Abstract
We developed polyamidoamine dendrimers conjugated with epidermal growth factor (EGF) for use in receptor-mediated delivery of therapeutics to cancer cells. Here, we demonstrate the utility of this approach to inhibit proliferation and migration of head and neck squamous carcinoma cells through targeting of EPS8, a key regulator of squamous carcinoma growth and motility. Use of EGF-dendrimers to deliver siRNA or shRNA against EPS8 resulted in inhibition of cell growth and reduction in cell motility. Moreover, more profound repression of the target protein was obtained with repeat exposure to the targeting reagent, and was consistent with the altered biological properties. Thus, targeting of EPS8 can be achieved with EGF-conjugated dendrimers delivering EPS8-specific RNAi therapeutics, leading to a reduction in the malignant phenotype of cells.
Keywords: Dendrimer, Oral cancer, Signal transduction, EGFR, Targeted therapy
1. Introduction
The use of nanoscale therapeutics has many advantages over existing modalities to treat human disease. Amongst these nanovectors, the well-defined and highly branched structure of dendrimers affords great flexibility for modification in terms of cell-specific targeting and delivery of a large payload of pharmacological or genetic therapeutics, or combinations thereof, as well as use in imaging applications. Previous studies have utilized a number of targeting moieties such as folate (Xu et al., 2017a,b, 2016), antibodies (Yuan et al., 2011), or growth factors to deliver nanoparticles to specific cell types (Yu et al., 2012), or even encapsulation within proteins such as ferritin (Fan et al., 2012), resulting in higher efficiency of uptake into cells (Wang and Thanou, 2010).
Whereas signal transduction through the epidermal growth factor receptor (EGFR) by EGF or related ligands is fundamental to epithelial cell growth and differentiation, many human malignancies of epithelial origin express the EGFR at greatly elevated levels. Although this contributes to tumor progression, it also provides an opportunity to utilize the differential EGFR expression between normal and malignant epithelia to deliver therapeutics to cancer cells with higher efficiency. In our previous work, we developed EGF-conjugated polyamidoamine (PAMAM) dendrimers and used NIH3T3 fibroblasts engineered to overexpress the human EGFR to demonstrate rapid uptake of nanoparticles in a ligand- and receptor-specific manner (Yuan et al., 2010), as well as delivery of siRNA with successful gene knockdown, but with minimal effects on key downstream signaling pathways.
EPS8 (EGFR pathway substrate 8) is a critical effector of EGF signaling to the actin cytoskeleton (Scita et al., 1999) as well as regulating expression of the FOXM1 transcription factor (Wang et al., 2010), with major influences on cell cycle regulation and squamous cell carcinoma development (Gemenetzidis et al., 2009). In this work, we tested whether targeted reduction of EPS8 by RNAi delivered by EGF-conjugated dendrimers would have implications for both cell growth and motility, two important aspects of the malignant phenotype.
2. Materials and methods
2.1. Materials
EDA core PAMAM dendrimer generation 4 (technical grade) was purchased from Dendritech (Midland, MI). Triglycine, N-hydroxysuccinimide (NHS), and 1-ethyl-3-[3-dimethylaminopropyl]carbodiimide hydrochloride (EDC) were purchased from Sigma-Aldrich (St. Louis, MO). Recombinant human EGF was purchased from Austral Biologicals (San Ramon, CA). Antibodies that recognize EPS8 (clone 15) and actin (sc-1616) were purchased from BD Biosciences (Carlsbad, CA) and Santa Cruz Biotechnologies Inc. (Santa Cruz, CA), respectively. Horseradish peroxidase-conjugated secondary antibodies were obtained from MP Biomedicals (Aurora, OH). TransIT keratinocyte transfection reagent (simply referred to as TransIT) was obtained from Mirus Bio (Madison, WI). EPS8 siRNA was purchased from Qiagen (Valencia, CA). EPS8 shRNA was prepared as described in our previous publication (Wang et al., 2010).
2.2. Synthesis of G4-EGF conjugates
The synthesis followed our previously reported procedure. Briefly, triglycine and EGF were activated with NHS/EDC, respectively. Afterwards, triglycine-NHS and EGF-NHS were conjugated to PAMAM dendrimer G4 in sequence as outlined in Scheme 1 (Yuan et al., 2010).
Scheme 1.
