SUMMARY
Background
Preclinical and clinical studies suggest that combinations of broadly neutralizing antibodies (bnAbs) targeting different HIV envelope epitopes may be required for protection. The Phase 1 trial HVTN 130/HPTN 089 (ClinicalTrials.gov NCT03928821) evaluated the safety, pharmacokinetics (PK) and functional activities of dual and triple anti-HIV bnAb combinations.
Methods
Adults without HIV were enrolled and randomized from July 31 to December 20, 2019 into three dual-bnAb treatment arms simultaneously, or the triple-bnAb arm, receiving 20 mg/kg of each antibody administered intravenously (IV) at four US sites (Fenway Health Clinical Research Site [CRS] in Boston, Harlem Prevention Center CRS in New York, Nashville CRS, and Columbia Physicians & Surgeons CRS in New York). Participants received a single dose of PGT121 + VRC07–523LS (n=6), PGDM1400 + VRC07–523LS (n=6), or 10–1074 + VRC07–523LS (n=6), and two doses of PGDM1400 + PGT121 + VRC07–523LS (n=9). Primary outcomes were safety, pharmacokinetics and neutralizing activity. Adverse events were determined by clinic staff monitoring, laboratory measures of safety, and participant diaries. bnAb serum concentrations were measured by binding antibody assays and serum neutralization titers by pseudovirus assays using viruses that were sensitive to a specific bnAb and viruses sensitive to multiple bnAbs in the combinations. PK parameters were estimated using two-compartment population PK models; combination bnAb neutralization titers were directly measured and assessed using different interaction models.
Findings
Twenty-seven participants were enrolled; median age was 26 (range 19–50), 16 participants were assigned female sex at birth, and 24 were non-Hispanic white. Infusions were well tolerated. There were no statistically significant differences in PK patterns between the dual and triple combinations of PGT121, PGDM1400 and VRC07–523LS. The median estimated elimination half-lives of PGT121, PGDM1400, 10–1074, and VRC07–523LS were 32·2, 25·4, 27·5, and 52·9 days, respectively. Neutralization coverage was greater in the triple- vs dual-bnAb arms. The Bliss-Hill multiplicative interaction model, which assumes complementary neutralization with no antagonism or synergism amongst the bnAbs, best described combination bnAb titers in the dual- and triple-bnAb arms.
Interpretation
No PK interactions amongst the bnAbs and no loss of complementary neutralization were observed in the dual and triple combinations. This study lays the foundation for designing future combination bnAb HIV prevention efficacy trials.
Funding
Funding was provided by the National Institute of Allergy and Infectious Diseases (NIAID), National Institute on Drug Abuse (NIDA), the National Institute of Mental Health (NIMH), and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD).
INTRODUCTION
HIV remains an important public health concern with estimated global prevalence of 37.9 million infected persons worldwide and 1·5 million new HIV infections in 2020 [1]. Despite extraordinary advances in HIV treatment, testing, and prevention, challenges to achieving meaningful and lasting impact on HIV incidence remain. Effective biomedical HIV prevention options that are safe, less dependent on individual adherence, and have sustained effectiveness are urgently needed. Passive immunization with monoclonal antibodies (mAb) is an important HIV prevention option that will potentially require dosing only a few times a year and eliminate the need for an individual’s daily or event-driven adherence. If the effectiveness and durability of mAbs are similar to other forms of long-acting preexposure prophylaxis (PrEP), they would offer additional choices in the prevention toolbox.
A minority of individuals living with HIV develop broadly neutralizing antibodies (bnAbs) within 2 to 3 years after infection. Over the last decade, dozens of such antibodies have been identified and their target sites and mechanisms of action have been described [2–4], paving the way to their evaluation as a passive immunization HIV prevention strategy. These antibodies bind to highly conserved regions of the HIV envelope (Env) such as the CD4 binding site (CD4bs) (e.g., 3BNC117, VRC07–523LS and N6), as well as other sites of vulnerability on the HIV-1 Env protein: V3 glycan (10–1074 and PGT121), the membrane-proximal external region (MPER) of gp41 (10E8), and the V2 glycan ( PGDM1400 and CAP256-VRC26.25) [3].
Over time, bnAbs with improved breadth and potency have been identified, and modified antibodies have been engineered to extend antibody half-life or augment effector function [2, 5, 6]. Two seminal, proof-of-concept Antibody Mediated Prevention (AMP) trials demonstrated that a single bnAb (VRC01) targeting the CD4bs could prevent infection with highly sensitive HIV strains [7]. Overall, prevention efficacy was low given the predominance of resistant strains [7]. These data suggest that similar to combination antiretroviral therapy, either a combination of complementary antibodies binding different epitopes, or a single molecule engineered to bind two or three epitopes (bispecific or trispecific bnAbs) [8], will be needed to increase breadth and potency and to limit viral resistance to ultimately enhance the prevention potential of these agents [9, 10].
Defining optimal combinations of bnAbs is an area of active investigation. While many studies reporting the safety of single monoclonal Ab therapy for HIV prevention in humans have been published [11–13], data on combination bnAbs remain limited. Early studies have suggested dual and triple combinations are safe and well tolerated [14–16]. The bnAbs VRC07–523LS, PGDM1400, PGT121, and 10–1074 were selected for this study based on their neutralization potency, breadth of HIV-1 strain coverage, and their complementarity. VRC07–523LS, which targets the CD4bs, was developed from a naturally occurring mAb and optimized to increase its breadth, potency, and half-life [17]. PGDM1400 protects against a large number of strains, targets the V2 glycan, and can prevent simian HIV (SHIV) transmission in nonhuman primates (NHP) [18, 19]. PGT121, which targets the V3 glycan, has high in vitro potency and confers protection in NHP challenge models [19, 20]. Similarly, 10–1074 (also targeting the V3 glycan) has high potency, blocks cell-free virus transmission, and protects against SHIV in NHP [20–22]. PGDM1400 and PGT121 have complementary neutralization profiles against global HIV-1 viruses [19, 23].
We conducted the HIV Vaccine Trials Network (HVTN) 130/HIV Prevention Trials Network (HPTN) 089 trial to study the safety, tolerability, pharmacokinetics, and antiviral properties of these four human bnAbs targeting different sites of Env administered in dual and triple combination therapy.
METHODS
Study design and participants
HVTN 130/HPTN 089 was a randomized, phase 1 study conducted at four centers in the US (Fenway Health Clinical Research Site [CRS] in Boston, Harlem Prevention Center CRS in New York, Nashville CRS, and Columbia Physicians & Surgeons CRS in New York) between July 31, 2019 and March 25, 2021. The primary objective was to evaluate the safety, tolerability, pharmacokinetics, and serum neutralizing activity of PGT121, PGDM1400, 10–1074 and VRC07–523LS antibodies when given sequentially in combinations of two (Treatment 1, 2 and 3: T1, T2, T3) and in a combination of three (Treatment 4; T4) bnAbs dosed intravenously (IV). Study products were administered sequentially in the order specified in Figure 1, with a 15- to 30-minute time interval between individual antibodies. Volunteers were eligible for participation if aged 18 to 50 years, without HIV-1, in good general health, and not likely to be exposed to HIV during the study period based on behavior reported within the 12 months before enrollment. Full eligibility criteria are presented in Supplemental Table 1 (appendix pg. 3).
Figure 1: Consort Diagram and specimen collection schedule.
