Abstract
Background and study aims The costs of reusable endoscope reprocessing have been evaluated, yet external validity of the findings remains challenging. The aim of this study was to assess the costs of purchase, maintenance, microbiological control, and reprocessing of a reusable duodenoscope per endoscopic retrograde cholangiopancreatography (ERCP) in France. Study findings exclude the costs of infection, downtime due to breakdown, reprocessing single-use material disposal, and device disposal, all of which should also be considered.
Materials and methods The study encompassed both observational and theoretical approaches. Observational data were collected in four hospitals, from December 2019 to December 2020, with an ad hoc survey, based on 2016 and 2018 national guidelines for duodenoscope reprocessing. Costs were modeled, using the same guidelines, assuming a mean workload of 223 ERCP/duodenoscope/year.
Results The mean observed cost of purchase, maintenance, microbiological control, reprocessing (human resources and consumables), and overhead (additional 35%) with a reusable duodenoscope was €80.23 (standard deviation €3.77) per ERCP. The corresponding mean theoretical cost was €182.71 for manual reprocessing without endoscope drying cabinet (EDC), €191.36 for manual reprocessing with EDC, €235.25 for automated endoscope reprocessing (AER) without EDC, and €253.62 for AER with EDC.
Conclusions Because procedures, equipment, volume activity, number of duodenoscopes, human resources, and internal work organizations are hospital-dependent, observed costs varied between hospitals. Theoretical costs were higher than observed costs, showing that the theoretical approach is not sufficient. Hypotheses to explain the difference between the two approaches include failing to measure some costs in the survey and challenges in guideline implementation.
Keywords: Cholangioscopy, Pancreatoscopy, Quality and logistical aspects, Hygiene, Pancreatobiliary (ERCP/PTCD), Quality and logistical aspects
Introduction
In France, each year, about 90,000 endoscopic retrograde cholangiopancreatographies (ERCPs) are performed with duodenoscopes 1 . Despite strict adherence to both manufacturer-issued reprocessing protocols and international guidelines on the high-level disinfection reprocessing workflow 2 3 4 , bacterial colonization of duodenoscopes leading to infections still occurs 5 6 7 . In France, the risk of duodenoscope-related infection was estimated to be one to three cases per million endoscopic procedures 8 or 25 infections in 6 years 9 —lower than in other countries 10 11 . Because the introduction of single-use duodenoscopes has been presented as a solution to avoid infections, assessing the costs of device purchase, maintenance, microbiological control, and reprocessing per ERCP with a reusable duodenoscope is timely.
The cost of reusable endoscope reprocessing has been evaluated in France and abroad 7 12 13 14 15 16 17 , yet external validity of the results remains challenging. Differences stem from the guidelines used, hospital internal organization, number of duodenoscopes, type and volume of activity, team composition, and reprocessing techniques 18 .
The aim of this study was to assess the costs of purchase, maintenance, microbiological control, and reprocessing (including storage) of a reusable duodenoscope, per ERCP. On the one hand, survey-based costs reflect real-world practices and some costs may be omitted or underestimated if, for instance, reprocessing guidelines are not strictly followed. On the other hand, guideline-based costs may be overestimated compared to real-world practices because guidelines aim to maximize security. Therefore, it appeared essential to have a dual approach: implementing a survey in hospitals to collect data on the real-world costs and estimating the costs using a theoretical approach partially based on the 2016 and 2018 national guidelines for duodenoscope reprocessing 8 19 .
Methods
The following costs were considered: purchase, maintenance, and reprocessing (including storage) ( Supplementary Table 1 ). The costs of infection, downtime due to breakdown or maintenance, reprocessing single-use material disposal, and device disposal were beyond the scope of this study.
Survey-based approach
Study centers and study period
Four French hospital centers were selected to ensure diversity of ownership: two university hospitals (a large one with 2,543 beds and a smaller one with 1,051 beds), one private hospital, and one private nonprofit hospital. Between December 1, 2019 and December 1, 2020, they completed a survey (Supplement Methods 1) to report the costs of duodenoscope reprocessing, microbiological controls, and associated costs of used equipment.
