Abstract
C4b-binding protein (C4BP) is a fluid-phase complement inhibitor that prevents uncontrolled activation of the classical and lectin complement pathways. As a complement inhibitor, C4BP also promotes apoptotic cell death, and is hijacked by microbes and tumors for complement evasion. Though initially characterized for its role in complement inhibition, there is an emerging recognition that C4BP functions in a complement-independent manner to promote cell survival, protect against autoimmune damage, and modulate the virulence of microbial pathogens. In this Brief Review, we summarize the structure and functions of human C4BP, with a special focus on activities that extend beyond the canonical role of C4BP in complement inhibition.
Keywords: Complement, C4b-binding protein, apoptosis, cancer, autoimmunity, microbial pathogenesis
Introduction
Complement is a system of proteins that aid or “complement” immune clearance of pathogens. Complement components are primarily synthesized by hepatocytes and secreted into circulation, but some components are also synthesized locally in tissues by immune cells, endothelial cells, and epithelial cells (1). Complement is activated through three distinct pathways (reviewed in (2)): the classical pathway, the mannose-binding lectin (MBL) pathway, and the alternative pathway. Regardless of the pathway of activation, each converges on the formation of the C3 convertase, an enzyme that cleaves C3 and C5 to produce effector molecules of complement.
Complement activation serves three primary functions in the control of infection. The first is opsonization, in which complement components coat the surface of the pathogen and tag it for recognition by phagocytes via their cognate complement receptors. Second is the production of the anaphylatoxins C3a and C5a, which creates a local inflammatory response that recruits leukocytes to the site of infection and promotes leukocyte activation via their cognate receptors. Third is the assembly of terminal complement components C5b-C9, which insert into lipid bilayers to form a cytolytic pore called the membrane attack complex (MAC) (3, 4). Direct bacteriolysis via MAC is a major method of complement control for Gram-negative bacteria and viruses (5, 6). On the other hand, Gram-positive bacteria, as well as fungi, are relatively resistant to direct cytolysis via a thick cell wall, and complement-mediated control of these organisms is primarily through opsonization and chemoattraction of leukocytes, which support phagocytic clearance (7–9).
Tightly regulated complement activation is important for human health and fitness. The complement cascade is intrinsically restrained by requiring the sequential cleavage of inactive precursors to generate effector molecules. As a second layer of control, membrane-bound and soluble proteinaceous complement inhibitors protect host cells from uncontrolled activation and subsequent damage.
C4b-binding protein (C4BP) is a prominent soluble regulator of the classical and mannose-binding lectin (MBL) pathways of complement activation. Beyond its canonical function in complement inhibition, C4BP has significant roles in other realms of human biology, some of which are complement-independent. In this Brief Review, we will summarize our current understanding and identify future areas for investigation for five roles of C4BP: 1) complement inhibition, 2) microbial complement resistance, 3) complement-independent modulation of microbial pathogenesis, 4) regulation of cell clearance and survival, and 5) control of excessive inflammation in cancer and chronic disease.
Complement inhibition by C4BP
Human C4BP is a glycoprotein complex present abundantly (~200 μg/mL) in healthy human serum (10). C4BP is mainly synthesized in the liver where it is secreted by hepatocytes into the bloodstream, but is also expressed in pancreatic islet cells and lung alveolar cells (11–13). While genetic deficiencies in some complement components have been described, there are no reported human deficiencies in C4BP, implying its importance to human biology (14).
C4BP exists as a multimer of C4BP α chains and a C4BP β chain, covalently linked by disulfide bonds at their C-termini (15–17). The assembly of these chains results in a structure, which when resolved by electron microscopy, has been described as spider- or octopus-like (16) (see Figure 1). Each α chain is 70 kDa and composed of 8 internal complement control protein (CCP) domain repeats, while the β chain is 45 kDa and composed of 3 CCP domains. CCP domains are numbered from most distal to the most proximal to the C-terminal disulfide bonds. Four C4BP isoforms have been reported: α7β1, α7β0, α6β1, and α6β0. The 570 kDa multimer α7β1 (7 α chains and 1 β chain) constitutes 80% of the C4BP complexes found in plasma. All β chain-containing C4BP isoforms exist in a high affinity complex with Protein S, a vitamin-K dependent anticoagulant (18–20), which interacts hydrophobically with the β chain CCP1 (21, 22).
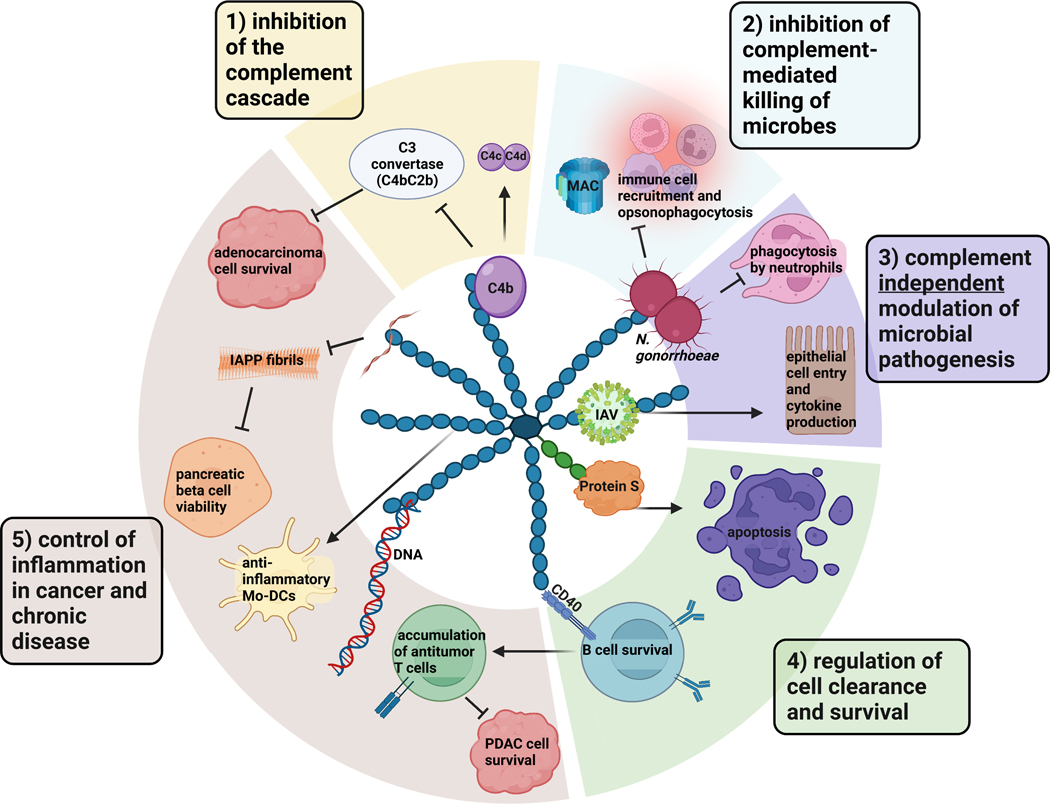
Figure 1. Functions of human C4BP.