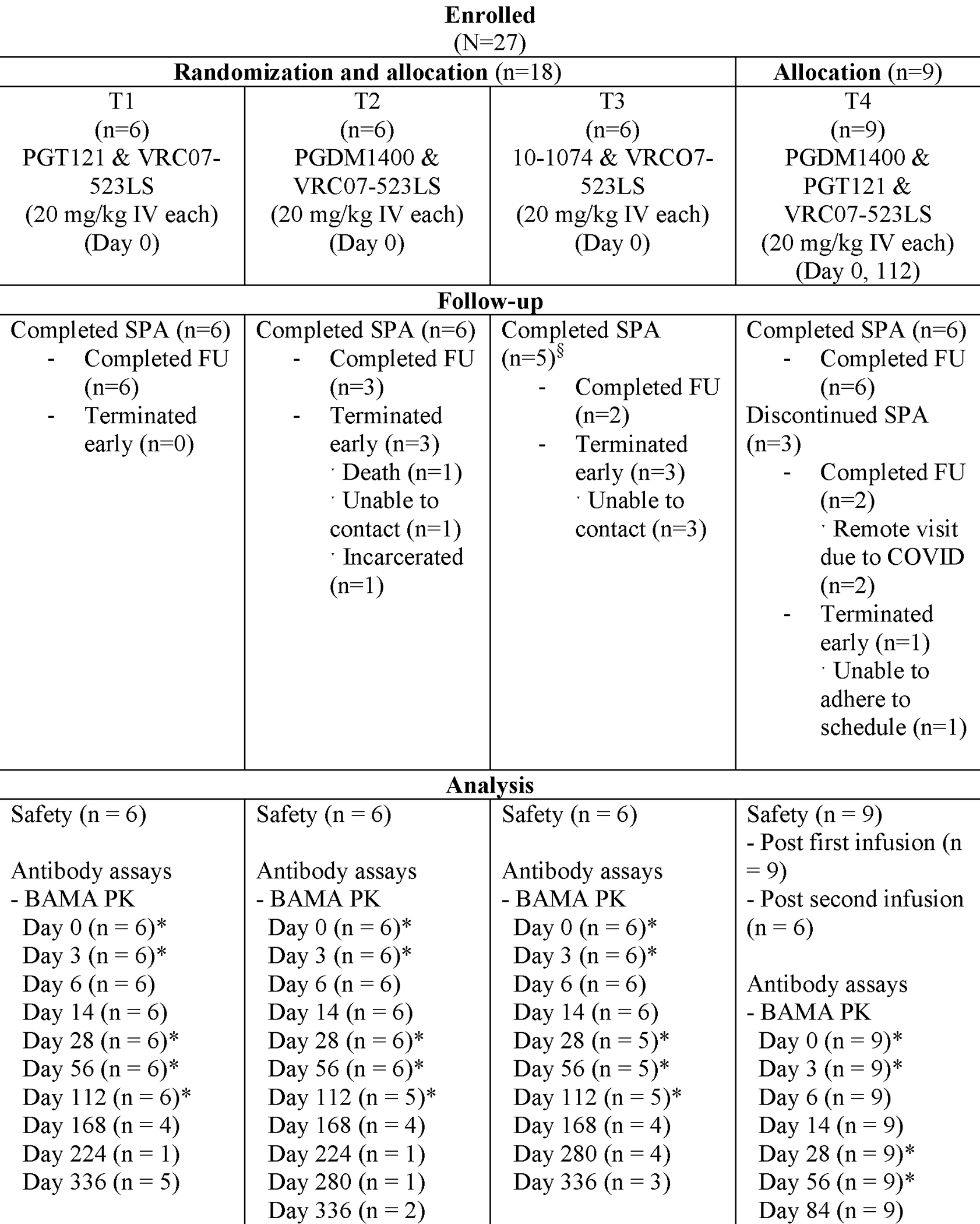
CONSORT, Consolidated Standards of Reporting Trials; IV, intravenous; BAMA, Binding Antibody Multiplex Assay; PK, pharmacokinetic; nAb, neutralizing antibody; SPA, study product administration; FU, follow-up.
The study was approved by the Institutional Review Boards of each participating CRS listed above. All participants provided written informed consent in English. The trial was overseen by the HVTN Safety Monitoring Board and registered with ClinicalTrials.gov (NCT03928821). All products were manufactured under current Good Manufacturing Practice standards.
Randomization and masking
Participants were randomized through a Web-based randomization system and the dual combination T1, T2, and T3 were randomized in blocks to ensure balance across groups. Enrollment of the triple combination T4 was contingent on review of safety data from T1–3 and thus T4 was not randomized with T1–3. Participants, clinical study staff and laboratory program staff were unblinded to participant treatment assignments.
Clinical and laboratory procedures
Clinical procedures and outcomes
Participants were screened for eligibility and randomly assigned to a treatment group within 56 days of screening. The first 18 participants were randomly assigned in a 1:1:1 ratio to sequential infusion of PGT121 and VRC07–523LS (T1), PGDM1400 and VRC07–523LS (T2), or 10–1074 and VRC07–523LS (T3) on day 0. Following review of safety data, an additional nine participants were assigned to T4 and received PGDM1400, PGT121, and VRC07–523LS on day 0 and again on day 112. All bnAbs were administered IV at a dose of 20 mg/kg. The final infusion in HVTN 130/HPTN 089 was administered to the last enrolled participant on March 9, 2020. The antibodies were infused over 60 minutes at the month 0 visit. Infusions of PGT121 and PGDM1400 at the month 4 visit occurred over 30 minutes, and VRC07–523LS at month 4 was infused over 15–30 minutes. The time interval between individual study products was 15 to 30 minutes.
Following study product administration (SPA), participants were monitored for at least 60 minutes at the study site to assess for solicited local and systemic adverse events (AEs), including pain/tenderness at the infusion site, fever, malaise, myalgia, headache, chills, arthralgia, nausea, urticaria, non-exertional dyspnea, non-exertional tachycardia, generalized pruritus, facial flushing, and unexplained diaphoresis. Participants subsequently completed daily diary entries to document AEs for three days following infusion. Focused physical exams and laboratory assays (complete blood count with differential, creatinine, alanine aminotransferase, urine dipstick, pregnancy testing, and HIV testing) were performed for safety monitoring at prespecified intervals throughout the study. Unsolicited AEs were documented throughout the duration of the study.
Although enrollment was completed when COVID-19 hit the United States in March 2020, follow-up was not completed, and 3 participants had not yet received their second (and final) infusion. As all attention turned to COVID-19 response, the protocol was amended to minimize study staff and participant risk of COVID-19 exposure: visit windows were extended and remote clinic visits were permitted (e.g., phone, text message, email, or other electronic means).
Laboratory methods
Serum PGT121, PGDM1400 and VRC07–523LS concentrations prior to and following administration were quantified by a validated binding antibody multiplex assay (BAMA) [24]; serum 10–1074 concentrations were quantified by a validated ELISA assay [14]. Serum bnAb concentrations were determined using standard curves for the corresponding mAb run on the same assay plate. Assays were conducted under Good Clinical Laboratory Practice (GCLP) standards, and performance of mAb standard curves and spiked quality control samples were tracked using Levey-Jennings charts.
Neutralizing antibody activity against HIV-1 was measured as a function of reductions in Tat-regulated luciferase (Luc) reporter gene expression in TZM-bl cells as described [25, 26]. The assay performed in TZM-bl cells measured neutralization titers against four Env-pseudotyped viruses that enables individual bnAb activities to be determined when multiple bnAbs were present (i.e., bnAb-specific viruses) (Supplementary Table 2, appendix pg. 3) in each infusion group. An additional panel of 12 Env-pseudotyped viruses each one selected as being highly susceptible to all antibodies used in this study (Supplementary Table 3, appendix pg. 4), was used to generate a rich dataset for validation of the predictive modeling and the assumption that combined effects of these three antibodies are mostly additive. The non-infused antibodies were assayed against this latter panel of viruses at concentrations and ratios that are consistent with their pharmacokinetics after intravenous infusion in normal healthy human volunteers as comparator values for the activity of post-infusion serum samples. Serum neutralization titer was defined as the serum dilution that reduced relative luminescence units (RLU) by 50% and 80% (ID50 and ID80) relative to the RLU in virus control wells (cells + virus only) after subtraction of background RLU (cells only).
