disorder. Recent studies by Goswami et al,11 and Mehr et al.12 suggest that profound activation of the innate immune system in the absence of an abnormal, antigen-specific T-cell response as the possible mechanism., They noted an increased activation marker expression of eosinophils, neutrophils, monocytes, and natural killer cells as well as increased IL10 (interleukin 10) 10 and IL8 (interleukin 8) in patients with positive OFC (oral food challenge) (Fig. 1). A current hypothesis suggests that ingestion of the trigger food leads to local inflammation that resembles a reaction to foodborne illness. Although the innate immune response might be the primary driver of the FPIES reaction, the cell-mediated response likely also contributes. A deficit in Transforming Growth Factor (TGF)-β1 response and an overzealous Tumor Necrosis Factor (TNF)-α response may be important factors in FPIES.13 Significant elevation in IL-17 released by T helper (Th)17 cells have been observed in patients with FPIES and links T-cell activation with neutrophil activation and de-margination.14 Jejunal biopsy specimens from patients with FPIES revealed increased numbers of IgM- and IgA-containing plasma cells, along with an increase of specific IgA and a decrease in specific IgG4 antibodies.13 However, humoral or antibody-mediated response seems to play little to no role in the pathophysiology of FPIES because no specific immunoglobulins to food antigens have been observed.11,12,14 Specific IgE antibody responses are generally not detected in FPIES.
Figure 1.
The pathophysiology of FPIES.
A role for antigen-specific CD4+CD25+ Treg cells (regulatory T cells) has been suggested in natural tolerance development because children who outgrow FPIES to cow’s milk protein have a higher frequency of cow’s milk protein specific Treg cells.13 It is postulated that the vomiting that occurs with FPIES is mediated by serotonin released by the enterochromaffin cells, which activate the vagus nerve and trigger the vomiting reflex (Fig. 1). Treatment of patients with the serotonin 5-HT3 (5-HT3 = 5-hydroxytryptamine 3) receptor antagonist, ondansetron, helps suppress vomiting triggered by FPIES challenge.15
DIAGNOSIS
FPIES is primarily a clinical diagnosis and is often missed or delayed due to its nonspecific symptoms. There is no single laboratory test or clinical biomarker for the definitive diagnosis of FPIES. Atopy patch tests have been evaluated in two small studies as a method to identify food sensitivities in patients with FPIES.16,17 The results from these studies were conflicting. Therefore, no recommendation with regard to the utility of atopy patch tests can be made.16,17
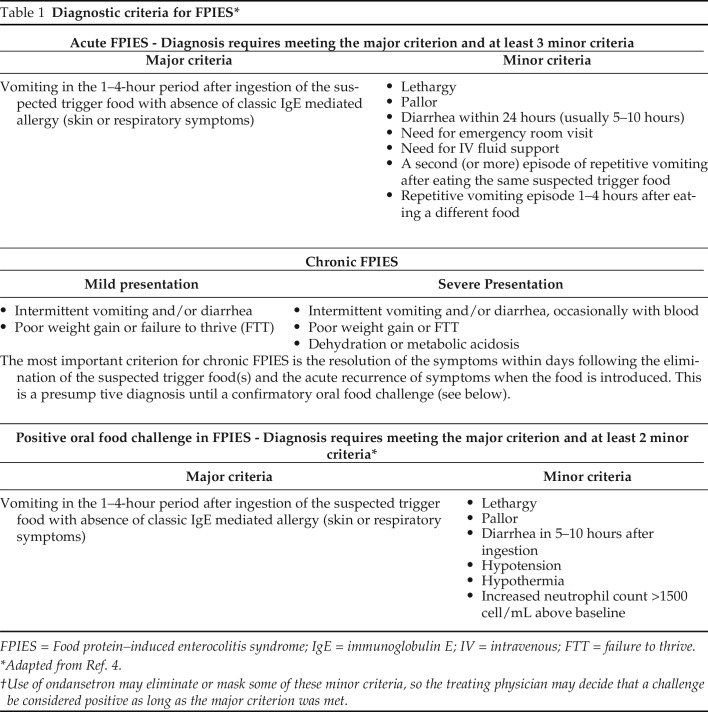
A detailed dietary history is essential to determine the causative agent. The classic constellation of symptoms begins with the trigger-food ingestion and symptomatic resolution on trigger-food elimination. The diagnostic criteria for acute and chronic FPIES based on the 2017 consensus guidelines are summarized in Table 1.4 Although oral food challenges are considered the criterion standard, they are not always necessary to confirm the diagnosis. Instead, they can be performed in cases of a diagnostic dilemma or to determine the acquisition of tolerance before reintroduction of the trigger food. Oral food challenges can also be used to search for safe alternatives to the trigger food.10
Table 1.