Survey
The survey questionnaire was based upon the 2016 and 2018 national guidelines [19,8] (with particular attention paid to the products used) and guidelines for hospital environmental control and videos directed by the Center for the Prevention of Healthcare-related Infections (CPIAS) of Nouvelle Aquitaine 20 21 22 . Data on the number of duodenoscopes and annual number of ERCPs and the duration of duodenoscope life cycles were collected.
Costs of purchase, maintenance, and microbiological control of the washing equipment (automated endoscope reprocessing machine, [AER]) and the storage equipment (endoscope drying cabinet, [EDC]) were excluded from the survey. Costs related to that "ancillary equipment" are difficult to compare from one hospital to another. Moreover, washing and storage equipment are not restricted to duodenoscopes and corresponding costs are small compared to the other costs involved in the cost of an ERCP. Neither were considered costs incurred by complying with procedures (training time, quality insurance, internal audits, time spent handling documentation for repair), structural costs, and costs incurred for biomedical operations. In all, 35% were added as overhead costs (15% and 20% for the facility 12 and quality assurance 7 , respectively).
Theoretical approach
This approach was based on national recommendations, guidelines released by the Ministry of Health 8 19 , associated Frequently Asked Questions, and CPIAS guidelines 20 21 22 .
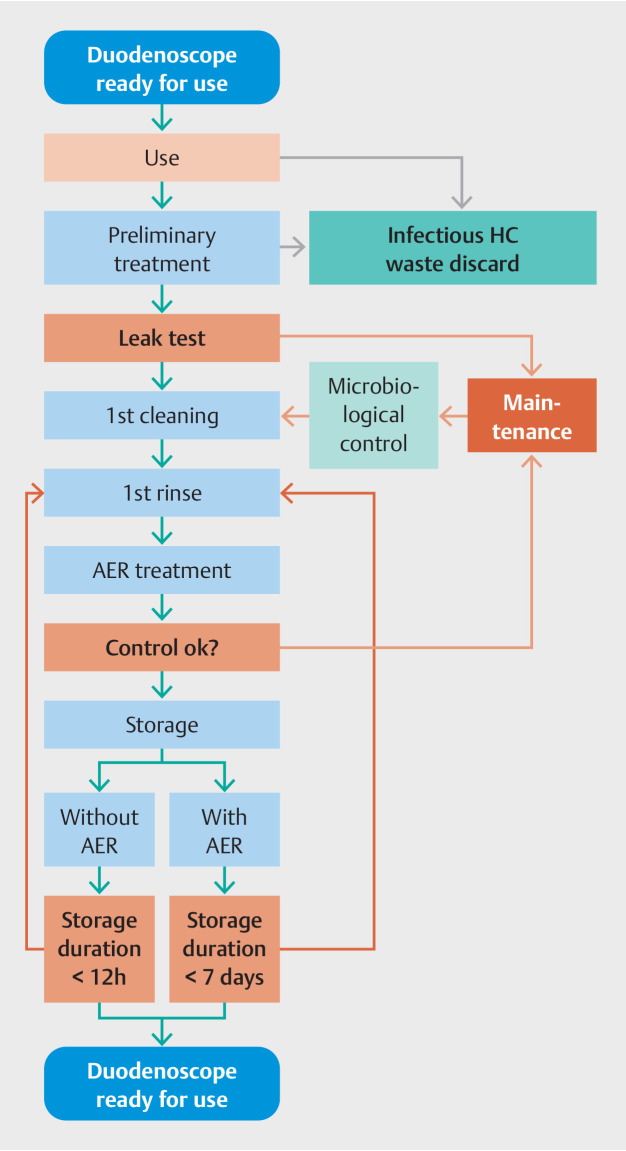
Fig. 1 presents the reprocessing steps and allows for the identification of corresponding needs and related costs. Consumables and operation time for duodenoscope reprocessing considered for the modeling cost assessment are shown in Supplementary Table 2 . The microbiological controls (with consumables and human resources) included controls of the duodenoscope, the water, the EDC, and the room environment. The number of microbiological controls per year are shown in Supplementary Table 3 . Absent EDC, if the duodenoscope is used ≥ 6 hours after reprocessing, a short reprocessing cycle is needed prior to use. Thus, one short reprocessing cycle per day was added to the reprocessing cost for reprocessing without EDC.
