Abstract
The latent viral reservoir (LVR) remains a major barrier to HIV-1 curative strategies. It is unknown whether receiving a liver transplant from a donor with HIV might lead to an increase in the LVR because the liver is a large lymphoid organ. We found no differences in intact provirus, defective provirus, or the ratio of intact to defective provirus between recipients with ART-suppressed HIV who received a liver from a donor with (n = 19) or without HIV (n = 10). All measures remained stable from baseline by 1 year posttransplant. These data demonstrate that the LVR is stable after liver transplantation in people with HIV.
Clinical Trials Registration. NCT02602262 and NCT03734393.
Keywords: HIV, HIV cure, latent viral reservoir, liver, organ transplant
We evaluated whether receiving a liver transplant might lead to a change in the HIV latent viral reservoir (LVR). There were no differences in LVR between recipients with HIV who received a liver from a donor with or without HIV.
The latent viral reservoir (LVR) remains one of the major barriers in curative strategies for people with human immunodeficiency virus (PWH) [1]. The LVR is established early in infection, augmented throughout periods of active viral replication, and persists primarily in resting CD4+ T cells, even in the presence of suppressive antiretroviral therapies [2]. Several strategies aimed at eliminating the LVR or inducing durable immunologic control of HIV have been evaluated in clinical trials, and while a small number of PWH have achieved durable antiretroviral therapy (ART)-free remission [3, 4], no broadly applicable and effective therapeutic approach targeting the LVR has been identified.
Previous studies have monitored the LVR over time in kidney transplant recipients with HIV who received organs from donors with and without HIV [5]. In these kidney recipients, despite a dramatic initial reduction in the LVR size among those receiving lymphocyte-depleting induction therapy, the reservoir reconstituted within 1 year, and there were no differences in the LVR size among those receiving lymphocyte nondepleting immunosuppression induction therapy or by allograft donor HIV status [5, 6]. However, one as yet unanswered question is whether the size of the LVR changes with liver transplantation from a donor with or without HIV into PWH. The liver is a secondary lymphoid organ that contains a substantial number of CD4+ T cells and also contains the largest population of tissue-resident macrophages in the body [7]. Accordingly, the liver may be a tissue reservoir of HIV, because HIV DNA and RNA have been found in human hepatocytes [8–11] and liver macrophages, even in the presence of suppressive ART [8, 10–12]. Consequently, the LVR could decrease among individuals who receive an HIV-negative allograft and increase among individuals who receive an HIV-positive allograft. The objective of this study was to evaluate changes in the LVR using the intact proviral DNA assay (IPDA) longitudinally in liver transplant recipients with ART-suppressed HIV participating in the HOPE in Action multicenter trial who received lymphocyte nondepleting therapies and allografts from donors with or without HIV.
METHODS
Study Populations and Procedures
Liver transplant recipients with HIV who received an organ from a deceased donor with or without HIV were longitudinally followed in the HOPE in Action multicenter, national clinical trial (clinicaltrials.gov, NCT02602262 and NCT03734393) [13]. Participant and donor inclusion criteria have been previously described [14]. Recipient CD4 cell count, viral load, and ART regimens at the time of transplantation were obtained by extraction from clinical records. Peripheral blood mononuclear cells (PBMCs) were collected from recipients and processed as previously described [14]. Briefly, PBMCs were collected at the time of transplant, prior to immunosuppression induction therapy (week 0), and at subsequent time points following transplant (approximately at 13, 26, and 52 weeks posttransplant, if applicable). All liver transplant recipients were given lymphocyte nondepleting immunosuppressive therapies. Participants had to have a minimum of 1 follow-up time point after transplant to be included in this study.
Laboratory Testing
IPDA was performed as previously described (Accelevir Diagnostics) [5]. Briefly, total CD4+ T cells were isolated from cryopreserved PBMCs via immunomagnetic selection, and genomic DNA was isolated. IPDA is a droplet digital polymerase chain reaction (ddPCR) assay that targets 2 regions (the packaging signal and envelope) in the HIV genome that are either frequently deleted or hypermutated in defective proviruses [15]. Droplets positive for both fluorescence signals are scored as intact, while 1 fluorescence signal can either indicate (1) a hypermutation and/or 3′ deletion, or (2) a 5′ defective provirus. Double-negative droplets contain either no provirus or defects in both amplicon regions [15]. Of the 87 samples, 82 amplified and the 5 samples that failed to amplify were thus excluded from the analysis.
