Abstract
Background
Persistence of viral reservoirs has been observed in people with human immunodeficiency virus (HIV), despite long-term antiretroviral therapy (ART), and likely contributes to chronic immune activation and inflammation. Obefazimod is a novel drug that inhibits human immunodeficiency virus type 1 (HIV-1) replication and reduces inflammation. Here we assess whether obefazimod is safe and might impact HIV-1 persistence, chronic immune activation, and inflammation in ART-suppressed people with HIV.
Methods
We evaluated obefazimod-related adverse events, changes in cell-associated HIV-1 DNA and RNA, residual viremia, immunophenotype, and inflammation biomarkers in blood and rectal tissue. We compared 24 ART-suppressed people with HIV who received daily doses of 50 mg obefazimod for 12 weeks (n = 13) or 150 mg for 4 weeks (n = 11) and 12 HIV-negative individuals who received 50 mg for 4 weeks.
Results
The 50- and 150-mg doses of obefazimod were safe, although the 150-mg dose showed inferior tolerability. The 150-mg dose reduced HIV-1 DNA (P = .008, median fold change = 0.6) and residual viremia in all individuals with detectable viremia at baseline. Furthermore, obefazimod upregulated miR-124 in all participants and reduced the activation markers CD38, HLA-DR, and PD-1 and several inflammation biomarkers.
Conclusions
The effect of obefazimod by reducing chronic immune activation and inflammation suggests a potential role for the drug in virus remission strategies involving other compounds that can activate immune cells, such as latency-reversing agents.
Keywords: HIV-1 persistence, immune activation, inflammation, miR-124, obefazimod (ABX464)
This study evaluates the impact of obefazimod, a molecule that interferes with RNA biogenesis, on reducing chronic immune activation and inflammation in people with HIV on suppressive antiretroviral therapy as well as its potential role in virus remission strategies.
Antiretroviral therapy (ART) has been successful in maintaining long-term viral suppression in people with human immunodeficiency virus (PWH). However, the persistence of latently infected cells carrying intact, inducible proviruses has been observed in different cellular and anatomical reservoirs [1–3]. Activation of latently infected cells can lead proviral DNA to be transcriptionally active and capable of producing new viral particles [4, 5], which may contribute to residual viremia and thus persistence of immune activation and inflammation, despite long-term ART suppression [6]. Because viral persistence is associated with a variety of biological mechanisms, the success of human immunodeficiency virus (HIV) remission strategies will most likely depend upon combinatorial strategies [7]. Thus, new antiretroviral drugs aimed at preventing viral reactivation from latently infected cells need to be explored, including those that target viral transcription, processing, and/or maturation of viral transcripts [8], which could simultaneously help reduce chronic immune activation and inflammation exhibited by ART-suppressed PWH.
Obefazimod (formerly ABX464) is an investigational small quinoline with a novel mechanism of HIV type 1 (HIV-1) inhibition based on interference with RNA biogenesis. In brief, by binding to the cap-binding complex (CBC), obefazimod targets viral Rev protein and prevents Rev-mediated nuclear export and maturation of unspliced viral RNA [9]. Obefazimod has been shown to be highly effective at inhibiting HIV-1 replication in human peripheral blood mononuclear cells (PBMCs) and macrophages in vitro. Moreover, obefazimod reduced viral replication in HIV-1–infected humanized mice and delayed viral rebound after drug discontinuation [9]. Furthermore, obefazimod demonstrated safety, tolerability, and potential reduction of viral replication in previous clinical trials [10–13].
Of note, the CBC targeted by obefazimod is also involved in the biogenesis of endogenous small noncoding RNAs (miRNAs) such as miR-124. During miRNA processing, obefazimod specifically enables a quick release of miR-124 through splicing of its long noncoding RNA precursor (lncRNA 0599-205) [14]. Through the targeting of STAT3 mRNA, miR-124 functions as a negative regulator of inflammation, attenuating the production of proinflammatory cytokines to help maintain homeostasis, and is reported to be a critical modulator of immunological and inflammatory disorders [15, 16]. Obefazimod has demonstrated strong anti-inflammatory effects by the selective induction of miR-124 in in vitro and in vivo studies, including in PWH [14, 16, 17]. Hence, due to these properties, this novel drug might be a promising therapeutic strategy to impact the HIV-1 reservoir and reduce chronic immune activation and inflammation in the context of sustained inflammation during ART, as well as in virus remission strategies involving other compounds that can activate immune cells. The present study aimed to further evaluate safety and impact of obefazimod on HIV-1 persistence and chronic immune activation and inflammation in blood and gut-associated lymphoid tissue (GALT) from ART-suppressed PWH.
METHODS
Study Design and Participants
This was a nonrandomized, open-label, phase 2b/2a clinical trial designed to evaluate safety, virological, and immunological parameters in 36 adult participants (24 PWH and 12 HIV-negative) at either 50 mg (n = 13) once-daily oral doses of obefazimod for 12 weeks (HIV50mg/12w) or 150 mg (n = 11) for 4 weeks (HIV150mg/4w) or, in the case of HIV-negative individuals (n = 12), 50 mg for 4 weeks (HN50mg/4w).
Seropositive participants were required to be on an integrase inhibitor–based regimen for at least 12 months prior to enrollment with undetectable plasma viremia (≤50 HIV-1 RNA copies/mL) during the 6 months prior to screening. In addition, they were required to have CD4+ T-cell counts >250 cells/μL since diagnosis with at least 600 cells/μL at screening. All study participants were recruited from the University Hospital Germans Trias i Pujol (Badalona, Spain) from April 2017 to December 2018, and all provided signed informed consent. The study protocol (ABX464-005) was approved by the hospital ethics committee (AC-16-047-CEIM) and the Spanish Agency of Medicines and Medical Products (EudraCT: 2016-002797-12) and registered at ClinicalTrials.gov (identifier NCT02990325).
