Abstract
Aims
Little is known about dynamic changes of the left atrial (LA) substrate over time in patients with atrial fibrillation (AF). This study aims to evaluate substrate changes following pulmonary vein isolation (PVI).
Methods and results
In our prospective observational study, consecutive patients undergoing first PVI-only and redo ablation were included. High-density maps of the two procedures were compared. Progression or regression was diagnosed if a significant concordant decrease or increase in bipolar voltages in ≥2 segments was observed, respectively. In 28 patients (61.2 ± 9.5 years, 39% female, 53.5% persistent AF), 111.013 voltage points from 56 high-density LA maps (1.982 points/patient) were analysed. Comparing the high-density maps of the first and second procedures, in the progression group (17 patients, 61%), there was a decrease in global (−35%, P < 0.001) and all regional voltages. In the regression group (11 patients, 39%), there was an increase in global (+43%, P < 0.001) and regional voltages. Comparing the progression with the regression group, the area of low-voltage zone (LVZ) increased (+3.5 vs. −4.5 cm2, P < 0.001) and LA activation time prolonged (+8.0 vs. −9.1 ms, P = 0.005). Baseline clinical parameters did not predict progression or regression. In patients with substrate progression, pulmonary veins (PVs) were more frequently isolated (P = 0.02) and the AF pattern at recurrence was more frequently persistent (P = 0.005).
Conclusion
Our study describes bidirectional dynamic properties of the LA substrate with concordant either progressive or regressive changes. Regression occurs with reduced AF burden after the first procedure, while progression is associated with persistent AF recurrence despite durable PV isolation. The dynamic nature of LA substrate poses questions about LVZ-based ablation strategies.
Keywords: Atrial fibrillation, Progression, Regression, Atrial substrate, Voltage mapping
Graphical Abstract
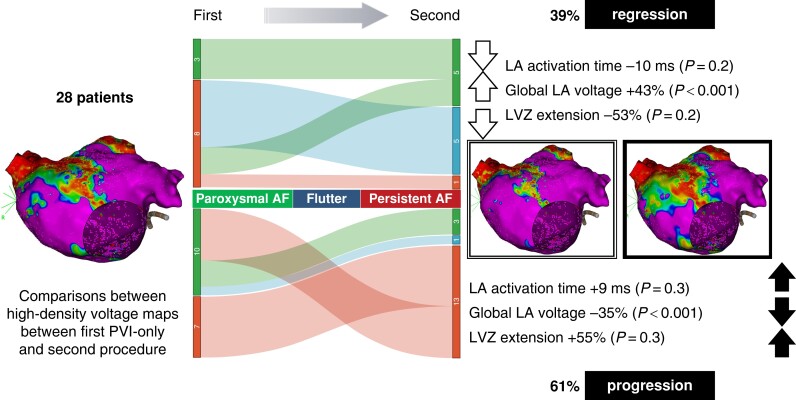
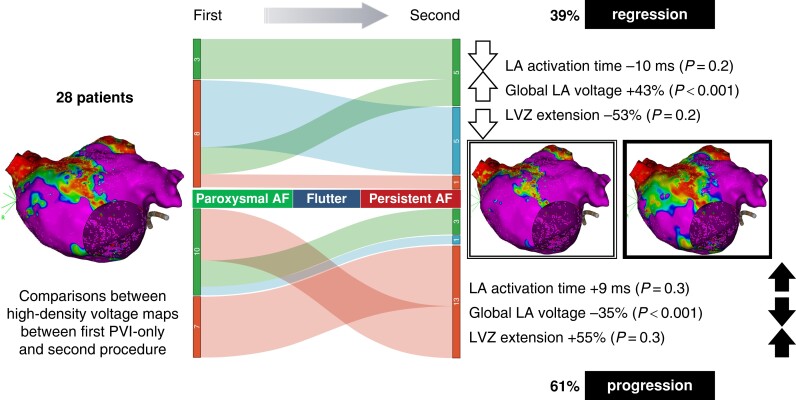
Graphical abstract.
Change in the percentage of paroxysmal AF (green), atrial flutter (blue), and persistent AF (red) in the regression group (upper central side) and progression group (lower central side) between the first and the second procedures. On the left side, the modifications in LA activation time, global LA voltage, and LVZ extension are described in the regression group (upper part) and in the progression group (lower part). AF, atrial fibrillation; LA, left atrium; LVZ, low-voltage zone; PVI, pulmonary vein isolation.
What’s new?
In most patients with recurrent atrial arrhythmias following pulmonary vein isolation (PVI), there’s a progression of atrial substrate marked by a consistent decrease in bipolar voltage in non-ablated left atrial (LA) regions. This progression correlates with an early recurrence of persistent atrial fibrillation (AF), often despite durable PVI.
In a few patients, particularly those noting a reduction in AF burden post-procedure, our study describes for the first time a regression of atrial substrate. This regression is linked to an increase in both global and regional bipolar voltage values, coupled with enhanced conduction velocity.
None of the initial clinical, electrocardiographic, or echocardiographic parameters could distinguish between patients showing progression vs. those with regression.