Fig. 1.
Reusable duodenoscope reprocessing steps. AER, automated endoscope reprocessing machine; HC, healthcare; microbiological control, controls (with consumables and human resources) of the duodenoscope, the water, the endoscope drying cabinet, and the room environment.
To account for hidden and structural costs, 35% overhead costs were added 7 12 .
Data sources
Mean consumables and equipment costs were estimated based on tariff data from three university hospitals (no fixed tariffs). The human resources costs were based on 2021 data provided by the University Hospital of Toulouse, the annual gross salary of a nurse, a laboratory technician, and a caregiver were €70,744.28, €55,365.46, and €47,553.97, respectively. According to the Ministry of Health, the gross annual salary of hospital doctors was €118,012 23 . Computations were based on 1,575 annual working hours.
Assumptions
The number, methods, and frequency of microbiological controls were based on the 2018 Good Practices for Microbiological Surveillance of the Environment in Health Care Institutions 24 . Based on 88,440 ERCP performed in 346 facilities (min-max 179–266 ERCP per facility) 1 , the mean annual number of ERCP per duodenoscope was 223 (corresponding to using a duodenoscope once per business day). This number was used as the denominator to compute the theoretical cost per ERCP. We assumed that the duodenoscope would undergo a short reprocessing cycle prior to use (except when stored in an EDC or sealed in a vacuum-dried envelope) and added the corresponding cost. Of note, additional microbiological controls may be needed after repair or maintenance but were not considered.
We followed the number of maintenance procedures recommended by the manufacturer and added one repair. The depreciation periods for the equipment were set at 4 years for duodenoscopes (although it is more than 4 years in public hospitals), 6 years for AERs and 8 years for EDCs. The costs of maintenance, purchase, and the product life cycle for a duodenoscope, an AER, and an EDC are presented in Supplementary Table 4 .
Outcome
The outcome was the mean cost of purchase, maintenance, and reprocessing (including storage) per ERCP with a reusable duodenoscope, in a real-world setting (survey-based approach) and from a theoretical viewpoint (guidelines-based model). Of note, the number of ERCP per duodenoscope was different in the survey-based and the guidelines-based approaches.
Statistical methods
Continuous data were summarized by their mean and standard deviation (SD) for the survey results. Costs are expressed in EUR2020.
Ethics
This study did not involve patient data and, therefore, approval from an ethics committee was not required.
Results
Survey-based approach
The four hospitals performed between 250 and 580 duodenoscopies per year and owned three or four duodenoscopes ( Table 1 ). Consequently, the annual number of ERCPs per duodenoscope varied from 62 to 193 between hospitals.
Table 1 Number of reusable duodenoscopes, annual number of ERCPs, and annual number of ERCPs per duodenoscope in each surveyed hospital.
| Hospital | Number of reusable duodenoscopes | Annual number of ERCPs | Annual number of ERCPs per duodenoscope |
| ERCP, endoscopic retrograde cholangiopancreatography. | |||
| A | 4 | 410 | 102.5 |
| B | 3 | 530 | 176.7 |
| C | 3 | 580 | 193.3 |
| D | 4 | 250 | 62.5 |
The average purchasing cost of a duodenoscope was around €30,000. Device life cycle and depreciation period varied between 4 and 6 years. In addition, the annual cost of maintenance was around €4,000 (identical in all hospitals). Consequently, the cost of purchase and maintenance per ERCP varied between €18.55 and €29.53 per hospital ( Table 2 ). Assuming five microbiological controls per year (four compulsory + one after maintenance), the mean cost of the microbiological control per duodenoscope use was €2.96. As for reprocessing, the cost of consumables was similar in all hospitals (mean €14.76), but the cost of human resources varied from one hospital to the other (from €13.50 to €20.88).
