Abstract
Introduction
Cannabidiol (CBD) has several potential benefits and therapeutic uses, especially in pain, inflammation, and anxiety. CBD has high hydrophobicity and very low solubility in water. CBD has also shown exceptionally low oral-gastrointestinal (oral-GI) bioavailability. In this study, we aimed to examine the oral gastrointestinal absorption and subsequent bioavailability of CBD in a nanoemulsion formulation prepared by Pressure BioSciences’ UltraShearTM technology.
Methods
CBD nanoemulsion (2%) was provided by Pressure BioSciences, Inc. (South Easton, MA), and CBD pharmacokinetic parameters were evaluated in male Sprague-Dawley rats using LC-MS/MS technology.
Results
Bioavailability of orally delivered CBD UltraShear nanoemulsion was calculated to be 18.6% at 6 h and 25.4% at 24 h. While oral-GI bioavailability is unsurprisingly limited by first-pass metabolism, it is nonetheless notable that CBD bioavailability for oral-GI UltraShear nanoemulsion CBD is roughly 3–4x higher than the typical bioavailability for oral-GI CBD delivered in oil solution or conventional edible formats.
Conclusion
This study has provided a compelling demonstration of unprecedented speed and efficiency of oral-GI CBD absorption of CBD UltraShear nanoemulsions, achieving 10% of levels achieved for direct IV injection within 30 min and 80% of IV levels in 24 h. Notably, within just the first hour post-administration, the bioavailability of oral CBD from UltraShear nanoemulsion formulation exceeded the typical 6% total CBD oral bioavailability benchmarks reported for CBD edibles and ultimately achieved 3–4X these levels within 6–24 h.
Keywords: Cannabidiol, UltraShear nanoemulsion, Bioavailability, Pharmacokinetics, Absorption
Introduction
Cannabidiol (CBD) is a non-psychotropic compound derived from the cannabis plant. CBD interacts subtly with the CB1 and CB2 receptors in the body, which bind strongly to THC and are responsible for its psychotropic effects [1]. CBD also interacts with certain serotonin receptors, vanilloid receptors, adenosine receptors, and ion channels, which may explain its potential therapeutic effects in a variety of medical conditions [2]. Epidiolex® is the first FDA-approved prescription CBD drug for the control of epileptic seizures associated with Lennox-Gastaut syndrome [3] and Dravet syndrome [4]. CBD enjoys common over-the-counter use for managing pain, inflammation, anxiety, stress, posttraumatic stress disorder, sleep disorders, and skin conditions. Early research suggests that CBD reduces spasticity in multiple sclerosis [5] and may lessen the severity of neurological symptoms associated with Alzheimer’s disease [6], Parkinson’s disease [7], and schizophrenia [8].
Despite its potential benefits, the therapeutic use of CBD is hampered by its high hydrophobicity and very low solubility in water. CBD has shown exceptionally low oral-gastrointestinal (oral-GI) bioavailability, with only about 6% of the bioactive compound reaching the systemic circulation [9, 10], limited by both
Low oral-GI absorption efficiency of current commercial preparations, and
Highly efficient transformation in first-pass metabolism (before reaching systemic circulation) into primary metabolites CBD-7-COOH and 7-OH-CBD among others.
Current commercial topical CBD products are generally reported to have even lower systemic bioavailability [11]. Improving the oral-GI bioavailability of CBD products is an active area of research. CBD capsules containing nanospheres of lyophilized powder in biodegradable polymer are in a phase 2 trial planned for the treatment of opioid addiction [12]. Preclinical studies of CBD oral cocrystals with tetramethylpyrazine are being carried out by Artelo Biosciences for the treatment of posttraumatic stress disorder, inflammatory bowel disease, and stroke [13].
CBD therapeutics must be specifically formulated according to their intended delivery route, which include inhalation, oral-GI, sublingual, buccal, intranasal, transdermal, and rectal, among others. For oral-GI delivery, significantly increased absorption and subsequent bioavailability of several lipophilic compounds encapsulated in nanoemulsions have been shown to correlate with oil droplet size reduction [14, 15]. Specifically, the intestinal absorption of CBD is markedly increased when delivered as an oral nanoemulsion where oil droplet size is extremely small [16].
Several different approaches to increase CBD solubility in water and to enhance its absorption and bioavailability have been undertaken including encapsulation into amphiphilic clathrate structures such as cyclodextrins [17]; combining the oil phase with dry powders of biodegradable polymer substances such as maltodextrin [18]; chemical modifications rendering higher polarity [19]; and preparation of oil-in-water nanoemulsions [20].
Nanoemulsions have recently attracted the attention of pharmaceutical formulation developers as effective media to deliver hydrophobic active ingredients via oral, transmucosal, and transdermal routes. Nanoemulsions are kinetically stable mixtures of oil droplets of submicron size coated by emulsifiers. Stability of nanoemulsions is a function of several factors; mainly, the choice of emulsifiers and the physical preparation method that define nanoemulsion droplet size and polydispersity. Emulsification requires blending immiscible oil and water phases using mechanical shear that can be achieved by rotor-stator homogenizers, ultrasonic equipment, and high-pressure homogenizers. Rotor-stator homogenizers are incapable of achieving sufficient shear rates to produce nanometer-size emulsions. Ultrasonic equipment, while capable of delivering enough energy to make nanoemulsions, tends to generate polydisperse nanoemulsions with a broad range of droplet sizes, which facilitates nanoemulsion breakdown. High polydispersity negatively affects nanoemulsion stability and interferes with filter sterilization.
High-pressure homogenization is a relatively new approach that uses pressures reaching or exceeding 30,000 psi to generate intense fluid shear forces producing highly monodisperse nanoemulsions of oil-encapsulated bioactives [21]. Currently, commercialized high-pressure homogenization technologies face operational efficiency limitations due to fouling or clogging in their shearing valve designs and significant downtime associated with clearing these valves and reestablishing operational qualification. Pressure BioSciences, Inc. has recently introduced UltraShear™ technology for high-pressure homogenization, delivering unprecedented shear rates of up to 109s−1 and incorporating a “dynamic valve” design featuring self-clearing capabilities while in continuous operation. This design innovation offers important benefits in operational efficiency improvement and economic viability for nanoemulsion production of CBD and a diverse range of other products requiring low droplet size, monodisperse, stable nanoemulsions for effective delivery of hydrophobic active compounds.
