ABSTRACT
Introduction
Posttraumatic stress disorder (PTSD) and depression are common in service members and veterans, and the response to currently available treatments is often modest at best. Recent studies suggest potential benefit with psychedelic-assisted therapies (PATs), particularly 3,4-methylenedioxymethamphetamine-assisted therapy for PTSD and psilocybin-assisted therapy for depression. This study examined beliefs and perceived barriers regarding PAT among service members and veterans to inform the delivery of these treatments if they are approved by the FDA.
Materials and Methods
Twenty-one service members and veterans (67% male, 81% White, and 43% active duty) with a history of traumatic brain injury and co-occurring cognitive and psychological symptoms completed a measure assessing baseline knowledge and views of PAT, read a brief psychoeducation regarding PAT, and then responded to questions related to their beliefs and perceived barriers to PAT.
Results
Before psychoeducation, participants reported a neutral view of psychedelic drugs (M = 2.76; range: 1-5), PAT (M = 3.33), and interest in PAT (M = 3.10). After psychoeducation, participants reported a significantly more positive view of psychedelic drugs (M = 3.24, P = .014) and interest in PAT (M = 3.67, P = .016). Overall, participants indicated that they would support PAT availability in medical settings if proven beneficial (M = 4.52; 5 = “agree strongly”) and they would support a loved one engaging in PAT (M = 4.29). The most frequently reported health concerns were concern of long-term effects (43%), fear of losing their mind (33%), fear of personality changes (33%), and fear of traumatic brain injury complications (24%). The most frequently endorsed barriers were time commitment, transportation, financial concerns, work, and childcare (33%-19%), with 48% reporting no barriers.
Conclusions
This is the first study to explore beliefs and perceived barriers regarding PAT among service members and veterans. These results indicate that military populations may be interested in PAT, particularly if psychoeducation and outreach regarding these treatments occurred. If FDA approved, it will be important to facilitate command support and address logistical barriers to ensure appropriate access within military contexts.
INTRODUCTION
Randomized controlled trials support the promise of psychedelic-assisted therapy (PAT), most notably, psilocybin and 3,4-methylenedioxymethamphetamine (MDMA) for depression and PTSD, respectively.1,2 In the first phase 3 trial, MDMA-assisted therapy for PTSD was found to be effective (67% of individuals randomized to MDMA no longer meeting criteria for PTSD).1 If the results of the next phase 3 study are positive, MDMA-assisted therapy is likely to be an FDA approved in 2023. Similarly, results from trials of psilocybin for major depressive disorder support its efficacy,2,3 with a phase 3 study planned to begin this year.4
Service members and veterans are in great need of treatment options for PTSD and depression, and PATs may be promising options for these populations. Indeed, evidence-based treatments for PTSD such as prolonged exposure and cognitive processing therapy are efficacious, yet many patients do not respond or dropout from treatment.5,6 Reduced effectiveness in service members and veterans, as opposed to civilians, may result from more complex presentations including a more extensive trauma history, co-occurring traumatic brain injury (TBI), and higher rates of psychiatric comorbidity.6 Thus, there are many military patients with treatment-resistant psychopathology, for whom a new treatment option may be beneficial.
Given the unique needs of service members and veterans, it is important to gauge their perspective on this potential treatment and to identify possible barriers to engaging in these novel interventions. Psychedelics and PAT have received an enormous amount of recent public attention and media coverage that could impact their views. Although recent media coverage has largely been positive, historical media coverage of psychedelics was often negative and included events such as Project MKUltra and unethical human experiments conducted by the Central Intelligence Agency to investigate if psychedelics could be used in psychological warfare.7,8 Further, the U.S. Military prohibits substance use, including psychedelics, and a positive drug screen can result in discharge from the military. Thus, given strong media portrayals, historical use by the military, and a general intolerance to substance use, it is essential to characterize the views that military members have of PATs to understand why some may be interested in, or resistant to, this putative future treatment.
