Abstract
Childhood dementia is a devastating and under-recognized group of disorders with a high level of unmet need. Typically monogenic in origin, this collective of individual neurodegenerative conditions are defined by a progressive impairment of neurocognitive function, presenting in childhood and adolescence. This scoping review aims to clarify definitions and conceptual boundaries of childhood dementia and quantify the collective disease burden.
A literature review identified conditions that met the case definition. An expert clinical working group reviewed and ratified inclusion. Epidemiological data were extracted from published literature and collective burden modelled.
One hundred and seventy genetic childhood dementia disorders were identified. Of these, 25 were analysed separately as treatable conditions. Collectively, currently untreatable childhood dementia was estimated to have an incidence of 34.5 per 100 000 (1 in 2900 births), median life expectancy of 9 years and prevalence of 5.3 per 100 000 persons. The estimated number of premature deaths per year is similar to childhood cancer (0–14 years) and approximately 70% of those deaths will be prior to adulthood. An additional 49.8 per 100 000 births are attributable to treatable conditions that would cause childhood dementia if not diagnosed early and stringently treated. A relational database of the childhood dementia disorders has been created and will be continually updated as new disorders are identified (https://knowledgebase.childhooddementia.org/).
We present the first comprehensive overview of monogenic childhood dementia conditions and their collective epidemiology. Unifying these conditions, with consistent language and definitions, reinforces motivation to advance therapeutic development and health service supports for this significantly disadvantaged group of children and their families.
Keywords: neurodegeneration, rare neurological diseases, progressive intellectual and neurological deterioration, progressive childhood encephalopathy, childhood-onset dementia
Dementia is usually considered a disease of the elderly but there are many genetic diseases that cause dementia in childhood. Elvidge et al. refine the definition of childhood dementia and determine that the collective incidence of currently untreatable childhood dementias equates to 34.5 per 100 000 (1 in 2900 births).
Introduction
Childhood dementia is a unifying taxonomy representing a heterogeneous collective of childhood neurodegenerative disorders. Defined as global neurocognitive decline with multiple developmental skill loss following a period of developmental progress,1 the hallmark of childhood dementia is enduring and progressive loss of previously acquired developmental skills, in contrast to static or transient intellectual loss.1,2 Moreover, childhood dementia can be distinguished from conditions such as intellectual disability, which reflect relative loss of developmental trajectory without regression.1
In contrast to adult-onset dementia, childhood dementia has received little recognition in the medical literature, nor the lay media; in part reflecting the low prevalence of individual constituent disorders and their typical classification on discrete pathological grounds, rather than under a broader superordinate clinical phenotype, a concept that is widely accepted for adult-onset dementia. Indeed, while the literature reveals a great many individual disorders causing dementia in childhood, ‘childhood dementia’ as a disease classification is unrecognized in the World Health Organisation’s (WHO) International Classification of Diseases (ICD), the Diagnostic and Statistical Manual of Mental Disorders (DSM) or any other diagnostic system worldwide. Furthermore, childhood dementia has not been considered in any dementia policies or strategies globally, for example, the WHO’s ‘Global action plan on the public health response to dementia 2017–2025’.3
Further impacting recognition is consensus toward inclusion criteria amongst the childhood dementia disorders and the language used to describe them. These disorders have previously been described as ‘progressive childhood encephalopathy’4,5 and ‘progressive intellectual and neurological deterioration’ (PIND)2 as well as childhood dementia,1 albeit using slight variations in their definition. However, none of the terms are ubiquitously employed as standard throughout the medical literature and this inconsistency of both language and definition is hampering efforts to understand the burden of disease, and to advocate for improved care and treatment of this group of patients.
Several studies have attempted to assess the prevalence and impact of childhood dementia. The Australian Childhood Dementia Study was a surveillance study through the Australian Paediatric Surveillance Unit between May 1993 and June 1995, which also included a brief clinician survey of the psychosocial impact. This study included children under 14 years of age with multiple losses of already attained development skills, duration of illness greater than 3 months, skill loss most likely due to CNS dysfunction, evidence of generalized brain dysfunction, a condition not explicable in terms of acute drug toxicity, prolonged ictal confusion or other causes of delirium and skill loss that is progressive, or almost certainly will be, progressive.1 A similar definition was used by the British Paediatric Surveillance Unit (BPSU) for their monitoring of neurocognitive decline in relation to variant Creutzfeldt-Jakob disease (vCJD), and in this context, the differential diagnosis of PIND was assessed. The term dementia was not used in the PIND study, which includes both acquired and genetic pathologies giving rise to progressive neurological deterioration with onset under 16 years of age.2 Other studies have examined incidence rates of ‘progressive childhood encephalopathy’, focusing on children presenting before 15 years of age with signs of progressive CNS disease associated with impairment of cognitive functioning.4,5
This is a large, complex and heterogeneous group of disorders and their rarity means that consistently reported data are not always available. This scoping review aims to firstly clarify working definitions and conceptual boundaries of childhood dementia. Second it synthesizes available published data on the epidemiology of the childhood dementia disorders to quantify the collective burden of childhood dementia and identifies gaps in available data.
In high and upper-middle income countries the primary monogenic causes constitute the greatest burden of childhood dementia, with a more predictable incidence than acquired diseases, such as subacute sclerosing panencephalitis (SSPE). Therefore, we hypothesized that although the monogenic childhood dementia disorders are individually rare and ultra-rare in frequency, including an increasing number of novel genetic origin, when considered collectively their incidence is high and associated with an extraordinary, shared burden of disease.
Literature search and analysis
Case definition
Here we define the primary monogenic childhood dementia disorders as: progressive neurocognitive decline, presenting before 18 years of age, as characterized by multiple losses of prior attained development skills in the context of generalized (not focally restricted) brain dysfunction, secondary to disease of monogenic aetiology.
Non-progressive causes of intellectual disability (e.g. head injury) and progressive acquired disorders such as uncorrected nutritional deficiencies and infectious encephalitides were excluded from this definition. Additionally, neurocognitive decline as a result of uncontrolled epilepsy (the primary epileptic encephalopathies) were considered distinct from the primary dementias. Conditions for which epilepsy is an adjunct feature, without necessarily impacting the neurodegenerative disease course, were considered appropriate for inclusion. Additionally, while polygenic susceptibility is recognized in relapsing-remitting and primary progressive multiple sclerosis, and other progressive neuroimmune and autoimmune neuroinflammatory conditions (e.g. anti-NMDA receptor encephalitis), these were excluded in the absence of a definitive monogenic aetiology.
Treatable disorders, such as neurometabolic encephalopathies, where episodic crises may result in cumulative cognitive impairment when untreated [e.g. urea cycle disorders and phenylketonuria (PKU)], were included. However, these were subject to independent cohort analysis, recognizing that in most contemporary health settings, early diagnosis and compliance with effective management mitigates progressive decline in such cases.
Review protocol
A literature review and Human Phenotype Ontology database search was conducted to compile a list of conditions that met the case definition. The searches were conducted between June and October 2021.