Introduction
In experimental models, atrial fibrillation (AF) was demonstrated to induce electrical and structural remodelling that promotes AF maintenance and recurrence.1,2 In this context, changes in atrial substrate are dynamic: electrical and contractile remodelling are reversible, while some structural changes may be irreversible.3,4 In human, a decrease in atrial voltages and appearance of low-voltage zones (LVZs) on electroanatomic mapping have been used as a surrogate for atrial fibrosis and may indicate progression of atrial substrate.5–11 In preliminary studies in patients with AF, progression of atrial substrate has been described in the right and left atria despite pulmonary vein isolation (PVI).6,7 The recovery of atrial electrical and structural changes (regression of atrial substrate) has recently been demonstrated in the right atria in patients with severe mitral stenosis treated with commissurotomy, in patients with obstructive sleep apnoea (OSAS) treated with continuous positive airway pressure (CPAP) and in patients with AF and unexplained cardiomyopathy treated with PVI.12–14 Additionally, in a small study of patients with persistent AF, limited remodelling of left atrial (LA) substrate has been reported short after PVI.15 Dynamic changes over time among patients experiencing AF recurrences after initial ablation are not well characterized.
The aim of the present study was (i) to compare LA bipolar voltage characteristics with high-density mapping between first and second ablation procedures in patients experiencing recurrent atrial tachyarrhythmia after PVI-only approach and (ii) to identify concordant either progressive, regressive, or unchanged bipolar voltage in different LA regions outside of the pulmonary vein (PVs) following PVI.
Methods
Study design
A single-centre prospective observational study was designed to identify changes in LA substrate over time with high-density multipolar mapping in patients undergoing first and redo AF ablation. Mapping was performed during sinus rhythm. The study was carried out according to the principles of the Declaration of Helsinki. All patients provided informed consent to participate, and the study protocol was approved by the ethical committee of the University Hospital of Antwerp.
Patient population
Between 2017 and 2022, patients undergoing catheter ablation for AF in the University Hospital of Antwerp were screened to fulfil the following inclusion criteria: (i) first ablation procedure with PVI-only approach followed by redo ablation procedure due to recurrent atrial fibrillation, atrial tachycardia, or atrial flutter (AF/AT/AFL) more than 3 months after the initial procedure and (ii) sufficient quality high-density multipolar voltage maps performed with the same types of 3D electro-anatomical mapping system and multipolar mapping catheter in both procedures.
High-density electro-anatomical mapping
All procedures were performed with the same electro-anatomical mapping system (CARTO®, Biosense Webster, Diamond Bar, CA, USA). Details of the PVI, mapping algorithm, and map analysis were previously described.16,17 Shortly, high-density electro-anatomical mapping involved the use of LASSO™ Circular Mapping Catheter (Biosense Webster®) for the first procedure and the use of PentaRay® Catheter (Biosense Webster®) at redo procedure. The automatic point collection was set at very strict parameters: location stability was set at maximum 2 mm and local activation time stability at 3 ms. Anatomical resolution was set at a high value,18 and fill threshold was set low to ensure dense mapping. Tissue proximity indication (TPI) was not utilized; however, at the end of the mapping phase, all points >5 mm distance from the anatomical shell were filtered and deleted. Low-voltage cut-off was set at <0.5 mV. The LVZ was identified as an area of at least 1 cm2 containing ≥3 neighbouring points with ≤10 mm distance. The LVZ area was measured with a measurement tool by manually encircling the area and was expressed in cm2. The extent of LVZ was defined as the percentage area of the LA surface. During offline analysis, all EGM signals were manually checked and numerically entered in an electronic database. The LA was divided into six regions: anterior, septal, lateral, inferior, PV antrum, and posterior LA (see Supplementary material online, Figure S1). To avoid confounding effect of ablation lesions from the first procedure, the PV antrum and posterior LA regions were excluded from the analysis. In each patient, voltage measurements of the four LA regions and extent of spontaneous LVZ area were compared between first and second procedures considering each segment in each patient as its own control. Progression was diagnosed in a patient when a significant concordant decrease in voltage measurements in at least two of the four segments between the first and second procedures was observed (progression group). A patient was classified into the regression group if a significant concordant increase in the bipolar voltage in at least two of the four segments was observed (regression group). If a significant change occurred in less than two segments or no change at all occurred, the patient was planned to be classified as unchanged (unchanged group). All patients underwent ipsilateral wide area circumferential PVI with the use of CF sensing irrigated tip ablation catheter (Smart-Touch®, Biosense Webster, Diamond Bar, CA, USA) and automatic ablation annotation module (Visi-Tag®, Biosense Webster, Diamond Bar, CA, USA).
Statistical analysis
Continuous variables are presented as mean ± standard deviation or median and interquartile range as appropriate. Comparisons between groups were undertaken with parametric (Student’s t-test) or non-parametric (Mann–Whitney U test) tests, respectively. The comparison between categorical variables was performed with the χ2 test and Fisher’s exact test. Event-free survival was estimated by the Kaplan–Meier method using the log-rank test. The SPSS 28.0.1.0 (IBM Corp, Armonk, New York, USA) was used for all statistical analyses.