Safety Assessments
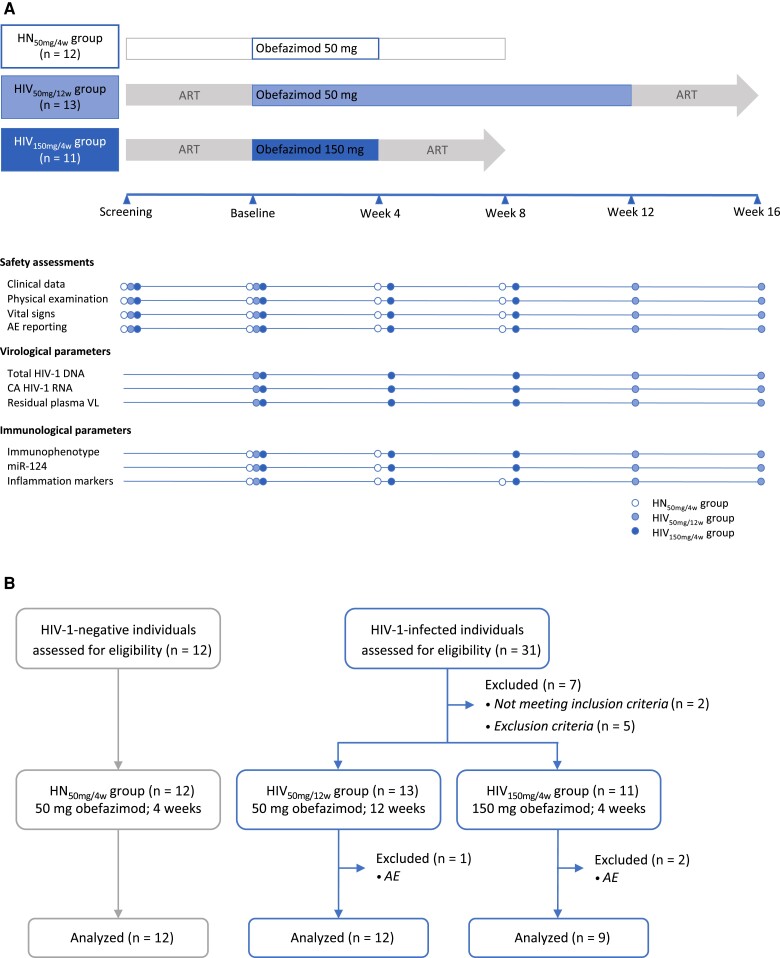
Safety and tolerability were evaluated based on individual values for clinical data (including signs and symptoms, laboratory toxicities, and clinical events), physical examinations, vital signs, and number and grade of treatment-emergent adverse events (AEs) at each visit as described in Figure 1A. The data and safety monitoring board reviewed the safety data to recommend, if appropriate, enrollment of additional individuals and the continuation of the study.
Figure 1.
Study design and flow diagram. A, Schedule of study events. B, Consort flow diagram. Abbreviations: ART, antiretroviral therapy; AE, adverse event; ART, antiretroviral therapy; CA, cell-associated; HIV-1, human immunodeficiency virus type 1; HIV50mg/12w, HIV infected taking once-daily oral obefazimod 50 mg for 12 weeks; HIV150mg/4w, HIV infected taking once-daily oral obefazimod 150 mg for 4 weeks; HN50mg/4w, HIV negative taking once-daily oral obefazimod 50 mg for 4 weeks; VL, viral load.
Sample Collection and Analysis
Blood and GALT samples [18] were collected at baseline, end of intervention (EoI), and 4 weeks after drug discontinuation (EoI + 4w) from each participant in accordance with the schedule of study events (Figure 1A). Total HIV-1 DNA [18], cell-associated (CA) HIV-1 RNA [19], and ultrasensitive residual viremia [20] were measured to estimate changes in viral persistence. To evaluate the state of immune activation, T-cell subset distribution and expression of cell surface activation markers were measured by flow cytometry. Moreover, miR-124 was quantified in rectal tissue to evaluate intestinal inflammation, and 26 inflammation biomarkers were measured in plasma to assess systemic inflammation. Specific details on these assays and statistical analysis can be found in the Supplementary Material.
RESULTS
Participant Characteristics and Disposition
Thirty-six participants were prospectively and sequentially enrolled into 3 groups as follows: 13 ART-suppressed PWH received 50 mg of obefazimod orally once daily for 12 weeks (HIV50mg/12w group), 11 ART-suppressed PWH received 150 mg for 4 weeks (HIV150mg/4w group), and 12 HIV-negative individuals received 50 mg for 4 weeks (HN50mg/4w group) as shown in Figure 1B. All study participants were White cisgender men with a median age of 44 years. Baseline demographic and clinical characteristics are summarized in Table 1. No differences between groups were observed, except for the time on viral suppression, which was longer in the HIV150mg/4w group than in the HIV50mg/12w group (P = .02). All participants were on suppressive integrase inhibitor–based ART at study initiation.
Table 1.
Baseline Demographic and Clinical Characteristics of the Study Population
| Characteristic | Dose of Once-Daily Oral Obefazimod |
P Values Between HIV+ Groups |
||
|---|---|---|---|---|
| HIV–, 50 mg for 4 wk (n = 12) | HIV+, 50 mg for 12 wk (n = 13) | HIV+, 150 mg for 4 wk (n = 11) | ||
| Age, y | 38 (26–46) | 41 (34–49) | 51 (43–53) | .06 |
| Sex, male, No. (%) | 12 (100) | 13 (100) | 11 (100) | 1.0 |
| Race, White, No. (%) | 12 (100) | 13 (100) | 11 (100) | 1.0 |
| CD4+ T-cell count | ||||
| Cells/μL | … | 1035 (694–1173) | 906 (781–1439) | .9 |
| Percentage | … | 39 (34–44) | 37 (33–45) | .5 |
| CD8+ T-cell count | ||||
| Cells/μL | … | 753 (554–870) | 773 (647–966) | .4 |
| Percentage | … | 30 (23–45) | 32 (27–42) | .5 |
| HIV-1 RNA, copies/mL | … | <50 | <50 | 1.0 |
| Nadir CD4+ T-cell count, cells/μL | … | 475 (354–655) | 443 (335–729) | .9 |
| Zenith VL, log10 HIV-1 RNA copies/mL | … | 4.5 (0.4–8.0) | 2.7 (0.7–4.6) | .7 |
| ART | ||||
| Months from diagnosis to ART | … | 4 (1–38) | 11 (2–77) | .4 |
| Years on ART | … | 5 (4–9) | 11 (7–23) | .02 |
| Integrase inhibitor–based ART, No. (%) | ||||
| Dolutegravir | … | 10 (77) | 9 (82) | … |
| Raltegravir | … | 3 (23) | 2 (18) | … |
Data are presented as median (interquartile range) unless otherwise indicated. P values ≤ .05 are shown in bold.
Abbreviations: ART, antiretroviral therapy; HIV–, human immunodeficiency virus negative; HIV+, human immunodeficiency virus positive; VL, viral load.
Obefazimod Is Safe Either Alone or in Combination With ART
Just over half of participants (53%) experienced any AE during obefazimod administration (Table 2). However, no AEs were severe (grade 3 or above), life-threatening, or disabling, and all symptoms and biochemical abnormalities normalized upon drug withdrawal. Only 3 participants, 1 in the HIV50mg/12w group and 2 in the HIV150mg/4w group, discontinued the study due to AEs and were excluded from subsequent analyses. The most frequently reported AEs were nervous system disorders (headache) (42%), gastrointestinal disorders (abdominal pain, diarrhea, and nausea) (39%), and musculoskeletal pain (back pain and myalgia) (19%). The total number of AEs was slightly higher at the 150-mg dose compared to the 50-mg dose (P = .3) (Table 2). Nevertheless, standard plasma viral load, and CD4+ and CD8+ T-cell counts showed no significant changes during obefazimod treatment, which suggests that obefazimod does not disrupt clinical control of HIV-1 infection and therefore does not negatively interact with ART (Supplementary Figure 1).
Table 2.