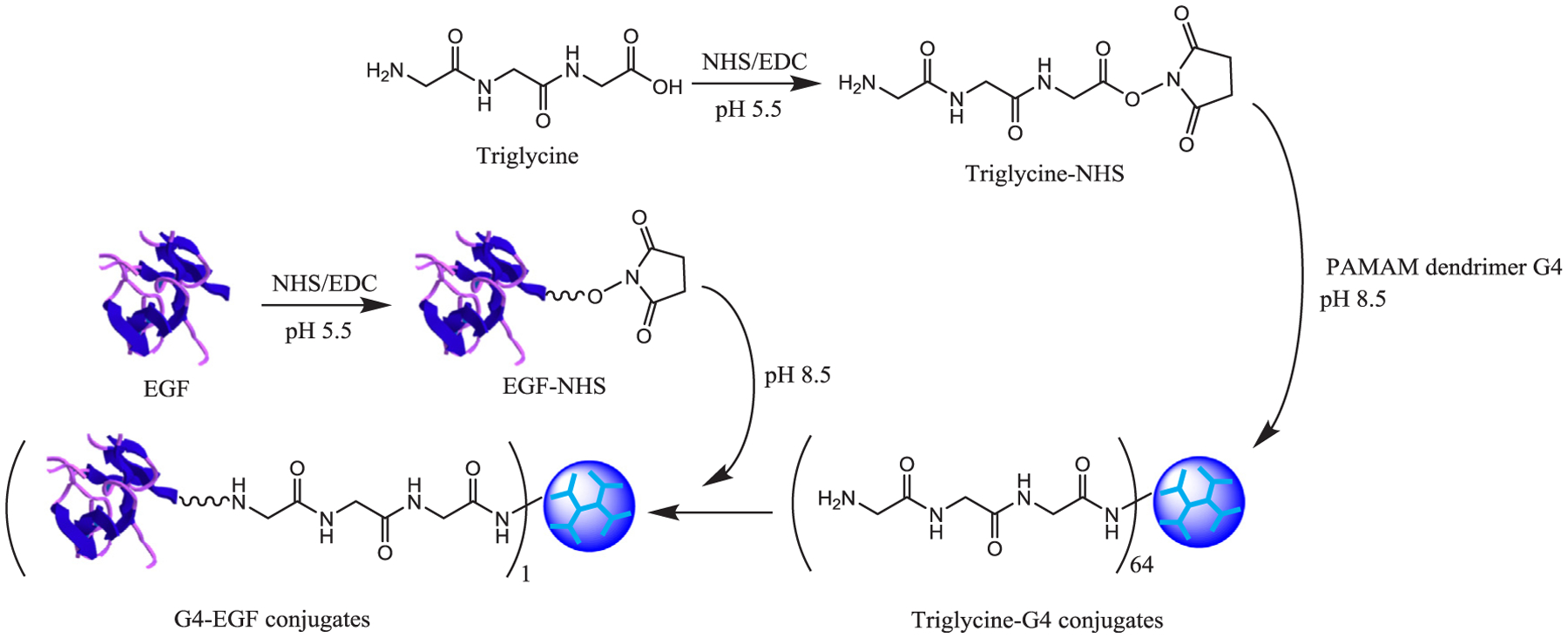
Synthesis of G4-EGF conjugates.
2.3. Cell culture
HN12 cells, derived from a metastatic squamous cell carcinoma, were cultured at 37 °C in a humidified atmosphere containing 10% CO2 and 90% air in Dulbecco’s Modification of Eagle’s Medium (DMEM) supplemented with 10% fetal bovine serum and penicillin-streptomycin (100 units/ml) (Wang et al., 2009).
2.4. Knocking down EPS8 in HN12 cells
HN12 cells were seeded at 2–10 × 103 cells/dish (or well), cultured overnight, and then incubated with one dose of EPS8 siRNA, EPS8 shRNA or non-targeting control (NTC) (4 μg in 60 mm dishes or 2 μg in 6-well plates), which was complexed with vectors (5 μL of TransIT, 10 μg of G4-EGF, or 10 μg of G4 in 60 mm dishes, or half quantities in 6-well plates) for 6 h. Afterwards, the media were replaced, and the cells were cultured until they reached 80% confluence. In multi-dose treatment, two additional doses were added to the cells for 6 h incubation every other day. Untreated cells were included as a control. The transfected cells were subjected to western blot analysis or used in scratch assays.
2.5. Western blot analysis
Cells were washed twice in ice-cold PBS, and lysed in 50 mM Hepes pH 7.5, 150 mM NaCl, 1 mM EDTA, 0.1% Tween-20, 1 mM PMSF, 20 μg/mL aprotinin, and 20 μg/mL leupeptin, on ice for 10 min, scraped immediately, and transferred to sterile 1.5 mL microcentrifuge tubes. The supernatant was transferred to a fresh microcentrifuge tube after 10 min of microcentrifugation at 10,000g at 4 °C. Cleared lysates were quantified using a modified Bradford assay (BCA; Bio-Rad, Hercules, CA), and equivalents amounts of protein were resolved by SDS-PAGE and then transferred to polyvinylidene difluoride (PVDF) membrane (Immobilon-P; Millipore, Billerica, MA). Membranes were blocked in 5% skimmed milk in TTBS (10 mM Tris-HCl, pH 7.6, 0.5% Tween-20, 150 mM NaCl) for 1 h at room temperature and then incubated in primary antibodies diluted 1:1000 in blocking buffer overnight at 4 °C. After washing in TTBS, bound primary antibodies were detected using horseradish peroxidase-conjugated secondary antibodies (MP Biomedicals, Solon, OH) and ECL Western Lightning (Perkin Elmer Inc., Waltham, MA).