The Human Phenotype Ontology (HPO) database (hpo.jax.org) was used to identify disorders for possible inclusion. Disorders associated with the following HPO terms were compiled: developmental regression, psychomotor deterioration, dementia, progressive psychomotor deterioration, progressive neurologic deterioration, progressive neurodegeneration, mental deterioration, intellectual deterioration, progressive cognitive decline, cognitive decline.
In addition, a PubMed search using the following query was conducted:
[(‘developmental regression’) OR (‘dementia’) OR (‘progressive neurodegeneration’) OR (‘mental deterioration’) OR (‘cognitive decline’)] AND (‘childhood’) AND (‘genetic’). The results were limited to ‘Child: birth-18 years’ and human studies only.
The flow diagram (Supplementary Fig. 1) describes the process to select childhood dementia disorders for the study. Duplicates were removed and disorders excluded due to onset over the age of 18, non-monogenic aetiology, no or minimal cognitive involvement or non-progressive course, focal brain dysfunction or classified as primary epileptic encephalopathies. Some disorders were excluded because so few cases have been described in the literature that it was not possible to judge whether they complied with the inclusion criteria. For those disorders where inclusion was unclear, an independent expert clinical working group (authors N.J.C.S., M.A.F., J.C. and collaborators R.W., C.E., B.D. and A.I.) was consulted to review and ratify inclusion.
Burden of disease data extraction
Incidence and life expectancy data were extracted from published literature for the final list of 170 conditions using PubMed and Google Scholar. If more than one source of incidence or life expectancy data was found, preference was given to large cohorts and Australian studies or those with similar ethnic diversity.
Where only a subset of patients with a condition met the eligibility criteria, the proportion of those patients was applied to the identified incidence estimate as detailed in Supplementary Table 1. Where the available information reported a range for either the incidence or life expectancy a simple average was taken.
The characteristics of the childhood dementia disorders were analysed based on accepted pathological classification of disease. Where some conditions fit into the classification of more than one subgroup, allocation was based on the more specific aetiological category. For example, metachromatic leukodystrophy was classified as a ‘lysosomal disorder of lipid metabolism and transport’ instead of the more general category, leukodystrophy, describing conditions that affect the white matter of the brain.
Data analysis
The incidence data from the literature were used to calculate the collective incidence of childhood dementia per 100 000 births. The numbers of children and persons expected to be living with a childhood dementia condition were estimated from the incidence, life expectancy and birth cohort data. Full methods are available in the Supplementary material.
Life expectancy for all of the childhood dementia disorders collectively was calculated from the life expectancies of each individual condition and weighted by their incidence.
An overall survival curve was derived from the incidence and life expectancy [mean ± standard deviation (SD)] of the individual conditions included in the analysis. Where the SD for the life expectancy was not available for a given disorder, it was estimated to be one-third of the mean (which was approximately the ratio of mean to SD observed in the disorders where both mean and SD were available).
Average ages of onset and diagnosis were calculated from data from the literature. A weighted average was calculated based on the incidence. Mean or median diagnostic delay was tabulated if available from the literature.
Results
One hundred and seventy genetic disorders or groups of disorders were identified that meet the inclusion criteria for childhood dementia. These disorders are caused by pathogenic variants in at least 200 individual genes, plus an additional multitude of genetic causes of mitochondrial disease (>200 genes).
From the original group of 170 disorders, 76 could not be included in the analysis due to insufficient incidence or life expectancy data (Supplementary Table 3). Another 25 disorders were analysed separately as treatable conditions (Supplementary Table 2).
Of note, estimation of mitochondrial disease incidence presented a unique, disease-specific challenge, given their genetic heterogeneity and pleiotropy. Childhood dementia can be a component of at least 12 clinical categories of mitochondrial disease (Supplementary Table 1) and can be caused by pathogenic variants in more than half of the over 300 genes known to underlie mitochondrial disease.6–8 Leigh syndrome alone can be caused by pathogenic variants in one of more than 89 different genes (both in the nuclear and mitochondrial genome).9 Consequently, published data were supplemented with diagnostic frequency and relevant genotype-phenotype data obtained from the Australian Laboratory that has acted as the major national referral laboratory for paediatric mitochondrial disease for several decades (updated from their previous epidemiological study,10 David Thorburn, 2020, personal communication).
Of the remaining 69 disorders, individual incidences varied from 0.03 per 100 000, as seen in MPS VII (Sly syndrome) and fucosidosis (type I and II) to 7 per 100 000 for the group of mitochondrial disorders (Supplementary Table 1). Collectively, the incidence is estimated to be 34.5 per 100 000 (1 in 2900 births) (Table 1). This equates to 107 births in 2021 in Australia, 240 in the UK and 1262 in the USA.
Table 1.
Collective epidemiology of childhood dementia disorders in high and upper-middle income countries
| Epidemiological outcome | Incidence per 100 000 births | Life expectancy average (years)a | Prevalence per 100 000 population | Prevalence per 100 000 children |
|---|---|---|---|---|
| All childhood dementia | 84.3 | 55.7 | 28.7 | 58.3 |
| Treatable childhood dementiab | 49.8 | 83.0 | 23.4 | 41.8 |
| Currently non-treatable childhood dementia | 34.5 | 16.3 | 5.3 | 16.5 |
Mean calculated from the life expectancies of each individual condition (Supplementary Tables 1 and 2) and weighted by their incidence. Note that a median life expectancy of 9 years was also calculated using survival curves for the currently non-treatable childhood dementia conditions.
Life expectancy similar to the general population with treatment, assuming early diagnosis and treatment compliance.
The mean life expectancy was estimated to be 16.3 years with a range from 1 to 52.4 years. This translates to an expected prevalence of 5.3 per 100 000 persons. In 2021 it is estimated that 1394, 3568 and 17 587 persons were living with childhood dementia in Australia, the UK and the USA, respectively. Around two-thirds are predicted to be under the age of 18: 969, 2367 and 12 162 children in Australia, the UK and the USA in 2021, respectively (Table 2).
Table 2.
Collective impact of currently non-treatable childhood dementia disorders in 2021 in example countries
| Epidemiological outcome 2021 | Australia | UK | USA |
|---|---|---|---|
| Births | 309 996 | 694 684 | 3 659 289 |
| Childhood dementia births | 107 | 240 | 1262 |
| Children living with childhood dementia (<18 years old) | 969 | 2367 | 12 162 |
| People living with childhood dementia | 1394 | 3568 | 17 587 |
| Premature deaths due to childhood dementia | 91 | 204 | 1077 |
| Premature deaths in children due to childhood dementia (<18 years old) | 74 | 166 | 873 |
| Years of life lost (YLL) | 1191 | 2669 | 14 059 |
| Years of life lost to disability (YLD) | 284 | 636 | 3349 |
Sources of population data are listed in the Supplementary material. Includes 69 conditions with data available, listed in Supplementary Table 1.
