Significance
Combined with the cocatalytic effect of single-atom molybdenum, single-atom cobalt catalysts achieved long-term and efficient degradation of organic pollutants and retained their catalytic ability even at pH = 1.0 or 13.0. The transfer of electrons from organic pollutants to cobalt sites achieved by single-atom molybdenum reduced the dissolution toxicity of metal ions, significantly expanded the range of pollutants that could be degraded by single-atom cobalt catalyst–PMS systems, and solved the electron loss of catalysts in heterogeneous Fenton-like reactions.
Keywords: environment remediation, molybdenum cocatalysis, single-atom catalyst, electron transfer
Abstract
Single-atom catalysts (SACs) are a promising area in environmental catalysis. We report on a bimetallic Co–Mo SAC that shows excellent performance in activating peroxymonosulfate (PMS) for sustainable degradation of organic pollutants with high ionization potential (IP > 8.5 eV). Density Functional Theory (DFT) calculations and experimental tests demonstrate that the Mo sites in Mo–Co SACs play a critical role in conducting electrons from organic pollutants to Co sites, leading to a 19.4-fold increase in the degradation rate of phenol compared to the CoCl2–PMS group. The bimetallic SACs exhibit excellent catalytic performance even under extreme conditions and show long-term activation in 10-d experiments, efficiently degrading 600 mg/L of phenol. Moreover, the catalyst has negligible toxicity toward MDA-MB-231, Hela, and MCF-7 cells, making it an environmentally friendly option for sustainable water treatment. Our findings have important implications for the design of efficient SACs for environmental remediation and other applications in biology and medicine.
The treatment of organic wastewater has become an increasingly critical issue in the long-term development of society (1, 2). The depletion of fossil fuels, environmental pollution, shortages of water, and other resources are prompting efforts to treat wastewater sustainably (3, 4). The reaction between Fe2+ and H2O2 proposed by Fenton in 1894 can produce strong oxidizing free radicals which have a broad application prospect in wastewater treatment (5). In recent years, a variety of catalysts for Fenton and Fenton-like reactions were presented to overcome inherent defects of traditional Fenton reactions. But the formation of iron cement, intolerance of inorganic salts in solution, narrow pH applicable range, and nonrecovery still bother researchers for now (6–10). In addition, organic molecules in wastewater are complex, and some with high ionization potential (IP) are hard to degrade through oxidation. This is because it takes a lot of energy to remove an electron from their outer layer, making it difficult to start the process of breaking them down.
Single-atom catalysts (SACs) with excellent chemical and physical properties are emerging as a new area in the catalysis community, and it has become the most active new frontier in the CO reduction, methane oxidation, hydroformylation of propylene, water-gas shift conversion, and many other fields beyond dispute (11–14). In the field of Fenton and Fenton-like environmental chemistry, SACs can indeed improve the activation rate of active oxides such as H2O2, peroxymonosulfate (PMS), and so on (15–20). However, the ≡Co(III) produced after the activation reaction needs to sostenuto consume PMS for its own reduction with slow reaction rate (21–23). The reduction and oxidation rate of Co sites in the PMS system is close to 30 times under certain conditions (Eqs. 1 and 2) (23). Furthermore, this process indirectly causes the continuous loss of electrons on the Co sites, which hinders the utilization of PMS and reactive oxygen species (ROSs) in the system. Therefore, the reaction between PMS and single-atom Co catalysts is a REDOX reaction instead of the catalytic process. This reaction leads to the short-term property of single-atom cobalt catalysts and blocks the persistent degradation of organic pollutants. In addition, transition metal SACs such as single-atom iron and cobalt are susceptible to extreme alkaline and acidic conditions. Recent studies indicated that the activity of transition metal SACs decreased in strongly alkaline or acidic solutions (20, 24–27), and the resistance of transition metal SACs in the complex environment is alarming. Therefore, it is necessary to develop a technology that can support the adaptable and long-term degradation of pollutants by single-atom cobalt catalyst at present.
| [1] |
| [2] |
This study reported on the development and testing of a single-atom catalyst, SAMCC0.5, which contained both Mo and Co sites. The results indicated that this catalyst was robust and versatile in its ability to degrade organic pollutants, with high resistance to interference from extreme pH levels and other ionic species. The results also suggested that the Mo sites acted as a bridge to facilitate electron transfer from the organic pollutants to the Co sites, a process supported by both theoretical calculations and degradation experiments using different model contaminants with varying IPs. The long-term stability and recyclability of SAMCC0.5 were also demonstrated through a 10-d, 30-round degradation experiment, where ROSs such as singlet oxygen (1O2) and high-valent cobalt were identified. Overall, these findings contributed to the advancement of sustainable and efficient catalytic technologies for environmental remediation.
Results
Synthesis and Characterization of SAMCC0.5.
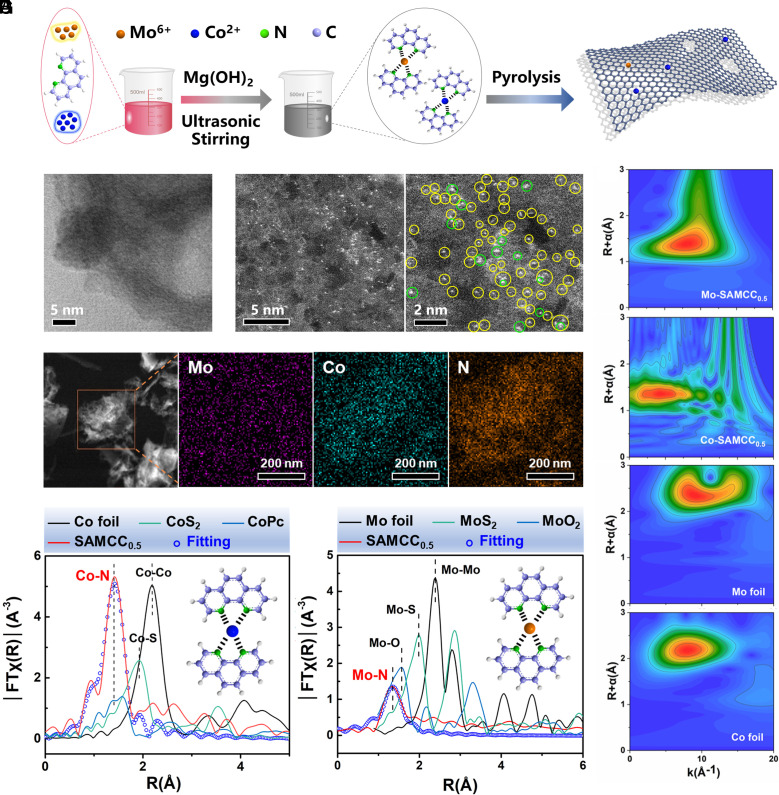
With a simple synthesis method (Fig. 1A), Co and Mo atoms coordinated with 1,10-phenanthroline in mixed solution, and then, the metal atoms were fixed together with the substrate after calcination. The proportion of Co and Mo elements in the catalyst was found to be crucial and was studied through SI Appendix, Figs. S1 and S2. Therefore, the ratio of the Co to Mo element in the raw material was the first to be studied in SI Appendix, Figs. S1 and S2. In the end, the best catalyst in this series was identified as SAMCC0.5. SAC only containing Co element was called Co–N–C.