C4BP, shown here as the major isoform composed of 7 α chains (blue) and 1 β chain (green) linked by disulfide bonds at their C termini, has diverse functions. 1) Canonically, C4BP inhibits the activation of the complement cascade by binding C4b, degrading C4b, and accelerating the decay of the C3 convertase to protect host tissues from uncontrolled complement activation. 2) Microbes hijack C4BP by binding it to their surfaces, where it functions to protect them from complement lysis and clearance by phagocytes, and 3) can modulate complement-independent microbial pathogenesis 4) C4BP promotes cell survival by engaging CD40 and controlled cell death via apoptosis. 5) C4BP controls inflammation in autoimmune diseases and cancer. IAPP = islet amyloid polypeptide; MAC = membrane attack complex; Mo-DC = monocyte-derived dendritic cell; PDAC = pancreatic ductal adenocarcinoma
As an acute-phase reactant, C4BP is transcriptionally upregulated during systemic reaction to infection or tissue injury (23). During the acute-phase response in humans, total plasma concentration of C4BP increases as much as 4-fold, and the C4BP isoforms lacking the β chain increase relative to those containing the β chain (24). The equilibrium between free Protein S (30%) and C4BP-bound Protein S (70%) is important for coagulation pathway homeostasis, and the preferential upregulation of the α7β0 isoform of C4BP during acute-phase conditions helps maintain this balance (24).
Canonically, C4BP inhibits complement activation at the level of the C3 convertase in three ways. First, as its name suggests, C4BP binds to fluid phase and cell surface-bound C4b (25) to prevent formation of the C3 convertase C4bC2b, which in the classical and mannose binding lectin (MBL) pathways requires C4b as a subunit. 4–5 C4b molecules are estimated to bind a single C4BP molecule at once (25). Second, once bound to C4b, C4BP acts as a cofactor for Factor I, which inactivates C4b by cleaving it. Cleavage of C4b generates C4c and C4d, which are functionally inactive, preventing convertase reconstitution. CCPs 1–3 of the C4BP α chain mediate binding to C4b and are required for cofactor activity (17, 26, 27). Third, C4BP accelerates the decay of the C3 convertase by destabilizing and dissociating C2b from the complex (28). Since all of these inhibitory activities are directed at the decay of the C3 convertase C4bC2b, C4BP inhibitory capacity is limited to the classical (28) and MBL (29) pathways in which this convertase is formed.
Exploitation of C4BP for microbial complement resistance
Complement evasion is an important pillar in the coevolution of microbes with humans, as nearly all successful human pathogens have developed strategies to circumvent complement killing (30). One such strategy is capturing and binding human C4BP to a microbial surface ligand to resist complement. Though some microorganisms bind sites on C4BP that overlap with that of C4b, the 7α C4BP multimer remains functionally active to bind and cleave C4b. Over 30 publications (reviewed elsewhere (12, 31); See Table 1) describe C4BP as a mechanism of complement resistance for microbes across taxonomical kingdoms and at a variety of infectious sites. For these microbes, the ability to bind C4BP often correlates with their pathogenic potential (reviewed in (31)). In this section, we highlight the recent developments in pathogens’ complement resistance mediated by C4BP.
Table 1:
C4BP-binding pathogens.
| Pathogen | C4BP-binding ligand(s) | Binding domain(s) on C4BP | |
|---|---|---|---|
| Bacterial | Bordetella pertussis | FHA(32) | CCP 1–2(32) |
|
| |||
|
Borrelia afzelii
|
43 kDa uncharacterized protein(33) | Unknown | |
|
Borrelia burgdorferi
| |||
| Borrelia garinii | |||
|
| |||
| Borrelia recurrentis | CihC(34) | Unknown | |
|
| |||
| Escherichia coli | OmpA(35) | CCP3(35) | |
|
| |||
| Nontypeable Haemophilus influenzae | Omp P5(36) | CCPs 2, 7(37) | |
|
| |||
| Leptospira interrogans | LigA, LigB, LcpA(38, 39) | CCPs 7, 8(39) | |
|
| |||
| Moraxella catarrhalis | UspA1, UspA2(40) | CCPs 2, 5, 7(40) | |
|
| |||
| Neisseria gonorrhoeae | Porin (Por1A, Por1B)(41) Pili(42) |
Porin- CCP1(41) Pili – CCPs 1, 2(42) |
|
|
| |||
| Neisseria meningitidis | PorA(43) | CCPs 2, 3, 6(43) | |
|
| |||
| Porphyromonas gingivalis | HrgpA(44) | CCPs 1, 6–7(44) | |
|
| |||
| Prevotella intermedia | Unknown | Unknown | |
|
| |||
| Salmonella enterica | Rck(45) | CCPs 7, 8(45) | |
|
| |||
| Staphylococcus aureus | SdrE/Bbp(46) | Unknown | |
|
| |||
| Streptococcus pneumoniae | LytA(47), PspA(48), PspC(49), PepO(50), Enolase(51) |
PspC – CCPs 2,3(49) PepO – CCP8(50) Enolase – CCPs 1,2,8(51) LytA, PspA – unknown |
|
|
| |||
| Streptococcus pyogenes | M proteins (M5(52), M22(53, 54), Protein H(55), M4(56)) |
Protein H(55), M4(56) - CCPs1, 2 | |
|
| |||
| Yersinia enterocolitica | YadA, Ail(57) | YadA – CCP 1–2(57) Ail – CCP 1–3(57) |
|
|
| |||
| Yersinia pestis | Ail(58) | CCPs 6, 8(58) | |
|
| |||
| Yersinia pseudotuberculosis | Ail(59) | CCPs 6, 7, 8(59) | |
|
| |||
| Viral | Flaviviruses | NS1(60) | CCPs 2,4,5,8(60) |
|
| |||
| Influenza A Virus | HA, NA, M1(61) | CCPs 4–5, 7–8(61) | |
|
| |||
| Eukaryotic | Aspergillus. fumigatus | Enolase(62) | Unknown |
|
| |||
| Candida albicans | Pra1(63) | Unknown ligand – CCPs 1, 2(64) Pra1 – CCPs 4, 7, 8(63) |
|
|
| |||
| Loa loa microfilariae | Unknown | Unknown | |
|
| |||
| Plasmodium falciparum | CSP(65) | CCP 1–2(65) | |
|
| |||
| Toxoplasma gondii | Unknown | Unknown | |
Binding of C4BP to the Gram-positive organism Streptococcus pyogenes (group A Streptococcus, GAS) is mediated by a highly variable N-terminal region of the streptococcal M protein family (52). 90% of M protein family members interact with C4BP(66). One member of the M protein family, Protein H, has antiphagocytic properties, which have been attributed to its ability to bind C4BP and the Fc region of human immunoglobulin (Ig-Fc) (54, 55). Binding to complement inhibitors is important for GAS virulence, as C4BP colocalizes with IgG and Protein H in tissues with necrotizing fasciitis caused by GAS (67), and human C4BP transgenic mice exhibit higher GAS burdens and pro-inflammatory cytokine production with enhanced mortality compared to controls without C4BP (68). Recently, a synergy between C4BP and IgG binding to Protein H has been elucidated. When bound to Protein H, Ig-Fc not only inhibits IgG opsonic activity, but also dimerizes Protein H on the bacterial surface, enabling it to bind more molecules of C4BP than when monomeric (67).