Donor viral load was evaluated using the Abbot Realtime viral load assay. The limit of detection was 40 copies per mL. Donor CD4 cell counts were measured using the T-cell subset assay (Becton Dickinson).
Statistics
All baseline and follow-up characteristics of the study population were summarized overall and by donor HIV status using descriptive statistics as previously described [5]. The primary outcomes evaluated included intact provirus, defective provirus, ratio of intact/defective provirus frequencies, and CD4+ T-cell counts per μL of blood stratified by donor allograft HIV status. The intact provirus and defective provirus frequencies were analyzed both by frequency per million CD4+ T cells and frequency per mL of blood, as previously described [5]. All values were log10 transformed, except the ratio of intact/defective provirus frequencies, to approximate normal distributions. The biomarkers were plotted over time with locally estimated scatterplot smoothing (LOESS) and t-based approximation of 95% confidence intervals (CIs). All analyses were performed in R 4.1 (R-Core Team) and Stata/MP, version 15.1 (StataCorp).
Study Approval
The trial and current study were approved by the Johns Hopkins University Institutional Review Board (IRB) and also IRBs at each center. All transplant recipients (participants) provided written informed consent.
RESULTS
Demographics of the Study Participants
There were 29 liver transplant recipients with a median age of 58 years old (interquartile range [IQR] = 57–64), and the majority were male (83%, n = 24) and white (59%, n = 17) (Table 1). Most recipients were on integrase inhibitor (INSTI)-containing ART (83%, n = 24), less than one-quarter were on nonnucleoside reverse transcriptase inhibitors (NNRTI)-containing ART (17%, n = 5), and only 1 (3%) was on protease inhibitor-based ART. Five received a simultaneous liver and kidney transplant. No recipients received lymphocyte-depleting induction immunosuppression.
Table 1.
Baseline Characteristics of Liver and Liver-Kidney Recipients by Donor HIV Status
| Characteristics | Total (n = 29) |
HIV D+/R+ (n = 19) | HIV D−/R+ (n = 10)a | P Value |
|---|---|---|---|---|
| Age, y, median (IQR) | 58 (57–64) | 57 (56–64) | 58 (57–62) | .82 |
| Male sex | 24 (83) | 16 (84) | 8 (80) | .78 |
| Race | ||||
| White | 17 (59) | 11 (58) | 6 (60) | .86 |
| African American | 8 (28) | 5 (26) | 3 (30) | |
| Asian | 1 (3) | 1 (5) | 0 (0) | |
| American Indian or Alaska Native | 1 (3) | 1 (5) | 0 (0) | |
| Not specified | 2 (7) | 1 (5) | 1 (10) | |
| Latino ethnicity | 6 (21) | 4 (21) | 2 (20) | |
| Duration of HIV infection, y, median (IQR) | 28 (15–32) | 30 (15–33) | 26 (9–29) | .16 |
| CD4+ T-cell count, median (IQR) | 278 (198–393) | 290 (198–427) | 273 (145–392) | .71 |
| HIV RNA < 200 copies/mL | 29 (100) | 19 (100) | 10 (100) | |
| Received SLK | 5 (17) | 5 (26) | 0 (0) | .07 |
| ART regimenb,c | ||||
| INSTI containing | 24 (83) | 16 (84) | 8 (80) | .78 |
| NRTI containing | 11 (38) | 8 (42) | 3 (30) | .52 |
| NNRTI containing | 5 (17) | 4 (21) | 1 (10) | .45 |
| PI containing | 1 (3) | 0 (0) | 1 (10) | .16 |
| Immunosuppressionb | ||||
| Induction regimen | ||||
| Corticosteroids only | 25 (86) | 15 (79) | 10 (100) | .29 |
| Basiliximab and corticosteroids | 3 (10) | 3 (16) | 0 (0) | |
| Basiliximab only | 1 (3) | 1 (5) | 0 (0) | |
| Maintenancec | ||||
| Tacrolimus | 27 (93) | 17 (89) | 10 (100) | .29 |
| Mycophenolate mofetil (MMF) | 22 (76) | 14 (74) | 8 (80) | .71 |
| Prednisone | 23 (79) | 13 (68) | 10 (100) | .05 |
| Everolimus | 1 (3) | 1 (5) | 0 (0) | .46 |
| Donor characteristics | ||||
| CD4+ T-cell count, median (IQR)d | … | 156 (112–272) | ND | |
| On ART | … | 11 (58) | NA | |
| HIV load <200 copies/mLe | … | 10 (53) | NA | |
| Not on ART | … | 8 (42) | NA | |
| HIV load, copies/mL, median (IQR) | … | 15 186 (760–290 222) | NA | |
Data are presented as median (IQR) for continuous measures, and No. (%) for categorical/binary measures. P values were calculated by Wilcoxon rank sum test while Pearson χ2 test was used for categorical and binary variables.