Generally, formulations with droplet sizes below 100 nm and appropriate use of emulsifier appear to be stable for extended periods of time when stored refrigerated or at room temperature. Moreover, these formulations are readily miscible with water and can be sterilized by microfiltration. Absorption of nanoemulsified active ingredients is expected to be considerably higher compared to the same hydrophobic molecules administered as tinctures or as solutions in oil mixed with powdered material such as maltodextrin. However, bioavailability of nanoemulsified CBD has not been sufficiently studied via any of its potential administration pathways. This study aimed to fill significant gaps in this understanding by examining the oral-GI absorption and subsequent bioavailability of CBD in a nanoemulsion formulation prepared by Pressure BioSciences’ UltraShear™ technology [22, 23].
Materials and Methods
Standards and Reagents
A solution of 10 mg/mL CBD standard in a mixture of ethanol:Kolliphor ELP:water (1:1:8) was used for intravenous injection and was prepared in-house. 7-OH-CBD and CBD-7-COOH as well as d3-CBD and tolbutamide were purchased from Cerilliant. Acetonitrile and other solvents were ACS grade.
CBD nanoemulsion (2%) was provided by Pressure BioSciences, Inc. (South Easton, MA, USA): CBD isolate (>98% pure CBD, Mile High Labs, Boulder, CO, USA) was dissolved 1:5 (w/w) in virgin hempseed oil (Jedwards International, Braintree, MA, USA) and combined 1:10 with a continuous phase containing glycerol, polysorbate 80, and deionized water. The final mixture, containing 20 mg/mL CBD, 10% hempseed oil, 10% glycerol, and 9% polysorbate 80, was pre-blended using a hand-held high-speed rotor-stator homogenizer (Omni International, a Perkin Elmer Company, Kennesaw, GA) at 10,000 RPM. The resulting crude emulsion was nanoemulsified by six consecutive passes through a BaroShear™ Mini high-pressure homogenizer (Pressure BioSciences). Resulting nanoemulsion showed a mean droplet size of about 60 nm and very low polydispersity index (PDI = 0.22, well within the <0.3 target range considered acceptable for lipid-based carriers in drug delivery applications), as characterized by a LiteSizer 500 dynamic light scattering instrument (Anton Paar USA, Ashland, VA, USA) [24]. The final CBD concentration of 20 mg/mL in the formulation was verified by solid-phase extraction followed by HPLC assay at ProVerde Laboratories (Milford, MA, USA).
Animal Studies
Male Sprague-Dawley rats (200–250 g) were used to evaluate CBD pharmacokinetic parameters. The animals were individually housed in filter-top cages and kept in the University of Mississippi Vivarium. All procedures were performed in compliance with the University of Mississippi Animal Care and Use Committee (IACUC) and following the National Institute of Health (NIH) guidelines (Protocol #22-003). Before initiation of the study, a jugular cannula was placed in each animal. All animals were fasted overnight and divided into two groups. Four animals were used in the oral-GI nanoemulsion test group and three animals in the intravenous control group. Zero-min time-point blood samples (200 µL) were drawn before drug administration. UltraShear CBD nanoemulsion formulation was given orally at 5 mg/animal (0.25 mL of a 20 mg/mL preparation) by gavage. The intravenous (IV) route dose was 1 mg/animal (0.1 mL of a 10 mg/mL mixture) given via the jugular cannula. Blood samples (200 µL–300 µL) were collected in Terumo microcentrifuge tubes (Capiject) coated with EDTA (0.78 mg/tube) at 5 min, 10 min, 15 min, 30 min, 1 h, 2 h, 4 h, 6 h, and 24 h for both orally and IV administered CBD formulations. The plasma was separated by centrifugation of the blood samples at 3,000 RPM for 5 min at 4°C (Legend Mach 1.6R, Thermo Sorvall). Plasma (100 µL) was stored at −20°C until analysis.
Plasma Extraction Method
A 50 µL aliquot of plasma from each blood collection time was transferred into 1.5-mL Eppendorf tubes. Samples were treated with 100 µL of 100% acetonitrile containing internal standard (20 ng/mL of d3-CBD or tolbutamide) and vortexed for 2 min. Samples were then centrifuged at 13,000 rpm for 10 min at 4°C, and 100 µL of supernatant was transferred into inserts in 1.0 mL vials and capped with polyethylene plugs prior to analysis using UPLC-MS/MS.
LC/MS/MS Analysis
Water Acquity (Xevo TQ-S) LC-MS/MS was used for the analyses of the plasma samples. The UPLC system was an Acquity UPLC® with BEH Phenyl Column (50 × 3.0 mm, 1.7 µm), and the injection volume was 2 µL. The column oven temperature was set to 40°C, while the sample cooler temperature was 10°C. Flow rate was 0.3 mL/min, and the total run time was 5 min. The mobile phase was acetonitrile and Milli-Q water containing 0.2% formic acid.
Table 1 shows the mass spectrometric conditions and the transitions for all analytes. Table 2 shows the HPLC conditions.
Table 1.
Mass spectrometric conditions and the transitions for all analytes and internal standard
| Analyte: CBD | IS: CBD-d3 | Analyte: CBD-7-COOH | Analyte: 7-OH-CBD | IS: tolbutamide | |
|---|---|---|---|---|---|
| MRM transitions | Q1 – m/z 315 | Q1 – m/z 318 | Q1 – m/z 343 | Q1 – m/z 329 | Q1 – m/z 269 |
| Q3 – m/z 193 | Q3 – m/z 134 | Q3 – m/z 230 | Q3 – m/z 267 | Q3 – m/z 169 | |
| Resolution – Q1 and Q3 | Unit | Unit | |||
| Capillary voltage, KV | 3.90 | 3.20 | |||
| Cone voltage, V | 40 | 40 | 40 | 46 | 40 |
| Collision energy, V | 20 | 18 | 26 | 26 | 16 |
| Ionization/polarity | Positive | Negative | |||
| Dwell time, ms | 0.025 | 0.025 | |||
| Ionization source | Electrospray ion source | Electrospray ion source | |||
| Desolvation gas flow, L/h | 800 | 800 | |||
| Source temperature | 500°C | 200°C | |||
| Sample preparation method | Protein precipitation | Protein precipitation | |||
| Retention time, min | 2.68 | 1.48 | 1.53 | 1.43 | |
| Calibration range | 5.60–560 ng/mL | 5.0–500 ng/mL | 5.0–500 ng/mL | NA | |
Table 2.