Results
Study population
A total of 28 patients (61.2 ± 9.5 years, 39% female, 53.5% persistent AF) were included. The time between first and second procedures was 1.2 (0.4–2.3) years. The baseline characteristics of the study population are shown in Table 1. Seventeen (61%) patients showed a significant decrease in bipolar voltage in ≥2 LA segments at the second procedure (progression group). In this group, five (29%) patients showed a significant decrease in all four segments, eight (47%) patients in three, and in four (24%) patients in two segments. Eleven (39%) patients had a significant increase in ≥2 segments and were classified into the regression group. Within this group, in three (27%) patients, a significant increase in voltage occurred in three segments and in eight (73%) patients in two segments. None of the patients showed a significant change in <2 segments or no change at all (unchanged group). The detailed segment analysis for each patient is depicted in Supplementary material online, Table S1. There was no significant difference in any baseline clinical characteristics between the progression and regression groups (Table 1).
Table 1.
Baselines characteristics of the study population
| Characteristics | Total N = 28 | Progression Group N = 17 | Regression Group N = 11 | P Value |
|---|---|---|---|---|
| Age, years | 61.2 ± 9.5 | 62.5 ± 8.4 | 58.8 ± 10.9 | 0.33 |
| Female sex | 11 (39.3%) | 9 (52.9%) | 2 (18.2%) | 0.11 |
| BMI | 26.3 ± 4.1 | 26.8 ± 2.9 | 25.6 ± 5.6 | 0.47 |
| Years of AF | 4.1 (2.3–7.2) | 3.9 (1.6–7.2) | 4.3 (3.2–7.1) | 0.36 |
| Persistent AF | 15 (53.5%) | 7 (41.2%) | 8 (72.7%) | 0.10 |
| History of HF | 8 (28.6%) | 6 (35.3%) | 2 (18.2%) | 0.42 |
| OSAS | 5 (17.8%) | 2 (11.8%) | 3 (27.3%) | 0.35 |
| Hypertension | 11 (39.3%) | 6 (35.3%) | 5 (45.5%) | 0.70 |
| Diabetes | 4 (14.3%) | 2 (11.8%) | 2 (18.2%) | 1 |
| Previous TIA/stroke | 3 (10.7%) | 3 (17.6%) | 0 (0%) | 0.26 |
| CHA2DS2-VASc score | 2 (1–3.75) | 2 (1–4) | 1 (1–3) | 0.28 |
| PR interval | 162.2 ± 60.2 | 161.1 ± 71.5 | 164.7 ± 26.9 | 0.89 |
| QRS interval | 104.4 ± 27.6 | 101.2 ± 25.2 | 109.3 ± 31.5 | 0.46 |
| LAVI (echo), mL/m2 | 37.0 ± 8.1 | 38.8 ± 8.1 | 34.3 ± 7.7 | 0.15 |
| LVEF (echo), % | 57.9 ± 7.6 | 57.5 ± 8.7 | 58.5 ± 5.6 | 0.72 |
| LVEF (echo) < 50% | 3 (10.7%) | 2 (11.8%) | 1 (9.1%) | 1 |
AF, atrial fibrillation; BMI, body mass index; echo, echocardiography; HF, heart failure; LAVI, left atrial volume index; LVEF, left ventricular ejection fraction; OSAS, obstructive sleep apnoea syndrome; TIA, transient ischaemic attack.
Per-point analysis
A total of 34.055 (1.216/patient) points from the first and 76.958 (2748/patient) points from the second procedure were collected. After exclusion of the PV and posterior LA segments and filtering internal points, 16.841 from the first and 21.682 bipolar voltage measurements from the second procedure were analysed. The comparison between global and regional voltage between the first and second procedures by per-point analysis is shown in Table 2. Considering the total study population, a significant decrease was observed in the global and all regional voltages between the first and second procedures. The comparison between global and regional voltage measurements between the first and second procedures by per-point analysis in the progression and regression groups is shown in Table 3. In the progression group, a significant 35% decrease in the global voltages was observed, while in the regression group, a significant 43% increase in the global voltages was observed.
Table 2.
Comparison of mean global and segmental bipolar voltage measurements between the first and second procedures by per-point analysis in the whole study population (n = 28)
| Characteristic | First Procedure | Second Procedure | P Value |
|---|---|---|---|
| Mean LA voltage, mV | 1.59 ± 1.53 | 1.36 ± 1.31 | <0.001 |
| Mean LA anterior wall voltage, mV | 1.30 ± 1.35 | 1.24 ± 1.22 | 0.02 |
| Mean LA septal wall voltage, mV | 1.15 ± 1.21 | 1.01 ± 1.05 | <0.001 |
| Mean LA lateral wall voltage, mV | 2.21 ± 1.80 | 1.84 ± 1.53 | <0.001 |
| Mean LA inferior wall voltage, mV | 1.66 ± 1.47 | 1.44 ± 1.25 | <0.001 |
LA, left atrial.
Table 3.