Fig. 1.
(A) Synthesis process of SAMCC0.5 and schematic diagram of the structure. (B) HRTEM image of SAMCC0.5. (C) Aberration-corrected HAADF-STEM image of SAMCC0.5: Yellow circles indicate single Co atoms, and green circles indicate single Mo atoms. The atomic number contrast indicates that the larger the atomic number, the brighter the image. Thus, atomic Mo will be brighter. (D) Elemental mapping for SAMCC0.5. (E) EXAFS in R-space and corresponding fitting curves for Co foil, CoS2, CoPc, and SAMCC0.5. (F) EXAFS in R-space and corresponding fitting curves for Mo foil, MoS2, MoO2, and SAMCC0.5. (G) WT-EXAFS for the Mo element of SAMCC0.5, Co element of SAMCC0.5, Mo foil, and Co foil.
Scanning electron microscope (SI Appendix, Fig. S3) and high-resolution transmission electron microscopy (HRTEM) images (Fig. 1B and SI Appendix, Fig. S4) showed that this catalyst was a porous particle with a rough surface and there was no aggregation of metal particles on the evenly distributed edge flakes. According to SI Appendix, Fig. S5, nitrogen adsorption and desorption isotherms of SAMCC0.5 and Co–N–C showed that their specific surface areas were 372.3731 m2/g and 306.2080 m2/g, respectively. Through infrared spectroscopy in SI Appendix, Fig. S6, the main chemical bonds of SAMCC0.5 and Co–N–C were C–N and C=C bonds, and there were some hydroxyl groups on the catalyst surface. The existence of C–N and C=C bonds indicated that the conjugate skeleton still existed in the SAMCC0.5 and Co–N–C. The high-angle annular dark-field (HAADF) STEM images in Fig. 1C revealed that the SAMCC0.5 sample was abundant in atomic Co and Mo sites, and most of the metal atoms were in the form of single atoms or several adjacent atoms. Mo atoms appeared slightly brighter than Co atoms in the high-angle annular dark-field image due to their larger atomic number. The ratio of Mo to Co atoms was estimated to be approximately 1:4. The X-ray diffraction (XRD) pattern, as shown in SI Appendix, Fig. S7, confirmed the absence of detectable amounts of crystalline Co or Mo phases, indicating that SAMCC0.5 was substantially free of metallic Co/Mo or cobalt/molybdenum oxides. The energy dispersive X-ray spectroscopy mapping images in Fig. 1D showed a homogeneous distribution of Mo (represented by purple), Co (represented by indigo), and N (represented by orange) elements, demonstrating a uniform distribution of Mo and Co atoms throughout the SAMCC0.5 particles. The results from the inductively coupled plasma atomic emission spectrometry (ICP-AES), as presented in SI Appendix, Table S1, showed that the Co and Mo content in the catalyst were approximately 0.74% and 0.19%, respectively. As a comparison, the content of the Co element in Co–N–C was 1.00%.
The chemical state and local environment of Mo and Co were further investigated by the X-ray adsorption near edge structure (XANES) and extended X-ray absorption fine structure (EXAFS) spectra. According to SI Appendix, Fig. S8, the Co and Mo K-edge X-ray absorption near-edge structure (XANES) and valence analysis suggested the valence state of Co in SAMCC0.5 including Co2+ and Co3+ states while the valence state of Mo was Mo6+, combining with k-space and fitted data of Co and Mo elements in SI Appendix, Figs. S9 and S10. As is shown in Fig. 1E, the k3-weighted Fourier-transformed extended X-ray absorption fine structure spectra (FT-EXAFS) showed that the Co element in SAMCC0.5 was mainly coordinated with nitrogen (N) atoms, with a bond length of 1.42 Å corresponding to the Co–N bond in CoPc. Meanwhile, no obvious peaks assigned to the Co–Co bond at 2.18 Å were observed, thus implying the absence of Co clusters or crystalline particles. Moreover, for the FT-EXAFS curves of the Mo element in Fig. 1F, the main peaks for SAMCC0.5 were located at 1.34 Å differing from the Mo–Mo bond located at 2.38 Å and were consistent with the location of the fitting curve for Mo–N bond. To assign the above peaks, the wavelet transform (WT) EXAFS in Fig. 1G was carried out for the SAMCC0.5 sample as well as the standards. Referring to the WT-EXAFS feature of CoPc in SI Appendix, Fig. S11, the peak at 1.42 Å on SAMCC0.5 could be assigned to the Co–N bond. Compared to the feature peak of Mo foil (2.38 Å, 8.66 Å−1) in WT-EXAFS, the presence of the peak of the Mo element in SAMCC (1.38 Å, 7.78 Å−1) excluded the existence of the Mo–Mo bond. Therefore, Mo atoms and Co atoms in SAMCC0.5 were mainly coordinated with N atom in SAMCC0.5.
Catalytic Effect Test.
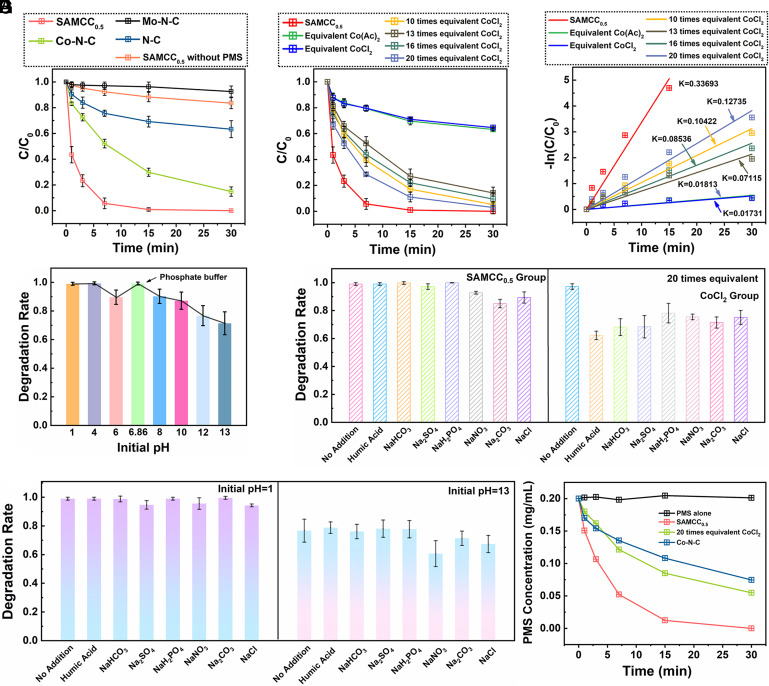
The catalytic activity of SAMCC0.5 for Fenton-like reactions was evaluated using phenol as a model pollutant. The results in Fig. 2A showed that the degradation rate of phenol reached 78% in 3 min with the addition of PMS. On the other hand, the degradation rate of phenol in the presence of Co–N–C, which did not contain Mo, only reached 28%. Additionally, Mo–N–C and N–C, which lacked Co or other metal elements, were unable to effectively activate PMS. According to the Zeta potential of catalysts under different pH in SI Appendix, Fig. S12, negative charge existed on the surface of catalysts and the addition of phenol did not change the chemical environment which mean that the adsorption of phenol hardly existed. At the same time, the degradation of phenol in the SAMCC0.5 system decreased sharply with the absence of PMS, which also indicated that SAMCC0.5 could not adsorb phenol.