Number of Participants With Adverse Events by Group
| Dose of Once-Daily Oral Obefazimod | ||||
|---|---|---|---|---|
| System Organ Class Preferred Term | HIV–, 50 mg for 4 wk (n = 12) | HIV+, 50 mg for 12 wk (n = 13) | HIV+, 150 mg for 4 wk (n = 11) | Total (N =36) |
| Any AE | 3 (25) | 7 (54) | 9 (82) | 19 (53) |
| Blood and lymphatic disorders | 0 (0) | 0 (0) | 1 (9) | 1 (3) |
| Thrombocytopenia | 0 | 0 | 1 | 1 |
| Gastrointestinal disorders | 2 (17) | 6 (46) | 6 (55) | 14 (39) |
| Abdominal pain | 0 | 2 | 2 | 4 |
| Abdominal pain upper | 1 | 1 | 1 | 3 |
| Constipation | 0 | 0 | 1 | 1 |
| Diarrhea | 0 | 2 | 1 | 3 |
| Dyspepsia | 1 | 0 | 0 | 1 |
| Flatulence | 0 | 1 | 0 | 1 |
| Nausea | 0 | 0 | 4 | 4 |
| General disorders and administrative site conditions | 0 (0) | 3 (23) | 3 (27) | 6 (17) |
| Asthenia | 0 | 0 | 1 | 1 |
| Chest pain | 0 | 0 | 1 | 1 |
| Feeling abnormal | 0 | 1 | 0 | 1 |
| Malaise | 0 | 1 | 1 | 2 |
| Thirst | 0 | 1 | 0 | 1 |
| Metabolism and nutrition disorders | 0 (0) | 0 (0) | 1 (9) | 1 (3) |
| Hyperamylasemia | 0 | 0 | 1 | 1 |
| Hyperlipasemia | 0 | 0 | 1 | 1 |
| Musculoskeletal and connective tissue disorders | 0 (0) | 1 (8) | 6 (55) | 7 (19) |
| Arthralgia | 0 | 0 | 1 | 1 |
| Back pain | 0 | 1 | 4 | 5 |
| Muscle spasms | 0 | 0 | 1 | 1 |
| Myalgia | 0 | 0 | 4 | 4 |
| Nervous system disorders | 2 (17) | 5 (39) | 8 (73) | 15 (42) |
| Headache | 2 | 4 | 8 | 14 |
| Migraine | 0 | 1 | 0 | 1 |
| Psychiatric disorders | 0 (0) | 0 (0) | 2 (18) | 2 (6) |
| Insomnia | 0 | 0 | 1 | 1 |
| Nightmare | 0 | 0 | 1 | 1 |
| Skin and subcutaneous tissue disorders | 0 (0) | 0 (0) | 2 (18) | 2 (6) |
| Folliculitis | 0 | 0 | 1 | 1 |
| Rash erythematous | 0 | 0 | 1 | 1 |
Data are presented as No. (%).
Abbreviation: AE, adverse event; HIV, human immunodeficiency virus; HIV–, human immunodeficiency virus negative; HIV+, human immunodeficiency virus positive.
Obefazimod Reduces HIV-1 Persistence
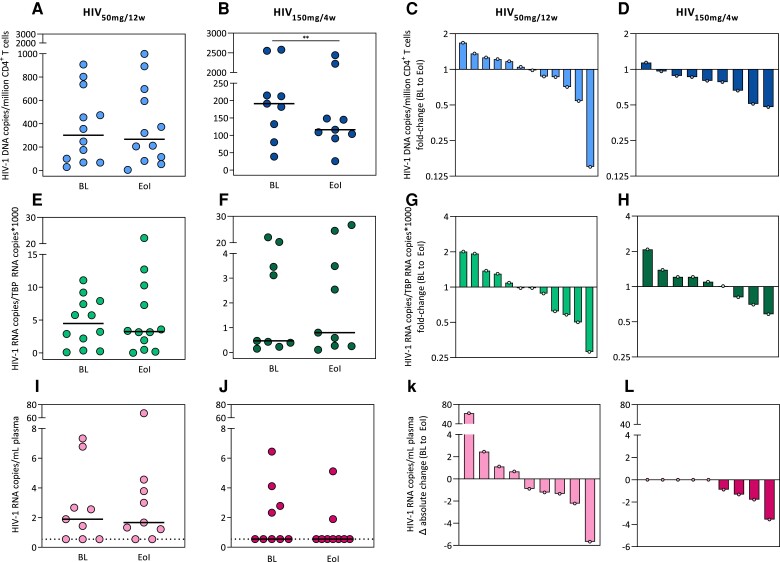
To evaluate the impact of obefazimod on proviral reservoir size, we measured total HIV-1 DNA in peripheral CD4+ T cells by droplet digital PCR (ddPCR). Total HIV-1 DNA was detected in all individuals. Data confirmed a significant reduction of total HIV-1 DNA at the 150-mg dose (P = .008, median fold-change = 0.6) (Figure 2A and 2B). Indeed, the number of individuals with decreased total HIV-1 DNA was higher at the 150-mg dose compared to the 50-mg dose (88.9% vs 50%, respectively; P = .159, Fisher exact test) (Figure 2C and 2D). However, this was a transient effect and total HIV-1 DNA returned to basal levels after drug discontinuation (Supplementary Figure 2A–D). These results suggest that obefazimod reduces total HIV-1 DNA in a dose-dependent manner, potentially impacting viral persistence.
Figure 2.
Effect of obefazimod on viral reservoir throughout the intervention period. A–D, Changes in total human immunodeficiency virus type 1 (HIV-1) DNA from peripheral CD4+ T cells measured by droplet digital PCR (ddPCR). E–H, Changes in cell-associated HIV-1 RNA from peripheral CD4+ T cells measured by reverse-transcription ddPCR. I–L, Changes in residual plasma viremia measured using an ultrasensitive viral load test. Undetectable plasma viral load was defined as <0.55 HIV-1 RNA copies/mL (broken line). Data from 3 individuals in the HIV-infected group taking 50 mg for 12 weeks were lost due to technical problems. A–B, E–F, I–J panels represent absolute values of each timepoint in each group. C–D, G–H, K–L panels represent waterfall charts to show the increase/decrease from baseline to end of obefazimod administration. Each symbol/shape represents a different individual. Horizontal lines in panels A–B, E–F, and I–J represent medians. Asterisks denote significant differences between timepoints detected using the Wilcoxon signed-rank test (**P ≤ .01). Abbreviations: BL, baseline; EoI, end of intervention; HIV-1, human immunodeficiency virus type 1; HIV50mg/12w, HIV infected taking once-daily oral obefazimod 50 mg for 12 weeks; HIV150mg/4w, HIV infected taking once-daily oral obefazimod 150 mg for 4 weeks; TBP, TATA-binding protein.
To assess the effect of obefazimod on viral transcription, we measured CA HIV-1 RNA in peripheral CD4+ T cells by reverse-transcription (RT) ddPCR. CA HIV-1 RNA was detected in all individuals. Data showed no consistent effect of obefazimod on viral transcription during the administration period (Figure 2E–2H). These data suggest that obefazimod did not significantly affect viral transcription initiation (as measured by our targeting of the 5′ end of the HIV-1 mRNA transcripts). However, CA HIV-1 RNA significantly increased after drug discontinuation at the 150-mg dose (P = .02, median fold change = 2.1) and the 50 mg (P = .02, median fold change = 1.2) (Supplementary Figure 2E and 2F). Indeed, the number of individuals who increased CA HIV-1 RNA after discontinuation was higher at the 150-mg dose compared to the 50-mg (100% vs 75%, respectively) (Supplementary Figure2G and 2H).