Comparison of global (four segments) and segmental bipolar voltage measurements between the first and second procedures in the progression and regression groups by per-point analysis
| Characteristic | First Procedure | Second Procedure | P Value |
|---|---|---|---|
| Points/patient | 601.4 ± 294.6 | 774.3 ± 370.0 | 0.06 |
| Progression group (n = 17 patients) | |||
| Global, mV | 1.76 ± 1.64 | 1.14 ± 1.15 | <0.001 |
| Anterior segment, mV | 1.38 ± 1.38 | 0.97 ± 1.01 | <0.001 |
| Septal segment, mV | 1.23 ± 1.22 | 0.87 ± 0.94 | <0.001 |
| Lateral segment, mV | 2.47 ± 1.92 | 1.54 ± 1.36 | <0.001 |
| Inferior segment, mV | 1.89 ± 1.62 | 1.25 ± 1.18 | <0.001 |
| Regression group (n = 11 patients) | |||
| Global, mV | 1.28 ± 1.27 | 1.83 ± 1.48 | <0.001 |
| Anterior segment, mV | 1.15 ± 1.27 | 1.65 ± 1.39 | <0.001 |
| Septal segment, mV | 1.01 ± 1.19 | 1.36 ± 1.21 | <0.001 |
| Lateral segment, mV | 1.71 ± 1.41 | 2.45 ± 1.68 | <0.001 |
| Inferior segment, mV | 1.27 ± 1.06 | 1.69 ± 1.30 | <0.001 |
Values in bold indicates significant differences. LA, left atrial.
Per-patient analysis
The extent of spontaneous LVZ, LA activation times, and volumes in the first and second procedures is shown in Table 4 and in Supplementary material online, Table S2. Comparing the first with the second procedure, in the progression group, the area of LVZ and the LA activation time increased non-significantly. In the regression group, the area of LVZ and LA activation time decreased non-significantly (Table 4). Comparing the progression group with the regression group, the change in area of LVZ and LA activation times was significant (+3.5 vs. −4.5 cm2, P < 0.001, and +8.0 vs. −9.1 ms, P = 0.005, respectively) (see Supplementary material online, Table S2 and Figure S2 and Figure 1).
Table 4.
Comparison of spontaneous low-voltage zone (LVZ, <0.5 mV) area, LA volume, and activation times between the first and second procedures in the progression and regression groups
| Characteristics | First Procedure | Second Procedure | P Value |
|---|---|---|---|
| Progression group (n = 17) | |||
| Area spontaneous LVZ, cm2 | 9.13 ± 13.24 | 14.01 ± 14.08 | 0.30 |
| % LVZ/total LA surface | 6.35 ± 9.45 | 9.82 ± 10.11 | 0.30 |
| Patients with >5% LVZ | 6 (35.3%) | 8 (47.1%) | 0.73 |
| LA activation time, ms | 109.94 ± 20.79 | 118.44 ± 22.26 | 0.27 |
| LA volume (EAM), mL | 157.83 ± 39.73 | 151.88 ± 32.19 | 0.08 |
| Regression group (n = 11) | |||
| Area spontaneous LVZ, cm2 | 14.21 ± 16.08 | 7.4 ± 7.02 | 0.21 |
| % LVZ/total LA surface | 8.55 ± 9.47 | 4.05 ± 4.00 | 0.16 |
| Patients with >5% LVZ | 5 (45.5%) | 3 (27.3%) | 0.66 |
| LA activation time, ms | 97.60 ± 17.48 | 87.60 ± 17.39 | 0.21 |
| LA volume (EAM), mL | 130.87 ± 36.42 | 141.05 ± 32.24 | 0.39 |
EAM, electroanatomic mapping; LA, left atrial; LVZ, low-voltage zone (calculated excluding the posterior wall and pulmonary veins).
Figure 1.
Change in the percentage of paroxysmal AF (green), atrial flutter (blue), and persistent AF (red) in the regression group (upper central side) and progression group (lower central side) between the first and the second procedures. On the right side, the modifications in LA activation time, global LA voltage, and LVZ extension are described in the regression group (upper part) and in the progression group (lower part). AF, atrial fibrillation; LA, left atrium; LVZ, low-voltage zone; PVI, pulmonary vein isolation.
Atrial tachyarrhythmia recurrence
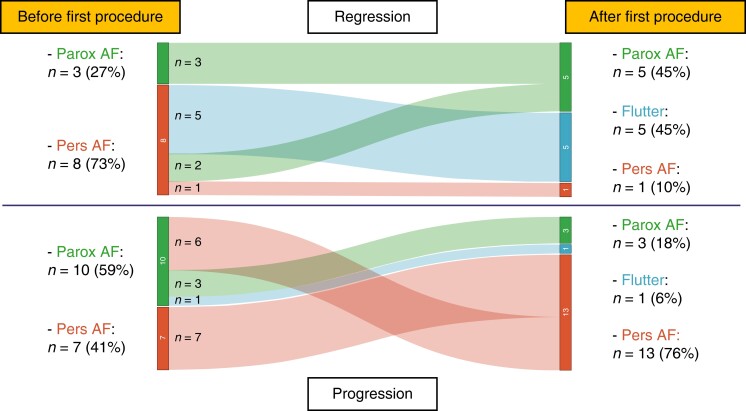
The type and evolution of atrial arrhythmia pattern (paroxysmal AF vs. persistent AF vs. atrial flutter) over time are depicted in Table 5 and Figure 2. Persistent AF recurrence with isolated PVs after the first procedure was significantly more frequent in the progression group (Table 5). Recurrence of paroxysmal AF or atrial flutter was significantly more frequent in the regression group. The time spent in AF after the first procedure is depicted in Supplementary material online, Figure S3. In the regression group, the time spent in sinus rhythm after the first procedure is longer compared with that in the progression group (210 vs. 97 days, P = 0.06). After the recurrence, patients in the regression group spend less time in AF before the second procedure compared with patients in the progression group (133 vs. 235 days, P = 0.10).