2.6. In vitro scratch assay
Scratch assays were carried out essentially as described previously (Yeudall et al., 2012). The transfected cells (2 × 105 cells/well) were seeded in duplicate in 12-well tissue culture plates in complete medium (DMEM containing 10% FBS) and cultured they reached 100% confluence. Three areas in each well were denuded by scratching with a pipette tip, and the width of each scratch was measured under a light microscope using Axiovision v4.7 software (Carl Zeiss Microimaging, Inc., Thornwood, NY). After 10 h, the width of each scratch was measured again, and migration rate was calculated.
2.7. Cell proliferation assay
Cells (5 × 103 cells/well) were seeded in triplicate in 12-well tissue culture plates in complete medium and cultured for 24 h, after which the medium was replaced with serum-free medium for 24 h. The cells were incubated for 3 days with RNAi complexed with vectors and then subjected to the MTT assay.
2.8. Statistical analysis
Data are expressed as mean ± standard deviation. One-way ANOVA was conducted followed by subgroup comparison using Student-Newman-Keuls Method. P < 0.05 was considered statistically significant.
3. Results
HN12 cells express the EGFR at elevated levels on the cell surface (Cardinali et al., 1995). In our previous work, we examined the uptake of G4.0 dendrimer labeled with quantum dots, with or without EGF as a targeting ligand, by HN12 cells by using fluorescence microscopy. The uptake of EGF-conjugated dendrimers was widespread, with most cells