To evaluate the impact of obefazimod on residual viremia, we measured plasma viral load (VL) using an ultrasensitive VL (usVL) test (limit of detection <1 HIV-1 RNA copy/mL). Residual viremia was detectable in 10 (56%) individuals at baseline (6 in the HIV50mg/12w group and 4 in the HIV150mg/4w group) (Figure 2I and 2J). Longitudinal analysis confirmed a substantial reduction of plasma HIV-1 RNA in all (100%) individuals who showed detectable residual viremia at baseline at the 150-mg dose compared to the 50-mg (56%) (Figure 2I–2L). Nonetheless, this effect was lost after drug discontinuation (Supplementary Figure 2I–2L). The very low levels of plasma HIV-1 RNA at baseline and high proportion of undetectable samples might have interfered to detect significant changes. Nevertheless, these results suggest that obefazimod reduces residual viremia in a dose-dependent manner.
Obefazimod Reduces Chronic Immune Activation in PWH
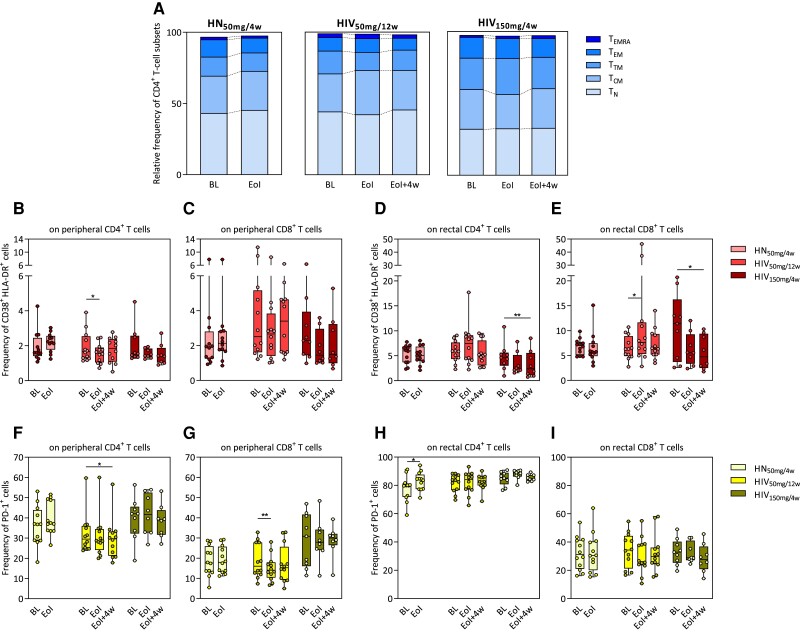
To assess the effect of obefazimod on immune activation, first we measured CD4+ T-cell subset distribution in PBMCs by flow cytometry. The dynamics of the CD4+ T-cell subsets did not significantly change throughout obefazimod administration, which suggests that there is no redistribution of cellular subsets (Figure 3A).
Figure 3.
Effect of obefazimod on T-cell activation. A, Upper graph shows the relative frequency of different CD4+ T-cell subsets in the study groups identified in peripheral blood mononuclear cells by flow cytometry. Bars represent medians of each subset. B–I, Immune activation levels determined as percentages of CD38+HLA-DR+ (B–E) and PD-1+ (F–I) cells analyzed both in peripheral and rectal CD4+ and CD8+ T-cell populations by flow cytometry. B and C, Frequency of CD38+HLA-DR+ cells from peripheral CD4+ and CD8+ T cells. D and E, Frequency of CD38+HLA-DR+ cells from rectal CD4+ and CD8+ T cells. F and G, Frequency of PD-1+ cells from peripheral CD4+ and CD8+ T cells. H and I, Frequency of PD-1+ cells from rectal CD4+ and CD8+ T cells. Median and interquartile range are represented. Asterisks denote significant differences between timepoints detected using the Wilcoxon signed-rank test (*P ≤ .05; **P ≤ .01). Abbreviations: BL, baseline; EoI, end of intervention; EoI + 4w, 4 weeks after end of intervention; HIV50mg/12w, HIV infected taking once-daily oral obefazimod 50 mg for 12 weeks; HIV150mg/4w, HIV infected taking once-daily oral obefazimod 150 mg for 4 weeks; HN50mg/4w, HIV negative taking once-daily oral obefazimod 50 mg for 4 weeks; TCM, central memory T cells; TEM, effector memory T cells; TEMRA, effector memory RA T cells; TN, naive T cells; TTM, transitional memory T cells.
Then, the dynamics of cell surface activation markers (CD38+HLA-DR+ and PD-1+ cells) were analyzed in peripheral and rectal CD4+ and CD8+ T cells. Data showed no baseline differences between PWH and HIV-negative individuals except for lower proportion of rectal CD4+PD-1+ cells in HIV-negative individuals compared to the HIV150mg/4w group (P = .04; Supplementary Table 1). Data confirmed a significant reduction of peripheral CD8+PD-1+ and CD4+CD38+HLA-DR+ cells at week 12 (EoI) and CD4+PD-1+ cells at week 16 (EoI + 4w) in the HIV50mg/12w group (P = .007, P = .04, and P = .02, respectively) (Figure 3B, 3F, and 3G; Supplementary Table 1). The fact that the reduction of immune activation in peripheral CD4+ and CD8+ T cells is only significant when obefazimod is given for at least 12 weeks suggests a lasting effect of obefazimod at reducing immune activation in ART-suppressed PWH. Of note, these results were reinforced by the findings in GALT, as we observed a significant reduction in rectal CD4+CD38+HLA-DR+ and CD8+CD38+HLA-DR+ cells at week 8 (EoI + 4w) in the HIV150mg/4w group (P = .008, median fold change = 1.9; and P = .02, median fold change = 2.4, respectively) (Figure 3D and 3E; Supplementary Table 1). However, obefazimod intervention transiently increased rectal CD8+CD38+HLA-DR+ cells in the HIV50mg/12w group (P = .03) and CD4+PD-1+ cells in HIV-negative individuals (P = .01) (Figure 3E and 3H; Supplementary Table 1). Altogether, these data suggest that obefazimod may reduce chronic immune activation in PWH.
Obefazimod Reduces Chronic Inflammation in PWH
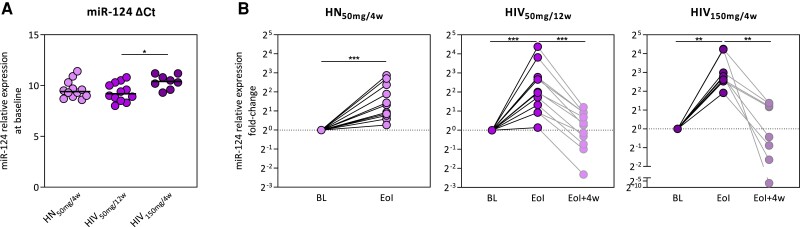
To further investigate the effect of obefazimod on intestinal and systemic inflammation, we measured expression of miR-124 in rectal tissue by qRT-PCR and plasma levels of 26 inflammation biomarkers using Luminex-based multiplex immunoassay and enzyme-linked immunosorbent assay. miR-124 was detected in all individuals. Basal expression of miR-124 was higher in the HIV150mg/4w group compared to the HIV50mg/12w (P = .02; Figure 4A), perhaps because they have been on suppressive ART longer. Nevertheless, miR-124 expression significantly increased in all (100%) participants from all groups (HN50mg/4w, P = .0005, median fold change = 2.2; HIV50mg/12w, P = .0005, median fold change = 4.0; HIV150mg/4w, P = .004, median fold change = 6.7) (Figure 4B). However, this effect was reversed after drug discontinuation. These data suggest that obefazimod may reduce intestinal inflammation by the selective upregulation of miR-124 in ART-suppressed PWH.
