Abstract
Five moderately thermophilic iron-oxidizing bacteria, including representative strains of the three classified species (Sulfobacillus thermosulfidooxidans, Sulfobacillus acidophilus, and Acidimicrobium ferrooxidans), were shown to be capable of reducing ferric iron to ferrous iron when they were grown under oxygen limitation conditions. Iron reduction was most readily observed when the isolates were grown as mixotrophs or heterotrophs with glycerol as an electron donor; in addition, some strains were able to couple the oxidation of tetrathionate to the reduction of ferric iron. Cycling of iron between the ferrous and ferric states was observed during batch culture growth in unshaken flasks incubated under aerobic conditions, although the patterns of oxidoreduction of iron varied in different species of iron-oxidizing moderate thermophiles and in strains of a single species (S. acidophilus). All three bacterial species were able to grow anaerobically with ferric iron as a sole electron acceptor; the growth yields correlated with the amount of ferric iron reduced when the isolates were grown in the absence of oxygen. One of the moderate thermophiles (identified as a strain of S. acidophilus) was able to bring about the reductive dissolution of three ferric iron-containing minerals (ferric hydroxide, jarosite, and goethite) when it was grown under restricted aeration conditions with glycerol as a carbon and energy source. The significance of iron reduction by moderately thermophilic iron oxidizers in both environmental and applied contexts is discussed.
Moderately thermophilic acidophilic bacteria that catalyze the dissimilatory oxidation of ferrous iron are distinct both phylogenetically and in aspects of their physiology. They differ from the known acidophilic mesophilic iron oxidizers (gram-negative, nonsporulating chemolithotrophic bacteria) and the extremely thermophilic iron oxidizers (certain archaea) in several fundamental ways, including cellular morphology (they are gram-positive rods that often form endospores) and growth temperature optima, which are typically 45 to 55°C (15). In addition, the moderately thermophilic iron-oxidizing acidophiles characteristically have a highly versatile metabolism (18) and may grow as autotrophs (e.g., in media containing ferrous iron or reduced sulfur), heterotrophs (e.g., on yeast extract), mixotrophs (e.g., in media containing both ferrous iron and glucose, in which both CO2 and glucose are used as carbon sources), or chemolithoheterotrophs (e.g., in ferrous iron-yeast extract medium, in which iron acts as the energy source and yeast extract is the carbon source). Isolates have been obtained from a range of thermal acidic environments, such as geothermal areas, self-heating mine waste spoils, and commercial mineral-processing operations (2a, 5, 14). There are currently two recognized genera of these bacteria. All but one Sulfobacillus species are iron- and sulfur-oxidizing, gram-positive, sporulating rods. Two such species have been described, Sulfobacillus thermosulfidooxidans and Sulfobacillus acidophilus, which may be distinguished by their different chromosomal DNA base compositions and by their abilities to grow autotrophically on reduced sulfur (16). The genus Acidimicrobium currently contains a single species, Acidimicrobium ferrooxidans. This organism differs from Sulfobacillus spp. by its greater capacity to fix CO2, by its lower tolerance of ferric iron, by its apparent lack of spore formation (although it is also gram positive), and by its chromosomal DNA base composition (4). Analysis of 16S rRNA sequences has also differentiated this moderate thermophile from Sulfobacillus spp. (9).
The small amount of energy associated with the oxidation of ferrous iron (−30 kJ mol−1 at pH 2) can serve as the exclusive source of energy for moderately thermophilic iron-oxidizing acidophiles when they are growing autotrophically with oxygen as the terminal electron acceptor. Under limited aeration conditions, ferric iron, which is often abundant and present in a soluble form in extremely acidic environments, is a thermodynamically attractive alternative electron sink (electrode potential [E′], +780 mV). Ferric iron reduction by mesophilic chemolithotrophic and heterotrophic acidophiles has been observed previously (5, 7, 17). Some moderately thermophilic, acidophilic, heterotrophic bacteria (Alicyclobacillus-like isolates) (5a) and the extremely thermophilic archaeon Sulfolobus acidocaldarius (3) can also reduce iron. While many neutrophilic microorganisms are also able to reduce ferric iron, the ability to conserve energy to support growth by coupling organic matter oxidation exclusively to ferric iron reduction appears to be more restricted among neutrophilic bacteria (11).
In this paper, we describe the dissimilatory reduction of ferric iron by representative isolates of different species of iron-oxidizing moderate thermophiles with both an organic electron donor (glycerol) and an inorganic electron donor (tetrathionate), and we also describe the reductive dissolution of ferric iron-containing minerals by a Sulfobacillus isolate.
MATERIALS AND METHODS
Bacterial strains and media.