Diagnostic criteria for FPIES*
FPIES = Food protein–induced enterocolitis syndrome; IgE = immunoglobulin E; IV = intravenous; FTT = failure to thrive.
Adapted from Ref. 4.
Use of ondansetron may eliminate or mask some of these minor criteria, so the treating physician may decide that a challenge be considered positive as long as the major criterion was met.
MANAGEMENT
Timely identification of the trigger-food and dietary elimination are the cornerstones to long-term management of FPIES. Supervised oral food challenges can be used when the causative agent cannot be identified by history alone. Treatment of accidental exposures should be initiated to reduce the morbidity associated with FPIES. Mild symptoms can be treated with oral rehydration and monitored for 4 to 6 hours after resolution of symptoms.4 Patients with moderate-to-severe cases require watchful observation and aggressive management5 because they can be life-threatening. Although the utility of steroids is not clearly understood in FPIES reactions, a single dose of intravenous methylprednisolone (1 mg/kg, maximum 60–80 mg) may be given for presumed inflammation in severe reactions.4 Intravenous, intramuscular, or oral ondansetron (0.15 mg/kg, maximum dose 16 mg/dose) administration has been used for symptomatic relief in children > 6 months of age.15 Treatment of coexisting issues, such as dyselectrolytemia, correction of the acid-base status, and methemoglobinemia should be initiated. Worsening clinical status, persistent or severe hypotension, hypovolemic shock, and respiratory distress warrant immediate escalation of care. At the time of discharge, formal parental education, anticipatory guidance, and an emergency action plan should be provided to the family.2
Most infants with FPIES safely tolerate the offending food allergen in breast milk. There are no formal recommendations for a maternal dietary elimination of a trigger-food protein in breast-feeding mothers, and breast-feeding should be encouraged in all infants who are asymptomatic.2 Early initiation of formula is recommended for infants who are unable to breast-feed and hypoallergenic formula should be used in those with cow’s milk FPIES.5 If the child's symptoms do not resolve or if there is evidence of failure to thrive, switching to an amino acid–based formula is advised.4
Trigger protein allergens must be completely removed from the child’s diet until a formal supervised oral food challenge is performed. It is equally essential to provide optimal variety in the diet to prevent nutritional deficiency and food aversion. The timing of the reintroduction of food is variable. Results of several studies suggest reintroduction of food 12–18 months after the last reaction.18 However, the severity of a previous reaction, type of food trigger, and comfort level of the caregiver should be taken into consideration.
NATURAL HISTORY
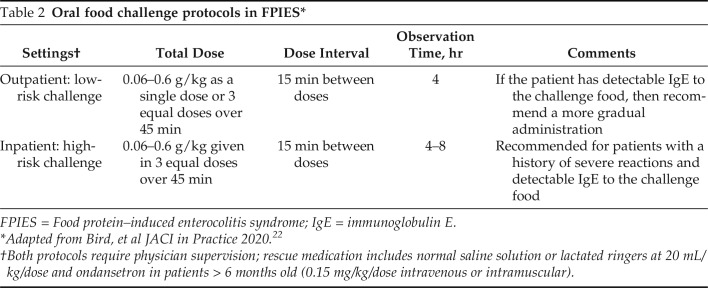
Clinical findings generally suggest that most infants with FPIES become tolerant to the offending food over time. The age of resolutions seems to depend on the trigger food and country of study. A prospective study from South Korea reported tolerance rates of 63.6% to cow’s milk and 91.7% to soy at 10 months.19 In contrast, Caubet et al.20 found that, on a retrospective chart review of 160 patients with FPIES, the median age of tolerance for cow’s milk was 5.1 years and for soy was 6.7 years. In general, tolerance was achieved at a significantly younger age in patients with rice and cow’s milk FPIES compared with those with fish and egg FPIES.21 The current recommendation in the United States is to consider oral food challenge after 12–18 months since the last reaction to determine the acquisition of tolerance Table 2.
Table 2.
Oral food challenge protocols in FPIES*
FPIES = Food protein–induced enterocolitis syndrome; IgE = immunoglobulin E.
