Abstract
For various ailments, natural remedies have been traditionally used. To defend against common disorders, medicinal plants are progressively used as nutritional supplements. Gingivitis and periodontitis are widespread and can affect most of the world's population. Gingivitis is a very common, nondestructive inflammatory disease of gums that causes redness and irritation of the gingiva (gums), but periodontitis causes permanent damage to teeth' subsidiary structures. Herbal medicines are getting popular for the treatment of such types of disorders due to being economical with their medicinal effectiveness, compatibility, and nontoxicity. Traditional chemical therapies can cause cell toxicity along with their disease-curing effects. In this article, we discussed the medicinal plants that can be used as an alternative for the treatment of gingivitis (early-stage gum disease) and periodontitis (chronic-stage gum disease).
1. Introduction
The mouth, akin to other areas of the digestive tract, teems with natural microflora, mostly harmless, and confers several benefits to the host. However, in the absence of proper oral hygiene, bacteria can accumulate beyond the levels compatible with natural defense. This leads to a shift in the level of the predominant microbiota far from those associated with oral health; such shift scan predisposes a site to more severe oral health problems like dental caries, gingivitis, periodontal diseases, etc. [1]. Gingivitis is a very common, nondestructive inflammatory disease of gums that causes redness and irritation of the gingiva (gums) and can lead to tooth loss also. Gingivitis disease generally occurs due to bacterial plaque (naturally occurring sticky film) built upon tooth shells and is called plaque-induced gingivitis. The disease can be cured with regular check-ups and proper oral hygiene. Symptoms of gingivitis include swollen, receding, and puffy gums, which sometimes become tender or bleed easily. Its treatment involves professional cleaning, oral rinses, and self-care with dental floss using eucalyptol/menthol/salicylic acid/thymol, fluoride/triclosan, tooth brushing, and oral hygiene.
However, untreated gingivitis can progress to a serious gum infection, periodontitis (Figure 1), in which the inflamed gums may start pulling away from the neck of the tooth and form gaps between the teeth and gums, usually called gum pockets or periodontal pockets. Ultimately, periodontitis can lead to shifts in the teeth' position; supporting bones can be lost, wobbling while chewing, and even the tooth may fall out [2]. Periodontitis is the progression of gingival inflammation at sites where collagen fibers detach pathologically from the cementum and the junctional epithelium migrates apically. Resorption of coronal portions of supporting alveolar bone of the tooth also takes place due to inflammatory events associated with connective tissue attachment loss [3].
Figure 1.
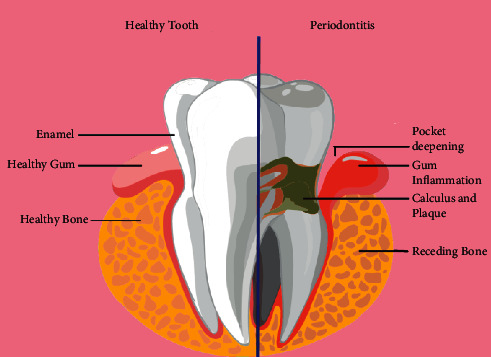
Depiction of periodontitis.
2. Pathogenesis of Periodontitis
In 1976, depending on experimental and histological results, Schroeder & Page classified the progress of periodontal plus gingival infection into four stages, primary, early, recognized (well known), and periodontitis as the advanced (highly developed) stage. These stages form the pedestal for a better understanding of the pathophysiology of disease development [4].
Advancements in technologies and research efforts have been escorted by better knowledge and understanding of the immune potential and also the involvement of various molecular and cellular processes during periodontitis infections (Figure 2) [5]. However, the accurate pathways for periodontitis from a preestablished infection in gingivitis are not entirely clear. Investigators have confirmed that some pathological measures are tied together with active commencement and declaration of inflammation, a molecular mechanism in periodontal tissues like other parts of the body [6]. The highly active inner epithelial layers in front of the dental surface do not have adequate keratinization, and also the turnover pace of the gingival epithelial film is very high. With its barricade functions, the epithelial layer is openly concerned with immune defense mechanisms by presenting chemokines and cytokines (e.g., interleukins). Besides every unique quality of the gingival layer or flexible tissues, dental surroundings are the solitary functional and living hard tissues in the whole body which communicate straight with the outer environment passing a sequence of related soft (i.e., connective tissue and epithelial) and hard (i.e., bone) tissues [7].
Figure 2.
Factors affecting periodontitis severity.
Numerous Gram-negative anaerobic and microaerophilic belonging to polymicrobial flora have already been allied with periodontitis. Noticeably, species belonging to “Red Complex,” comprise Treponema denticola, Porphyromonas gingivalis, and Tannerella forsythia. These species signify a distinctive group of pathogens in periodontitis that adhere to host surfaces, coaggregate, and invade buccal epithelial cells directly from the mouth [8, 9]. Basically, that skill depends on adhesions that identify and interact with host rudiments such as extracellular matrix (ECM) components or protein expression on epithelial cell surfaces [10]. Host cell attack by periodontal-pathogens is well thought out to be an important virulence means for evasion of the body's defense mechanism and for developing reservoirs vital in recurrent infections [11]. These pathogens induce a local inflammatory response through antigen stimulation and liberate toxic products when the obstacle to bacterial colonization and attack is overcome. The defense reaction includes activation of both natural and acquired immunity with penetration of the gingival tissues adjacent to the sulcular space with neutrophils and the expression of antibodies by B cells. In an attempt to defeat the microbial load, epithelial cells, leukocytes, osteoblasts, periodontal ligament fibroblasts, and dendritic cells liberate cytokines and chemokines together with IL-1, IL-6, CXCL-8, TNF-α, in addition to others as proteases, inflammatory mediators, matrix metalloproteinases (MMPs), and prostaglandins [12]. Despite the primary protection, these inflammatory molecules and proteases also cause a breakdown of the most important tooth-supporting structures. Thus, the periodontal tissue gets swollen or inflamed, which leads to rigorous histological changes like damage to connective tissue and bone, apical relocation of the junctional epithelium, deepening of the periodontal pockets, and, ultimately, tooth loss [5, 13].
3. Immune Response Regulators on Molecular Level
The attachment of cementum and the periodontal ligaments makes a highly exclusive structure not established somewhere else. Also, it will not be shocking to spot overlap of unusual tasks accredited to periodontal cells and tissues. For instance, active participation of gingival along with periodontal ligament fibroblasts in the inflammatory response through the generation of cytokines, as discussed earlier [14]. Similarly, their highly bouncy ruling of tissue with the fibroblast in the periodontium via expression of multiple receptors for identification of profibrotic stimuli and proinflammatory stimuli (other than those in the skin as well as lung fibroblasts) [15]. Such a vibrant and sole environment furthermore provides an extremely complex system of interactions throughout the process of inflammation. The basic molecular origin for such proceedings is being elucidated here. As discussed over, inflammation develops an ordinary defense means utilized by the body to escape pathogens and reply to damage. With that, inflammation resolution is too a natural means for bringing back homeostatic equilibrium and is nearly paralleled by the commencement of the inflammatory reaction [16]. Although both mechanisms offer the idea of neutralizing each other, in reality, similar types of cells and molecular commencement designs equally control the commencement of inflammatory reactions and deactivation. [17] Thus, the whole procedure of physiopathological conversion with its phases presents a very vibrant, overlapping, moreover, to a certain extent, redundant sequence of actions. The description of a cascade of events in contrast to reductionist cellular activities and events allows the researchers to split the body's reply to various exogenous plus endogenous insults. An innovative transition is promising by which such bundles of information could be correlated through a deeper perception (Figure 3) [7].
Figure 3.
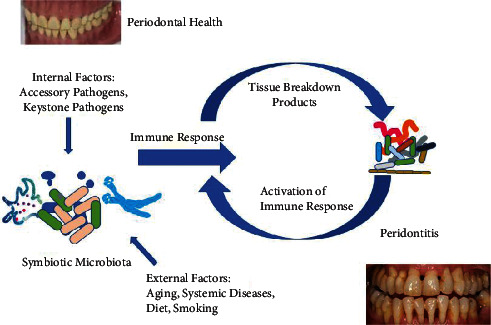
Pathogenesis in periodontitis and immune response on dysbiosis.