HPLC conditions
| Mobile phase | Solvent A: acetonitrile |
| Solvent B: 0.2% formic acid in Milli-Q water | |
| Chromatographic method | Gradient |
| Flow rate | 0.3 mL/min |
| Column | Acquity UPLC® BEH Phenyl (50 × 3.0 mm, 1.7 µm) |
| Injection volume | 2 µL |
| Run time | 5.0 min |
| Sample cooler temperature | 10°C |
| Column oven temperature | 40°C |
| Rinsing solution | MeOH:Milli-Q water (80:20, v/v) |
Pharmacokinetics Calculations
Tables 3 and 4 show the pharmacokinetic raw data. The area under curve (AUC) was determined using the trapezoidal method. CBD total absorption in either ng/mL or as AUC is calculated as CBD equivalents (CBDE) with adjustment for molecular weight (MW) differences of CBD metabolites versus CBD with the following equation:
Table 3.
Time versus plasma concentration (ng/mL) of CBD and metabolites, CBD-7-COOH and 7-OH-CBD, after oral administration of 5 mg CBD nanoemulsion
| Rat 1 | Rat 2 | Rat 3 | Rat 4 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CBD | CBD-7-COOH | 7-OH-CBD | CBD | CBD-7-COOH | 7-OH-CBD | CBD | CBD-7-COOH | 7-OH-CBD | CBD | CBD-7-COOH | 7-OH-CBD | |
| 0 min | BLQ | BLQ | BLQ | BLQ | BLQ | BLQ | BLQ | BLQ | BLQ | BLQ | BLQ | BLQ |
| 5 min | BLQ | 19.5 | 6.5 | BLQ | 10.85 | BLQ | 10.86 | 11.03 | BLQ | 8.67 | 39.48 | 7.52 |
| 10 min | 7.7 | 192.79 | BLQ | 12.98 | 124.78 | 10.66 | 19.76 | 61.72 | 7.98 | 7.64 | 170.62 | 8.6 |
| 15 min | 19.67 | 1,052.24 | 6.04 | 19.98 | 394.48 | 6.77 | 53.78 | 206.66 | 13.21 | 67.47 | 430.81 | 28.75 |
| 30 min | 147.99 | 1,943.91 | 28.75 | 28.21 | 338.66 | 26.39 | 147.16 | 481.66 | 48.1 | 442.85 | 781.5 | 60.99 |
| 1 h | 326.29 | 759.87 | 58.01 | 20.36 | 221.14 | BLQ | 144.57 | 745.11 | 49.97 | 252.32 | 755.78 | 55.02 |
| 2 h | 111 | 704.5 | 31.21 | 111.35 | 413.16 | 27.39 | 100.23 | 315.1 | 20.96 | 125.51 | 377.25 | 36.45 |
| 4 h | 54.41 | 291.65 | 55.73 | 46.38 | 334.28 | 10.75 | 60.4 | 350.28 | 16.86 | 98.73 | 368.14 | 32.23 |
| 6 h | 42.32 | 194.4 | 28.4 | 36.78 | 278.51 | 19.53 | 13.82 | 473.84 | 16.48 | 83.24 | 259 | 18.42 |
| 24 h | 7.92 | 64.81 | BLQ | BLQ | 26.4 | BLQ | BLQ | 48.74 | 5.5 | BLQ | BLQ | BLQ |
Below limit of quantitation (BLQ), lower limits of quantitation (LLOQ): CBD 5.6 ng/mL, CBD-7-COOH 5.0 ng/mL, 7-OH-CBD 5.0 ng/mL.
Table 4.
Time versus plasma concentrations (ng/mL) profile of CBD and metabolites, CBD-7-COOH and 7-OH-CBD, in plasma after IV administration of 1 mg control CBD
| Time | Rat 5 | Rat 6 | Rat 7 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| CBD | CBD-7-COOH | 7-OH-CBD | CBD | CBD-7-COOH | 7-OH-CBD | CBD | CBD-7-COOH | 7-OH-CBD | |
| 0 min | BLQ | BLQ | BLQ | BLQ | BLQ | BLQ | BLQ | BLQ | BLQ |
| 5 min | 842 | 306 | BLQ | 849 | 546 | 40.1 | 563 | 428 | 124 |
| 10 min | 588 | 477 | BLQ | 969 | 175 | BLQ | 743 | 553 | 212 |
| 15 min | 484 | 243 | BLQ | 641 | 310 | BLQ | 670 | 880 | BLQ |
| 30 min | 202 | 678 | BLQ | 203 | 743 | BLQ | 391 | 589 | 33.6 |
| 1 h | 117 | 178 | BLQ | 154 | 225 | BLQ | 142 | 158 | 30.3 |
| 2 h | 41.9 | 65.4 | 13.3 | 62.4 | 66.6 | BLQ | 93.3 | 119 | 17.2 |
| 4 h | 15.4 | 20.1 | BLQ | 21.9 | 38.9 | BLQ | 54.6 | 63.3 | BLQ |
| 6 h | 10.3 | BLQ | BLQ | 8.66 | 23.3 | BLQ | 28.1 | BLQ | BLQ |
| 24 h | BLQ | 12.7 | BLQ | 7.54 | BLQ | BLQ | N/A | N/A | N/A |
Below limit of quantitation (BLQ), lower limits of quantitation (LLOQ): CBD 5.6 ng/mL, CBD-7-COOH 5.0 ng/mL, 7-OH-CBD 5.0 ng/mL.
N/A, data not available.