Anti-drug antibodies (ADAs) against 10–1074, PGDM1400, PGT121, or VRC07–523LS were detected and quantified using a qualified bridging electrochemiluminescence assay as previously described [27]. Assays were conducted under Good Clinical Laboratory Practice (GCLP) standards. Samples were tested in duplicate along with a panel of anti-idiotype and negative controls and data accepted based on meeting pre-established criteria.
Outcomes
The primary clinical objective was to evaluate safety and tolerability of the bnAbs administered in various combinations via sequential intravenous (IV) infusion. The primary endpoints included frequency of local and systemic solicited adverse events (AEs), laboratory measures of safety, unsolicited AEs, and serious AEs were recorded by clinicians at every visit as described above. The primary laboratory objectives included evaluating serum concentrations of each bnAb and serum neutralizing activity against bnAb-specific and the global panel of viruses. A complete list of primary, secondary and exploratory objectives and endpoints is described in Supplementary Table 4 (appendix pg. 5).
Statistical and pharmacokinetics analysis
All data were analyzed according to participants’ assigned treatment in the modified intent-to-treat (MITT) cohort that included all enrolled participants receiving the first infusion. For each bnAb, serum concentrations following IV administration exhibited bi-exponential decay and were described by an open 2-compartment disposition model with first-order elimination from the central compartment. The model was parameterized in terms of four parameters: clearance from the central compartment (CL, L/day), volume of the central compartment (Vc, L), inter-compartmental distribution clearance (Q, L/day) and volume of the peripheral compartment (Vp, L). An exponential between-individual random effect was considered for CL, Vc, Q, and Vp based on patterns observed in the data. Nonlinear mixed effects modeling with the stochastic approximation of expectation-maximization (SAEM) estimation method was employed. For each bnAb used in the triple combination (PGT121, PGDM1400 and VRC07–523LS), PK data collected from both the dual-administration and triple-administration were pooled for the PK modeling; the effect of dual- vs. triple-administration on each of the PK parameter was tested using the likelihood ratio test in the PK model. For 10–1074, only PK data from the dual combination was used for the modeling. PK modeling was performed using Monolix (Version 2019R1. Antony, France: Lixoft SAS, 2019); all other analyses used SAS or R3.5.1. Combination bnAb neutralization interactions were assessed using the Maximum, Additive, and Bliss-Hill models [28]. The Concordance Correlation Coefficient (CCC) was used to quantify the agreement between observed and estimated combination neutralization titers under each of the interaction models.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, or data interpretation.
RESULTS
Participant demographics and infusion frequency
Twenty-seven healthy participants without HIV enrolled between July 31, 2019 and December 20, 2019 at four US-based CRS affiliated with the HVTN and HPTN. Duration of follow-up was 12 months and 16 months for participants receiving dual and triple combination bnAb therapy, respectively, and all visits were completed by March 25, 2021 (Table 1). Participant median age was 26 and 16/27 (59%) were assigned female at birth (Table 1). Seventy percent (19/27) identified as White (non-Hispanic), 2 (7%) identified as Black, and 3 (11%) as Hispanic or Latino. Data unavailability was mostly caused by missed visits or remote visits due to the COVID-19 pandemic (Supplementary Table 5, appendix pg. 6).
Table 1.
Demographic characteristics of HVTN 130/HPTN 089 participants.
| T1 (n=6) | T2 (n=6) | T3 (n=6) | T4 (n=9) | Total (N=27) | ||
|---|---|---|---|---|---|---|
| Sex at birth, n (%) | Female | 5 (83⋅3%) | 3 (50⋅0%) | 3 (50⋅0%) | 5 (55⋅6%) | 16 (59⋅3%) |
| Male | 1 (16⋅7%) | 3 (50⋅0%) | 3 (50⋅0%) | 4 (44⋅4%) | 11 (40⋅7%) | |
| Gender1, n (%) | Female | 3 (50⋅0%) | 3 (50⋅0%) | 3 (50⋅0%) | 4 (44⋅4%) | 13 (48⋅1%) |
| Male | 2 (33⋅3%) | 3 (50⋅0%) | 3 (50⋅0%) | 3 (33⋅3%) | 11 (40⋅7%) | |
| Other2 | 1 (16⋅7%) | 0 (0⋅0%) | 1 (16⋅7%) | 2 (22⋅2%) | 4 (14⋅8%) | |
| Age, years, median (range) | 25 (21, 25) | 29 (21, 50) | 28 (25, 49) | 30 (19, 49) | 26 (19, 50) | |
| Weight, kg, median (range) | 72⋅3 (55⋅7, 84⋅4) | 61⋅6 (55⋅8, 74⋅4) | 67⋅8 (51⋅3, 77⋅6) | 71⋅2 (53⋅5, 86⋅4) | 68⋅0 (51⋅3, 86⋅4) | |
| Ethnicity, n (%) | Hispanic or Latino | 0 (0⋅0%) | 1 (16⋅7%) | 0 (0⋅0%) | 2 (22⋅2%) | 3 (11⋅1%) |
| Not Hispanic or Latino | 6 (100⋅0%) | 5 (83⋅3%) | 6 (100⋅0%) | 7 (77⋅8%) | 24 (88⋅9%) | |
| Race, n (%) | Black | N/A4 | N/A | 2 (33⋅3%) | 0 (0⋅0%) | 2 (7⋅4%) |
| White | 5 (83⋅3%) | 3 (50⋅0%) | 4 (66⋅7%) | 7 (77⋅8%) | 19 (70⋅4%) | |
| Other3 | 1 (16⋅7%) | 3 (50⋅0%) | N/A | 2 (22⋅2%) | 6 (22⋅2%) | |
Participants could self-report more than one gender identity, thus percentages may total more than 100%.
Other categories: transgender male, transgender female, gender queer, gender variant or gender nonconforming, self-identify, and/or prefer not to answer.
Other categories: Asian, Native Hawaiian/other Pacific Islander, American Indian/Alaska Native, other, multiracial.
Participants are not required to answer these questions, so counts may not match the number of participants. Missingness is not included explicitly as it is minimal.
Six of six (100%) participants each in T1 & T2 and 5/6 (83%) participants in T3 received a single IV dose of either PGT121, PGDM1400 or 10–1074 followed by VRC07–523LS, respectively. Nine of nine (100%) participants in T4 received a first IV dose of VRC07–523LS administered sequentially with PGDM1400 and PGT121 and 6/9 (67%) received the second IV dose 4 months later; 3/9 (33%) participants missed the second dose due to CRS operational disruptions from the SARS-CoV-2 pandemic with one participant missing a visit and two participants undergoing remote visits, which precluded study product administration and collection of samples (Figure 1). A total of 26 participants received at least one dose of VRC07–523LS at 20 mg/kg.
Safety and tolerability
Overall, IV administration of study products was generally well tolerated with no related serious AEs (SAEs), unexpected reactions, or safety events warranting study pause. Nineteen (70%) participants reported 52 unsolicited AEs, the majority of which were grade 1 (31 in 16 participants, 60%) or grade 2 (20 in 13 participants, 48%) in severity. The most commonly reported AEs were upper respiratory tract infection in four participants (15%), decreased platelet count in three (11%; one grade 2 at day 112, the rest grade 1/mild), grade 1 pyrexia in two (7%, during study follow up, unrelated to study product), COVID-19 in two (7%; one grade 1 and one grade 2), urinary tract infection in two (7%), and increased alanine aminotransferase in two participants (7% occurring at day 14 and 112). In 18 participants (67%), 51 unsolicited events were of mild or moderate severity. There was one SAE of death due to respiratory failure in a participant in T2 with an undisclosed pre-existing history of asthma who had received his last SPA more than 7 months earlier. This SAE was assessed by the Principal Investigator as not related to study products.
