Abstract
Cheddar cheese was manufactured with either Lactobacillus salivarius NFBC 310, NFBC 321, or NFBC 348 or L. paracasei NFBC 338 or NFBC 364 as the dairy starter adjunct. These five strains had previously been isolated from the human small intestine and have been characterized extensively with respect to their probiotic potential. Enumeration of these strains in mature Cheddar cheese, however, was complicated by the presence of high numbers (>107 CFU/g of cheese) of nonstarter lactic acid bacteria, principally composed of lactobacilli which proliferate as the cheese ripens. Attempts to differentiate the adjunct lactobacilli from the nonstarter lactobacilli based on bile tolerance and growth temperature were unsuccessful. In contrast, the randomly amplified polymorphic DNA method allowed the generation of discrete DNA fingerprints for each strain which were clearly distinguishable from those generated from the natural flora of the cheeses. Using this approach, it was found that both L. paracasei strains grew and sustained high viability in cheese during ripening, while each of the L. salivarius species declined over the ripening period. These data demonstrate that Cheddar cheese can be an effective vehicle for delivery of some probiotic organisms to the consumer.
Cheddar cheese may offer certain advantages as a carrier of probiotic microorganisms. Having a higher pH than the more traditional probiotic foods (e.g., yogurts and fermented milks), it may provide a more stable milieu to support their long-term survival. Furthermore, the matrix of the cheese and its relatively high fat content may offer protection to probiotic bacteria during passage through the gastrointestinal tract. However, among the most important criteria when considering Cheddar cheese as a probiotic food is that the microorganisms be able to survive the relatively long ripening time of at least 6 months and/or that they grow in the cheese over this period. During the ripening period, generally performed at 2 to 16°C (21), a number of nonpathogenic bacteria, chiefly lactobacilli (Lactobacillus plantarum, L. casei, and L. brevis) and pediococci (Pediococcus pentosaceus) proliferate in the maturing cheese (5). Many of these nonstarter lactic acid bacteria (NSLAB) have complex proteolytic systems, which have been associated with the maturation process. Consequently, lactobacilli with defined proteolytic systems have been deliberately added as adjuncts to cheese milk in order to influence cheese maturation (4, 26, 27, 40). The results of such an approach can be variable depending on the particular strain used. At present, a number of Lactobacillus adjuncts employed for the improvement of flavor are commercially available for cheeses, including Cheddar.
There are relatively few reports concerning cheese as a carrier of probiotic organisms, even though there are a small number of probiotic cheeses currently on the market worldwide. In 1994, Dinakar and Mistry (10) incorporated Bifidobacterium bifidum into Cheddar cheese as a starter adjunct. This strain survived well in the cheese and retained a viability of approximately 2 × 107 CFU/g of cheese even after 6 months of ripening, without adversely affecting cheese flavor, texture, or appearance. This example suggested that Cheddar could provide a suitable environment for the maintenance of probiotic organisms at high levels over long periods. In another study, bifidobacteria were used in combination with L. acidophilus strain Ki as a starter in Gouda cheese manufacture (12). In this case, there was a significant effect on cheese flavor in the resultant product after 9 weeks of ripening, possibly due to acetic acid production by the bifidobacteria.
In order to exert a probiotic effect, cultures must maintain their viability in food products through to the time of consumption, which for Cheddar cheese is many months after manufacture. The potential health-promoting effects achieved by the consumption of dairy products containing probiotic organisms, such as Lactobacillus and Bifidobacterium spp., have resulted in intensive research efforts in recent years (for reviews, see references 11, 25, and 35). The products which have received the most attention in this regard include fermented milks, such as yogurt and buttermilk, as well as unfermented milks with cultures added (3, 33, 36–38) together with frozen desserts such as ice cream and frozen yogurt (6, 14, 23). The importance of these probiotic-containing products, commonly regarded as functional foods, in the maintenance of health and well-being is becoming a key factor affecting consumer choice. This has resulted in rapid growth and expansion of the market for such products, in addition to increased commercial interest in exploiting their proposed healthful attributes.
This study investigates the performance of a number of Lactobacillus strains, including L. salivarius and L. paracasei, when employed as adjuncts in Cheddar cheese, over 8 months of ripening. These strains had previously been isolated from healthy human intestine and characterized in detail with regard to their probiotic potential (8). In this respect, these strains have been shown to be acid and bile tolerant, adherent to human epithelial cells, and nonpathogenic and to have desirable antibiogram profiles. A prerequisite to the enumeration of these strains from cheese was the development of a reliable genetic fingerprinting method to distinguish them from the resident flora. The results demonstrate that the probiotic L. paracasei species used in this study are particularly suitable for Cheddar cheese applications. They grow to numbers in excess of 108 CFU/g and remain at this level even after 8 months of ripening, while their presence has negligible effects on cheese composition, flavor, and aroma.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The probiotic Lactobacillus strains used in this study had previously been isolated from the human gastrointestinal tract and were obtained from University College Cork, Cork, Ireland, under a restricted materials transfer agreement. These strains were identified as L. salivarius (subsp. salivarius) and L. paracasei (subsp. paracasei) by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (PAGE) analysis of total cell protein (31) and were designated L. salivarius NFBC 310, NFBC 321, and NFBC 348 and L. paracasei NFBC 338 and NFBC 364. NSLAB Lactobacillus strains (L. curvatus DPC 2042 and DPC 2081, L. plantarum DPC 2102 and DPC 2142, and L. casei subsp. casei DPC 2047 and DPC 2103) which had previously been isolated from 8-week-old commercial Cheddar cheeses were obtained from the culture collection of the Dairy Products Research Centre. All Lactobacillus strains were routinely cultured in MRS broth (9) (Difco Laboratories, Detroit, Mich.) under anaerobic conditions (anaerobic jars with Anaerocult A gas packs; Merck, Darmstadt, Germany) at 30 and 37°C for NSLAB and probiotic strains, respectively. Lactococcus lactis subsp. cremoris strains 227 and 223, obtained from C. Hansen’s Laboratories (Little Island, Cork, Ireland) in the form of freeze-dried pellets, were used as starters for cheesemaking. These were grown overnight at 21°C in heat-treated (90°C for 30 min) 10% (wt/vol) reconstituted skim milk (RSM).