Given that several complement proteins are synthesized in the airway epithelium(69), respiratory tract pathogens including Bordetella pertussis and non-typeable Haemophilus influenzae have evolved strategies to evade complement activity. Recently, the OmpA family outer membrane protein, Omp protein 5 (P5) was identified as a H. influenzae ligand for C4BP (36). Along with its polysaccharide capsule, which also confers protection from complement, P5 expression and C4BP binding correlate with H. influenzae resistance to serum (36).
Neisseria gonorrhoeae has one of the most well-studied complement-evasion strategies involving C4BP. N. gonorrhoeae uses its porin and pili to bind human C4BP on its surface. C4BP exhibits cofactor activity in the inactivation of C4b by Factor I (41), markedly inhibiting complement fixation on N. gonorrhoeae. C4BP binding is strongly correlated with N. gonorrhoeae serum resistance, with isolates and mutants that cannot bind C4BP showing sensitivity to serum-mediated lysis (41, 70). The Ram and Blom groups are taking advantage of C4BP binding by N. gonorrhoeae to develop new gonorrhea therapeutics. They engineered a chimeric molecule with C4BP α chain CCPs 1 and 2 fused to the constant portion of IgM, which was multimerized to a hexamer (C4BP-IgM), and found it outcompeted native C4BP binding to the gonococcal surface (71). The C4BP-IgM chimera increases complement activation and subsequent serum bactericidal activity against strains MS11, 1291, 15253, FA1090, and 20 of 26 tested clinical isolates, and it enhances clearance of N. gonorrhoeae from the genital tract of mice that are transgenic for human C4BP (71). C4BP-IgM in conjunction with normal human serum also increased the sensitivity of laboratory strain FA1090 to antibiotics and restored sensitivity to azithromycin for two azithromycin-resistant gonococcal strains, by promoting complement activation, pore formation, and antibiotic entry into the bacterial cell (72). While a similarly generated C4BP-IgG fusion binds gonococci, it does not outcompete native C4BP (71). These studies suggest that C4BP-IgM and antibiotics may be used synergistically to successfully combat drug-resistant gonorrhea.
Flaviviruses (reviewed in (73)) and eukaryotic pathogens also exploit C4BP for complement resistance. The nonstructural protein NS1 from Dengue, West Nile, and yellow fever viruses is displayed on the surface of infected cells and also released into solution. NS1 binds C4BP in solution and recruits it back to the plasma membrane of the infected cell. Binding of C4BP inhibits complement activation on virions and infected cells, allowing evasion of complement control(60). Recent studies show that the opportunistic fungus Aspergillus fumigatus binds C4BP via its enolase, protecting it from complement activation (62, 64). Binding of C4BP for complement evasion also extends to protozoan parasites with primarily intracellular lifestyles that have brief but critical extracellular phases in the blood early in infection. The sporozoite stage of the Plasmodium falciparum parasite resists classical complement activation induced by malarial hyperimmune IgG by binding C4BP via the major surface circumsporozoite protein (CSP) (65). Toxoplasma gondii reduces MAC formation on its surface by binding C4BP and the analogous alternative pathway regulator Factor H(74). The contribution of C4BP to T. gondii survival in vivo, or the ability of C4BP and Factor H to work cooperatively on the surface of the T. gondii, remain open questions (74).
The apparent convergent evolution of diverse C4BP binding ligands and the evolutionary distant pathogens to which they belong (Table 1) underscores the importance of complement resistance via C4BP to microbial pathogenesis.
Complement cascade-independent modulation of microbial pathogenesis
C4BP has recently been implicated in two complement-independent roles related to the interaction of infectious organisms and host cells. Varghese and colleagues reported that C4BP defends against Influenza A virus subtype H1N1 (IAV) without relying on regulation of complement (61). IAV infects through oral or nasal cavities, then hemagglutinin binds to sialic acids in the lung epithelium, where viral particles are endocytosed. C4BP binds to the IAV envelope proteins hemagglutinin, neuraminidase, and matrix protein 1, interactions that mapped to CCP domains 4, 5, 7, and 8. Binding C4BP inhibited the entry of H1N1 pseudotyped particles into lung epithelial cells. Moreover, in line with the concept of C4BP as an anti-inflammatory molecule, C4BP suppressed the pro-inflammatory cytokine storm driven by IAV. IL-12, TNF-α, and NFκB levels were significantly downregulated in C4BP-treated, H1N1-challenged lung epithelial cells. Interestingly, C4BP was also found to bind H3N2 subtype IAV, but promoted viral endocytosis and upregulated proinflammatory cytokine production by lung epithelial cells for this subtype. The authors speculated that the opposing effects of C4BP on H1N1 and H3N2 IAV could be attributed to the structural differences between surface proteins in the two subtypes. Overall, their findings implicate C4BP as an important regulator of IAV replication efficacy by modulating entry into cells, an observation that warrants further study.
Evidence for C4BP functioning independently of complement to benefit a bacterial pathogen was recently uncovered in N. gonorrhoeae. Binding of C4BP to the bacterial surface enhanced its resistance to killing by neutrophils, by limiting neutrophil activation and phagocytosis of N. gonorrhoeae (75). These effects were independent of complement, as shown using serum-free conditions, heat-inactivated serum, and complement component 3 (C3)-depleted serum (75). Curiously, the suppressive activities of C4BP were restricted to N. gonorrhoeae that interacted with neutrophil carcinoembryonic antigen-related cell adhesion molecules (CEACAMs), but not other phagocytic receptors. Given the diverse pathogens that are reported to bind C4BP, many of which encounter phagocytic immune cells during infection (Table 1), it is important to examine if the complement-independent effects of C4BP on N. gonorrhoeae extend to these other organisms. The findings with IAV and N. gonorrhoeae reveal that microbial hijacking of C4BP can affect pathogenesis in ways beyond its long-appreciated role in complement resistance.
Regulation of cell survival and clearance
In serum, C4BP complexes with the vitamin K-dependent glycoprotein and anticoagulant Protein S, which tailors complement deposition and phagocytosis during homeostatic cell clearance to prevent excessive complement activation and inflammation. Protein S binds to negatively charged phospholipids on apoptotic cells including neutrophils (76–78). During early apoptosis, the binding of free Protein S stimulates phagocytosis of apoptotic cells by macrophages (79), which is important for apoptotic cell clearance. During mid to late apoptosis, complement activation is initiated on the apoptotic cell surface, and the C4BP-Protein S complex binds (80). In contrast to Protein S alone, C4BP-Protein S does not promote phagocytosis (81), and instead is thought to benefit the host by replacing the membrane-bound regulators lost during apoptosis, and by limiting C3 and C9 deposition to avoid complement activation, prevent necrosis, and promote controlled cell death via apoptosis (80).