Two participants (7%) developed two AEs that were assessed as related to the study products; these resolved without residual effects. One participant in T3 developed mild and episodic bilateral hand paresthesia the evening of SPA, which resolved the following day. One participant in T2 developed a mild infusion-related reaction (chills, mild upper back muscle pain, mild joint pain in wrists, and a mild headache), which resolved within one hour.
Solicited local and systemic reactions in dual and triple combination treatment arms were mild to moderate and are summarized in Supplementary Figure 1 (appendix pg. 15).
Pharmacokinetics
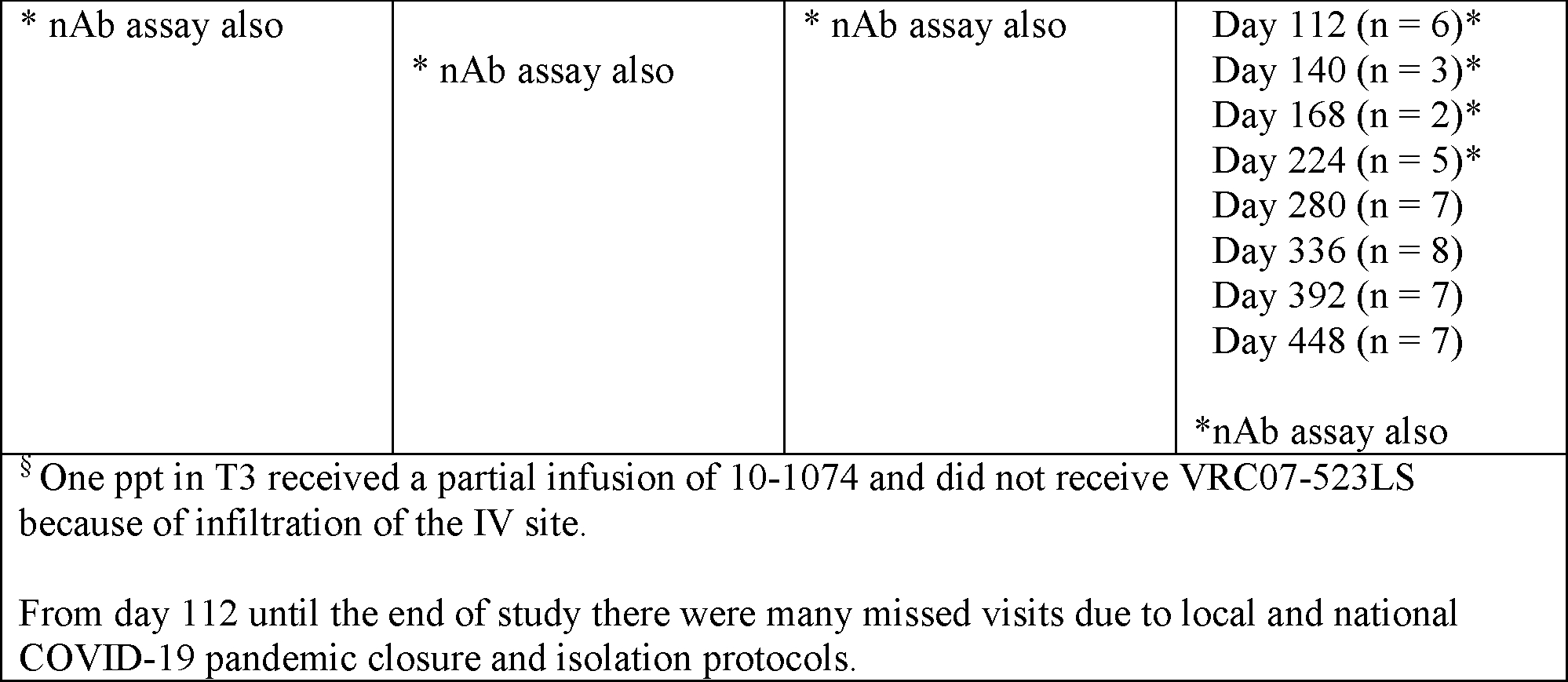
The observed antibody concentrations across treatment arms and at every timepoint following infusion are displayed in Figure 2 and summarized in Supplementary Table 6 (appendix pg. 7). The median elimination half-life estimates were 32·2 days (mean = 33·0 days, 95% CI 28·9–36·5) for PGT121, 25.4 days (mean = 25·7 days, 95% CI 23·9–27·7) for PGDM1400, 27·5 days (mean = 30·1 days, 95% CI 27·7–33·0) for 10–1074, and 52·9 days (mean = 53·3 days, 95% CI 49·5–57·8) for VRC07–523LS (Supplementary Table 7, appendix pg. 9)). More details of other PK parameters are provided in Supplementary Tables 9–12 (appendix pg. 11–14). There were no statistically significant differences in any PK parameters for PGT121, PGDM1400 and VRC07–523LS between dual-combination T1 and T2 compared to triple-combination T4. Coadministration in dual or triple combination did not affect individual antibody PK (Supplementary Table 7, appendix pg. 9). The median elimination half-life of VRC07–523LS in the triple combination (T4), 54·9 days (standard deviation (SD) = 6·7), was not significantly different than in the dual combinations (T1, T2 or T3): T1, 53·2 days (SD = 6·5); T2, 50·6 days (SD = 4·7); and T3, 51·8 days (SD = 8·3) (Supp Figure 2, appendix pg. 16).
Figure 2: Observed antibody serum concentrations with 90% prediction interval from the population PK model.
Observed (symbol) and predicted (line) concentrations of each bnAb. Serum concentration was measured by BAMA and 10–1074 by ELISA. Shaded areas denote 90% prediction interval. One T3 participant received partial infusion of 10–1074 and no VRC07–523LS.