Five strains of moderately thermophilic iron oxidizers were used in the experimental work (Table 1); three of these (TH1, TH3, and ALV) were kindly provided by Paul Norris (Warwick University, Warwick, United Kingdom), and the other two (THWX and YTF1) were isolated from environmental samples by one of us (5). Three of these bacteria (ALV, THWX, and YTF1) have been identified on the basis of physiological characteristics and chromosomal DNA base composition as strains of S. acidophilus, and the other two have been identified as strains of S. thermosulfidooxidans (TH1) and A. ferrooxidans (TH3) (4, 5a, 17). Bacteria were maintained in a liquid medium containing 10 mM ferrous sulfate, 0.02% (wt/vol) yeast extract, and basal salts and adjusted to pH 2.0 (with H2SO4) and were grown at 45°C (5). Cultures were purified by isolating single colonies of bacteria grown on ferrous iron overlay medium (6). Plating was also used regularly to monitor the purity of cultures.
TABLE 1.
Moderately thermophilic acidophilic bacteria used in the present study
| Strain | Origin | Species | Reference(s) |
|---|---|---|---|
| ALV | Self-heating coal spoil heap, Alvecote, Warwickshire, United Kingdom | S. acidophilus | 16 |
| THWX | Self-heating coal spoil heap, Wrexham, Clywd, United Kingdom | S. acidophilus | 5 |
| YTF1 | Frying Pan Hot Spring, Yellowstone National Park, Wyo. | S. acidophilus | 5 |
| TH1 | Thermal spring, Iceland | S. thermosulfidooxidans | 2a, 16 |
| TH3 | Copper leach dump, Kennecott Chino Mine, New Mexico | A. ferrooxidans | 4, 14 |
Iron reduction by heterotrophically and mixotrophically grown bacteria.
The moderate thermophiles were grown in liquid media containing 10 mM glycerol, 20 mM ferric (or ferrous) sulfate, 0.02% (wt/vol) yeast extract, and basal salts (100 ml of medium in 250-ml Ehrlenmeyer flasks). Ferrous and ferric sulfate stock solutions (1 M; pH 1.8) were filter sterilized and added to the separately heat-sterilized medium which had been adjusted to pH 2.0 with sulfuric acid. Cultures were incubated either shaken (150 rpm) or unshaken at 45°C. Cultures were swirled prior to removal of samples for iron analysis to ensure thorough mixing. Ferric iron reduction by heterotrophically grown bacteria incubated under anaerobic conditions was assessed by growing the moderate thermophiles in 20 mM ferric sulfate–glycerol–yeast extract medium in 25-ml universal bottles. Prior to autoclaving, the iron-free medium was deoxygenated with N2, and after ferric sulfate and inoculum were added, the bottles were filled with sterile medium and sealed. Large numbers of cultures were prepared so that when measurements had been taken (and oxygen had been introduced), they could be discarded. Reduction of ferric iron was monitored by measuring the increase in the ferrous iron concentration relative to the concentration in sterile cell-free control cultures. A separate control culture inoculated with heat-killed S. acidophilus YTF1 was also used.
Iron reduction by autotrophically grown bacteria.
Experiments were conducted to assess whether the moderately thermophilic acidophiles were able to couple the oxidation of tetrathionate to the reduction of ferric iron when they were grown under anaerobic conditions. A medium containing 5 mM potassium tetrathionate, 20 mM ferric sulfate, and basal salts and supplemented with trace elements (5) was prepared, and cultures were grown under anaerobic conditions, as described above. Ferric iron reduction was measured by determining the increase in ferrous iron concentrations.
Ferric iron-coupled growth in anaerobically incubated cultures.
To test whether iron reduction supported anaerobic growth of the bacteria, the moderate thermophiles were inoculated onto a solid medium containing 25 mM ferric sulfate, 10 mM glycerol, 0.02% yeast extract, and basal salts, adjusted to pH 2.5, and gelled with Sigma type 1 agarose at a final concentration of 0.5%. A second solid medium, containing 25 mM ferrous sulfate rather than ferric sulfate, was used as a control. Plates were inoculated and incubated either aerobically or anaerobically (Oxoid anaerobic system; Unipath, Basingstoke, United Kingdom) at 45°C.
Correlations between biomass yield and ferric iron reduction were also assessed in liquid medium. S. acidophilus YTF1, S. thermosulfidooxidans TH1, and A. ferrooxidans TH3 were each grown in a medium containing 10 mM glycerol, 0.02% yeast extract, and basal salts (pH 2.0) and supplemented with different concentrations of ferric sulfate (5, 10, and 25 mM). Control cultures containing either 25 mM ferrous sulfate or no added iron were also prepared. Cultures (20 ml in 25-ml universal tubes) were incubated under anaerobic conditions (Oxoid anaerobic jars) at 45°C for up to 7 days. Microbial biomasses were determined by measuring the optical densities (at 600 nm) of harvested cells resuspended in basal salts solution and by cell counting by using a Thoma bacterial counting chamber and phase-contrast microscopy (magnification, ×400). Ferric iron reduction in these cultures was determined by measuring concentrations of ferrous iron at the end of incubation.