Based on technological advancements (high investigative methods), “Omics” of molecular signatures have exposed how the transcriptome, proteome, peptidome, and lipidome regulate our body's response to microbes. All advances are swiftly accepted in periodontal remedies due to the linkage of systemic and local actions that happen during the process of inflammation. In medicine, they have an obvious purpose of detecting targets at the molecular level that can be used for the development of new therapies. Although at extreme compartmental phase and strong dependence on dissect chain of cell actions, a molecular reach to some definite events for the period of inflammation is warranted. Even if the inflammatory response is defensive but fails to remove noxious matter released by neutrophils by way of phagocytosis, it fails to remove inflammatory apoptotic cells, and a hindrance during apoptosis is a feature of the chronic and pathological disease [16]. Partial removal of leukocytes as of a lesion insusceptible person results in breakdown to fix acute inflammation, which leads to serious illness and fibrosis [18]. This failure to sort out acute inflammation and tissue come back to homeostasis causes neutrophil-mediated damage and persistent inflammation also [19]. The above-said destruction of the personal tissues of human beings is a major source of inflammatory illnesses, such as asthma, cancers, arthritis, and periodontal and cardiovascular disorders [20]. Therefore, the above data specify that inflammation resolution is very significant in support of the prevention of chronic diseases. It also specifies that the opportunity window for treatment based on the resolution of the inflammatory procedure depends upon the performance of prime cells of our body's defense system, like phagocytes. With that, the major objective of defense system reaction is to first identify and then remove pathogens, otherwise unfamiliar invaders including parasites (like worms), microorganisms (namely fungi, bacteria, and viruses, commonly called germs), cancer cells along with transplanted tissues as well as organs [21]. Any classic immune defense has four gears, namely, inducers of inflammation, sensors of detection, downstream intermediaries, and, at last, aimed tissues that get affected. The kind and extent of activation of inflammatory reply both rely upon the personality of triggers of inflammation (including parasitic, bacterial, and viral) and its resolution also [22]. In reply to an inflammatory or infectious cause, only two classes of immune reactions occur: that is innate along adaptive. From them, the native (innate) immunity already presents at birth time and need not be obtained via exposure to attackers [23]. Therefore, it gives quick action to pathogens. Also, its components respond to every strange attacker in the same way, spotting only a restricted quantity of identical materials (antigens) or patterns present on different foreign microbes. For that reason, pattern detection is also a means for the activation of native immunity. Thus ontogenically, innate resistance is the simplest, prime means and has been reserved all through the development of host response in the natural world like animals [24]. Still, the very simple species of animals have the only type of immunity or defense means, signifying the value of innate immune defense mechanisms for their continued existence. This type of evolutionary insight emphasizes not only the perfection but also the complexity that the cells, as well as the molecular means of innate immunity or defense, have approached. Innate (natural) immunity, distinct adaptive immunity, does not memorize specific foreign substances or antigens, has no remembrance of the encounters, and also does not offer any defense against potential infection or illness in the future [25]. Innate immunity works through the employment of resistant cells, identification and elimination of foreign substances, complement system activation, and adaptive immune system activation [26]. The cellular components of the above-discussed innate immunity are phagocyte cells (monocytes, neutrophils, and macrophages). These cells elicit the discharge of some mediators, for example, cytokines, which in turn activate acute phase response and the complement system. This activation supports the antibodies for pathogens removal or spots them for ruin via another cell. Besides the nonspecific natural defense mechanism, our body is also talented in attaining an extra specific and adaptive response to inflammation or injury. In that case, pathogens are predictable, thereby giving a stronger response to pathogens there again in the future. Apart from that, acquired immunity (specific or adaptive) is certainly not there at birth; since it is acquired and merely established in complex animal species [27–30]. As a human being's resistant system destroys unfamiliar kinds of stuff (antigens), then the compounds of the adaptive or specific immune system find out the greatest way to bother every antigen plus start to build up a memory intended for that microbe. Adaptive immunity is also known as specific immunity for the reason that it tails or sits attack to a particular antigen earlier encountered due to its talent to find out, adapt, and memorize [31]. The adaptive immune system takes time to build up following the initial experience with a new microbe or (antigen). After that, the same antigens are memorized, and subsequent responses to the antigen are more rapid and efficient than earlier responses that occurred from the very first exposure. After any wound or inflammation, an explosion of antigen-specific T-cells and B-cells occurs. Former cells identify that foreign antigen and particularly goal it, which in turn encourages B-cells to release antibodies in opposition to that attacker. Both B-and T-cells help macrophages and moreover lend a helping hand to produce slaughter cells that scale a response (Figure 3) [7].
4. Treatment Approaches
Periodontal treatment is often used to treat inflamed tissues, decline the count of pathogenic microbes and decrease unhealthy pockets. Multiple treatment and chemical therapies, along with the systemic introduction of antibiotics, are a choice of medical measures being used now [32]. Usual treatment comprises scaling-calculus and plaque elimination, cure clear the tender, soft tissue and also root arrangement-necrotic tissues removal from the root surface. The above-discussed periodontal ailments are united with microbacterial infections; for this reason, antimicrobial healing is assumed to be a suitable method for recovering inflamed tissues [33]. One of the major problems tied with conventional curing via systemic antibiotics administration is the supply of drugs all over the body, in reality not requisite and may also supply noxious problems. The flow of therapeutic agents in the human body is decreased with the aid of a confined drug release scheme. Numerous antimicrobials are of use for the treatment of periodontal diseases. A few examples of local drug delivery systems are irrigating solutions, mouth rinses, and sustained-release medical devices. Some examples of periodontal local drug delivery devices for the targeted release of antimicrobial agents are compacts as well as strips, fibers (monolithic and hollow), nanoparticles, films, gels, microparticles, etc. [34]. Although various chemicals commercially exist, they can modify oral microbiota with some negative effects such as tooth staining, vomiting, diarrhea, etc. Therefore, other products and some ordinary phytochemicals obtained from plant life broadly utilized in conventional medicine concluded as the best substitute for synthetic compounds. In contrast to diseases caused by microbes and the rising resistance to chemicals, natural and herbal compounds of folk medicine have been widely used for many years in each custom all over the world [35–37]. The exercise of medicine has evolved over many pathogens. At present, used therapeutic agents like antibiotics, in addition to antiviral agents, have enhanced interest in the discovery of novel anti-infective compound [38].
According to World Health Organization (WHO), approximately three-quarters of the total world's population rely upon plants and their extracts for various healthcare ideas [39]. They are used as antibacterial agents because of their ability to penetrate and cause damage to the cell walls of Gram-positive in addition to Gram-negative bacteria resulting in bacterial cell destruction. Owing to these advantageous properties of natural and herbal goods like antibacterial, anesthetic, anti-inflammatory, anticariogenic, dentistry, and astringents effect have proved the path above [40]. Around 5,00,000 plant genera going on throughout the world, of that just 1% have been studied phytochemically, seem to have an immense perspective to discover new composites from these types of plant life [38]. Frequently used phytochemicals are flavonoids, alkaloids, tannins, terpenoids, etc. The antimicrobial performance of those phytochemicals is meant to be most valuable for periodontal ailments. The major trouble is the lack of data regarding the effect of the herb on the oral environment, their system of action, and their side effects [41]. The rationale of this check is to introduce various current examples of conventional remedial plant phytochemicals that have been used to reduce oral pathogens expansion and decrease dental plaque development and oral disease symptoms [38].
5. Medicinal Plants Used to Treat Gingivitis and Periodontitis
5.1. Curcuma longa L. (Turmeric)
Turmeric, also known as Haldi, is obtained from rhizomes of Turmeric (Curcuma longa L.) as a palatable orange-yellow spice and has curcumin as the main constituent. The plant has a height of 3 feet with lance-fashioned leaves and consists of yellow color flowers which develop in a fleshy rhizome as well as in stems under the ground. Inside the rhizome, there is an orange pulp holding turmeric powder of medicinal properties [42].
Acute as well as chronic swelling can be treated with curcumin. It diminishes inflammation by decreasing histamine levels [43]. It also has counterinflammatory responses, which are very similar to the action of the steroid, having no harmful effects. Inflammation can be rapidly decreased by using turmeric. Turmeric water (consisting of 5 g turmeric powder along with two cloves, only two dry guava leaves in 200 g of water, and then boiled) can be used as a mouth rinse to decrease swelling and pain. It can also be lessened by applying roasted, ground turmeric on painful teeth. Gingivitis and periodontitis can be treated with a paste consisting of 1 teaspoon full turmeric, 1⁄2 teaspoon full salt, and 1⁄2 tsp mustard oil on the surface of teeth and gums two times a day [44–46]. Turmeric has high antimicrobial characteristics to reduce bacterial growth like Lactobacillus, Streptococci, Staphylococci, etc. A study made by Lee et al. shows that Curcuma longa L. essential oil reduces growth as well as production of acid of Streptococcus mutants by 0.5–4 mg/mL, therefore, having anticariogenic characteristics [47].
It has the maximum potential to inhibit cancers that occur due to chemotherapy, which is used to take care of previous cancers. It reduces metastasis and also inhibits the carcinogens in smoking and chewing tobacco. Also, the maximum yield of antioxidants can be obtained from turmeric and its different varieties [48].
Turmeric solution is used as a mouth rinse for oral purposes. “Curenext” is a gel applied topically and is used to treat the signs of plaque-induced periodontal symptoms. “Curenext” consists of 10 mg/g Curcuma longa L. extract. It is used to treat periodontitis and is also useful in plaque-associated gingivitis [49]. In a clinical trial done by Kumar et al., herbal dentifrice showed 87–95%, 80–95%, and 70–72% decline in plaque, dental calculus, and gingivitis, respectively, with a treatment of 15 days [50].
Turmeric mouthwash can be used in traditional plaque control methods. Here, dissolve 10 mg curcumin in 100 ml purified water. Peppermint oil is used as a flavoring agent [45, 46].
Using 1% curcumin as a subgingival irrigant results in a considerable fall in bleeding upon probing and redness once compared with the saline group and chlorhexidine. It is widely used as adjunctive therapy for periodontitis patients. It leads to improved resolution of inflammatory signs than saline irrigation and chlorhexidine, thus, selectively inhibiting the inflammatory mediators and causing contraction by decreasing vascular engorgement and inflammatory edema of connective tissues. It also increases the healing of wounds due to transforming growth factor transcription and fibronectin [48].
Gums pain can be reduced by rubbing down the painful teeth utilizing roasted and ground turmeric to give some relief from periodontitis and gingivitis. Topical applications: Apply a paste that is to be prepared to contain turmeric 1 tsp, 1⁄2 tsp of salt, in addition to 1⁄2 tsp of mustard oil. It is also suggested to massage the gums and teeth twice daily [44]. The latest study of nano-curcumin particles within chitosan film shows potential periodontal disease treatment [51].
