One of the most profound challenges associated with biomarker development in neurological diseases is the specific capture of neuronally-derived molecules in the massive excess of chemically complex biofluids such as serum. This is especially important for α-synuclein because almost all of blood α-synuclein is in the circulation in a free form, mainly derived from peripheral sources such as red blood cells. This explains why blood-based measurements of unfractionated α-synuclein are not clinically useful. A promising alternative is the isolation of neuronally-derived extracellular vesicles (EVs) using the neuronal antigen L1CAM (L1EVs). We previously developed an improved immunocapture method to isolate L1EVs directly from serum with minimal fouling and reported that L1EV α-synuclein is increased in the serum of patients with Parkinson’s disease (PD) but not controls or patients with alternative proteinopathies using >700 samples from four cohorts internationally.1,2 Additional studies also found increased EV-associated α-synuclein levels in PD compared to controls.3-8
Blommer et al.9 recently reported in Brain that L1EV α-synuclein is reduced in PD compared to controls and in PD with cognitive impairment compared to PD with normal cognition in one cohort from New Zealand (n = 273). Intriguingly, the authors reported in their supplementary material that the mean EV α-synuclein levels in PD with normal cognition (PD-N) is 3502.2 pg/ml versus 3099.9 pg/ml for controls (i.e. higher in PD than controls), which is in contrast to some of their own conclusions. The authors reported a concentration of L1EV α-synuclein in the range of 2000–3500 pg/ml, which is substantially higher than the threshold value (∼10–15 pg/ml) that was previously shown to separate PD from controls by us and others.1-4 Although the authors have thoroughly demonstrated that they successfully immunocapture L1EVs, their methodology involves the ExoQuick kit, a polymer-based precipitation assay that is prone to non-specific binding of contaminant proteins or vesicles. It is noteworthy that studies employing the ExoQuick kit in EV isolation from serum or plasma are inconsistent in terms of absolute L1EV-associated α-synuclein concentrations and reported relatively high levels.5,8,9 In contrast to Blommer et al.,9 other studies using this method found increased L1EV-associated α-synuclein in PD.5,8
α-Synuclein is highly abundant in a free form in blood with a tendency to aggregate and to bind non-specifically to most surfaces including magnetic beads or polymers that are used in the ExoQuick assay. Therefore, we believe that the use of ExoQuick specifically in the study of EV-associated α-synuclein is likely to confound the results due to non-specific precipitation of free circulating α-synuclein monomers and/or oligomers. We used the HiScreen Capto Core 700 column to separate EVs from the bulk of free proteins and estimated the levels of α-synuclein in total EV-associated fractions in serum from healthy volunteers without employing immunocapture. Based on the UV spectra that measured total protein abundance, we identified a small early-elution peak, followed by a second larger peak, which depicts abundant free serum proteins. We collected 10 separate fractions (F) spanning across these two peaks (F1 to F10) as shown in Fig. 1A. The stacked spectra were obtained from n = 10 subjects, with comparable column retention time and elution peaks, demonstrating the high reproducibility of the fractionation process. Using nanoparticle tracking analysis (NTA) we found that EVs were enriched in F2 to F5 with no nanoparticles detected in subsequent fractions, as shown in Fig. 1B. To further define the molecular profile of EVs that were detected in F2–F5 we performed immunoblotting for CD9, which is a generic EV surface marker and syntenin-1, which is an internal EV cargo protein and the best available marker for exosomes, which are endosome-derived EVs.1 Based on immunoblotting, CD9 and syntenin-1 bands were enriched in F3 and F4 and not in the late fractions (Fig. 1C). Thus, size exclusion chromatography using the HiScreen Capto Core 700 column successfully separates EVs from the bulk of free serum proteins with EVs eluting primarily in F3 and F4. Under these conditions, most α-synuclein in serum as measured by electrochemiluminescence (MSD platform) was detected in a free form in late fractions (n = 6 replicates) with only a small amount eluted in F3 and F4 (Fig. 1D). Importantly α-synuclein in the EV-associated fractions F3 and F4 was very consistent across biological replicates (43.46 ± 19.99 pg per 1 ml of serum) despite considerable variability in the free α-synuclein in fractions F5–F8 (4716.47 ± 4973.63 pg per 1 ml of serum), which may be caused by haemolysis or contamination from other peripheral sources. Similar results to our findings in serum were previously reported in plasma.7 In these experiments, parallel measurements of CD81 using electrochemiluminescence, which is more sensitive than immunoblotting, identified this generic EV marker mostly in F3 and F4, in line with our earlier conclusions above using immunoblotting for CD9 and syntenin-1. Thus, α-synuclein levels in the EV-enriched fractions which include both neuronal (L1EVs) and non-neuronal EVs are much lower than free α-synuclein and what was reported by Blommer et al. in L1EVs.
Figure 1.