The relative absorption A(OGI/IV) was determined using the following equation:
where OGI = oral-GI, and IV = intravenous. The relative bioavailability F(OGI/IV) was determined using only unmetabolized CBD levels, using the following equation:
Results and Discussion
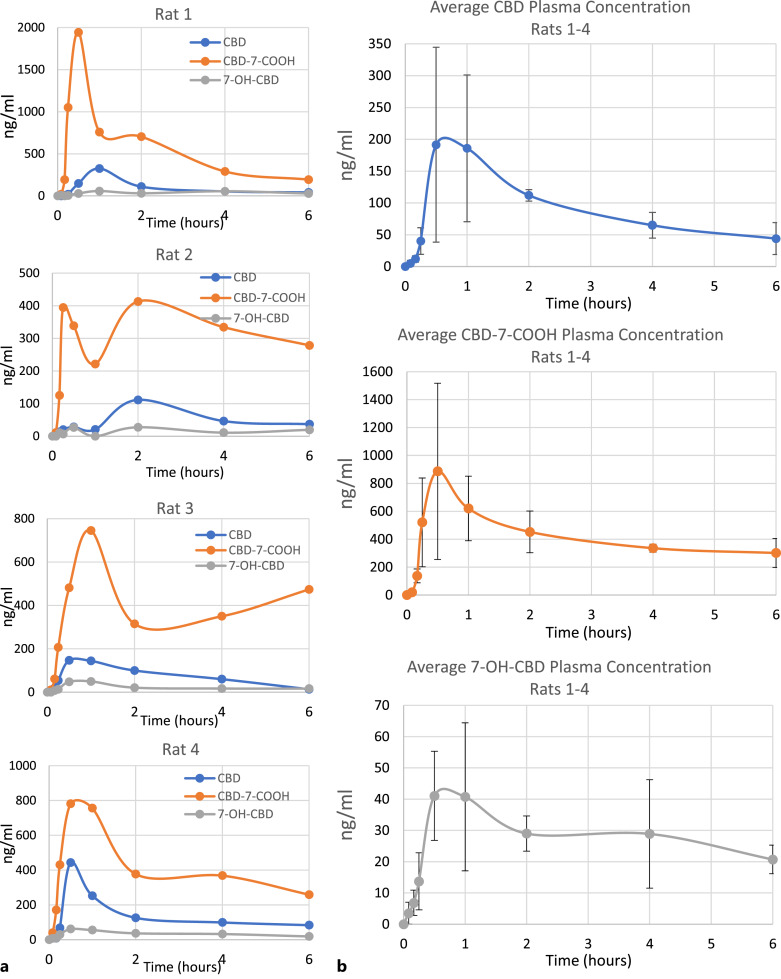
The plasma concentrations of CBD, CBD-7-COOH, and 7-OH-CBD were evaluated through time intervals from 5 min to 24 h in 4 rats following oral-GI administration of CBD UltraShear nanoemulsion (Table 3; Fig. 1). In three out of four animals individually and for all four animals on average, the maximum plasma CBD concentration was reached between 30 min and 1 h after oral administration. For the fourth animal, the plasma CBD concentration peak was measured at 2 h. CBD was detected in plasma for up to 6 h in all animals. By 24 h, the plasma CBD level was below limit of quantation (LOQ) in 3 out of 4 animals. In 3 out of 4 animals, the maximum CBD-7-COOH concentration was observed between 15 min and 30 min and for the fourth animal at 1 h. In three out of four animals, the maximum 7-OH-CBD concentration was observed between 30 min and 1 h and for the fourth animal at 2 h. Fast absorption of the parent CBD compound and rapid first-pass metabolism of oral-GI CBD from the UltraShear nanoemulsion formulation are indicated by the very early appearance, and higher combined levels, of the CBD-7-COOH and 7-OH-CBD metabolites relative to levels of the parent CBD molecule.
Fig. 1.
Time versus concentration profile of CBD and its metabolites, CBD-7-COOH and 7-OH-CBD, in plasma after oral administration of 5 mg CBD UltraShear nanoemulsion. a Individual results are shown for each of 4 rats tested. b Average of all rats, with standard deviation brackets (n = 4). Lower limits of quantitation (LLOQ): CBD 5.6 ng/mL, CBD-7-COOH 5.0 ng/mL, 7-OH-CBD 5.0 ng/mL. Values BLQ were rounded to zero for purposes of averaging.
It is noted that there is high interanimal variability in CBD metabolism (as expected) and a second maximum for CBD-7-COOH and an increase in the metabolite level after 2 h in animal 4. It is thought that the second maximum in animals 1, 2, and 3 might be the result of dumping of metabolites in the bile into the general circulation and thus the rise. On the other hand, the rise in CBD-7-COOH in animal 4 could be the result of more delayed absorption through the normal enterohepatic circulation than through the lymphatic system compared to the other animals.