A close parallel to PAT is esketamine, which was approved in 2019 by the FDA for treatment-resistant depression. In recent qualitative work, investigators found that patients with a history of depression had many beliefs about the promise and pitfalls of its use for depression. In particular, patients were broadly enthusiastic about it as a treatment option but had several potential concerns including safety and side effects, practical considerations of engaging in the treatment, and if the experience of rapid change in mood would be “jarring.”9,10 Understanding treatment perceptions among potential patients is key to improving trust and engagement in treatment, given research indicating that knowledge and beliefs of treatment options for mental health disorders are broadly predictive of seeking treatment.11
The present study consisted of a sample of service members and veterans with a history of TBI and co-occurring cognitive and psychological symptoms. They were asked a series of questions regarding familiarity with PAT, as well as their views and interest in PAT before and after reading a brief description of PAT. Participants were also asked a series of questions regarding their beliefs of the potential benefits or downsides of PAT and potential barriers to engaging in PAT. Although we did not have specific hypotheses given the novelty and qualitative nature of these questions, we anticipated a wide range of familiarity and views of PAT.
METHODS
Procedure
Participants were recruited from several channels including a referral program for current or former service members and civilians with TBI.12 Interested individuals contacted the study team and completed a phone screening to determine eligibility. Individuals were eligible if they were active duty, veteran, or retired service members, TRICARE eligible reservists, or national guard members over 18 years of age with mild or moderate TBI greater than 3 months ago according to the Ohio State University Traumatic Brain Injury Identification Method (OSU-TBI) scoring criteria.13 Of note, the OSU-TBI criterion was formally added after the enrollment of the first six participants. Exclusion criteria included lack of English fluency sufficient to complete study measures.
Participants completed three study appointments, spaced approximately 4 weeks apart, including self-report assessments of demographics, mental health, and executive functioning; performance-based tasks of executive functioning; and interviews on TBI history and depression. This study focused on data exclusively from the baseline session. The majority of participants completed the PAT questions at the baseline session, with the exception of four participants who completed the questions after their final study appointment because of the questions being added after the initial start of the study.
Trained research staff administered study protocols. Participants completed informed consent at the beginning of their first study appointment and were compensated $90 for study participation with the exception of active duty individuals who were not paid because of restrictions on receiving compensation while on duty with DoD research funds. The study was approved by the Institutional Review Board of Uniformed Services University.
Measures
Psychedelic Perceptions Survey
Participants completed a self-report questionnaire on perceptions of PAT (see Supplementary Material for the full questionnaire). Participants answered initial questions to rate (ranging 1-5) familiarity with psychedelic drugs and PAT as well as interest in PAT. Then, participants were provided brief-written psychoeducation on psychedelic drugs and PAT and asked the same questions. Additionally, participants completed items assessing perceptions and views of PAT with response options ranging from 1 (“disagree strongly”) to 5 (“agree strongly”). Finally, participants were asked to indicate barriers and concerns related to engaging in PAT in the domains of logistical barriers (e.g., time commitment, work, and childcare), stigma (fear of judgment, fear of legal consequences), and health concerns (fear of TBI complications, existing health conditions).
Demographics
Participants completed a self-report questionnaire on race, ethnicity, sex, age, income, military status, and military branch.
Depression
Depression was assessed with the Montgomery–Asberg Depression Rating Scale (MADRS), a validated 10-item semi-structured interview of depression symptoms with a 0-6 rating scale per item,14 and the Patient Health Questionnaire-9 (PHQ-9), a well-validated, widely used nine-item self-report questionnaire of major depressive disorder.15 PHQ-9 respondents used a self-report rating scale ranging from 0 (“not at all”) to 4 (“nearly every day”) for each symptom.
Anxiety
Anxiety was assessed with the Generalized Anxiety Disorder Screener GAD-7, a validated seven-item self-report measure of symptoms of generalized anxiety disorder.16 Respondents used a scale ranging from 0 (“not at all”) to 3 (“nearly every day”) for each symptom.
Post-traumatic stress disorder
Post-traumatic stress disorder was assessed with the PTSD Checklist (PCL-5), a validated 20-item self-report measure assessing Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, symptom criteria for PTSD.17 Respondents used a self-report rating scale ranging from 0 (“not at all”) to 4 (“extremely”) for each symptom.
Traumatic brain injury
Traumatic brain injury and concussion were assessed with the OSU-TBI, a semi-structured interview.13 Several questions were added to ascertain whether the injury occurred during military service or deployment.