Fig. 2.
(A) Degradation rate of phenol when SAMCC0.5, Co–N–C, Mo–N–C and N–C were used as catalysts. (B) Degradation rate of phenol when 300 mg L−1 SAMCC0.5 and different equivalents of CoCl2 were used as catalysts. (C) Apparent kinetic constants of (B). (D) SAMCC0.5 catalytic degradation rate of phenol under different pH environments. (E) SAMCC0.5 and different equivalents of CoCl2 catalytic degradation rate of phenol with interference of 0.2 M salts and humic acid. (F) SAMCC0.5 catalytic degradation rate of phenol with interference of 0.2 M salts and humic acid with pH = 1 and 13. (G) Decomposition rate of PMS with the catalytic effect of SAMCC0.5, Co–N–C and 20 times equivalent CoCl2. Dosage: PMS: 200 mg L−1, reaction solution: 100 mL, catalyst: 300 mg L−1, reaction time: 30 min.
The use of Co2+ as a classical catalyst for PMS activation in Fenton-like reactions is widely established. However, few heterogeneous catalysts can match the efficiency of PMS activation of Co2+, let alone surpass it. To further demonstrated the effectiveness of phenol degradation by the SAMCC0.5–PMS system, experiments were conducted using Co(Ac)2 and CoCl2 as comparison catalysts. The results in Fig. 2 B and C showed that phenol was not efficiently degraded when using Co(Ac)2 and CoCl2 equivalent to the Co element in SAMCC0.5. The apparent kinetic constant of the SAMCC0.5–PMS system was 19.4 times higher than that of the CoCl2–PMS system. Even when the concentration of CoCl2 was increased 20 times, the degradation rate of phenol was still not as efficient as the SAMCC0.5–PMS system. The use of Co3O4 as a catalyst in a Fenton-like reaction also showed limited effectiveness in oxidizing phenol, as shown in SI Appendix, Fig. S13. These results further demonstrated the unique advantage of the single-atom catalyst in addressing environmental pollution.
The SAMCC0.5–PMS system was a modified version of the traditional Fenton reaction and has been used to treat phenol solutions under different pH conditions and buffer systems. It was found in Fig. 2D that the pH of the reaction solution had little effect on the catalytic activity of SAMCC0.5, and the reaction rate did not decrease when phosphate buffer was used as the reaction solution (SI Appendix, Fig. S14). When pH was set to 12 or 13, the degradation rate of phenol decreased slightly, and the pH after reaction was 11.68 and 12.70, respectively (Fig. 2D). According to the pH of reaction solution after reaction, the whole process of reaction was under an alkaline environment, and the decline of the degradation rate of phenol here was probably due to the ineffective self-decomposition of PMS under alkaline environment (28, 29). To sum up, Fenton-like reaction dominated by the SAMCC0.5–PMS system has greatly escaped the disadvantage of a narrow pH range of application.
Inspired by phosphate buffer, Na2CO3, NaHCO3, humic acid, and other salts were used as interferences in the reaction system, and further activity tests were carried out. It can be seen from Fig. 2E that most of the salts and humic acid could not cause the attenuation of the activity of the SAMCC0.5–PMS system, and the degradation rate of phenol in the SAMCC0.5–PMS system interestingly became faster when humic acid was added as is shown in SI Appendix, Fig. S15. In the traditional Fenton reaction, ·OH and SO4·−, which are the main ROSs in the traditional Fenton system, easily interact with a variety of anions to generate other free radicals with weaker oxidizing potential (30, 31). In Fig. 2E, the degradation rate of 20 times equivalent CoCl2–PMS system declined when salts and humic acid were added. Among these, humic acid, Na2SO4, and NaHCO3 disturbed the degradation indeed. For single-atom Co catalysts, Co sites were the electron-rich region in the catalyst and cannot strongly attract various anions (18, 32). While Co2+ as a transition metal ion has many vacant orbitals, which is easy to coordinate with anions and results in the inactivation of Co2+. In addition, it is suggested that the nonradical ROSs rather than free radicals may dominate in the SAMCC0.5–PMS system, which could hardly be consumed by the anions. In order to further verify the stability and anti-interference ability of SAMCC0.5 in complex actual water treatment scenarios, the degradation ability of the SAMCC0.5–PMS system was tested under extreme pH conditions in Fig. 2F. Surprisingly, the degradation effect of the SAMCC0.5–PMS system was not affected by the addition of various salts, no matter at pH = 1 or 13. After the addition of various salts, the degradation rate of phenol was all close to 100% at pH = 1. And the degradation rate of phenol at pH = 13 was not very different from that before the addition of inorganic salts, although the degradation rate of phenol could not reach 100%. Therefore, the SAMCC0.5–PMS system maintains good resistance to interference even under extreme pH conditions. In order to further investigate the performance of the SAMCC0.5–PMS system in complex environments, phosphate buffer solutions with different anions were used as reaction solutions. In SI Appendix, Fig. S16, it could be clearly seen that even in the complex environment of buffer solution, the SAMCC0.5–PMS system was still not affected by various anionic interferences. The character that had great anti-interference capability under wide pH environment made this system promising in the future application of actual waste treatment. Moreover, in order to further verify the ability of the catalyst to degrade pollutants in practical applications, activity tests were carried out with river water from different locations as the reaction solution. The results, shown in SI Appendix, Fig. S17, demonstrated that the catalyst was highly effective even when tested with river water from the Huangpu River in Shanghai, the Changjiang River in Nanjing, the Bailang River in Shandong, the Zhujiang River in Guangzhou, and running water from Shanghai. The catalyst was able to effectively degrade phenol in all these water samples, indicating its strong potential for practical use.
Therefore, the results of the degradation of phenol demonstrated that the SAMCC0.5–PMS system outperformed traditional Fenton and Fenton-like reaction systems. The superior performance could be attributed to the higher activation rate of PMS in the SAMCC0.5 system, as indicated by the Iodine reduction reaction. This was further supported by the results presented in Fig. 2G and SI Appendix, Fig. S18, which showed a significantly higher PMS activation rate in the SAMCC0.5 system compared to the other two catalysts.
Possible Degradation Pathways.
The degradation products of phenol in the SAMCC0.5–PMS system were analyzed using gas chromatography–mass spectrometry (GC–MS), as illustrated in SI Appendix, Fig. S19. Three of the four resonance products of phenol were easy to be oxidized, and the products were catechol and hydroquinone (m/z = 110) (33, 34). Both catechol and hydroquinone were found to be more easily oxidized than phenol due to their lower ionization potential. Further oxidation of catechol and hydroquinone led to the formation of o-quinone and p-quinone (m/z = 108), followed by the oxidation of these compounds into C6H6O4 (m/z = 142), C6H4O6 (m/z = 172) and C4H4O4 (m/z = 116). Ultimately, these organic compounds were oxidized to C2H2O4 (m/z = 142), which was easily converted into CO2 and H2O.
Catalytic Mechanism of the SAMCC0.5–PMS System.