Table 5.
Atrial fibrillation, atrial tachycardia, and flutter recurrence following the first and second procedures in the total study population and in the progression and regression groups
| Characteristic | Total N = 28 | Progression Group N = 17 | Regression Group N = 11 | P Value |
|---|---|---|---|---|
| Persistent AF before first procedure | 15 (53.5%) | 7 (41.2%) | 8 (72.7%) | 0.10 |
| Persistent AF before second procedure | 14 (50%) | 13 (76.5%) | 1 (9.1%) | 0.005 |
| Paroxysmal AF before second procedure | 8 (28.6%) | 3 (17.6%) | 5 (45.5%) | 0.005 |
| AFL before second procedure | 6 (21.4%) | 1 (5.9%) | 5 (45.5%) | 0.005 |
| Days between first procedure and first AF/AFl recurrence | 157.5 (71.25–471.5) |
97 (22.5–378.5) |
210 (110–800) |
0.06 |
| Isolated PV at second procedure | 13 (46.4%) | 11 (64.7%) | 2 (18.2%) | 0.02 |
| AF/AFl recurrence after second procedure | 7/22 (31.8%) | 5/13 (38.5%) | 2/9 (22.2%) | 0.64 |
Values in bold indicates significant differences. AF, atrial fibrillation; AFL, atrial flutter; PV, pulmonary veins.
Figure 2.
Comparison of atrial fibrillation patterns before and after the first PVI procedure between the progression and regression groups. AF, atrial fibrillation; Parox, paroxysmal; Pers, persistent.
Discussion
The main findings of the current study are as follows: (i) in most patients with recurrent atrial arrhythmias following first PVI, progression of the LA substrate (decrease in mean and regional voltages) occurs. This is accompanied by an increase in LVZ area and slower atrial activation time. (ii) However, even among patients with AF recurrence, regression (increase in mean and regional voltages) of the LA substrate can be observed in up to 40% of the patients. These changes are concordant with a decrease in LVZ area and faster atrial activation time. (iii) None of the baseline clinical, electrocardiographic, or echocardiographic parameters are able to differentiate between patients with progression and regression. The dynamic substrate changes are associated with the AF burden after the first procedure: recurrence as persistent AF with durable PVI occurs more often in the progression than in the regression group.
Progression of atrial substrate
Experimental models have shown early reversal of electrical remodelling; however, the reversibility of structural changes in animal experiments has not been clearly demonstrated.2 In dogs, 2 weeks after cardioversion of 8 weeks of AF combined with mitral regurgitation, no regression of structural abnormalities was observed.3 In the goat model of 16 weeks of lone AF, 16 weeks after cardioversion structural remodelling was only partial.19 Gap junctions were recovered, and connexin 40 expression was normalized, but atrial myocytes remained abnormal. In human, in 11 patients without apparent structural heart disease 10 months following successful PVI, Teh et al.6 reported decreased bipolar voltage, no improvement or further slowing of conduction, and further prolongation of regional refractoriness in the right atrium despite reversal of LA dilatation. In a more recent larger study of 113 patients undergoing redo catheter ablation and high-density mapping for recurrent AF, Huo et al.7 reported similar findings. Sixteen months following PVI and substrate modification ablation approach, 32% of the study population showed de novo LVZ. Patients with de novo LVZ were older and had lower EF and more persistent AF recurrence. In patients without LVZ, global bipolar voltage decreased significantly. Based on the results, in agreement with the right atrial study by Teh et al., universal progression of atrial substrate with new LVZs in a significant proportion of patients presenting for redo ablation with recurrent AF was concluded. However, in the study by Huo et al., patients with previous radio or chemotherapy were excluded, another multipolar mapping catheter was used, no minimal area (of 1 cm2) definition for LVZ was considered, segmental mean voltages in all patients and not per-patient analysis were used for comparison, LA appendage was omitted from the analysis, and at the first procedure LVZ homogenization and at the second procedure additional AF induction and atrial premature complexes ablation were performed. In our current study, using a per-patient analysis (in which each patient serves as his or her own control) comparing all collected points, we confirm that in 60% of patients presenting for redo ablation, progression of atrial substrate occurs. We also report that patients presenting with persistent AF after first PVI-only procedure are more likely to show substrate progression. However, we did not find the reported high proportion of patients with de novo LVZ. This difference may be due to the fact that we used criteria of a minimal LVZ area of 1 cm2 and ≥3 neighbouring points to avoid inclusion of few low-voltage points of very small areas due to low contact with the circular or star-shaped mapping catheter. In our study, we did not identify any clinical or echocardiographic parameter to identify patients with progression. However, this may be due to the low number of patients in the subgroups and needs further evaluation. Additionally, it should be noted that both in our study and the study by Huo et al. only patients with recurrent AF were included. This may lead to significant overestimation of substrate progression in the general AF population. However, repeated invasive diagnostic electro-anatomical mapping of the LA over time in patients without AF recurrence on a large scale is challenging due to ethical constrains.
