Abstract
Traumatic brain injury (TBI) is common but little is known why up to a third of patients have persisting symptoms. Astrogliosis, a pathophysiological response to brain injury, may be a potential therapeutic target, but demonstration of astrogliosis in the brain of humans with TBI and persistent symptoms is lacking.
Astroglial marker monoamine oxidase B (MAO-B) total distribution volume (11C-SL25.1188 VT), an index of MAO-B density, was measured in 29 TBI and 29 similarly aged healthy control cases with 11C-SL25.1188 PET, prioritizing prefrontal cortex (PFC) and cortex proximal to cortical convexity. Correlations of PFC 11C-SL25.1188 VT with psychomotor and processing speed; and serum blood measures implicated in astrogliosis were determined.
11C-SL25.1188 VT was greater in TBI in PFC (P = 0.00064) and cortex (P = 0.00038). PFC 11C-SL25.1188 VT inversely correlated with Comprehensive Trail Making Test psychomotor and processing speed (r = −0.48, P = 0.01). In participants scanned within 2 years of last TBI, PFC 11C-SL25.1188 VT correlated with serum glial fibrillary acid protein (r = 0.51, P = 0.037) and total tau (r = 0.74, P = 0.001).
Elevated 11C-SL25.1188 VT argues strongly for astrogliosis and therapeutics modifying astrogliosis towards curative phenotypes should be tested in TBI with persistent symptoms. Given substantive effect size, astrogliosis PET markers should be applied to stratify cases and/or assess target engagement for putative therapeutics targeting astrogliosis.
Keywords: astrogliosis, traumatic brain injury, positron emission tomography, monoamine oxidase B, comprehensive trail making test
Using PET, Koshimori et al. reveal elevated astrogliosis in traumatic brain injury patients with persistent symptoms, even years after their last injury. Developing therapeutics to target harmful aspects of inflammation may help in reducing chronic traumatic brain injury symptoms.
Introduction
With lifetime prevalence of ∼20%, traumatic brain injury (TBI) is an important public health problem.1 Most disability is attributable to persistent, chronic symptoms in approximately one-third of cases, including severe headaches, excessive sensitivity to stimuli, dizziness and cognitive impairment.1,2 Presently there are no established pharmacological treatments for persistent symptoms.2 Arguably the main barrier to developing such is a lack of targetable processes broadly identifiable across a high proportion of TBI cases with persistent symptoms.
Astrogliosis is a pathophysiological response to injury for which there are promising therapeutic interventions.3,4 Unfortunately, it is unknown whether there is astrogliosis in the brain of humans with TBI and persistent symptoms. Post-mortem studies demonstrate astrogliosis in late stage chronic traumatic encephalopathy (CTE)5 representing 5% of TBI.1,2 However, about 85% of TBI cases, initially categorized as mild-to-moderate, occur in community settings and involve one to a few injuries with approximately a third of these progressing to severe persistent symptoms.1,2 Studies of astrogliosis in human TBI are either not representative of this latter group, have concurrent neurological sequelae confounding interpretation, or are too small. For example, the leading investigation of astrogliosis in TBI is observation in surgical tissue of nine severe TBI participants almost all of whom had subdural/epidural hygromas or haematomas.6 Other data include elevated glial fibrillary acidic protein (GFAP, an astrogliosis marker) immunoreactivity in prefrontal cortex (PFC) of 14 TBI participants with 11 years of repeated brain injury, mostly from college level American football, compared to seven control subjects.7 One imaging investigation of monoamine oxidase B (MAO-B), a marker of astrogliosis, applied deuterium labelled 11C-l-deprenyl PET in seven TBI cases with persistent seizures and nine control subjects.8 This study demonstrated feasibility but is inconclusive given the small sample size, uncertain effects of concurrent seizures, and low radiotracer sensitivity (Supplementary material).9
11C-SL25.1188 is a newer PET MAO-B radioligand with outstanding properties, including a very high specific binding to non-displaceable binding ratio (>8), high reversibility, high brain uptake and high selectivity for MAO-B; and no brain penetrant radioactive metabolites.10,11 The total distribution volume, 11C-SL25.1188 VT, an index of MAO-B density measured with this method, is highly correlated with MAO-B concentration in post-mortem human brain (r2 > 0.90) and very reproducible.10 MAO-B is applied as a marker of astrogliosis because MAO-B expression is increased in reactive astrocytes; MAO-B density is highly correlated with other markers of astroglial activation across neuropsychiatric diseases such as GFAP, vimentin and heat shock protein 27; and MAO-B overexpression in astrocytes of transgenic mice creates astrogliosis.12–15 In addition, MAO-B is mainly located in astrocytes throughout the brain with the chief exception of the midbrain.16