Neutralization antibody concentrations
Neutralization function predicted by PK data
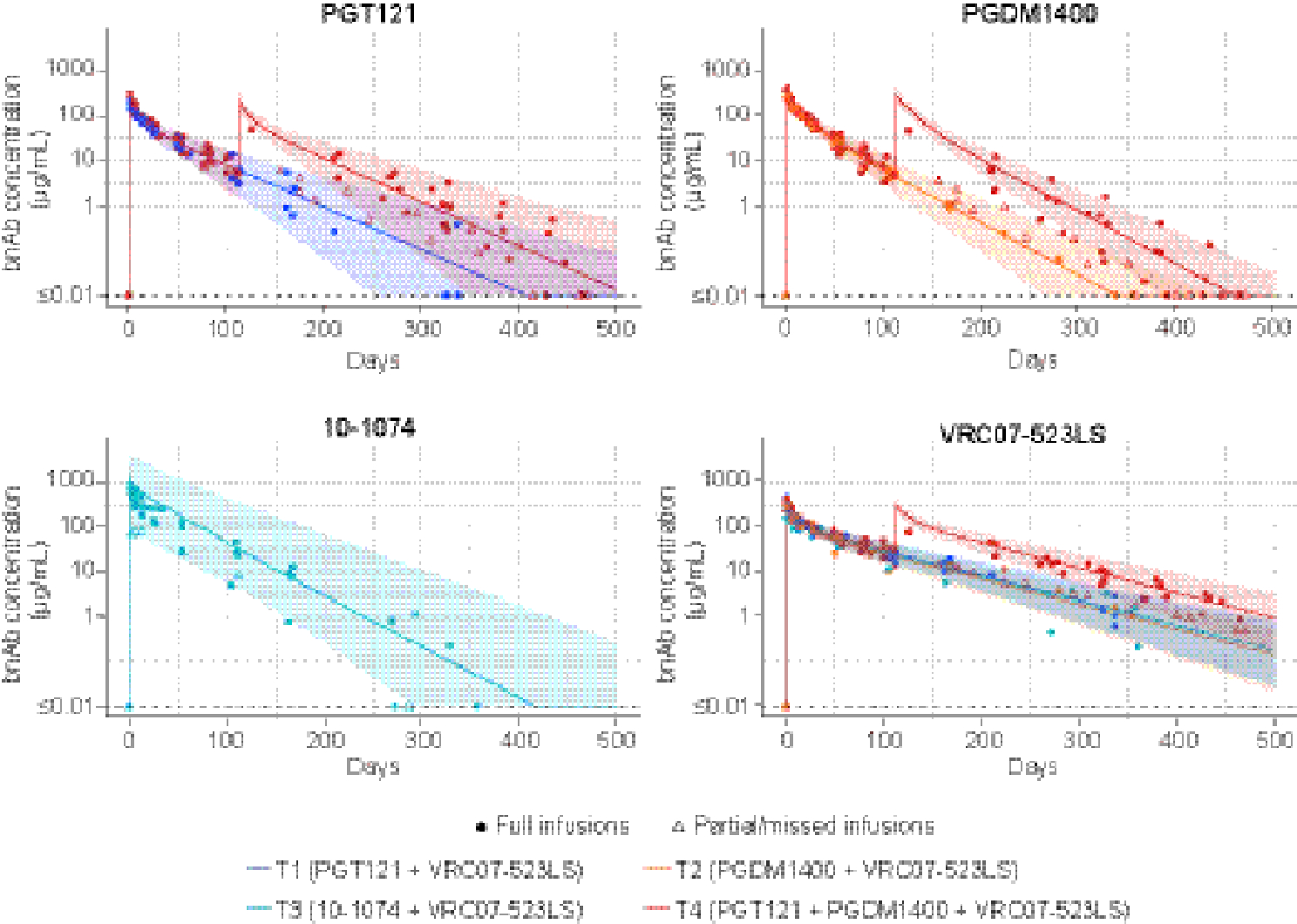
An important objective of the study was to assess how much of the neutralization activity was retained after passive administration of the bnAbs via estimating neutralization-effective antibody serum concentration as the product of the observed ID80 (or ID50) neutralization titer and the in vitro neutralization potency IC80 (or IC50) of the clinical lot of the bnAbs against the same virus. The neutralization-effective concentration of VRC07–523LS was 324·2 μg/mL, 257·8 μg /mL, 169·7 μg /mL and 193·4 μg /mL at the peak time-point (day 3 after infusion) in T1-T4, respectively (Figure 3). These values were similar to those measured by BAMA, suggesting that neutralization function for each bnAb in the combinations was largely maintained in serum as predicted by binding antibody assay-based PK concentrations. Similar neutralization-effective serum concentrations were observed for all dual and triple bnAb combinations. ID50 neutralizing titers showed the same pattern (Supp Figure 3, appendix pg. 17).
Figure 3. Geometric mean ID80 neutralization-effective serum concentrations of each bnAb in dual and triple-bnAb combinations.
x axis: time in days following infusions; y axis: TZM-bl assay derived concentration. Neutralization-effective serum concentrations of each bnAb were calculated by multiplying the ID80 neutralization titer of the serum sample against a bnAb-specific isolate by the IC80 of the clinical lot of the bnAb against the corresponding isolate.
Predicting bnAb dual and triple combination neutralization titers
In vitro models suggest that administering bnAbs in combination produces more potent and broader neutralization than administering a single bnAb [10, 23, 29]. Additionally, the triple combination of PGT121 + PGDM1400 + VRC07–523LS has been predicted to produce neutralization superior to any of the dual combinations [30]. Among participants who received all scheduled product administrations, we measured serum neutralization against a panel of 12 Env pseudoviruses in which each virus was highly susceptible to all four bnAbs. As expected, participants in the triple combination (T4) had greater magnitude of neutralizing activity against the panel than those from the dual-bnAb combination arms (T1, T2, T3) (Figure 4). This pattern was seen at each timepoint through day 112, and ID50 titer showed a similar trend (Supp Figure 4, appendix pg. 18).
Figure 4. Individual participant and group-average ID80 neutralization magnitude and breadth against a 12 multi-clade virus panel at 3 (Panel A), 28 (Panel B) and 112 days (Panel C) after product administration.
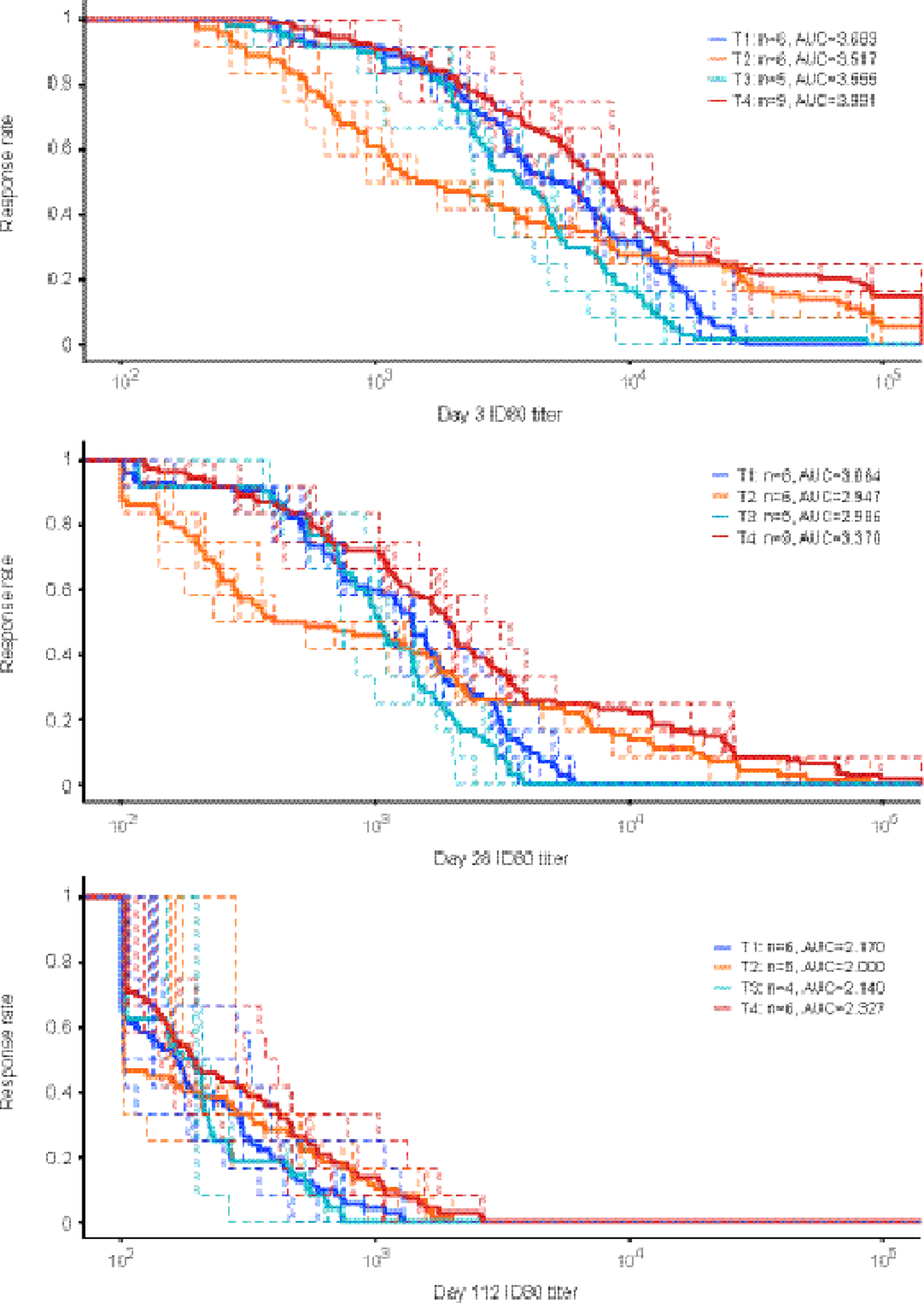
Participants received all scheduled product administrations. Dashed curves are for individual participant and solid curves are for group average. Area under the curve (AUC) are calculated for the group average magnitude-breadth curve. Env-pseudotyped virus panel includes: 0330.v4.c3; 3426.v5.c17; 377.v4.c09; AC10.0.29; Ce1176_A3; DU156.12; DU172.17; PVO.4; RHPA4259.7; SC422661.8; T263–8; and TRO.11. x-axis: neutralization ID80 titer. Y-axis: fraction of isolates with neutralization ID80 titer great then t.