Abbreviations: ART, antiretroviral therapy; D, donor; HIV, human immunodeficiency virus; INSTI, integrase strand transfer inhibitor; IQR, interquartile range; ND, not done; NA, not applicable; NNRTI, nonnucleoside reverse transcriptase inhibitors; PI, protease inhibitor; R, recipient; RNA, ribonucleic acid; SLK, simultaneous liver-kidney transplant.
Of 10 D−/R+, 3 donors had a false-positive HIV test.
Immunosuppression and ART regimens were reported as active prescriptions at 4 weeks posttransplant.
Percentages may add up to more than 100 because an individual can fall under more than 1 category.
Of 19 D+/R−, 2 donors had missing information on CD4+ T-cell count.
Of 19 D+/R−, 1 donor had missing information on HIV load.
Of the 29 participants, 19 received a transplant from a donor with HIV and 10 from a donor without HIV. Of the 19 donors with HIV, 11 were on ART and 10 of the 11 had suppressed HIV RNA <200 copies/mL. The remaining 8 donors had untreated HIV with median HIV-1 RNA 15 186 (IQR = 760–290 222) copies/mL. There were no significant differences between recipients according to liver donor HIV status, except that a greater proportion of recipients of livers from donors without HIV received prednisone maintenance therapy.
Longitudinal Trajectories in the Latent Viral Reservoir
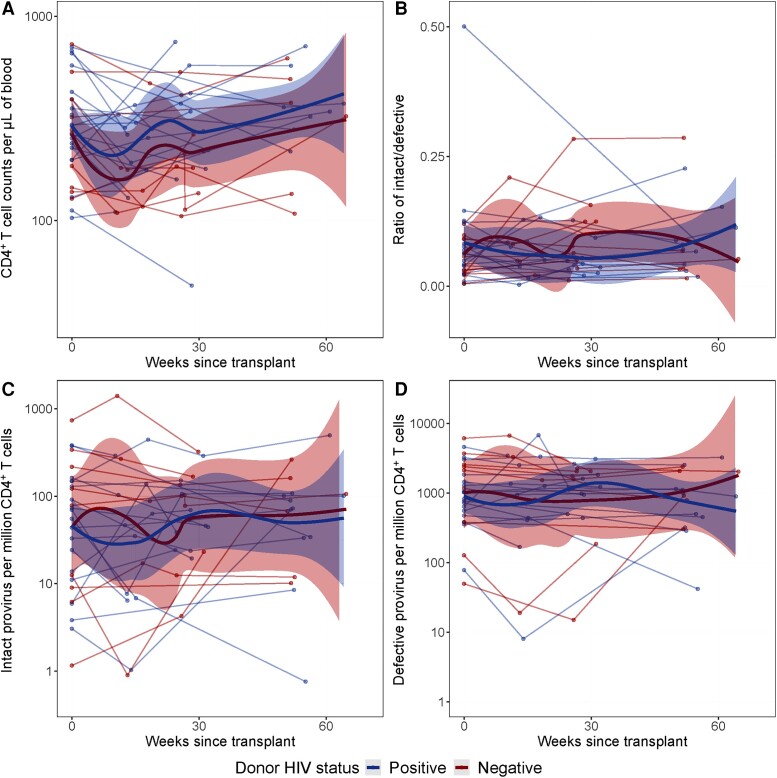
Total CD4+ T-cell counts in participants who received livers from donors with or without HIV declined slightly at weeks 13 and 30, but overall returned to baseline by 1 year posttransplant (Figure 1A). However, the ratio of intact to defective provirus did not significantly alter over time, and no differences were observed between those who received livers from donors with or without HIV (Figure 1B). Levels of intact provirus per million CD4+ T cells or per mL of blood had an initial slight increase in those who received livers from donors without HIV but declined by around week 25. Levels returned to near baseline in both groups by 1 year, and no differences between those who received livers from donors with or without HIV were observed (Figure 1C and Supplementary Figure 1A). Levels of defective provirus per million CD4+ T cells did not dramatically change from baseline to 1 year posttreatment in either group (Figure 1D and Supplementary Figure 1B). A sensitivity analysis was performed among recipients who received only a liver transplant (ie, removing the 5 participants who received a simultaneous liver-kidney transplant), but no differences were observed between the primary analysis and the sensitivity analysis (Supplementary Figure 2). A second sensitivity analysis was performed comparing recipients who received livers from viremic (viral load ≥ 200 copies/mL) and non-viremic donors (viral load < 200 copies/mL). By 1 year posttransplant, no differences were observed between the viremic and non-viremic donors (Supplementary Figure 3).