labeled, whereas the cells incubated with dendrimers unconjugated to the targeting ligand remained largely negative, suggesting that the ligand-receptor interaction greatly facilitated uptake (Yuan et al., 2010). Our studies have suggested that overexpression of EPS8 plays a number of key roles in the biology of oral cancer cells (Wang et al., 2010, 2009; Miyazaki et al., 2006). We have also demonstrated the utility of EGF-conjugated dendrimers for delivery of siRNA to tumor cells (Yuan et al., 2010). Therefore, in this work, we sought to determine the ability of EGF-conjugated dendrimers carrying a payload of EPS8-specific siRNA to alter the biological properties of HN12 cells. As shown in Fig. 1, EPS8 expression was reduced by delivery of siRNA by either unconjugated or EGF-conjugated dendrimers by 45–50%, and was improved over the control transfection reagent (12% reduction in expression compared to untreated cells). Multiple delivery by G4-EGF improved reduction in EPS8 expression compared to single treatment (50% reduction versus 40%). Cell motility, as judged by scratch assay also showed a decrease that was most pronounced when cells were treated three times with G4-EGF carrying EPS8 siRNA (Fig. 2).
Fig. 1.
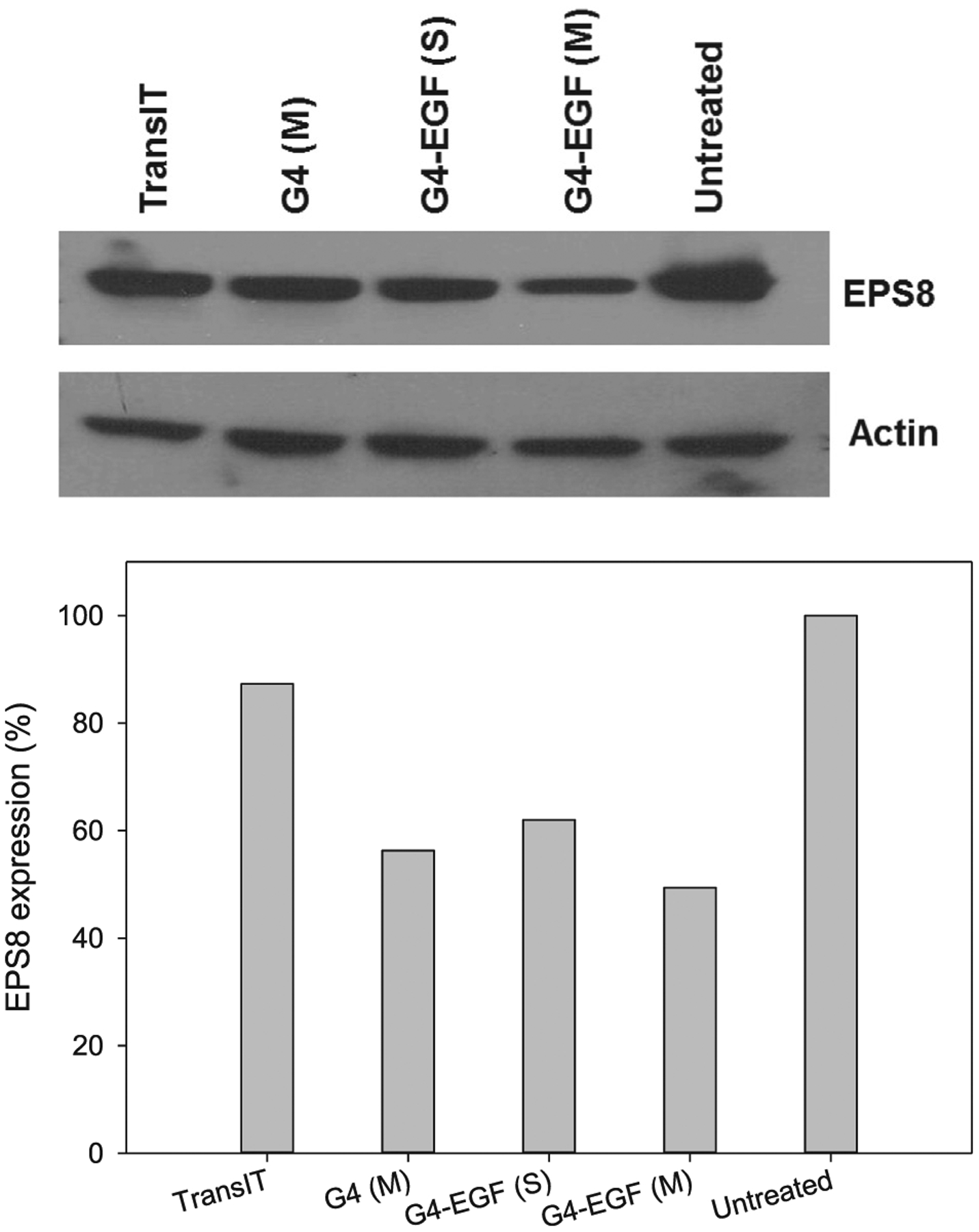
Western blot analysis of EPS8 knockdown in HN12 cells by EPS8 siRNA mediated by different vectors. S: single-dose treatment; M: multi-dose treatment.
Fig. 2.
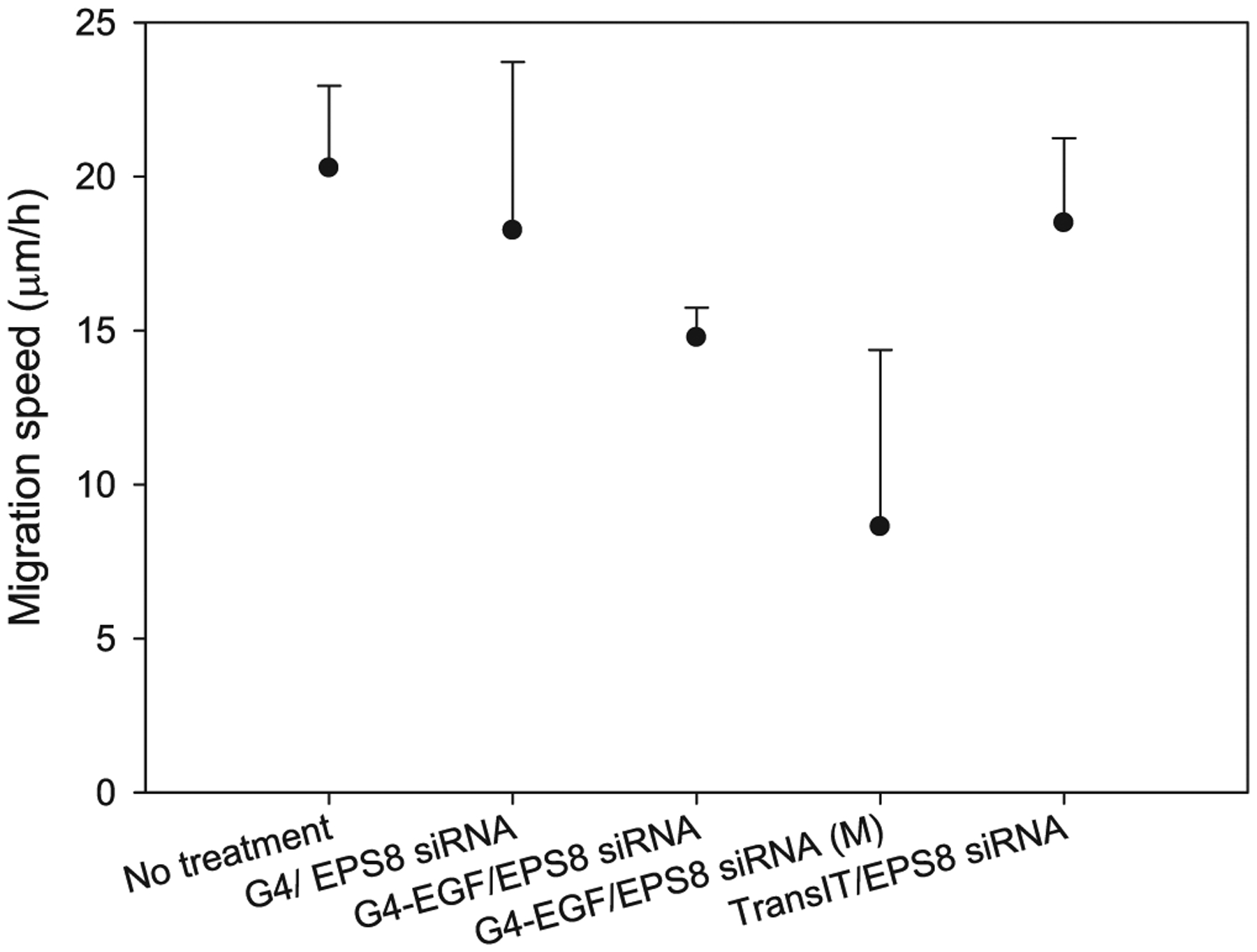
Cell migration assessment after EPS8 knockdown in HN12 cells by EPS8 siRNA.