Adapted from Bird, et al JACI in Practice 2020.22
Both protocols require physician supervision; rescue medication includes normal saline solution or lactated ringers at 20 mL/kg/dose and ondansetron in patients > 6 months old (0.15 mg/kg/dose intravenous or intramuscular).
A subset of patients with FPIES can develop a positive skin-prick test result and/or detectable serum levels of food-specific IgE to their FPIES trigger, termed atypical FPIES. Approximately 25% of those with atypical FPIES can transition from an FPIES phenotype to an IgE-mediated allergic reaction. It is unclear what role IgE may play in the remaining patients with evidence of sensitization. Caubet et al.20 noted that those with cow’s milk FPIES and detectable cow’s milk–specific IgE often do not outgrow their FPIES. Further studies are needed to elucidate the relationship between FPIES and the development of IgE-mediated food allergy.
CONCLUSION
A 5-month-old boy presents with recurrent vomiting and watery diarrhea 2 hours after his second ingestion of cow’s milk-based formula. His pediatrician suspects FPIES and requests confirmatory testing. Which of the following statements is correct?
Skin-prick test to milk will determine his allergy status
He will have FPIES his whole life
He will need to have an epinephrine autoinjector prescription
He should undergo an oral food challenge in 12–18 months
He is at risk to have FPIES to other food
Answer: D. Most patients will outgrow FPIES within 12–18 months.
Pearls
FPIES is a clinical diagnosis, and often a diagnosis of exclusion.
Since FPIES is a non-IgE mediated disease process, routine allergy testing via skin prick test or a blood test to measure food IgE antibodies is not commonly performed.
Some patients exhibit coexisting IgE-mediated food allergies and testing can be considered on a case-to-case basis.
The majority of infants with FPIES tolerate the offending allergen through breastfeeding without the need for a maternal dietary elimination. Routine avoidance of foods in an asymptomatic child is not recommended in breastfeeding mothers.
Most children outgrow FPIES by 3-4 years of age. Supervised oral food challenge to assess acquisition of tolerance is recommended 12-18 months since the last reaction.
Strict exclusion of trigger protein, adherence to individualized diet plan, and proper medical attention can help the child grow and thrive despite this condition.
Pitfalls
FPIES is commonly mistaken for other conditions, such as viral gastroenteritis, leading to a delay in diagnosis.
There are no definite screening or confirmatory tests for the disease entity.
There are no definitive therapeutic modalities to expedite symptomatic resolution.
Challenges to FPIES management includes the development of oral aversion or apathy to certain texture, nutritional deficiencies, and potential for failure to thrive.
The incidence and prevalence of FPIES is rising which nec essitates more effective educational tools for health care providers and families.
A multidisciplinary approach involving a dietician and/or occupatio nal therapist can be favorable in developing feeding skills.
Footnotes
The authors have no conflicts of interest to declare pertaining to this article
No external funding sources reported
REFERENCES
- 1. Gonzalez-Delgado P, Muriel J, Jimenez T, et al. Food protein-induced enterocolitis syndrome in adulthood: clinical characteristics, rognosis, and risk factors. J Allergy Clin Immunol Pract. 2022; 10:2397–2403. [DOI] [PubMed] [Google Scholar]
- 2. Katz Y, Goldberg MR, Rajuan N, et al. The prevalence and natural course of food protein-induced enterocolitis syndrome to cow's milk: a large-scale, prospective population-based study. J Allergy Clin Immunol. 2011; 127:647–653.e1-e3. [DOI] [PubMed] [Google Scholar]
- 3. Mehr S, Frith K, Barnes EH, et al. Food protein-induced enterocolitis syndrome in Australia: a population-based study, 2012-2014. J Allergy Clin Immunol. 2017; 140:1323–1330. [DOI] [PubMed] [Google Scholar]