Dissolution of ferric iron-containing minerals.
Three solid-phase ferric iron minerals were prepared in the laboratory. Amorphous ferric hydroxide [Fe(OH)3] was prepared by the method of Lovley and Phillips (12); a 0.4 M solution of FeCl3 was neutralized to pH 7.0 with NaOH, and the resulting precipitate was collected by centrifugation, washed twice with distilled water, and dried at 40°C. Jarosite [KFe3(SO4)2(OH)6] was prepared biologically by using the mesophilic iron-oxidizing acidophile Thiobacillus ferrooxidans ATCC 23270 (10); the bacterium was grown in liquid medium containing 100 mM ferrous sulfate and 200 mM potassium sulfate (in basal salts). After 10 days, the bacteria and precipitates were removed by filtration, and the clear orange-brown liquor was kept at room temperature for an additional 4 to 5 weeks, during which jarosite precipitated. Goethite (αFeOOH) was synthesized by the method of Atkinson et al. (1); sodium hydroxide pellets were added to a 0.4 M solution of ferric chloride to give a final pH of 12.0, the solution was aged for 1 week (at room temperature) and then heated at 90°C for 16 h, and the resulting precipitate was collected and washed thoroughly to remove excess chloride. The identities and purities of all of the synthesized ferric iron minerals were confirmed by X-ray diffraction analysis.
The dissolution of the three ferric iron-containing minerals by four of the moderate thermophiles (strains ALV, YTF1, TH1, and TH3) was assessed by monitoring changes in both ferrous iron and total iron concentrations in cultures grown heterotrophically under limited aeration conditions (100 ml of medium in unshaken 100-ml conical flasks). Liquid media containing 10 mM glycerol, 0.02% yeast extract, and basal salts (adjusted to pH 2.0) were supplemented with 0.1% (wt/vol) amorphous ferric hydroxide, 0.1% (wt/vol) jarosite, or 0.1% (wt/vol) goethite and incubated for up to 37 days at 45°C.
Iron analysis.
The ferrous iron concentrations in shake flask experiments were determined titrimetrically by using 1 mM potassium permanganate. The ferrous iron concentrations in cultures incubated anaerobically and cultures containing ferric iron minerals were measured colorimetrically by the ferrozine assay (13). The latter method provided greater sensitivity and did not suffer from interference with tetrathionate (as the titrimetric method did). Although the ferrozine assay requires a neutral pH, it was found to be appropriate for use with cultures of acidophilic microorganisms due to the adequate buffering capacity of the HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) buffer in the reagent solution. The total soluble iron concentrations were measured by atomic absorbance spectroscopy (Pye Unicam SP 2900), and the ferric iron concentration was estimated from the difference between the total iron and ferrous iron concentrations.
RESULTS
Oxidoreduction of iron by mixotrophically and heterotrophically grown bacteria.
All five strains of the three species of moderately thermophilic acidophilic bacteria examined were able to reduce ferric iron. As with mesophilic acidophilic heterotrophic bacteria, iron reduction did not require strictly anoxic conditions, although one obvious problem in assessing this phenomenon with microorganisms that also have the capacity to catalyze the oxidation of iron is that any ferrous iron produced may be reoxidized in the presence of O2. However, ferrous iron oxidation by the moderate thermophiles was found to be completely inhibited when the bacteria were grown under strictly anoxic conditions, which allowed iron reduction to be more readily assessed. In contrast, no reduction of ferric iron was detected when cultures were grown in shake flasks, in which the conditions were aerobic. Control cultures, which either were cell free or contained heat-killed cells of S. acidophilus YTF1, displayed no (<1 mM over 200 h) oxidation or reduction of iron, indicating that the iron transformations observed were biological rather than abiotic.