5.2. Aloe vera (L.) Burm.f
Aloe vera (L.) Burm.f. plant has been widely known and used for many years because of its skincare and medicinal characteristics. The given name Aloe vera (L.) Burm.f. is derived from “Alloeh,” the Arabic word, which means “shining bitter material,” while “vera” means “true” in Latin. Around 2000 years ago, Greek scientists proved Aloe vera (L.) Burm.f. the same as the worldwide panacea. It is also termed “the plant of immortality” by Egyptians. Nowadays, the plant has been used for a variety of dermatology conditions [52]. Aloe vera (L.) Burm.f. is a cactus-like plant and has been used for traditional medico-purposes for thousands of years. Leaves can be split into two main basic products: the latex, an acidic yellow liquid under the leaf epidermis, and the gel, a pale and tasteless material present within the leaf's innermost part. Both contain numerous biologically active compounds, generally anthraquinones and polysaccharides (the most active is acemannan). Scientific research gives favor to the uses of Aloe vera (L.) Burm.f. in cosmetic moisturizers, toothpaste, flavoring agents, preservatives in fresh products, and medicine for human beings and animals. It seems to care for diverse conditions because of its wound remedial, antibacterial, anti-inflammatory, immunity, antioxidant, laxative, antifungal, antiviral, antidiabetic, antitumor effects, etc. In addition, these applications can also be included in the animal's diet to develop their benefits to the utmost extent [53].
Leaves of Aloe vera (L.) Burm.f. plant encompasses three different deposits. The external layer consists of 15–20 cells of a broad defensive layer capable of synthesizing proteins and carbohydrates. Its active components include anthraquinones, chromones, polysaccharides, and enzymes. The anthraquinones and chromones are responsible for anticancer activity, anti-inflammatory, and evacuation. The elements Al, B, Ba, Ca, Fe, Mg, Na, P, Si, etc. have also been reported to be present in Aloe vera gel [54, 55].
Aloe vera (L.) Burm.f. is widely used in treating dental diseases. It is extremely useful in gingivitis and periodontitis disease [56]. It also controls inflammation and gingival bleeding and also acts as a potent antiseptic. It has antifungal properties and is widely used in the cure of aphthous lesions and denture stomatitis, fractured and cracked mouth corners. It also has different anti-inflammatory compounds. Aloe vera (L.) Burm.f. contains carboxypeptidase that helps in reducing pain by deactivating bradykinin to 67% and it also has components that retard oxidation of arachidonic acid and anti-PG synthesis characteristics, which might decrease inflammation [57–59]. It also shows the inhibition of free oxygen radicals and reduction of the substitute and chemical way of complement activity [60]. In 2017, Moghaddam et al. proposed a study that signifies the effectiveness of local use of Aloe vera (L.) Burm.f. gel into chronic periodontitis patients as an addition to root planning and scaling [61, 62]. Abdelmonem et al., in their study, proved that scaling and root planning, in addition to subgingival administration of Aloe vera (L.) Burm.f. gel causes enhancement of the periodontal state. It can also be used as an adjuvant for local drug delivery systems due to its different benefits like ease of applicability with leased equipment, low cost, and no side effects [63]. A study was reported in 2016 for the evaluation of the efficiency of Aloe vera (L.) Burm.f. mouthwash, as well as chlorhexidine, depends upon periodontal safety, stating that it is effective, like chlorhexidine, in lessening the soreness of gingivitis and plaque [64].
5.3. Ocimum sanctum L. (Tulsi)
Ocimum sanctum L. (synonym of Ocimum tenuiflorum L.) has a chemical composition that is extremely complex and contains a variety of compounds as well as nutrients that are mainly biologically active. Ocimum sanctum L. leaves have antibacterial characteristics, which are present as essential oils. The five major constituents of essential oils are caryophyllene oxide, caryophyllene, clemene, eugenol, germacrene-A, etc. Oleanolic acid, ursolic acid, rosmarinic acid, etc., are some other biologically active compounds that are found in phytochemical's forms. These types of essential oils, as well as biologically active compounds, are effective against Gram-positive and Gram-negative bacteria because of their antibacterial characteristics [65, 66]. They can damage the cytoplasmic membrane by stimulating the release of cellular potassium. These types of mechanisms which are effective besides systemic disease-causing bacteria may also act toward the periodontal pathogen Aggregatibacter actinomycetemcomitans in human dental plaque. A clinical study conducted by Gupta et al. found the efficiency of Ocimum sanctum L. mouth rinses in decreasing plaque and gingivitis like chlorhexidine [66]. In 2016, Mallikarjun et al. conducted an in vitro study and examined the antimicrobial effectiveness of Tulsi leaf extricate on A. actinomycetemcomitans [67]. The immunomodulatory effect of Tulsi has also been studied and acts by increasing the interferon level, T helper cells, and interleukin-4, which will support the host response to infections [62, 68]. Hosadurga et al. studied that 2% tulsi (Ocimum sanctum L.) gel was useful in the cure of experimental periodontitis [69].
5.4. Salvia officinalis L. (Sage)
Sage is a herb that belongs to the Lamiaceae family. Sage can be found in fields, gardens, as well as along roadsides, etc. It covers various herbal species such as Salvia officinalis L., Salvia lavandulaefolia (Spanish sage), Mentha × piperita L. (menthol), Commiphora myrrha Engl., Matricaria chamomilla L., Eugenia caryophyllus (Spreng.) Bullock & S.G.Harrison (synonym of Syzygium aromaticum (L.) Merr. & L.M.Perry) (Myrtaceae), Carum carvi L. (Umbelliferae), and Echinacea purpurea (L.) Moench, but the two main common species are Salvia officinalis L. and Salvia lavandulaefolia (Spanish sage). Sage has antiseptic, aromatic, astringent, and antispasmodic properties [70]. So, as a mouth rinse, Sage deals efficiently with various throat infections, tonsils, mouth ulcers, and gum diseases, like gingivitis. It has been recommended for stomatitis, sore throat, gingivitis, and periodontal infections [71]. Various essential oils obtained from sage show antifungal, antibacterial, and antiviral properties, and also it has been used for its anti-inflammatory effect in pharyngitis, stomatitis, and tonsils.
For remedy: Add 3 g chopped leaves of sage to 150 ml warm water and boil it for 10 minutes. Then filter it to get clear liquid [72]. The filtrate can be used as a mouth rinse many times a day. An additional prescription for oral rinse is to take two tablespoons of chopped leaves of sage in half a liter of water, lid them and start boiling, and then it is left enclosed for 15 minutes. After that, filter the solution to get a clear liquid. The filtrate can be used for gargling or mouth rinsing various times a day for about 5 to 10 minutes.
Narayanan and Thangavelu reported a considerable improvement in gingival índices with regular use of mouth rinse and also concluded that they could be used on a regular basis as adjunctive therapy to decrease gingival swelling. Intake of sage tea is not suggested for pregnant and lactating women, but gargling and mouth rinsing can be advised [71, 73].
5.5. Matricaria chamomilla L. (Chamomile)
Matricaria chamomilla L. is a commonly known plant. It belongs to the Asteraceae family and is a highly favored traditional medicine for scientific research and use. It consists of various therapeutically active compounds. Flavonoids, Sesquiterpenes, polyacetylenes, and coumarins are the most significant constituents of the chamomile drug. It is widely used as an anti-inflammatory, antiseptic, and ingredient of mouthwash for the avoidance and treatment of throat and gum infections like gingivitis and periodontal, etc. For medicinal purposes, capsules, tablets, tinctures, and lotions of chamomile are available on the market [74, 75]. Lucena et al. reported a decline in gingivitis bleeding index by chamomile remedy. Accordingly, the mouthwash of Matricaria chamomilla L. extract can reduce the bleeding index in a patient suffering from gingivitis or chronic periodontitis [76]. Another study was performed by Batista et al. using pomegranate and chamomile mouthwashes, which efficiently reduced bleeding of gingiva in periodontal disease. They proposed that both the extracts consist of antimicrobial and anti-inflammatory characteristics parallel to chlorhexidine 0.12% solution. In this contrast, the combination could also be used as additional curative agents to restore and sustain dental health [77]. Allergic reactions have also been reported to chamomile, which was followed by skin allergy after topical appliance and bronchial constriction through systemic administration [78, 79]. In a study, Aggarwal and Chaudhary suggested that the mouth rinse of Matricaria chamomilla L. is effective for clinical and microbiological illustration for chronic periodontitis. Its outcomes are equal to the gold standard chlorhexidine mouth wash; hence, chamomile mouth rinse can be a potential remedial mediator for chronic periodontitis [80, 81].
5.6. Mentha × Piperita L. (Peppermint)
Mentha × piperita L. belongs to the family Lamiaceae. Peppermint has been used to lessen tooth pain by using cotton balls saturated with peppermint oil and then placing them in the tooth hollow space. Peppermint oil, while applied in the vicinity, produces an analgesic effect. Peppermint leaves capsules and tablets, 4 to 6 gm per day, after dilution, can also be used as a mouth wash to minimize inflammation of the gingival after the healing of periodontitis [50, 72]. Peppermint leaves, as well as essential oils, are used for the production of gels and mouth rinses that would control the periodontal bacteria [82].