Table 2 Cost of duodenoscope purchase, maintenance, microbiological controls, and reprocessing, per ERCP, using the survey-based approach.
| Hospital | Duodenoscope purchase and maintenance (€) (1) | Microbiological controls (€) (2) | Reprocessing (3) | Overhead 35% of (1) + (2) + (3) | Total (€) | |
| Consumables (€) | Human resources (€) | |||||
| SD, standard deviation. *The cost of duodenoscope purchase and maintenance for Hospital D was not communicated; the mean cost at the other hospitals was used. † The cost of microbiological controls for Hospitals B and D was not communicated; the mean costs at the other hospitals were used. | ||||||
| A | 24.40 | 3.95 | 14.48 | 19.20 | 21.71 | 83.74 |
| B | 18.55 | 2.96 | 17.18 | 20.88 | 20.85 | 80.42 |
| C | 29.53 | 1.97 | 13.17 | 16.60 | 21.44 | 82.71 |
| D | 24.16 | 2.96 | 14.22 | 13.50 | 19.19 | 74.03 |
| Mean (±SD) | 24.16 (±4.49) | 2.96 (±0.99) | 14.76 (±1.48) | 17.55 (±2.79) | 20.80 (±0.98) | 80.23 (±3.77) |
In all, the average observed cost of purchase, maintenance, microbiological control, reprocessing, and overhead was €80.23 (±3.77, 95% confidence interval [76.54–83.92]) per ERCP.
Theoretical approach
For each ERCP, the purchase and maintenance costs were €60.54 for the duodenoscope, €49.33 for the AER, and €5.32 for the EDC ( Table 3 ). The cost of using an EDC was €6.80. The costs of the microbiological controls were €2.99 for the duodenoscope, €2.81 for the water, €1.59 for the EDC, and €1.25 for the room environment. The cost of all microbiological controls was small (from €4.24 to €8.64) compared to the cost of purchase and maintenance, and reprocessing. The total cost of one reprocessing (consumables and human resources), considering whether the hospital performed it manually or with AER, with or without EDC, ranged from €57.34 (AER processing without EDC) to €78.33 (manual reprocessing without EDC).
Table 3 Cost of duodenoscope purchase, maintenance, microbiological controls, and reprocessing, per ERCP, using the theoretical approach.
| Procedure | Duodenoscope, EDC, and AER purchase and maintenance (€) (1) | Microbiological controls (€) (2) | Reprocessing (3) | Overhead 35% of (1) + (2) + (3) | Total (€) | |
| Consumables (€) | Human resources (€) | |||||
| EDC, endoscope drying cabinet; AER, automated endoscope reprocessing. Overhead was defined as 15% for facility + 20% for quality assurance. *Includes additional reprocessing per day to take into consideration duodenoscope use for more than 6 hours after reprocessing. | ||||||
| Manual reprocessing without EDC* | 60.54 | 4.24 | 49.76 | 28.57 | 47.37 | 182.71 |
| Manual reprocessing with EDC | 65.86 | 5.83 | 45.89 | 24.17 | 49.61 | 191.36 |
| AER without EDC* | 109.87 | 7.05 | 42.14 | 15.20 | 60.99 | 235.25 |
| AER with EDC | 115.19 | 8.64 | 47.83 | 19.05 | 65.75 | 253.62 |
In all, the average theoretical cost of purchase, maintenance, microbiological control, reprocessing, and overheads per ERCP was €182.71 for manual reprocessing without EDC, €191.36 for manual reprocessing with EDC, €235.25 for AER reprocessing without EDC, and €253.62 for AER reprocessing with EDC.
Discussion
Based on direct observations in four hospitals, the mean cost of purchase, maintenance, microbiological control, reprocessing, and overhead per ERCP with a reusable duodenoscope was €80.23 and fairly consistent across hospitals. Based on a theoretical, guidelines-based approach, this mean cost was estimated to vary between €182.71 and €253.62, depending on whether an EDC and an AER were used or not.