Regression of atrial substrate
We report for the first time that up to 40% of patients show regression of atrial substrate after PVI, in spite of AF recurrence. In a preliminary study by John et al.12, a significant increase in tissue voltage and recovery of conduction velocity in the right atria was reported for the first time in 14 patients with severe mitral stenosis with and without AF 6 months after successful mitral valve commissurotomy. More recently, in a long-term follow-up substudy of the CAMERA-MRI study, repeat right atrial mapping was performed in 15 patients with unexplained cardiomyopathy and AF at initial presentation and left ventricular (LV) systolic function recovery and >90% AF burden reduction at 23 months following AF ablation. An increase in global, septal, and posterior RA bipolar voltage and a decrease in the proportion of complex fractionated electrograms was reported.13 In the SLEEP-AF study, by the same group, 24 patients with OSAS were randomized to CPAP vs. no therapy.14 The right atrial mapping study at 6 months showed higher global bipolar voltage, faster conduction velocity, and a decrease in the proportion of complex fractionated electrograms in the CPAP group as compared with the control group. Based on these studies in the human right atria, in patients successfully treated for severe mitral stenosis with commissurotomy, AF causing tachycardiomyopathy, or OSAS treated by CPAP, regression of atrial substrate was suggested. Additionally, in a more recent study of 23 patients with persistent AF, all patients underwent left atrial voltage mapping at the first PVI and 2 months later at a protocol-mandated remapping study irrespective of AF recurrence. In this study, a significant decrease in the LA volume and increase in the LA conduction velocities were reported. However, a significant increase in the bipolar voltage was only reported in the posterior wall and not in other LA regions. Our current study is the first study to report in human an increase in voltage in the whole LA in a significant proportion of patients following PVI. We hypothesize that the reason for the contradictory results from the previous similar right and left trial studies is due to the different methodology. In our study, we analysed all points collected with high-density mapping in non-ablated regions, but we used an initial ‘per-patient’ analysis comparing in each patient all points collected in the same region between the first and second procedures separately, thus the first procedure of each patient serving as his or her own control. As most patients show progression, comparing all collected points per region in a ‘per-point analysis’ in the total population shows a decrease in voltages; thus, also in our study, only progression is seen, similar to the two previous studies (Table 2). However, the per-patient analysis we are proposing allows the identification of the smaller but still significant proportion of patients showing regression of atrial substrate. Additionally, as compared with the only left atrial study reporting limited regression (only in the posterior LA), it should be noted that we performed mapping more than 1 year after PVI and that we excluded the posterior region. Two months may not be sufficient time to observe neither progression nor regression, and left posterior LA voltages may be influenced by wide area PVI. Our findings have significant clinical implications. Targeting all LVZ zones or transition zones with ablation at a first procedure for persistent AF may not be necessary as these regions in some patients may regress spontaneously. Additionally, it explains our previous findings from the MASH AF II study, suggesting that in paroxysmal AF >5% and in persistent AF only >15% LVZ burden signals a worse outcome after PVI-only approach.16 These findings support the hypothesis that not all LVZ zones are equally arrhythmogenic in all patients.20 Especially in persistent AF when the AF burden decreases, some LVZ areas due to AF-induced reversible structural remodelling may spontaneously regress. Our findings may also explain the negative results of some of the LVZ-based ablation approach studies (STABLE-SR I21 and II studies) in which transition zones at the first procedure of persistent AF were also targeted with focal ablations. We were not able to identify any baseline parameter to identify patients showing regression, suggesting reversible atrial structural remodelling. Patients with recurrent paroxysmal arrhythmias and flutter following first PVI were more likely to belong to the regression group, likely due to the decrease in AF burden facilitating reversion of AF-induced reversible structural remodelling Otherwise, persistent AF recurrences were more frequent in the progression group in the setting of isolated PVs due to non-PV substrate. Whether the progression of the substrate itself or the more advanced atrial substrate at baseline is the cause of the recurrences remains unclear. A representative figure depicting the progression of atrial substrate between the first and second procedures is present in the online supplementary material (see Supplementary material online, Figure S4). Larger studies are needed to better characterize and identify patients undergoing regression or progression of atrial substrate following PVI.
Limitations
The main limitation of our study is the low number of patients in the subgroups. However, the manual offline analysis and editing of maps with >35 000 points is time-consuming and cumbersome. The development of automatic tools may overcome the challenges and allow larger studies. The definition of regression or progression of the atrial substrate based on the use of only two segments is arbitrary. The remapping at the redo procedure was more dense compared with the first procedure due to the need for identifying reconnection/gap sites around PVs and previous PVI ablation lines and low-voltage sites for substrate modification. Furthermore, the use of PentaRay® Catheter (Biosense Webster®) at redo procedure allowed to collect a greater number of points compared with LASSO™ Circular Mapping Catheter (Biosense Webster®) adopted in the first procedure. However, both have 20 1 mm electrodes separated by 2 mm inter-electrode spacing; thus, no difference in the quality of signal recorded was expected. Furthermore, if there was a bias from the use of two different catheters, it would result in a unidirectional shift between the first and second maps, characterized by either all progression or all regression. However, our findings demonstrate a balanced trend in both directions, resulting in a heterogeneous population, thereby affirming the reliability of data collection. Low-voltage regions are a surrogate marker for atrial fibrosis, but histological validation is missing, and amyloidosis or other infiltrative aetiologies cannot be excluded. Spatial distribution and extent of LVZ depend largely on spontaneous rhythm and site and frequency of atrial pacing, as well as the mapping catheter and inter-electrode spacing. However, all patients were mapped in spontaneous sinus rhythm without pacing, using catheters with the same electrode size, inter-electrode spacing, and automatic acquisition settings validated in our previous studies. Multipolar catheters may be prone to suboptimal contact in several LA regions. Only patients with recurrent arrhythmias following first PVI procedure were included; thus, the proportion of patients showing regression is likely underestimated. Finally, continuous monitoring was not available in our study and no direct conclusion on AF burden can be drawn.