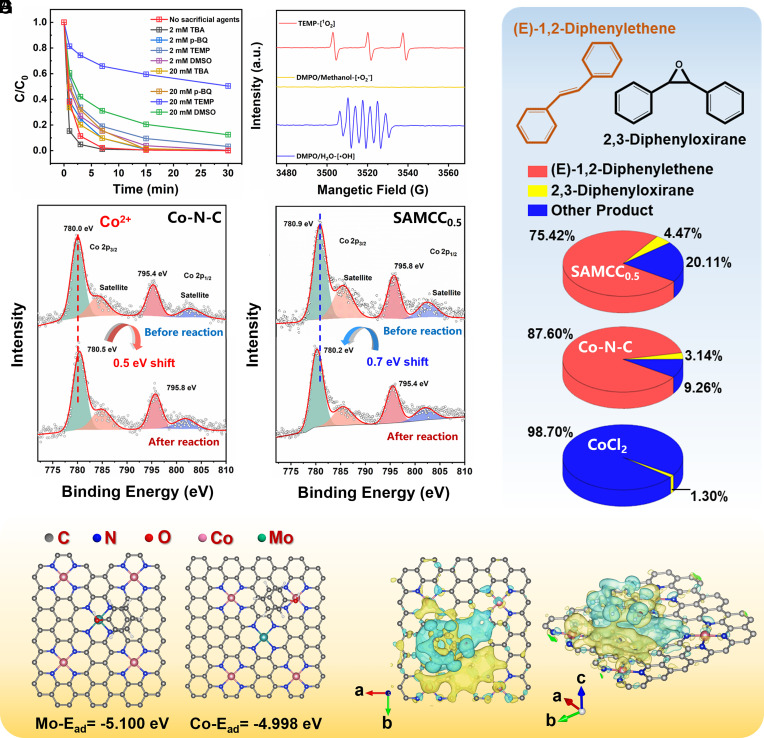
In the study of the Co2+–PMS system, researchers have long believed that the system is dominated by the presence of ·OH and ·SO4−. In order to evaluate the role of ROSs in the SAMCC0.5–PMS system, four sacrificial agents were employed in Fig. 3A. Tert-butanol was utilized as a limiting agent for ·OH and ·SO4−, while p-benzoquinone was employed to restrict the presence of ·O2−. However, neither of these agents showed significant inhibitory effects when used at concentrations of 2 mM or 20 mM in the SAMCC0.5–PMS system. The use of 2,2,6,6-tetramethylpiperidine (TEMP) and dimethyl sulfoxide (DMSO) as sacrificial agents for 1O2 and high-valent cobalt in the SAMCC0.5–PMS system was found to have a significant inhibitory effect on the reaction. Both 20 mM TEMP and DMSO were shown to significantly decrease the rate of degradation of the system. The results of this experiment were consistent with the results of the electron paramagnetic resonance (EPR) test in Fig. 3B. When TEMP was utilized as the capture agent in the experiment, a distinct triplet peak corresponding to 1O2 was observed. On the other hand, the use of 2,2-dimethyl-3,4-dihydro-2H-pyrrole 1-oxide (DMPO) as the capture agent resulted in a sevenfold peak in the EPR test, which was identified as the deep oxidation product of DMPO, (5,5-dimethyl-2-oxo-pyrroline-1-oxyl, DMPOX). It is important to note that the presence of DMPOX does not provide specific information about the types of ROSs present in the system, as various oxides can lead to the conversion of DMPO into DMPOX (35, 36). Here, ·OH was detected through the fluorescence method. According to SI Appendix, Fig. S20, there was a weak peak at 410 nm, which meant that the content of ·OH in the SAMCC0.5–PMS system was small. Combining with the results of sacrificial agent experiments and EPR test, the active oxygen species dominated in the SAMCC0.5–PMS system was still high-valent cobalt and 1O2.
Fig. 3.
(A) Sacrificial agent experiments. Dosage: catalyst: 300 mg L−1PMS: 200 mg L−1, aqueous solution: 100 mL, reaction time: 30 min. (B) EPR tests for the SAMCC0.5–PMS system. (C) Proportion of product in (E)-1,2-Diphenylethene probe experiment with different system. (D and E) XPS results of the Co element in the Co–N–C and SAMCC0.5 before and after reaction. (F) Adsorption energy of phenol on the Co and Mo sites. (G) Differential charge of the system after the adsorption of phenol on the Mo site.
In order to investigate the presence of high-valent cobalt, (E)-1,2-diphenylethene was utilized as a probe molecule in degradation experiments (Fig. 3C and SI Appendix, Table S2). High-valent cobalt, which is characterized by its ability to carry out an oxygen atom transfer reaction, can oxidize (E)-1,2-diphenylethene to produce 2,3-diphenyloxirane. Although other ROSs can also react with (E)-1,2-diphenylethene, the products produced by these reactions are complex and can be differentiated from 2,3-diphenyloxirane. The probe experiment with (E)-1,2-diphenylethene showed the presence of 2,3-diphenyloxirane in both the SAMCC0.5–PMS and Co–N–C–PMS systems. Although the proportions of 2,3-Diphenyloxirane in the products of these two systems were only 4.47% and 3.14% respectively, the low conversion rate of (E)-1,2-diphenylethene suggested the presence of a certain amount of high-valent cobalt. For the CoCl2–PMS system, 2,3-Diphenyloxirane occupied a very small proportion in the product, while most of the reaction products were the outcome of (E)-1,2-Diphenylethene reacting with free radicals. Therefore, high-valent cobalt was negligible compared to other ROSs in the CoCl2–PMS system.
In accordance with the above findings, it has been determined that 1O2 was generated as a result of the interaction between a single-atom cobalt site and PMS. Conversely, PMS has the capability to oxidize the cobalt site, leading to the formation of high-valent cobalt in its oxidation state. This accepted series of reactions serve as a basis for understanding the generation of ROSs (Eqs. 3–6) (37–39).
| [3] |
| [4] |
| [5] |
| [6] |
From Fig. 2A, it was clear that the Mo–N–C catalyst alone failed to effectively activate PMS. However, the introduction of Mo enhanced the interaction between Co sites and PMS, resulting in a substantial improvement in phenol degradation. This highlighted the crucial role that Mo played as a cocatalyst in the SAMCC0.5–PMS system. To further explore the role of Mo in the cocatalytic process, X-ray photoelectron spectroscopy (XPS) was conducted on samples of SAMCC0.5 and Co–N–C before and after the reaction. XPS analysis of the Co element in Co–N–C as shown in Fig. 3D revealed a shift in the peak position of the Co element toward higher energy levels by 0.5 eV as a result of oxidation after its reaction with PMS. Notably, the XPS results of the Co element in SAMCC0.5 in Fig. 3E showed a shift of 0.7 eV toward lower energy levels, indicating that the Co site in the SAMCC0.5–PMS system has received electrons. However, the Mo, C, and N elements in SAMCC0.5 did not change significantly before and after the reaction as is shown in SI Appendix, Fig. S21, and the Mo element always existed in the valence state of +6. It indicated that the source of electrons transferred to the Co site was not the Mo element. Meanwhile, PMS with strong oxidizing properties can hardly reduce the Co element, and there was no obvious trace of reaction between PMS and Mo element. As such, the source of electrons was likely to be the residual organic phenol molecule present in the system.