The main objective of this study was to compare 11C-SL25.1188 VT between TBI cases with persistent symptoms and control subjects; and secondarily, to evaluate the relationship of PFC 11C-SL25.1188 VT to symptoms of psychomotor speed and cognitive processing in TBI. More specifically, it is hypothesized that greater 11C-SL25.1188 VT is present in the PFC and cortex regions proximal to cortical convexity in a community-based sample of TBI with persistent symptoms, a group typically reporting mild-to-moderate symptoms initially after injury. Although this TBI group lacks investigations of astrogliosis in brain, persistence of symptoms could indicate ongoing injury and/or response to injury; astrogliosis is reported in CTE, severe surgical cases6 as well as some animal models of machine-induced concussions.17 The secondary hypothesis is that slowed psychomotor speed is correlated with greater PFC 11C-SL25.1188 VT. Slowed psychomotor speed is common in chronic TBI and diseases with astrogliosis in PFC.18 Moreover, transgenic mice with globally greater MAO-B protein expression and associated astrogliosis including PFC demonstrate decreased rates of activity and movement.15 Among neuropsychological tests of psychomotor speed, the Comprehensive Trail Making Test (CTMT), requiring movement speed in the context of avoiding distractors, is prioritized because it is demonstrably affected in TBI, including separation from malingering.18 Additional hypotheses are that PFC 11C-SL25.1188 VT correlates with serum level of proteins often expressed in reactive astrocytes with the primary measure being GFAP and secondarily S100 calcium-binding protein β (S100β) and S100 calcium-binding protein A8 (S100A8) (detailed in the Supplementary material).19
Materials and methods
Participants
This study was conducted from 1 December 2018 to 4 February 2022 at CAMH, Toronto, Canada. Fifty-eight participants aged 18 to 66, including 29 participants with TBI and 29 similarly aged control subjects, completed the study (Table 1 and Supplementary material). Participants were recruited from CAMH, Southern Ontario through organizations supporting people with TBI and advertisement (Supplementary material). All participants provided written informed consent. The protocol and informed consent forms were approved by the Research Ethics Board of the CAMH and Health Canada.
Table 1.
Demographic/clinical history and traumatic brain injury cases
| Characteristics | Patients | Healthy |
|---|---|---|
| Age,* mean (SD), y | 36 (12) | 35 (13) |
| Sex*, n (%) | ||
| Males | 4 (14) | 10 (34) |
| Females | 25 (86) | 19 (66) |
| Multiple TBIs, n (%) | 21 (72) | NA |
| Number of TBIs, mean (SD) | 3 (3) | NA |
| Duration since last TBI, mean (SD), y | 2 (1) | NA |
| Duration since most severe TBI, mean (SD), y | 7 (1) | NA |
| Loss of consciousnessa, n (%)b | ||
| No loss of consciousness | 14 (50) | NA |
| <1 h | 8 (29) | NA |
| 1–24 h | 1 (3) | NA |
| Unknown | 5 (18) | NA |
| Duration of confusiona, n (%) | ||
| None | 5 (17) | NA |
| <1 day | 9 (31) | NA |
| 1 day–1 month | 5 (17) | NA |
| >1 month | 10 (35) | NA |
| Cause of injury, n (%)c | ||
| Sports | 17 (59) | NA |
| Motor vehicle accident | 14 (48) | NA |
| Falling | 12 (41) | NA |
| Pedestrian hit by vehicle | 5 (17) | NA |
| Physical abuse | 3 (10) | NA |
| Fight/assault | 1 (3) | NA |
| Other | 7 (24) | NA |
| Health services used, n (%)d | ||
| Emergency department | 18 (62) | NA |
| Family doctor | 24 (83) | NA |
| Walk-in clinic | 7 (24) | NA |
| Neurologist | 14 (48) | NA |
| Psychiatrist | 7 (24) | NA |
| Psychologist | 10 (34) | NA |
| Rehabilitation services | 15 (52) | NA |
| Alternative therapies | 21 (72) | NA |
| Other | 4 (14) | NA |
NA = not applicable; SD = standard deviation; TBI = traumatic brain injury; y = years.
Self-reported from most serious incident.
n = 28 able to remember.
The number of injuries surpasses the number of participants because participants endured on average three injuries.
The number of health services visited surpasses the number of subjects because participants each visited multiple services.
P > 0.10.
Main inclusion criteria for TBI participants were that the last TBI occurred within the past 5 years and that there were continuing symptoms; documented on the Brain Injury Screening Questionnaire (BISQ), Head Injury Questionnaire (HIQ), and a standardized self report of TBI symptoms from a University of Toronto affiliated clinic (for descriptions of questionnaires, see the Supplementary material). Main inclusion criteria for healthy participants were good health based on a structured health questionnaire, no history of TBI and medication-free. Exclusion criteria common to TBI and healthy were no history of neurological disease (except TBI symptoms in the TBI group), current substance use disorder, substance use within the previous month (including marijuana) further verified by urine drug screen, cigarette smoking, and use of an MAO-B inhibitor in the previous 4 weeks (Supplementary material).