Bile and temperature tolerance.
To investigate the tolerance of both the probiotic and NSLAB Lactobacillus isolates to bile, overnight MRS broth cultures of each of the Lactobacillus strains were serially diluted in maximum recovery diluent (Oxoid Ltd., Basingstoke, Hampshire, United Kingdom) and appropriate dilutions were pour plated on MRS agar with 0, 0.1, 0.3, 0.5, 1.0, or 3.0% porcine bile (Sigma Chemical Co., Poole, Dorset, United Kingdom). After 3 days of incubation, the plates were examined, and where colonies were present, their numbers and sizes were recorded. Temperature tolerance of the probiotic lactobacilli was investigated by pour plating appropriate dilutions of overnight cultures on LBS agar (34) (Becton Dickinson, Cockeysville, Md.) and incubating the plates anaerobically both at 37°C (which is the optimum temperature of growth for these strains) and at 42°C. Colony numbers obtained after 5 days were compared. In the same way, the temperature tolerance of these strains and NSLAB, following isolation from Cheddar cheese, was also investigated.
Genetic fingerprinting by RAPD-PCR.
Randomly amplified polymorphic DNA (RAPD)-PCR analysis was carried out on each of the probiotic Lactobacillus strains and on cultures grown from Lactobacillus colonies isolated from Cheddar cheese. Genomic DNA was isolated from 1.5 ml of overnight MRS broth cultures by using a modification of the method of Hoffman and Winston (13). This procedure utilizes shearing with glass beads to lyse the cells and was modified as outlined by Coakley et al. (7). One microliter of the extracted DNA was used in subsequent PCR amplifications, which were performed in a total volume of 25 μl in a Perkin-Elmer (Norwalk, Conn.) DNA Thermal Cycler. The method was employed essentially as described by Coakley et al. (7) and used a single primer of arbitrary nucleotide sequence (5′ ATGTAACGCC 3′), obtained from Pharmacia Biotech (Uppsala, Sweden). DNA was amplified for 35 cycles using the following temperature profile: denaturing at 93°C for 1 min, annealing at 36°C for 1 min, and polymerization at 72°C for 1 min. Taq DNA polymerase (0.625 U; Bioline) was added to the reaction mixture during the first denaturation step (hot start). Between 5 and 10 μl of the PCR mixture was analyzed on a 1.5% (wt/vol) agarose (Sigma) gel with ethidium bromide staining. A 100-bp ladder (Pharmacia) was used as a molecular weight standard. Gels were run for approximately 3 h at 100 V, and the DNA was visualized by UV transillumination.
Cheddar cheese manufacture.
Laboratory-scale cheesemaking trials (trials 1 and 2) were performed initially with 25 liters of pasteurized whole milk in each cheese vat. To limit contamination with wild lactobacilli, these cheeses were manufactured under controlled bacteriological conditions, as described by McSweeney et al. (28). A 1.5% inoculum of the mixed-strain starter culture was used, and in each trial one vat (vat 1) acted as a control to which starter only was added. To each of the experimental vats, one probiotic Lactobacillus strain, grown overnight in 10% RSM, was added as an adjunct to the starter culture. In trial 1, the probiotic adjuncts L. salivarius NFBC 348 and L. paracasei NFBC 364 were added at an inoculum level of 0.1% to vats 2 and 3, respectively. In the second trial, L. salivarius NFBC 310 (vat 2), L. salivarius NFBC 321 (vat 3), and L. paracasei NFBC 338 (vat 4) were inoculated at a level of 0.2%. Cheddar cheeses were then manufactured according to standard procedures as follows. Filter-sterilized rennet (C. Hansen’s Laboratories) was added at a concentration of 0.07 ml/liter 35 min after starter and adjunct addition, and the curd was cut approximately 40 min later. Curds were cooked to 39°C, pitched at pH 6.1, and milled at approximately pH 5.3. Salt was added at a rate of 2.8% (wt/wt), and the curds were placed in molds and pressed at approximately 200 kPa overnight. The cheeses were removed from the molds, vacuum packed, and ripened at 8°C for approximately 8.5 months. Subsequently, two pilot-scale cheesemaking trials (trials 3 and 4) were performed with two of the adjunct Lactobacillus strains which were found to maintain high viability in the laboratory-scale cheeses during ripening. In each trial, two vats, one experimental and one control, each containing 450 liters of standardized (fat/protein ratio = 1) pasteurized whole milk, were used. As in the laboratory-scale trials, a 1.5% inoculum of the starters 223 and 227 was added to each vat. In addition, in each trial the experimental vat (vat 2) contained a 0.1% inoculum of either L. paracasei NFBC 364 (trial 3) or NFBC 338 (trial 4) added as a starter adjunct. The cheesemaking procedure was as previously described for the laboratory-scale cheeses except that the salting level was 2.7% and the curds were pressed overnight at approximately 413 kPa.
Cheese compositional analysis.
Grated cheese samples were analyzed in duplicate for salt content by a potentiometric method (15), for fat content by the Gerber method (17), for moisture content by oven-drying at 102°C (16), and for protein content on a LECO FP-428 nitrogen determinator. The pH of a slurry, prepared by blending 12 ml of H2O with 20 g of grated cheese, was measured by using a standard pH meter (Radiometer, Copenhagen, Denmark).
Bacteriological analyses of cheeses.