We also tested the ability of EGF-conjugated and unconjugated dendrimers to inhibit cell proliferation and migration through the delivery of a plasmid that directs expression of EPS8-specific shRNA (Wang et al., 2009). As shown in Fig. 3, cell proliferation was reduced by EPS8 shRNA delivered either by unconjugated or EGF-conjugated dendrimers, although G4-EGF was more effective in this regard. Plasmids encoding a non-targeting control shRNA had no significant effect on proliferation. When we tested the effects on cell migration, we found that G4-EGF combined with EPS8-specific shRNA plasmid had a profound effect on motility across the denuded area of the culture dish (Fig. 4). Taken together, our data suggest that EGF-conjugated dendrimers are effective at delivering RNAi molecules to EGFR-expressing cancer cells, with resultant decreases in cell growth and motility.
Fig. 3.
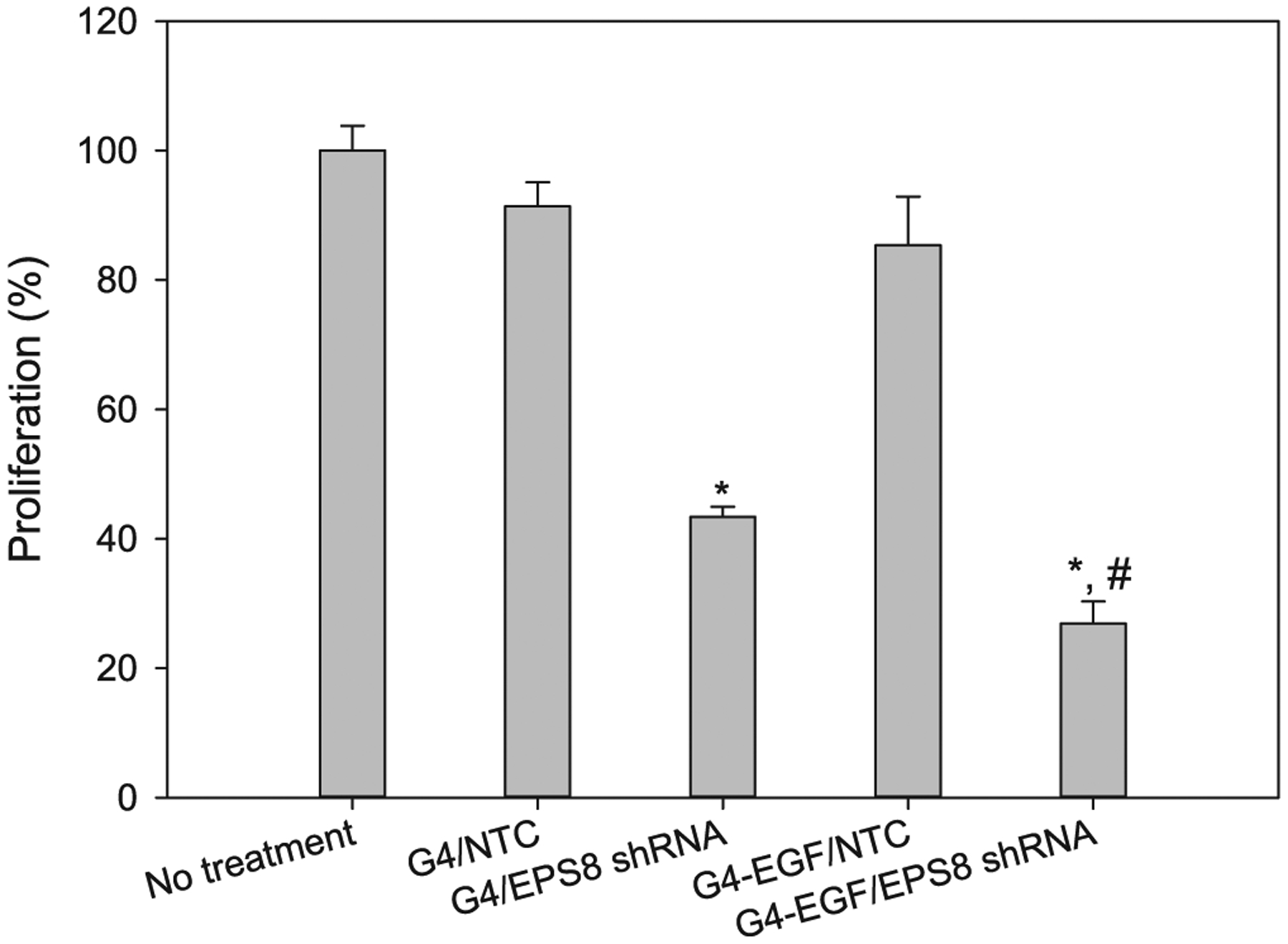
Cell proliferation assessment following treatment with EPS8 shRNA. NTC: non-targeting control sequence. *p < 0.001 versus no treatment, G4/NTC and G4-EGF/NTC; #p < 0.05 versus G4/EPS8 shRNA.