Neuroimaging
A 3D high-resolution research tomograph (HRRT; CPS/Siemens) PET scanner system acquired imaging data for 90 min as previously described (Supplementary material).10 During the emission PET scan, concurrent arterial sampling was done using an automatic blood sampling system and manual sampling. For delineation of regions of interest (ROI), an MRI image (Discovery MR750 3.0T GE scanner) was acquired. 11C-SL25.1188 VT was calculated using a two-tissue compartment model previously validated for 11C-SL25.1188 PET.10
Neuropsychological tests
In addition to the aforementioned BISQ, HIQ and structured questions, participants with TBI completed neuropsychological tests, covering different domains by trained staff (Supplementary material). The prioritized test was the CTMT Trail 1, 2 and 3, items identified in factor analysis, which assess psychomotor speed in the context of varying degrees of distractors.20
Serum markers
Blood serum levels of GFAP and NDGR2 were measured with a sandwich ELISA; and S100β, S100A8, MCP-1 and T-tau were measured by a customized multiplex assay (R&D system) using the LUMINEX platform (Supplementary material).
Statistical analyses
Initial analyses compared 11C-SL25.1188 VT between TBI participants and healthy controls, starting with whole PFC using ANOVA. Additional analyses applied repeated measures ANOVA (rmANOVA) and mixed effects models evaluating group and region as fixed effects; and for mixed effects models, participant as a random effect. Second analyses included cortex regions proximal to cortical convexity including prefrontal, parietal, temporal and occipital cortices; and third analyses included other grey matter regions including hippocampus, anterior cingulate cortex (ACC), ventral striatum, dorsal putamen, thalamus and midbrain. Where significance of rmANOVA and mixed effects models were the same, one result is reported (see the Supplementary material for additional detail).
The relationship between 11C-SL25.1188 VT in subregions of PFC with the CTMT Trail 1, 2 and 3, as well as tests corresponding to other domains of cognitive functioning were examined using Pearson correlation analysis (Supplementary material). Serum level of markers associated with astrogliosis and/or astrocytes were correlated with PFC 11C-SL25.1188 VT applying Pearson correlation analysis (two-tailed, α threshold = 0.05). In addition, since blood level of some markers like GFAP and T-tau vary over 2 to 5 years post-injury,21 an exploratory sub-analysis of the relationship between blood serum markers and PFC 11C-SL25.1188 VT was done, including cases who had their last TBI within 2 years of their 11C-SL25.1188 PET scan.
Results
Fifty-eight participants, 29 with TBI and 29 healthy controls were included in the analyses (Supplementary material). Across the TBI and healthy control groups, age was similar and there were no significant differences in sex (Table 1). Symptoms at time of TBI were mild-to-moderate severity.
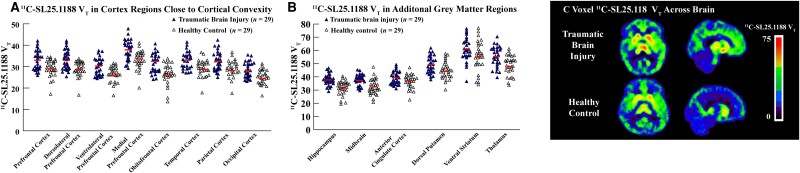
11C-SL25.1188 VT in PFC was significantly elevated in TBI compared to control groups [F(1,56) = 13.10, P = 0.00064] (Fig. 1A and C and Table 2). Consistent with such, 11C-SL25.1188 VT was greater in TBI than control groups across subregions of PFC [F(1,56) = 13.40, P = 0.00056] (Fig. 1A and Table 2). 11C-SL25.1188 VT was also significantly elevated in TBI participants compared to healthy controls in PFC, temporal, parietal and occipital cortices [F(1,56) = 14.33, P = 0.00038] (Fig. 1A and Table 2). 11C-SL25.1188 VT in PFC and parietal, temporal and occipital cortices were highly intercorrelated with correlation coefficients ∼r = 0.90 (Supplementary material). Group differences were also found in hippocampus, dorsal putamen, thalamus and midbrain [F(1,56) = 8.14, P = 0.0060] (Fig. 1B and Table 2).
Figure 1.
Comparison of 11C-SL25.1188 total distribution volume in traumatic brain injury and healthy controls. (A) Plots of 11C-SL25.1188 total distribution volume with group means in cortical and prefrontal regions. A bar represents a group mean. Prefrontal cortex (PFC): F(1,56) = 13.10, P = 0.00064; PFC subregions: F(1,56) = 13.40, P = 0.00056; cortex regions (PFC, temporal cortex, parietal cortex and occipital cortex): F(1,56) = 14.33, P = 0.00038. (B) Plots of 11C-SL25.1188 total distribution volume with group means in subcortical regions (hippocampus, midbrain, anterior cingulate cortex, dorsal putamen, ventral striatum and thalamus): F(1,56) = 8.14, P = 0.0060. A bar represents a group mean. (C) Parametric maps of 11C-SL25.1188 total distribution volume of one representative participant from each group. MAO-B = monoamine oxidase B; VT = total distribution volume.