C4BP influences cell survival in a complement-independent manner by interacting with CD40, a receptor found on diverse cell types including antigen presenting cells and epithelial cells. In tonsillar tissue, CD40 on B cells directly binds the α-chain of C4BP in a manner that mimics signaling from CD40 ligand (CD40L) (82). C4BP induces B cell proliferation, upregulates CD54 and CD86 expression, with IL-4 stimulation induces isotype switching to IgE, and promotes signaling through NFκB and p38 MAP kinase (82). However, C4BP binds to a distinct site on CD40 and does not compete with CD40L. In germinal centers where CD40L is not detectable, C4BP may phenocopy CD40 ligation to promote B cell survival. C4BP similarly modulates epithelial cell survival in the bile duct. Here, C4BP complexes with soluble CD40L, preventing it from ligating to CD40, which abrogates apoptosis of cholangiocytes and permits cell survival (83). Since increased apoptosis of cholangiocytes is implicated in diseases such as primary biliary cirrhosis, C4BP is an important down-regulator of cholangiocyte apoptosis, and critical for biliary duct integrity. Thus, C4BP plays a significant role in the regulation of cell survival, in a complement-independent manner.
Modulation of inflammation in cancer and chronic disease
C4BP is a regulator of excessive inflammation in chronic disease, which protects healthy host cells. However, C4BP can also protect tumor cells from host immune cell clearance. In this way, C4BP has both beneficial and detrimental functions relating to inflammation in chronic disease.
The α chain of C4BP exhibits complement-independent antitumor immunity in the pancreas, where its expression is correlated with tumor regression and more favorable outcomes for pancreatic ductal adenocarcinoma (PDAC). In vivo mouse models have shown that, similarly to B cells in the tonsil, the α7β0 form of C4BP (hereafter called C4BPα) binds to CD40 on B cells and other antigen presenting cells in the pancreas. This promotes accumulation of antitumor T cells at the periphery of PDACs (84). In another antitumor capacity, C4BPα is expressed intracellularly in colorectal cancer cells. Expression of C4BPα with certain mutations drives NFκB-dependent apoptosis in the tumor cells, and correlates with improved patient survival outcomes (85).
However, C4BP can also be tumor-promoting when functioning as a complement inhibitor. C4BP protects ovarian adenocarcinoma cells from complement activation by binding the surface via CCP4, and retaining functional cofactor activity for Factor I- mediated inactivation of C4b (86). In another tumor-promoting function, C4BPα expression is induced by the oncogenic Hepatitis B virus. C4BPα binds to the surface of hepatocellular carcinoma cells, thereby protecting the cells from complement-dependent cytotoxicity and promoting hepatoma survival (87).
C4BP also exhibits anti-inflammatory activity in the context of autoimmunity. Extracellular DNA elicits autoantibodies and complement and is implicated in autoimmune disorders such as rheumatoid arthritis and systemic lupus erythematosus (SLE). C4BP binds DNA via a positively charged patch of amino acids in α chain CCP2, capturing free DNA at the necrotic cells surface, and thereby limiting the inflammatory potential of necrosis (88).
C4BP helps limit the development of autoimmunity in SLE, a disease in which uncontrolled inflammation leads to tissue damage, often in the kidney (lupus nephritis). Underscoring the importance of C4BP in SLE, C4BP lacking the β chain (C4BP (β−)) protects lupus-prone mice from nephritis by downregulating immunopathogenic cell infiltration into the kidney (89). Moreover, individuals with active lupus flares have lower levels of C4BP in plasma (90, 91). Additionally, the CCP6 domain of C4BP(β−) reprograms monocyte-derived dendritic cells (Mo-DCs) isolated from lupus nephritis patients from a pro-inflammatory to an anti-inflammatory phenotype, as shown by downregulation of surface activation markers and pro-inflammatory cytokines TNF-α and IL-12 (92, 93). Interestingly, the β chain interferes with this function, but a multimer made of solely CCP6 and the oligomerization domains of C4BP is sufficient to recapitulate the function of limiting of lupus nephritis in animal models (89, 93). Since β chain-deficient forms of C4BP are upregulated during the acute-phase, this may represent a mechanism by which C4BP protects the kidney in the context of SLE.
While most C4BP is produced in the liver, C4BP is also secreted from the islet cells of the pancreas, where it has a cytoprotective effect. Here, C4BP binds to islet amyloid polypeptide (IAPP) (11), a protein that is co-secreted with insulin. For individuals with type 2 diabetes, IAPP leads to the formation of amyloid deposits, which induce inflammasome activation of beta cells (94). C4BP localizes to these deposits and neutralizes the activity of IAPP, which blocks fibrillation of IAPP and prevents IAPP-mediated IL-1β production and IAPP-induced NOD-like receptor protein 3 (NLRP3) inflammasome activation, protecting beta cell function and viability (94). Recently, evidence of C4BP as an inhibitor of NLRP3 inflammasome activation has been extended beyond IAPP. C4BP binds to and co-internalizes into human primary macrophages with monosodium urate crystals and silica (drivers of inflammation in gout and silicosis, respectively), where it prevents NLRP3 inflammasome activation by protecting against lysosomal damage (95). In these ways, C4BP is a critical modulator of inflammation, whether to host benefit or detriment, in chronic conditions of diverse etiology.
Conclusions
C4BP is emerging as a broad-acting molecule with diverse functions (see Figure 1). Its contribution to controlling inflammation can have beneficial or deleterious effects for human health, as C4BP protects healthy host cells, tumor cells, and pathogens alike. In an extension of the well-known ability of C4BP to protect microorganisms from complement-mediated killing, recent reports characterize C4BP as a complement-independent modulator of virulence for pathogenic microorganisms.
Open questions remain about how C4BP may modulate the efficacy of immunotherapies that rely on complement activation to kill malignant cells or pathogens, such as rituximab for B cell malignancies or vaccines for pathogens (96). There is promising evidence that C4BP may be harnessed in modified forms to be used therapeutically, such as C4BP-IgM for treatment of N. gonorrhoeae (71, 72) or M. catarrhalis (97), or as a CCP6 multimer for the treatment of SLE (93). Furthermore, there may be unappreciated complement-independent effects of C4BP for other microbes. For example, C4BP reduces invasion of both H1N1 IAV and N. gonorrhoeae, raising the question of whether C4BP may broadly inhibit interactions of pathogens with host cells. The contributions of C4BP to homeostasis and chronic conditions of infectious and non-infectious etiologies will continue to be uncovered, revealing new perspectives on the balance between complement-dependent and complement-independent activities of C4BP.
Acknowledgements
We apologize to authors whose work we could not include due to space constraints. We thank Evan Lamb for careful reading of this manuscript. Figure 1 was created with BioRender using the University of Virginia School of Medicine’s academic license.
This work was supported by NIH R01AI097312 and R21AI157539 (AKC). LMW was supported in part by NIH T32 AI007046 and the University of Virginia School of Medicine Wagner Fellowship. The funders had no role in the design and conduct of the manuscript, in the collection, analysis, and interpretation of the data reviewed in the manuscript, or in the preparation, review, or approval of the manuscript.