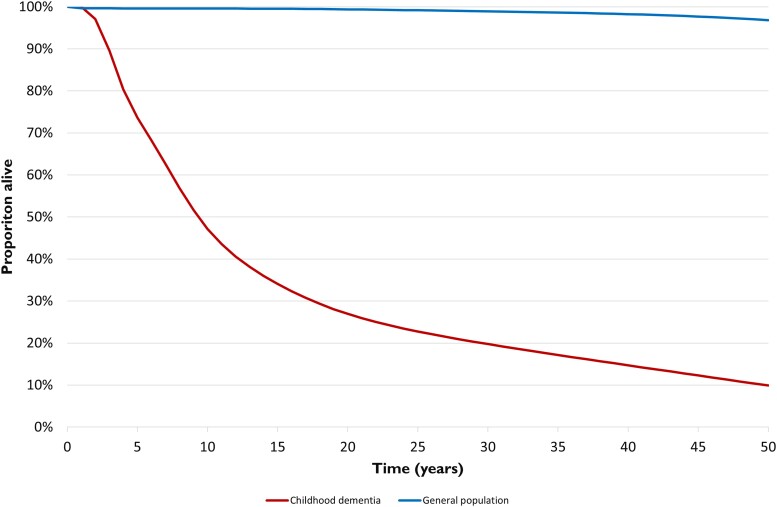
Overall survival over time (Fig. 1) was estimated from the incidence and life expectancy (mean ± SD) of the individual conditions included in the analysis. Median survival was 9 years of age, with approximately one-third (29%) of those born with a childhood dementia condition reaching adulthood (18 years of age) and 10% reaching the age of 50 (compared to 99.5% at 18 years and 96.8% at age 50 in a general population).11
Figure 1.
Overall survival of the cohort born with currently untreatable childhood dementia.
Based on the incidence of, and survival curves for childhood dementia presented in Fig. 1, it is estimated that 91 Australians, 204 Britons and 1077 Americans (USA) will have died prematurely due to childhood dementia in 2021. The years of life lost (YLL) due to premature mortality and years lived with disability (YLD) for Australia, the USA and the UK are presented in Table 2.
Data on age of onset and diagnosis were available for 52 and 46 disorders accounting for 78% and 71% of births, respectively (Supplementary Table 4). The mean (weighted for incidence) age of onset is 2.5 years (range 0 to 13.1 years) and age of diagnosis is 4 years (range 0.15 to 35.5 years). Median or mean time to diagnosis was reported for 19 conditions with more than half of these taking two or more years to reach a diagnosis after onset (range 0.13 to 16 years). In three studies the median time to diagnosis was six or more years.
Disorders that were identified as treatable were analysed separately. Treatable disorders are defined as those that are typically diagnosed early enough for successful treatment and average life expectancy does not usually differ from that of the general population in high and upper-middle income countries. This included some large groups of disorders—urea cycle disorders, organic acidurias and amino acidopathies and some individual disorders—biotinidase deficiency, cobalamin c disease, holocarboxylase synthetase deficiency, Wilson disease and chronic infantile neurological cutaneous and articular (CINCA) syndrome. An additional 49.8 per 100 000 births are attributable to these conditions that would cause childhood dementia if not diagnosed early and stringently treated (Supplementary Table 2).
The 69 currently untreatable childhood dementia disorders with incidence data available (Supplementary Table 1) were grouped by aetiopathological classifications and incident data used to calculate the proportion of births in each category (Table 3). The largest group of conditions was those designated ‘diseases not otherwise categorized’, which accounts for 27% of births and includes disparate disorders such as Rett syndrome and juvenile Huntington disease. This was followed by the lysosomal disease and mitochondrial disorder categories, accounting for 22% and 20% of births, respectively.
Table 3.
Functional classifications of currently non-treatable childhood dementia conditions
| Aetiopathological classification | % of childhood dementia births |
|---|---|
| Inborn errors of metabolism (65.6% of births) | |
| Lysosomal disorders (22.4% of births) | |
| Lysosomal disorders of lipid metabolism and transport | 10.1 |
| Mucopolysaccharidoses | 7.3 |
| Glycoproteinosis | 1.4 |
| Other lysosomal diseases | 3.6 |
| Other disorders of lipid metabolism and transport | 0.7 |
| Disorders of amino acid and other organic acid metabolism | 6.7 |
| Vitamin-responsive inborn errors of metabolism | 4.8 |
| Disorders of mineral absorption and transport | 1.0 |
| Peroxisomal disease | 9.5 |
| Mitochondrial disorders | 20.3 |
| Other inborn errors of metabolism | 0.1 |
| Leukodystrophies not otherwise categorized | 6.6 |
| Neurodegeneration with brain iron accumulation | 0.6 |
| Diseases not otherwise categorised | 27.3 |
Includes 69 conditions with data available, listed in Supplementary Table 1.
Discussion
This paper reconceptualizes the classification of childhood dementia disorders. This group of disorders is notoriously complex with simultaneous neurodegenerative and neurodevelopmental processes at play and considerable variability both within and between disorders in terms of aetiology, onset and rate of progression. However, these disorders have in common devastating and progressive neurocognitive decline and a severely shortened life expectancy. This scoping review has refined the definition of genetic childhood dementia and lists the major disorders and groups of disorders. In addition, the collective characteristics of childhood dementia are reported in terms of incidence, prevalence, age of onset and diagnosis and life expectancy.
Definition and classification of the childhood dementia disorders
This review identified 170 disorders caused by pathogenic variants in at least 200 individual genes (plus an additional multitude of genetic causes of mitochondrial disease) as fitting the case definition. Other studies have identified between 28 and 220 disorders causing progressive cognitive decline in childhood, although there are differences in definition and grouping of disorders.1,2,4,12
Delineating the boundaries of the childhood dementia disorders was challenging and relied on an experienced panel of clinicians to ratify their inclusion or exclusion. One such challenge was that some of the disorders have a highly variable phenotype and only a subset develop dementia in childhood, for example, cerebrotendinous xanthomatosis, mitochondrial disorders and congenital disorders of glycosylation. For these disorders, the clinicians erred towards inclusion if a significant proportion of the diagnoses would experience childhood dementia, they were marked as variable, and this variability was considered in the data analysis. In addition, some disorders such as Zellweger syndrome are so severe, and have a large impact on neurodevelopment that the neurocognitive decline begins from a low baseline of development. Similarly, these disorders were highlighted as such.