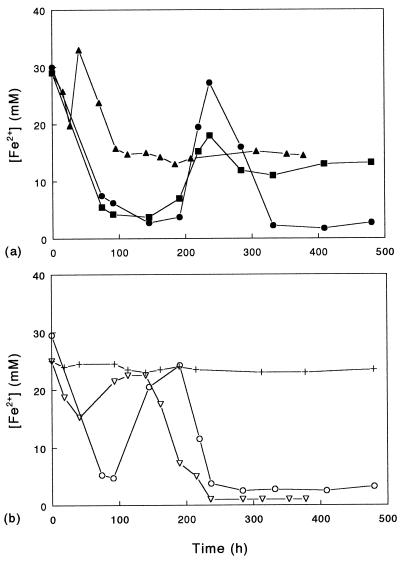
When the organisms were grown under conditions under which the aeration status of cultures was not controlled (nonshaken cultures), cycles of oxidoreduction of iron were observed (Fig. 1). Under the culture conditions used in this experiment, growth of the moderate thermophiles was predicted to be mixotrophic. The ferrous iron concentrations recorded were sometimes greater than the concentration added to the culture medium due to carryover of ferrous iron from the inoculum. The results presented below are means from duplicate cultures and are representative of data obtained from several separate experiments, so that the trends noted (lag periods, etc.) are considered meaningful. While all five isolates displayed basically similar patterns of oxidoreduction, there were subtle differences between bacterial species and between strains. For example, two of the three S. acidophilus strains, ALV and THWX, displayed similar rates (although different magnitudes) of oxidoreduction. In contrast, net iron reduction began in cultures of the Yellowstone isolate (YTF1) after a far shorter incubation period, and this was followed by a second phase of net oxidation; a ferrous/ferric ratio of about 1:1 was subsequently maintained in S. acidophilus YTF1 cultures over ca. 300 h. Cycles of oxidoreduction of iron were also observed under such growth conditions when iron was provided initially as ferric iron rather than ferrous iron (data not shown).
FIG. 1.
Oxidoreduction of iron by strains of moderately thermophilic, acidophilic bacteria grown in nonshaken cultures (100 ml of medium/250-ml conical flask) containing 25 mM ferrous sulfate, 10 mM glycerol, and 0.02% (wt/vol) yeast extract (pH 2.0) and incubated at 45°C. Symbols: •, S. acidophilus ALV; ▪, S. acidophilus THWX; ▴, S. acidophilus YTF1; ○, S. thermosulfidooxidans TH1; ▿, A. ferrooxidans TH3; +, cell-free control.
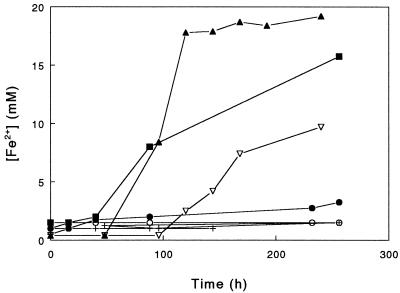
Growth of moderate thermophiles in glycerol medium containing ferric sulfate rather than ferrous sulfate is necessarily heterotrophic, at least until ferrous iron is generated by the reductive process. Figure 2 shows the reduction of ferric iron in cultures incubated under anaerobic conditions. Again, differences between species and between strains were apparent. Only three of the bacteria (S. acidophilus YTF1 and THWX and A. ferrooxidans TH3) were able to reduce ferric iron under these conditions, and both the greatest rate and the greatest extent of iron reduction were displayed by the Yellowstone isolate (YTF1).
FIG. 2.
Reduction of ferric iron by strains of moderately thermophilic, acidophilic bacteria grown in anaerobic cultures containing 20 mM ferric sulfate, 10 mM glycerol, and 0.02% (wt/vol) yeast extract (pH 2.0) and incubated at 45°C. Symbols: •, S. acidophilus ALV; ▪, S. acidophilus THWX; ▴, S. acidophilus YTF1; ○, S. thermosulfidooxidans TH1; ▿, A. ferrooxidans TH3; +, cell-free control.
Oxidoreduction of iron by autotrophically grown bacteria.
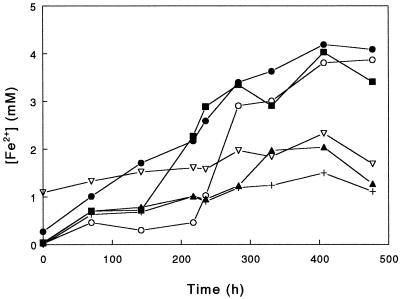
Attempts to grow the moderate thermophiles autotrophically on tetrathionate as the electron donor and ferric iron as the sole electron acceptor were unsuccessful with both A. ferrooxidans TH3 and S. acidophilus YTF1. No reduction of iron or cell growth was observed on either of the two occasions on which this experiment was performed. In contrast, ferric iron reduction was observed with the other two strains of S. acidophilus (ALV and THWX) and with S. thermosulfidooxidans TH1 grown under anoxic conditions (Fig. 3). While there was some limited abiotic oxidation of tetrathionate by ferric iron (Fig. 3), it was apparent that these three bacteria were able to couple tetrathionate oxidation and ferric iron reduction. Phase-contrast microscopy indicated that the numbers of cells in these cultures were greater at the end of incubation than at the start of incubation, although no quantitative data were recorded. The maximum ferrous iron concentration measured in these cultures (approximately 4 mM) was considerably less than the maximum concentration in corresponding heterotrophically grown cultures.
FIG. 3.