The hypothesis was verified using DFT calculations. The adsorption energy of the model organic pollutant (phenol) on Mo/Co sites in SAMCC0.5 was calculated in Fig. 3F, and the results showed that the adsorption energy of phenol to the Mo site was −5.100 eV, which was more favorable than that to the Co site (−4.998 eV). This suggested that phenol molecules are more likely to interact with Mo sites compared to Co sites. To further support the hypothesis, the differential charge of the system when phenol was adsorbed on the Mo site was calculated, as illustrated in Fig. 3G. The yellow regions indicated areas of increased electron density, while the cyan regions indicated areas of electron deficiency. The electron transfer path of the SAMCC0.5–PMS system could be described as follows: phenol adsorbed on the Mo site on the catalyst surface injected its electrons into Mo, which were then transferred to the Co sites through the conjugated framework, resulting in an increase in the electron cloud density of Co after the reaction. This suggested that the electrons used for activating on the Co site primarily come from the organic pollutant of phenol and that Co itself did not lose electrons, thereby enabling cyclic degradation of phenol. These calculation results were basically consistent with our hypothesis. The previous experiment revealed an intriguing phenomenon, where the addition of humic acid and tert-butanol did not diminish the degradation rate of phenol. Rather, humic acid and tert-butanol served as electron donors, facilitating the transfer of electrons to the Mo sites and increasing the likelihood of electrons flowing to the electron-deficient Co sites. This, in turn, allowed the Co sites to sustainably activate PMS by continuously reducing itself. According to the results obtained, it could be stated that the Mo sites played a crucial role as a bridge that facilitated electron transfer from organic pollutants to the Co sites throughout the entire conjugate structure. Additionally, the results of the electrochemical impedance spectroscopy performed on SAMCC0.5 and Co–N–C also provided support for this conclusion. As shown in SI Appendix, Fig. S22, SAMCC0.5 displayed a significantly lower electrochemical impedance compared to Co–N–C, indicating that electrons were able to flow more efficiently on SAMCC0.5 and reinforcing the role of the Mo site as an electron conduit. AgNO3 was added as a sacrificial agent to the SAMCC0.5–PMS system to demonstrated that electron transfer in the reaction system only existed on the surface of the catalyst. In SI Appendix, Fig. S23, both 2 mM and 20 mM AgNO3 could not inhibit the degradation reaction. This indicated that electron transfer hardly occurred in the reaction solution.
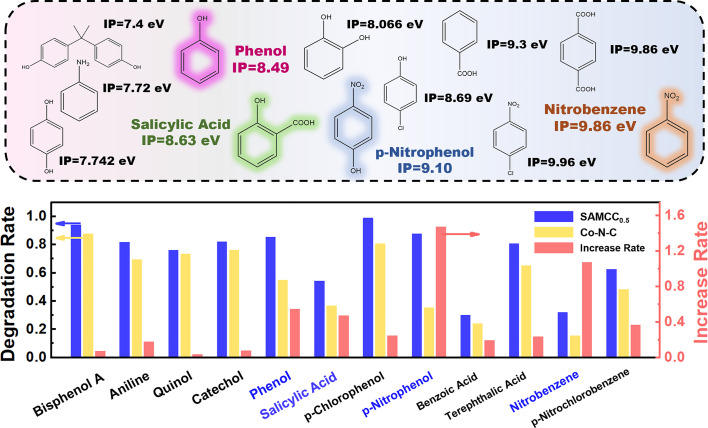
Different model organic pollutants differ in the ability of electron donation. To further prove the previous hypothesis, we further determined the range of organic pollutants that Mo sites can offer a cocatalytic effect in the degradation, with the 5-min degradation effect of various pollutants. The adsorption of various model pollutants by SAMCC0.5 and Co–N–C was first excluded in SI Appendix, Fig. S24. The adsorption capacity of various model pollutants was less than 20%, and the degradation rate part of subsequent experiments had excluded the adsorption part. Depending on the IP of different model pollutants in Fig. 4 (40–43), SAMCC0.5 had little difference in the degradation effect of pollutants with low IP compared with Co–N–C. Starting from phenol (IP = 8.49 eV), the cocatalytic effect of Mo sites on pollutants was significantly improved. Previous studies have indicated that high-valent cobalt systems exhibit improved degradation efficiency for pollutants with low IP due to their relatively weak oxidation potential (44, 45). As a result, for low IP pollutants, Co–N–C systems could effectively degrade these contaminants on their own, and the electron-donating effect of the Mo sites in this system was not pronounced. On the other hand, for pollutants with higher IP, high-valent cobalt systems were less effective in degradation. In this case, the interaction between the pollutant and the Mo site acted as an electron donor, leading to faster regeneration of ROSs in the system and increased resistance to the pollutant. Therefore, the cocatalytic influence of Mo sites in SAMCC0.5 was particularly pronounced during the oxidation of pollutants with high IP. In other words, the presence of Mo in SAMCC0.5 might broaden the scope of model pollutants that could be oxidized by the single-atom Co catalyst–PMS system, and this scope of pollutants was likely related to their IP. However, there were specific conditions that must be met for this cocatalytic process to occur, such as the adsorption of pollutant molecules on Mo sites. The electron donation effect of Mo sites on pollutants could be effectively applied to substances with an IP ranging from 8.5 to 9.8 eV or even higher. The adsorption of pollutant molecules on Mo sites is an important factor in facilitating the transfer of electrons. However, the strength of the cocatalytic effect of Mo sites is not solely determined by the IP value. Other factors, such as the spatial arrangement of the pollutant molecules and the electronegativity at the adsorption site, also play a significant role in this electron donation process.
Fig. 4.
Degradation rate and increase rate of different model pollutants in the comparison between the SAMCC0.5–PMS system and the Co–N–C–PMS system. Dosage: catalyst: 300 mg L−1PMS: 200 mg L−1, aqueous solution: 100 mL, reaction time: 5 min.
Biotoxicity Test of SAMCC0.5.
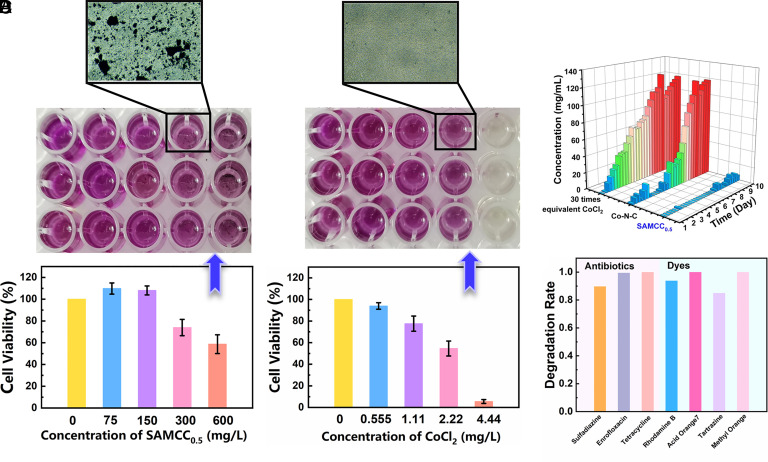
In evaluating the suitability of SAMCC0.5 as a catalyst in water treatment, its biological toxicity is of paramount importance. To assess this, the effect of SAMCC0.5 on human myeloid leukemia mononuclear cells (THP-1), human breast cancer cells (MCF-7 and MDA-MB-231), and Hela cells was evaluated by in vitro cytotoxicity test experiments. The duration of these experiments was set at 48 h, and the concentration of viable cells was determined using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay at a concentration of 0.5 mg/mL. The results were presented in Fig. 5 A and B and SI Appendix, Figs. S25 and S26. The biotoxicity of CoCl2 was found to be substantial in a dose-dependent manner. At 4.44 mg/L CoCl2, cell viability was found to be generally less than 5%. Notably, the addition of SAMCC0.5 resulted in a surprising outcome, with high survival rates observed in all cell lines. The survival rate of THP-1 cells remained close to 60% even when the cobalt concentration in SAMCC0.5 was elevated to match that of 4.44 mg/L CoCl2, at a dose of 600 mg/L (Fig. 5A). The results of our experiments demonstrated that SAMCC0.5 exhibited low biotoxicity toward MDA-MB-231, Hela, and McF-7 cells, in comparison to the commonly used CoCl2 (SI Appendix, Figs. S25 and S26). This made SAMCC0.5 an environmentally friendly option for water treatment. Furthermore, SAMCC0.5 exhibited higher PMS activation activity than previously reported cobalt catalysts, further highlighting its potential for sustainable use in environmental and biological contexts. The combination of low toxicity and high activity presented a compelling case for the utilization of SAMCC0.5 in these fields.