Combination antibody neutralization interactions were assessed in serum samples using three different mathematical modeling approaches (additive, maximum and Bliss-Hill) that, in vitro, enabled predicting the bnAb combination neutralization magnitude and breadth using single bnAb data [23, 29]. We observed good agreement with a high concordance correlation coefficient (CCC > 0·9 mostly) between the observed and predicted combination serum neutralization titers against a 12-virus panel for participants in T4 (Figure 5, Supp Figure 5 [appendix pg. 19], Supp Table 8 [appendix pg. 10]). bnAb combination serum neutralization titers were best predicted by the Bliss-Hill model based on individual bnAb neutralization titers. Similar performances of the Bliss-Hill model were observed for the dual combination arms.
Figure 5. Concordance between observed and estimated combination bnAb neutralization ID80 titers against a 12 multi-clade virus panel in T4 participants.
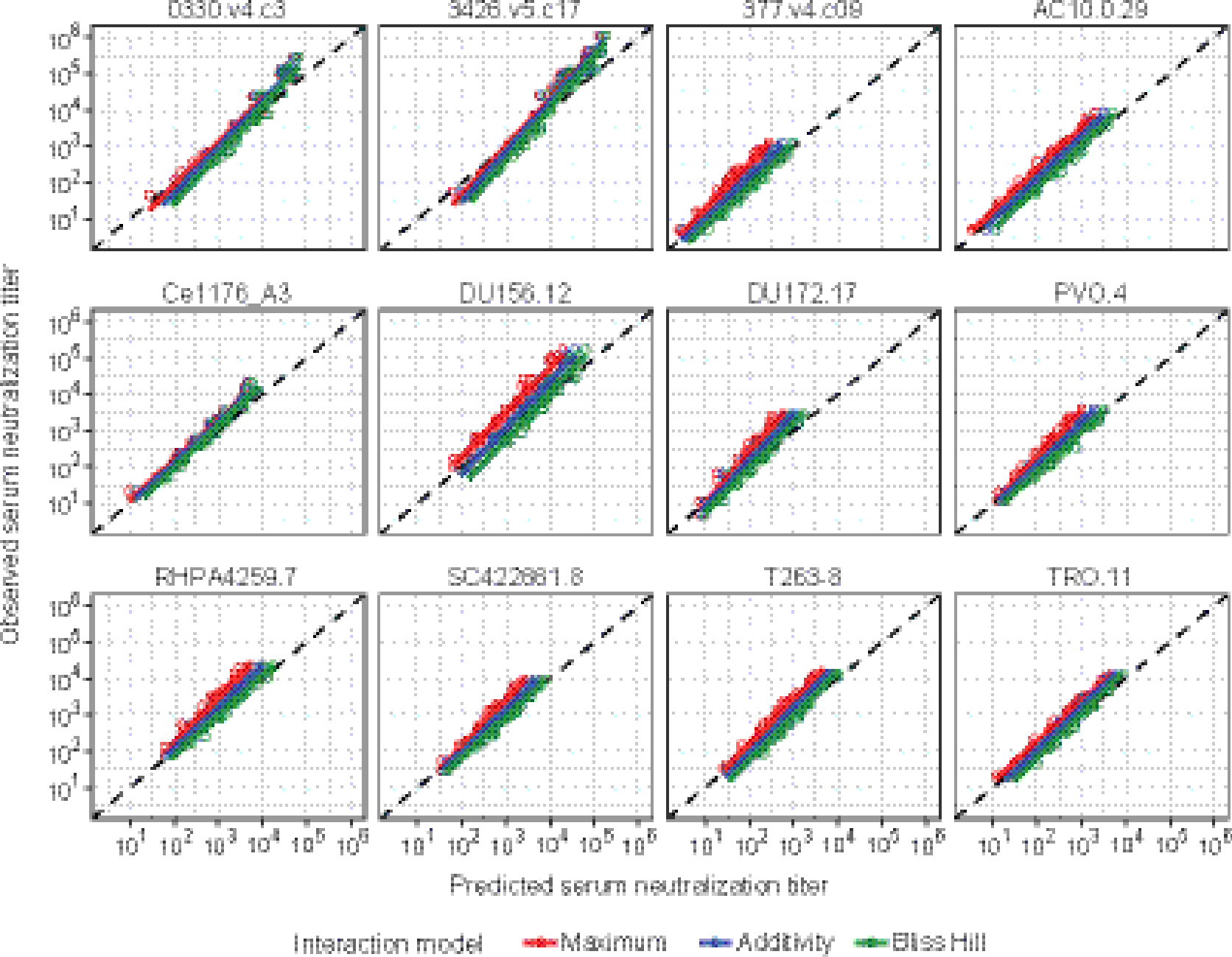
Y-axis: observed neutralization ID80 titer at 3, 28 and 112 days after product administration in serum samples of T4 participants who received the triple bnAb combination PGT121+PGDM1400+VRC07–523LS. X-axis: estimated combination neutralization ID50 titer under the Maximum (red color), Additive (blue) and Bliss-Hill (green) neutralization interaction model. Circles denote individual data points. Solid lines denote the best fit linear regression line. Dashed line denote the identity Y=X line (indicating perfect agreement).
Anti-drug antibodies
Serum samples for participants were evaluated for anti-drug antibodies (ADA) prior to treatment and then at several follow-up visits. No treatment-induced or boosted ADAs against any of the four drug products were observed (Supp Figure 6, appendix pg. 20).
DISCUSSION
This study provides important data about the safety and tolerability of dual and triple combination of bnAbs when administered sequentially via IV infusion and is among the first to describe the use of triple bnAb combinations. All combinations and infusions were generally well tolerated with no serious adverse events attributed to the study products. This is similar to prior reports evaluating dual or triple bnAb combinations for HIV prevention or for treatment in persons living with HIV, and adds to the body of critical data as we move toward future bnAb efficacy trials. Additionally, the data reflect the challenges of conducting an interventional clinical trial for HIV prevention in the setting of the COVID-19 pandemic.
We noted that PK patterns were consistent for each bnAb between the dual or triple combinations, and these were similar to previous reports [31], even accounting for different study population, timepoints, and dose. For example, another Phase 1 study evaluating single-dose pharmacokinetics of VRC07–523LS administered via different routes and doses reported an estimated median elimination half-life of 54·8 days [31], which is similar to what we report in our study (52·9 days). The finding that coadministration in dual or triple combination did not affect the PK features of the individual bnAbs suggests that there was no clear evidence of PK interactions between any of the antibodies.