Figure 4.
Effect of obefazimod on miR-124 expression. A, Baseline mir-124 expression of the study population measured in rectal tissue by reverse-transcription quantitative polymerase chain reaction. B, Fold changes in miR-124 expression throughout intervention and subsequent discontinuation of the study groups. Horizontal lines in panel A represent medians. Asterisks denote significant differences between timepoints detected using the Wilcoxon signed-rank test (*P ≤ .05; **P ≤ .01; ***P ≤ .001). Abbreviations: ΔCt, change in threshold cycle; BL, baseline; EoI, end of intervention; EoI + 4w, 4 weeks after end of intervention; HIV50mg/12w, HIV infected taking once-daily oral obefazimod 50 mg for 12 weeks; HIV150mg/4w, HIV infected taking once-daily oral obefazimod 150 mg for 4 weeks; HN50mg/4w, HIV negative taking once-daily oral obefazimod 50 mg for 4 weeks.
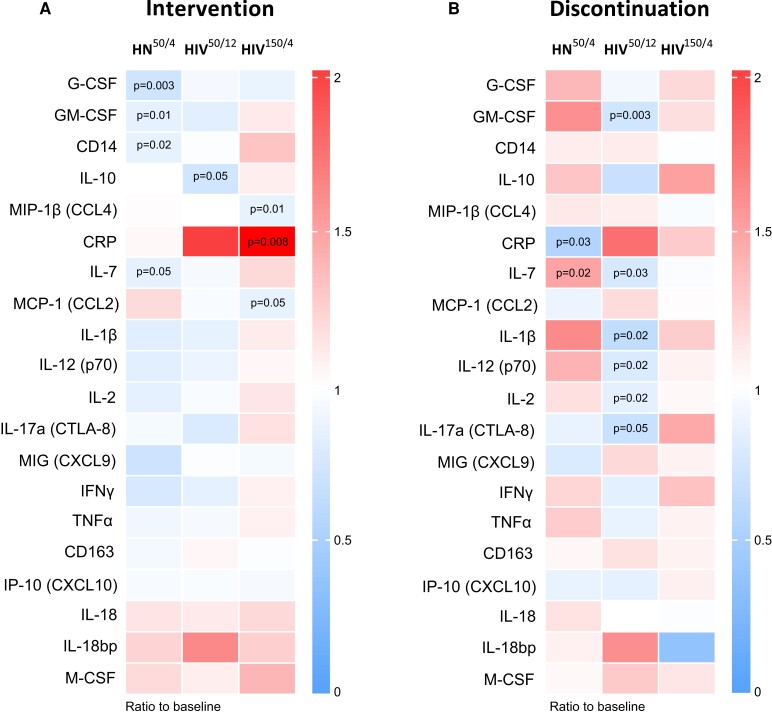
Regarding soluble inflammation biomarkers, interleukin (IL) 4, IL-6, IL-8, IL-12 (p40), IL-22, and liver-type fatty acid-binding protein were excluded from the analysis due to the high proportion of undetectable samples. Among the remaining 20 biomarkers, data showed a significant downregulation of granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), CD14, and IL-7 in HIV-negative individuals (P = .003, P = .01, P = .02, and P = .05, respectively); IL-10 in the HIV50mg/12w group (P = .05); and macrophage inflammatory protein-1beta (MIP-1β; CCL4) and monocyte chemoattractant protein-1 (MCP-1; CCL2) in the HIV150mg/4w group (P = .01 and P = .05, respectively) throughout obefazimod administration (Figure 5A). These results reinforce the anti-inflammatory effect of obefazimod in both PWH and HIV-negative individuals. Unexpectedly, C-reactive protein (CRP) significantly increased in the HIV150mg/4w group (P = .008), although mean values were always <10 mg/L, the upper limit of the normality range. In addition, CRP significantly decreased in all groups after drug withdrawal (HN50mg/4w, P = .03; HIV50mg/12w, P = .03; and HIV150mg/4w, P = .004; Supplementary Figure 3B). Nevertheless, significant differences remained present between PWH and HIV-negative individuals either at baseline or after obefazimod administration, such as the expression of G-CSF, IL-7, tumor necrosis factor–α, IL-18 bp, MIP-1β (CCL4), MCP-1 (CCL2), and CRP (Supplementary Figure 4). This fact supports the evidence that PWH exhibit chronic inflammation, which seems to be higher in the HIV150mg/4w group, perhaps due to the longer time living with HIV-1 and undergoing ART.
Figure 5.
Effect of obefazimod on inflammation. A, Heat map representing median fold changes in plasma levels of inflammation biomarkers throughout obefazimod intervention (baseline to end of intervention). B, Heat map representing median fold changes in plasma levels of inflammation biomarkers from baseline to 4 weeks after obefazimod discontinuation. Decrease and increase in median fold changes are shown in blue and red, respectively. C-reactive protein is outside the defined range. Median increase/decrease comparisons between timepoints were performed using the Wilcoxon signed-rank test. P values are shown. Abbreviations: CRP, C-reactive protein; G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte-macrophage colony-stimulating factor; HIV50/12, HIV infected taking once-daily oral obefazimod 50 mg for 12 weeks; HIV150/4, HIV infected taking once-daily oral obefazimod 150 mg for 4 weeks; HN50/4, HIV negative taking once-daily oral obefazimod 50 mg for 4 weeks; IFN-γ, interferon gamma; IL, interleukin; IP-10, interferon-γ inducible protein-10; MCP-1, monocyte chemoattractant protein-1; M-CSF, macrophage colony-stimulating factor; MIG, monokine induced by gamma interferon; MIP-1β, macrophage inflammatory protein-1beta; TNF-α, tumor necrosis factor alpha.
Of note, the tendency to reduce inflammation was even greater 4 weeks after drug discontinuation in PWH, especially when obefazimod was administered at 50 mg for 12 weeks, showing a significant downregulation of GM-CSF, IL-7, IL-1β, IL-12 (p70), IL-2, and IL-17α (CTLA-8) (P = .003, P = .03, P = .02, P = .02, P = .02, and P = .05, respectively) (Figure 5B). Overall, these results suggest that obefazimod may reduce chronic inflammation in ART-suppressed PWH, and that longer duration of the intervention may enhance lasting anti-inflammatory effects.
DISCUSSION
In this study we evaluated the safety and tolerability in addition to the impact of obefazimod on viral persistence, and chronic immune activation and inflammation in ART-suppressed PWH. We confirm previous findings showing that obefazimod is safe and well tolerated when used in combination with ART in PWH [13]. Furthermore, we observed a reduction in total HIV-1 DNA, residual viremia, and cell-activation and inflammation markers in blood and GALT. Overall, this study suggests that obefazimod may have a triple in vivo effect by reducing HIV-1 persistence, chronic immune activation, and chronic inflammation in PWH.