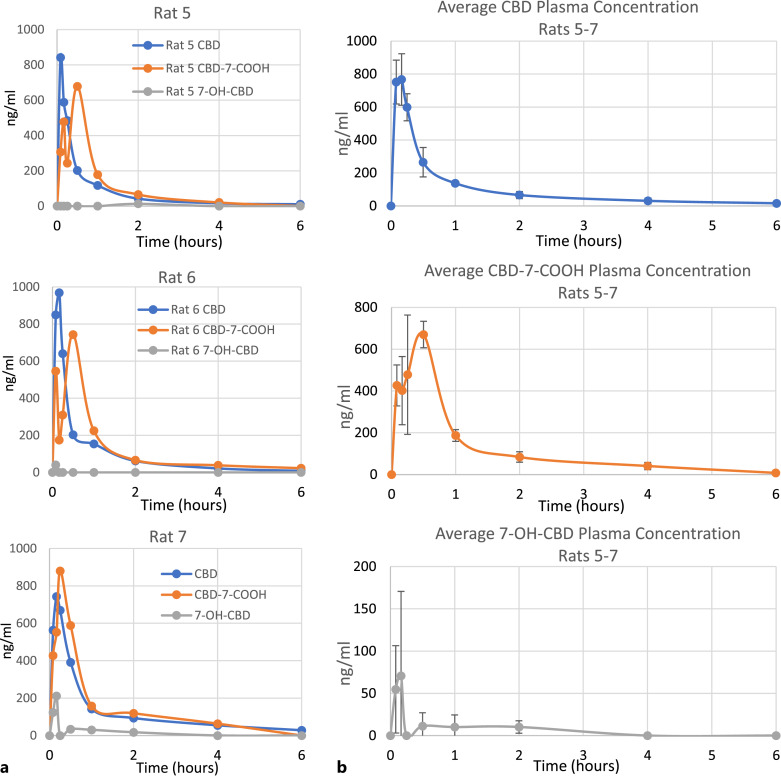
For a comparative “gold standard” baseline, CBD concentration over time was also measured in 3 additional rats that received 1 mg of intravenous control CBD via jugular cannula (Table 4; Fig. 2). CBD was detected in plasma for up to 6 h in all animals after IV injection. After 24 h, plasma CBD was below LOQ in 2 out of 3 animals. It is noted that a maximum CBD-7-COOH concentration was initially found at 5–10 min followed by another maximum at 15–30 min. However, a secondary maximum in CBD-7-COOH concentration was also observed in the rats receiving oral-GI administration of nanoemulsion, which suggests that the explanation may lie in common biological pathways at work in both sets of animals, such as discussed above regarding the interanimal variability in the oral-GI rats. The maximum CBD-7-COOH concentration was observed between 15 and 30 min and remained above LOQ for only 4–6 h. Only low levels of 7-OH-CBD were sporadically detected after IV administration. In contrast to the rapid conversion of CBD to CBD-7-COOH observed in the oral treatment group, the plasma levels of CBD and CBD-7-COOH are roughly equivalent in the IV group, reflective of the fact that IV administration circumvents the first-pass metabolism encountered in the rats receiving CBD via the oral-GI route.
Fig. 2.
Time versus concentration profile of CBD and CBD-7-COOH in plasma after IV administration of 1 mg control CBD. a Individual results are shown for each of 3 rats tested. b Average of all rats, with standard deviation brackets (n = 3). Lower limits of quantitation (LLOQ): CBD 5.6 ng/mL, CBD-7-COOH 5.0 ng/mL, 7-OH-CBD 5.0 ng/mL. Values BLOQ were rounded to zero for purposes of averaging.
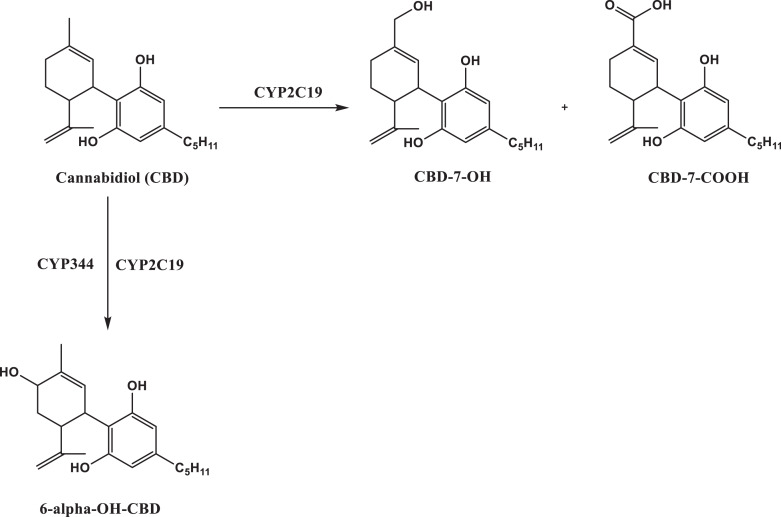
Figure 3 illustrates the primary liver metabolism of CBD. CBD metabolism is thought to go through at least 9 different pathways involving several cytochrome P450 enzymes, resulting in formation of approximately 40 distinct metabolites [25, 26]. The most significant metabolic pathways are thought to involve CYP2C19 enzyme and result in hydroxylation at positions 6 or 7. CYP3A4 also contributes to hydroxylation at position 6. The present study focused only on quantitative analysis of the parent CBD compound and its most prominently observed metabolites: 7-OH-CBD and its immediate downstream metabolite: CBD-7-COOH.
Fig. 3.
Liver metabolism of CBD to its primary carboxy and hydroxy metabolites.
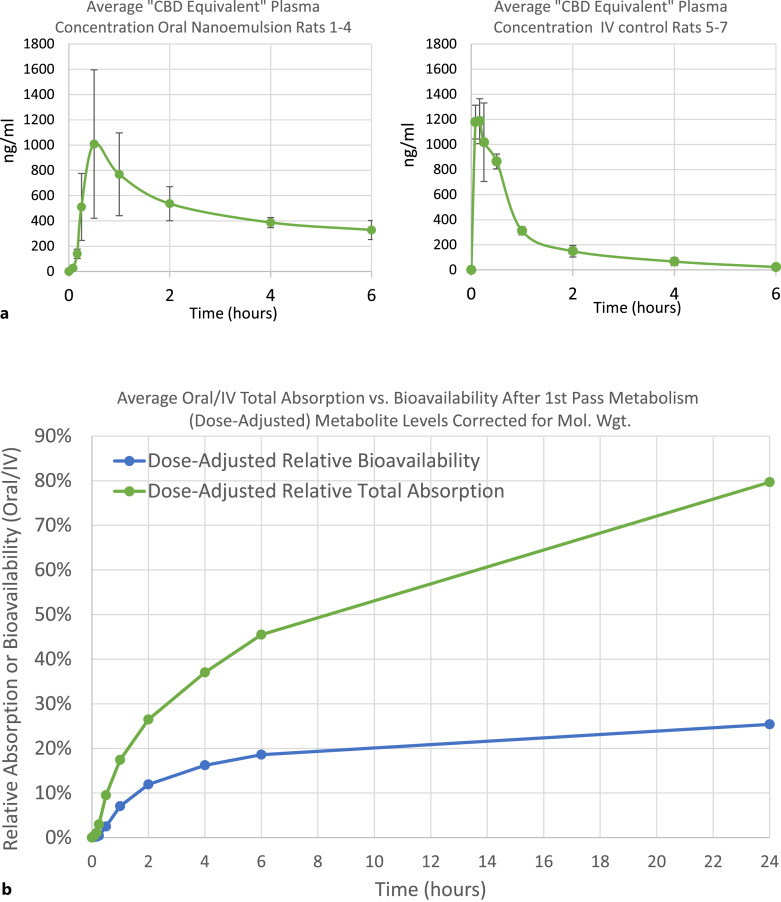
Considering the oral-GI administration route studied herein, it is extremely significant to note that plasma levels of CBD and/or its metabolites were easily detectable at the very first testing point in the plasma of all four animals, just 5 min after oral administration of the UltraShear nanoemulsion, and were sharply increased (including metabolites) in the plasma of all animals at 10 min. The rapid attainment of measurable levels of not just CBD but also its conversion into much higher levels of the carboxy and/or hydroxy metabolite products in the plasma of all four animals already at the 5 and 10 min time points, and the achievement of Cmax for the average total of CBD and metabolites combined at 30 min, clearly indicates extremely rapid absorption of the UltraShear nanoemulsion in the gut. Indeed, comparing the efficiency of oral-GI absorption of CBD UltraShear nanoemulsion in rats 1–4 versus IV injection in rats 5–7 (both populations were assessed in “CBD equivalents,” measuring parent CBD combined with metabolites after correction for MW differences, to ignore the rapid CBD conversions in first-pass metabolism for the oral-GI route), the average Oral/IV relative total absorption of CBD equivalents (A(OGI/IV)) exceeded 45% at 6 h and reached nearly 80% by 24 h (Tables 5–8; Fig. 4).