The neutralization function was maintained as predicted by the PK data and the complementary neutralization magnitude and breadth of bnAbs, with no antagonism or synergism amongst the bnAbs, were maintained in this study. We also confirmed that the neutralization coverage was the greatest in the triple bnAb combination compared to any of the dual combinations. The Day 112 concentration decays were not at very high titers, but we believe this issue can be resolved with the improved half-life LS versions of the V2 and V3 bnAbs [32, 33]. At Days 3 and 28 we observed ID80 titers of ~200, which was recently shown to be sufficient for prevention of HIV acquisition [33]. The average serum concentrations at 16 weeks after the second IV infusion at a dose level of 20 mg/kg were predicted to be 1.6, 1.8 and 3.4 mcg/mL, respectively, for PGDM1400, PGT121, and VRC07–523LS. These trough concentration levels were believed to confer sufficient neutralization against diverse panels of viruses. Based on the protective neutralization threshold defined in the AMP study [33], these concentration levels collectively confer protection against a virus with in vitro neutralization sensitivity of IC80 around 0.01 to 0.04 mcg/mL.
We found that combination bnAb neutralization titers can be predicted using individual bnAb neutralization titers, which validates the in vitro models. The ability to accurately predict the breadth and potency of antibody combinations without experimental validation is important to future rapid and iterative identification of optimal combinations, especially in resource limited settings.
Our study did have some limitations. Due to COVID-19 imposed operational restrictions, several participants had remote visits and therefore samples were missing from timepoints, including 3 out of the 9 participants in Group 4 having missed their second infusion. This reduced sample size influences the precision (not necessarily the accuracy) of our study results; however, we do not believe the interpretation of the presented results is majorly impacted. Specifically, for the evaluation of the safety endpoints, data after the first infusion were available from all 27 participants, and available from 6 (out of 9) participants in the triple-bnAb group (T4) after the second infusion. Importantly, 2 of the 3 participants who missed the second infusion in T4 did attend at least one additional clinical/blood collection visit. Therefore, for lab endpoints including bnAb concentrations and neutralization titers, we were able to account for the actual number of infusions that each participant received, and the actual visit and specimen collection dates based on all available data. Another drawback is that, as noted above, a third of the participants in T4 did not receive the second triple bnAb infusion due to disruption caused by COVID-19 pandemic. It is important to note that the panel of viruses used in our neutralization assays were chosen to be sensitivity to the four antibodies, and may not represent the diversity of circulating strains. Lastly, even though the point estimates of the PK parameters for the dual and triple combinations did not seem to differ in a clinically meaningful way, it is important to highlight the small sample size of the study. Given the sample size, we only had power to detect large differences in PK or neutralization between the dual and triple combinations, as well as overall safety. These results, therefore, will need to be further investigated in on-going (NCT04212091, NCT05184452) and future bnAb trials evaluating LS formulations of all four antibodies in dual and triple combinations.
These data, building on AMP correlates results, provide an important basis for the prediction of prevention efficacy of the triple-mAb combination, and inform the field as we seek to identify optimal antibody combinations for HIV prevention. It is expected that future studies will evaluate mAb combinations incorporating LS-modified versions of all 4 antibodies, which are expected to evolve to improve function and administration, in preparation for a planned efficacy trial.
Supplementary Material
RESEARCH IN CONTEXT.
Evidence before this study
Passive immunization with broadly neutralizing antibodies (bnAbs) to prevent HIV acquisition is a rapidly expanding field. A single antibody targeting the CD4 binding site on the HIV envelope conferred protection against HIV strains susceptible to the antibody in the AMP trials, albeit prevention efficacy was limited due to circulating strains resistant to VRC01. To address this issue, several bnAbs are being tested alone or in combinations for safety, tolerability, and pharmacokinetic properties in Phase 1 trials as well as in nonhuman primate models. Studies assessing neutralization coverage and potential interactions of different bnAb combinations are important to guide the selection of optimal combinations and further inform the HIV prevention field. We performed a systematic search in PubMed and clinicaltrials.gov for the search terms PGT121, PGDM1400, 10–1074, and VRC07–523LS through October 2022 and assessed publications for quality of evidence. The present study was designed to evaluate the safety and tolerability, neutralization activity, as well as pharmacokinetics of dual and triple antibody combinations that target three different envelope regions in people living without HIV.
Added value of this study
This Phase 1 study demonstrated the feasibility of combining anti-HIV bnAbs targeting different sites on the HIV envelope in people living without HIV. It also demonstrated that the dual- and triple-antibody combinations were as effective as the individual antibodies at neutralization, thus justifying a combination approach going forward for additional monoclonal antibody studies for HIV prevention. The fact that modeling predicted the combination neutralization titer based on the single antibody titers will be valuable for future trial design of different antibody combinations.
Implications of all the available evidence
Our data demonstrate that a combination of three bnAbs can achieve the breadth and titer indicative of advanced clinical development. Addition of Fc mutations to increase the half-life of the V2 and V3 loop directed antibodies to match that of VRC07–523LS should enhance the feasibility of this type of intravenous regimen for global prevention of HIV.
ACKNOWLEDGMENTS
We’d like to thank the study participants. We’d also like to thank Kelvin Chiong, Lu Zhang, Mark Sampson, Martina Wesley, Caroline Brackett, and Nicole Yates Ph.D. for help with lab assays. Overall support for the HIV Prevention Trials Network (HPTN) is provided by the National Institute of Allergy and Infectious Diseases (NIAID), Office of the Director (OD), National Institutes of Health (NIH), National Institute on Drug Abuse (NIDA), the National Institute of Mental Health (NIMH), and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) under Award Numbers UM1AI068619 (HPTN LOC), UM1AI068617 (HPTN SDMC), and UM1AI068613 (HPTN LC). Additional funding includes National Institutes of Health grants UM1 AI068614 (HVTN LOC), UM1 AI068635 (HVTN SDMC), and UM1 AI068618 (HVTN LC), UM1 AI069412, UM1 AI069439, UM1 AI069470.
Footnotes
DECLARATION OF INTERESTS
MES received NIH funding to the institution to conduct bnAb prevention studies.
BJ is a part-time employee and equity holder of Leyden Laboratories B.V., a company developing pandemic prevention therapeutics. All other authors have no conflicts to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Magdalena E. Sobieszczyk, Columbia University Irving Medical Center, New York, NY, USA.
Sharon Mannheimer, Mailman School of Public Health, Columbia University, New York, NY, USA.
Carmen A. Paez, Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Center, Seattle, WA, USA
Chenchen Yu, Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Center, Seattle, WA, USA.
Theresa Gamble, FHI 360, Durham, NC, USA.
Deborah A. Theodore, Columbia University Irving Medical Center, New York, NY, USA
Wairimu Chege, National Institute of Allergy and Infectious Diseases, Rockville, MD, USA.
Margaret Yacovone, National Institute of Allergy and Infectious Diseases, Rockville, MD, USA.
Brett Hanscom, Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Center, Seattle, WA, USA.
Jack Heptinstall, Duke University School of Medicine, Durham, NC, USA.
Kelly E. Seaton, Duke University School of Medicine, Durham, NC, USA
Lily Zhang, Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Center, Seattle, WA, USA.
Maurine D. Miner, Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Center, Seattle, WA, USA
Amanda Eaton, Duke University School of Medicine, Durham, NC, USA.
Joshua A. Weiner, Thayer School of Engineering, Dartmouth College, Hanover, NH, USA
Kenneth Mayer, Fenway Institute, Boston, MA, USA.
Spyros Kalams, Vanderbilt University Medical Center, Nashville, TN, USA.
Kathryn Stephenson, Ragon Institute of MGH, MIT and Harvard, Cambridge, MA, USA.
Boris Julg, Ragon Institute of MGH, MIT and Harvard, Cambridge, MA, USA.
Marina Caskey, Rockefeller University, New York, NY, USA.
Michel Nussenzweig, Rockefeller University, New York, NY, USA.
Lucio Gama, Vaccine Research Center, National Institute of Health, Bethesda, MD, USA.
