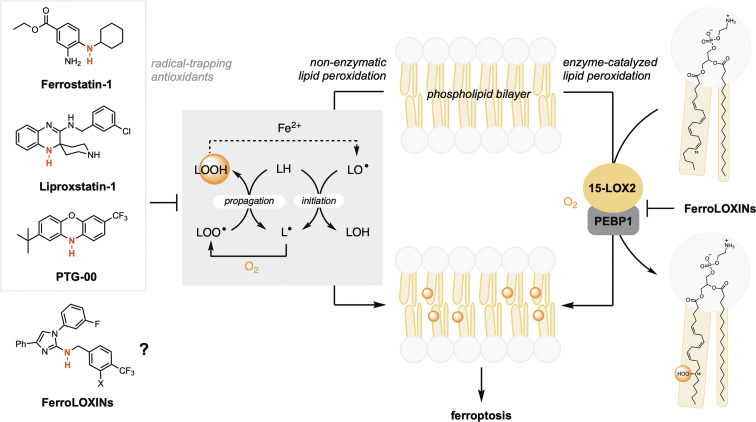
Ferroptosis (1) is an iron-dependent form of cell death associated with the accumulation of lipid peroxidation products, which eventually results in membrane permeabilization and osmolysis. It is believed to link the long-observed increase in lipid peroxidation products to the tissue damage observed in many acute and chronic pathological contexts, including neurodegeneration, ischemic stroke, and traumatic brain injury. As such, ferroptosis has emerged as an important target for therapeutic development. Distinct from apoptosis and other forms of cell death, ferroptosis is triggered when the capacity of the cell to detoxify (phospho)lipid hydroperoxides is overcome (2, 3). Lipid hydroperoxides arise due to autoxidation, a chain reaction propagated by reactions of lipid-derived free radicals (4), as well as by enzyme-catalyzed processes (Fig. 1, Middle) (5). Hydroperoxides derived from polyunsaturated lipids esterified to phosphatidyl-ethanolamine (PE) are believed to be particularly important in ferroptosis execution (6). In PNAS, Dar et al. (7) report the first attempt to develop inhibitors designed to target an enzyme-protein complex that oxidizes PE lipids.
Fig. 1.
Unrestrained peroxidation of membrane lipids drives ferroptotic cell death. Inhibition of both nonenzymatic lipid peroxidation (autoxidation) and enzyme-catalyzed lipid peroxidation may suppress ferroptosis. Newly reported FerroLOXins have been designed to inhibit oxidation of phosphatidylethanolamine lipids by a 15-LOX2/PEBP1 complex. Since FerroLOXins are aromatic amines, such as the canonical RTA inhibitors ferrostatin-1, liproxstatin-1, and PTG-00, they may also prevent nonenzymatic lipid peroxidation.
Of the limited number of enzymes that directly catalyze lipid peroxidation, the 15-lipoxygenases (15-LOX1 and 15-LOX2) have been most often implicated in ferroptosis execution (8–11). Lipoxygenases (LOXs) are nonheme iron-containing enzymes that catalyze oxygenation of free arachidonic acid and some related polyunsaturated fatty acids in a largely regioselective and stereoselective manner (5). Although LOXs have been found to be dispensable for ferroptosis both in vitro (12) and in vivo (2, 13, 14), cells which express substantial levels may be sensitized to ferroptosis induction (12). To date, only LOX inhibitors with off-target radical-trapping antioxidant (RTA) activity have been reported to suppress ferroptosis (6, 15). RTAs suppress ferroptosis by trapping the lipid-derived free radicals that propagate nonenzymatic lipid peroxidation (16, 17) and comprise the overwhelming majority of ferroptosis inhibitors yet identified, including the archetype ferroptosis inhibitors ferrostatin-1 (1) and liproxstatin-1 (2), and the more potent phenoxazine PTG-00 (Fig. 1, Left) (18). LOX inhibitors lacking RTA activity have been shown only to desensitize cells to ferroptosis (12).
Based on the previous observation that 15-lipoxygenase-2 (15-LOX2), when complexed with PE binding protein-1 (PEBP1) (19), can catalyze the oxygenation of polyunsaturated lipids esterified to PE (Fig. 1, Right), Dar et al. sought to develop inhibitors with specificity for the complex. Starting from scaffolds of known 15-LOX2 inhibitors, and the results of computations suggesting that association with PEBP1 increases the volume of the catalytic site of 15-LOX2, they added substituents to increase affinity for the complex over 15-LOX2 alone. Cell-based assays (in human bronchial epithelial cells) revealed that derivatives which featured an N–H bond adjacent to the imidazole substituent were good ferroptosis suppressors. Increasing the lipophilicity of the compounds, particularly via inclusion of fluoroalkyl substituents, afforded the most potent inhibitors (EC50~ 100 nM).