Comparison of survey results in four hospitals
Even if hospitals follow guidelines, their processes may not coincide because of differences in their internal organizations. While the cost of one ERCP was fairly homogeneous among the four hospitals, differences were noted in some items. For instance, Hospital B had a lower purchasing cost for the duodenoscope. Hospital D hired less qualified staff for the multiple tasks, hence the cost of human resources in the duodenoscope reprocessing was lower than in the other hospitals. Other factors impact duodenoscope reprocessing costs, including volume of activity, number of devices, and equipment.
Comparison of survey-based and theoretical approaches
The average theoretical costs were two to three times higher than the average survey-based costs, showing that the theoretical approach in itself is not sufficient. On the face of it, the higher theoretical costs were explained by the higher costs of purchase and maintenance, microbiological controls, and consumables for the reprocessing considered in the model. The average theoretical costs per ERCP were higher despite assuming a higher annual number of ERCPs per duodenoscope compared to the numbers found with the survey. In any case, the optimal duodenoscope reprocessing modality remains to be determined 25 . For example, maximum time between device reprocessing and use is 6 hours in France but 4 hours in Belgium 26 . In addition, recommendations are sometimes contradictory and difficult to implement 27 . For all these reasons, it is therefore difficult to ascertain, even theoretically, the cost of an ERCP. The difference between the theoretical and the observed costs also implies that not all costs can be captured in a survey (some costs were estimated and not measured, costs were reported by staff and not collected by an external independent worker), even based on guidelines.
Finally, the difference in the results of the two approaches could mean that, despite laudable efforts from the hospitals, guidelines were not fully implemented.
Comparison of our results with other studies
Previous French studies have examined the cost of reprocessing reusable select endoscopes (including bronchoscopes 12 , fiberscopes 13 14 , soft endoscopes 15 , and choledochoscopes 16 ) but not duodenoscopes. The cost of reprocessing flexible endoscopes has been investigated in 14 healthcare institutions in the United States to explore the real-world impact of strengthened guidelines on reprocessing time and cost 17 . Although the study did not account for every aspect of reprocessing, the minimum and maximum costs were $114.07 and $280.71 per reprocessing, which is higher than the survey-based and theoretical costs of reprocessing we found. In a second US study, assuming 200 ERCPs per year, the per-procedure cost with a reusable duodenoscope was $232 without considering infections 11 . The per-procedure cost rose to $732 and $2,107 when factoring in infection rates of 0.4% to 1.5%, respectively (with the cost of treating cholangitis of $125,000). In a third US study, assuming 650 ERCPs per year performed with three duodenoscopes (or 217 ERCPs per duodenoscope), the per-procedure cost (including purchase, repair and maintenance, reprocessing, 20% overhead, and a 1% infection risk) was $960.24 7 . The estimated cost for treating a duodenoscope-related infection was $47,181, leading to a per-procedure infection cost ranging from $471.81 to $566.17 for 1% and 1.2% infection risks, respectively. A difference in the costs between the United States and France is to be expected and can be explained by differences between guidelines, labor costs, and device costs. At any rate, our study confirms that cost studies should be conducted on a national basis before making national decisions on payment of procedures, reimbursement for devices, and the types of devices to be preferred (single-use versus reusable).
Future of duodenoscope reprocessing
Despite the best possible reprocessing, patient cross-contamination is still possible with reusable duodenoscopes 28 . Enhanced reprocessing protocols may further reduce patient risk of exposure to contaminated duodenoscopes, yet they significantly increase the cost of performing ERCPs. Future innovation should focus on approaches that can ensure patient safety while maintaining the ability to perform ERCP in a cost-effective manner 29 . Acknowledging the challenges of eliminating all contamination during reprocessing and maintaining the duodenoscope contaminant-free during storage, the US Food and Drug Administration issued a safety communiqué recommending transitioning to duodenoscopes with innovative designs that allow more effective reprocessing, including single-use sterile parts 30 . In select cases deemed at high risk of cross-contamination or when the quality of high-level disinfection cannot be enforced with the highest level of confidence, SUU present an alternative which is already available and may deserve consideration. However, our study—especially the survey findings—demonstrated a relatively low cost for duodenoscope reprocessing and, if microbiological and environmental concerns are added, the debate is not over. So far, single-use duodenoscopes might be more appropriate in limited cases, such as highly-infectious or immunocompromised patients, and when duodenoscopes are unavailable due to maintenance, repair, or bacteriological surveillance and after hours when trained staff is unavailable for reprocessing.