Analyses
Analyses were conducted in R version 3.5.1.18 Descriptive statistics were calculated for the demographic variables and the PAT questionnaire. Summary scores were calculated for the MADRS, PHQ-9, GAD-7, and PCL-5. Paired samples t-tests were conducted to evaluate the change in views of psychedelics and PAT before and after reading a brief description of PAT.
RESULTS
Participant Characteristics
The sample consisted of 21 participants between the ages of 31 and 65 years (M = 46.7). The sample was 67% male and predominantly White (81%) followed by Black/African American (14%). 43% were active duty and 57% were veterans. Twelve participants screened positive for depression on the PHQ-9, eight screened positive for PTSD on the PCL-5, five screened positive for generalized anxiety on the GAD-7, and 17 participants had a history of mild TBI. Further participant characteristics are provided in Table I.
TABLE I.
Sample Characteristics
| Characteristic | Frequency/M (SD) | Percent/Range |
|---|---|---|
| Male | 14 | 66.67% |
| Race | ||
| White | 17 | 80.95% |
| Black/African American | 3 | 14.29% |
| Multiracial | 1 | 4.76% |
| Not Hispanic or Latino | 20 | 95.24% |
| Age | 46.67 (SD = 10.69) | 31-65 |
| Income | ||
| $45,000-$59,999 | 4 | 19.05% |
| $60,000-$74,999 | 4 | 19.05% |
| $75,000-$89,999 | 1 | 4.76% |
| $90,000-$104,999 | 1 | 4.76% |
| $105,000-$119,999 | 3 | 14.29% |
| >$120,000 | 8 | 38.10% |
| Military status | ||
| Veteran | 12 | 57.14% |
| Active duty | 9 | 42.86% |
| Branch | ||
| Air Force | 1 | 4.76% |
| Army | 16 | 76.19% |
| Army Reserves | 1 | 4.76% |
| Marine Corps | 1 | 4.76% |
| Navy | 2 | 9.52% |
| TBI classification | ||
| Mild | 17 | 80.95% |
| Moderate | 3 | 14.29% |
| Severea | 1 | 4.76% |
| MADRS score | 17.81 (SD = 7.48) | 4-29b |
| PHQ-9 total | 11.43 (SD = 5.30) | 4-22c |
| GAD-7 score | 7.95 (SD = 5.54) | 2-19d |
| PCL-5 | 28.19 (SD = 14.44) | 8-65e |
| Familiarity with psychedelic drugs | 2.19 (SD = 1.08) | 1-4 |
| Familiarity with PAT | 1.48 (SD = 0.98) | 1-4 |
Abbreviations: GAD-7:Generalized Anxiety Disorder-7; MADRS:Montgomery–Asberg Depression Rating Scale; PAT:psychedelic-assisted therapy; PCL-5:PTSD Checklist-5; PHQ-9:Patient Health Questionnaire-9; TBI:traumatic brain injury.
This participant enrolled before the implementation of the OSU-TBI inclusion criteria for mild or moderate TBI only.
Two participants scored no depression, 12 participants scored within the mild depression range (7-19), and seven participants scored within the moderate depression range (20-34).
12 participants scored a 10 or higher indicating a positive screen for depression.
Five participants scored a 10 or higher indicating a positive screen for generalized anxiety.
Eight participants scored a 31 or higher indicating a positive screen for PTSD.
Familiarity and Views of Psychedelics and Psychedelic-assisted Therapy
Overall, participants reported being slightly familiar with psychedelic drugs (M = 2.19, 2 = “slightly familiar”) and not familiar at all with PAT (M = 1.48, 1 = “not familiar at all”). Before psychoeducation on PAT, participants reported a neutral view of psychedelic drugs (M = 2.76; 3 = “neutral”), PAT (M = 3.33), and interest in PAT (M = 3.10). After psychoeducation on PAT, participants reported a significantly more positive view of psychedelic drugs (M = 3.24, P = .014) and of interest in PAT (M = 3.67, P = .016). Their interest in PAT trended toward an increase (M = 3.38, P = .055). Further details are provided in Figure 1.
FIGURE 1.