Viability of lactobacilli (both probiotic adjuncts and NSLAB) in the inoculated cheese milk and in the cheeses during ripening was determined on LBS agar after 5 days of anaerobic incubation at 30°C, while starter lactococci were enumerated on LM17 agar (39) after 3 days of incubation at 30°C. Coliforms in cheese milk and cheeses were enumerated on violet red bile agar (VRBA; Oxoid) after incubation at 37°C for 24 h. Cheeses were aseptically sampled in duplicate for bacteriological analysis at intervals during ripening. Cheese samples were emulsified in sterile 2% (wt/vol) trisodium citrate and diluted in maximum-recovery diluent, and appropriate dilutions were pour plated. After one, three, and six monthly intervals, 18 individual Lactobacillus colonies from each cheese were randomly selected from the LBS agar plates for RAPD-PCR analysis.
Sensory evaluation of Cheddar cheese.
Cheeses were graded blindly after 3 and 6 months of ripening by a commercial grader from a local cheese manufacturing plant. The cheeses were graded for the characteristics “flavor/aroma” and “body/texture,” with maximum scores of 45 and 40, respectively. Minimum scores of 38 and 31 for flavor/aroma and body/texture, respectively, are required for commercial Cheddar cheese.
Assessment of proteolysis in Cheddar cheese.
Cheeses were analyzed by urea-PAGE using the stacking gel system of Andrews (1). Cheese samples were prepared by dispersing 10 mg of grated cheese in 1 ml of sample buffer, and 10 μl of this sample was applied to the gel. Sodium caseinate (5 μl) was used as a standard for comparative purposes, and gels were stained by the direct-staining procedure of Blakesley and Boezi (2).
Water-soluble extracts (pH 4.6) of each of the cheeses were prepared according to the method of Kuchroo and Fox (22) and freeze-dried. The size distribution of peptides in these freeze-dried extracts was determined by size exclusion high-performance liquid chromatography (HPLC), using a TSK G2000 SW (Beckman Instruments Ltd., High Wycombe, Buckinghamshire, United Kingdom) gel permeation column (7.5 nm by 60 cm) fitted to a Waters HPLC system (Waters Chromatography Division, Milford, Mass.). The column was eluted at a flow rate of 1 ml/min with 30% acetonitrile containing 0.1% trifluoroacetic acid. The freeze-dried water-soluble extracts were reconstituted (3 mg/ml) in HPLC-grade water and filtered through a Whatman 0.2-μm-pore-size filter, and 20 μl of the filtered extract was applied to the column. Column eluates were continually monitored at 214 nm. Data were collected by using a PC Minichrom system (VG Data Systems, Altrircham, Cheshire, United Kingdom), and the results were compared to a previously prepared calibration curve.
Individual free amino acids (FAA) in the water-soluble extracts were determined by using a Beckman System 6300 High Performance Analyzer (Beckman Instruments Ltd.) equipped with a Beckman P-N 338052 Na+ column (12 by 0.5 cm) as described by Lynch et al. (26). Amino acid concentrations were expressed as micrograms per milliliter of cheese extract, which was subsequently converted to micrograms per gram of cheese.
RESULTS AND DISCUSSION
Probiotic strain identification and enumeration.
A prerequisite to the successful enumeration of added probiotic strains is their selective enumeration from the natural, often complex microflora found in food products. Since NSLAB can reach levels up to 107 to 108 CFU/g in cheese during ripening (30), the present study initially focused on evaluating a number of methods aimed at selectively distinguishing the lactobacilli added as starter adjuncts from these NSLAB.
(i) Bile and temperature tolerance of Lactobacillus adjuncts.
Both the probiotic adjuncts and NSLAB Lactobacillus strains varied considerably with regard to their ability to tolerate bile, and therefore selections based on bile tolerance were not a useful means of distinguishing the probiotic adjunct lactobacilli from the NSLAB lactobacilli during the ripening of Cheddar cheese. Similarly, temperature tolerance of the two groups of lactobacilli was found to be nonselective, although it has been shown that NSLAB isolated from Irish Cheddar cheeses do not grow at 45°C (19) but that some of the human-derived probiotic lactobacilli may withstand such temperatures (20).
(ii) RAPD-PCR analysis.
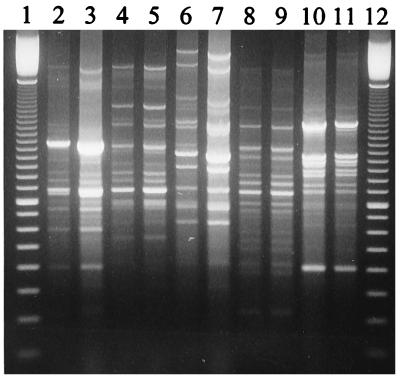
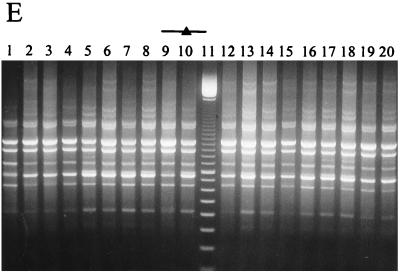
Consequently, the RAPD method, which involves PCR using an arbitrary primer, was used to generate DNA fingerprints for each of the probiotic strains. Each of the Lactobacillus strains generated reproducible discrete DNA fingerprints (Fig. 1) which were found to be substantially different from those of representative NSLAB lactobacilli. Thus, the RAPD method proved to be a successful means of identifying the probiotic organisms and demonstrated potential as a means of selective identification of these strains from the NSLAB flora in Cheddar cheese.
FIG. 1.
RAPD-PCR-generated DNA fingerprints for probiotic Lactobacillus strains L. salivarius NFBC 310 (lanes 2 and 3), L. salivarius NFBC 321 (lanes 4 and 5), L. paracasei NFBC 338 (lanes 6 and 7), L. salivarius NFBC 348 (lanes 8 and 9), and L. paracasei NFBC 364 (lanes 10 and 11). Lanes 1 and 12 contain 100-bp ladders.
Incorporation of Lactobacillus species into Cheddar cheese.