α-Synuclein concentration in all extracellular vesicles isolated from serum by size exclusion chromatography. (A) Size exclusion chromatography spectra of 10 eluted fractions (F) from 1 ml of serum. (B) Extracellular vesicles were measured in each fraction using nanoparticle tracking analysis. (C) Immunoblotting for syntenin-1 (Synt-1) and CD9 in each fraction after total protein precipitation using methanol-chloroform extraction. (D) Profiling of each unprocessed fraction with duplexed MSD assay demonstrates that CD81 is detected in early fractions, which also contain very low levels of α-synuclein (<50 pg/ml) whereas later fractions are enriched in free α-synuclein (up to ∼3000 pg/ml).
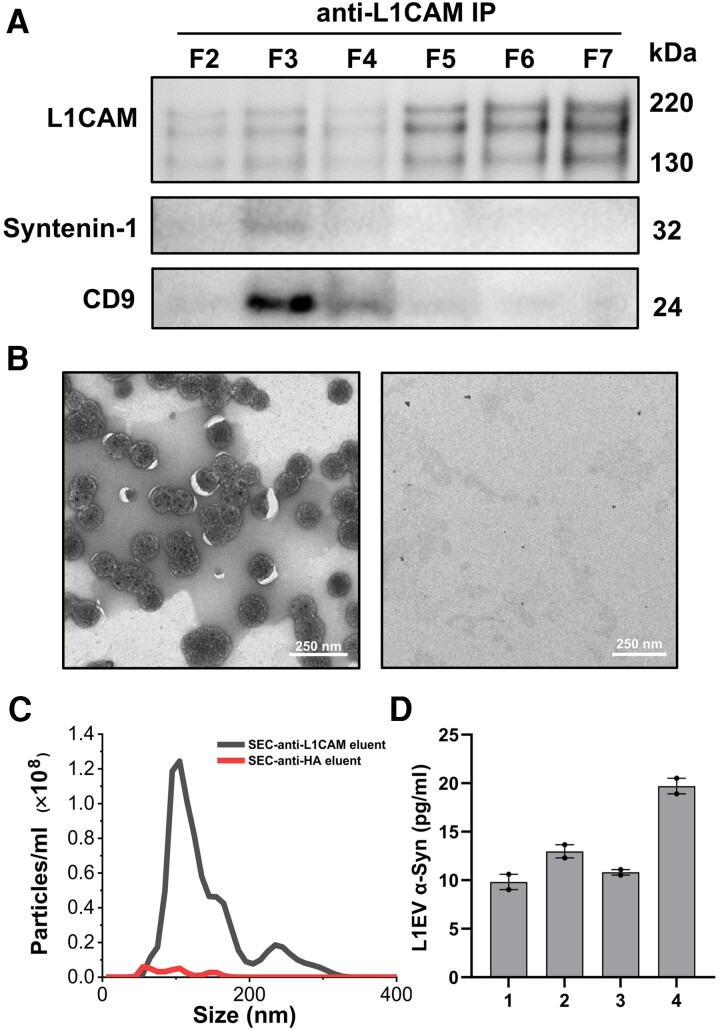
We then assessed how much α-synuclein in the EV-enriched fractions is found in the association with the L1EV subpopulation. We performed anti-L1CAM immunocapture using pCBMA-coated beads followed by immunoblotting in each fraction from F2 to F7 that span over the two peaks across the size exclusion chromatography spectra. Although we detected full-length L1CAM (220 kDa) in all tested fractions, only in F3 and F4 was L1CAM isolated in association with CD9 and syntenin-1 (Fig. 2A). This was confirmed by negative staining transmission electron microscopy after EV elution with glycine buffer (pH 2.8), demonstrating that the L1CAM-immunocaptured particles from F2–F4 had the typical cup-shaped morphology of EVs and measured ∼100 nm (Fig. 2B). Thus, although a large amount of L1CAM is found free in blood as previously reported, a small fraction is found in association with EVs. Based on NTA, the total number of eluted L1EVs with a typical peak size at ∼110 nm (i.e. area under the curve in Fig. 2C) was calculated to constitute ∼ 8% of total serum EVs from the corresponding fractions (F2–F4 in Fig. 1B) assuming elution efficiency was complete. Under these conditions, L1CAM immunocapture from pooled fractions F2–F4 followed by α-synuclein measurements using electrochemiluminescence revealed that L1EV-associated α-synuclein is 13.33 ± 4.45 pg per ml of serum based on four healthy volunteers each tested in duplicate (Fig. 2D). This is in line with our previous estimates of α-synuclein concentration in serum L1EVs from healthy controls1,2,10 and much lower than what Bloomer et al.9 reported in Brain (3099.9 ± 2986 pg/ml).
Figure 2.