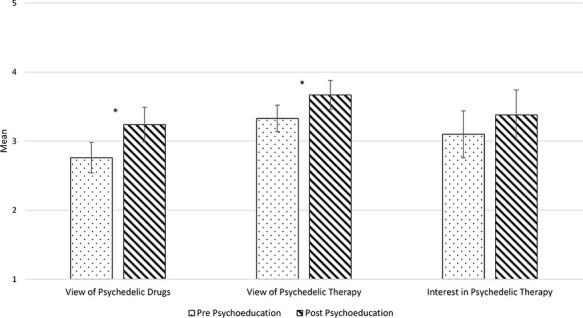
View of psychedelics and psychedelic-assisted therapy. Mean and SEs bars are presented for responses before and after brief psychoeducation on psychedelic on psychedelic-assisted therapy. Higher responses indicate more positive views and greater interest.*P < .05.
Attitudes and Beliefs Regarding PAT
Overall, participants agreed (M = 4.52; 5 = “agree strongly”) that they would support PAT availability in medical settings if proven beneficial and that they would support a loved one doing PAT (M = 4.29; 4 = “agree somewhat”). Participants reported neutral and somewhat positive attitudes of being able to trust therapists during PAT (M = 3.86; 4 = “agree somewhat”). Participants did not agree that it was important for a psychedelic-assisted therapist to be from their same race/ethnicity (M = 1.76; 2 = “disagree somewhat”) or gender (M = 2.00). Further details are provided in Figure 2.
FIGURE 2.
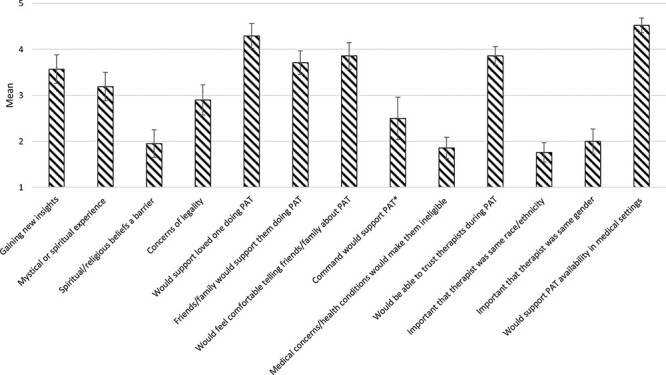
Attitudes and beliefs regarding psychedelic-assisted therapy. Mean and SEs bar are presented for responses. Response options range from 1 (“disagree strongly”) to 5 (“agree strongly”). *Only active duty participants were included for this item.
Barriers, Stigma, and Health Concerns
The most frequently endorsed logistical barrier was time commitment (33%), followed by transportation, financial concerns, work, and childcare (29%-19%), with about half endorsing no barriers (48%). The most frequently reported stigma barriers were fear of workplace consequences (29%) and fear of judgment (24%). The most frequently reported health concerns were concern of long-term effects (43%), fear of losing mind (33%), fear of personality changes (33%), and fear of TBI complications (24%). A third (33%) of the sample reported no stigma or health concerns related to engaging in PAT. Further details are provided in Figure 3.
FIGURE 3.
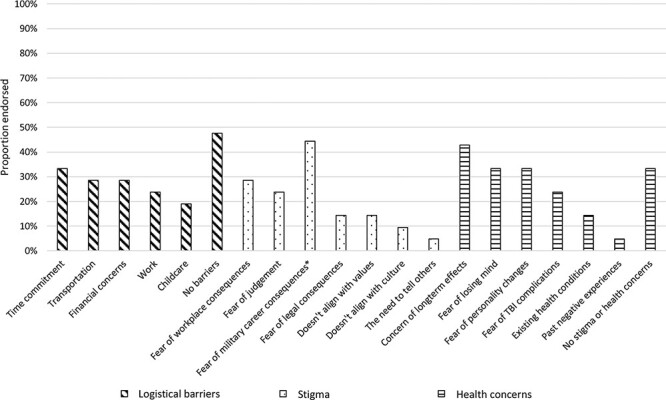
Logistical barriers, stigma, and health concerns related to psychedelic-assisted therapy. *Only active duty participants were included in this item.