Initially, laboratory-scale cheese trials were performed under microbiologically controlled conditions (thus limiting development of high numbers of NSLAB during ripening) to assess the performance of five probiotic Lactobacillus strains in Cheddar cheese. Firstly, for inoculation purposes, the performance of these strains in RSM was investigated. None of the strains performed well in milk (levels of only 107 to 108 CFU/ml were achieved), and all were subsequently found to be nonproteolytic or only weakly proteolytic (data not shown). Thus, when 0.1 to 0.2% inocula of these L. salivarius and L. paracasei strains were used as starter adjuncts, relatively low levels (104 to 105 CFU/ml of milk) were obtained in the experimental vats during cheese manufacture. All adjunct lactobacilli were found to survive during cheesemaking, and acid development during the process was unaffected by the presence of these strains.
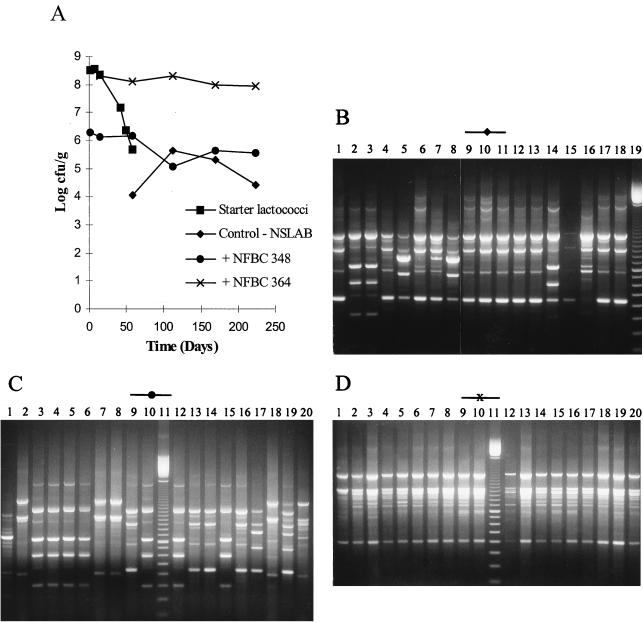
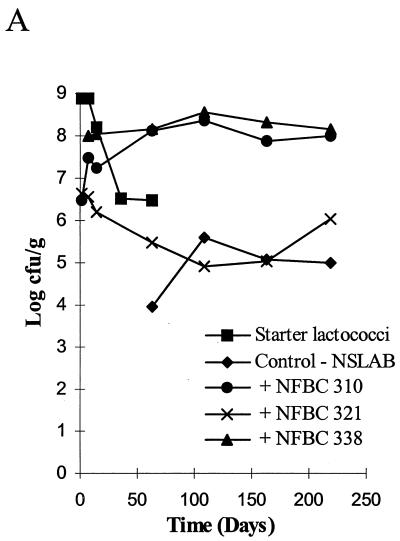
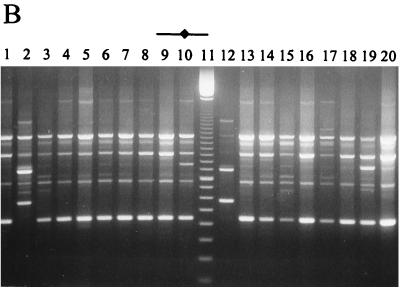
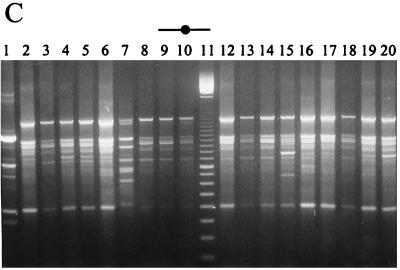
Microbiology of the Cheddar cheeses during ripening and the RAPD-PCR profiles of Lactobacillus isolates from the ripened cheeses are shown in Fig. 2 to 4. It should be noted that day 0 (Fig. 2A, 3A, and 4A) represents the day of cheese manufacture, while lanes 1 (Fig. 2C and D, Fig. 3C to E, and Fig. 4B) represent the RAPD-PCR profile of the probiotic strain added to the experimental cheeses during manufacture. Results demonstrate that cheese made with NFBC 364 and NFBC 338 L. paracasei adjuncts (Fig. 2A and 3A) contained high levels of these probiotic strains after 8 months of ripening; with final counts of 9.2 × 107 and 1.4 × 108 CFU/g achieved, respectively. This was confirmed following comparison of the RAPD-PCR fingerprints generated for L. paracasei NFBC 364 and NFBC 338 (respectively, Fig. 2D and 3E, lanes 1) and those obtained for lactobacilli isolated from the cheeses (Fig. 2D and 3E, lanes 2 to 10 and 12 to 20) which were found to be identical. In contrast, although lactobacilli grew to high levels (108 CFU/g) in the cheese to which strain NFBC 310 was added (Fig. 3A) and subsequently remained at this level throughout ripening, these lactobacilli (Fig. 3C, lanes 2 to 10 and 12 to 20) were identified by RAPD-PCR as NSLAB. Levels of lactobacilli in cheeses with L. salivarius adjuncts NFBC 348 and NFBC 321 (Fig. 2A and 3A) declined to 1.2 × 105 and 8.6 × 104 CFU/g, respectively, after 4 months of ripening, although these levels did increase slightly to reach final levels of 3.5 × 105 and 1.1 × 106 CFU/g, respectively, after 8 months of ripening. Interestingly, the genetic fingerprints of isolates taken from each of these cheeses after 6 months revealed that these lactobacilli were predominantly NSLAB (Fig. 2C and 3D, respectively). Thus, the L. salivarius strains used in this study did not maintain viability in Cheddar cheeses during ripening. Furthermore, many of the NSLAB isolated from the cheeses in which the adjunct strain declined and from the control cheese to which no probiotic adjunct was added yielded identical PCR-generated DNA fingerprints (compare Fig. 3B, lanes 3 to 9 with, Fig. 3D, lanes 12 to 18). This suggests that the DNA was obtained from identical strains and shows a predominance of certain Lactobacillus strains in the NSLAB population of these cheeses.