Reduction of ferric iron by strains of moderately thermophilic, acidophilic bacteria grown in anaerobic cultures containing 20 mM ferric sulfate and 5 mM potassium tetrathionate (pH 2.0) and incubated at 45°C. Symbols: •, S. acidophilus ALV; ▪, S. acidophilus THWX; ▴, S. acidophilus YTF1; ○, S. thermosulfidooxidans TH1; ▿, A. ferrooxidans TH3; +, cell-free control.
Anaerobic growth of iron-oxidizing moderate thermophiles.
All five of the acidophilic bacteria used in the present study grew well on overlaid ferrous iron solid media (6); however, overlaid media were inappropriate for assessing ferric iron-coupled growth of the moderate thermophiles, as the indigenous bacterium Acidiphilium sp. strain SJH also reduces ferric iron on these plates (7). Only three of the strains (S. acidophilus ALV, S. thermosulfidooxidans TH1, and A. ferrooxidans TH3) were able to grow on agarose-gelled nonoverlaid media. Growth of these bacteria occurred on solid media containing either ferric iron or ferrous iron when they were incubated aerobically; ferrous iron was oxidized under these conditions, but there was no reduction of ferric iron. In contrast, when the same cultures were incubated anaerobically, growth was apparent only on the plates which contained ferric iron. Small (diameter, 1 to 2 mm) colonies were visible after 8 days of incubation. The initially orange medium became increasingly bleached around the developing bacterial colonies, indicating that ferric iron reduction was occurring.
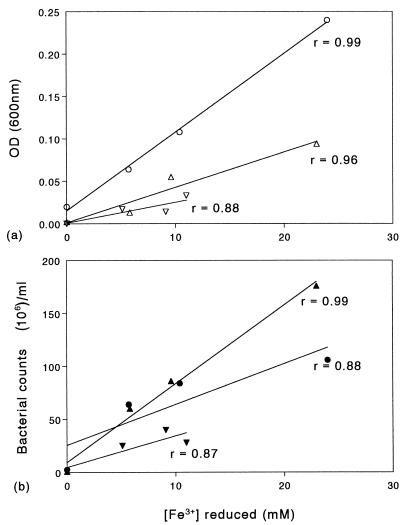
More definitive evidence that ferric iron reduction could support the growth of moderately thermophilic iron-oxidizing bacteria under anaerobic conditions came from studies performed in liquid media (Fig. 4). Under the conditions used, both S. acidophilus YTF1 and A. ferrooxidans TH3 reduced up to 25 mM ferric iron, although more limited reduction (ca. 11 mM) was observed with S. thermosulfidooxidans TH1. Biomass yields, measured either as optical densities of resuspended bacteria or from cell counts, were closely correlated with the amount of ferric iron reduced. The biomass yields in anaerobic cultures which contained 25 mM ferrous iron were similar to the biomass yields obtained in control cultures to which no iron had been added (data not shown).
FIG. 4.
Relationship between ferric iron reduction and biomass yields, as determined by optical densities of resuspended bacteria (a) and counting of moderately thermophilic iron-oxidizing bacterial cells grown under anaerobic conditions (b). Symbols: ○ and •, S. acidophilus YTF1; ▿ and ▾, S. thermosulfidooxidans TH1; ▵ and ▴, A. ferrooxidans TH3. The linear correlation coefficients (r values) are shown. OD (600 nm), optical density at 600 nm.
Dissolution of ferric iron minerals by moderate thermophiles.
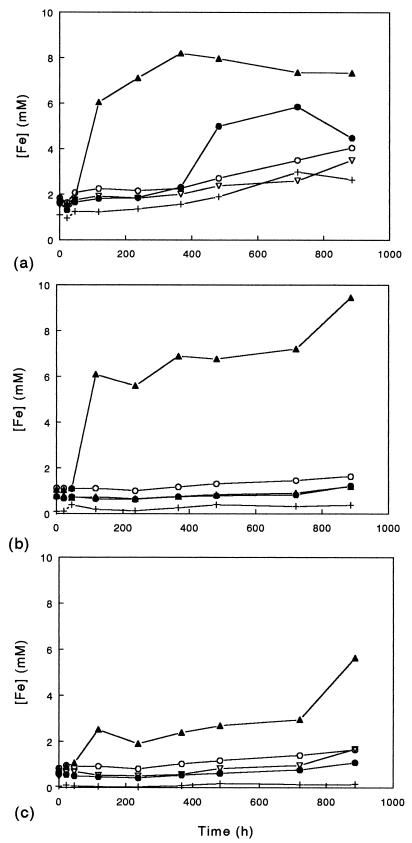
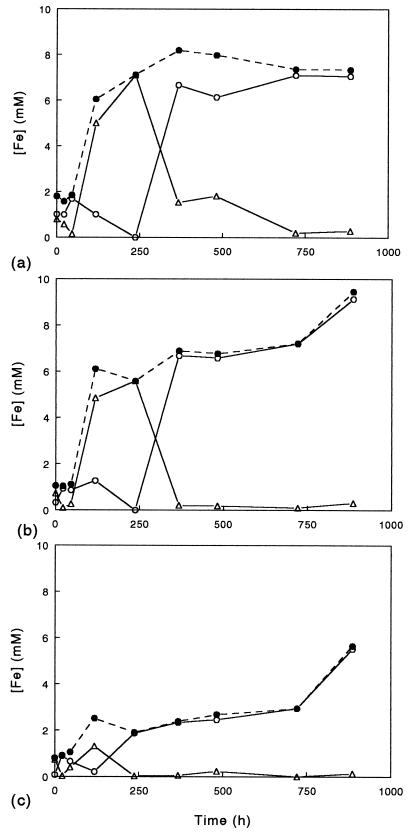