References
- 1.Lubbers R, van Essen MF, van Kooten C, and Trouw LA. 2017. Production of complement components by cells of the immune system. Clin. Exp. Immunol 188: 183–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Merle NS, Church SE, Fremeaux-Bacchi V, and Roumenina LT. 2015. Complement system part I – molecular mechanisms of activation and regulation. Front. Immunol 6:262. DOI: 10.3389/fimmu.2015.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Janeway CA, Travers P, Walport M, and Shlomchik MJ. 2001. The complement system and innate immunity. Immunobiology: The Immune System in Health and Disease. 5th edition. New York: Garland Science. [Google Scholar]
- 4.Merle NS, Noe R, Halbwachs-Mecarelli L, Fremeaux-Bacchi V, and Roumenina LT. 2015. Complement system part II: role in immunity. Front. Immunol 6: 257. DOI: 10.3389/fimmu.2015.00257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heesterbeek DAC, Angelier ML, Harrison RA, and Rooijakkers SHM. 2018. Complement and bacterial infections: from molecular mechanisms to therapeutic applications. J. Innate Immun. 10: 455–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agrawal P, Nawadkar R, Ojha H, Kumar J, and Sahu A. 2017. Complement evasion strategies of viruses: an overview. Front. Microbiol 8: 1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kozel TR 1996. Activation of the complement system by pathogenic fungi. Clin. Microbiol. Rev 9: 34–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laarman A, Milder F, van Strijp J, and Rooijakkers S. 2010. Complement inhibition by gram-positive pathogens: molecular mechanisms and therapeutic implications. J. Mol. Med. (Berlin) 88: 115–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown EJ 1985. Interaction of Gram-positive microorganisms with complement. In Bacteria and Complement: Current Topics in Microbiology and Immunology Loos M, ed. Springer, Berlin, Heidelberg. 159–187. [DOI] [PubMed] [Google Scholar]
- 10.Marcovina SM, Zoppo A, Viganó-D’Angelo S, Di Cola G, and D’Angelo A. 1991. Determination of serum levels of complement component C4b-binding protein: influence of age and inflammation. Int. J. Clin. Lab. Res 21: 171–175. [DOI] [PubMed] [Google Scholar]
- 11.Sjölander J, Byman E, Kulak K, Nilsson SC, Zhang E, Krus U, Westermark GT, Storm P, King BC, Renström E, and Blom AM. 2016. C4b-binding protein protects β-cells from islet amyloid polypeptide-induced cytotoxicity. J. Biol. Chem 291: 21644–21655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ermert D, and Blom AM. 2016. C4b-binding protein: The good, the bad and the deadly. Novel functions of an old friend. Immunol. Lett 169: 82–92. [DOI] [PubMed] [Google Scholar]
- 13.Chung LP, Bentley DR, and Reid KB. 1985. Molecular cloning and characterization of the cDNA coding for C4b-binding protein, a regulatory protein of the classical pathway of the human complement system. Biochem. J 230: 133–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mollah F, and Tam S. 2022. Complement deficiency. In StatPearls [Internet], StatPearls Publishing, Treasure Island (FL). https://www.ncbi.nlm.nih.gov/books/NBK557581/. [PubMed] [Google Scholar]
- 15.Villoutreix BO, Blom AM, Webb J, and Dahlbäck B. 1999. The complement regulator C4b-binding protein analyzed by molecular modeling, bioinformatics and computer-aided experimental design. Immunopharmacol. 42: 121–134. [DOI] [PubMed] [Google Scholar]
- 16.Hofmeyer T, Schmelz S, Degiacomi MT, Dal Peraro M, Daneschdar M, Scrima A, van den Heuvel J, Heinz DW, and Kolmar H. 2013. Arranged sevenfold: structural insights into the C-terminal oligomerization domain of human C4b-binding protein. J. Mol. Biol 425: 1302–1317. [DOI] [PubMed] [Google Scholar]
- 17.Blom AM, Kask L, and Dahlbäck B. 2001. Structural requirements for the complement regulatory activities of C4BP. J. Biol. Chem 276: 27136–27144. [DOI] [PubMed] [Google Scholar]
- 18.Dahlbäck B, and Stenflo J. 1981. High molecular weight complex in human plasma between vitamin K-dependent protein S and complement component C4b-binding protein. Proc. Natl. Acad. Sci 78: 2512–2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hillarp A, and Dahlbäck B. 1988. Novel subunit in C4b-binding protein required for protein S binding. J. Biol. Chem 263: 12759–12764. [PubMed] [Google Scholar]
- 20.Dahlbäck B 2011. C4b-binding protein: a forgotten factor in thrombosis and hemostasis. Semin. Thromb. Hemost 37: 355–361. [DOI] [PubMed] [Google Scholar]
- 21.Hessing M, Vlooswijk RA, Hackeng TM, Kanters D, and Bouma BN. 1990. The localization of heparin-binding fragments on human C4b-binding protein. J. Immunol 144: 204–208. [PubMed] [Google Scholar]
- 22.Blom AM, Covell DG, Wallqvist A, Dahlbäck B, and Villoutreix BO. 1998. The C4b-binding protein–protein S interaction is hydrophobic in nature. Biochim. Biophys. Acta (BBA) - Prot. Struct. Mol. Enzymol 1388: 181–189. [DOI] [PubMed] [Google Scholar]
- 23.Saeki T, Hirose S, Nukatsuka M, Kusunoki Y, and Nagasawa S. 1989. Evidence that C4b-binding protein is an acute phase protein. Biochem. Biophys. Res. Commun 164: 1446–1451. [DOI] [PubMed] [Google Scholar]
- 24.Frutos P. G. de, Alim RIM, Härdig Y, Zoller B, and Dahlbâck B. 1994. Differential regulation of α and β chains of C4b-binding protein during acute-phase response resulting in stable plasma levels of free anticoagulant Protein S. Blood 84: 815–822. [PubMed] [Google Scholar]
- 25.Scharfstein J, Ferreira A, Gigli I, and Nussenzweig V. 1978. Human C4-binding protein. I. Isolation and characterization. Exp. Med 148: 207–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blom AM, Webb J, Villoutreix BO, and Dahlbäck B. 1999. A cluster of positively charged amino acids in the C4BP α-chain is crucial for C4b binding and Factor I cofactor function. J. Biol. Chem 274: 19237–19245. [DOI] [PubMed] [Google Scholar]
- 27.Härdig Y, Hillarp A, and Dahlbäck B. 1997. The amino-terminal module of the C4b-binding protein α-chain is crucial for C4b binding and factor I-cofactor function. Biochem. J 323: 469–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barnum SR 1991. C4b-binding protein, a regulatory protein of complement. Immunol. Res 10: 28–42. [DOI] [PubMed] [Google Scholar]