Our study showed a reduction in total HIV-1 DNA in peripheral CD4+ T cells and a trend toward decreased residual plasma viremia when obefazimod was administered at 150 mg for 4 weeks in ART-suppressed PWH. This effect might indirectly happened by cellular toxicity or changes in cellular distribution, since central and transitional memory CD4+ T cells are the major cellular reservoirs for HIV [5]. However, no toxicity related to obefazimod affecting CD4+ T-cell counts has been previously described [12] nor observed in our study (Supplementary Figure 1), and no redistribution of memory CD4+ T-cell subsets was observed either (Supplementary Table 1). Therefore, our results suggest that obefazimod directly impacts the proviral reservoir size in a dose-dependent manner. Previous published studies with obefazimod have already confirmed a dose-dependent efficacy of the drug [12]. Indeed, similar trend in total HIV-1 DNA had previously been reported in PWH receiving 150 mg dose of obefazimod for 4 weeks [13] but, comparatively, our study provides statistically significant changes in total HIV-1 DNA and a greater proportion of responders. Furthermore, in a recently reported substudy of the 150-mg dose of obefazimod [21], intact proviral DNA was measured and showed a similar decreasing trend without reaching statistical significance. Altogether these data suggest that obefazimod decreases the total proviral reservoir and may also decrease the intact proviral reservoir when administered in combination with ART [21]. This finding has important implications given the failure of multiple interventions to achieve a measurable reduction of the viral reservoir size in other studies. Nevertheless, in both studies the effect on proviral DNA reversed after drug discontinuation, so it is tempting to speculate that obefazimod's ability to interfere with cell activation and inflammation might contribute to reducing T-cell proliferation as well.
In our study, we did not observe changes in the CA HIV-1 RNA during obefazimod treatment. However, as our assay is designed to measure all mRNAs resulting from HIV-1 transcription initiation and not specifically further mature RNA forms such as elongated or spliced molecules, this might have impeded the detection of the impact of obefazimod in late steps of HIV-1 RNA biogenesis. To further investigate the viral transcription profile throughout obefazimod administration, we made an in-depth substudy of different viral transcripts in which we only analyzed samples from the HIV150mg/4w group given the dose-dependent efficacy of the drug [21]. This substudy showed that obefazimod decreases viral transcription initiation and may decrease viral transcription elongation [21]. Nevertheless, both studies showed an increase in viral transcription after drug discontinuation, like the increase in total HIV-1 DNA after drug withdrawal, newly suggesting that obefazimod withdrawal might also increase viral transcription through associated changes in immune cell activation and inflammation. These findings suggest that obefazimod may impact specific forms of HIV transcription but lacks lasting effect. Overall, our study showed the potential effect of obefazimod at reducing the persistence of the HIV-1 reservoir in PWH despite suppressive ART.
So far, no study has investigated the effect of obefazimod on immune activation in blood nor rectal tissue in ART-suppressed PWH. In agreement with previous published data, we reported higher frequency of activated T cells in rectal tissue compared to blood [22, 23]. Indeed, the highest proportion of CD4+PD-1+ T cells was found within the rectal tissue, mainly in the HIV150mg/4w group who had been living with HIV and taking long-term ART. Importantly, our study shows for the first time the lasting effect of obefazimod at reducing immune activation in ART-suppressed PWH by reducing the expression of cell surface activation markers (ie, CD38, HLA-DR, and PD-1), that occurs in peripheral and rectal CD4+ and CD8+ T cells during HIV-1 infection, which is associated with a progressive loss of effector function [24, 25]. Therefore, it may have applicability for the treatment of chronic immune activation exhibited by PWH despite long-term ART suppression.
Consistent with previous studies [14, 16, 26], we confirmed the strong anti-inflammatory effect of obefazimod in ART-suppressed PWH. Obefazimod upregulated the anti-inflammatory miR-124 in all HIV-1–infected participants undergoing ART. miR-124 is a critical modulator of inflammation and immunity that is reported to provide therapeutic restitution of physiological pathways lost in multiple inflammatory diseases [15]. By the selective induction of miR-124, obefazimod demonstrates anti-inflammatory effects in both preclinical models and patients with ulcerative colitis (UC) [17, 27, 28], and is currently in phase 3 trials in UC, and phase 2 trials in Crohn disease and rheumatoid arthritis [29]. The role of miR-124 in HIV-1 infection has only been preliminarily explored. Thus, it has been suggested that HIV-1 Tat could induce microglial activation via the promoter DNA hypermethylation of miR-124, leading to the exacerbated release of proinflammatory cytokines [30], a molecular mechanism that could be involved in neuroinflammation associated with HIV-1–associated neurocognitive disorders [31].
Consistent with miR-124 upregulation, obefazimod represses overexpression of some HIV-inductive soluble inflammation biomarkers, such as G-CSF, GM-CSF, CD14, IL-7, IL-10, MIP-1β (CCL4), and MCP-1 (CCL2). These effects on the immune system are reminiscent of some, but not all, of those observed in serum samples from UC patients treated with obefazimod (Tazi, personal communication), which emphasizes that inflammatory bowel diseases and HIV infection share some similarities, but that the inflammation seen in HIV-infected patients is very specific and that there are significant differences in terms of immunopathological changes between these 2 conditions [32]. CRP was transiently upregulated only after obefazimod administration at the 150-mg dose (P = .008), but the magnitude of change was not clinically meaningful. Actually, no transient elevation of CRP has been observed to date in >800 UC patients given 50 mg of obefazimod [29], and when a Spearman correlation of CRP with all variables included in this study was analyzed, no significant correlation was found that could explain this transient effect (data not shown).
In contrast with miR-124 upregulation, which was reversed after drug discontinuation, the greatest attenuation of inflammation biomarkers was observed 4 weeks after drug discontinuation, such as downregulation of GM-CSF, IL-7, IL-1β, IL-12 (p70), IL-2, and IL-17α (CTLA-8), when obefazimod was administered for a longer period. Altogether, our results show the broad anti-inflammatory effect of obefazimod and suggest that it might be a good candidate to treat the chronic inflammation that ART-suppressed PWH exhibit as well as other inflammatory diseases.
Among the study limitations, we should acknowledge the small number of participants that may preclude statistical power of several results; the restricted demographics (all White men), which could hide ethnic- and gender-based differences in response to the intervention; and differences in total time of HIV infection and undergoing ART between the HIV50mg/12w and HIV150mg/4w groups that might mask differences between the 50- and 150-mg doses of treatment with obefazimod.
It is tempting to speculate that based on the present results, intermediate doses of obefazimod and longer treatment periods than those used in the present study could result in a sustained impact on viral persistence and chronic immune activation and inflammation, while limiting drug-related adverse effects. Of note, obefazimod was not administered to patients with UC at a daily dose >50 mg. Moreover, the present results also support the search of alternative drugs that target viral transcription, processing, and/or maturation of viral transcripts with potential to eliminate or silence viral persistence and simultaneously suppress virus-induced immune activation and inflammation [33].