DISCUSSION
This is the first study to explore beliefs and perceived barriers regarding PAT among service members and veterans. The primary findings were that participants on average were only slightly familiar, had neutral views, and were somewhat interested in PAT. The positivity of their views of PAT, and to a lesser extent their interest, increased after reading a brief description of PAT. They endorsed a wide range of views regarding the potential benefits or downsides of PAT as well as potential fears and barriers related to engaging in the treatment. With regard to barriers to participating, more than half of participants identified at least one barrier to engaging in PAT. The top three barriers were time commitment, transportation, and financial concerns.
Participants expressed a diversity of positive and negative views of aspects of PAT. They were on average neutral in their views, but there were some deviations. In particular, there was strong agreement in support of PAT, if proven beneficial, being made available in medical settings to help with mental health problems. The lowest endorsement was of command supporting them in engaging in PAT. On the other hand, participants broadly endorsed very few legal or career concerns from engaging in PAT. Real or perceived career ramifications are an important consideration because it is not yet clear if and how PAT may achieve approval and be integrated in the Military Health System, including the logistics of implementing this resource intensive treatment modality, how it may impact military readiness, and what stigma soldiers may experience in pursuing PAT versus other treatment options. Notably, the 2023 National Defense Authorization Act, in its current form,19 passed two amendments through the House that specifically authorize the DoD to study PAT for active duty service members, suggesting that there may be increasing interest in the potential of PAT for service members.
Many participants endorsed fear regarding perceived effects and risks of PAT. In particular, several participants reported fear of negative long-term effects, losing their mind, and personality change. The concern over negative long-term effects may in part be related to specific concerns over TBI-related issues, as more than a third endorsed a fear of TBI complications. Notably, psilocybin, MDMA, and other psychedelics have been found to promote neuroplasticity in the form of neuritogenesis and synaptogenesis,20 but it is yet unclear to what extent these findings may translate to outcomes of safety and efficacy in those with TBI. The first study of psilocybin for migraines found that migraine frequency and intensity were significantly reduced compared to placebo after a single dose with no unexpected or serious adverse events.21 The first study of psilocybin for postconcussive headaches is currently underway (NCT03806985).22 Altogether, further investigation is warranted to study the safety and efficacy of psychedelics for the treatment of TBI and related conditions.
This study has notable limitations. First, this study asked jointly about MDMA- and psilocybin-assisted psychotherapy, and thus it was not possible to parse substance-specific beliefs that patients may have. It will be important for future studies to explore beliefs regarding separate substances and formats of PAT administration (e.g., group versus individual) as well as the impact of reading descriptions of PAT that include different framing and emphases on the potential benefits and side effects. Second, this study was underpowered to explore differences in views between service members and veterans. Third, this study has a small sample size and thus should not be seen as being broadly representative of the views of active duty service members and veterans. Fourth, the reported results in this set of individuals who volunteered to take part in this assessment may not generalize to other groups of veterans and soldiers who may have different sociodemographic or clinical characteristics and who may be more reticent to consider novel therapies. It will be important for future studies to build on this pilot study to comprehensively evaluate beliefs regarding PAT in a large, diverse cohort of patients and key stakeholders (e.g., Military Health System providers). Despite these limitations, this study was the first of its kind to provide a voice to service members and veterans regarding PAT.
In conclusion, this study offers important insights into the views on PAT among service members and veterans with a history of head injury and co-occurring cognitive or psychological symptoms. While their familiarity and views were varied, many expressed both interest and hesitation with respect to PAT. The results highlight the need to give careful consideration to patient views of novel treatments for longstanding conditions, particularly given research indicating that treatment expectations impact intervention effectiveness.23 It is therefore important that expectations and concerns of patients are anticipated and addressed in future psychoeducational materials and PAT treatment protocols. Furthermore, military-specific concerns or barriers need to be further evaluated and addressed if these treatments are FDA approved, given the identified concern of command support and logistical barriers.
Supplementary Material
ACKNOWLEDGMENTS
None declared.
Contributor Information
Joshua C Gray, Department of Medical and Clinical Psychology, Uniformed Services University, Bethesda, MD 20814, USA.
Mikela Murphy, Department of Medical and Clinical Psychology, Uniformed Services University, Bethesda, MD 20814, USA.