Fig. 4.
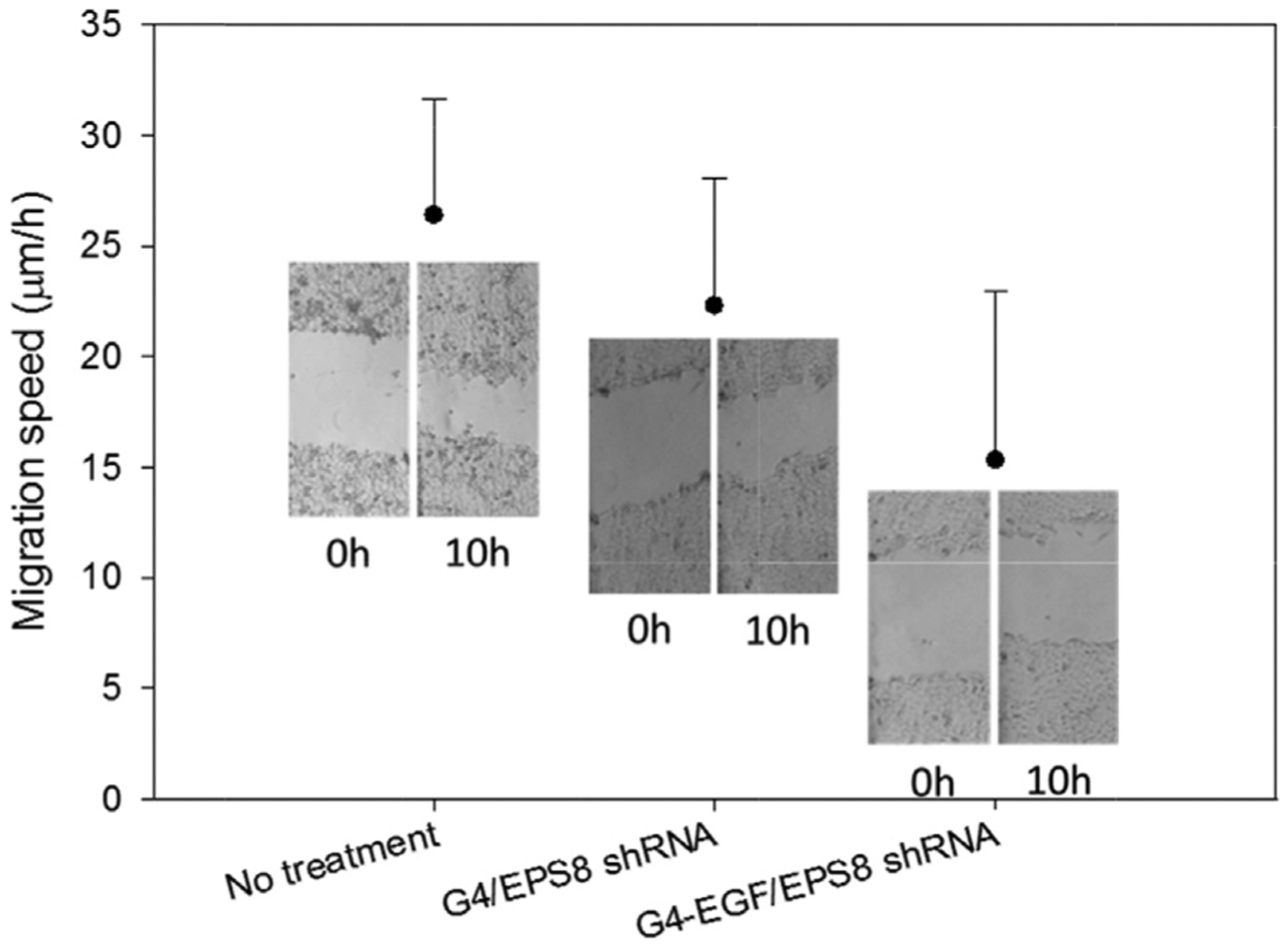
Cell migration assessment after EPS8 knockdown in HN12 cells by EPS8 shRNA. Insets are transmittance images of cells in the scratch assay at the indicated time points.