For other disorders, neurocognitive decline is overshadowed by more prominent features, for example the ataxia and immune dysfunction typical of ataxia-telangiectasia. However, recognition of the cognitive decline, even if less striking than other childhood dementia disorders, has a significant impact for some children and may enable interventions to improve quality of life.13 Conversely it is important to recognize that children with dementia have a range of comorbidities depending on the subtype (e.g. bone, joint, gastrointestinal, cardiac, respiratory) that have significant impacts on quality of life, and must not be overlooked. Likewise, degeneration of neural networks in adult-onset dementia may mediate diverse phenotypes not isolated to neurocognitive decline, including weakness, spasticity, bulbar issues and movement disorders. A continuum has been described with amyotrophic lateral sclerosis and frontotemporal dementia14 and Parkinson’s disease and Lewy body dementia.15
The inclusion of Rett syndrome was also controversial as this is largely considered a progressive neurodevelopmental disorder, and not neurodegenerative. It is characterized by rapid developmental regression in girls aged 1 to 4 years followed by a pseudostationary phase into adulthood where the cognitive decline appears to stabilize.16 Affected adults experience progressive neurological decline, particularly in the motor domain17 but assessment of cognitive ability is challenging due to profound physical disabilities.18 Rett syndrome was included in the PIND study2 and the Australian Childhood Dementia Study1 but was excluded in studies of progressive childhood encephalopathy in Norway4 and Sweden.5
More disorders will be identified as fitting the criteria for childhood dementia as new evidence emerges. A salient example is infantile-onset Pompe disease (IOPD), a neuromuscular disorder, which if left untreated, causes death in the first year of life due to heart failure. Early treatment with enzyme replacement therapy (ERT) in countries with newborn screening programmes are significantly improving patient survival. However, the ERT is unable to cross the blood–brain barrier and there is increasing evidence of CNS involvement and cognitive decline in these children.19
The aetiopathological classification of the disorders allows the identification of overlap of disease pathophysiology, with the possibility that common pathways may be affected, which suggests that in some circumstances single therapeutics could treat multiple clinical conditions. The majority are classified as inborn errors of metabolism (IEMs) (66% of births) with the largest subcategories within this group being lysosomal disorders and mitochondrial disorders. This is in line with other studies that reported 62.5–75.0% of all PIND cases are caused by IEMs.12
This study focused on the primary monogenic causes as they constitute the greatest burden of childhood dementia in high and upper-middle income countries and for the purposes of modelling, they have a more predictable incidence than acquired diseases such as SSPE. It is important to acknowledge that SSPE, caused by the persistence of the measles virus in the CNS, is a significant cause of childhood dementia in countries where immunization rates are low, such as sub-Saharan Africa and Southeast Asia.20 In high and upper-middle income countries, SSPE is very rare21; however, immunization rates are declining leading to an increase in SSPE incidence.22
Three broad groups of disorders (urea cycle disorders, organic acidurias and amino acidopathies; at least 21 disorders) and four individual disorders were analysed as an independent cohort (Supplementary Table 2) as they were considered treatable with a life expectancy similar to the general population assuming early diagnosis and enduring treatment compliance.23–29 As such, in high and upper-middle income countries, these children would generally not have a childhood dementia phenotype. Most of these disorders are included in newborn screening programmes so are detected presymptomatically and can be treated with relatively simple interventions such as dietary restrictions, supplements and widely available medications. However, despite this, there inevitably will be a small proportion of children (<10%) who will die either in the newborn period or later because of an acute metabolic crisis.30,31 In addition, within this group of treated individuals, response to treatment is variable, and individuals can have a number of symptoms and disease-associated morbidity. These disorders, even phenylketonuria, which has been screened in newborns since the 1960s, can also remain undiagnosed and untreated, including in high and upper-middle income countries due to immigration from countries without newborn screening.32
Incidence and prevalence
Whilst individually rare, the collective incidence of the monogenic causes of childhood dementia included in this study equates to 34.5 per 100 000 (1 in 2900 births). This is higher than the incidence of cystic fibrosis in Australia (1 in 3139 births)33 and more than three times higher than spinal muscular atrophy (1 in 11 000 births).34 Surveillance studies with a similar case definition to ours have reported incidence rates between 10 and 60 per 100 000 births.2,4,5 The incidence of PINDs in the UK was estimated to be ∼10 per 100 000 live births and incidence rates of progressive childhood encephalopathy in Norway4 and Sweden5 estimated an incidence of 60 and 58 per 100 000 live births, respectively. The Australian Childhood Dementia Study did not report a birth incidence but the cumulative 2-year prevalence of childhood dementia for children under 15 years was 5.6 per 100 000.1 The disparity in part reflects case definition and methodology of the respective studies.
It is reasonable to assume that the incidence of childhood dementia is comparable in high and upper-middle income countries with similar consanguinity rates,5 and this paper uses incidence data from a wide range of such countries including Australia, UK, USA and western Europe. However, some ethnic groups have higher incidence of these disorders. The Ashkenazi Jewish population, for example, has a very high carrier rate of Canavan disease, Tay Sachs disease, Niemann-Pick disease type A and mucolipidosis type IV,35 which gives parents with Ashkenazi Jewish ancestry an ∼2-fold higher risk of having a baby born with a childhood dementia disorder. Preconception carrier screening programmes in Jewish communities in Australia, Canada, USA and Israel, have resulted in markedly reduced incidence of these disorders.36,37 A UK study found higher rates of PIND in people of South Asian descent38 and an Australian study found a higher prevalence of mitochondrial disorders in people of Lebanese descent.10 As shown by the success of the carrier screening in the Ashkenazi Jewish population and emerging evidence from the Australian Reproductive Genetic Carrier Screening Project (Mackenzie’s Mission), this could significantly reduce the incidence of childhood dementia in the future.
The incidence and prevalence values calculated here are likely to be an under-estimation due to the lack of available data for these rare and ultra-rare diseases. Incidence and life expectancy data were only available for just over half of the 170 identified disorders (55%) and this is an ever-expanding group with an increasing number of ultra-rare and novel single patient disorders discovered. There is also likely to be a sizeable undiagnosed population. In the PIND study, 531 children of 2255 (23.5%) had been investigated without a diagnosis being made or were still under investigation2 and in the Australian Childhood Dementia Study, 17 of 80 (21%) were undiagnosed or had an uncertain diagnosis.1
In countries without newborn screening programmes for treatable conditions, such as PKU and limited access to specialist healthcare, it is expected that there would be a substantial increase in childhood dementia burden. We estimated an additional incidence of 49.8 per 100 000 births (Supplementary Table 2) to give a total incidence of 84.3 per 100 000 (1 in 1190) births. In these countries, where access to interventions to manage the disease manifestations is also typically limited, the life expectancy would be expected to be lower for all of the childhood dementia disorders, but few publications are available on the epidemiology of childhood dementia in low and lower-middle income countries. Further research is needed to understand the impact of childhood dementia globally.
Age of onset and diagnosis
Our data show a mean age of onset of 2.5 years when weighted for incidence (range birth to 13 years). This is in line with other studies—in the PIND study 81% presented before 5 years of age2 and in Norway 71.4% of the cases presented during the first year of life.4
Making generalizations about age of diagnosis is more difficult as this can be greatly influenced by the year of diagnosis and the expertise of the local clinicians. Of concern is the often-lengthy delay to diagnosis after the onset of symptoms—more than half of 19 studies reported two or more years to reach a diagnosis after onset and in three studies, diagnosis took six or more years from onset on average. This delay is partly due to the non-specific nature of presenting symptoms that could indicate more common conditions such as autism, but it is likely that a lack of awareness of this group of conditions and clear diagnostic and referral pathways are contributing factors. With new technology, such as whole genome sequencing, it is hoped that the delays are decreasing but data are not yet available on emerging trends, and even in many advanced countries, there is not widespread, equitable and timely access to this technology.