In PNAS, Dar et al. report the first attempt to develop inhibitors designed to target an enzyme-protein complex that oxidizes PE lipids.
Consistent with the premise, these inhibitors suppressed oxygenation of arachidonic acid esterified to PE by the combination of 15-LOX2 and PEBP1 in a liposome model (by ~20 to 25%), but had no measurable effect on 15-LOX2 alone. Computational studies suggest that the enhanced inhibition of the 15-LOX2/PEBP1 complex over 15-LOX2 may result from blocking O2 access to the active site, dislodging the oxidizable sidechain of the phospholipid from the active site, and favoring binding of the nonoxidizable sidechain. Further studies demonstrated that two of the compounds, coined FerroLOXIN-1 and FerroLOXIN-2, were unable to rescue cells from necroptosis, pyroptosis, or apoptosis—suggesting specificity for ferroptosis, similarly to the archetype inhibitors ferrostatin-1 and liproxstatin-1. Similar experiments in four additional cell lines (intestinal epithelial cells Caco2 and FHs 74 Int) and cancer cells (HT-1080 and A375) suggest that ferroptosis suppression by FerroLOXINs is general.
Since ferroptosis suppression by the FerroLOXINs was observed in cells that have been reported to not express 15-LOX2 (e.g., HT-1080s) (20), it is possible that they possess off-target RTA activity in addition to their ability to target the 15-LOX2/PEBP1 complex. It is perhaps not a coincidence that the FerroLOXINs possess aromatic amine moieties—the key chemical functionality that features in ferrostatin-1, liproxstatin-1, and PTG-00. Future work should address this point, by carrying out studies to assess the ability of FerroLOXINs to prevent nonenzymatic lipid peroxidation in phospholipid bilayers, as well as to validate 15-LOX2/PEBP1 as the target in cells which do express 15-LOX2 by demonstrating the loss of antiferroptotic activity when the genes encoding 15-LOX2 and/or PEBP1 are knocked down/out.
Regardless of the precise mechanism of ferroptosis suppression, the FerroLOXINs were found to be active in vivo as demonstrated by the suppression of radiation damage in mice. Radiation damage has long been associated with augmented levels of lipid peroxidation, suggesting a role of ferroptosis in the associated tissue damage. Indeed, the most common ferroptosis inducers are known to synergize with ionizing radiation, and genetic and biochemical hallmarks of ferroptosis are observed in radiation-treated cells (21, 22). Moreover, RTAs such as ferrostatin-1 are good radioprotectants (21, 22). Similarly, FerroLOXIN treatment (25 mg/kg 24 h after irradiation) significantly prolonged the survival of both female and male mice. This was associated with the protection of the intestinal epithelium, a particularly radiosensitive tissue. The levels of PE-derived hydroperoxides were correspondingly reduced, linking the protective effect with suppression of lipid peroxidation. In contrast, markers of apoptotic and necroptotic cell death were unaffected by FerroLOXIN treatment—again implicating ferroptosis specifically in radiation damage.
Of the many individual proteins and metabolic pathways which have been found to modulate a cell’s sensitivity to ferroptosis (23), LOXs are unique because they directly contribute to the cellular pool of (phospho)lipid hydroperoxides. Accordingly, they can seed nonenzymatic lipid peroxidation when their reaction products undergo one-electron reduction by labile iron in the same manner as nonenzymatically produced (phospho)lipid hydroperoxides. Since ferroptosis occurs once lipid peroxidation can no longer be managed by the cell, preventing the accumulation of hydroperoxides of any origin—enzymatic or not—should contribute to ferroptosis suppression. Thus, LOXs and/or their complexes with scaffolding proteins such as PEBP1 present a unique point of intervention upstream of, and/or complementary to, RTAs. Moreover, they may afford a means to achieve tissue specificity in the fight against ferroptosis, given that LOXs are not expressed at meaningful levels in all cells and tissues (24). It will be exciting to see whether further research establishes the FerroLOXINs as first-in-class inhibitors that can provide such a proof of principle and whether they can be developed into therapeutics for ferroptosis-related disease.
Acknowledgments
Author contributions
D.A.P. wrote the paper.
Competing interests
D.A.P. is a scientific cofounder of Prothegen Inc. The company seeks to develop therapeutics targeting ferroptosis-related disease. D.A.P. owns stock in Prothegen Inc. and has patent filings related to ferroptosis inhibitors.
Footnotes
See companion article, “Discovering selective antiferroptotic inhibitors of the 15LOX/PEBP1 complex noninterfering with biosynthesis of lipid mediators,” 10.1073/pnas.2218896120.