Figure 1.
Longitudinal trajectories of CD4 T+ cell count, and intact and defective provirus following liver transplant. A, CD4+ T-cell counts per µL of blood, (B) ratios of intact to defective virus, (C) intact provirus per million CD4+ T cells, and (D) defective provirus per million CD4 T-cells were measured longitudinally from time since transplant. Each line represents an individual, and each dot represents a specific time point. Bolded red and blue lines represent the locally estimated scatterplot smoothing (LOESS) curve. Shaded areas represent the 95% confidence interval of the LOESS curves.
DISCUSSION
The persistence of the LVR remains the largest barrier in HIV curative strategies. Because of its size and function as a secondary lymphoid organ and its potential to harbor a significant number of HIV-infected cells, we examined whether transplantation of a liver from a donor with HIV or without HIV affects the size of the LVR in the recipient. Our study was designed to answer this question, because we could compare the size of the LVR longitudinally after liver transplantation from donors with or without HIV. Here, liver transplant recipients with HIV had a slight decline in CD4+ T-cell counts, but the ratio of intact to defective provirus did not change dramatically. No differences were observed in recipients who received a liver from a donor with or without HIV. By 1 year posttransplant, CD4+ T-cell counts, intact provirus, and defective provirus were all comparable to baseline levels. This study demonstrates that the LVR is stable after transplantation of large lymphoid organs, such as the liver, in people with ART-suppressed HIV.
Studies have shown that kidney transplant patients who received lymphocyte nondepleting therapies had a slight reduction of CD4+ T-cell counts, and intact and defective provirus initially, but levels returned to baseline [5]. Liver transplant recipients in this study receiving lymphocyte nondepleting therapies show similar results. The ratio of intact to defective provirus did not change over time, suggesting that latently infected cells are able to be maintained even in larger lymphoid organ transplantations. However, liver macrophages have been shown to have higher infective levels than hepatocytes, and future studies should evaluate whether the LVR changes in these cellular reservoirs [8, 11].
There are a few limitations to this study. All participants were on suppressive ART and therefore the findings cannot be generalized to transplant recipients who interrupt HIV treatment. Moreover, this study only assessed proviruses within CD4+ T cells that are in circulation. Therefore, it is possible that if other cells populations were included in the analysis (eg, liver-resident CD4+ T cells), changes in the size of the LVR posttransplant may have been observed. When using the IPDA, not all samples had amplification, and thus some had to be excluded (n = 5). Participants receiving liver transplants were only given lymphocyte nondepleting therapies, and results could not be compared to those who received lymphocyte depleting therapies.
In conclusion, we found that PWH who received a large lymphoid organ transplantation, such as a liver, have similar intact and proviral trajectories compared to patients who received kidney transplants. Moreover, the ratio of intact/defective provirus did not change overtime in liver patients who received lymphocyte nondepleting therapies and no differences were observed among recipients of livers from donors with or without HIV. It is reassuring that the LVR does not seem to increase with this novel transplantation practice.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Sarah E Benner, Department of Pathology, Johns Hopkins School of Medicine, Baltimore, Maryland, USA.
Xianming Zhu, Department of Pathology, Johns Hopkins School of Medicine, Baltimore, Maryland, USA.
Sarah Hussain, Department of Medicine, Division of Infectious Diseases, Johns Hopkins School of Medicine, Baltimore, Maryland, USA.
Sander Florman, Recanati/Miller Transplantation Institute, Icahn School of Medicine at Mount Sinai, New York, New York, USA.
Yolanda Eby, Department of Pathology, Johns Hopkins School of Medicine, Baltimore, Maryland, USA.