- 29.Suankratay C, Mold C, Zhang Y, Lint TF, and Gewurz H. 1999. Mechanism of complement-dependent haemolysis via the lectin pathway: role of the complement regulatory proteins. Clin. Exp. Immunol 117: 442–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lambris JD, Ricklin D, and Geisbrecht BV. 2008. Complement evasion by human pathogens. Nat. Rev. Microbiol 6: 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hovingh ES, van den Broek B, and Jongerius I. 2016. Hijacking complement regulatory proteins for bacterial immune evasion. Front. Microbiol 7: 2004. DOI: 10.3389/fmicb.2016.02004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berggård K, Lindahl G, Dahlbäck B, and Blom AM. 2001. Bordetella pertussis binds to human C4b-binding protein (C4BP) at a site similar to that used by the natural ligand C4b. Eur. J. Immunol 31: 2771–2780. [DOI] [PubMed] [Google Scholar]
- 33.Pietikäinen J, Meri T, Blom AM, and Meri S. 2010. Binding of the complement inhibitor C4b-binding protein to Lyme disease borreliae. Mol. Immunol 47: 1299–1305. [DOI] [PubMed] [Google Scholar]
- 34.Grosskinsky S, Schott M, Brenner C, Cutler SJ, Simon MM, and Wallich R. 2010. Human complement regulators C4b-binding protein and C1 esterase inhibitor interact with a novel outer surface protein of Borrelia recurrentis. PLOS Negl. Trop. Dis 4: e698. DOI: 10.1371/journal.pntd.0000698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prasadarao NV, Blom AM, Villoutreix BO, and Linsangan LC. 2002. A novel interaction of outer membrane protein A with C4b binding protein mediates serum resistance of Escherichia coli K11. J. Immunol 169: 6352–6360. [DOI] [PubMed] [Google Scholar]
- 36.Thofte O, Bettoni S, Su Y-C, Thegerström J, Jonsson S, Mattsson E, Sandblad L, Martí S, Garmendia J, Blom AM, and Riesbeck K. 2021. Nontypeable Haemophilus influenzae P5 binds human C4b-binding protein, promoting serum resistance. J. Immunol 207: 1566–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hallström T, Jarva H, Riesbeck K, and Blom AM. 2007. Interaction with C4b-binding protein contributes to nontypeable Haemophilus influenzae serum resistance. J. Immunol 178: 6359–6366. [DOI] [PubMed] [Google Scholar]
- 38.Barbosa AS, Monaris D, Silva LB, Morais ZM, Vasconcellos SA, Cianciarullo AM, Isaac L, and Abreu PAE. 2010. Functional characterization of LcpA, a surface-exposed protein of Leptospira spp. that binds the human complement regulator C4BP. Infect. Immun 78: 3207–3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Breda LCD, Hsieh C-L, Valencia MMC, da Silva LB, Barbosa AS, Blom AM, Yung-Fu C, and Isaac L. 2015. Fine mapping of the interaction between C4b-binding protein and outer membrane proteins LigA and LigB of pathogenic Leptospira interrogans. PLOS Negl. Trop. Dis 9: e0004192. DOI: 10.1371/journal.pntd.0004192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nordström T, Blom AM, Forsgren A, and Riesbeck K. 2004. The emerging pathogen Moraxella catarrhalis interacts with complement inhibitor C4b binding protein through ubiquitous surface proteins A1 and A21. J. Immunol 173: 4598–4606. [DOI] [PubMed] [Google Scholar]
- 41.Ram S, Cullinane M, Blom AM, Gulati S, McQuillen DP, Monks BG, O’Connell C, Boden R, Elkins C, Pangburn MK, Dahlback B, and Rice PA. 2001. Binding of C4b-binding protein to porin: a molecular mechanism of serum resistance of Neisseria gonorrhoeae. J. Exp. Med 193: 281–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blom AM, Rytkonen A, Vasquez P, Lindahl G, Dahlback B, and Jonsson AB. 2001. A novel interaction between type IV pili of Neisseria gonorrhoeae and the human complement regulator C4B-binding protein. J. Immunol 166: 6764–6770. [DOI] [PubMed] [Google Scholar]
- 43.Jarva H, Ram S, Vogel U, Blom AM, and Meri S. 2005. Binding of the complement inhibitor C4bp to serogroup B Neisseria meningitidis. J. Immunol 174: 6299–6307. [DOI] [PubMed] [Google Scholar]
- 44.Potempa M, Potempa J, Okroj M, Popadiak K, Eick S, Nguyen K-A, Riesbeck K, and Blom AM. 2008. Binding of complement inhibitor C4b-binding protein contributes to serum resistance of Porphyromonas gingivalis. J. Immunol 181: 5537–5544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ho DK, Tissari J, Järvinen HM, Blom AM, Meri S, and Jarva H. 2011. Functional recruitment of human complement inhibitor C4b-binding protein to outer membrane protein Rck of Salmonella. PLoS One 6: e27546. DOI: 10.1371/journal.pone.0027546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hair PS, Foley CK, Krishna NK, Nyalwidhe JO, Geoghegan JA, Foster TJ, and Cunnion KM. 2013. Complement regulator C4BP binds to Staphylococcus aureus surface proteins SdrE and Bbp inhibiting bacterial opsonization and killing. Results Immunol. 3: 114–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ramos-Sevillano E, Urzainqui A, Campuzano S, Moscoso M, González-Camacho F, Domenech M, Rodríguez de Córdoba S, Sánchez-Madrid F, Brown JS, García E, and Yuste J. 2015. Pleiotropic effects of cell wall amidase LytA on Streptococcus pneumoniae sensitivity to the host immune response. Infect. Immun 83: 591–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haleem KS, Ali YM, Yesilkaya H, Kohler T, Hammerschmidt S, Andrew PW, Schwaeble WJ, and Lynch NJ. 2018. The pneumococcal surface proteins PspA and PspC sequester host C4-binding protein to inactivate complement C4b on the bacterial surface. Infect. Immun 87: e00742–18. DOI: 10.1128/iai.00742-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dieudonné-Vatran A, Krentz S, Blom AM, Meri S, Henriques-Normark B, Riesbeck K, and Albiger B. 2009. Clinical isolates of Streptococcus pneumoniae bind the complement inhibitor C4b-binding protein in a PspC allele-dependent fashion. J. Immunol 182: 7865–7877. [DOI] [PubMed] [Google Scholar]
- 50.Agarwal V, Sroka M, Fulde M, Bergmann S, Riesbeck K, and Blom AM. 2014. Binding of Streptococcus pneumoniae endopeptidase O (PepO) to complement component C1q modulates the complement attack and promotes host cell adherence. J. Biol. Chem 289: 15833–15844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Agarwal V, Hammerschmidt S, Malm S, Bergmann S, Riesbeck K, and Blom AM. 2012. Enolase of Streptococcus pneumoniae binds human complement inhibitor C4b-binding protein and contributes to complement evasion. J. Immunol 189: 3575–3584. [DOI] [PubMed] [Google Scholar]
- 52.Johnsson E, Thern A, Dahlbäck B, Hedén LO, Wikström M, and Lindahl G. 1996. A highly variable region in members of the streptococcal M protein family binds the human complement regulator C4BP. J. Immunol 157: 3021–3029. [PubMed] [Google Scholar]