In conclusion, obefazimod impacts chronic immune activation and inflammation in ART-suppressed PWH, further contributing to changes in viral persistence. It would be interesting to better define the drug-associated mechanisms that counteract persistent activation/inflammation, either by decreasing HIV antigen expression and presumably reducing T-cell proliferation, or by selectively upregulating miR-124 through its specific interaction with the CBC. The overall effect of obefazimod suggests a potential role for the drug in context of sustained inflammation during ART, as well as in virus remission strategies involving other compounds that can activate immune cells, such as latency-reversing agents.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Silvia Bernal, IrsiCaixa AIDS Research Institute, Badalona, Spain; Department of Infectious Diseases and Immunity, School of Medicine, University of Vic–Central University of Catalonia, Vic, Spain.
Maria C Puertas, IrsiCaixa AIDS Research Institute, Badalona, Spain; Consorcio Centro de Investigación Biomédica en Red de Enfermedades Infecciosas, Instituto de Salud Carlos III, Madrid, Spain.
Sara Morón-López, IrsiCaixa AIDS Research Institute, Badalona, Spain; Consorcio Centro de Investigación Biomédica en Red de Enfermedades Infecciosas, Instituto de Salud Carlos III, Madrid, Spain.
Ross D Cranston, Department of Infectious Diseases, Hospital Clinic of Barcelona, University of Barcelona, Barcelona, Spain.
Víctor Urrea, IrsiCaixa AIDS Research Institute, Badalona, Spain.
Judith Dalmau, IrsiCaixa AIDS Research Institute, Badalona, Spain.
María Salgado, IrsiCaixa AIDS Research Institute, Badalona, Spain; Consorcio Centro de Investigación Biomédica en Red de Enfermedades Infecciosas, Instituto de Salud Carlos III, Madrid, Spain; Germans Trias i Pujol Research Institute, Badalona, Spain.
Cristina Gálvez, IrsiCaixa AIDS Research Institute, Badalona, Spain.
Itziar Erkizia, IrsiCaixa AIDS Research Institute, Badalona, Spain.
Ian McGowan, Department of Medicine, University of Pittsburgh, Pittsburgh, Pennsylvania, USA.
Didier Scherrer, Abivax, Paris, France.
Boris Revollo, Fundació Lluita contra les Infeccions, Badalona, Spain; Department of Infectious Diseases, University Hospital Germans Trias i Pujol, Badalona, Spain.
Guillem Sirera, Fundació Lluita contra les Infeccions, Badalona, Spain; Department of Infectious Diseases, University Hospital Germans Trias i Pujol, Badalona, Spain.
José Ramón Santos, Fundació Lluita contra les Infeccions, Badalona, Spain; Department of Infectious Diseases, University Hospital Germans Trias i Pujol, Badalona, Spain.
Bonaventura Clotet, IrsiCaixa AIDS Research Institute, Badalona, Spain; Department of Infectious Diseases and Immunity, School of Medicine, University of Vic–Central University of Catalonia, Vic, Spain; Consorcio Centro de Investigación Biomédica en Red de Enfermedades Infecciosas, Instituto de Salud Carlos III, Madrid, Spain; Fundació Lluita contra les Infeccions, Badalona, Spain; Department of Infectious Diseases, University Hospital Germans Trias i Pujol, Badalona, Spain.
Roger Paredes, IrsiCaixa AIDS Research Institute, Badalona, Spain; Department of Infectious Diseases and Immunity, School of Medicine, University of Vic–Central University of Catalonia, Vic, Spain; Consorcio Centro de Investigación Biomédica en Red de Enfermedades Infecciosas, Instituto de Salud Carlos III, Madrid, Spain; Fundació Lluita contra les Infeccions, Badalona, Spain; Department of Infectious Diseases, University Hospital Germans Trias i Pujol, Badalona, Spain.
Javier Martinez-Picado, IrsiCaixa AIDS Research Institute, Badalona, Spain; Department of Infectious Diseases and Immunity, School of Medicine, University of Vic–Central University of Catalonia, Vic, Spain; Consorcio Centro de Investigación Biomédica en Red de Enfermedades Infecciosas, Instituto de Salud Carlos III, Madrid, Spain; Germans Trias i Pujol Research Institute, Badalona, Spain; Catalan Institution for Research and Advanced Studies, Barcelona, Spain.
Notes
Author contributions. All authors made substantial contributions to the conception or design of the work, or the data acquisition, analysis, or interpretation of the work. R. D. C., I. M., J. D., D. S., R. P., and J. M.-P. contributed to the study conception and design. J. R. S., B. R., G. S., and B. C. contributed to the inclusion and clinical follow-up of the patients. S. B. contributed to data acquisition, analysis, and interpretation, and drafted the original manuscript including figures and tables. S. B. and M. C. P. contributed to nucleic acid analysis. S. B. and I. E. contributed to flow cytometry analysis; S. B. and S. M.-L. contributed to GALT sample processing. S. B. and M. S. contributed to residual viremia analysis. S. B. and C. G. contributed to measurement of soluble inflammation biomarkers. V. U. contributed to statistical analysis. J. M.-P., S. M.-L., and M. C. P. reviewed the manuscript critically. All authors have approved the final version submitted for publication.
Acknowledgments. The authors thank the study participants who made this study possible. We thank A. Nieto for the patients’ recruitment and clinical management; R. Ayén, L. Gómez, and E. Grau for sample processing and cryopreservation; M. A. Fernandez and G. Requena from Cytometry Core Facility at the Research Institute Germans Trias i Pujol; and A. Hernández from the microbiology department of the University Hospital Germans Trias i Pujol, for their contributions to this publication.
Disclaimer. The funders had no role in data collection and analysis, decision to publish, or preparation of the manuscript.
Financial support. Abivax sponsored the clinical study and contributed to the design of the clinical trial. S. M.-L. was supported by the EU Gilead Research Scholars Program award 2021 and the grant 2020 BP 00046 from the Catalan Agency of Management of University and Research Grants. Funding to pay the Open Access publication charges for this article was provided by IrsiCaixa AIDS Research Institute.