Table 5.
Area under curve (AUC) calculations of oral CBD UltraShear nanoemulsion 5 mg/rat results at 6 h
| CBD AUC | Relative bioavailability, % | CBD-7-COOH (CBD equivalents) AUC | 7-OH-CBD (CBD equivalents) AUC | Total CBD equivalents AUC | Relative total absorption, % | |
|---|---|---|---|---|---|---|
| Rat 1 | 621.8 | 20.60 | 2,917.9 | 230.8 | 3,770.4 | 57.90 |
| Rat 2 | 326.8 | 10.80 | 1,698.8 | 89.5 | 2,115.1 | 32.50 |
| Rat 3 | 460.1 | 15.20 | 2,128.8 | 133.2 | 2,722.1 | 41.80 |
| Rat 4 | 836.9 | 27.70 | 2,202 | 197.8 | 3,236.6 | 49.70 |
| Avg (n = 4) | 561.4 | 18.60 | 2,236.9 | 162.8 | 2,961.1 | 45.50 |
| Std Dev | 190.3 | 6.30 | 437.7 | 55 | 613.1 | 9.40 |
Relative bioavailability of CBD from oral-GI UltraShear nanoemulsion (F(OGI/IV)) after 1st pass metabolism. Relative total absorption of CBD equivalents from oral-GI UltraShear nanoemulsion (A(OGI/IV)) includes metabolites after 1st pass metabolism.
Table 8.
Area under curve (AUC) calculations of IV CBD Control 1 mg/rat results at 24 h
| CBD AUC | Dose-adjusted (5X) CBD AUC | CBD-7-COOH (CBD equivalents) AUC | 7-OH-CBD (CBD equivalents) AUC | Total CBD equivalents AUC | Dose-adjusted (5X) total CBD equivalents AUC | |
|---|---|---|---|---|---|---|
| Rat 5 | 560.0 | 2,799.8 | 654.5 | 19.0 | 1,233.4 | 6,167.0 |
| Rat 6 | 741.9 | 3,709.6 | 850.6 | 3.2 | 1,595.7 | 7,978.3 |
| Rat 7* | 1,003.9 | 5,019.7 | 765.8 | 84.8 | 1,854.6 | 9,272.8 |
| Avg (n = 3) | 768.6 | 3,843.0 | 757.0 | 35.6 | 1,561.2 | 7,806.0 |
| Std Dev | 182.2 | 911.2 | 80.3 | 35.3 | 254.8 | 1,273.8 |
*24-h data missing for rat 7: gap-filled with 0’s to complete 24-h AUC calculation.
Fig. 4.
a Average “CBD Equivalent” plasma concentration for rats administered CBD via oral UltraShear nanoemulsion versus via IV injection, including metabolites adjusted for MW differences versus CBD. b Comparison of average oral/IV total absorption of “CBD equivalents” versus bioavailability for “CBD only” after first-pass metabolism.
Table 7.
Area under curve (AUC) calculations of oral CBD UltraShear Nanoemulsion 5 mg/rat results at 24 h
| CBD AUC | Relative bioavailability, % | CBD-7-COOH (CBD equivalents) AUC | 7-OH-CBD (CBD equivalents) AUC | TOTAL CBD equivalents AUC | Relative total absorption, % | |
|---|---|---|---|---|---|---|
| Rat 1 | 1,073.9 | 27.9 | 4,964.0 | 474.0 | 6,512.0 | 83.4 |
| Rat 2 | 657.9 | 17.1 | 4,105.7 | 256.7 | 5,020.3 | 64.3 |
| Rat 3 | 584.5 | 15.2 | 6,254.0 | 321.5 | 7,159.9 | 91.7 |
| Rat 4 | 1,586.1 | 41.3 | 4,246.5 | 355.5 | 6,188.0 | 79.3 |
| Avg (n = 4) | 975.6 | 25.4 | 4,892.6 | 351.9 | 6,220.0 | 79.7 |
| Std Dev | 398.8 | 10.4 | 850.7 | 78.9 | 776.0 | 9.9 |
Relative bioavailability of CBD from oral-GI UltraShear nanoemulsion (F(OGI/IV)) after 1st pass metabolism. Relative total absorption of CBD equivalents from oral-GI UltraShear nanoemulsion (A(OGI/IV)) includes metabolites after 1st pass metabolism.