Conclusions
In more than 60% of patients presenting for redo AF ablation after PVI-only approach, a significant decrease in voltage in non-ablated LA regions was identified suggesting progression of LA substrate. In close to 40% of patients, a significant increase in the voltage was observed identifying regression of LA substrate. Patients in the progression group tend to develop more extensive substrate and slower LA activation time. None of the clinical, electrocardiographic, or echocardiographic baseline parameters is able to differentiate between patients with progression and regression. Recurrent persistent AF frequently despite durable PVI was associated with progression. In these patients, the progression may be the cause of AF recurrence or the consequence of persistent AF recurrence and further remodelling. Our study is the first to report bidirectional dynamic properties of the LA substrate with concordant either progressive or regressive changes in different atrial regions outside of the PVs, confirming heterogeneity of the AF patient population referred for ablation. A better understanding of the dynamic changes of the LA substrate could help to differentiate between patients with reversible and despite ablation progressive irreversible atrial structural remodelling.
Supplementary Material
Contributor Information
Lorenzo Marcon, Cardiology Department, University Hospital Antwerp, Wilrijkstraat 10, Edegem, Antwerp 2650, Belgium; Heart Rhythm Management Center, Postgraduate Program in Cardiac Electrophysiology and Pacing, European Reference Networks Guard-Heart, Universitair Ziekenhuis Brussel-Vrije Universiteit Brussel,1090 Brussels, Belgium; Department of Clinical Electrophysiology and Cardiac Pacing, Centro Cardiologico Monzino IRCCS, Milan, Italy.
Marco Bergonti, Cardiology Department, University Hospital Antwerp, Wilrijkstraat 10, Edegem, Antwerp 2650, Belgium; Division of Cardiology, Cardiocentro Ticino Institute, Ente Ospedaliero Cantonale, Lugano, Switzerland.
Francesco Spera, Cardiology Department, University Hospital Antwerp, Wilrijkstraat 10, Edegem, Antwerp 2650, Belgium.
Johan Saenen, Cardiology Department, University Hospital Antwerp, Wilrijkstraat 10, Edegem, Antwerp 2650, Belgium.
Wim Huybrechts, Cardiology Department, University Hospital Antwerp, Wilrijkstraat 10, Edegem, Antwerp 2650, Belgium.
Hielko Miljoen, Cardiology Department, University Hospital Antwerp, Wilrijkstraat 10, Edegem, Antwerp 2650, Belgium.
Olivier Van Leuven, Cardiology Department, University Hospital Antwerp, Wilrijkstraat 10, Edegem, Antwerp 2650, Belgium.
Lien Vandaele, Cardiology Department, University Hospital Antwerp, Wilrijkstraat 10, Edegem, Antwerp 2650, Belgium.
Anouk Wittock, Anesthesiology Department, University Hospital Antwerp, Antwerp, Belgium.
Hein Heidbuchel, Cardiology Department, University Hospital Antwerp, Wilrijkstraat 10, Edegem, Antwerp 2650, Belgium; University of Antwerp, Faculty Medicine and Health Sciences, Universiteitsplein 1, Wilrijk, Antwerpen 2610, Belgium.
Andrea Sarkozy, Cardiology Department, University Hospital Antwerp, Wilrijkstraat 10, Edegem, Antwerp 2650, Belgium; Heart Rhythm Management Center, Postgraduate Program in Cardiac Electrophysiology and Pacing, European Reference Networks Guard-Heart, Universitair Ziekenhuis Brussel-Vrije Universiteit Brussel,1090 Brussels, Belgium; University of Antwerp, Faculty Medicine and Health Sciences, Universiteitsplein 1, Wilrijk, Antwerpen 2610, Belgium.
Supplementary material
Supplementary material is available at Europace online.
Data availability
All relevant data are within the manuscript and its online supplementary material.