FIG. 2.
(A) Survival of lactobacilli and starter during cheese ripening in trial 1 laboratory-scale Cheddar cheeses manufactured under microbiologically controlled conditions. (B to D) RAPD-PCR profiles of a representative number of Lactobacillus isolates from each of the cheeses. Lanes 1 (C and D) show the RAPD profile of each probiotic Lactobacillus strain added to the cheese at manufacture, while a 100-bp ladder is shown in lane 19 (B) or 11 (C and D) and all other lanes show RAPD profiles of Lactobacillus isolates from 6-month (180-day)-ripened cheeses.
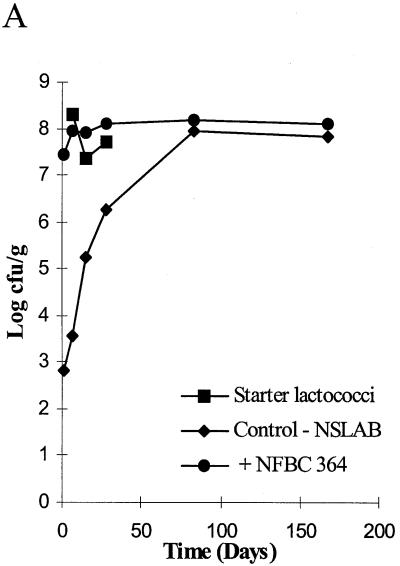
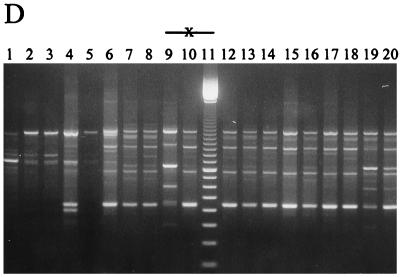
FIG. 4.
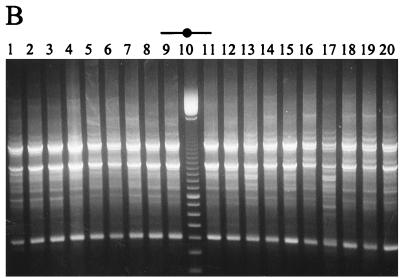
(A) Survival of lactobacilli and starter during cheese ripening in trial 3 pilot-scale Cheddar cheeses. (B) RAPD-PCR profiles of a representative number of Lactobacillus isolates from vat 2 cheese to which L. paracasei NFBC 364 was added during manufacture. Lane 1 shows the RAPD profile of the added strain. A 100-bp ladder is shown in lane 10, while lanes 2 to 9 and 11 to 20 show RAPD profiles of Lactobacillus isolates from the 6-month (180-day)-ripened cheese.
FIG. 3.
(A) Survival of lactobacilli and starter during cheese ripening in trial 2 laboratory-scale Cheddar cheeses manufactured under microbiologically controlled conditions. (B to E) RAPD-PCR profiles of a representative number of Lactobacillus isolates from each of the cheeses. Lanes 1 (C to E) show the RAPD profile of each probiotic Lactobacillus strain added to the cheese at manufacture, while a 100-bp ladder is shown in lanes 11 (B to E) and all other lanes show RAPD profiles of Lactobacillus isolates from 6-month (180-day)-ripened cheeses.
Subsequently, pilot-scale cheese trials were performed, in which only the two L. paracasei strains, NFBC 338 and NFBC 364, that survived to high levels in the laboratory-scale trials were incorporated into Cheddar cheese. These strains were added at inocula of 1.7 × 105 and 8.9 × 105 CFU/ml of cheese milk, respectively. Thereafter, both NFBC 364 (Fig. 4A) and NFBC 338 grew in the cheese from initial numbers of 2.7 × 107 and 1.1 × 107 CFU/g, respectively, to reach levels between 2.9 × 108 and 1.5 × 108 CFU/g after 3 months of ripening, and viability was sustained at this level for the remainder of the ripening period. These results were confirmed for both pilot-scale trials by RAPD-PCR of cheese isolates as described above. Only the data for isolates from cheese manufactured with NFBC 364 are shown (Fig. 4B).
Taken together, the data from the laboratory- and pilot-scale cheese trials provide molecularly based evidence for the persistence in Cheddar cheese of strains selected for their potential as probiotics. In order to appreciate the beneficial effects of probiotic foods, it has been proposed that viable probiotic organisms should be present at levels of at least 107 viable cells per gram or milliliter of product (18). The probiotic-containing cheeses developed as part of the present study contained levels up to 108 CFU/g of cheese, thus satisfying these criteria for a probiotic food product.
It should also be noted that lactococcal starter numbers in the control cheeses of all trials showed a typical decline during the ripening period (Fig. 2A, 3A, and 4A). However, due to the growth of lactobacilli on the LM17 medium used to enumerate these starter organisms, it was possible to monitor starter organisms only in the cheeses to which no adjunct lactobacilli had been added, and then it was possible only in the early stages of ripening.
Although lactobacilli have previously been added as adjuncts to Cheddar cheese and have subsequently been found to remain at high levels throughout maturation (4, 26, 28), no definitive identification method was used in these previous studies to distinguish the adjunct lactobacilli from the natural flora of the cheese. However, in our study RAPD-PCR analysis, when used as an identification method, was capable of determining that probiotic L. paracasei strains grew and maintained high viability (108 CFU/g) in cheese, while the particular L. salivarius adjunct strains used did not appear to be suited for such an application. Furthermore, in the present study, survival of these probiotic Lactobacillus strains at high numbers in Cheddar cheese was achieved with a relatively small inoculum (0.1 to 0.2%) in the cheese vat and without altering the cheesemaking process in any way. This was possible because these strains were added as starter adjuncts and were not therefore necessary for acid production during cheesemaking. In a study conducted by Gomes et al. (12), bifidobacteria and L. acidophilus were used as the sole starters in Gouda cheese manufacture, requiring relatively large inocula (3%) of both strains and adaptation of cheesemaking technology. Thus, our approach for incorporation of probiotic organisms into Cheddar cheese offers certain advantages to industry; no alteration of existing cheesemaking technology and low cost due to the low inoculum required.