Reinaldo E Fernandez, Department of Medicine, Division of Infectious Diseases, Johns Hopkins School of Medicine, Baltimore, Maryland, USA.
Darin Ostrander, Department of Medicine, Division of Infectious Diseases, Johns Hopkins School of Medicine, Baltimore, Maryland, USA.
Meenakshi Rana, Department of Medicine, Icahn School of Medicine at Mount Sinai, New York, New York, USA.
Shane Ottmann, Department of Surgery, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
Jonathan Hand, Department of Infectious Diseases, Ochsner Health, New Orleans, Louisiana, USA.
Jennifer C Price, Department of Medicine, University of California San Francisco, San Francisco, California, USA.
Marcus R Pereira, Department of Medicine, Columbia University Irving Medical Center, New York, New York, USA.
David Wojciechowski, Division of Nephrology, University of Texas Southwestern, Dallas, Texas, USA.
Jacques Simkins, Department of Medicine/Division of Infectious Diseases, University of Miami School of Medicine, Miami, Florida, USA.
Valentina Stosor, Departments of Medicine and Surgery, Divisions of Infectious Diseases and Organ Transplantation, Northwestern University Feinberg School of Medicine, Chicago, Illinois, USA.
Sapna A Mehta, Department of Medicine, New York University Grossman School of Medicine, New York University Langone Health, New York, New York, USA.
Saima Aslam, Department of Medicine, Division of Infectious Diseases and Global Public Health, University of California San Diego, La Jolla, California, USA.
Maricar Malinis, Department of Internal Medicine, Yale University School of Medicine, New Haven, Connecticut, USA.
Ghady Haidar, Department of Medicine, Division of Infectious Diseases, University of Pittsburgh, Pittsburgh, Pennsylvania, USA.
Allan Massie, Department of Surgery, New York University Grossman School of Medicine, New York University Langone Health, New York, New York, USA.
Melissa L Smith, Department of Biochemistry and Molecular Genetics, University of Louisville, Louisville, Kentucky, USA.
Jonah Odim, Division of Extramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland, USA.
Megan Morsheimer, Division of Extramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland, USA.
Thomas C Quinn, Department of Medicine, Division of Infectious Diseases, Johns Hopkins School of Medicine, Baltimore, Maryland, USA; Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland, USA.
Gregory M Laird, Accelevir Diagnostics, Baltimore, Maryland, USA.
Robert Siliciano, Department of Medicine, Division of Infectious Diseases, Johns Hopkins School of Medicine, Baltimore, Maryland, USA.
Ashwin Balagopal, Department of Medicine, Division of Infectious Diseases, Johns Hopkins School of Medicine, Baltimore, Maryland, USA.
Dorry L Segev, Department of Surgery, New York University Grossman School of Medicine, New York University Langone Health, New York, New York, USA.
Christine M Durand, Department of Medicine, Division of Infectious Diseases, Johns Hopkins School of Medicine, Baltimore, Maryland, USA.
Andrew D Redd, Department of Medicine, Division of Infectious Diseases, Johns Hopkins School of Medicine, Baltimore, Maryland, USA; Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland, USA.
Aaron A R Tobian, Department of Pathology, Johns Hopkins School of Medicine, Baltimore, Maryland, USA; Department of Medicine, Division of Infectious Diseases, Johns Hopkins School of Medicine, Baltimore, Maryland, USA.
Notes
Acknowledgments. A special thank you to the organ donors, families, and recipients who made this study possible. We also thank Diane Brown, Natasha Watson, and the HOPE in Action laboratory and clinical trial teams for their hard work and assistance with this project. We thank Kristen Ritter, Mignot Mathias, Miriam Hays, and Hanna Marks for their work on sample processing and virologic testing with this project. We also thank Northwestern University, Leah Goudy, RN and Michelle Callegari; University of Miami School of Medicine, Isabel Vital and Lissettt Moni; University of Pittsbugh, Kailey Hughes Kramer; University of California San Diego, Phirum Nguyen and Jeff Mills; New York University Lagone Health, Rebecca Dieter PharmD; and Ochsner Health, Angela Smith, MBA.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases (NIAID) (grant numbers 1R01AI120938, U01AI134591, U24AI143502, and U01AI138897); the National Institute of Diabetes, Digestive and Kidney Diseases (grant number 1R01DK131926); and the Division of Intramural Research, NIAID, National Institutes of Health.