4. Discussion
In the present study, we have used a gene knockdown approach together with targeted dendrimer nanoparticles to inhibit the expression of EPS8 in oral cancer cells. EPS8 has been reported to be over-expressed in around one third of head and neck cancers, and to correlate with lymph node metastasis (Yap et al., 2009). Our previous work has indicated the importance of EPS8 in squamous carcinogenesis as modest overexpression is sufficient to enhance cell growth and motility in vitro, and to impart a tumorigenic phenotype to otherwise non-tumorigenic cells in vivo (Wang et al., 2010, 2009). Thus, targeting a protein involved in these key aspects of malignancy is likely to have significant impact as a therapeutic approach.
Using EGF-conjugated dendrimers, we found that there was enhanced gene knockdown compared to either unconjugated dendrimers or a commercial transfection reagent. Moreover, a single application using EGF-conjugated dendrimers produced similar inhibition of gene expression to multiple applications using unconjugated nanoparticles. Coupled with rapid uptake into cells, this suggests that ligand-conjugated dendrimers likely represent a highly efficient means to deliver therapeutic agents to target cells.
Many squamous cell carcinomas express high levels of the EGFR compared to normal keratinocytes (Cardinali et al., 1995; Ozanne et al., 1986; Partridge et al., 1988; Prime et al., 1994), making it an attractive gateway into the cancer cell. However, a major concern with the use of EGF as a targeting ligand is the potential for stimulation of growth-promoting pathways, which would be undesirable in cancer treatment. One report has indicated the potential for this (Thomas et al., 2008), but our previous work investigating multiple signaling components downstream of the EGFR did not demonstrate pathway activation following exposure of cells to EGFR-conjugated dendrimers (Yuan et al., 2010). Moreover, EGFR-overexpressing cancer cells may even respond to EGF by growth inhibition (Fan et al., 1995; Jakus and Yeudall, 1996). Nevertheless, it is likely that cancer cells will exhibit a range of biological responses to EGFR engagement, and caution is worthwhile when considering the use of EGF-conjugated anti-cancer therapeutics.
In summary, we have shown that multiple-dose targeting of EPS8 with siRNA or shRNA, delivered by EGF-conjugated dendrimers, is a feasible approach to reduce malignant properties of head and neck cancer cells that overexpress this protein. Moreover, the use of growth factor conjugated dendrimers provides rapid and enhanced uptake into cancer cells expressing elevated levels of the EGFR on the cell surface compared to unconjugated vectors. Ligand-receptor targeting protocols will likely result in a more profound, and possibly more specific, therapeutic response of cancer cells.
Acknowledgement
This work was supported, in part, by the National Science Foundation (CAREER award CBET0954957).
Footnotes
Conflict interest
The authors declare no conflict interest.