Cheese compositional analysis.
The composition of the cheese was generally found to be within the range typical for Cheddar (Table 1). Atypical values for salt in moisture, fat, and pH were obtained for some of the trial 1 cheeses, which reflects the difficulties in controlling the cheesemaking parameters (i.e., temperature) at laboratory scale. In contrast, all the compositional analysis values obtained for the pilot-scale trials were generally within the typical range for Cheddar. Thus, the comparable values observed for control and experimental cheeses (Table 1) indicate that incorporation of probiotic lactobacilli as starter adjuncts, and their survival at high numbers, had no direct effect on cheese composition.
TABLE 1.
Composition and sensory evaluation of control and probiotic Cheddar cheesesa
| Cheese | % Moisture | % Salt | % S/Mb | % Fat | % Protein | pH | Flavor/aroma scorec | Body/texture scored |
|---|---|---|---|---|---|---|---|---|
| Trial 1 | ||||||||
| Control | 38.28 | 1.53 | 4.0 | 31.5 | 26.33 | 5.4 | 38 | 33 |
| NFBC 348 | 38.24 | 1.70 | 4.45 | 32.0 | 26.63 | 5.2 | 38 | 33 |
| NFBC 364 | 39.89 | 1.23 | 3.08 | 31.0 | 25.79 | 5.3 | 39 | 32 |
| Trial 2 | ||||||||
| Control | 37.48 | 1.64 | 4.38 | 33.0 | 26.5 | 5.2 | 37 | 33 |
| NFBC 310 | 35.73 | 1.81 | 5.07 | 33.0 | 26.99 | 5.1 | 39 | 32 |
| NFBC 321 | 37.22 | 1.61 | 4.33 | 33.0 | 27.27 | 5.1 | 38 | 33 |
| NFBC 338 | 38.01 | 1.71 | 4.55 | 33.0 | 27.27 | 5.1 | 38 | 32 |
| Trial 3 | ||||||||
| Control | 34.88 | 2.05 | 5.88 | 34.5 | 26.17 | 5.4 | 39 | 33 |
| NFBC 364 | 35.14 | 1.80 | 5.12 | 35.0 | 26.42 | 5.3 | 38 | 33 |
Compositions are presented as means of results of duplicate analyses conducted at 14 days. Sensory evaluation was conducted at 6 months. Probiotic cheeses contained the indicated Lactobacillus adjunct strains.
Salt in moisture.
Maximum score = 45; minimum commercial score = 38.
Maximum score = 40; minimum commercial score = 31.
Sensory evaluation.
With the exception of the control cheese of trial 2, all cheeses could be described as commercial grade with respect to sensory criteria, after 6 months of ripening, having achieved minimum scores of 38 and 31 for flavor/aroma and body/texture, respectively (Table 1). Lactobacillus adjuncts have previously been reported to improve Cheddar cheese flavor (4, 26, 28), although in some cases they were responsible for flavor defects (24, 32). In this study, laboratory-scale cheeses with high levels of Lactobacillus adjuncts were found to have flavor and texture comparable to those of control cheeses, indicating that addition of these probiotic lactobacilli to Cheddar cheese had no adverse effects on sensory criteria. Furthermore, when production was repeated on a larger scale, sensory parameters remained unaffected by the presence of high levels of these adjuncts.
Proteolysis in laboratory-scale Cheddar cheeses.
Urea-PAGE patterns of whole cheese samples after 8 months of ripening were typical for Cheddar and did not show any differences in the extents of primary proteolysis between the control cheeses and those manufactured with adjunct lactobacilli (data not shown). Similarly, others (26, 28) have shown that adjunct lactobacilli have no effect on PAGE electophoretograms, which is not surprising, as proteolysis at this level is due to the action of plasmin and rennet and is not influenced by the activity of cheese flora (29). The molecular mass distribution of peptides in water-soluble extracts from the cheeses (as measured by size exclusion HPLC) serves as a further indication of the extent of proteolysis in the cheeses during ripening; the greater the extent of proteolysis, the higher the level of low-molecular-mass peptides generated. After 6 months of ripening, these low-molecular-mass peptides (<500 Da) were found to have accumulated to high levels in all cheeses (data not shown). Moreover, similar levels were detected in the control and experimental cheeses, even in those cheeses which had high levels of survival of L. paracasei NFBC 338 and 364 adjuncts, indicating that the extent of proteolysis in the cheeses, as demonstrated by generation of small peptides, was not affected by adjunct addition.