Major vital phenolic constituent in species of Mentha, like flavonoids, consists of a wide variety of pharmacological activities such as cytoprotective, antioxidant, antiulcer, anti-inflammatory, etc. [83]. Peppermint tea is considered a nontoxic drink for normal intake. Peppermint oil can produce consciousness in the stomach in many cases [84]. Fayed reported that peppermint is one of the most potent and extremely safe drugs used to cure because of its effectual antibacterial activity against carcinogenic bacteria. It leads to brilliant potential in this field for its vast therapeutic benefits and human safety with no significant harmful effects as well as contraindications [85].
5.7. Melaleuca alternifolia Cheel (Tea Tree)
Melaleuca alternifolia Cheel belongs to the family Myrtaceae. Usually, a nonsurgical periodontal remedy has been verified to be an effective cure for patients suffering from chronic periodontitis. Tea tree oil (TTO) can also be used as an additive to periodontal therapy in patients suffering from chronic periodontitis. [86]. It is capable of applying directly to the puffy gums to get fast relief from pain. The mouth rinse is used to lessen swelling and it has been frequently used in endodontics as well as in the treatment of necrotic pulp [87, 88]. Melaleuca alternifolia Cheel has exposed excellent efficiency in microbial biofilm control, with a considerable decrease in the bleeding index of the gingiva. [89]. Taalab et al. reported the antimicrobial characteristics of the tea tree and essential oils gel in the production of microbial biofilm [90]. TTO possesses broad-spectrum antiviral, anti-inflammatory, antioxidant, antimicrobial, and antifungal effects. The intrapocket use of TTO has been proved clinically and biochemically (Melaleuca alternifolia Cheel) as gel additive to scale as well as root plan (SRP) in stage 2 (moderate) periodontitis treatment and to compare biochemical levels with clinical reactions. TTO has been confirmed to decrease the in vitro inflammatory cytokines production, signifying its possibility as a remedial agent mainly for inflammatory ailments, like periodontal disease, using host response modulation [91]. Ripari et al. studied and verified that the oil of tea tree produced good results in the probing depth, plaque index as well as BOP evaluation; moreover, it did not directly test alteration as well as dental dyschromia [92].
5.8. Echinacea (Purple Coneflower)
It is an herb that belongs to the family of Asteraceae. Echinacea can enhance the immune response. Its components work simultaneously toward white blood cell (macrophages and lymphocytes) activity. The mouth rinse with chamomile, Echinacea, sage, and mint oil is used for gingivitis and periodontal disease cure. Kumar et al. described the anti-inflammatory and antibacterial activity of Echinacea in their study [50]. Abadi et al. found remarkable results of chlorhexidine mouthwash as well as Echinacea against microbial flora for incubated patients which were hospitalized in the intensive care unit. Their results show that the Echinacea solution is much more efficient in diminishing the oral microbial flora. Due to the antibacterial and anti-inflammatory properties of the herb Echinacea, it can be recommended as a substitute for chlorhexidine [93].
5.9. Rosmarinus officinalis L. (Rosemary)
Rosemary belongs to the family Lamiaceae. It consists of volatile oils having antifungal as well as antibacterial characteristics. It was also found to be effective against chronic candidiasis. Rosemary volatile oil must be given by mouth in diluted form. Previous studies regarding rosemary's essential oil characteristics have verified its antioxidative and antimicrobial properties [94]. Santoyo et al. studied the antimicrobial nature of rosemary and reported that successive 5 essential oil constituents are responsible: verbenone, α-pinene, borneol, 1,8-cineole, and camphor. Borneol provides a good response as compared to verbenone and camphor [95]. Valones et al. concluded that toothpaste based on rosemary was able to decrease biofilm and bleeding of gingival [96].
5.10. Trifolium pratense L. (Red Clover)
It belongs to the family Fabaceae. Red clover mouthwash is generally applied for the cure of periodontal syndrome as well as gingivitis. Its flowers and leaves can also be used for gel formulation possessing antibacterial activity [97]. Ramos et al. reported the anti-inflammatory characteristics of dried-out extract of red clover during in vivo as well as in vitro studies [98].
5.11. Gaultheria procumbens L. (Wintergreen)
It refers to the Ericaceae family. It has good antiseptic as well as astringent characteristics. Cotton roll saturated with wintergreen oil is mainly applied for short-term aid and also it acts as a medication to treat sore throat as well as swelling of gums. Nikoli et al. have revealed wintergreen essential oil antimicrobial activity against a wide variety of Gram-positive as well as Gram-negative bacteria and fungi, and it also possesses antioxidant properties [99].
5.12. Berberis vulgaris L. (Barberry)
It refers to the family Berberidaceae. Alkaloid berberine is obtained from Berberis vulgaris L. It has been commonly added to mouth rinses and toothpaste due to its antimicrobial properties. The gel is widely applied as an effective additive for oral biofilm control and also reduces swelling of the gingiva in children [100]. Barberry juice consists of vitamin C and enhances the response of the host immune and stimulates the absorption of iron. Palombo confirmed that alkaloids like berberine were highly efficient against bacteria like Aggregatibacter actinomycetemcomitans and Porfyromonas gingivalis. Berberine also lowers the Aggregatibacter actinomycetemcomitans and Porfyromonas gingivalis collagenase activity [101]. Makarem and Asodeh reported that berberine gel decreases oral biofilm near to 57% and gingival index near to 34%. Various studies have demonstrated that it has frequent pharmacological therapeutic characteristics such as anti-inflammatory, synthase effects of anti-inducible nitric oxide, and anticyclooxygenase. It is highly recommended that it might be used to reduce the degradation of periodontal tissue throughout the matrix for metalloproteinase regulation during periodontal disease progression [100].
5.13. Cimicifuga racemosa (L.) Nutt. (Black Cohosh)
Black Cohosh consists of major ingredients like acetylacetone, cimidenol, cycloartenol-based triterpenes, deoxyactein, 26 deoxy acetol, and cimicifugaside. It has an anti-inflammatory effect. Various studies have been made using its anti-inflammatory characteristics in curing periodontitis, although there is no evidence of it. It is advised not to be taken during pregnancy and lactation and children below 12 years of age. Slight gastrointestinal distress and headache are several harmful effects of black cohosh.
Dosage-daily dosage: 40–60% isopropyl alcohol and ethanol extracts of the drug equivalent to 40 mg of the drug [102].
5.14. Syzygium aromaticum (L.) Merr. & L. M. Perry (Clove Oil)
Clove oil consists of essential oils, β-caryophyllene, eugenol, and eugenol acetate. It has anti-inflammatory, analgesic, antioxidant, antibacterial, and antiviral properties. It has been used to get relief from a toothache, in the treatment of periodontitis, as an anesthetic agent, and also to cure bleeding of gums. Use it with carefulness in children, pregnant as well as lactating women. It is also available as mouthwash, tincture (1 : 5, 25% ethanol), and lozenges [103]. Voleti et al. evaluated the gel formulation of clove oil for various parameters like pH, antimicrobial activity, drug content, spreadability, extrudability, etc. In vitro experiments verified that the formulation is a suitable dosage form for periodontitis treatment. Clove oil formulation exhibited an inhibition zone of about 22.05 ± 0.04 mm [104].
5.15. Oenothera biennis L. (Evening Primrose)
The various chemical constituents found in evening primrose are gamma-linolenic acid (8–10%), stearic acid (1.5–3.5%), palmitic acid (7–10%), linoleic acid (cis-linoleic acid) (65–80%), oleic acid (6–11%), triterpene alcohols, sterols, etc.
These have antiulcer and antiallergic activity. It is used in dental caries and orthodontic tooth movement. It has various side effects such as headache, diarrhea, nausea, loose stools, etc. [41].
5.16. Allium sativum L. (Garlic)
Garlic has been used since ancient times to suppress the growth of bacteria, fungi, and viruses. Garlic has various chemical constituents such as diallyl sulfide, S-acetylcysteine, alliin, ajoene, B vitamins, dithiin, minerals, enzymes, proteins, etc. It has antiseptic, bacteriostatic, antibacterial, antihelminthic, antifungal, and antiviral effects. Various investigations have been made with garlic to cure dental caries as well as periodontitis [105]. Shetty et al. concluded that there was preliminary confirmation for the antimicrobial activity of garlic extracts against A. actinomycetemcomitans, periodontal pathogens, and P. gingivalis. Its action against P. gingivalis also includes total protease activity inhibition, and thus increases the possibility that garlic may have curative use for periodontitis and other oral infections [106]. The use of garlic in oral remedy was shown to have potential results against Actinobacillus actinomycetemcomitans, Porphyromonas gingivalis, etc. that were found in periodontitis. Moreover, in vivo studies have demonstrated that mouth rinse having garlic extract is capable to cure of Streptococcus mutans bacteria by falling their total count within saliva. A partial interest in garlic as an oral antibacterial agent has developed as it appears that the bacterial resistance to garlic is much less than usual antibiotics [107].
5.17. Zingiber officinale Roscoe (Ginger)
Several components present in ginger are sesquiphellandrene, 1–4% essential oils, alcohols, bisabolene, zingiberene, curcumin, oleoresins, monoterpene aldehydes, etc. It has analgesic, antibacterial as well as anti-inflammatory characteristics. It is also used to get relief from a toothache, as a sialagogue, usually in the oral thrush treatment. It may also retard the lethal effects of the chemotherapeutic agent cyclophosphamide. It should not be used in pregnancy and biliary disease-suffering patients. Since ginger can interfere with the clotting of blood, it should be used carefully in patients with anticoagulant therapies such as heparin and coumadin. Ginger supplementation given with a nonsurgical periodontal remedy can reduce oxidative anxiety and enhance the periodontal anti-inflammatory action, and can enhance antioxidant enzymes serum level. Therefore, it has been recommended that ginger supplementation along with nonsurgical periodontal therapy may be more effective in systemic inflammation control in type-II diabetes mellitus patients along with chronic periodontitis [108].