References
- Cardinali M, Pietraszkiewicz H, Ensley JF, Robbins KC, 1995. Tyrosine phosphorylation as a marker for aberrantly regulated growth-promoting pathways in cell lines derived from head and neck malignancies. Int. J. Cancer 61 (1), 98–103. [DOI] [PubMed] [Google Scholar]
- Fan K, Cao C, Pan Y, Lu D, Yang D, Feng J, Song L, Liang M, Yan X, 2012. Magnetoferritin nanoparticles for targeting and visualizing tumour tissues. Nat. Nanotechnol 7 (7), 459–464. [DOI] [PubMed] [Google Scholar]
- Fan Z, Lu Y, Wu X, DeBlasio A, Koff A, Mendelsohn J, 1995. Prolonged induction of p21Cip1/WAF1/CDK2/PCNA complex by epidermal growth factor receptor activation mediates ligand-induced A431 cell growth inhibition. J. Cell Biol 131 (1), 235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemenetzidis E, Bose A, Riaz AM, Chaplin T, Young BD, Ali M, Sugden D, Thurlow JK, Cheong SC, Teo SH, Wan H, Waseem A, Parkinson EK, Fortune F, Teh MT, 2009. FOXM1 upregulation is an early event in human squamous cell carcinoma and it is enhanced by nicotine during malignant transformation. PLoS One 4 (3), e4849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakus J, Yeudall WA, 1996. Growth inhibitory concentrations of EGF induce p21 (WAF1/Cip1) and alter cell cycle control in squamous carcinoma cells. Oncogene 12 (11), 2369–2376. [PubMed] [Google Scholar]
- Miyazaki H, Patel V, Wang H, Ensley JF, Gutkind JS, Yeudall WA, 2006. Growth factor-sensitive molecular targets identified in primary and metastatic head and neck squamous cell carcinoma using microarray analysis. Oral Oncol. 42 (3), 240–256. [DOI] [PubMed] [Google Scholar]
- Ozanne B, Richards CS, Hendler F, Burns D, Gusterson B, 1986. Over-expression of the EGF receptor is a hallmark of squamous cell carcinomas. J. Pathol 149 (1), 9–14. [DOI] [PubMed] [Google Scholar]
- Partridge M, Gullick WJ, Langdon JD, Sherriff M, 1988. Expression of epidermal growth factor receptor on oral squamous cell carcinoma. Br. J. Oral Maxillofac. Surg 26 (5), 381–389. [DOI] [PubMed] [Google Scholar]
- Prime SS, Game SM, Matthews JB, Stone A, Donnelly MJ, Yeudall WA, Patel V, Sposto R, Silverthorne A, Scully C, 1994. Epidermal growth factor and transforming growth factor alpha characteristics of human oral carcinoma cell lines. Br. J. Cancer 69 (1), 8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scita G, Nordstrom J, Carbone R, Tenca P, Giardina G, Gutkind S, Bjarnegard M, Betsholtz C, Di Fiore PP, 1999. EPS8 and E3B1 transduce signals from Ras to Rac. Nature 401 (6750), 290–293. [DOI] [PubMed] [Google Scholar]
- Thomas TP, Shukla R, Kotlyar A, Liang B, Ye JY, Norris TB, Baker JR, 2008. Dendrimer-epidermal growth factor conjugate displays superagonist activity. Biomacromolecules 9 (2), 603–609. [DOI] [PubMed] [Google Scholar]
- Wang H, Patel V, Miyazaki H, Gutkind JS, Yeudall WA, 2009. Role for EPS8 in squamous carcinogenesis. Carcinogenesis 30 (1), 165–174. [DOI] [PubMed] [Google Scholar]
- Wang H, Teh M-T, Ji Y, Patel V, Firouzabadian S, Patel AA, Gutkind JS, Yeudall WA, 2010. EPS8 upregulates FOXM1 expression, enhancing cell growth and motility. Carcinogenesis 31, 1132–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Thanou M, 2010. Targeting nanoparticles to cancer. Pharmacol. Res 62 (2), 90–99. [DOI] [PubMed] [Google Scholar]
- Xu L, Kittrell S, Yeudall WA, Yang H, 2016. Folic acid-decorated polyamidoamine dendrimer mediates selective uptake and high expression of genes in head and neck cancer cells. Nanomedicine 11 (22), 2959–2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Bai Q, Zhang X, Yang H, 2017b. Folate-mediated chemotherapy and diagnostics: an updated review and outlook. J. Control. Release [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Yeudall WA, Yang H, 2017a. Folic acid-decorated polyamidoamine dendrimer exhibits high tumor uptake and sustained highly localized retention in solid tumors: its utility for local siRNA delivery. Acta Biomater. 57, 251–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap LF, Jenei V, Robinson CM, Moutasim K, Benn TM, Threadgold SP, Lopes V, Wei W, Thomas GJ, Paterson IC, 2009. Upregulation of Eps8 in oral squamous cell carcinoma promotes cell migration and invasion through integrin-dependent Rac1 activation. Oncogene 28 (27), 2524–2534. [DOI] [PubMed] [Google Scholar]
- Yeudall WA, Vaughan CA, Miyazaki H, Ramamoorthy M, Choi MY, Chapman CG, Wang H, Black E, Bulysheva AA, Deb SP, Windle B, Deb S, 2012. Gain-of-function mutant p53 upregulates CXC chemokines and enhances cell migration. Carcinogenesis 33 (2), 442–451. [DOI] [PubMed] [Google Scholar]
- Yu MK, Park J, Jon S, 2012. Targeting strategies for multifunctional nanoparticles in cancer imaging and therapy. Theranostics 2 (1), 3–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Q, Lee E, Yeudall WA, Yang H, 2010. Dendrimer-triglycine-EGF nanoparticles for tumor imaging and targeted nucleic acid and drug delivery. Oral Oncol. 46 (9), 698–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Q, Fu Y, Kao WJ, Janigro D, Yang H, 2011. Transbuccal delivery of CNS therapeutic nanoparticles: synthesis, characterization, and in vitro permeation studies. ACS Chem. Neurosci 2 (11), 676–683. [DOI] [PMC free article] [PubMed] [Google Scholar]