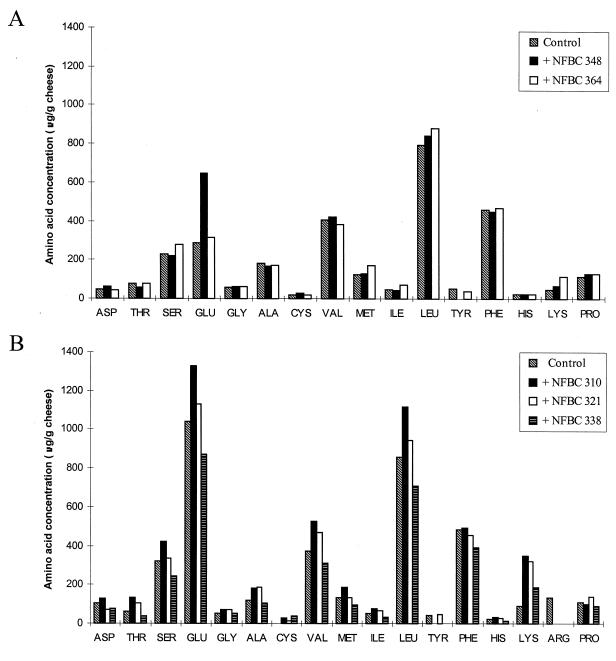
However, higher levels of individual FAA were detected in some of the cheeses made with added lactobacilli, after 6 months of ripening (Fig. 5). Most notably, concentrations of glutamic acid, methionine, leucine, and lysine (trial 1) (Fig. 5A) in addition to valine (trial 2) (Fig. 5B) were higher in the cheeses made with added lactobacilli than in the control cheese to which no adjunct had been added. This was found to be true even for the cheeses in which the Lactobacillus adjuncts declined during ripening. This may be accounted for by the release of intracellular peptidases as the organisms died and lysed. Thus, in general the results suggest that the adjunct lactobacilli, whether they survived to high levels or not, did contribute to proteolysis in the cheese, as demonstrated by increased formation of FAA. Similarly, addition of both lactobacilli and bifidobacteria to Cheddar cheese has previously been shown to increase proteolysis at the level of FAA formation (4, 26, 28, 32). In contrast, in a study by Dinakar and Mistry (10) bifidobacteria were found not to alter proteolysis in Cheddar cheese; however, FAA were not measured in that study.
FIG. 5.
Concentration of individual FAA in water-soluble extracts of 6-month-old control and experimental Cheddar cheeses of trial 1 (A) and trial 2 (B).
Conclusions.
The results of the present study demonstrate that probiotic L. paracasei strains incorporated into Cheddar cheese proved particularly suitable as starter adjuncts. These strains were found to grow and proliferate to high cell numbers in cheese over 8 months of ripening, even when added at a relatively small inoculum. Furthermore, RAPD-PCR proved extremely useful in distinguishing these probiotic adjuncts from NSLAB. Moreover, the results from the control cheese suggest the predominance of certain NSLAB strains. While proteolysis during cheese ripening was influenced by the adjuncts at the level of FAA formation, cheese flavor, texture, and appearance were not affected. Incorporation of these probiotic adjuncts to Cheddar cheese, as conducted in this study, can be achieved without alteration of the cheesemaking technology, thus making this system attractive for commercial exploitation. The results of the present study indicate that Cheddar cheese offers potential as an effective vehicle for delivery of these strains to the consumer.
ACKNOWLEDGMENTS
The technical assistance of Finbar Drinan, Helen Slattery, and Eddie Mulholland is gratefully acknowledged. We thank Pat Fenton, Dairygold, Mitchelstown, Co. Cork, Ireland, for sensory analyses.
This work was supported by the European Research and Development Fund. G.G. was supported by a Teagasc Walsh Fellowship.
REFERENCES
- 1.Andrews A T. Proteinases in normal bovine milk and their action on the caseins. J Dairy Res. 1983;50:45–55. doi: 10.1017/s0022029900032519. [DOI] [PubMed] [Google Scholar]
- 2.Blakesley R W, Boezi J A. A new staining technique for proteins in polyacrylamide gels using Coomassie Brilliant Blue G250. Anal Biochem. 1977;82:580–581. doi: 10.1016/0003-2697(77)90197-x. [DOI] [PubMed] [Google Scholar]
- 3.Bourlioux R, Pochart P. Nutritional and health properties of yogurt. World Rev Nutr Diet. 1988;56:217–258. doi: 10.1159/000416229. [DOI] [PubMed] [Google Scholar]
- 4.Broome M C, Krause D A, Hickey M W. The use of non-starter lactobacilli in Cheddar cheese manufacture. Aust J Dairy Technol. 1990;45:67–73. [Google Scholar]
- 5.Chapman H R, Sharpe M E. Microbiology of cheese. In: Robinson R K, editor. Dairy microbiology. London, United Kingdom: Applied Science Publishers; 1981. pp. 157–243. [Google Scholar]
- 6.Christiansen P S, Edelsten D, Kristiansen J R, Nielsen E W. Some properties of ice cream containing Bifidobacterium bifidum and Lactobacillus acidophilus. Milchwissenschaft. 1996;51:502–504. [Google Scholar]
- 7.Coakley M, Ross R P, Donnelly D. Application of the polymerase chain reaction to the rapid analysis of brewery yeast strains. J Inst Brew. 1996;102:349–354. [Google Scholar]
- 8.Collins, J. K., and G. Thornton. Selection of probiotic strains for human applications. Int. Dairy J., in press.