Table 2.
Comparing regional 11C-SL25.1188 VT between patients with TBI and healthy controls
| ROI | 11C-SL25.1188 VTa | Effect sizeb | Per cent differencec | Mean difference (95% CI) | P-valued | |
|---|---|---|---|---|---|---|
| Patients (n = 29) |
Healthy (n = 29) |
|||||
| PFC | 33.06 (4.48) | 29.00 (3.91) | 1.03 | 14.00 | 4.07 (1.81–6.32) | 0.00064 |
| DLPFC | 33.20 (4.58) | 29.37 (3.64) | 1.05 | 13.04 | 3.83 (1.66–6.01) | 0.00084 |
| VLPFC | 30.03 (4.04) | 26.76 (3.67) | 0.89 | 12.22 | 3.27 (1.24–5.30) | 0.0021 |
| MPFC | 37.65 (4.80) | 33.67 (4.36) | 0.91 | 11.82 | 3.98 (1.57–6.40) | 0.0017 |
| OFC | 31.49 (4.51) | 26.92 (4.84) | 0.94 | 16.98 | 4.57 (2.11–7.03) | 0.00046 |
| Temporal cortex | 32.68 (4.01) | 28.89 (3.78) | 0.98 | 13.12 | 3.79 (1.71–5.87) | 0.00058 |
| Parietal cortex | 32.38 (4.45) | 28.69 (3.87) | 0.93 | 12.86 | 3.69 (1.50–5.89) | 0.0014 |
| Occipital cortex | 28.38 (3.62) | 24.51 (3.33) | 1.14 | 15.79 | 3.87 (2.02–5.73) | 0.00010 |
| Hippocampus | 37.90 (4.37) | 32.24 (5.64) | 1.00 | 17.56 | 5.66 (3.01–8.32) | <0.0001 |
| Midbrain | 37.66 (4.20) | 32.70 (5.90) | 0.84 | 15.17 | 4.96 (2.26–7.65) | 0.00051 |
| ACC | 39.28 (5.01) | 36.82 (5.12) | 0.48 | 6.68 | 2.46 (–0.21–5.12) | 0.070 |
| Dorsal putamen | 49.21 (6.83) | 45.53 (6.63) | 0.56 | 8.08 | 3.38 (0.14–7.22) | 0.042 |
| Ventral striatum | 60.24 (9.15) | 56.47 (11.59) | 0.33 | 6.68 | 3.77 (–1.73–9.26) | 0.18 |
| Thalamus | 55.19 (7.80) | 49.05 (7.41) | 0.83 | 12.52 | 6.14 (2.14–10.15) | 0.0033 |
ACC = anterior cingulate cortex; CI = confidence interval; DLPFC = dorsolateral prefrontal cortex; MPFC = medial prefrontal cortex; OFC = orbitofrontal cortex; PFC = prefrontal cortex; ROI = region of interest; TBI = traumatic brain injury; VLPFC = ventrolateral prefrontal cortex; VT = total distribution volume.
Mean (standard deviation, SD).
(Mean of patients with TBI − Mean of healthy controls) / SD of healthy controls.
[(Mean of patients with TBI − Mean of healthy controls) / Mean of healthy controls] × 100.
ANOVA.
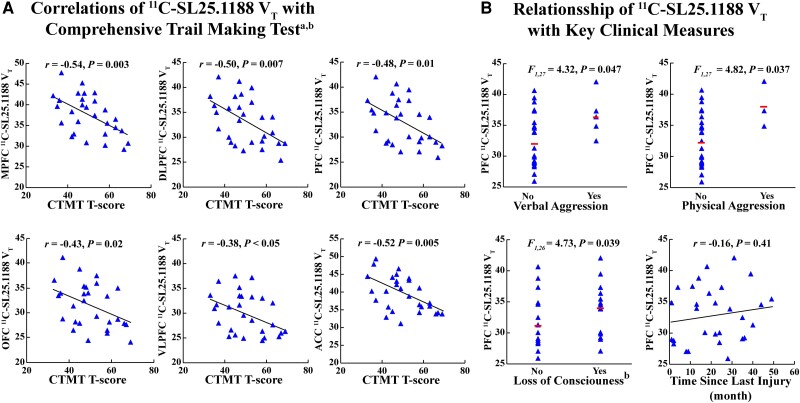
In TBI participants, greater 11C-SL25.1188 VT in PFC and ACC was associated with slower psychomotor and processing speed measured with mean T-scores of CTMT Trails 1, 2 and 3 (PFC: r = −0.48, P = 0.010; ACC: r = −0.52, P = 0.0048) (see Fig. 2A for PFC subregions). 11C-SL25.1188 VT did not significantly correlate with other test scores inclusive of psychomotor speed or other exploratory tests, except between immediate recall on the Hopkins Verbal Learning Test (HVLT-R) and 11C-SL25.1188 VT in the ACC (r = −0.38, P = 0.045) (Supplementary material).