Solubilization of amorphous ferric hydroxide, jarosite, and goethite by the moderately thermophilic bacteria is shown in Fig. 5. Only S. acidophilus YTF1 caused significant dissolution of all three minerals, although there was also a detectable increase in the soluble iron concentration in cultures of S. acidophilus ALV which contained amorphous ferric hydroxide. When estimates were determined on the basis of the amounts of mineral ferric iron originally present, we calculated that 78% of the amorphous mineral, 100% of the jarosite, and 50% of the goethite were solubilized after 900 h. However, since there was some limited evaporation from these cultures (resulting in some concentration of soluble iron), these figures probably overestimate the true extent of mineral dissolution. The speciation of iron in mineral-containing cultures of S. acidophilus YTF1 is shown in Fig. 6. Cultures containing each of the three minerals showed similar patterns; there was an initial increase in soluble ferric iron due to the oxidation of ferrous iron present in the inoculum, which was followed by a phase of reductive dissolution of the mineral during which soluble iron was predominantly in the ferrous form and a third phase in which all (or almost all in the case of amorphous ferric hydroxide) of the ferrous iron was oxidized to ferric iron. Interestingly, while most mineral dissolution occurred when YTF1 was catalyzing net reduction of ferric iron, there appeared to be continued dissolution of jarosite and goethite during the later oxidative phase.
FIG. 5.
Dissolution of amorphous ferric hydroxide (a), jarosite (b), and goethite (c) by moderately thermophilic, acidophilic bacteria. Cultures were grown under microaerobic conditions in media (pH 2.0) containing 10 mM glycerol and 0.02% (wt/vol) yeast extract; each of the media also contained one of the ferric iron-containing minerals at a concentration of 0.1%, and the media were incubated at 45°C. Symbols: •, S. acidophilus ALV; ▴, S. acidophilus YTF1; ○, S. thermosulfidooxidans TH1; ▿, A. ferrooxidans TH3; +, cell-free control.
FIG. 6.
Speciation of soluble iron in cultures of S. acidophilus YTF1 grown under microaerobic conditions in media (pH 2.0) containing 10 mM glycerol and 0.02% (wt/vol) yeast extract and supplemented with 0.1% (wt/vol) amorphous ferric hydroxide (a), 0.1% (wt/vol) jarosite (b), or 0.1% (wt/vol) goethite (c). Symbols: •, total iron; ▵, ferrous iron; ○, ferric iron.
DISCUSSION
One of the characteristics of moderately thermophilic iron-oxidizing acidophilic bacteria is their highly versatile metabolic capabilities in terms of energy acquisition and carbon dioxide fixation. The results presented here illustrate another aspect of the metabolic diversity of these organisms. The ability of iron-oxidizing moderate thermophiles to use ferric iron as an electron acceptor (with either organic or inorganic electron donors) in anoxic cultures implies that Sulfobacillus spp. and A. ferrooxidans are facultative anaerobes rather than strict aerobes. Evidence that bacterial growth occurs under such conditions came from the development of colonies on solid media which contained ferric iron but not ferrous iron and from the observation that bacterial yields were closely correlated with the amounts of ferric iron reduced when the moderate thermophiles were grown in the absence of oxygen. The fact that neither cell-free controls nor controls inoculated with heat-killed cells of the most active iron-reducing moderate thermophile (S. acidophilus YTF1) showed evidence of ferric reduction indicates that the observed reduction phenomenon was not due to any medium components or to any reducing chemical transferred with the inocula.