- 9.de Man J C, Rogosa M, Sharpe M E. A medium for the cultivation of lactobacilli. J Appl Bacteriol. 1960;23:130–135. [Google Scholar]
- 10.Dinakar P, Mistry V V. Growth and viability of Bifidobacterium bifidum in Cheddar cheese. J Dairy Sci. 1994;77:2854–2864. doi: 10.3168/jds.S0022-0302(94)77225-8. [DOI] [PubMed] [Google Scholar]
- 11.Fuller R. Probiotic foods. Current use and future developments. Int Food Ingred. 1993;3:23–26. [Google Scholar]
- 12.Gomes A M P, Malcata F X, Klaver F A M, Grande H G. Incorporation and survival of Bifidobacterium sp. strain Bo and Lactobacillus acidophilus strain Ki in a cheese product. Neth Milk Dairy J. 1995;49:71–95. [Google Scholar]
- 13.Hoffman C S, Winston F. A ten-minute DNA preparation from yeast efficiently releases autonomous plasmids for transformations of Escherichia coli. Gene. 1987;57:267–272. doi: 10.1016/0378-1119(87)90131-4. [DOI] [PubMed] [Google Scholar]
- 14.Holcomb J E, Frank J F, McGregor J U. Viability of Lactobacillus acidophilus and Bifidobacteria bifidum in soft-serve frozen yogurt. Cult Dairy Prod J. 1991;30:4–5. [Google Scholar]
- 15.International Dairy Federation. Cheese and processed cheese. Determination of chloride content: potentiometric titration method. International Dairy Federation standard 88. Brussels, Belgium: International Dairy Federation; 1979. [Google Scholar]
- 16.International Dairy Federation. Determination of the total solids content (cheese and processed cheese). International Dairy Federation standard 4A. Brussels, Belgium: International Dairy Federation; 1982. [Google Scholar]
- 17.Irish Standard. Determination of the percentage fat in cheese. Irish standard 69. Dublin, Ireland: Institute for Industrial Research and Standards; 1955. [Google Scholar]
- 18.Ishibashi N, Shimamura S. Bifidobacteria: research and development in Japan. Food Technol. 1993;46:126–135. [Google Scholar]
- 19.Jordan K N, Cogan T M. Identification and growth of non-starter lactic acid bacteria in Irish Cheddar cheese. Ir J Agric Food Res. 1993;32:47–55. [Google Scholar]
- 20.Kandler O, Weiss N. Regular, non-sporing gram-positive rods. In: Sneath P H A, et al., editors. Bergey’s manual of determinative bacteriology. Vol. 2. Baltimore, Md: The Williams & Wilkins Co.; 1989. p. 1208. [Google Scholar]
- 21.Kosikowski F. Cheese and fermented milk foods. Ann Arbor, Mich: Edwards Brothers Inc.; 1977. pp. 228–260. [Google Scholar]
- 22.Kuchroo C N, Fox P F. Soluble nitrogen in Cheddar cheese: comparison of extraction procedures. Milchwissenschaft. 1982;37:331–335. [Google Scholar]
- 23.Laroia S, Martin J H. Effect of pH on survival of Bifidobacterium bifidum and Lactobacillus acidophilus in frozen fermented dairy desserts. Cult Dairy Prod J. 1991;26:13–21. [Google Scholar]
- 24.Lee B H, Laleye L C, Simard R E, Munsch M H, Holley R A. Influence of homofermentative lactobacilli on the microflora and soluble nitrogen components in Cheddar cheese. J Food Sci. 1990;55:391–397. [Google Scholar]
- 25.Lee Y K, Salminen S. The coming of age of probiotics. Trends Food Sci Technol. 1995;6:241–245. [Google Scholar]
- 26.Lynch C M, McSweeney P L H, Fox P F, Cogan T M, Drinan F D. Manufacture of Cheddar cheese with and without adjunct lactobacilli under controlled microbiological conditions. Int Dairy J. 1996;6:851–867. [Google Scholar]
- 27.Marth E H. Microbiological and chemical aspects of Cheddar cheese ripening. A review. J Dairy Sci. 1963;46:869–890. [Google Scholar]
- 28.McSweeney P L H, Walsh E M, Fox P F, Cogan T M, Drinan F D, Castelo-Gonzalez M. A procedure for the manufacture of Cheddar cheese under controlled bacteriological conditions and the effect of adjunct lactobacilli on cheese quality. Ir J Agric Food Res. 1994;33:183–192. [Google Scholar]
- 29.O’Keeffe R B, Fox P F, Daly C. Contribution of rennet and starter proteases to proteolysis in Cheddar cheese. J Dairy Res. 1976;43:97–107. [Google Scholar]
- 30.Peterson S D, Marshall R T. Non-starter lactobacilli in Cheddar cheese: a review. J Dairy Sci. 1990;73:1395–1410. [Google Scholar]
- 31.Pot B, Hertel C, Ludwig W, Descheemaeker P, Kersters K, Schleifer K H. Identification and classification of Lactobacillus acidophilus, L. gasseri, and L. johnsonii strains by SDS-PAGE and rRNA-targeted oligonucleotide probe hybridization. J Gen Microbiol. 1993;139:513–517. doi: 10.1099/00221287-139-3-513. [DOI] [PubMed] [Google Scholar]
- 32.Puchades R, Lemieux L, Simard R D. Evolution of free amino acids during the ripening of Cheddar cheese containing added lactobacilli strains. J Food Sci. 1989;54:885–888. [Google Scholar]
- 33.Reuter G. Bifidobacteria as components of yogurt-like products. Bifidobacteria Microflora. 1990;9:107–118. [Google Scholar]
- 34.Rogosa M, Mitchell J A, Wiseman R T. A selective medium for the isolation and enumeration of oral and fecal lactobacilli. J Bacteriol. 1951;62:132–133. doi: 10.1128/jb.62.1.132-133.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sanders M E. Lactic acid bacteria as promoters of human health. In: Goldberg I, editor. Functional foods: designer foods, pharmafoods and neutraceuticals. New York, N.Y: Chapman and Hall; 1994. pp. 294–322. [Google Scholar]
- 36.Sanders M E, Walker D C, Walker K M, Aoyama K, Klaenhammer T R. Performance of commercial cultures in fluid milk applications. J Dairy Sci. 1996;79:943–955. doi: 10.3168/jds.S0022-0302(96)76445-7. [DOI] [PubMed] [Google Scholar]
- 37.Shah N P. Bifidobacteria: characteristics and potential for application in fermented milk products. Milchwissenschaft. 1997;52:16–20. [Google Scholar]
- 38.Tamime A Y, Marshall V M, Robinson R K. Microbiological and technological aspects of milks fermented by bifidobacteria. J Dairy Res. 1995;62:151–187. doi: 10.1017/s002202990003377x. [DOI] [PubMed] [Google Scholar]
- 39.Terzaghi B E, Sandine W E. Improved medium for lactic streptococci and their bacteriophages. Appl Microbiol. 1975;29:807–813. doi: 10.1128/am.29.6.807-813.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trepanier G, Simmard R E, Lee B H. Lactic acid bacteria in relation to accelerated maturation of Cheddar cheese. J Food Sci. 1991;56:1238–1240. [Google Scholar]