Dan H. Barouch, Ragon Institute of MGH, MIT and Harvard, Cambridge, MA, USA
Margaret E. Ackerman, Thayer School of Engineering, Dartmouth College, Hanover, NH, USA
Georgia D. Tomaras, Duke University School of Medicine, Durham, NC, USA
Yunda Huang, Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Center, Seattle, WA, USA.
David Montefiori, Duke University School of Medicine, Durham, NC, USA.
DATA SHARING
All data will be publicly available upon publication.
REFERENCES
- 1.UNAIDS. Global HIV & AIDS statistics - Fact sheet. Accessed August 18, 2021. [Google Scholar]
- 2.Karuna ST, Corey L. Broadly Neutralizing Antibodies for HIV Prevention. Annu Rev Med 2020; 71: 329–46. [DOI] [PubMed] [Google Scholar]
- 3.Mahomed S, Garrett N, Baxter C, Abdool Karim Q, Abdool Karim SS. Clinical Trials of Broadly Neutralizing Monoclonal Antibodies for Human Immunodeficiency Virus Prevention: A Review. J Infect Dis 2021; 223(3): 370–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walsh SR, Seaman MS. Broadly Neutralizing Antibodies for HIV-1 Prevention. Front Immunol 2021; 12: 712122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaudinski MR, Coates EE, Houser KV, et al. Safety and pharmacokinetics of the Fc-modified HIV-1 human monoclonal antibody VRC01LS: A Phase 1 open-label clinical trial in healthy adults. PLoS Med 2018; 15(1): e1002493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roopenian DC, Akilesh S. FcRn: the neonatal Fc receptor comes of age. Nat Rev Immunol 2007; 7(9): 715–25. [DOI] [PubMed] [Google Scholar]
- 7.Corey L, Gilbert PB, Juraska M, et al. Two Randomized Trials of Neutralizing Antibodies to Prevent HIV-1 Acquisition. N Engl J Med 2021; 384(11): 1003–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Padte NN, Yu J, Huang Y, Ho DD. Engineering multi-specific antibodies against HIV-1. Retrovirology 2018; 15(1): 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Julg B, Liu PT, Wagh K, et al. Protection against a mixed SHIV challenge by a broadly neutralizing antibody cocktail. Science translational medicine 2017; 9(408). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kong R, Louder MK, Wagh K, et al. Improving neutralization potency and breadth by combining broadly reactive HIV-1 antibodies targeting major neutralization epitopes. J Virol 2015; 89(5): 2659–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaudinski MR, Houser KV, Doria-Rose NA, et al. Safety and pharmacokinetics of broadly neutralising human monoclonal antibody VRC07–523LS in healthy adults: a phase 1 dose-escalation clinical trial. Lancet HIV 2019; 6(10): e667–e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mayer KH, Seaton KE, Huang Y, et al. Safety, pharmacokinetics, and immunological activities of multiple intravenous or subcutaneous doses of an anti-HIV monoclonal antibody, VRC01, administered to HIV-uninfected adults: Results of a phase 1 randomized trial. PLoS Med 2017; 14(11): e1002435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takuva S, Karuna ST, Juraska M, et al. Infusion Reactions After Receiving the Broadly Neutralizing Antibody VRC01 or Placebo to Reduce HIV-1 Acquisition: Results From the Phase 2b Antibody-Mediated Prevention Randomized Trials. J Acquir Immune Defic Syndr 2022; 89(4): 405–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen YZ, Butler AL, Millard K, et al. Safety, pharmacokinetics, and immunogenicity of the combination of the broadly neutralizing anti-HIV-1 antibodies 3BNC117 and 10–1074 in healthy adults: A randomized, phase 1 study. PLoS One 2019; 14(8): e0219142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mahomed S, Garrett N, Capparelli EV, et al. Safety and pharmacokinetics of monoclonal antibodies VRC07–523LS and PGT121 administered subcutaneously for HIV prevention. J Infect Dis 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Julg B, Stephenson KE, Wagh K, et al. Safety and antiviral activity of triple combination broadly neutralizing monoclonal antibody therapy against HIV-1: a phase 1 clinical trial. Nature Medicine 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rudicell RS, Kwon YD, Ko SY, et al. Enhanced potency of a broadly neutralizing HIV-1 antibody in vitro improves protection against lentiviral infection in vivo. J Virol 2014; 88(21): 12669–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Julg B, Tartaglia LJ, Keele BF, et al. Broadly neutralizing antibodies targeting the HIV-1 envelope V2 apex confer protection against a clade C SHIV challenge. Science translational medicine 2017; 9(406). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sok D, van Gils MJ, Pauthner M, et al. Recombinant HIV envelope trimer selects for quaternary-dependent antibodies targeting the trimer apex. Proc Natl Acad Sci U S A 2014; 111(49): 17624–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scheid JF, Mouquet H, Ueberheide B, et al. Sequence and structural convergence of broad and potent HIV antibodies that mimic CD4 binding. Science 2011; 333(6049): 1633–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gautam R, Nishimura Y, Pegu A, et al. A single injection of anti-HIV-1 antibodies protects against repeated SHIV challenges. Nature 2016; 533(7601): 105–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malbec M, Porrot F, Rua R, et al. Broadly neutralizing antibodies that inhibit HIV-1 cell to cell transmission. The Journal of experimental medicine 2013; 210(13): 2813–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wagh K, Bhattacharya T, Williamson C, et al. Optimal Combinations of Broadly Neutralizing Antibodies for Prevention and Treatment of HIV-1 Clade C Infection. PLoS Pathog 2016; 12(3): e1005520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wesley MS, Chiong KT, Seaton KE, et al. Validation of a Triplex Pharmacokinetic Assay for Simultaneous Quantitation of HIV-1 Broadly Neutralizing Antibodies PGT121, PGDM1400, and VRC07–523-LS. Front Immunol 2021; 12: 709994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Montefiori DC. Measuring HIV neutralization in a luciferase reporter gene assay. Methods Mol Biol 2009; 485: 395–405. [DOI] [PubMed] [Google Scholar]
- 26.Sarzotti-Kelsoe M, Bailer RT, Turk E, et al. Optimization and validation of the TZM-bl assay for standardized assessments of neutralizing antibodies against HIV-1. J Immunol Methods 2014; 409: 131–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bharadwaj P, Riekofski C, Lin S, et al. Implementation of a three-tiered approach to identify and characterize anti-drug antibodies raised against HIV-specific broadly neutralizing antibodies. J Immunol Methods 2020; 479: 112764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mayer BT, deCamp AC, Huang Y, et al. Optimizing clinical dosing of combination broadly neutralizing antibodies for HIV prevention. PLoS Comput Biol 2022; 18(4): e1010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wagh K, Seaman MS, Zingg M, et al. Potential of conventional & bispecific broadly neutralizing antibodies for prevention of HIV-1 subtype A, C & D infections. PLoS Pathog 2018; 14(3): e1006860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stephenson KE, Wagh K, Korber B, Barouch DH. Vaccines and Broadly Neutralizing Antibodies for HIV-1 Prevention. Annu Rev Immunol 2020; 38: 673–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walsh SR, Gay C, Karuna ST, et al. Safety and single-dose pharmacokinetics of VRC07–523LS administered via different routes and doses. J Int AIDS Soc 2021; 24: 8–9. [Google Scholar]
- 32.Julg B, Stephenson KE, Wagh K, et al. Safety and antiviral activity of triple combination broadly neutralizing monoclonal antibody therapy against HIV-1: a phase 1 clinical trial. Nat Med 2022; 28(6): 1288–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gilbert PB, Huang Y, deCamp AC, et al. Neutralization titer biomarker for antibody-mediated prevention of HIV-1 acquisition. Nat Med 2022; 28(9): 1924–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data will be publicly available upon publication.