5.18. Azadirachta indica A.Juss. (Neem)
The various chemical compounds found in Azadirachta indica A.Juss. are azadirachtin, nimbin, nimbidiol, nimbidin, salannin, quercetin, sodium nimbinate, genin, etc. Neem leaves consist of carbohydrates, essential amino acids, proteins, fluoride, carotenoid, fibers, calcium, etc. Neem exhibits antihelminthic, anti-inflammatory, antiviral, antifungal, analgesic, antitumor, antimicrobial, antibacterial, antioxidant, antipyretic as well as anticarcinogenic activity. Different studies have stated that leaves of neem are used in periodontitis, gingivitis, and dental caries treatment.
Neem bark, as well as leaf extracts, are efficiently used to prevent cavities and diseases related to gums. Neem mouthwashes are used as a remedy for tooth decomposition, sore gums, and oral infections. It also prevents bleeding gums. Twigs of the neem tree are mainly used as chewing sticks by people all over India [109]. 0.19% Azadirachta indica A.Juss. has considerable anti-inflammatory characteristics. Thus, it can be used as mechanical therapy to treat plaque-induced gingivitis. In an examination, Chatterjee et al. studied that neem extract mouth rinse is as similarly effective as chlorhexidine in reducing symptoms of periodontal infection. Several results are reliable with a previous study that neem-based mouth rinse is highly effective and that it may also be used as an optional therapy in the cure of periodontal ailments [110].
5.19. Terminalia Chebula Retz. (Haritaki)
Triphala consists of various chemical constituents like gallic acid methyl ester, tannins, chebulagic acid, corilagin, chebulinic acid, cerulenin, punicalagin, gallic acid, terchebulin, and terminalic acid. Flavonols of concern comprise rutin, quercetin, and isoquercitrin. It has antihelminthic, antioxidant, antimicrobial, anti-inflammatory, and astringent properties. Various studies have demonstrated it can be successfully used in dental caries, ulcerated gums, and bleeding. It is contraindicated in children below 12 years of age, lactating and pregnant women [111].
Phytochemical constituents of various plants are discussed in Table 1.
Table 1.
Various plants and their Chemical Constituents.
| Sr. no. | Name | Botanical name | Part used | Phytochemical constituents | References |
|---|---|---|---|---|---|
| 1 | Turmeric | Curcuma longa L. | Rhizome and stem | Curcumin, vanillic acid | [43–45] |
| 2 | Aloe vera | Aloe vera (L.) Burm.f. | Plant | Aloin, chromone | [52–55] |
| 3 | Tulsi | Ocimum sanctum L. (synonym of Ocimum tenuiflorum L.) | Leaf | Copaene, ursolic acid | [65, 66] |
| 4 | Sage | Salvia officinalis L. | Leaves | 8-Cineole, camphor, Α-thujone, Β-thujone, borneol, and viridiflorol | [70, 71, 112] |
| 5 | Chamomile | Matricaria recutita L. (synonym of matricaria chamomilla blanco) | Flowers | Sabinene, bisabolol | [74–76] |
| 6 | Peppermint | Mentha × piperita L. | Leaves | Menthol, carvone | [50, 72, 83] |
| 7 | Tea tree | Melaleuca alternifolia cheel | Leaves | Terpinene, terpineol | [86, 89, 90] |
| 8 | Purple coneflower | Echinacea | Whole plant | Ketoalkenes, caffeic acid derivatives, polysaccharides, and glycoproteins | [50, 93] |
| 9 | Rosemary | Rosmarinus officinalis L. | Leaves | Camphene, borneol | [94–96] |
| 10 | Red clover | Trifolium pratense L. | Flower and leaves | Pratensein, biochanin A | [97, 98] |
| 11 | Wintergreen | Gaultheria procumbens L. | Leaves | Methyl salicylate, ethyl salicylate | [99] |
| 12 | Barberry | Berberis vulgaris L. | Dates | Berberine, palmatine | [100, 101] |
| 13 | Black cohosh | Cimicifuga racemosa (L.) nutt. | Root | Fukinolic acid, isoferulic acid | [101] |
| 14 | Clove oil | Syzygium aromaticum (L.) merr. & L.M.Perry | Buds, leaves and stems | Eugenol, methyleugenol, isoeugenol | [103, 113] |
| 15 | Evening primrose | Oenothera biennis L. | Seeds | G-linolenic, stearic acid (1.5–3.5%), palmitic acid (7–10%), linoleic acid (cis-linoleic acid) (65–80%), oleic acid (6–11%), acid (Cis-G-Linolenic acid) (8–14%), triterpene alcohols, sterols | [41], |
| 16 | Garlic | Allium sativum L. | Bulb | Organosulfur compounds, saponins, phenolic compounds, and polysaccharides | [105, 106, 114] |
| 17 | Ginger | Zingiber officinale roscoe | Rhizomes | Gingerols, shogaols, and paradols, 6-gingerol, 8-gingerol, and 10-gingerol | [108, 115] |
| 18 | Neem | Azadirachta indica A.Juss. | Leaves, bark, fruits, seeds | Azadirachtin, nimbolinin, nimbin, nimbidin, nimbidol, sodium nimbinate, gedunin, salannin, and quercetin | [11, 109, 116] |
| 19 | Haritaki | Terminalia chebula retz. | Bark, roots, stems, leaves, fruits | Gallic acid methyl ester, tannins, chebulagic acid, corilagin, chebulinic acid, cerulenin, punicalagin, gallic acid, terchebulin, and terminalic acid, flavonols | [111, 117] |
6. Marketed Formulation
To cure and prevent dental problems, various types of herbal formulations are used, such as toothpaste, mouthwash, tooth tablets, irrigants, and dental powders. These formulations consist of a single or a blend of herbal constituents. In the market, different formulations of various discussed plants are available. Some of the marketed formulations and their ingredients are listed in Table 2.
Table 2.
Different marketed formulations.
| Sr. No. | Category | Marketed formulations | Ingredients |
|---|---|---|---|
| (1) | Toothpaste | Sensodyne herbal multi care | Eucalyptus & fennel extracts |
| Colgate anticavity toothpaste herbal | Eucalyptus, tea tree oil, chamomile, myrrh, sag | ||
| Himalaya whitening antiplaque toothpaste | Turmeric, coconut oil | ||
| Dabur herbal toothpaste basil | Basil | ||
| Dabur herbal toothpaste-neem | Neem | ||
| Himalaya, botanique, toothpaste | Neem, pomegranate, and triphala | ||
|
| |||
| (2) | Mouthwash | ||
| Cur-Q-Fresh mouthwash | Turmeric (nanocurcumin), tulsi, eucalyptus oil, clove, thymol, tea tree oil, mint, and honey | ||
| Amarantha herbal mouth Wash | Lavanga oil, triphala extract, gandhapura oil, nimba extract | ||
| Tea tree therapy mouthwash | Tea tree oil | ||
| Ornament herbal mouthwash | Amla, licorice, neem, tulsi, cardamom | ||
| Oro-T oral rinse | Malaki (Phyllanthus emblica L.), bibhitaki (Terminalia bellirica (gaertn.) roxb.), and haritaki (Terminalia Chebula retz.) | ||
|
| |||
| (3) | Tooth tablets | Ningen curcumin crush and brush sugar-free toothpaste tablets–100 | Mentha × piperita L., calcium carbonate. Citric acid, longa extract. Syzygium aromaticum (L.) merr. & L.M.Perry. |
| Sudanta tooth tabs | Mayaphal, lavanga, maricha, bakul, dalchini | ||
|
| |||
| (4) | Irrigant | Under the gums irrigant concentrate | Peppermint, eucalyptus, lavender, cinnamon bark, thyme, Echinacea, gotu kola |
|
| |||
| (5) | Tooth powder | Vicco vajradanti ayurvedic tooth powder | Babhul (bark), bakul (bark), jambhul (bark), lavang, manjishtha, bor, acrod, akkal kadha, jeshthamadh, ajwan, dalchini, khair, patang, harada vajradanti, anantmul, amala, behada, kavab – Chini, maifal. |
| Dabur lal dant manjan | Clove oil, pudina satva & karpura (camphor), pippali, tomar beej (Zanthoxylum alatum wall. (Synonym of Zanthoxylum armatum DC.)) | ||
| Kairali dasanakanthi choornam | Kairali dasanakanthi choornam contains arimedastwak (Acacia leucophloea (roxb.) willd. (Synonym of Vachellia leucophloea (roxb.) maslin, seigler & ebinger)); yashti (Glycyrrhiza glabra L.); darvi (Berberis aristata DC.); khadirasara (Acacia catechu (L.f.) willd. (Synonym of Senegalia catechu (L.f.) P.J.H.Hurter & mabb.)); gairika (red ochre); maricha (Piper nigrum L.); krishna (Piper longum L.); jatikosa (Myristica fragrans houtt.); jatiphala (Myristica fragrans houtt.); kaunti (Piper cubeba L.f.); lavanga (Syzygium aromaticum (L.) merr. & L.M.Perry); ela (Elettaria cardamomum (L.) maton); twak (Cinnamomum verum J.Presl); karpoora (Cinnamomum camphora (L.) J.Presl), himambusara (Rosa centifolia L.). | ||
| K.P. Namboodiris ayurvedic tooth powder-strong | Amla, pepper, ginger, clove, cinnamon, licorice. | ||
| Patanjali divya dant manjan | Akarkara, neem, pippali, black salt, babool | ||
7. Conclusion
Herbal medicines have been used all over the world for the cure of various ailments due to their being available, cheap, nontoxic, and efficient. Herbal extracts are used in the form of gel, dentifrice, solutions, ointments, etc., for the prevention and cure of oral diseases. The phytochemicals of herbal medicine are useful for the prevention and cure of gingivitis and periodontitis due to their anti-inflammatory, antibacterial, antifungal, and antioxidative properties. Dentists are working on finding a novel and effective alternative for the healing of such a destructive disease. Studying the treatment approaches made in past can be proved helpful in the case of dentistry.