Fig. 5.
(A) Cell viability of THP-1 under different concentrations of SAMCC0.5. Cell culture time: 48 h. The concentration of viable cells with 0.5 mg/mL 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) as inspection agent and cells in the culture medium were observed under a microscope. (B) Cell viability of THP-1 under different concentrations of CoCl2. Cell culture time: 48 h. (C) Long-term degradation tests of totally 600 mg L−1 phenol with 300 mg L−1 SAMCC0.5, 300 mg L−1 Co–N–C, and 30 times equivalent CoCl2 as catalyst. Dosage: PMS: 200 mg L−1 every round, phenol: 20 mg L−1 every round, aqueous solution: 100 mL. (D) Degradation rate of different model pollutants in the SAMCC0.5–PMS system. Dosage: catalyst: 300 mg L−1PMS: 200 mg L−1, aqueous solution: 100 mL, reaction time: 30 min, model pollutants: 20 mg L−1.
Universality and Stability Testing.
The stability of the SAMCC0.5 and Co–N–C catalysts was rigorously evaluated through a series of experiments, including cycle tests, long-term degradation experiments, and relevant assessments. To assess the residual metal ions present in the reaction solution, ICP-AES analysis was employed. The results, as reported in SI Appendix, Table S3, showed that the residual Co and Mo ions in the solution after the reaction were only 0.39 mg/L and 0.20 mg/L, respectively, for SAMCC0.5. This was much lower than the equivalent CoCl2 experiment group, which had residual Co ions of 20.10 mg/L. Compared to SAMCC0.5, Co–N–C and Mo–N–C exhibited higher loss of metal ions (0.63 mg/L Co ions for Co–N–C and 2.5 mg/L Mo ions for Mo–N–C) after the reaction. In the cycle tests, SAMCC0.5 and Co–N–C were filtered out of the reaction solution after each cycle and washed thoroughly with deionized water. According to SI Appendix, Fig. S27, the SAMCC0.5 catalyst demonstrated consistent reaction rates during the first two cycles of the five-cycle experiment. Although there was a slight decrease in reaction activity in the latter three cycles, attributed to the impact of the washing process on the catalyst's performance, the SAMCC0.5 still exhibited good catalytic activity. Conversely, the cycle experiment for the Co–N–C catalyst in SI Appendix, Fig. S28, indicated a declining reactivity with each successive cycle, reflecting its inherently short-lived activity.
In comparison, the long-term experiment provided a clearer illustration of the catalyst’s stability in the degradation of organic pollutants within the reaction solution, offering a more comprehensive evaluation of its performance. As shown in Fig. 5C, the results of the 10-d long-term degradation experiments with 600 mg/L phenol revealed that the SAMCC0.5–PMS system demonstrated superior performance compared to the CoCl2–PMS and Co–N–C–PMS systems. The latter two systems exhibited a significant decline in reactivity after 3 and 13 rounds of testing, respectively, whereas the SAMCC0.5–PMS system retained high reactivity over 30 rounds of experiments. The slow reduction of Co3+ produced after activation was found to hinder the effective and rapid activation of PMS in the latter stages of the reaction, particularly in the CoCl2–PMS system. In the case of the Co–N–C–PMS system, a similar decline in reactivity was observed. The unique advantage of the SAMCC0.5–PMS system lied in the Mo element, which played a crucial role in the Co2+/Co3+ cycle. In the SAMCC0.5–PMS system, the reduction of Co sites was facilitated by the transfer of electrons from organic pollutants via the Mo sites and conjugated skeleton. The SAMCC0.5, with its unique feature, maintained high activity throughout the extended degradation process, which provided a significant advantage compared to catalysts lacking a cocatalytic system. Moreover, the SAMCC0.5–PMS system exhibited broad universality in its ability to degrade a wide range of pollutants. This was evidenced by the results of the degradation experiments with complex model pollutants, as shown in Fig. 5D. Within 30 min, the degradation rate of various antibiotics and dyes, including sulfadiazine, enrofloxacin, tetracycline, Rhodamine B, acid orange 7, tartrazine, and methyl orange, reached 90%. This demonstrated the system's ability to effectively oxidize organic compounds with complex structures. In order to further verify the performance of the SAMCC0.5–PMS system in pollutants degradation, actual industrial wastewater was used as a reaction raw material and reaction solution in SI Appendix, Table S4, with chemical oxygen demand (COD) as the index. For the sample from biochemical wastewater, the COD decreased by 127 mg/L. And for the actual wastewater from Beijing Construction Engineering Group Co., Ltd., the COD decreased by 95 mg/L after treatment.
Conclusions
In this article, we synthesized bimetallic site SACs that were well suited to environmental remediation and explored the interactions between metal sites and pollutants. The individual distribution of metal sites in SAMCC0.5 ensured both excellent stability and high reactivity. The addition of Mo sites significantly increased the catalytic performance of Co–N–C in the initial 10 min of the reaction, resulting in a 19.4-fold increase in the degradation rate of phenol compared to the CoCl2–PMS group. Furthermore, the degradation efficacy of SAMCC0.5 was not impacted by variations in pH or the presence of ions, even at extreme pH = 1 or 13. In the 10-d long-term experiments, SAMCC0.5 exhibited stable, long-term activation, as a result of the cocatalytic effect of Mo sites, leading to the complete degradation of 600 mg/L of phenol. And the cocatalytic effect of Mo sites was proved to be the guidance of the electron transportation from pollutants to Co sites. The electron transfer facilitated by Mo sites was particularly well suited for pollutants with IP ranging from 8.5 to 9.8 eV or higher. This cocatalytic effect of Mo sites represented a significant advancement in the study of cocatalysts, offering a unique perspective compared to previous work on the cocatalytic effect of the Mo element. Furthermore, this research provides valuable insights into the interaction between different metal sites in SACs, leading to increased metal utilization and reduced biotoxicity of the catalyst. With its environmentally friendly and low biotoxicity properties, SAMCC0.5 holds great potential for a range of applications in fields such as environmental remediation, biology, medicine, and catalysis.
Materials and Methods
Preparation of Catalysts.