References
- 1. Schotten U, Verheule S, Kirchhof P, Goette A. Pathophysiological mechanisms of atrial fibrillation: a translational appraisal. Physiol Rev 2011;91:265–325. [DOI] [PubMed] [Google Scholar]
- 2. Allessie M, Ausma J, Schotten U. Electrical, contractile and structural remodeling during atrial fibrillation. Cardiovasc Res 2002;54:230–46. [DOI] [PubMed] [Google Scholar]
- 3. Everett TH, Li H, Mangrum JM, McRury ID, Mitchell MA, Redick JAet al. Electrical, morphological, and ultrastructural remodeling and reverse remodeling in a canine model of chronic atrial fibrillation. Circulation 2000;102:1454–60. [DOI] [PubMed] [Google Scholar]
- 4. Calkins H, Hindricks G, Cappato R, Kim Y-H, Saad EB, Aguinaga Let al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm 2017;14:e275–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ravelli F, Masè M, Cristoforetti A, Avogaro L, D’Amato E, Tessarolo Fet al. Quantitative assessment of transmural fibrosis profile in the human atrium: evidence for a three-dimensional arrhythmic substrate by slice-to-slice histology. Europace 2023;25:739–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Teh AW, Kistler PM, Lee G, Medi C, Heck PM, Spence SJet al. Long-term effects of catheter ablation for lone atrial fibrillation: progressive atrial electroanatomic substrate remodeling despite successful ablation. Heart Rhythm 2012;9:473–80. [DOI] [PubMed] [Google Scholar]
- 7. Huo Y, Kronborg MB, Richter U, Guo J, Ulbrich S, Zedda AMet al. Electrophysiological findings during atrial fibrillation reablation: extending from pulmonary vein reconnection to sequential bipolar voltage map information. J Cardiovasc Electrophysiol 2020;31:885–94. [DOI] [PubMed] [Google Scholar]
- 8. Al-Kaisey AM, Parameswaran R, Joseph SA, Kistler PM, Morton JB, Kalman JM. Extensive right atrial free wall low-voltage zone as the substrate for atrial fibrillation: successful ablation by scar homogenization. Europace 2021;23:59–64. [DOI] [PubMed] [Google Scholar]
- 9. Akoum N, Mahnkopf C, Kholmovski EG, Brachmann J, Marrouche NF. Age and sex differences in atrial fibrosis among patients with atrial fibrillation. Europace 2018;20:1086–92. [DOI] [PubMed] [Google Scholar]
- 10. Junarta J, Siddiqui MU, Riley JM, Dikdan SJ, Patel A, Frisch DR. Low-voltage area substrate modification for atrial fibrillation ablation: a systematic review and meta-analysis of clinical trials. Europace 2022;24:1585–98. [DOI] [PubMed] [Google Scholar]
- 11. Van Leuven O, Bergonti M, Spera FR, Ferrero TG, Nsahlai M, Bilotta Get al. Gender-related differences in atrial substrate in patients with atrial fibrillation. Am J Cardiol 2023;203:451–8. [DOI] [PubMed] [Google Scholar]
- 12. John B, Stiles MK, Kuklik P, Brooks AG, Chandy ST, Kalman JMet al. Reverse remodeling of the atria after treatment of chronic stretch in humans: implications for the atrial fibrillation substrate. J Am Coll Cardiol 2010;55:1217–26. [DOI] [PubMed] [Google Scholar]
- 13. Sugumar H, Prabhu S, Voskoboinik A, Young S, Gutman SJ, Wong GRet al. Atrial remodeling following catheter ablation for atrial fibrillation-mediated cardiomyopathy: long-term follow-up of CAMERA-MRI study. JACC Clin Electrophysiol 2019;5:681–8. [DOI] [PubMed] [Google Scholar]
- 14. Nalliah CJ, Wong GR, Lee G, Voskoboinik A, Kee K, Goldin Jet al. Impact of CPAP on the atrial fibrillation substrate in obstructive sleep apnea: the SLEEP-AF study. JACC Clin Electrophysiol 2022;8:869–77. [DOI] [PubMed] [Google Scholar]
- 15. Maille B, Das M, Hussein A, Shaw M, Chaturvedi V, Williams Eet al. Reverse electrical and structural remodeling of the left atrium occurs early after pulmonary vein isolation for persistent atrial fibrillation. J Interv Card Electrophysiol Int J Arrhythm Pacing 2020;58:9–19. [DOI] [PubMed] [Google Scholar]
- 16. Bergonti M, Spera FR, Ferrero TG, Nsahlai M, Bonomi A, Tijskens Met al. Characterization of atrial substrate to predict the success of pulmonary vein isolation: the prospective, multicenter MASH-AF II (multipolar atrial substrate high density mapping in atrial fibrillation) study. J Am Heart Assoc 2023;12:e027795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rodríguez-Mañero M, Valderrábano M, Baluja A, Kreidieh O, Martínez-Sande JL, García-Seara Jet al. Validating left atrial low voltage areas during atrial fibrillation and atrial flutter using multielectrode automated electroanatomic mapping. JACC Clin Electrophysiol Elsevier Inc 2018;4:1541–52. [DOI] [PubMed] [Google Scholar]
- 18. Sim I, Bishop M, O’neill M, Williams SE. Left atrial voltage mapping: defining and targeting the atrial fibrillation substrate. J Interv Card Electrophysiol 2019;56:213–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ausma J, van der Velden HMW, Lenders M-H, van Ankeren EP, Jongsma HJ, Ramaekers FCSet al. Reverse structural and gap-junctional remodeling after prolonged atrial fibrillation in the goat. Circulation 2003;107:2051–8. [DOI] [PubMed] [Google Scholar]
- 20. Starek Z, Di Cori A, Betts TR, Clerici G, Gras D, Lyan Eet al. Baseline left atrial low-voltage area predicts recurrence after pulmonary vein isolation: WAVE-MAP AF results. Europace 2023;25:euad194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yang B, Jiang C, Lin Y, Yang G, Chu H, Cai Het al. STABLE-SR (electrophysiological substrate ablation in the left atrium during sinus rhythm) for the treatment of nonparoxysmal atrial fibrillation: a prospective, multicenter randomized clinical trial. Circ Arrhythm Electrophysiol 2017;10:e005405. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data are within the manuscript and its online supplementary material.