Contributor Information
Rajeev K. Singla, Email: rajeevsingla26@gmail.com.
Md. Mominur Rahman, Email: mominur.ph@gmail.com.
Data Availability
All data are included within the text.
Conflicts of Interest
Rajeev K Singla is having honorary-based association with iGlobal Research and Publishing Foundation, New Delhi, India, which declares that there are no conflicts of interest. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Authors' Contributions
Neeraj Rani, Sonia Narwal, Tanushree, and Nitish Kumar drafted the manuscript. Rajeev K. Singla and Md. Mominur Rahman have revised the manuscript.
References
- 1.Kharaeva Z. F., Mustafaev M. S., Khazhmetov A. V., et al. Antibacterial and anti-inflammatory effects of toothpaste with Swiss medicinal herbs towards patients suffering from gingivitis and initial stage of periodontitis: from clinical efficacy to mechanisms. Dentistry Journal . 2020;8(1):p. 10. doi: 10.3390/dj8010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alawad M. A. Gingivitis: An Overall View for Undergraduate . Buraydah Saudi Arabia: Qassim University; 2018. [Google Scholar]
- 3.Savage A., Eaton K. A., Moles D. R., Needleman I. A systematic review of definitions of periodontitis and methods that have been used to identify this disease. Journal of Clinical Periodontology . 2009;36(6):458–467. doi: 10.1111/j.1600-051x.2009.01408.x. [DOI] [PubMed] [Google Scholar]
- 4.Page R. C. Journal of Clinical Periodontology . 1986;13(5):345–355. doi: 10.1111/j.1600-051x.1986.tb01471.x. [DOI] [PubMed] [Google Scholar]
- 5.Trindade F., Oppenheim F. G., Helmerhorst E. J., Amado F., Gomes P. S., Vitorino R. Uncovering the molecular networks in periodontitis. Proteomics-Clinical Applications . 2014;8(9-10):748–761. doi: 10.1002/prca.201400028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ortiz F. R., Sfreddo C. S., Coradini A. G. M., Fagundes M. L. B., Ardenghi T. M. Gingivitis influences oral health-related quality of life in adolescents: findings from a cohort study. Revista Brasileira de Epidemiologia . 2020;23:12. doi: 10.1590/1980-549720200051.e200051 [DOI] [PubMed] [Google Scholar]
- 7.Kurgan S., Kantarci A. Molecular basis for immunohistochemical and inflammatory changes during progression of gingivitis to periodontitis. Periodontology 2000 . 2018;76(1):51–67. doi: 10.1111/prd.12146. [DOI] [PubMed] [Google Scholar]
- 8.Page R. C., Kornman K. S. The pathogenesis of human periodontitis: an introduction. Periodontology 2000 . 1997;14(1):9–11. doi: 10.1111/j.1600-0757.1997.tb00189.x. [DOI] [PubMed] [Google Scholar]
- 9.Kinane D. F. Causation and pathogenesis of periodontal disease. Periodontology 2000 . 2001;25(1):8–20. doi: 10.1034/j.1600-0757.2001.22250102.x. [DOI] [PubMed] [Google Scholar]
- 10.Amano A. Bacterial adhesins to host components in periodontitis. Periodontology 2000 . 2010;52(1):12–37. doi: 10.1111/j.1600-0757.2009.00307.x. [DOI] [PubMed] [Google Scholar]
- 11.Di Benedetto A., Gigante I., Colucci S., Grano M. Periodontal disease: linking the primary inflammation to bone loss. Clinical and Developmental Immunology . 2013;2013:7. doi: 10.1155/2013/503754.503754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kornman K. S. Mapping the pathogenesis of periodontitis: a new look. Journal of Periodontology . 2008;79(8):1560–1568. doi: 10.1902/jop.2008.080213. [DOI] [PubMed] [Google Scholar]
- 13.Ahuja A. Gingival desquamation seen with oral massaging of zandu balm-a rare clinical case report. Journal of Dental Panacea . 2014;1:40–44. [Google Scholar]
- 14.Spite M., Serhan C. N. Novel lipid mediators promote resolution of acute inflammation: impact of aspirin and statins. Circulation Research . 2010;107(10):1170–1184. doi: 10.1161/circresaha.110.223883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flavell S. J., Hou T. Z., Lax S., Filer A. D., Salmon M., Buckley C. D. Fibroblasts as novel therapeutic targets in chronic inflammation. British Journal of Pharmacology . 2008;153(S1):S241–S246. doi: 10.1038/sj.bjp.0707487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freire M. O., Van Dyke T. E. Natural resolution of inflammation. Periodontology 2000 . 2013;63(1):149–164. doi: 10.1111/prd.12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fontana J. M., Alexander E., Salvatore M. Translational research in infectious disease: current paradigms and challenges ahead. Translational Research . 2012;159(6):430–453. doi: 10.1016/j.trsl.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Z., Yang H., Tachado S. D., et al. Phosphatase-mediated crosstalk control of ERK and p38 MAPK signaling in corneal epithelial cells. Investigative Ophthalmology & Visual Science . 2006;47(12):5267–5275. doi: 10.1167/iovs.06-0642. [DOI] [PubMed] [Google Scholar]
- 19.Van Dyke T. E. The management of inflammation in periodontal disease. Journal of Periodontology . 2008;79(8s):1601–1608. doi: 10.1902/jop.2008.080173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kantarci A., Oyaizu K., Dyke T. E. V. Neutrophil‐mediated tissue injury in periodontal disease pathogenesis: findings from localized aggressive periodontitis. Journal of Periodontology . 2003;74(1):66–75. doi: 10.1902/jop.2003.74.1.66. [DOI] [PubMed] [Google Scholar]
- 21.Alberts B., Johnson A., Lewis J., Raff M., Roberts K., Walter P. The adaptive immune system. Molecular Biology of the Cell . 2002;4:1363–1402. [Google Scholar]
- 22.Medzhitov R., Janeway C. A., Jr Innate immune recognition and control of adaptive immune responses. Seminars in Immunology . 1998;10(5):351–353. doi: 10.1006/smim.1998.0136. [DOI] [PubMed] [Google Scholar]
- 23.Akira S. Innate immunity and adjuvants. Philosophical Transactions of the Royal Society B: Biological Sciences . 2011;366(1579):2748–2755. doi: 10.1098/rstb.2011.0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rossa C., Jr, Liu M., Kirkwood K. L. A dominant function of p38 mitogen-activated protein kinase signaling in receptor activator of nuclear factor-κB ligand expression and osteoclastogenesis induction by Aggregatibacter actinomycetemcomitans and Escherichia coli lipopolysaccharide. Journal of Periodontal Research . 2008;43(2):201–211. doi: 10.1111/j.1600-0765.2007.01013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gold S. I. Periodontics. The past. Part (I). Early sources. Journal of Clinical Periodontology . 1985;12(2):79–97. doi: 10.1111/j.1600-051x.1985.tb01367.x. [DOI] [PubMed] [Google Scholar]
- 26.Bayne C. J. Origins and evolutionary relationships between the innate and adaptive arms of immune systems. Integrative and Comparative Biology . 2003;43(2):293–299. doi: 10.1093/icb/43.2.293. [DOI] [PubMed] [Google Scholar]
- 27.Pearson G., Robinson F., Beers Gibson T., et al. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocrine Reviews . 2001;22(2):153–183. doi: 10.1210/er.22.2.153. [DOI] [PubMed] [Google Scholar]
- 28.Fraser I. D. C., Germain R. N. Navigatin g the network: signaling cross-talk in hematopoietic cells. Nature Immunology . 2009;10(4):327–331. doi: 10.1038/ni.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hajishengallis G. Toll gates to periodontal host modulation and vaccine therapy. Periodontology 2000 . 2009;51(1):181–207. doi: 10.1111/j.1600-0757.2009.00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hasturk H., Abdallah R., Kantarci A., et al. Resolvin E1 (RvE1) attenuates atherosclerotic plaque formation in diet and inflammation-induced atherogenesis. Arteriosclerosis, Thrombosis, and Vascular Biology . 2015;35(5):1123–1133. doi: 10.1161/atvbaha.115.305324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cooper M. D., Alder M. N. The evolution of adaptive immune systems. Cell . 2006;124(4):815–822. doi: 10.1016/j.cell.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 32.Friedman M., Golomb G. New sustained release dosage form of chlorhexidine for dental use: I. Development and kinetics of release. Journal of Periodontal Research . 1982;17(3):323–328. doi: 10.1111/j.1600-0765.1982.tb01160.x. [DOI] [PubMed] [Google Scholar]
- 33.Steinberg D., Friedman M. Drug Delivery Devices: Fundamentals and Applications . New York, NY, USA: Marcel Dekker; 1998. Sustained release drug delivery devices for treatment of dental diseases. [Google Scholar]