Early diagnosis is not only critical for the delivery of equitable and quality care, but it also plays a key role in the development of much needed therapeutic interventions for children with dementia. Early diagnosis and intervention before the onset of irreversible neuropathology gives the best chance of successful treatment.39 For example, although the vast majority of Hurler syndrome (MPS I) patients (83.6%) display symptoms by 1 month of age, diagnosis was not until a median of 9.3 months40 and it has been demonstrated that haematopoietic stem cell transplantation (HSCT) during the first 9 months of life is associated with typical cognitive development, while those that are transplanted later show long-term deficits.41 In addition, currently many childhood dementia clinical trials are limited to pre- or early symptomatic children—those that are most likely to respond to treatment. For example, recruitment for gene therapy clinical trials for Sanfilippo syndrome type A (MPS IIIA) was limited to those under 2 years of age (NCT02716246, NCT04201405), but the average age of diagnosis is 4 years.42 Therefore, clinical trial recruitment relies on younger siblings being identified, which is slow and heartbreaking for the older sibling. It increases the cost of trials, discourages pharmaceutical industry investment, and delays wider access to treatments. Expanding and improving newborn screening programmes and rapid and reliable diagnostic pathways for childhood dementia disorders would help to break this dichotomy and restore reproductive confidence for families.
Mortality
The mean life expectancy for currently untreatable childhood dementia was calculated to be 16.3 years and survival analysis predicted the median to be ∼9 years. Some children die in infancy, for example Gaucher disease type 2, whereas for some disorders survival into the fifth and sixth decade is not uncommon, for example Rett syndrome.
It is estimated that 91 Australians, 204 Britons and 1077 Americans (USA) died in 2021 due to childhood dementia, respectively. For comparison 92 children aged 0–14 years die annually in Australia from childhood cancer,43 260 in Britain44 and 1050 in the USA.45
These statistics not only highlight the need for greater investment in therapeutics but are useful for the planning of improvements to current palliative care services.
Treatments
Besides the disorders identified as treatable (listed in Supplementary Table 2), for the remaining 145 disorders, at present, there is a scarcity of effective and available treatments. Most management is hence directed at symptom management, such as anti-epileptic and behaviour-modifying medications. Interventions for optimizing mobility, nutrition and communication are available via physiotherapy, occupational therapy and speech therapy.
Some disorders in this list such as cerebrotendinous xanthomatosis46 and X-linked adrenoleukodystrophy (X-ALD)47 could be considered treatable if they were diagnosed prior to the onset of symptoms, or at least very early in the disease course, but in most high and upper-middle income countries this is not usually the case. Newborn screening for X-ALD has been implemented in some regions,48 which may improve outcomes for these patients.
Childhood dementia conditions in the lysosomal disorder category have some specific treatment strategies. Five ERT medications are currently approved for use; however, four of these therapies are only given intravenously and are unable to cross the blood–brain barrier. This renders them largely ineffective for CNS disease processes and does not address the dementia aspects of these conditions.49–52 The fifth product, Brineura® (cerliponase alfa), delivered intraventricularly, targets a type of Batten’s disease (CLN2). It has been shown to slow progression of motor and language deficits53 although no long-term studies have been performed to date. HSCT is a treatment option in some cases for a select number of disorders including metachromatic leukodystrophy,54 Krabbe disease,55 MPS I (Hurler syndrome)56 and MPS II (Hunter syndromes)57 and X-ALD,58 but has proved ineffective in multiple other disorders.59,60 HSCT is most effective if performed pre-symptomatically and risks versus benefits must be weighed up on an individual case basis, as the procedure is associated with significant morbidity and mortality.61
Multiple gene therapy technologies are showing promise, and at least 28 clinical trials of gene therapies for childhood dementia disorders are currently active (as of June 2021, clinicaltrials.gov). Two gene therapies have received regulatory authorization—Skysona (Bluebird Bio) for cerebral X-ALD (approved in the USA and EU) and Libmeldy (Orchard Therapeutics) for metachromatic leukodystrophy (approved in the EU). However, reimbursement and access to these expensive therapies remains a challenge.
Other therapeutic approaches being researched for the childhood dementia disorders target neurodegenerative mechanisms such as neuroinflammation, mitochondrial dysfunction, lipid accumulation, lysosomal dysfunction and neuroprotective pathways with small molecule drugs. Despite research that suggests overlapping disease mechanisms among the various childhood dementia disorders,62,63 drug development and clinical trials to date have focussed on one disorder in isolation. Drawing attention to the childhood dementia disorders and their common attributes will lead to concurrent research of multiple disorders and therefore enhanced efficiencies and greater benefit for children with dementia.
Limitations
Limitations of this scoping review can be attributed to the nature of the childhood dementia disorders. Given the rarity of individual diseases and the paucity of epidemiological data, a combination of mean, median and estimated incidence and life expectancy data from multiple populations was used. For variable disorders with multiple phenotypes, assumptions were made as outlined in Supplementary Table 1. However, data acquisition was restricted to peer reviewed and population verified data as best possible to give an estimate of the scale of the childhood dementia population in high and upper-middle income countries. High quality, up to date studies collecting data on the incidence, natural history, life expectancy and costs of childhood dementia are required to provide a more comprehensive analysis of the burden of childhood dementia.
Several studies have attempted to quantify the economic and societal burden of childhood dementia, either collectively64 or individually,65–67 however the evidence is scarce. There is no health state directly applicable to childhood dementia in the WHO Global Health Estimates to allow accurate estimation of disability-adjusted life years (DALYs), nor comprehensive measurement of healthcare expenditure and indirect costs for this group of diseases. Investment in data capture from health systems is warranted, in most jurisdictions this will require improvements in how these disorders are coded, data linkage infrastructure and increased consistency in the use of phenotyping terms (HPO/SNOMED).
Conclusions and future prospects
This scoping review highlights the importance of grouping these conditions as a phenotypic syndrome, rather than individually rare diseases, in keeping with the approach to adult dementia. By unifying these conditions, we have highlighted the many unmet needs of this significantly disadvantaged group of children and young people and the data presented here will enable advocacy for systemic change in treatment, care and support for them and their families.
To improve recognition of childhood dementia and outcomes for patients, consistent language and definitions are needed for this group of disorders. The term ‘childhood dementia’ has not been widely adopted despite being described in medical literature since the mid-20th century68 and the term being increasingly associated with individual conditions, especially Batten disease,69 Sanfilippo syndrome,70 Lafora disease,71 Hurler syndrome72 and Alexander disease.73 Patient organizations are adopting the term and individual families are using it to raise awareness and understanding of their child’s condition on social media and in the community. Dementia support organizations are beginning to recognize childhood dementia and include affected families in their service provision.74,75 More research is needed to understand attitudes and any barriers associated with the term, but it appears there is a groundswell of support for bringing these conditions together under the childhood dementia umbrella to improve outcomes for these children and their families.