Figure 2.
Associations between 11C-SL25.1188 total distribution volume and cognitive and clinical measures. (A) Associations between T-scores of CTMT and 11C-SL25.1188 VT in the entire PFC, PFC subregions and ACC. (B) Associations between clinical measures including verbal and physical aggression, loss of consciousness, and time since last injury (month) and PFC 11C-SL25.1188 VT. ACC = anterior cingulate cortex; CTMT = Comprehensive Trail Making Test; DLPFC = dorsolateral prefrontal cortex; MAO-B = monoamine oxidase B; MPFC = medical prefrontal cortex; OFC = orbitofrontal cortex; PFC = prefrontal cortex; VLPFC = ventrolateral prefrontal cortex; VT = total distribution volume.
aMean T-Scores of CTMT Trail 1, 2, and 3.
b n = 28.
Variation in symptom severity was modest, so exploratory analyses investigated relationships between PFC 11C-SL25.1188 VT and presence of symptoms reported with the HIQ, showing that PFC 11C-SL25.1188 VT was associated with presence of verbal aggression [ANOVA, F(1,27) = 4.32, P = 0.047] and physical aggression [ANOVA, F(1,27) = 4.82, P = 0.037] (Fig. 2B). In addition, reported loss of consciousness (presence/absence) after a previous TBI was associated with greater PFC 11C-SL25.1188 VT [ANOVA, F(1,26) = 4.73, P = 0.039; n = 28 able to remember]. There were no significant correlations of PFC 11C-SL25.1188 VT (Fig. 2B); or parietal, temporal or occipital cortices with duration since injury.
Exploratory analyses found significant correlations between MCP-1 (r = −0.41, P = 0.03) and T-tau (r = 0.39, P = 0.042) with PFC 11C-SL25.1188 VT (Supplementary Fig. 1). In those who had their last injury within 2 years of scanning, there were significant correlations between PFC 11C-SL25.1188 VT with GFAP (r = 0.51, P = 0.037; Supplementary Fig. 1); and T-tau (r = 0.74, P = 0.001; Supplementary Fig. 1).
Discussion
This is the first study to detect strong evidence of astrogliosis in the brain of TBI with persistent symptoms from a community-based sample and this has key implications for developing therapeutics, including using astrogliosis based imaging for target engagement. 11C-SL25.1188 VT was significantly elevated with almost half of TBI participants having a greater level than the range of healthy controls in PFC; cortex regions proximal to cortical convexity; as well as hippocampus. Although participants were sampled up to 5 years after their last TBI, the 11C-SL25.1188 VT marker of astrogliosis was still elevated. Since most TBI are consequent to mild-to-moderate severity events, this study has broad clinical relevance.
While astrogliosis is heterogeneous with a mix of protective and pathological roles, in chronicity, pathological roles tend to be more concerning4 and it is plausible that amidst possible protective roles, there is ongoing harm from astrogliosis labelled with MAO-B. MAO-B is a major source of hydrogen peroxide in astrocytes; and astrogliosis associated with greater MAO-B level and/or activity, whether transgenically induced, stimulated with lipopolysaccharide, or sampled from tissue adjacent to amyloid plaques, is associated with greater production of hydrogen peroxide and reactive oxygen species.15,22,23 Widespread astrogliosis associated with overexpression of MAO-B in rodents causes adverse behavioural sequelae of reduced speed, activity and exploratory behaviour, as well as induction of Parkinson’s disease pathology.15 Furthermore, MAO-B labelled astrocytes are implicated in fostering greater amyloid accumulation based on their location, presence and timing.13,24,25 In the present study, the distribution of 11C-SL25.1188 VT elevation encompasses regions affected by astrogliosis in Alzheimer’s disease and Parkinson’s disease, raising the possibility that one mechanism through which TBI creates risk for these diseases is via MAO-B labelled astrogliosis. Moreover, greater MAO-B density itself is implicated in pathological events like production of aldehydes, impairment of mitochondrial function, and creation of neurotoxic metabolites.9,15,16,22
There are a number of examples of pathological roles of astrogliosis associated with greater expression of MAO-B, but there may be other roles of such astroglia that are helpful and that only through testing astroglial targeting medications would the risk-benefit profile of such interventions be ascertained in TBI with persistent symptoms. Potential approaches to ascertain whether targeting aspects of astrogliosis in chronically symptomatic TBI has therapeutic benefit includes reducing potentially harmful functions like overexpression of MAO-B through MAO-B inhibition with medications like KDS2010 or rasagiline. Targeting other downstream effects like production of reactive oxygen species and more generalized inflammatory sequelae via antioxidant medications like n-acetylcysteine or celecoxib could also be studied.3,26 Enhancing curative roles of