Relative bioavailability (F(OGI/IV)) of the orally administered UltraShear CBD nanoemulsion was calculated compared to the dose-adjusted AUC values derived from control rats that received intravenous CBD in a conventional (i.e., not nanoemulsion) formulation. Based on these results, the relative bioavailability of orally delivered CBD UltraShear nanoemulsion was calculated to be 18.6% at 6 h and 25.4% at 24 h. While oral-GI bioavailability is unsurprisingly limited by first-pass metabolism, it is nonetheless notable that CBD bioavailability for oral-GI UltraShear nanoemulsion CBD is roughly 3–4X higher than the typical bioavailability for oral-GI CBD as reported in the introduction above.
Relevant pharmacokinetic parameters for the oral-GI CBD UltraShear nanoemulsion and control IV groups are presented in Tables 6 and 8. The average half-life of CBD in plasma is similar in rats receiving oral nanoemulsion (5.3 +/− 1.5 h) compared to IV injection of the conventional formulation (4.4 +/− 0.9 h). While standard deviation and %CV data have been calculated and presented for mathematical completeness in the analyses herein, it should be noted that for population sizes of 3 and 4 animals, respectively, the standard deviation and %CV results are of limited utility.
Table 6.
Area under curve (AUC) calculations of IV CBD Control 1 mg/rat results at 6 h
| CBD AUC | Dose-adjusted (5X) CBD AUC | CBD-7-COOH (CBD equivalents) AUC | 7-OH-CBD (CBD equivalents) AUC | TOTAL CBD equivalents AUC | Dose-adjusted (5X) total CBD equivalents AUC | |
|---|---|---|---|---|---|---|
| Rat 5 | 467.3 | 2,336.3 | 554.2 | 19.0 | 1,040.4 | 5,202.2 |
| Rat 6 | 596.1 | 2,980.6 | 666.7 | 3.2 | 1,265.9 | 6,329.7 |
| Rat 7 | 751.0 | 3,755.2 | 765.8 | 84.8 | 1,601.7 | 8,008.3 |
| Avg (n = 3) | 604.8 | 3,024.0 | 662.2 | 35.6 | 1,302.7 | 6,513.4 |
| Std dev | 116.0 | 580.1 | 86.5 | 35.3 | 230.6 | 1,152.9 |
Conclusions
This study has provided a compelling demonstration of unprecedented speed and efficiency of oral-GI CBD absorption of CBD UltraShear nanoemulsions, achieving 10% of levels achieved for direct IV injection within 30 min and 80% of IV levels in 24 h. Notably, within just the first hour post-administration, the bioavailability of oral-GI CBD UltraShear nanoemulsions in systemic circulation following first-pass metabolism exceeded the typical 6% total oral-GI CBD bioavailability benchmarks reported for CBD edibles and ultimately achieved 3–4X these levels within 6–24 h.
While it is anticipated that rapid first-pass metabolism in the oral-GI route limits the bioavailability of CBD in systemic circulation, it is clear that the CBD UltraShear nanoemulsion effectuates extremely fast total absorption and correspondingly higher parent CBD survival through first-pass metabolism. Indeed, this study indicated that the total absorption of CBD UltraShear nanoemulsion, including the majority of the CBD converted into metabolites by first-pass metabolism immediately following GI absorption, reached 45–50% within 6–8 h and 80% in 24 h of the total CBD bioavailability measured from IV injection. This observation offers great promise for possible rapid absorption and increased bioavailability via alternative non-oral-GI delivery, including potential sublingual, buccal, intranasal, rectal, and possibly transdermal routes. These results strongly invite additional testing of alternative delivery routes. Notably, in our hands, the most successful existing commercial CBD formulations in sesame seed oil or other vegetable oils (including Epidiolex®) exhibit bioavailability of <10% in the same cannulated Sprague-Dawley rat animal model [27].
Acknowledgments
Authors appreciate the support provided by the CORE facility of the COBRE project at the University of Mississippi which is supported by the National Institute of General Medical Sciences of the National Institutes of Health under award number P30GM122733. The content is solely the responsibility of the authors and does not represent the official views of COBRE or NIH. The authors also thank Dr. Elsayed Ibrahim for reviewing the manuscript.
Statement of Ethics
This study protocol was reviewed and approved by the University of Mississippi Animal Care and Use Committee (IACUC) and following the National Institute of Health (NIH) guidelines, approval number 22-003.
Conflict of Interest Statement
The authors declare no conflicts of interest.
Funding Sources
This study was partially supported by Pressure BioSciences Incorporated, South Easton, MA.
Author Contributions
Mahmoud ElSohly conceptualized and designed the study, interpreted the data, performed statistical analysis of the data, reviewed, edited, and directed the final approval of the manuscript. Waseem Gul and Iram Shahzadi interpreted the data, performed statistical analysis of the data, drafted, reviewed, edited, and performed the final approval of the manuscript.
Funding Statement
This study was partially supported by Pressure BioSciences Incorporated, South Easton, MA.
Data Availability Statement
Data might be available from the corresponding author on reasonable request.