- 4. Nowak-Węgrzyn A, Chehade M, Groetch ME, et al. International consensus guidelines for the diagnosis and management of food protein-induced enterocolitis syndrome: executive summary-Workgroup Report of the Adverse Reactions to Foods Committee, American Academy of Allergy, Asthma & Immunology. J Allergy Clin Immunol. 2017; 139:1111–1126.e4. [DOI] [PubMed] [Google Scholar]
- 5. Michelet M, Schluckebier D, Petit L-M, et al. Food protein-induced enterocolitis syndrome - a review of the literature with focus on clinical management. J Asthma Allergy. 2017; 10:197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ocak M, Akarsu A, Sahiner UM, et al. Phenotypes and natural history of food protein-induced enterocolitis syndrome in the east Mediterranean region. Allergy Asthma Proc. 2020; 41:420–427. [DOI] [PubMed] [Google Scholar]
- 7. Lopes JP, Cox AL, Baker MG, et al. Peanut-induced food protein-induced enterocolitis syndrome (FPIES) in infants with early peanut introduction. J Allergy Clin Immunol Pract. 2021; 9:2117–2119. [DOI] [PubMed] [Google Scholar]
- 8. Akashi M, Hayashi D, Kajita N, et al. Recent dramatic increase in patients with food protein-induced enterocolitis syndrome (FPIES) provoked by hen's egg in Japan. J Allergy Clin Immunol Pract. 2022; 10:1110–1112.e2. [DOI] [PubMed] [Google Scholar]
- 9. Ruffner MA, Ruymann K, Barni S, et al. Food protein-induced enterocolitis syndrome: insights from review of a large referral population. J Allergy Clin Immunol Pract. 2013; 1:343–349. [DOI] [PubMed] [Google Scholar]
- 10. Ocak M, Akarsu A, Sahiner UM, et al. Food protein-induced enterocolitis syndrome: current practices in oral food challenge. Allergy Asthma Proc. 2021; 42:343–349. [DOI] [PubMed] [Google Scholar]
- 11. Goswami R, Blazquez AB, Kosoy R, et al. Systemic innate immune activation in food protein-induced enterocolitis syndrome. J Allergy Clin Immunol. 2017; 139:1885–1896.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mehr S, Lee E, Hsu P, et al. Innate immune activation occurs in acute food protein-induced enterocolitis syndrome reactions. J Allergy Clin Immunol. 2019; 144:600–602.e2. [DOI] [PubMed] [Google Scholar]
- 13. Berin MC. Immunopathophysiology of food protein-induced enterocolitis syndrome. J Allergy Clin Immunol. 2015; 135:1108–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Berin MC, Lozano-Ojalvo D, Agashe C, et al. Acute FPIES reactions are associated with an IL-17 inflammatory signature. J Allergy Clin Immunol. 2021; 148:895–901.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Holbrook T, Keet CA, Frischmeyer-Guerrerio PA, et al. Use of ondansetron for food protein-induced enterocolitis syndrome. J Allergy Clin Immunol. 2013; 132:1219–1220. [DOI] [PubMed] [Google Scholar]
- 16. Fogg MI, Brown-Whitehorn TA, Pawlowski NA, et al. Atopy patch test for the diagnosis of food protein-induced enterocolitis syndrome. Pediatr Allergy Immunol. 2006; 17:351–355. [DOI] [PubMed] [Google Scholar]
- 17. Jarvinen KM, Caubet J-C, Sickles L, et al. Poor utility of atopy patch test in predicting tolerance development in food protein-induced enterocolitis syndrome. Ann Allergy Asthma Immunol. 2012; 109:221–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jarvinen KM, Nowak-Wegrzyn A. Food protein-induced enterocolitis syndrome (FPIES): current management strategies and review of the literature. J Allergy Clin Immunol Pract. 2013; 1:317–322. [DOI] [PubMed] [Google Scholar]
- 19. Hwang J-B, Sohn SM, Kim AS. Prospective follow-up oral food challenge in food protein-induced enterocolitis syndrome. Arch Dis Child. 2009; 94:425–428. [DOI] [PubMed] [Google Scholar]
- 20. Caubet JC, Ford LS, Sickles L, et al. Clinical features and resolution of food protein-induced enterocolitis syndrome: 10-year experience. J Allergy Clin Immunol. 2014; 134:382–389. [DOI] [PubMed] [Google Scholar]
- 21. Lee E, Campbell DE, Barnes EH, et al. Resolution of acute food protein-induced enterocolitis syndrome in children. J Allergy Clin Immunol Pract. 2017; 5:486–488.e1. [DOI] [PubMed] [Google Scholar]
- 22. Bird JA, Leonard S, Groetch M, et al. Conducting an Oral Food Challenge: An Update to the 2009 Adverse Reactions to Foods Committee Work Group Report. J Allergy Clin Immunol Pract. 2020 Jan; 8:75–90.e17. [DOI] [PubMed] [Google Scholar]