Childhood dementia is a chronic, life-limiting condition that requires increasingly complex care, often over many years, and in some cases decades. Children with dementia lack defined care pathways when compared to other childhood diseases, ultimately impacting on their quality of care and long-term health outcomes. In one study, clinicians judged the impact of childhood dementia upon day-to-day family functioning was ‘marked’ or ‘extreme’ in the majority of families.1 Additionally, emerging research shows that the impacts on mental health and overall quality of life for carers of children with dementia are severe and health and social systems are not effectively meeting the needs of families.76,77 This highlights the need for the collection of real-world health system data globally and further research into the psychosocial impacts of childhood dementia on families and their experiences of health and social care systems. This will provide the evidence needed to address care and support needs.
Given the significant incidence, prevalence, mortality and lack of effective treatments, more attention and resources need to be channelled towards childhood dementia to develop new treatments, and improve patient management, service planning and provision.
A database of the key characteristics of the childhood dementia disorders listed in this paper is available at www.childhooddementia.org/knowledgebase.
Supplementary Material
Appendix 1
The members of the Childhood Dementia Working Group who contributed to the authorship of this paper are: David R. Thorburn, Gail Hilton, Ellie Van Velsen, Danielle Cini, Briana Davis, Richard Webster, Carolyn J. Ellaway and Anita Inwood.
Further details are provided in the Supplementary material.
Contributor Information
Kristina L Elvidge, Childhood Dementia Initiative, Brookvale, NSW 2100, Australia.
John Christodoulou, Brain and Mitochondrial Research Group, Murdoch Children’s Research Institute, Royal Children's Hospital, Parkville, Victoria 3052, Australia; Department of Paediatrics, University of Melbourne, Parkville, Victoria 3010, Australia.
Michelle A Farrar, Department of Neurology, Sydney Children's Hospital Network, Randwick, NSW 2031, Australia; Discipline of Paediatrics, School of Clinical Medicine, UNSW Medicine and Health, Sydney, NSW 2052, Australia.
Dominic Tilden, THEMA Consulting Pty Ltd, Pyrmont, NSW 2009, Australia.
Megan Maack, Childhood Dementia Initiative, Brookvale, NSW 2100, Australia.
Madeline Valeri, THEMA Consulting Pty Ltd, Pyrmont, NSW 2009, Australia.
Magda Ellis, THEMA Consulting Pty Ltd, Pyrmont, NSW 2009, Australia.
Nicholas J C Smith, Discipline of Paediatrics, University of Adelaide, Women's and Children's Hospital, North Adelaide, South Australia 5006, Australia; Department of Neurology and Clinical Neurophysiology, Women’s and Children’s Health Network, North Adelaide, South Australia 5006, Australia.
the Childhood Dementia Working Group:
David R Thorburn, Gail Hilton, Ellie Van Velsen, Danielle Cini, Briana Davis, Richard Webster, Carolyn J Ellaway, and Anita Inwood
Data availability
The authors confirm that the data supporting the findings of this study are available within the article, its Supplementary material and deposited in the Childhood Dementia Knowledgebase: https://knowledgebase.childhooddementia.org/.
Funding
The research conducted at the Murdoch Children’s Research Institute (MCRI) was supported by the Victorian Government’s Operational Infrastructure Support Program. The Chair in Genomic Medicine awarded to John Christodoulou is generously supported by The Royal Children’s Hospital Foundation. M.A.F. received grant support from the National Health and Medical Research Council of Australia: Investigator grant (APP1194940). Disclosed funding relates to general support as disclosed by the collaborating authors and is not specific to this study. K.L.E., N.J.C.S., D.T., M.M., M.V. and M.E. have no specific funding disclosure for this work.
Competing interests
K.L.E., M.M. and G.H. are current employees of Childhood Dementia Initiative. N.J.C.S., M.A.F. and J.C. are non-pecuniary members of the Scientific and Medical Advisory Committee for the Childhood Dementia Initiative.
Supplementary material
Supplementary material is available at Brain online.
References
- 1. Nunn K, Williams K, Ouvrier R. The Australian childhood dementia study. Eur Child Adolesc Psychiatry. 2002;11:63–70. [DOI] [PubMed] [Google Scholar]
- 2. Verity C, Baker E, Maunder P, Pal S, Winstone AM. Differential diagnosis of progressive intellectual and neurological deterioration in children. Dev Med Child Neurol. 2021;63:287–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organisation . Global action plan on the public health response to dementia 2017–2025. 7 December 2017. Accessed 8 September 2022. https://apps.who.int/iris/rest/bitstreams/1092215/retrieve
- 4. Stromme P, Kanavin OJ, Abdelnoor M, et al. Incidence rates of progressive childhood encephalopathy in Oslo, Norway: A population based study. BMC Pediatr. 2007;7:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Uvebrant P, Lanneskog K, Hagberg B. The epidemiology of progressive encephalopathies in childhood. I. Live birth prevalence in west Sweden. Neuropediatrics. 1992;23:209–211. [DOI] [PubMed] [Google Scholar]
- 6. Zeviani M, Viscomi C. Mitochondrial neurodegeneration. Cells. 2022;11:637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Thompson K, Collier JJ, Glasgow RIC, et al. Recent advances in understanding the molecular genetic basis of mitochondrial disease. J Inherit Metab Dis. 2020;43:36–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gorman GS, Chinnery PF, DiMauro S, et al. Mitochondrial diseases. Nat Rev Dis Primer. 2016;2:16080. [DOI] [PubMed] [Google Scholar]
- 9. Rahman J, Noronha A, Thiele I, Rahman S. Leigh map: A novel computational diagnostic resource for mitochondrial disease. Ann Neurol. 2017;81:9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Skladal D, Halliday J, Thorburn DR. Minimum birth prevalence of mitochondrial respiratory chain disorders in children. Brain J Neurol. 2003;126(Pt 8):1905–1912. [DOI] [PubMed] [Google Scholar]
- 11. Australian Bureau of Statistics . Life tables, 2018–2020. Published 4 November 2021. Accessed 22 February 2023. https://www.abs.gov.au/statistics/people/population/life-tables/2018-2020
- 12. Warmerdam HAG, Termeulen-Ferreira EA, Tseng LA, et al. A scoping review of inborn errors of metabolism causing progressive intellectual and neurologic deterioration (PIND). Front Neurol. 2020;10:1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hoche F, Daly MP, Chutake YK, Valera E, Sherman JC, Schmahmann JD. The cerebellar cognitive affective syndrome in ataxia-telangiectasia. Cerebellum Lond Engl. 2019;18:225–244. [DOI] [PubMed] [Google Scholar]