Potential conflicts of interest. J. M.-P. received grant support and consultancy fees from Abivax; research grants paid to their institution from Grifols during the conduct of the study; unrestricted research grants to their institution from MSD, ViiV Healthcare, Gilead Sciences, and AstraZeneca; consulting fees from MSD; and payment for educational activities from Janssen and Gilead, all outside the submitted work. I. M. is the Chair of the Abivax scientific advisory board and has shares in the company. D. S. is Abivax vice president of R&D and has shares in the company. M. C. P. received research grant from Gilead Sciences, outside the submitted work. S. M.-L. received a research scholars program award from Gilead Sciences, outside the submitted work. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Chun TW, Fauci AS. HIV reservoirs: pathogenesis and obstacles to viral eradication and cure. AIDS 2012; 26:1261–8. [DOI] [PubMed] [Google Scholar]
- 2. Cohn LB, Chomont N, Deeks SG. The biology of the HIV-1 latent reservoir and implications for cure strategies. Cell Host Microbe 2020; 27:519–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Halvas EK, Joseph KW, Brandt LD, et al. . HIV-1 viremia not suppressible by antiretroviral therapy can originate from large T cell clones producing infectious virus. J Clin Invest 2020; 130:5847–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fischer M, Wong JK, Russenberger D, et al. . Residual cell-associated unspliced HIV-1 RNA in peripheral blood of patients on potent antiretroviral therapy represents intracellular transcripts. Antivir Ther 2002; 7:91–103. [PubMed] [Google Scholar]
- 5. Chomont N, El-Far M, Ancuta P, et al. . HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat Med 2009; 15:893–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Utay NS, Hunt PW. Role of immune activation in progression to AIDS. Curr Opin HIV AIDS 2016; 11:131–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Deeks SG, Archin N, Cannon P, et al. . Research priorities for an HIV cure: International AIDS Society global scientific strategy 2021. Nat Med 2021; 27:2085–98. [DOI] [PubMed] [Google Scholar]
- 8. Yukl SA, Kaiser P, Kim P, et al. . HIV latency in isolated patient CD4+ T cells may be due to blocks in HIV transcriptional elongation, completion, and splicing. Sci Transl Med 2018; 10:eaap9927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Campos N, Myburgh R, Garcel A, et al. . Long lasting control of viral rebound with a new drug ABX464 targeting Rev-mediated viral RNA biogenesis. Retrovirology 2015; 12:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Scherrer D, Rouzier R, Noel Barrett P, et al. . Pharmacokinetics and tolerability of ABX464, a novel first-in-class compound to treat HIV infection, in healthy HIV-uninfected subjects. J Antimicrob Chemother 2017; 72:820–8. [DOI] [PubMed] [Google Scholar]
- 11. Scherrer D, Rouzier R, Cardona M, et al. . Randomized trial of food effect on pharmacokinetic parameters of ABX464 administered orally to healthy male subjects. Antimicrob Agents Chemother 2017; 61:e01288-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Steens J-M, Scherrer D, Gineste P, et al. . Safety, pharmacokinetics, and antiviral activity of a novel HIV antiviral, ABX464, in treatment-naive HIV-infected subjects in a phase 2 randomized, controlled study. Antimicrob Agents Chemother 2017; 61:e00545-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rutsaert S, Steens J-M, Gineste P, et al. . Safety, tolerability and impact on viral reservoirs of the addition to antiretroviral therapy of ABX464, an investigational antiviral drug, in individuals living with HIV-1: a phase IIa randomised controlled study. J Virus Erad 2019; 5:10–22. [PMC free article] [PubMed] [Google Scholar]
- 14. Vautrin A, Manchon L, Garcel A, et al. . Both anti-inflammatory and antiviral properties of novel drug candidate ABX464 are mediated by modulation of RNA splicing. Sci Rep 2019; 9:792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Qin Z, Wang P-Y, Su D-F, Liu X. miRNA-124 in immune system and immune disorders. Front Immunol 2016; 7:406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chebli K, Papon L, Paul C, et al. . The anti-HIV candidate Abx464 dampens intestinal inflammation by triggering il-22 production in activated macrophages. Sci Rep 2017; 7:4860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vermeire S, Hébuterne X, Tilg H, et al. . Induction and long-term follow-up with ABX464 for moderate-to-severe ulcerative colitis: results of phase IIa trial. Gastroenterology 2021; 160:2595–98.e3. [DOI] [PubMed] [Google Scholar]
- 18. Morón-López S, Puertas MC, Gálvez C, et al. . Sensitive quantification of the HIV-1 reservoir in gut-associated lymphoid tissue. PLoS One 2017; 12:e0175899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Puertas MC, Salgado M, Morón-López S, et al. . Effect of lithium on HIV-1 expression and proviral reservoir size in the CD4+ T cells of antiretroviral therapy suppressed patients. AIDS 2014; 28:2157–9. [DOI] [PubMed] [Google Scholar]
- 20. Martínez-Bonet M, Puertas MC, Fortuny C, et al. . Establishment and replenishment of the viral reservoir in perinatally HIV-1-infected children initiating very early antiretroviral therapy. Clin Infect Dis 2015; 61:1169–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Moron-Lopez S, Bernal S, Wong JK, Martinez-Picado J, Yukl SA. ABX464 decreases the total human immunodeficiency virus (HIV) reservoir and HIV transcription initiation in CD4+ T cells from antiretroviral therapy–suppressed individuals living with HIV. Clin Infect Dis 2021; 74:2044–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Khoury G, Fromentin R, Solomon A, et al. . Human immunodeficiency virus persistence and T-cell activation in blood, rectal, and lymph node tissue in human immunodeficiency virus-infected individuals receiving suppressive antiretroviral therapy. J Infect Dis 2017; 215:911–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yukl SA, Gianella S, Sinclair E, et al. . Differences in HIV burden and immune activation within the gut of HIV-positive patients receiving suppressive antiretroviral therapy. J Infect Dis 2010; 202:1553–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hunt PW, Martin JN, Sinclair E, et al. . T cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus–infected patients with sustained viral suppression during antiretroviral therapy. J Infect Dis 2003; 187:1534–43. [DOI] [PubMed] [Google Scholar]
- 25. Day CL, Kaufmann DE, Kiepiela P, et al. . PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 2006; 443:350–4. [DOI] [PubMed] [Google Scholar]
- 26. Tazi J, Begon-Pescia C, Campos N, et al. . Specific and selective induction of miR-124 in immune cells by the quinoline ABX464: a transformative therapy for inflammatory diseases. Drug Discov Today 2021; 26:1030–9. [DOI] [PubMed] [Google Scholar]
- 27. Vermeire S, Hébuterne X, Napora P, et al. . OP21 ABX464 is safe and efficacious in a proof-of-concept study in ulcerative colitis patients. J Crohns Colitis 2019; 13:S014–5. [Google Scholar]
- 28. Vermeire S, Sands B, Tilg H, et al. . ABX464 (obefazimod) for moderate to severe active ulcerative colitis: a randomised, placebo-controlled phase 2b induction trial and 48-week extension. Lancet Gastroenterol Hepatol 2022; 7:1024–35. [DOI] [PubMed] [Google Scholar]
- 29. Daien C, Krogulec M, Gineste P, et al. . Safety and efficacy of the miR-124 upregulator ABX464 (obefazimod, 50 and 100 mg per day) in patients with active rheumatoid arthritis and inadequate response to methotrexate and/or anti TNFα therapy: a placebo-controlled phase II study. Ann Rheum Dis 2022; 81:1076–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Periyasamy P, Thangaraj A, Guo M-L, et al. . Epigenetic promoter DNA methylation of miR-124 promotes HIV-1 tat-mediated microglial activation via MECP2-STAT3 axis. J Neurosci 2018; 38:5367–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Buch S, Periyasamy P, Guo M. Involvement of epigenetic promoter DNA methylation of miR-124 in the pathogenesis of HIV-1–associated neurocognitive disorders. Epigenetics Insights 2018; 11:251686571880690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Alzahrani J, Hussain T, Simar D, et al. . Inflammatory and immunometabolic consequences of gut dysfunction in HIV: parallels with IBD and implications for reservoir persistence and non-AIDS comorbidities. EBioMedicine 2019; 46:522–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pasternak AO, Berkhout B. The splice of life: does RNA processing have a role in HIV-1 persistence? Viruses 2021; 13:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.