- 34.Lakshmi T., Geetha R. V., Ramamurthy J. G., Anand V. A., Roy A., Vishnupriya V. S. Unfolding gift of nature–Herbs for the management of periodontal disease: a comprehensive review. Journal of Pharmacy Research . 2011;4:2576–2580. [Google Scholar]
- 35.Park K. M., You J. S., Lee H. Y., Baek N. I., Hwang J. K., Kuwanon G. An antibacterial agent from the root bark of Morus alba against oral pathogens. Journal of Ethnopharmacology . 2003;84(2-3):181–185. doi: 10.1016/s0378-8741(02)00318-5. [DOI] [PubMed] [Google Scholar]
- 36.Chung J. Y., Choo J. H., Lee M. H., Hwang J. K. Anticariogenic activity of macelignan isolated from Myristica fragrans (nutmeg) against Streptococcus mutans. Phytomedicine . 2006;13(4):261–266. doi: 10.1016/j.phymed.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 37.Prabu G. R., Gnanamani A., Sadulla S. Guaijaverin-a plant flavonoid as potential antiplaque agent against Streptococcus mutans. Journal of Applied Microbiology . 2006;101(2):487–495. doi: 10.1111/j.1365-2672.2006.02912.x. [DOI] [PubMed] [Google Scholar]
- 38.Kala B. S., Gunjan C., Disha N., Shobha P. Treatment of periodontal disease-a herbal approach. International Journal of Pharmaceutical Sciences Review and Research . 2015;33(2):126–136. [Google Scholar]
- 39.Ashu Agbor M., Naidoo S. Ethnomedicinal plants used by traditional healers to treat oral health problems in Cameroon. Evidence-based Complementary and Alternative Medicine . 2015;2015:10. doi: 10.1155/2015/649832.649832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ali A. K., Rajani E. R., Rahman S. A., Kiran M., Krishnaveni L. Ayurvedic dentistry: an unsung past to a bright future. Journal of Orofacial Research . 2020;9:57–62. [Google Scholar]
- 41.Anushri M., Yashoda R., Puranik M. P. Herbs: a good alternatives to current treatments for oral health problems. Dermatitis . 2015;7:9–12. [Google Scholar]
- 42.Nagpal M., Sood S. Role of curcumin in systemic and oral health: an overview. Journal of Natural Science, Biology and Medicine . 2013;4(1):p. 3. doi: 10.4103/0976-9668.107253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ammon H. P., Safayhi H., Mack T., Sabieraj J. Mechanism of antiinflammatory actions of curcumine and boswellic acids. Journal of Ethnopharmacology . 1993;38(2-3):113–119. doi: 10.1016/0378-8741(93)90005-p. [DOI] [PubMed] [Google Scholar]
- 44.Çıkrıkçı S., Mozioglu E., Yılmaz H. Biological activity of curcuminoids isolated from Curcuma longa. Records of Natural Products . 2008;2:19–24. [Google Scholar]
- 45.Chaudhari A., Karhadkar V., Waghmare P., Jamkhande A. S. Comparative evaluation of turmeric and chlorhexidine gluconate mouthwash in prevention of plaque formation and gingivitis: a clinical and microbiological study. The Journal of Contemporary Dental Practice . 2011;12(4):221–224. doi: 10.5005/jp-journals-10024-1038. [DOI] [PubMed] [Google Scholar]
- 46.Suhag A., Dixit J., Dhan P. Role of curcumin as a subgingival irrigant: a pilot study. Periodontal Practice Today . 2007;4(2) [Google Scholar]
- 47.Lee K. H., Kim B. S., Keum K. S., et al. Essential oil of Curcuma longa inhibits Streptococcus mutans biofilm formation. Journal of Food Science . 2011;76(9):H226–H230. doi: 10.1111/j.1750-3841.2011.02427.x. [DOI] [PubMed] [Google Scholar]
- 48.Monisha K., Ramamurthy J. Role of curcumin in periodontal disease-a review. Drug Invention Today . 2019;11(5) [Google Scholar]
- 49.Sharma V., Kalsi D. S. Effects of topical application of Curcuma longa extract in the treatment of early periodontal diseases. Indian Journal of Dental Sciences . 2016;8:118–123. doi: 10.4103/0976-4003.191725. [DOI] [Google Scholar]
- 50.Kumar P., Ansari S. H., Ali J. Herbal remedies for the treatment of periodontal disease – a patent review. Recent Patents on Drug Delivery and Formulation . 2009;3:221–228. doi: 10.2174/187221109789105603. [DOI] [PubMed] [Google Scholar]
- 51.Mazzarino L., Borsali R., Lemos-Senna E. Mucoadhesive films containing chitosan-coated nanoparticles: a new strategy for Buccal curcumin release. Journal of Pharmaceutical Sciences . 2014;103(11):3764–3771. doi: 10.1002/jps.24142. [DOI] [PubMed] [Google Scholar]
- 52.Surjushe A., Vasani R., Saple D. G. Aloe vera: a short review. Indian Journal of Dermatology . 2008;53(4):p. 163. doi: 10.4103/0019-5154.44785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Christaki E. V., Florou-Paneri P. C. Aloe vera: a plant for many uses. Journal of Food Agriculture and Environment . 2010;8(2):245–249. [Google Scholar]
- 54.Sahu P. K., Giri D. D., Singh R., et al. Therapeutic and medicinal uses of; Aloe vera a review. Pharmacology &Pharmacy . 2013;04(8):599–610. doi: 10.4236/pp.2013.48086. [DOI] [Google Scholar]
- 55.Brown J. P. A review of the genetic effects of naturally occurring flavonoids, anthraquinones and related compounds. Mutation Research: Reviews in Genetic Toxicology . 1980;75(3):243–277. doi: 10.1016/0165-1110(80)90029-9. [DOI] [PubMed] [Google Scholar]
- 56.Grindlay D., Reynolds T. The Aloe vera phenomenon: a review of the properties and modern uses of the leaf parenchyma gel. Journal of Ethnopharmacology . 1986;16(2-3):117–151. doi: 10.1016/0378-8741(86)90085-1. [DOI] [PubMed] [Google Scholar]
- 57.Tello C. G., Ford P., Iacopino A. M. In vitro evaluation of complex carbohydrate denture adhesive formulations. Quintessence International . 1998;29(9):585–593. [PubMed] [Google Scholar]
- 58.Fujita K., Teradaira R., Nagatsu T. Bradykinase activity of aloe extract. Biochemical Pharmacology . 1976;25(2):p. 205. doi: 10.1016/0006-2952(76)90292-6. [DOI] [PubMed] [Google Scholar]
- 59.Bautista-Pérez R., Segura-Cobos D., Vázquez-Cruz B. In vitro antibradykinin activity of Aloe barbadensis gel. Journal of Ethnopharmacology . 2004;93(1):89–92. doi: 10.1016/j.jep.2004.03.030. [DOI] [PubMed] [Google Scholar]
- 60.Hart L. A., van Enckevort P. H., van Dijk H., Zaat R., De Silva K., Labadie R. Two functionally and chemically distinct immunomodulatory compounds in the gel of Aloe vera. Journal of Ethnopharmacology . 1988;23(1):61–71. doi: 10.1016/0378-8741(88)90115-8. [DOI] [PubMed] [Google Scholar]
- 61.Moghaddam A. A., Radafshar G., Jahandideh Y., Kakaei N. Clinical evaluation of effects of local application of Aloe vera gel as an adjunct to scaling and root planning in patients with chronic periodontitis. Journal of Dental Medicine . 2017;18(3):165–172. [PMC free article] [PubMed] [Google Scholar]
- 62.Dayakar M., Pai P., Nath A. R., Ashwini G. Phytotherapeutics in the management of periodontal disease-A review. SRM Journal of Research in Dental Sciences . 2019;10(2):p. 82. doi: 10.4103/srmjrds.srmjrds_41_18. [DOI] [Google Scholar]
- 63.Abdelmonem H. M., Khashaba O. H., Al-Daker M. A., Moustafa M. D. Effects of aloe vera gel as an adjunctive therapy in the treatment of chronic periodontitis: a clinical and microbiologic study. Mansoura Journal of Dentistry . 2014;1:11–19. [Google Scholar]
- 64.Al‐Maweri S. A., Nassani M. Z., Alaizari N., et al. Efficacy of Aloe vera mouthwash versus chlorhexidine on plaque and gingivitis: a systematic review. International Journal of Dental Hygiene . 2020;18(1):44–51. doi: 10.1111/idh.12393. [DOI] [PubMed] [Google Scholar]
- 65.Mondal S., Mirdha B. R., Mahapatra S. C. The science behind sacredness of Tulsi (Ocimum sanctum Linn.) Indian Journal of Physiology & Pharmacology . 2009;53(4):291–306. [PubMed] [Google Scholar]
- 66.Gupta D., Bhaskar D. J., Gupta R. K., et al. A randomized controlled clinical trial of Ocimum sanctum and chlorhexidine mouthwash on dental plaque and gingival inflammation. Journal of Ayurveda and Integrative Medicine . 2014;5(2):109–116. doi: 10.4103/0975-9476.131727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mallikarjun S., Rao A., Rajesh G., Shenoy R., Pai M. Antimicrobial efficacy of Tulsi leaf (Ocimum sanctum) extract on periodontal pathogens: an in vitro study. Journal of Indian Society of Periodontology . 2016;20:50. doi: 10.4103/0972-124x.175177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mondal S., Varma S., Bamola V. D., et al. Double-blinded randomized controlled trial for immunomodulatory effects of tulsi (Ocimum sanctum Linn.) leaf extract on healthy volunteers. Journal of Ethnopharmacology . 2011;136(3):452–456. doi: 10.1016/j.jep.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 69.Hosadurga R. R., Rao S. N., Jose J., et al. Evaluation of the efficacy of 2% Ocimum sanctum gel in the treatment of experimental periodontitis. International Journal of Pharmaceutical Investigation . 2015;5(1):p. 35. doi: 10.4103/2230-973x.147231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kitić D. V., Milašin J. M., Obradović R. R., Bojović M. D., Simonović A. A. Periodontal disease and phytotherapy. International Journal of Dental Hygiene . 2015;10:1–4. [Google Scholar]