- 14. Gelon PA, Dutchak PA, Sephton CF. Synaptic dysfunction in ALS and FTD: Anatomical and molecular changes provide insights into mechanisms of disease. Front Mol Neurosci. 2022;15:1000183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jellinger KA, Korczyn AD. Are dementia with Lewy bodies and Parkinson’s disease dementia the same disease? BMC Med. 2018;16:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gold WA, Krishnarajy R, Ellaway C, Christodoulou J. Rett syndrome: A genetic update and clinical review focusing on comorbidities. ACS Chem Neurosci. 2018;9:167–176. [DOI] [PubMed] [Google Scholar]
- 17. Banerjee A, Miller MT, Li K, Sur M, Kaufmann WE. Towards a better diagnosis and treatment of rett syndrome: A model synaptic disorder. Brain. 2019;142:239–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ahonniska-Assa J, Polack O, Saraf E, et al. Assessing cognitive functioning in females with rett syndrome by eye-tracking methodology. Eur J Paediatr Neurol. 2018;22:39–45. [DOI] [PubMed] [Google Scholar]
- 19. Hsu YK, Chien YH, Shinn-Forng Peng S, et al. Evaluating brain white matter hyperintensity, IQ scores, and plasma neurofilament light chain concentration in early-treated patients with infantile-onset pompe disease. Genet Med. 2023;25:27–36. [DOI] [PubMed] [Google Scholar]
- 20. Mekki M, Eley B, Hardie D, Wilmshurst JM. Subacute sclerosing panencephalitis: Clinical phenotype, epidemiology, and preventive interventions. Dev Med Child Neurol. 2019;61:1139–1144. [DOI] [PubMed] [Google Scholar]
- 21. Campbell C, Levin S, Humphreys P, Walop W, Brannan R. Subacute sclerosing panencephalitis: Results of the Canadian paediatric surveillance program and review of the literature. BMC Pediatr. 2005;5:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lam T, Ranjan R, Newark K, et al. A recent surge of fulminant and early onset subacute sclerosing panencephalitis (SSPE) in the United Kingdom: An emergence in a time of measles. Eur J Paediatr Neurol. 2021;34:43–49. [DOI] [PubMed] [Google Scholar]
- 23. Ah Mew N, Simpson KL, Gropman AL, et al. Urea cycle disorders overview. In: Adam MP, Everman DB, Mirzaa GM, et al., eds. Genereviews®. University of Washington; 1993. https://www.ncbi.nlm.nih.gov/books/NBK1217/ [Google Scholar]
- 24. Wajner M. Neurological manifestations of organic acidurias. Nat Rev Neurol. 2019;15:253–271. [DOI] [PubMed] [Google Scholar]
- 25. Saleem H, Simpson B. Biotinidase deficiency. In: Statpearls. StatPearls Publishing; 2022. https://www.ncbi.nlm.nih.gov/books/NBK560607/ [Google Scholar]
- 26. Donti TR, Blackburn PR, Atwal PS. Holocarboxylase synthetase deficiency pre and post newborn screening. Mol Genet Metab Rep. 2016;7:40–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Martinelli D, Deodato F, Dionisi-Vici C. Cobalamin C defect: Natural history, pathophysiology, and treatment. J Inherit Metab Dis. 2011;34:127–135. [DOI] [PubMed] [Google Scholar]
- 28. Czlonkowska A, Litwin T, Dusek P, et al. Nature reviews disease primers article: Wilson disease. Nat Rev Dis Primer. 2018;4:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Finetti M, Omenetti A, Federici S, Caorsi R, Gattorno M. Chronic infantile neurological cutaneous and articular (CINCA) syndrome: A review. Orphanet J Rare Dis. 2016;11:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mew NA, Krivitzky L, McCarter R, Batshaw M, Tuchman M. Clinical outcomes of neonatal onset proximal versus distal urea cycle disorders do not differ. J Pediatr. 2013;162:324–329.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wilcken B, Haas M, Joy P, et al. Expanded newborn screening: Outcome in screened and unscreened patients at age 6 years. Pediatrics. 2009;124:e241–e248. [DOI] [PubMed] [Google Scholar]
- 32. van Wegberg AMJ, Trefz F, Gizewska M, et al. Undiagnosed phenylketonuria can exist everywhere: Results from an international survey. J Pediatr. 2021;239:231–234.e2. [DOI] [PubMed] [Google Scholar]
- 33. Massie RJH, Curnow L, Glazner J, Armstrong DS, Francis I. Lessons learned from 20 years of newborn screening for cystic fibrosis. Med J Aust. 2012;196:67–70. [DOI] [PubMed] [Google Scholar]
- 34. Shih STF, Keller E, Wiley V, Farrar MA, Wong M, Chambers GM. Modelling the cost-effectiveness and budget impact of a newborn screening program for spinal muscular atrophy and severe combined immunodeficiency. Int J Neonatal Screen. 2022;8:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Risch N, Tang H, Katzenstein H, Ekstein J. Geographic distribution of disease mutations in the ashkenazi Jewish population supports genetic drift over selection. Am J Hum Genet. 2003;72:812–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Triggs-Raine BL, Feigenbaum AS, Natowicz M, et al. Screening for carriers of tay-sachs disease among ashkenazi Jews. A comparison of DNA-based and enzyme-based tests. N Engl J Med. 1990;323:6–12. [DOI] [PubMed] [Google Scholar]
- 37. Kaback MM. Population-based genetic screening for reproductive counseling: The tay-sachs disease model. Eur J Pediatr. 2000;159(Suppl 3):S192–S195. [DOI] [PubMed] [Google Scholar]
- 38. Devereux G, Stellitano L, Verity C, Nicoll A, Will R, Rogers P. Variations in neurodegenerative disease across the UK: Findings from the national study of progressive intellectual and neurological deterioration (PIND). Arch Dis Child. 2004;89:8–12. [PMC free article] [PubMed] [Google Scholar]
- 39. Sevin C, Deiva K. Clinical trials for gene therapy in lysosomal diseases with CNS involvement. Front Mol Biosci. 2021;8:624988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kiely BT, Kohler JL, Coletti HY, Poe MD, Escolar ML. Early disease progression of hurler syndrome. Orphanet J Rare Dis. 2017;12:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Poe MD, Chagnon SL, Escolar ML. Early treatment is associated with improved cognition in hurler syndrome. Ann Neurol. 2014;76:747–753. [DOI] [PubMed] [Google Scholar]
- 42. Chin SJ, Fuller M. Prevalence of lysosomal storage disorders in Australia from 2009 to 2020. Lancet Reg Health West Pac. 2022;19:100344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Australian Institute of Health and Welfare . Australia’s children: Cancer incidence and survival. Updated 25 February 2022. Accessed 2 December 2022. https://www.aihw.gov.au/reports/children-youth/australias-children/contents/health/cancer-incidence-survival
- 44. Public Health England . Children, teenagers and young adults UK cancer statistics report 2021. Published February 2021. Accessed 2 December 2022. http://www.ncin.org.uk/view?rid=4272
- 45. The American Cancer Society medical and editorial content team . Key Statistics for Childhood Cancers. American Cancer Society. Revised 12 January 2023. Accessed 2 December 2022. https://www.cancer.org/cancer/cancer-in-children/key-statistics.html
- 46. DeBarber AE, Kalfon L, Fedida A, et al. Newborn screening for cerebrotendinous xanthomatosis is the solution for early identification and treatment. J Lipid Res. 2018;59:2214–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mahmood A, Raymond GV, Dubey P, Peters C, Moser HW. Survival analysis of haematopoietic cell transplantation for childhood cerebral X-linked adrenoleukodystrophy: A comparison study. Lancet Neurol. 2007;6:687–692. [DOI] [PubMed] [Google Scholar]