Strengths and limitations
This study’s strengths are its double approach (survey-based and theoretical) and that multiple costs have been considered and detailed.
This study also has some limitations. First, the survey was completed by the hospital staff, which could have an impact on the answers. Second, it failed to consider the cost of infections, which would increase the cost of ERCP— because it is difficult to distinguish infection related to preexisting contamination of the duodenoscope and infection related to the act itself (bacteremia, for example) 11 . The cost of infections is extremely variable from one case to another and should include the direct expenditures (treatments, extended hospital stay) and indirect costs (crisis management, degradation of the institution's image). Then, some costs were covered by the overhead (facility and quality insurance), but other costs were beyond the scope of this study but merit attention and should be integrated into the cost of ERCP (disposal of trash generated by the reprocessing and the duodenoscope 17 , environmental impact of reprocessing, downtime due to breakdown or maintenance and its consequence, device end-of-life disposal). The findings may not be representative on a national level, because the types of duodenoscope used, staff employed, and reprocessing materials and methods vary greatly. Participation of other hospitals might have yielded different results (in one direction or the other), given that some perform fewer than 250 ERCPs per year and others more than 580. The model should have been replicated for varied mean number of ERCPs per duodenoscope. Some data could not be obtained from two hospitals.
Conclusions
The estimated costs of purchase, maintenance, microbiological control, and reprocessing of a reusable duodenoscope per ERCP were significantly different with the theoretical and observational approaches. The costs found with the theoretical approach were much higher. The costs found with the observational approach were consistent between hospitals and fairly low. This shows that using several approaches (each having its own limitations) is necessary to assess costs associated with duodenoscopes. These findings can feed the discussion on the positioning of single-use duodenoscopes.
Acknowledgement
We thank the following investigators in the centres: Vincent Philippe and Audrey Folcher (Bordeaux University Hospital Centre, Bordeaux, France), and Maud Mehring (Toulouse Clinique Pasteur, Toulouse, France). We thank Joannie Lortet-Tieulent (ORCID 0000-0002-8816-7475) for writing assistance (Heva, Lyon, France).
Footnotes
Conflict of Interest DT declares a leadership role in EURO-PHARMAT (president and treasurer) and that the institution received funding from Boston Scientific. FD and IDZ declare having received consulting fees from Boston Scientific. JH declares having received consulting fees from Boston Scientific and Mylan. BN declares having received training sessions funded by Boston Scientific. FP declares having participated in advisory boards and having received consulting fees from Boston Scientific. GV declares a leadership role as Lead of the research committee of French endoscopic digestive society and having received consulting fees from Ambu, Boston Scientific, Fujifilm, Pentax and Tillotts. TP declares having received consulting fees from Boston Scientific, Olympus, Norgine and Ipsen, as well as payment or honoraria for educational event from Ipsen. ELD, VM and CP declare no conflicts of interest.