astroglial function like increasing extracellular glutamate clearance to avoid neurotoxicity, such as with riluzole, is another potential direction. Whereas most clinical trials for TBI address acute injury,26 these interventions would be for chronically persistent symptoms. Moreover, the prominent elevation of cortex 11C-SL25.1188 VT, with an effect size ∼1, represents a breakthrough for astrogliosis PET neuroimaging to assess target engagement of novel therapeutics. Therapeutics assessed could include those that directly lower astrogliosis labelled with MAO-B or those that reduce ongoing injury that indirectly elicits such astrogliosis. Although 11C-SL25.1188 has outstanding radioligand qualities, other potential astroglial PET imaging agents include imidazoline-2 receptor binding 11C-BU9900825 and MAO-B binding 18F-SBMT-1.27
Several clinical measures correlated with greater PFC 11C-SL25.1188 VT, including decrements in psychomotor functioning with visual scanning on the CTMT as well as greater problems with verbal and physical aggression. Correlations do not prove causality but it is established that injury to PFC is associated with CTMT impairment and predisposes to aggressive behaviour, which could account for these changes and elevated in 11C-SL25.1188 VT. Also, exploratory analyses found relationships between regional 11C-SL25.1188 VT and level of several serum blood markers, including a positive association with serum T-tau, a negative correlation with MCP-1, and in a subset of participants, whose previous TBI was within 2 years of scanning, a positive correlation with GFAP (and T-tau) (see Supplementary material regarding speculative mechanisms). These symptom measures and blood markers could be considered for stratifying in future clinical trials of astrogliosis targeting interventions for TBI, applying cut-offs corresponding to PFC 11C-SL25.1188 VT outside the range of health.
Some limitations should be addressed. First, as is standard with PET imaging studies, 11C-SL25.1188 VT reflects radioligand binding to MAO-B plus non-displaceable binding. However, based on blocking studies in humans, non-displaceable binding in the cortex is less than 15%10 and unlikely to account for a mean ∼15% elevation in cortex 11C-SL25.1188 VT as this would require elevations in non-displaceable binding exceeding 150%. Second, while MAO-B is mainly found in astrocytes within regions sampled, the midbrain is an exception because of substantial contributions of MAO-B from cell bodies of serotonergic neurons of the raphe and dopaminergic neurons in the substantia nigra.16 Third, TBI is heterogeneous so the best interpretation of elevated 11C-SL25.1188 VT may be that about half of TBI participants exhibit much more highly elevated cortical 11C-SL25.1188 VT (∼30% to 40%) diluted by TBI participants with lesser change, rather than the interpretation that all TBI cases on average have elevated 11C-SL25.1188 VT. Finally, although not statistically significant, there were more females in the TBI than control group; however, sex was not a significant predictor of 11C-SL25.1188 VT and even if sex were added as a covariate, group differences were similarly significant.
In summary, strong evidence for elevated gliosis in TBI with persistent symptoms in a community-based sample is presented with greater 11C-SL25.1188 VT in PFC, cortices proximal to cortical convexity, as well as hippocampus, thalamus and midbrain. Given the potentially adverse effects of MAO-B labelled astrocytes and implication of ongoing injury, this argues for testing therapeutic candidates that reduce harmful aspects of astrogliosis and/or shift astrogliosis towards curative roles in TBI with persistent symptoms. Moreover, astroglial-based PET imaging should be applied as markers of target engagement for novel therapeutics that impact MAO-B labelled astrogliosis.
Supplementary Material
Acknowledgements
Infrastructure support included the Azrieli Foundation for radiochemistry laboratories; and the Canadian Foundation for Innovation and the Ontario Ministry for Innovation for PET scanner, MRI scanner, and cyclotron infrastructure. The authors acknowledge the invaluable contributions of the study participants as well as research staff at Centre for Addiction and Mental Health.
Contributor Information
Yuko Koshimori, Brain Health Imaging Centre, Azrieli Centre for Neuro-Radiochemistry, and Campbell Family Mental Health Research Institute, CAMH, Toronto, M5T 1R8, Canada.
Michael D Cusimano, Neurosurgery, St. Michael’s Hospital, University of Toronto, Toronto, M5B 1W8, Canada.
Erica L Vieira, Molecular Neurobiology and Campbell Family Mental Health Research Institute, CAMH, Toronto, M5T 1R8, Canada; Department of Psychiatry, University of Toronto, Toronto, M5T 1R8, Canada.
Pablo M Rusjan, Douglas Research Centre and Department of Psychiatry, McGill University, Montreal, H3A 1A1, Canada.