First, 1,10-phenanthroline and Co(CH3COO)2 were dissolved in 20 mL ethanol, and (NH4)2MoO4 was dissolved in 5 mL deionized water. After mixing the above solution, the solution was treated with ultrasound for 30 min. In the second step, Mg(OH)2 was added to the mixed solution and treated with ultrasound for 30 min. Subsequently, the mixture was placed in a vacuum oven to dry after rotary evaporation. The dried sample was then ground and calcined in a tube furnace at 700 °C for 120 min. The product was stirred in 1M of HNO3 for 4 h to leach Mg(OH)2, MgO, and any possible metal or oxide. After washing with ultrapure water five times, the solid was dried at 60 °C overnight. Here, we name the resulting catalyst SAMCC, and the amount of relevant raw materials in catalyst synthesis was indicated in SI Appendix, Figs. S1 and S2. In the synthesis of Co–N–C, (NH4)2MoO4 was not used while other processing methods were consistent. In the synthesis of Mo–N–C, Co(CH3COO)2 was not used while other processing methods were consistent. In the synthesis of N–C, Co(CH3COO)2 was replaced by the same amount of acetic acid, and other metal salts were not added.
Catalytic Effect Test in Fenton-Like Reaction.
Typically, 30 mg catalyst was added into 100 mL phenol solution (20% wt. %). Then, NaOH and H2SO4 were added to adjust pH if needed, and various inorganic salts were also added at this time if needed. Twenty mg of PMS was added at the beginning of the experiment. After sampling at different time points, it was filtered with a filter head, and then, methanol was added to quench the reaction. The concentration of phenol was detected by HPLC.
Cell Culture and Cell Viability Assay.
The Hela and MDA-MB-231 cell lines were obtained from China Center for Type Culture Collection (CCTCC). These cell lines were cultured in Dulbecco’s modified Eagle’s medium (DMEM; KeyGEN Biotech) with 10% fetal bovine serum (FBS; ExCell Bio). The cells were maintained at 37 °C in a humidified incubator with 95% air and 5% CO2.
Cells (5 × 105 cells/well) were seeded in 96-well plates overnight and then treated with various concentrations of tested samples. The concentrations were 0, 75, 150, 300, and 600 mg, respectively, for SAMCC0.5. The concentrations were 0, 0.555, 1.11, 2.22, and 4.44 mg, respectively, for CoCl2. After 48 h, the original medium was removed, and 100 μL fresh medium containing 0.5 mg/mL 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was added to each well for 4 h at 37 °C. Then, the medium was replaced with 150 μL of DMSO to dissolve the formazan. Absorbance was measured at 570 and 630 nm using a microplate reader (Molecular Devices). All the experiments were performed in three independent replicates.
Statistical Analysis.
All data were gained directly from the source experiment and processed using Origin. All experiments were carried out in duplicate. Full details for DFT computations, control experiments, and materials characterizations were introduced in SI Appendix.
Supplementary Material
Appendix 01 (PDF)
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos. 22176060 and 41876189) and sponsored by the Program of Shanghai Academic/Technology Research Leader (23XD1421000). Project supported by Shanghai Municipal Science and Technology Major Project (Grant No. 2018SHZDZX03) and the Program of Introducing Talents of Discipline to Universities (B16017). Science and Technology Commission of Shanghai Municipality (20DZ2250400). The Fundamental Research Funds for the Central Universities (222201717003). We thank Research Center of Analysis and Test of East China University of Science and Technology for the help on the characterization.
Author contributions
M.X. designed research; Z.C. performed research; F.A., Y.Z., and M.X. contributed new reagents/analytic tools; Z.C., Z.L., W.L., and M.X. analyzed data; and Z.C. and M.X. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission.
Data, Materials, and Software Availability
All study data are included in the article and/or SI Appendix.
Supporting Information
References
- 1.Zhang M.-H., Dong H., Zhao L., Wang D.-X., Meng D., A review on Fenton process for organic wastewater treatment based on optimization perspective. Sci. Total Environ. 670, 110–121 (2019). [DOI] [PubMed] [Google Scholar]
- 2.Sumpter J. P., Johnson A. C., Lessons from endocrine disruption and their application to other issues concerning trace organics in the aquatic environment. Environ. Sci. Technol. 39, 4321–4332 (2005). [DOI] [PubMed] [Google Scholar]
- 3.Li W.-W., Yu H.-Q., He Z., Towards sustainable wastewater treatment by using microbial fuel cells-centered technologies. Energy Environ. Sci. 7, 911–924 (2014). [Google Scholar]
- 4.Liu W., et al. , Efficient hydrogen production from wastewater remediation by piezoelectricity coupling advanced oxidation processes. Proc. Natl. Acad. Sci. U.S.A. 120, e2218813120 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liang S., et al. , Fe2+/HClO reaction produces FeIVO2+: An enhanced advanced oxidation process. Environ. Sci. Technol. 54, 6406–6414 (2020). [DOI] [PubMed] [Google Scholar]
- 6.Goswami A., Jiang J.-Q., Petri M., Treatability of five micro-pollutants using modified Fenton reaction catalysed by zero-valent iron powder (Fe (0)). J. Environ. Chem. Eng. 9, 105393 (2021). [Google Scholar]
- 7.Lu S., Liu L., Demissie H., An G., Wang D., Design and application of metal-organic frameworks and derivatives as heterogeneous Fenton-like catalysts for organic wastewater treatment: A review. Environ. Int. 146, 106273 (2021). [DOI] [PubMed] [Google Scholar]
- 8.Du M., et al. , Sustainable activation of peroxymonosulfate by the Mo (IV) in MoS2 for the remediation of aromatic organic pollutants. Chin. Chem. Lett. 31, 2803–2808 (2020). [Google Scholar]
- 9.Dong C., et al. , Research progress of photocatalysis based on highly dispersed titanium in mesoporous SiO2. Chin. Chem. Lett. 30, 853–862 (2019). [Google Scholar]
- 10.Zhen Y., et al. , Facile preparation of α-MnO2 nanowires for assembling free-standing membrane with efficient Fenton-like catalytic activity. Chin. Chem. Lett. 34, 107664 (2023). [Google Scholar]
- 11.Wang A., Li J., Zhang T., Heterogeneous single-atom catalysis. Nat. Rev. Chem. 2, 65–81 (2018). [Google Scholar]
- 12.Zhang H., Liu G., Shi L., Ye J., Single-atom catalysts: Emerging multifunctional materials in heterogeneous catalysis. Adv. Energy Mater. 8, 1701343 (2018). [Google Scholar]
- 13.Ding S., Hülsey M. J., Perez-Ramirez J., Yan N., Transforming energy with single-atom catalysts. Joule 3, 2897–2929 (2019). [Google Scholar]
- 14.Beniya A., Higashi S., Towards dense single-atom catalysts for future automotive applications. Nat. Catal. 2, 590–602 (2019). [Google Scholar]