- 48. Moser AB, Seeger E, Raymond GV. Newborn screening for X-linked adrenoleukodystrophy: Past, present, and future. Int J Neonatal Screen. 2022;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Stirnemann J, Vigan M, Hamroun D, et al. The French gaucher’s disease registry: Clinical characteristics, complications and treatment of 562 patients. Orphanet J Rare Dis. 2012;7:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Harmatz P, Cattaneo F, Ardigò D, et al. Enzyme replacement therapy with velmanase alfa (human recombinant alpha-mannosidase): Novel global treatment response model and outcomes in patients with alpha-mannosidosis. Mol Genet Metab. 2018;124:152–160. [DOI] [PubMed] [Google Scholar]
- 51. Guffon N, Heron B, Chabrol B, Feillet F, Montauban V, Valayannopoulos V. Diagnosis, quality of life, and treatment of patients with hunter syndrome in the French healthcare system: A retrospective observational study. Orphanet J Rare Dis. 2015;10:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cadaoas J, Boyle G, Jungles S, et al. Vestronidase alfa: Recombinant human β-glucuronidase as an enzyme replacement therapy for MPS VII. Mol Genet Metab. 2020;130:65–76. [DOI] [PubMed] [Google Scholar]
- 53. Schulz A, Ajayi T, Specchio N, et al. Study of intraventricular cerliponase alfa for CLN2 disease. N Engl J Med. 2018;378:1898–1907. [DOI] [PubMed] [Google Scholar]
- 54. Boucher AA, Miller W, Shanley R, et al. Long-term outcomes after allogeneic hematopoietic stem cell transplantation for metachromatic leukodystrophy: The largest single-institution cohort report. Orphanet J Rare Dis. 2015;10:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yoon IC, Bascou NA, Poe MD, Szabolcs P, Escolar ML. Long-term neurodevelopmental outcomes of hematopoietic stem cell transplantation for late-infantile krabbe disease. Blood. 2021;137:1719–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gardin A, Castelle M, Pichard S, et al. Long term follow-up after haematopoietic stem cell transplantation for mucopolysaccharidosis type I-H: A retrospective study of 51 patients. Bone Marrow Transplant. 2023;58:295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sreekantam S, Smith L, Stewart C, et al. Efficacy of early haematopoietic stem cell transplantation versus enzyme replacement therapy on neurological progression in severe hunter syndrome: Case report of siblings and literature review. Mol Genet Metab Rep. 2022;32:100881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Chiesa R, Boelens JJ, Duncan CN, et al. Variables affecting outcomes after allogeneic hematopoietic stem cell transplant for cerebral adrenoleukodystrophy. Blood Adv. 2022;6:1512–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lund TC, Cathey SS, Miller WP, et al. Outcomes after hematopoietic stem cell transplantation for children with I-cell disease. Biol Blood Marrow Transplant. 2014;20:1847–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Welling L, Marchal JP, van Hasselt P, van der Ploeg AT, Wijburg FA, Boelens JJ. Early umbilical cord blood-derived stem cell transplantation does not prevent neurological deterioration in mucopolysaccharidosis type III. JIMD Rep. 2015;18:63–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Boelens JJ, Orchard PJ, Wynn RF. Transplantation in inborn errors of metabolism: Current considerations and future perspectives. Br J Haematol. 2014;167:293–303. [DOI] [PubMed] [Google Scholar]
- 62. Alessenko AV, Albi E. Exploring sphingolipid implications in neurodegeneration. Front Neurol. 2020;11:437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Pará C, Bose P, Pshezhetsky AV. Neuropathophysiology of lysosomal storage diseases: Synaptic dysfunction as a starting point for disease progression. J Clin Med. 2020;9:616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tilden D, Valeri M, Ellis M. Childhood dementia in Australia: Quantifying the burden on patients, carers, the healthcare system and our society. THEMA Consulting Pty Ltd; 2020. [Google Scholar]
- 65. Imrie J, Galani C, Gairy K, Lock K, Hunsche E. Cost of illness associated with niemann-pick disease type C in the UK. J Med Econ. 2009;12:219–229. [DOI] [PubMed] [Google Scholar]
- 66. Hendrie D, Bebbington A, Bower C, Leonard H. Measuring use and cost of health sector and related care in a population of girls and young women with rett syndrome. Res Autism Spectr Disord. 2011;5:901–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wyatt K, Henley W, Anderson L, et al. The effectiveness and cost-effectiveness of enzyme and substrate replacement therapies: A longitudinal cohort study of people with lysosomal storage disorders. Health Technol Assess Winch Engl. 2012;16:1–543. [DOI] [PubMed] [Google Scholar]
- 68. Girard PF, Kohler C, Thevenin M. The main clinical aspects of childhood dementia; their differential and etiological diagnosis. J Med Lyon. 1945;26:761–774. [PubMed] [Google Scholar]
- 69. McShane A, Mole SE. Sex bias and omission exists in batten disease research: Systematic review of the use of animal disease models. Biochim Biophys Acta Mol Basis Dis. 2022;1868:166489. [DOI] [PubMed] [Google Scholar]
- 70. Muschol N, Giugliani R, Jones SA, et al. Sanfilippo syndrome: Consensus guidelines for clinical care. Orphanet J Rare Dis. 2022;17:391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Brewer MK, Machio-Castello M, Viana R, et al. An empirical pipeline for personalized diagnosis of lafora disease mutations. iScience. 2021;24:103276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Katja K, Inga V, Ramona L, Almuth C, Maria MN. Mucopolysaccharidosis type I due to maternal uniparental disomy of chromosome 4 with partial isodisomy of 4p16.3p15.2. Mol Genet Metab Rep. 2020;25:100660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Wilson BA, Vargha-Khadem F, Florschutz G. Alexander’s disease and the story of louise. Neuropsychol Rehabil. 2018;28:199–207. [DOI] [PubMed] [Google Scholar]
- 74. Dementia Support Australia . Childhood Dementia Support. Accessed 3 April 2023. https://www.dementia.com.au/childhood-dementia-support
- 75. Dementia Australia . Childhood dementia. Accessed 3 April 2023. https://www.dementia.org.au/node/95018
- 76. Donnell M, Elvidge KL, Hilton G. State of Childhood Dementia 2022. Childhood Dementia Initiative; 2022. https://d1iap1m2kaw9nt.cloudfront.net/b7dc3a5c0c178b50a3ce1f40e7cdab54.pdf
- 77. Djafar JV, Johnson AM, Elvidge KL, Farrar MA. Childhood dementia: A collective clinical approach to advance therapeutic development and care. Pediatr Neurol. 2023;139:76–85. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article, its Supplementary material and deposited in the Childhood Dementia Knowledgebase: https://knowledgebase.childhooddementia.org/.