Supporting information
References
- 1.Agence Technique de l’Information sur l’Hospitalisation . [1] Agence Technique de l’Information sur l’Hospitalisation. Consommation et production de soins. ScanSanté. 2020. https://www.scansante.fr/applications/analyse-de-l-offre-de-soin?secteur=MCO https://www.scansante.fr/applications/analyse-de-l-offre-de-soin?secteur=MCO
- 2.Beilenhoff U, Biering H, Blum R et al. Prevention of multidrug-resistant infections from contaminated duodenoscopes: Position Statement of the European Society of Gastrointestinal Endoscopy (ESGE) and European Society of Gastroenterology Nurses and Associates (ESGENA) Endoscopy. 2017;49:1098–1106. doi: 10.1055/s-0043-120523. [DOI] [PubMed] [Google Scholar]
- 3.Day LW, Muthusamy VR, Collins J et al. Multisociety guideline on reprocessing flexible GI endoscopes and accessories. Gastrointest Endosc. 2021;93:11–3.3E7. doi: 10.1016/j.gie.2020.09.048. [DOI] [PubMed] [Google Scholar]
- 4.Petersen BT, Cohen J, Hambrick RD et al. Multisociety guideline on reprocessing flexible GI endoscopes: 2016 update. Gastrointest Endosc. 2017;85:282–2940. doi: 10.1016/j.gie.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 5.Rauwers AW, Voor In ’t Holt AF, Buijs JG et al. Nationwide risk analysis of duodenoscope and linear echoendoscope contamination. Gastrointest Endosc. 2020;92:681–6910. doi: 10.1016/j.gie.2020.05.030. [DOI] [PubMed] [Google Scholar]
- 6.Balan GG, Sfarti CV, Chiriac SA et al. Duodenoscope-associated infections: a review. Eur J Clin Microbiol Infect Dis Off Publ Eur Soc Clin Microbiol. 2019;38:2205–2213. doi: 10.1007/s10096-019-03671-3. [DOI] [PubMed] [Google Scholar]
- 7.Travis HS, Ehlers LH, Thornton J. The total cost of reuseable duodenoscopes - are single-use duodenoscopes the future of ERCP? Pharmacoeconomics Open Access. 2020;5:1–3. [Google Scholar]
- 8.Ministère des solidarités et de la santé . INSTRUCTION N° DGOS/PF2/DGS/VVS1/PP3/2018/195 du 2 août 2018 relative à l’actualisation du traitement des endoscopes souples thermosensibles à canaux de type duodénoscope au sein des structures de soins. 2018.
- 9.Soing-Altrach S. Signalement d’infections nosocomiales liées à l’endoscopie via e-SIN et la matériovigilence: 2012–2018. Lett Signal. 2019;13:8–9. [Google Scholar]
- 10.Vos M, Kwakman J, Bruno M. Risk estimate of duodenoscope-associated infections in The Netherlands. Infect Control Hosp Epidemiol. 2020;41:s436–s437. [Google Scholar]
- 11.Bang JY, Sutton B, Hawes R et al. Concept of disposable duodenoscope: at what cost? Gut. 2019;68:1915–1917. doi: 10.1136/gutjnl-2019-318227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coutron C. Montpellier, FR: Ecole des Hautes Etudes en Santé Publique; 2015. Analyse médico-économique relative au traitement des endoscopes au CHRU de Montpellier. [Google Scholar]
- 13.Debraine C, Touratier S, Faure P Fibroscope réutilisable versus usage unique Analyse des coûts en réanimation. Poster at JNFDM Montpellier Euro-Pharmat. 2013.
- 14.Robert J, Solans V, Sehier J Etude de minimisation des coûts des vidéo-endoscopes réutilisables et à usage unique Quelle évolution des dépenses selon le nombre d’examens? Poster at JNFDM Euro-Pharmat Saint Malo. 2017.
- 15.Allainmat-Lemercier A, Taurin S, Mehault L et al. Coût de la prise en charge en stérilisation des endoscopes souples au CHU de Rennes. Etude économique à 2 ans. Stérilisation Cent. 2017;1:19–26. [Google Scholar]
- 16.Saly M. Limoges, FR: Université de Limoges; 2018. Traitement des dispositifs médicaux réutilisables thermosensibles: mise en place d’une stérilisation basse température au CHU de Bordeaux. [Google Scholar]
- 17.Ofstead C, Quick M, Eiland J A glimpse at the true cost of reprocessing endoscopes: results of a pilot project. IAHCSMM Communique January/February. 2017.