- 15.Li X., et al. , Single cobalt atoms anchored on porous N-doped graphene with dual reaction sites for efficient Fenton-like catalysis. J. Am. Chem. Soc. 140, 12469–12475 (2018). [DOI] [PubMed] [Google Scholar]
- 16.Wang B., et al. , A site distance effect induced by reactant molecule matchup in single-atom catalysts for fenton-like reactions. Angew. Chem. Int. Ed. 134, e202207268 (2022). [DOI] [PubMed] [Google Scholar]
- 17.Xu J., et al. , Organic wastewater treatment by a single-atom catalyst and electrolytically produced H2O2. Nat. Sustain. 4, 233–241 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xiong Y., et al. , Single-atom Fe catalysts for Fenton-Like reactions: Roles of different N species. Adv. Mater. 34, 2110653 (2022). [DOI] [PubMed] [Google Scholar]
- 19.Chen F., et al. , Molecular engineering toward pyrrolic N-rich M-N4 (M= Cr, Mn, Fe Co, Cu) single-atom sites for enhanced heterogeneous fenton-like reaction. Adv. Funct. Mater. 31, 2007877 (2021). [Google Scholar]
- 20.Yin Y., et al. , Boosting Fenton-like reactions via single atom Fe catalysis. Environ. Sci. Technol. 53, 11391–11400 (2019). [DOI] [PubMed] [Google Scholar]
- 21.Chen M., et al. , Efficient degradation of organic pollutants by low-level Co2+ catalyzed homogeneous activation of peroxymonosulfate. J. Hazard. Mater. 371, 456–462 (2019). [DOI] [PubMed] [Google Scholar]
- 22.Hu P., Long M., Cobalt-catalyzed sulfate radical-based advanced oxidation: A review on heterogeneous catalysts and applications. Appl. Catal. B. 181, 103–117 (2016). [Google Scholar]
- 23.Rivas F., Gimeno O., Borallho T., Aqueous pharmaceutical compounds removal by potassium monopersulfate. Uncatalyzed and catalyzed semicontinuous experiments. Chem. Eng. J. 192, 326–333 (2012). [Google Scholar]
- 24.Lu X., et al. , Bioinspired copper single-atom catalysts for tumor parallel catalytic therapy. Adv. Mater. 32, 2002246 (2020). [DOI] [PubMed] [Google Scholar]
- 25.Liu X., Pei Y., Cao M., Yang H., Li Y., Highly dispersed copper single-atom catalysts activated peroxymonosulfate for oxytetracycline removal from water: Mechanism and degradation pathway. Chem. Eng. J. 450, 138194 (2022). [Google Scholar]
- 26.Qi Y., et al. , Novel lignin-based single atom catalysts as peroxymonosulfate activator for pollutants degradation: Role of single cobalt and electron transfer pathway. Appl. Catal. B. 286, 119910 (2021). [Google Scholar]
- 27.Zhao K., et al. , Enhanced chlorinated pollutant degradation by the synergistic effect between dechlorination and hydroxyl radical oxidation on a bimetallic single-atom catalyst. Environ. Sci. Technol. 55, 14194–14203 (2021). [DOI] [PubMed] [Google Scholar]
- 28.Yang Y., Banerjee G., Brudvig G. W., Kim J.-H., Pignatello J. J., Oxidation of organic compounds in water by unactivated peroxymonosulfate. Environ. Sci. Technol. 52, 5911–5919 (2018). [DOI] [PubMed] [Google Scholar]
- 29.Ball D. L., Edwards J. O., The kinetics and mechanism of the decomposition of Caro’s acid I. J. Am. Chem. Soc. 78, 1125–1129 (1956). [Google Scholar]
- 30.Lipczynska-Kochany E., Sprah G., Harms S., Influence of some groundwater and surface waters constituents on the degradation of 4-chlorophenol by the Fenton reaction. Chemosphere 30, 9–20 (1995). [DOI] [PubMed] [Google Scholar]
- 31.Sánchez-Polo M., Ocampo-Pérez R., Rivera-Utrilla J., Mota A. J., Comparative study of the photodegradation of bisphenol A by HO, SO4− and CO3−/HCO3 radicals in aqueous phase. Sci. Total Environ. 463, 423–431 (2013). [DOI] [PubMed] [Google Scholar]
- 32.Liang X., et al. , Coordination number dependent catalytic activity of single-atom cobalt catalysts for fenton-like reaction. Adv. Funct. Mater. 32, 2203001 (2022). [Google Scholar]
- 33.Al-Nu’airat J., et al. , Reaction of phenol with singlet oxygen. Phys. Chem. Chem. Phys. 21, 171–183 (2019). [DOI] [PubMed] [Google Scholar]
- 34.Zhou Y., et al. , Activation of peroxymonosulfate by phenols: Important role of quinone intermediates and involvement of singlet oxygen. Water Res. 125, 209–218 (2017). [DOI] [PubMed] [Google Scholar]
- 35.Yang T., Fan S., Li Y., Zhou Q., Fe-N/C single-atom catalysts with high density of Fe-Nx sites toward peroxymonosulfate activation for high-efficient oxidation of bisphenol A: Electron-transfer mechanism. Chem. Eng. J. 419, 129590 (2021). [Google Scholar]
- 36.Lee H., et al. , Activation of persulfates by carbon nanotubes: Oxidation of organic compounds by nonradical mechanism. Chem. Eng. J. 266, 28–33 (2015). [Google Scholar]
- 37.Huang B.-C., Huang G.-X., Jiang J., Liu W.-J., Yu H.-Q., Carbon-based catalyst synthesized and immobilized under calcium salt assistance to boost singlet oxygen evolution for pollutant degradation. ACS Appl. Mater. Interfaces. 11, 43180–43187 (2019). [DOI] [PubMed] [Google Scholar]
- 38.Zong Y., et al. , Unraveling the overlooked involvement of high-valent cobalt-oxo species generated from the cobalt (II)-activated peroxymonosulfate process. Environ. Sci. Technol. 54, 16231–16239 (2020). [DOI] [PubMed] [Google Scholar]
- 39.Liu X., et al. , Graphene oxide-supported three-dimensional cobalt–nickel bimetallic sponge-mediated peroxymonosulfate activation for phenol degradation. ACS ES&T Eng. 1, 1705–1714 (2021). [Google Scholar]
- 40.Wang Q., et al. , Two-dimensional ultrathin perforated Co3O4 nanosheets enhanced PMS-Activated selective oxidation of organic micropollutants in environmental remediation. Chem. Eng. J. 427, 131953 (2022). [Google Scholar]
- 41.da Silva A. R. L., Dos Santos A. J., Martínez-Huitle C. A., Electrochemical measurements and theoretical studies for understanding the behavior of catechol, resorcinol and hydroquinone on the boron doped diamond surface. RSC Adv. 8, 3483–3492 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Joshi R., Ghanty T. K., Mukherjee T., Substituent effect on ionization potential, O-H bond dissociation energy and intra-molecular hydrogen bonding in salicylic acid derivatives. J. Mol. Struct. 948, 47–54 (2010). [Google Scholar]
- 43.Lide D. R., CRC Handbook of Chemistry and Physics (CRC Press, vol. 85, 2004). [Google Scholar]
- 44.Liu B., et al. , Peroxymonosulfate activation by cobalt (II) for degradation of organic contaminants via high-valent cobalt-oxo and radical species. J. Hazard. Mater. 416, 125679 (2021). [DOI] [PubMed] [Google Scholar]
- 45.Liu B., et al. , Insights into the oxidation of organic contaminants by Co (II) activated peracetic acid: The overlooked role of high-valent cobalt-oxo species. Water Res. 201, 117313 (2021). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Data Availability Statement
All study data are included in the article and/or SI Appendix.