References
- 1.Dixon S. J., et al. , Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 149, 1060–1072 (2012), 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angeli J. P. F., et al. , Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat. Cell Biol. 16, 1180–1191 (2014), 10.1038/ncb3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seiler A., et al. , Glutathione peroxidase 4 senses and translates Oxidative stress into 12/15-lipoxygenase dependent- and AIF-mediated cell death. Cell Metab. 8, 237–248 (2008), 10.1016/j.cmet.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 4.Yin H., Xu L., Porter N. A., Free radical lipid peroxidation: Mechanisms and analysis. Chem. Rev. 111, 5944–5972 (2011), 10.1021/cr200084z. [DOI] [PubMed] [Google Scholar]
- 5.Kuhn H., Banthiya S., Leyen K. V., Mammalian lipoxygenases and their biological relevance. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 1851, 308–330 (2015), 10.1016/j.bbalip.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kagan V. E., et al. , Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat. Chem. Biol. 13, 81–90 (2017), 10.1038/nchembio.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dar H. H., et al. , Discovering selective antiferroptotic inhibitors of the 15LOX/PEBOP1 complex noninterfering with biosynthesis of lipid mediators. Proc. Natl. Acad. Sci. U.S.A. 120, e2218896120 (2023), 10.1073/pnas.2218896120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stockwell B. R., et al. , Ferroptosis: A regulated cell death nexus linking metabolism, redox biology, and disease. Cell 171, 273–285 (2017), 10.1016/j.cell.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conrad M., Pratt D. A., The chemical basis of ferroptosis. Nat. Chem. Biol. 15, 1137–1147 (2019), 10.1038/s41589-019-0408-1. [DOI] [PubMed] [Google Scholar]
- 10.Jiang X., Stockwell B. R., Conrad M., Ferroptosis: Mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Bio. 22, 266–282 (2021), 10.1038/s41580-020-00324-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang D., Chen X., Kang R., Kroemer G., Ferroptosis: Molecular mechanisms and health implications. Cell Res. 31, 107–125 (2021), 10.1038/s41422-020-00441-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shah R., Shchepinov M. S., Pratt D. A., Resolving the role of lipoxygenases in the initiation and execution of ferroptosis. ACS Cent. Sci. 4, 387–396 (2018), 10.1021/acscentsci.7b00589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brütsch S. H., et al. , Expression of inactive glutathione peroxidase 4 leads to embryonic lethality, and inactivation of the Alox15 gene does not rescue such knock-in mice. Antioxid. Redox Signal. 22, 281–293 (2015), 10.1089/ars.2014.5967. [DOI] [PubMed] [Google Scholar]
- 14.Matsushita M., et al. , T cell lipid peroxidation induces ferroptosis and prevents immunity to infection. J. Exp. Med. 212, 555–568 (2015), 10.1084/jem.20140857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang W. S., et al. , Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc. Natl. Acad. Sci. U.S.A. 113, E4966–E4975 (2016), 10.1073/pnas.1603244113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ingold K. U., Pratt D. A., Advances in radical-trapping antioxidant chemistry in the 21st century: A kinetics and mechanisms perspective. Chem. Rev. 114, 9022–9046 (2014), 10.1021/cr500226n. [DOI] [PubMed] [Google Scholar]
- 17.Zilka O., et al. , The radical-trapping antioxidant activity of ferrostatin-1 and liproxstatin-1 suggests that lipid peroxidation (autoxidation) drives ferroptotic cell death. Sci. ACS Cent. 3, 232–243 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farmer L. A., et al. , Intrinsic and extrinsic limitations to the design and optimization of inhibitors of lipid peroxidation and associated cell death. J. Am. Chem. Soc. 144, 14706–14721 (2014), 10.1021/jacs.2c05252. [DOI] [PubMed] [Google Scholar]
- 19.Wenzel S. E., et al. , PEBP1 wardens ferroptosis by enabling lipoxygenase generation of lipid death signals. Cell 171, 628–641.e26 (2017), 10.1016/j.cell.2017.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dixon S. J., et al. , Pharmacological inhibition of cystine–glutamate exchange induces endoplasmic reticulum stress and ferroptosis. eLife 3, e02523 (2014), 10.7554/elife.02523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ye L. F., et al. , Radiation-induced lipid peroxidation triggers ferroptosis and synergizes with ferroptosis inducers. ACS Chem. Biol. 15, 469–484 (2020), 10.1021/acschembio.9b00939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lei G., et al. , The role of ferroptosis in ionizing radiation-induced cell death and tumor suppression. Cell Res. 30, 146–162 (2020), 10.1038/s41422-019-0263-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dixon S. J., Pratt D. A., Ferroptosis: A flexible constellation of related biochemical mechanisms. Mol. Cell 83, 1030–1042 (2023), 10.1016/j.molcel.2023.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karlsson M., et al. , A single–cell type transcriptomics map of human tissues. Sci. Adv. 7, eabh2169 (2021), 10.1126/sciadv.abh2169. [DOI] [PMC free article] [PubMed] [Google Scholar]