Stephen J Kish, Brain Health Imaging Centre, Azrieli Centre for Neuro-Radiochemistry, and Campbell Family Mental Health Research Institute, CAMH, Toronto, M5T 1R8, Canada; Department of Psychiatry, University of Toronto, Toronto, M5T 1R8, Canada; Department of Pharmacology and Toxicology, University of Toronto, Toronto, M5S 1A8, Canada.
Neil Vasdev, Brain Health Imaging Centre, Azrieli Centre for Neuro-Radiochemistry, and Campbell Family Mental Health Research Institute, CAMH, Toronto, M5T 1R8, Canada; Department of Psychiatry, University of Toronto, Toronto, M5T 1R8, Canada.
Sho Moriguchi, Brain Health Imaging Centre, Azrieli Centre for Neuro-Radiochemistry, and Campbell Family Mental Health Research Institute, CAMH, Toronto, M5T 1R8, Canada.
Isabelle Boileau, Brain Health Imaging Centre, Azrieli Centre for Neuro-Radiochemistry, and Campbell Family Mental Health Research Institute, CAMH, Toronto, M5T 1R8, Canada; Department of Psychiatry, University of Toronto, Toronto, M5T 1R8, Canada; Department of Pharmacology and Toxicology, University of Toronto, Toronto, M5S 1A8, Canada.
Thomas Chao, Institute of Mental Health, Department of Psychiatry, University of British Columbia, Vancouver, V6T 2A1, Canada.
Zahra Nasser, Brain Health Imaging Centre, Azrieli Centre for Neuro-Radiochemistry, and Campbell Family Mental Health Research Institute, CAMH, Toronto, M5T 1R8, Canada.
M Ishrat Husain, Brain Health Imaging Centre, Azrieli Centre for Neuro-Radiochemistry, and Campbell Family Mental Health Research Institute, CAMH, Toronto, M5T 1R8, Canada; Department of Psychiatry, University of Toronto, Toronto, M5T 1R8, Canada; Department of Pharmacology and Toxicology, University of Toronto, Toronto, M5S 1A8, Canada.
Khunsa Faiz, Department of Diagnostic Radiology, Hamilton Health Sciences, McMaster University, Hamilton, L8S 4K1, Canada.
Joeffre Braga, Brain Health Imaging Centre, Azrieli Centre for Neuro-Radiochemistry, and Campbell Family Mental Health Research Institute, CAMH, Toronto, M5T 1R8, Canada; Department of Pharmacology and Toxicology, University of Toronto, Toronto, M5S 1A8, Canada.
Jeffrey H Meyer, Brain Health Imaging Centre, Azrieli Centre for Neuro-Radiochemistry, and Campbell Family Mental Health Research Institute, CAMH, Toronto, M5T 1R8, Canada; Department of Psychiatry, University of Toronto, Toronto, M5T 1R8, Canada; Department of Pharmacology and Toxicology, University of Toronto, Toronto, M5S 1A8, Canada.
Data availability
Data will be made available for each figure that contains the main findings (Supplementary material).
Funding
Primarily, Canadian Institutes of Health Research; some from the Canadian Institute for Military and Veteran Health Research. Data collection for this project and dissemination of the data presented in this manuscript was supported primarily by a Project Grant funded by the Canadian Institutes of Health Research and partially by the Canadian Institute for Military and Veteran Health Research. N.V., I.B. and J.H.M. were supported by the Canada Research Chair program. J.B. was supported by a Doctoral Award funded by the Canadian Research Institutes of Health Research.
Competing interests
The authors report no competing interests.
Supplementary material
Supplementary material is available at Brain online.