Iron reduction by moderate thermophiles does not require strictly anoxic conditions, as illustrated by its occurrence in unshaken flask cultures incubated under air. Reduction of ferric iron by some strains of mesophilic heterotrophic acidophiles has been found to be more rapid and extensive when the bacteria are grown under (micro)aerobic conditions than when they are grown under anaerobic conditions (7). Although the current experiments did not involve monitoring or control of dissolved oxygen concentrations, other experimental work involving S. acidophilus THWX and YTF1 grown in fermentor cultures has indicated that these bacteria may display net ferric iron reduction when dissolved oxygen concentrations are as high as 40 to 60% of the maximum value (2, 8). On the basis of previous work (8), the phases of oxidoreduction of iron observed when the moderate thermophiles were grown unshaken at atmospheric partial O2 pressure can be interpreted as follows: (i) an initial phase of net iron oxidation due to the presence of dissolved oxygen, which became increasingly depleted due to bacterial metabolism, resulted in (ii) a second phase characterized by net iron reduction during which bacterial activity declined or ceased and dissolved oxygen levels increased (by diffusion), which gave rise to (iii) a second phase of net iron oxidation. The more immediate reduction of iron observed with strain YTF1 than with the other two strains of S. acidophilus (ALV and THWX) was probably related to the much higher growth rate of the Yellowstone isolate (5).
While all five moderate thermophiles reduced ferric iron in unshaken flask cultures incubated aerobically, two strains (S. acidophilus ALV and S. thermosulfidooxidans) failed to do so in corresponding liquid media which were incubated anaerobically on both occasions that the experiment was performed. The reason for this is not clear, particularly since both of these bacteria reduced iron and formed colonies when they were cultured anaerobically on glycerol-ferric iron solid medium and also reduced ferric iron in anaerobic liquid media which contained tetrathionate. In contrast, the reasons why S. acidophilus YTF1 and A. ferrooxidans TH3 failed to reduce iron and also showed no evidence of cell number increases in anaerobic ferric iron-tetrathionate medium are more readily understood. A. ferrooxidans has been reported to have a very poor capacity to oxidize reduced sulfur compounds (4), and whereas S. acidophilus has been reported to oxidize elemental sulfur, it is a “heterotrophically inclined” bacterium that requires exogenous organic carbon for good growth (5). For the three moderate themophiles that did couple tetrathionate oxidation to ferric iron reduction, the maximum ferric iron concentration observed, about 4 mM, was far lower than the maximum ferric iron concentration indicated by the following equation, the stoichiometry of which predicts that all of the ferric iron present (20 mM) should have been reduced with an initial tetrathionate concentration of 5 mM: S4O62− + 10H2O + 14Fe3+→4HSO4− + 16H+ + 14Fe2+. However, in the closed systems used in this experiment, autotrophic growth of the bacteria would have been severely limited by the availability of CO2. It would be interesting to ascertain if enhanced CO2 concentrations promote more extensive tetrathionate-coupled ferric iron reduction under anaerobic conditions.
Only one of the four moderately thermophilic isolates tested, S. acidophilus YTF1, solubilized amorphous ferric hydroxide, jarosite, and goethite. This reflected the fact that this acidophile had a greater propensity than the other organisms to reduce soluble ferric iron by using glycerol as an electron donor, as noted in previous experiments. The fact that jarosite was solubilized more rapidly and more extensively than goethite reflects the different thermodynamic stabilities of these two minerals; the same trend was found in similar experiments performed with mesophilic acidophilic heterotrophs (2). The mechanism of ferric mineral solubilization by YTF1 appears to involve reductive dissolution. As indicated in the following equation, the reduction of soluble ferric iron by the bacteria should result in the equilibrium between solid-phase iron(III) and soluble-phase iron(III) (which is normally heavily biased in favor of the mineral form) being shifted somewhat, accelerating the dissolution of the mineral: Fe3+solid phase↔ Fe3+soluble→Fe2+ (by bacterial reduction). In the case of jarosite, most of the mineral was dissolved during the net iron reduction phase (i.e., when most or all of the soluble iron was present as ferrous iron). However, the further dissolution (also observed with goethite) which occurred when there was no net ferric iron reduction suggests that there is a possible second mechanism for accelerated mineral dissolution by S. acidophilus YTF1. This later phase of mineral solubilization might have resulted from the production of metabolites, such as organic acids, by the moderate thermophile, although this hypothesis has yet to be tested.
The ability of moderately thermophilic iron-oxidizing bacteria to use ferric iron as an alternative electron acceptor allows them to exploit environments which experience intermittent or continuous oxygen depletion. The lower solubility of oxygen in thermal environments than in those of lower temperatures implies that the organisms in thermal environments are more likely to experience oxygen limitation. Examples of thermal environments are the bulk central cores of heap leaching and mineral waste dumps, which are normally microaerobic or anoxic and which may be maintained at elevated temperatures by exothermic mineral oxidation reactions in the surface zones, and acidic geothermal areas (such as solfatara fields). In both situations, reduced sulfur compounds may be more important electron donors than organic compounds, as these environments often contain low concentrations of dissolved carbon. Ferric iron reduction by moderate thermophiles also has potential for improving commercial mineral processing, particularly in tank reactors, the majority of which operate at 40 to 50°C. Although these reactors are normally vigorously aerated (to promote mineral oxidation), the possibility of controlling aeration to promote occasional anoxic conditions could be considered as a means of removing passivation layers of jarosite and other ferric mineral deposits which accumulate during bioleaching of ores, thereby facilitating more efficient ore oxidation and metal recovery.