- 53.Berggård K, Johnsson E, Morfeldt E, Persson J, Stålhammar-Carlemalm M, and Lindahl G. 2001. Binding of human C4BP to the hypervariable region of M protein: a molecular mechanism of phagocytosis resistance in Streptococcus pyogenes. Mol. Microbiol 42: 539–551. [DOI] [PubMed] [Google Scholar]
- 54.Carlsson F, Berggård K, Stålhammar-Carlemalm M, and Lindahl G. 2003. Evasion of phagocytosis through cooperation between two ligand-binding regions in Streptococcus pyogenes M protein. J. Exp. Med 198: 1057–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ermert D, Weckel A, Agarwal V, Frick I-M, Björck L, and Blom AM. 2013. Binding of complement inhibitor C4b-binding protein to a highly virulent Streptococcus pyogenes M1 strain Is mediated by protein H and enhances adhesion to and invasion of endothelial cells. J. Biol. Chem 288: 32172–32183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jenkins HT, Mark L, Ball G, Persson J, Lindahl G, Uhrin D, Blom AM, and Barlow PN. 2006. Human C4b-binding protein, structural basis for interaction with streptococcal M protein, a major bacterial virulence factor. J. Biol. Chem 281: 3690–3697. [DOI] [PubMed] [Google Scholar]
- 57.Kirjavainen V, Jarva H, Biedzka-Sarek M, Blom AM, Skurnik M, and Meri S. 2008. Yersinia enterocolitica serum resistance proteins YadA and Ail bind the complement regulator C4b-binding protein. PLOS Pathog. 4: e1000140. DOI: 10.1371/journal.ppat.1000140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ho DK, Skurnik M, Blom AM, and Meri S. 2014. Yersinia pestis Ail recruitment of C4b-binding protein leads to factor I-mediated inactivation of covalently and noncovalently bound C4b. Eur. J. Immunol 44: 742–751. [DOI] [PubMed] [Google Scholar]
- 59.Ho DK, Riva R, Kirjavainen V, Jarva H, Ginström E, Blom AM, Skurnik M, and Meri S. 2012. Functional recruitment of the human complement inhibitor C4BP to Yersinia pseudotuberculosis outer membrane protein Ail. J. Immunol 188: 4450–4459. [DOI] [PubMed] [Google Scholar]
- 60.Avirutnan P, Hauhart RE, Somnuke P, Blom AM, Diamond MS, and Atkinson JP. 2011. Binding of Flavivirus non-structural protein NS1 to C4b binding protein modulates complement activation. J. Immunol 187: 424–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Varghese PM, Murugaiah V, Beirag N, Temperton N, Khan HA, Alrokayan SH, Al-Ahdal MN, Nal B, Al-Mohanna FA, Sim RB, and Kishore U. 2020. C4b binding protein acts as an innate immune effector against Influenza A Virus. Front. Immunol 11: 585361. DOI: 10.3389/fimmu.2020.585361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dasari P, Koleci N, Shopova IA, Wartenberg D, Beyersdorf N, Dietrich S, Sahagún-Ruiz A, Figge MT, Skerka C, Brakhage AA, and Zipfel PF. 2019. Enolase from Aspergillus fumigatus is a moonlighting protein that binds the human plasma complement proteins Factor H, FHL-1, C4BP, and plasminogen. Front. Immunol 10: 2573. DOI: 10.3389/fimmu.2019.02573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Luo S, Blom AM, Rupp S, Hipler U-C, Hube B, Skerka C, and Zipfel PF. 2011. The pH-regulated antigen 1 of Candida albicans binds the human complement inhibitor C4b-binding protein and mediates fungal complement evasion. J. Biol. Chem 286: 8021–8029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Meri T, Blom AM, Hartmann A, Lenk D, Meri S, and Zipfel PF. 2004. The hyphal and yeast forms of Candida albicans bind the complement regulator C4b-binding protein. Infect. Immun 72: 6633–6641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Khattab A, Rezola M, Barroso M, Kyrklund M, Pihlajamaa T, Freitag TL, van Gemert G-J, Bousema T, Permi P, Turunen O, Sauerwein R, Luty AJF, and Meri S. 2022. Hijacking the human complement inhibitor C4b-binding protein by the sporozoite stage of the Plasmodium falciparum parasite. Front. Immunol 13: 1051161. DOI: 10.3389/fimmu.2022.1051161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Buffalo CZ, Bahn-Suh AJ, Hirakis SP, Biswas T, Amaro RE, Nizet V, and Ghosh P. 2016. Conserved patterns hidden within group A Streptococcus M protein hypervariability are responsible for recognition of human C4b-binding protein. Nat. Microbiol 1: 16155. DOI: 10.1038/nmicrobiol.2016.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ermert D, Weckel A, Magda M, Mörgelin M, Shaughnessy J, Rice PA, Björck L, Ram S, and Blom AM. 2018. Human IgG increases virulence of Streptococcus pyogenes through complement evasion. J. Immunol 200: 3495–3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ermert D, Shaughnessy J, Joeris T, Kaplan J, Pang CJ, Kurt-Jones EA, Rice PA, Ram S, and Blom AM. 2015. Virulence of Group A Streptococci is enhanced by human complement inhibitors. PLOS Pathog. 11: e1005043. DOI: . 10.1371/journal.ppat.1005043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kulkarni HS, Liszewski MK, Brody SL, and Atkinson JP. 2018. The complement system in the airway epithelium: An overlooked host defense mechanism and therapeutic target? J. Allergy Clin. Immunol 141: 1582–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Garvin LE, Bash MC, Keys C, Warner DM, Ram S, Shafer WM, and Jerse AE. 2008. Phenotypic and genotypic analyses of Neisseria gonorrhoeae isolates that express frequently recovered PorB PIA variable region types suggest that certain P1a porin sequences confer a selective advantage for urogenital tract infection. Infect. Immun 76: 3700–3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bettoni S, Shaughnessy J, Maziarz K, Ermert D, Gulati S, Zheng B, Mörgelin M, Jacobsson S, Riesbeck K, Unemo M, Ram S, and Blom AM. C4BP-IgM protein as a therapeutic approach to treat Neisseria gonorrhoeae infections. JCI Insight 4: e131886. DOI: 10.1172/jci.insight.131886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bettoni S, Maziarz K, Stone MRL, Blaskovich MAT, Potempa J, Bazzo ML, Unemo M, Ram S, and Blom AM. 2021. Serum complement activation by C4BP-IgM fusion protein can restore susceptibility to antibiotics in Neisseria gonorrhoeae. Front. Immunol 12: 726801. DOI: 10.3389/fimmu.2021.726801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Murugaiah V, Varghese PM, Beirag N, DeCordova S, Sim RB, and Kishore U. 2021. Complement proteins as soluble pattern recognition receptors for pathogenic viruses. Viruses 13: 824. DOI: 10.3390/v13050824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sikorski PM, Commodaro AG, and Grigg ME. 2020. Toxoplasma gondii recruits Factor H and C4b-binding protein to mediate resistance to serum killing and promote parasite persistence in vivo. Front. Immunol 10: 3105. DOI: 10.3389/fimmu.2019.03105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Werner LM, Alcott A, Mohlin F, Ray JC, Dufrisne MB, Smirnov A, Columbus L, Blom AM, and Criss AK. 2023. Neisseria gonorrhoeae co-opts C4b-binding protein to enhance complement-independent survival from neutrophils. PLOS Pathog. 19: e1011055. DOI: 10.1371/journal.ppat.1011055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Oates AM, and Salem HH. 1991. The binding and regulation of protein S by neutrophils. Blood Coagul. Fibrinolysis 2: 601–607. [DOI] [PubMed] [Google Scholar]