α-Synuclein concentration in the L1CAM-positive serum extracellular vesicle subpopulation. (A) Immunocapture using anti-L1CAM antibodies from Fractions (F) 2 to 7 covering the two identified peaks on the size exclusion chromatography (SEC) spectra shows that full-length L1CAM was present in all fractions but co-immunoprecipitated with EV proteins syntenin-1 and CD9 only in F3 and F4 as shown by immunoblotting. (B) L1CAM immunocaptured EVs from Fractions 2 to 4 visualized with transmission electron microscopy (left). No EVs were detected with anti-HA antibodies used as control (right). (C) Nanoparticle tracking analysis of EVs eluted following L1CAM immunocapture from Fractions 2 to 4. (D) α-Synuclein concentration in the EV subpopulation immunocaptured with anti-L1CAM antibodies from pooled Fractions 2 to 4 (n = 4 healthy volunteers tested, two replicates for each individual).
The unusually high concentration of L1EV-associated α-synuclein reported by Blommer et al.9 is most likely explained by non-specific contamination with free α-synuclein, including potentially aggregated forms, casting doubt on the validity of their conclusions. For these reasons, we believe that the use of ExoQuick to concentrate EVs is not suitable for the quantification of blood-derived EV-associated α-synuclein in PD biomarker studies. This issue demonstrates the need for standardization of EV immunocapture protocols across laboratories and validation of any reported results in independent cohorts.
Supplementary Material
Contributor Information
Shijun Yan, Nuffield Department of Clinical Neurosciences, John Radcliffe Hospital, University of Oxford, Oxford OX3 9DU, UK; Kavli Institute for Nanoscience Discovery, Dorothy Crowfoot Hodgkin Building, University of Oxford, Oxford OX1 3QU, UK.
Cheng Jiang, Nuffield Department of Clinical Neurosciences, John Radcliffe Hospital, University of Oxford, Oxford OX3 9DU, UK; Kavli Institute for Nanoscience Discovery, Dorothy Crowfoot Hodgkin Building, University of Oxford, Oxford OX1 3QU, UK.
Jason J Davis, Department of Chemistry, University of Oxford, Oxford OX1 3QZ, UK.
George K Tofaris, Nuffield Department of Clinical Neurosciences, John Radcliffe Hospital, University of Oxford, Oxford OX3 9DU, UK; Kavli Institute for Nanoscience Discovery, Dorothy Crowfoot Hodgkin Building, University of Oxford, Oxford OX1 3QU, UK.
Data availability
Data are available upon request to the corresponding author.
Funding
The work was funded by grants to G.K.T. from the Michael J Fox Foundation, the Galen and Hilary Weston Foundation and the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre. G.K.T is supported by an MRC Senior Clinical Fellowship (MR/V007068/1).
Competing interests
The authors report no competing interests.
Supplementary material
Supplementary material is available at Brain online.
References
- 1. Jiang C, Hopfner F, Katsikoudi A, et al. Serum neuronal exosomes predict and differentiate Parkinson's disease from atypical parkinsonism. J Neurol Neurosurg Psychiatry. 2020;91:720–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jiang C, Hopfner F, Berg D, et al. Validation of α-synuclein in L1CAM-immunocaptured exosomes as a biomarker for the stratification of parkinsonian syndromes. Mov Disord. 2021;36:2663–2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shi M, Liu C, Cook TJ, et al. Plasma exosomal alpha-synuclein is likely CNS-derived and increased in Parkinson's disease. Acta Neuropathol. 2014;128:639–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cerri S, Ghezzi C, Sampieri M, et al. The exosomal/total alpha-synuclein ratio in plasma is associated with glucocerebrosidase activity and correlates with measures of disease severity in PD patients. Front Cell Neurosci. 2018;12:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dutta S, Hornung S, Kruayatidee A, et al. α-Synuclein in blood exosomes immunoprecipitated using neuronal and oligodendroglial markers distinguishes Parkinson's disease from multiple system atrophy. Acta Neuropathol. 2021;142:495–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Niu M, Li Y, Li G, et al. A longitudinal study on alpha-synuclein in plasma neuronal exosomes as a biomarker for Parkinson's disease development and progression. Eur J Neurol. 2020;27:967–974. [DOI] [PubMed] [Google Scholar]
- 7. Stuendl A, Kraus T, Chatterjee M, et al. α-Synuclein in plasma-derived extracellular vesicles is a potential biomarker of Parkinson's disease. Mov Disord. 2021;36:2508–2518. [DOI] [PubMed] [Google Scholar]
- 8. Zhao ZH, Chen ZT, Zhou RL, et al. Increased DJ-1 and alpha-synuclein in plasma neural-derived exosomes as potential markers for Parkinson's disease. Front Aging Neurosci. 2019;10:438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Blommer J, Pitcher T, Mustapic M, et al. Extracellular vesicle biomarkers for cognitive impairment in Parkinson's disease. Brain. 2023;146:195–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fu Y, Jiang C, Tofaris GK, Davis JJ. Facile impedimetric analysis of neuronal exosome markers in Parkinson's disease diagnostics. Anal Chem. 2020;92:13647–13651. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon request to the corresponding author.