ACKNOWLEDGMENTS
We thank Paul Norris (Warwick University, Warwick, United Kingdom) for providing cultures of moderately thermophilic bacteria and Kevin Hallberg (University of Wales, Bangor, United Kingdom) for constructive criticism of the manuscript.
T.A.M.B. is grateful to the Biological and Biotechnology Research Council (United Kingdom) for providing a research studentship.
REFERENCES
- 1.Atkinson R J, Posner A M, Quirk J P. Crystal nucleation and growth in hydrolysing iron(III) chloride solutions. Clays Clay Miner. 1977;25:9–56. [Google Scholar]
- 2.Bridge, T. A. M., and D. B. Johnson. Unpublished data.
- 2a.Brierley J A, Norris P R, Kelly D P, Le Roux N W. Characteristics of a moderately thermophilic and acidophilic iron-oxidizing Thiobacillus. Eur J Appl Microbiol. 1978;5:291–299. [Google Scholar]
- 3.Brock T D, Gustafson J. Ferric iron reduction by sulfur- and iron-oxidizing bacteria. Appl Environ Microbiol. 1976;32:567–571. doi: 10.1128/aem.32.4.567-571.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clark D A, Norris P R. Acidimicrobium ferrooxidans gen. nov., sp. nov.: mixed-culture ferrous iron oxidation with Sulfobacillus species. Microbiology. 1996;141:785–790. doi: 10.1099/00221287-142-4-785. [DOI] [PubMed] [Google Scholar]
- 5.Ghauri M A, Johnson D B. Physiological diversity amongst some moderately thermophilic iron-oxidising bacteria. FEMS Microbiol Ecol. 1991;85:327–334. [Google Scholar]
- 5a.Johnson, D. B. Unpublished data.
- 6.Johnson D B. Selective solid media for isolating and enumerating acidophilic bacteria. J Microbiol Methods. 1995;23:205–218. [Google Scholar]
- 7.Johnson D B, McGinness S. Ferric iron reduction by acidophilic heterotrophic bacteria. Appl Environ Microbiol. 1991;57:207–211. doi: 10.1128/aem.57.1.207-211.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson D B, Ghauri M A, McGinness S. Biogeochemical cycling of iron and sulphur in leaching environments. FEMS Microbiol Rev. 1993;11:63–70. [Google Scholar]
- 9.Lane D J, Harrison A P, Jr, Stahl D, Pace B, Giovannoni S J, Olsen G J, Pace N R. Evolutionary relationships among sulfur- and iron-oxidizing eubacteria. J Bacteriol. 1992;174:269–278. doi: 10.1128/jb.174.1.269-278.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lazaroff N, Sigal W, Wasserman A. Iron oxidation and precipitation of ferric hydroxysulfates by resting Thiobacillus ferrooxidans. Appl Environ Microbiol. 1982;43:924–938. doi: 10.1128/aem.43.4.924-938.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lovley D R. Microbial reduction of iron, manganese, and other metals. Adv Agron. 1995;54:175–231. [Google Scholar]
- 12.Lovley D R, Phillips E J P. Organic matter mineralization with reduction of ferric iron in anaerobic sediments. Appl Environ Microbiol. 1986;51:683–689. doi: 10.1128/aem.51.4.683-689.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lovley D R, Phillips E J P. Rapid assay for microbially reducible ferric iron in aquatic sediments. Appl Environ Microbiol. 1987;53:1536–1540. doi: 10.1128/aem.53.7.1536-1540.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Norris P R, Barr D W. Growth and iron oxidation by acidophilic moderate thermophiles. FEMS Microbiol Lett. 1985;28:221–224. [Google Scholar]
- 15.Norris P R, Johnson D B. Acidophilic microorganisms. In: Horikoshi K, Grant W D, editors. Extremophiles: microbial life in extreme environments. New York, N.Y: Wiley-Liss; 1998. pp. 133–154. [Google Scholar]
- 16.Norris P R, Clark D A, Owen J P, Waterhouse S. Characteristics of Sulfobacillus acidophilus sp. nov. and other moderately thermophilic mineral sulphide-oxidizing bacteria. Microbiology. 1996;141:775–783. doi: 10.1099/00221287-142-4-775. [DOI] [PubMed] [Google Scholar]
- 17.Pronk J T, Johnson D B. Oxidation and reduction of iron by acidophilic bacteria. Geomicrobiol J. 1992;10:153–171. [Google Scholar]
- 18.Wood A P, Kelly D P. Autotrophic and mixotrophic growth of three thermo-acidophilic iron-oxidizing bacteria. FEMS Microbiol Lett. 1985;20:107–112. [Google Scholar]