- 77.Furmaniak-Kazmierczak E, Hu CY, and Esmon CT. 1993. Protein S enhances C4b binding protein interaction with neutrophils. Blood 81: 405–411. [PubMed] [Google Scholar]
- 78.Webb JH, Blom AM, and Dahlbäck B. 2003. The binding of protein S and the protein S-C4BP complex to neutrophils is apoptosis dependent. Blood Coagul. Fibrinolysis 14: 355–359. [DOI] [PubMed] [Google Scholar]
- 79.Anderson HA, Maylock CA, Williams JA, Paweletz CP, Shu H, and Shacter E. 2003. Serum-derived protein S binds to phosphatidylserine and stimulates the phagocytosis of apoptotic cells. Nat. Immunol 4: 87–91. [DOI] [PubMed] [Google Scholar]
- 80.Trouw LA, Bengtsson AA, Gelderman KA, Dahlbäck B, Sturfelt G, and Blom AM. 2007. C4b-binding protein and Factor H compensate for the loss of membrane-bound complement Inhibitors to protect apoptotic cells against excessive complement attack. J. Biol. Chem 282: 28540–28548. [DOI] [PubMed] [Google Scholar]
- 81.Kask L, Trouw LA, Dahlbäck B, and Blom AM. 2004. The C4b-binding protein-protein S complex inhibits the phagocytosis of apoptotic cells. J. Biol. Chem 279: 23869–23873. [DOI] [PubMed] [Google Scholar]
- 82.Brodeur SR, Angelini F, Bacharier LB, Blom AM, Mizoguchi E, Fujiwara H, Plebani A, Notarangelo LD, Dahlback B, Tsitsikov E, and Geha RS. 2003. C4b-binding protein (C4BP) activates B cells through the CD40 receptor. Immunity 18: 837–848. [DOI] [PubMed] [Google Scholar]
- 83.Williams KT, Young SP, Negus A, Young LS, Adams DH, and Afford SC. 2007. C4b binding protein binds to CD154 preventing CD40 mediated cholangiocyte apoptosis: a novel link between complement and epithelial cell survival. PLoS One 2: e159. DOI: 10.1371/journal.pone.0000159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sasaki K, Takano S, Tomizawa S, Miyahara Y, Furukawa K, Takayashiki T, Kuboki S, Takada M, and Ohtsuka M. 2021. C4b-binding protein α-chain enhances antitumor immunity by facilitating the accumulation of tumor-infiltrating lymphocytes in the tumor microenvironment in pancreatic cancer. J. Exp. Clin. Cancer Res. 40: 212. DOI: 10.1186/s13046-021-02019-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Olcina MM, Kim RK, Balanis NG, Li CG, von Eyben R, Graeber TG, Ricklin D, Stucki M, and Giaccia AJ. 2020. Intracellular C4BPA levels regulate NF-κB-dependent apoptosis. iScience 23: 101594. DOI: 10.1016/j.isci.2020.101594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Holmberg MT, Blom AM, and Meri S. 2001. Regulation of complement classical pathway by association of C4b-binding protein to the surfaces of SK-OV-3 and Caov-3 ovarian adenocarcinoma cells. J. Immunol 167: 935–939. [DOI] [PubMed] [Google Scholar]
- 87.Feng G, Li J, Zheng M, Yang Z, Liu Y, Zhang S, Ye L, Zhang W, and Zhang X. 2016. Hepatitis B virus X protein up-regulates C4b-binding protein α through activating transcription factor Sp1 in protection of hepatoma cells from complement attack. Oncotarget 7: 28013–28026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Trouw LA, Nilsson SC, Gonçalves I, Landberg G, and Blom AM. 2005. C4b-binding protein binds to necrotic cells and DNA, limiting DNA release and inhibiting complement activation. J. Exp. Med 201: 1937–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Luque A, Serrano I, Ripoll E, Malta C, Gomà M, Blom AM, Grinyó JM, Rodríguez de Córdoba S, Torras J, and Aran JM. 2020. Noncanonical immunomodulatory activity of complement regulator C4BP(β-) limits the development of lupus nephritis. Kidney Int. 97: 551–566. [DOI] [PubMed] [Google Scholar]
- 90.Daha MR, Hazevoet HM, Hermans J, van Es LA, and Cats A. 1983. Relative importance of C4 binding protein in the modulation of the classical pathway C3 convertase in patients with systemic lupus erythematosus. Clin. Exp. Immunol 54: 248–252. [PMC free article] [PubMed] [Google Scholar]
- 91.Abdelghany WM, Salah M, Saleh WA, Dahy OM, and Helmy R. 2022. Activity of Protein S-C4b binding protein and total TFPI levels in Egyptian SLE patients: a cross-sectional study. Maced. J. Med. Sci 10: 803–811. [Google Scholar]
- 92.Olivar R, Luque A, Naranjo-Gómez M, Quer J, García de Frutos P, Borràs FE, Rodríguez de Córdoba S, Blom AM, and Aran JM. 2013. The α7β0 isoform of the complement regulator C4b-binding protein induces a semimature, anti-inflammatory state in dendritic cells. J. Immunol 190: 2857–2872. [DOI] [PubMed] [Google Scholar]
- 93.Serrano I, Luque A, Mitjavila F, Blom AM, Rodríguez de Córdoba S, Vega MC, Torras J, and Aran JM. 2022. The hidden side of complement regulator C4BP: dissection and evaluation of its immunomodulatory activity. Front Immunol 13: 883743. DOI: 10.3389/fimmu.2022.883743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kulak K, Westermark GT, Papac-Milicevic N, Renström E, Blom AM, and King BC. 2017. The human serum protein C4b-binding protein inhibits pancreatic IAPP-induced inflammasome activation. Diabetologia 60: 1522–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bierschenk D, Papac-Milicevic N, Bresch IP, Kovacic V, Bettoni S, Dziedzic M, Wetsel RA, Eschenburg S, Binder CJ, Blom AM, and King BC. 2023. C4b-binding protein inhibits particulate- and crystalline-induced NLRP3 inflammasome activation. Front Immunol 14. DOI: 10.3389/fimmu.2023.1149822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Golay J, and Taylor RP. 2020. The role of complement in the mechanism of action of therapeutic anti-cancer mAbs. Antibodies (Basel) 9: 58. DOI: 10.3390/antib9040058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Laabei M, Colineau L, Bettoni S, Maziarz K, Ermert D, Riesbeck K, Ram S, and Blom AM. 2020. Antibacterial fusion proteins enhance Moraxella catarrhalis killing. Front. Immunol 11: 2122. DOI: 10.3389/fimmu.2020.02122. [DOI] [PMC free article] [PubMed] [Google Scholar]