Sierra E Carter, Department of Psychology, Georgia State University, Atlanta, GA 30303, USA.
Matthew W Johnson, Department of Psychiatry and Behavioral Sciences, Johns Hopkins University School of Medicine, Baltimore, MD 21205, USA.
MAJ Aaron S Wolfgang, U.S. Army Medical Department, Brooke Army Medical Center, Fort Sam Houston, TX 78234, USA; Department of Psychiatry, Uniformed Services University, Bethesda, MD 20814, USA; Department of Psychiatry, Yale School of Medicine, New Haven, CT 06511, USA.
COL Michael J Roy, Department of Medicine and Center for Neuroscience and Regenerative Medicine, Uniformed Services University, Bethesda, MD 20814, USA.
Jessica L Maples-Keller, Department of Psychiatry and Behavioral Sciences, Emory University School of Medicine, Atlanta, GA 30329, USA.
SUPPLEMENTARY MATERIAL
Supplementary Material is available at Military Medicine online.
FUNDING
J.L.M.-K. has received funding from the Building Interdisciplinary Research Careers in Women’s Health of the National Institutes of Health under Award Numbers K12HD085850 and UL1TR002378 (Georgia Clinical and Translational Science Alliance) and received support from the Wounded Warrior Project and the Infinite Hero Foundation. J.L.M.-K. has received funding and consulting payments from COMPASS Pathways.
CONFLICT OF INTEREST STATEMENT
M.W.J. is in advisory relationships with the following organizations regarding the medical development of psychedelics and related compounds: AJNA Labs LLC., AWAKN Life Sciences Inc., Beckley Psychedelic Ltd., Field Trip Psychedelics Inc., Mind Medicine Inc., and Otsuka Pharmaceutical Development & Commercialization Inc.
DATA AVAILABILITY
The data underlying this article will be shared on reasonable request to the corresponding author.
CLINICAL TRIAL REGISTRATION
Not applicable.
INSTITUTIONAL REVIEW BOARD (HUMAN SUBJECTS)
The study was approved by the Institutional Review Board of Uniformed Services University.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE
Not applicable.
INDIVIDUAL AUTHOR CONTRIBUTION STATEMENT
J.C.G. designed the research and drafted the manuscript. S.E.C., J.L.M.-K., and J.C.G. designed the Psychedelic Perceptions Survey. J.C.G. and M.M. collected and analyzed the data. All authors reviewed, edited, and approved the final manuscript.
INSTITUTIONAL CLEARANCE
This manuscript was approved by the Uniformed Services University Office of External Affairs.
REFERENCES
- 1. Mitchell JM, Bogenschutz M, Lilienstein A, et al. : MDMA-assisted therapy for severe PTSD: a randomized, double-blind, placebo-controlled phase 3 study. Nat Med 2021; 27(6): 1025–33.doi: 10.1038/s41591-021-01336-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Carhart-Harris R, Giribaldi B, Watts R, et al. : Trial of psilocybin versus escitalopram for depression. N Engl J Med 2021; 384(15): 1402–11.doi: 10.1056/NEJMoa2032994. [DOI] [PubMed] [Google Scholar]
- 3. Davis AK, Barrett FS, May DG, et al. : Effects of psilocybin-assisted therapy on major depressive disorder. JAMA Psychiatry 2021; 78(5): 481–9.doi: 10.1001/jamapsychiatry.2020.3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. COMPASS Pathways announces phase 3 pivotal program design for COMP360 in treatment resistant depression at capital markets day. Available at https://compasspathways.com/compass-pathways-announces-phase-3-pivotal-program-design-for-comp360-in-treatment-resistant-depression-at-capital-markets-day/, published 2022; accessed November 7, 2022.