References
- 1. Maas AIR, Menon DK, Adelson PD, et al. . Traumatic brain injury: Integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. 2017;16:987–1048. [DOI] [PubMed] [Google Scholar]
- 2. Levin HS, Diaz-Arrastia RR. Diagnosis, prognosis, and clinical management of mild traumatic brain injury. Lancet Neurol. 2015;14:506–517. [DOI] [PubMed] [Google Scholar]
- 3. Forman HJ, Zhang H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat Rev Drug Discov. 2021;20:689–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sofroniew MV. Astrocyte reactivity: Subtypes, states, and functions in CNS innate immunity. Trends Immunol. 2020;41:758–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McKee AC, Stein TD, Kiernan PT, Alvarez VE. The neuropathology of chronic traumatic encephalopathy. Brain Pathol. 2015;25:350–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Castejon OJ. Morphological astrocytic changes in complicated human brain trauma. A light and electron microscopic study. Brain Inj. 1998;12:409–427; discussion 407. [DOI] [PubMed] [Google Scholar]
- 7. Babcock KJ, Abdolmohammadi B, Kiernan PT, et al. . Interface astrogliosis in contact sport head impacts and military blast exposure. Acta Neuropathol Commun. 2022;10:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fowler JS, Volkow ND, Cilento R, Wang GJ, Felder C, Logan J. Comparison of brain glucose metabolism and monoamine oxidase B (MAO B) in traumatic brain injury. Clin Positron Imaging. 1999;2:71–79. [DOI] [PubMed] [Google Scholar]
- 9. Meyer JH, Cervenka S, Kim M-J, Kreisl WC, Henter ID, Innis RB. Neuroinflammation in psychiatric disorders: PET imaging and promising new targets. Lancet Psychiatry. 2020;7:1064–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rusjan PM, Wilson AA, Miler L, et al. . Kinetic modeling of the monoamine oxidase B radioligand 11C-SL25.1188 in human brain with high-resolution positron emission tomography. J Cereb Blood Flow Metab. 2014;34:883–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vasdev N, Sadovski O, Garcia A, et al. . Radiosynthesis of 11C-SL25.1188 via 11C-CO2 fixation for imaging monoamine oxidase B. J Labelled Comp Radiopharm. 2011;54:678–680. [Google Scholar]
- 12. Ekblom J, Jossan SS, Oreland L, Walum E, Aquilonius SM. Reactive gliosis and monoamine oxidase B. J Neural Transm Suppl. 1994;41:253–258. [DOI] [PubMed] [Google Scholar]
- 13. Saura J, Luque JM, Cesura AM, et al. . Increased monoamine oxidase B activity in plaque-associated astrocytes of Alzheimer brains revealed by quantitative enzyme radioautography. Neuroscience. 1994;62:15–30. [DOI] [PubMed] [Google Scholar]
- 14. Tong J, Rathitharan G, Meyer JH, et al. . Brain monoamine oxidase B and A in human parkinsonian dopamine deficiency disorders. Brain. 2017;140:2460–2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mallajosyula JK, Kaur D, Chinta SJ, et al. . MAO-B elevation in mouse brain astrocytes results in Parkinson's pathology. PLoS One. 2008;3:e1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Saura J, Bleuel Z, Ulrich J, et al. . Molecular neuroanatomy of human monoamine oxidases A and B revealed by quantitative enzyme radioautography and in situ hybridization histochemistry. Neuroscience. 1996;70:755–774. [DOI] [PubMed] [Google Scholar]
- 17. Villapol S, Byrnes KR, Symes AJ. Temporal dynamics of cerebral blood flow, cortical damage, apoptosis, astrocyte-vasculature interaction and astrogliosis in the pericontusional region after traumatic brain injury. Front Neurol. 2014;5:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Woods DL, Wyma JM, Herron TJ, Yund EW. The effects of aging, malingering, and traumatic brain injury on computerized trail-making test performance. PLoS One. 2015;10:e0124345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cristovao JS, Gomes CM. S100 proteins in Alzheimer’s disease. Front Neurosci. 2019;13:463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Riccio CA, Blakely A, Yoon M, Reynolds CR. Two-factor structure of the comprehensive trail-making test in adults. Appl Neuropsychol Adult. 2013;20:155–158. [DOI] [PubMed] [Google Scholar]
- 21. Shahim P, Politis A, van der Merwe A, et al. . Time course and diagnostic utility of NfL, tau, GFAP, and UCH-L1 in subacute and chronic TBI. Neurology. 2020;95:e623–e636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Akiyama S, Tatsuhiro A, Takahashi C, Ohya R, Ano Y. B-lactolin, a monoamine oxidase B inhibitor lactopeptide, suppresses reactive oxygen Species production in lipopolysaccharide-stimulated astrocytes. Appl Sci. 2021;11:3034. [Google Scholar]
- 23. Borroni E, Bohrmann B, Grueninger F, et al. . Sembragiline: A novel, selective monoamine oxidase type B inhibitor for the treatment of Alzheimer’s disease. J Pharmacol Exp Ther. 2017;362:413–423. [DOI] [PubMed] [Google Scholar]
- 24. Rodriguez-Vieitez E, Ni R, Gulyas B, et al. . Astrocytosis precedes amyloid plaque deposition in Alzheimer APPswe transgenic mouse brain: A correlative positron emission tomography and in vitro imaging study. Eur J Nucl Med Mol Imaging. 2015;42:1119–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Livingston NR, Calsolaro V, Hinz R, et al. . Relationship between astrocyte reactivity, using novel 11C-BU99008 PET, and glucose metabolism, grey matter volume and amyloid load in cognitively impaired individuals. Mol Psychiatry. 2022;27:2019–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bergold PJ. Treatment of traumatic brain injury with anti-inflammatory drugs. Exp Neurol. 2016;275:367–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Harada R, Hayakawa Y, Ezura M, et al. . 18F-SMBT-1: A selective and reversible PET tracer for monoamine oxidase-B imaging. J Nucl Med. 2021;62:253–258. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available for each figure that contains the main findings (Supplementary material).