- 18.Thaker AM, Muthusamy VR, Sedarat A et al. Duodenoscope reprocessing practice patterns in U.S. endoscopy centers: a survey study. Gastrointest Endosc. 2018;88:316–32200. doi: 10.1016/j.gie.2018.04.2340. [DOI] [PubMed] [Google Scholar]
- 19.Ministère des affaires sociales et de la santé . INSTRUCTION N° DGOS/PF2/DGS/VSS1/2016/220 du 4 juillet 2016 relative à relative au traitement des endoscopes souples thermosensibles à canaux au sein des lieux de soins. 2016.
- 20.CPIAS Nouvelle-Aquitaine . Les prélèvements d’environnement: sur endoscopes. https://www.youtube.com/watch?v=d68CYZtafOA https://www.youtube.com/watch?v=d68CYZtafOA
- 21.CPIAS Nouvelle-Aquitaine . Traitement manuel des endoscopes non autoclavables. 2017. https://www.youtube.com/watch?v=VxsUzKwj_Eo https://www.youtube.com/watch?v=VxsUzKwj_Eo
- 22.Société Française d'Hygiène Hospitalière (SF2H) . Les contrôles microbiologiques en endoscopie. https://www.youtube.com/watch?v=iNl6rN0gInY https://www.youtube.com/watch?v=iNl6rN0gInY
- 23.Direction générale de l’offre de soins . Guide pour le suivi de la masse salariale. Ministère chargé de la santé, Paris. 2014. https://solidarites-sante.gouv.fr/IMG/pdf/dgos_guide_suivi_masse_salariale_2014.pdf https://solidarites-sante.gouv.fr/IMG/pdf/dgos_guide_suivi_masse_salariale_2014.pdf
- 24.Centre d’appui pour la Prévention des Infections Associées aux Soins de Nouvelle Aquitaine . Surveillance microbiologique de l’environnement 2016. CPIAS Nouv Aquitaine. https://www.cpias-nouvelle-aquitaine.fr/publication/surveillance-microbiologique-de-lenvironnement-2016 https://www.cpias-nouvelle-aquitaine.fr/publication/surveillance-microbiologique-de-lenvironnement-2016
- 25.Barakat MT, Banerjee S. Novel algorithms for reprocessing, drying and storing endoscopes. Gastrointest Endosc Clin N Am. 2020;30:677–691. doi: 10.1016/j.giec.2020.06.003. [DOI] [PubMed] [Google Scholar]
- 26.Conseil Supérieur de la Santé . Recommandations en matière de prévention des infections et de prise en charge des endoscopes et dispositifs médicaux endocavitaires thermosensibles Actualisation et élargissement des précédentes recommandations (CSS 8355 - 2010). Bruxelles, Belgium: Conseil Supérieur de la Santé. 2019.
- 27.Rubin ZA, Kim S, Thaker AM et al. Safely reprocessing duodenoscopes: current evidence and future directions. Lancet Gastroenterol Hepatol. 2018;3:499–508. doi: 10.1016/S2468-1253(18)30122-5. [DOI] [PubMed] [Google Scholar]
- 28.Rauwers AW, Voor In ’t Holt AF, Buijs JG et al. High prevalence rate of digestive tract bacteria in duodenoscopes: a nationwide study. Gut. 2018;67:1637–1645. doi: 10.1136/gutjnl-2017-315082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bomman S, Kozarek RA, Thaker AM et al. Economic burden of enhanced practices of duodenoscopes reprocessing and surveillance: balancing risk and cost containment. Endosc Int Open. 2021;9:E1404–E1412. doi: 10.1055/a-1515-2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.US Food and Drug Admistration . The FDA is Recommending transition to duodenoscopes with innovative designs to enhance safety: FDA safety communication. https://www.fda.gov/medical-devices/safety-communications/fda-recommending-transition-duodenoscopes-innovative-designs-enhance-safety-fda-safety-communication https://www.fda.gov/medical-devices/safety-communications/fda-recommending-transition-duodenoscopes-innovative-designs-enhance-safety-fda-safety-communication
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.