Potential conflicts of interest. J. H. has received research grant funding paid to the institution from Pfizer, Janssen, and Scynexis. J. C. P. has received research grants from Gilead, Merck, AbbVie, VIR Biotechnology, Genentech, and Zydus. V. S. has received research grant support paid to institution from Eli Lilly & Company and consultant for DiaSorin, SpA. S. A. has received research grant funding paid to the institution from Contrafect Corporation, Armata Pharmaceuticals, and Adaptive Phage Therapeutics; consultant for BioMx (honoraria), and Phico Therapeutics (unpaid); and is a member of the medical advisory board for Pherecydes Pharmaceuticals (honoraria). G. H. is a recipient of research grants from Allovir, Karius, and AstraZeneca; serves on the scientific advisory boards of Karius and AstraZeneca; and has received honoraria from MDOutlook and MAD-ID/ID Connect. G. M. L. holds equity in Accelevir Diagnostics. M. R. P. is a recipient of research grants from Pfizer, Merck, and Takeda paid to the institution; and is a consultant for Takeda (honoraria) and Union Therapeutics (honoraria). C. M. D. receives honoraria from Gilead Sciences for serving on a grant review committee. D. L. S. reports serving as a consultant and receiving honoraria for speaking from Sanofi, Novartis, CSL Behring, Jazz Pharmaceuticals, Veloxis, Mallincrodt, and Thermo Fisher Scientific. Aspects of the intact proviral DNA assay are the subject of patent application filed by Johns Hopkins University with R. F. S. as an inventor and licensed to AccelevirDx. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Finzi D, Hermankova M, Pierson T, et al. . Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science 1997; 278:1295–300. [DOI] [PubMed] [Google Scholar]
- 2. Siliciano JD, Kajdas J, Finzi D, et al. . Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nat Med 2003; 9:727–8. [DOI] [PubMed] [Google Scholar]
- 3. Hütter G, Nowak D, Mossner M, et al. . Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N Engl J Med 2009; 360:692–8. [DOI] [PubMed] [Google Scholar]
- 4. Gupta RK, Abdul-Jawad S, McCoy LE, et al. . HIV-1 remission following CCR5Δ32/Δ32 haematopoietic stem cell transplantation. Nature 2019; 568:244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Benner SE, Eby Y, Zhu X, et al. . The effect of induction immunosuppression for kidney transplant on the latent HIV reservoir. JCI Insight 2022; 7:e162968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Durand CM, Zhang W, Brown DM, et al. . A prospective multicenter pilot study of HIV-positive deceased donor to HIV-positive recipient kidney transplantation: HOPE in Action. Am J Transplant 2021; 21:1754–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Crispe IN. Immune tolerance in liver disease. Hepatology 2014; 60:2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cao Y, Dieterich D, Thomas PA, Huang Y, Mirabile M, Ho DD. Identification and quantitation of HIV-1 in the liver of patients with AIDS. AIDS 1992; 6:65–70. [DOI] [PubMed] [Google Scholar]
- 9. Schrager LK, D'Souza MP. Cellular and anatomical reservoirs of HIV-1 in patients receiving potent antiretroviral combination therapy. JAMA 1998; 280:67–71. [DOI] [PubMed] [Google Scholar]
- 10. Zerbato JM, Avihingsanon A, Singh KP, et al. . HIV DNA persists in hepatocytes in people with HIV-hepatitis B co-infection on antiretroviral therapy. eBioMedicine 2023; 87:104391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Housset C, Lamas E, Courgnaud V, et al. . Presence of HIV-1 in human parenchymal and non-parenchymal liver cells in vivo. J Hepatol 1993; 19:252–8. [DOI] [PubMed] [Google Scholar]
- 12. Kandathil AJ, Sugawara S, Goyal A, et al. . No recovery of replication-competent HIV-1 from human liver macrophages. J Clin Invest 2018; 128:4501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Durand CM, Florman S, Motter JD, et al. . HOPE in Action: a prospective multicenter pilot study of liver transplantation from donors with HIV to recipients with HIV. Am J Transplant 2022; 22:853–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bonny TS, Kirby C, Martens C, et al. . Outcomes of donor-derived superinfection screening in HIV-positive to HIV-positive kidney and liver transplantation: a multicentre, prospective, observational study. Lancet HIV 2020; 7:e611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bruner KM, Wang Z, Simonetti FR, et al. . A quantitative approach for measuring the reservoir of latent HIV-1 proviruses. Nature 2019; 566:120–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.