References
- 1. Pertwee R. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: Δ9-tetrahydrocannabinol, cannabidiol and Δ9-tetrahydrocannabivarin. Br J Pharmacol. 2008 Jan;153(2):199–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Devinsky O, Cilio MR, Cross H, Fernandez-Ruiz J, French J, Hill C, et al. Cannabidiol: pharmacology and potential therapeutic role in epilepsy and other neuropsychiatric disorders. Epilepsia. 2014 Jun;55(6):791–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Thiele EA, Marsh ED, French JA, Mazurkie- wicz-Beldzinska M, Benbadis SR, Joshi C, et al. Cannabidiol in patients with seizures associated with Lennox-Gastaut syndrome (GWPCARE4): a ran- domised, double-blind, placebo-controlled phase 3 trial. Lancet. 2018 Mar;391(10125):1085–96. [DOI] [PubMed] [Google Scholar]
- 4. Devinsky O, Cross JH, Laux L, Marsh E, Miller I, Nabbout R, et al. Trial of cannabidiol for drug-resistant seizures in the Dravet syndrome. N Engl J Med. 2017 May;376(21):2011–20. [DOI] [PubMed] [Google Scholar]
- 5. Rudroff, T, Sosnoff, J. Cannabidiol to improve mobility in people with multiple sclerosis. Front Neurol. 2018 Mar 22;9:183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Outen JD, Burhanullah MH, Vandrey R, Amjad H, Harper DG, Patrick RE, et al. Cannabinoids for agitation in Alzheimer’s disease. Am J Geriatr Psychiatry. 2021;29(12):1253–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chagas MH, Zuardi AW, Tumas V, Pena-Pereira MA, Sobreira ET, Bergamaschi MM, et al. Effects of cannabidiol in the treatment of patients with Parkinson's disease: an exploratory double-blind trial. J Psychopharmacol. 2014 Nov;28(11):1088–98. [DOI] [PubMed] [Google Scholar]
- 8. McGuire P, Robson P, Cubala WJ, Vasile D, Morrison PD, Barron R, et al. Cannabidiol (CBD) as an adjunctive therapy in schizophrenia: a multicenter randomized controlled trial. Am J Psychiatry. 2018 Mar 1;175(3):225–31. [DOI] [PubMed] [Google Scholar]
- 9. Millar SA, Maguire RF, Yates AS, O'Sullivan SE. Towards better delivery of cannabidiol (CBD). Pharmaceuticals. 2020 Aug 28;13(9):219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Perucca E, Bialer M. Critical aspects affecting cannabidiol oral bioavailability and metabolic elimination, and related clinical implications. CNS drugs. 2020 Aug;34(8):795–800. [DOI] [PubMed] [Google Scholar]
- 11. Pagano S, Coniglio M, Valenti C, Federici MI, Lombardo G, Cianetti S, et al. Biological effects of Cannabidiol on normal human healthy cell populations: systematic review of the literature. Biomed Pharmacother. 2020 Dec 1;132:110728. [DOI] [PubMed] [Google Scholar]
- 12. Aphios Pharma LLC . Aphios pharma LLC to develop FDA-approved, cannabis-based drug for opioid addiction and other CNS disorders. Available from: https://aphios.com/investors/investors-overview/aphiospharma-llc/[Accessed March 1, 2023]. [Google Scholar]
- 13. Biosciences A. Proprietary cocrystal program for PTSD, cancer, IBD, stroke, and other rare diseases. Available from: https://artelobio.com/pipeline/art12-11/[Date Accessed March 1, 2023]. [Google Scholar]
- 14. Onodera T, Kuriyama I, Andoh T, Ichikawa H, Sakamoto Y, Lee-Hiraiwa E, et al. Influence of particle size on the in vitro and in vivo anti-inflammatory and anti-allergic activities of a curcumin lipid nanoemulsion. Int J Mol Med. 2015 Jun 1;35(6):1720–8. [DOI] [PubMed] [Google Scholar]
- 15. Affandi MMM, Julianto T, Majeed ABA. Enhanced oral bioavailability of astaxanthin with droplet size reduction. Food Sci Technol Res. 2012;18(4):549–54. [Google Scholar]
- 16. Nakano Y, Tajima M, Sugiyama E, Sato VH, Sato H. Development of a novel nanoemulsion formulation to improve intestinal absorption of cannabidiol. Med Cannabis Cannabinoids. 2019 Sep 24;2(1):35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhu P, Lv P, Zhang Y, Liao R, Liu J, Guo R, et al. Self-assembly system based on cyclodextrin for targeted delivery of cannabidiol. Front Chem. 2021 Nov 8;9:754832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang C, Li J, Sun Y, Wang C, Guo M. Fabrication and characterization of a cannabidiol-loaded emulsion stabilized by a whey protein-maltodextrin conjugate and rosmarinic acid complex. J Dairy Sci. 2022 Aug 1;105(8):6431–46. [DOI] [PubMed] [Google Scholar]
- 19. Moreno-Sanz G, Ferreiro Vera C, Sánchez-Carnerero C, Nadal Roura X, Sánchez de Medina Baena V. Biological activity of Cannabis sativa L. Extracts critically depends on solvent polarity and decarboxylation. Separations. 2020 Oct 20;7(4):56. [Google Scholar]
- 20. Smejkal G, Lazarev A, Ting E, Hollister J, Schumacher R. New CBD nano-emulsions remain stable for more than a year. Analytical Cannabis; 2021. https://www.analyticalcannabis.com/news/new-cbd-nano-emulsions-remain-stable-for-more-than-a-year-say-scientists-313502. [Google Scholar]
- 21. Banerjee A, Binder J, Salama R, Trant JF. Synthesis, characterization, and stress-testing of a robust quillaja saponin stabilized oil-in-water phytocannabinoid nanoemulsion. J Cannabis Res. 2021 Sep 23;3(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Smejkal GB, Ting EY, Nambi Arul Nambi K, Schumacher RT, Lazarev AV. Characterization of astaxanthin nanoemulsions produced by intense fluid shear through a self-throttling nanometer range annular orifice valve-based high-pressure homogenizer. Molecules. 2021 May 12;26(10):2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Janahar JJ, Marciniak A, Balasubramaniam VM, Jimenez-Flores R, Ting E. Effects of pressure, shear, temperature, and their interactions on selected milk quality attributes. J Dairy Sci. 2021 Feb;104(2):1531–47. [DOI] [PubMed] [Google Scholar]
- 24. Danaei M, Dehghankhold M, Ataei S, Hasanzadeh Davarani F, Javanmard R, Dokhani A, et al. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics. 2018 May 18;10(2):57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Harvey DJ, Mechoulam R. Metabolites of cannabidiol identified in human urine. Xenobiotica. 1990 Mar;20(3):303–20. [DOI] [PubMed] [Google Scholar]
- 26. Jiang R, Yamaori S, Takeda S, Yamamoto I, Watanabe K. Identification of cytochrome P450 enzymes responsible for metabolism of cannabidiol by human liver microsomes. Life Sci. 2011 Aug 1;89(5–6):165–70. [DOI] [PubMed] [Google Scholar]
- 27. Ashour E, Gul W, Elsohly M. Pharmacokinetic studies of CBD from CBD oil formulations. Manuscript in preparation.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data might be available from the corresponding author on reasonable request.