- 5. Edwards‐Stewart A, Smolenski DJ, Bush NE, et al. : Posttraumatic stress disorder treatment dropout among military and veteran populations: a systematic review and meta‐analysis. J Trauma Stress 2021; 34(4): 808–18.doi: 10.1002/jts.22653. [DOI] [PubMed] [Google Scholar]
- 6. Steenkamp MM, Litz BT, Marmar CR: First-line psychotherapies for military-related PTSD. JAMA 2020; 323(7): 656–7.doi: 10.1001/jama.2019.20825. [DOI] [PubMed] [Google Scholar]
- 7. Petry LG, Sharma M, Wolfgang AS, Ross DA, Cooper JJ: Any questions? A sober look at MDMA. Biol Psychiatry 2021; 90(3): e7–8.doi: 10.1016/j.biopsych.2021.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gup T: The coldest. The Washington Post. Available at https://www.washingtonpost.com/archive/lifestyle/magazine/2001/12/16/the-coldest/83f56312-8cca-481f-af17-5d8eb0356612/, published December 16, 2001; accessed August 10, 2022. [Google Scholar]
- 9. Jilka S, Murray C, Wieczorek A, Griffiths H, Wykes T, McShane R: Exploring patients’ and carers’ views about the clinical use of ketamine to inform policy and practical decisions: mixed-methods study. BJPsych Open 2019; 5(5): e62.doi: 10.1192/bjo.2019.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jilka S, Odoi CM, Wilson E, Meran S, Simblett S, Wykes T: Ketamine treatment for depression: qualitative study exploring patient views. BJPsych Open 2021; 7(1): e32.doi: 10.1192/bjo.2020.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jorm AF: Mental health literacy: empowering the community to take action for better mental health. Am Psychol 2012; 67(3): 231–43.doi: 10.1037/a0025957. [DOI] [PubMed] [Google Scholar]
- 12. Joshi S, Dunbar K, Taylor P, et al. : Streamlining participant recruitment for TBI and PTSD research studies. Mil Med 2017; 182(S1): 124–7.doi: 10.7205/MILMED-D-16-00282. [DOI] [PubMed] [Google Scholar]
- 13. Corrigan JD, Bogner J: Ohio State University Traumatic Brain Injury Identification Method. In: Kreutzer JS, DeLuca J and Caplan B, eds. Encyclopedia of Clinical Neuropsychology. Springer; 2018:2502–4.doi: 10.1007/978-3-319-57111-9_9053. [DOI] [Google Scholar]
- 14. Williams JBW, Kobak KA: Development and reliability of a structured interview guide for the Montgomery Asberg depression rating scale (SIGMA). Br J Psychiatry 2008; 192(1): 52–8.doi: 10.1192/bjp.bp.106.032532. [DOI] [PubMed] [Google Scholar]
- 15. Kroenke K, Spitzer RL, Williams JBW: The PHQ-9 validity of a brief depression severity measure. J Gen Intern Med 2001; 16(9): 606–13.doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Spitzer RL, Kroenke K, Williams JBW, Löwe B: A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med 2006; 166(10): 1092–7.doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 17. Blevins CA, Weathers FW, Davis MT, Witte TK, Domino JL: The posttraumatic stress disorder checklist for DSM-5 (PCL-5): development and initial psychometric evaluation. J Trauma Stress 2015; 28(6): 489–98.doi: 10.1002/jts.22059. [DOI] [PubMed] [Google Scholar]
- 18. R Core Team : R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria, 2022. Available at https://www.R-project.org/.
- 19. H.R.7900 : National Defense Authorization Act for Fiscal Year. 2023. Available at https://www.congress.gov/bill/117th-congress/house-bill/7900/amendments; Accessed November 4, 2022.
- 20. Ly C, Greb AC, Cameron LP, et al. : Psychedelics promote structural and functional neural plasticity. Cell Rep 2018; 23(11): 3170–82.doi: 10.1016/J.CELREP.2018.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schindler EAD, Sewell RA, Gottschalk CH, et al. : Exploratory controlled study of the migraine-suppressing effects of psilocybin. Neurotherapeutics 2021; 18(1): 534–43.doi: 10.1007/s13311-020-00962-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. ClinicalTrials.gov : Effects of psilocybin in concussion headache. Available at https://clinicaltrials.gov/ct2/show/NCT03806985, published 2022; accessed August 2, 2022.
- 23. Aday JS, Heifets BD, Pratscher SD, Bradley E, Rosen R, Woolley JD: Great expectations: recommendations for improving the methodological rigor of psychedelic clinical trials. Psychopharmacol 2022; 239(6): 1989–2010.doi: 10.1007/s00213-022-06123-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.