- 71.Narayanan N., Thangavelu L. Salvia officinalis in dentistry. Dent. Hypotheses . 2015;6(1):p. 27. [Google Scholar]
- 72.Blumenthal M., Busse W. R., Goldberg A., Rister R., Hall T., Riggins C. The Complete German Commission E Monographs: Therapeutic Guide to Herbal Medicines . Austin, TX, USA: American Botanical Council; 1998. [Google Scholar]
- 73.Pistorius A., Willershausen B., Steinmeier E. M., Kreisler M. Efficacy of subgingival irrigation using herbal extracts on gingival inflammation. Journal of Periodontology . 2003;74(5):616–622. doi: 10.1902/jop.2003.74.5.616. [DOI] [PubMed] [Google Scholar]
- 74.Singh O., Khanam Z., Misra N., Srivastava M. K. Chamomile (Matricaria chamomilla L.): an overview. Pharmacognosy Reviews . 2011;5(9):p. 82. doi: 10.4103/0973-7847.79103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Srivastava J. K., Pandey M., Gupta S. Chamomile, a novel and selective COX-2 inhibitor with anti-inflammatory activity. Life Sciences . 2009;85(19-20):663–669. doi: 10.1016/j.lfs.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.de Lucena R. N., Lins R. D., Ramos I. N., Cavalcanti A. L., Gomes R. C., Maciel M. D. Estudo clínico comparativo do efeito anti-inflamatório da Matricaria recutita e da clorexidina em pacientes com gengivite crônica. Brazilian Journal of Medical and Biological Research . 2009 [Google Scholar]
- 77.Batista A. L. A., Diógenes Alves Uchôa Lins R., de Souza Coelho R., do Nascimento Barbosa D., Moura Belém N., Alves Celestino F. J. Clinical efficacy analysis of the mouth rinsing with pomegranate and chamomile plant extracts in the gingival bleeding reduction. Complementary Therapies in Clinical Practice . 2014;20(1):93–98. doi: 10.1016/j.ctcp.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 78.Brown D. J., Stevens H. D. Herbal Prescriptions for Better Health: Your Up-To-Date Guide to the Most Effective Herbal Treatments . Shreveport, LA, USA: Prima Pub; 1996. [Google Scholar]
- 79.Foti C., Nettis E., Panebianco R., Cassano N., Diaferio A., Pia D. P. Contact urticaria from Matricaria chamomilla. Contact Dermatitis . 2000;42(6):360–361. [PubMed] [Google Scholar]
- 80.Agarwal A., Chaudhary B. Clinical and microbiological effects of 1% Matricaria chamomilla mouth rinse on chronic periodontitis: a double-blind randomized placebo controlled trial. Journal of Indian Society of Periodontology . 2020;24(4):354–361. doi: 10.4103/jisp.jisp_441_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Taheri J. B., Azimi S., Rafieian N., Akhavan Zanjani H. Herbs in dentistry. International Dental Journal . 2011;61(6):287–296. doi: 10.1111/j.1875-595x.2011.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mimica-Dukic N., Bozin B. Mentha L. species (Lamiaceae) as promising sources of bioactive secondary metabolites. Current Pharmaceutical Design . 2008;14(29):3141–3150. doi: 10.2174/138161208786404245. [DOI] [PubMed] [Google Scholar]
- 83.Priyah K. S., Sheeba S., Ganapathy D., Kanniappan N. Role of essential plant oils in the treatment of periodontal and oral diseases 2018. Journal of Pharmacy Research . 2018;12(1):24–28. [Google Scholar]
- 84.Fayed M. A., Mentha Piperita L. A. Promising dental care herb mainly against cariogenic bacteria. Universal Journal of Pharmaceutical Research . 2019;4(3):33–38. [Google Scholar]
- 85.Elgendy E. A., Zineldeen D., Ali S. M. Effect of local application of tea tree (Melaleuca alternifolia) oil gel on long pentraxin level used as an adjunctive treatment of chronic periodontitis: a randomized controlled clinical study. Journal of Indian Society of Periodontology . 2013;17(4):p. 444. doi: 10.4103/0972-124x.118314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jorgensen M. G., Slots J. Responsible use of antimicrobials in periodontics. Journal-California Dental Association . 2000;28(3):185–193. [PubMed] [Google Scholar]
- 87.Soukoulis S., Hirsch R. The effects of a tea tree oil-containing gel on plaque and chronic gingivitis. Australian Dental Journal . 2004;49(2):78–83. doi: 10.1111/j.1834-7819.2004.tb00054.x. [DOI] [PubMed] [Google Scholar]
- 88.Offenbacher S. Periodontal diseases: pathogenesis. Annals of Periodontology . 1996;1(1):821–878. doi: 10.1902/annals.1996.1.1.821. [DOI] [PubMed] [Google Scholar]
- 89.Saxer U. P., Stäuble A., Szabo S. H., Menghini G. Effect of mouthwashing with tea tree oil on plaque and inflammation. Schweizerische Monatsschrift für Zahnmedizin . 2003;113(9):985–996. [PubMed] [Google Scholar]
- 90.Taalab M. R., Mahmoud S. A., Moslemany R. M. E., Abdelaziz D. M. Intrapocket application of tea tree oil gel in the treatment of stage 2 periodontitis. BMC Oral Health . 2021;21(1):239–240. doi: 10.1186/s12903-021-01588-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Williams A. C., Barry B. W. Terpenes and the lipid-protein-partitioning theory of skin penetration enhancement. Pharmaceutical Research . 1991;8(1):17–24. doi: 10.1023/a:1015813803205. [DOI] [PubMed] [Google Scholar]
- 92.Ripari F., Cera A., Freda M., Zumbo G., Zara F., Vozza I. Tea tree oil versus chlorhexidine mouthwash in treatment of gingivitis: a pilot randomized, double blinded clinical trial. European Journal of Dermatology . 2020;14(1):055–062. doi: 10.1055/s-0040-1703999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Abadi M. S., Ghaznavirad E. Comparing the effect of Echinacea and chlorhexidine mouthwash on oral health in patients hospitalized in intensive care units. Journal of Complementary Medicine Research . 2012;2(3):222–234. [Google Scholar]
- 94.Bozin B., Mimica-Dukic N., Samojlik I., Jovin E. Antimicrobial and antioxidant properties of rosemary and sage (Rosmarinus officinalis L. and Salvia officinalis L., Lamiaceae) essential oils. Journal of Agricultural and Food Chemistry . 2007;55(19):7879–7885. doi: 10.1021/jf0715323. [DOI] [PubMed] [Google Scholar]
- 95.Santoyo S., Cavero S., Jaime L., Ibanez E., Senorans F. J., Reglero G. Chemical composition and antimicrobial activity of Rosmarinus officinalis L. essential oil obtained via supercritical fluid extraction. Journal of Food Protection . 2005;68(4):790–795. doi: 10.4315/0362-028x-68.4.790. [DOI] [PubMed] [Google Scholar]
- 96.Valones M. A. A., Silva I. C. G., Gueiros L. A. M., Leão J. C., Caldas Jr A. F., Carvalho A. A. T. Clinical assessment of rosemary-based toothpaste (Rosmarinus officinalis Linn.): a randomized controlled double-blind study. Brazilian Dental Journal . 2019;30(2):146–151. doi: 10.1590/0103-6440201902164. [DOI] [PubMed] [Google Scholar]
- 97.Kumar G., Jalaluddin M. D., Rout P., Mohanty R., Dileep C. L. Emerging trends of herbal care in dentistry. Journal of Clinical and Diagnostic Research: Journal of Clinical and Diagnostic Research . 2013;7(8):1827–1829. doi: 10.7860/JCDR/2013/6339.3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ramos G. P., Apel M. A., Morais C. B. D., et al. In vivo and in vitro anti-inflammatory activity of red clover Trifolium pratense dry extract. Revista Brasileira de Farmacognosia . 2012;22(1):176–180. doi: 10.1590/s0102-695x2011005000200. [DOI] [Google Scholar]
- 99.Nikolić M., Marković T., Mojović M., et al. Chemical composition and biological activity of Gaultheria procumbens L. essential oil. Industrial Crops and Products . 2013;49:561–567. doi: 10.1016/j.indcrop.2013.06.002. [DOI] [Google Scholar]
- 100.Makarem A., Asodeh N. K. Efficacy of barberry aqueous extracts dental gel on control of plaque and gingivitis. Acta Medica Iranica . 2007;44:91–94. [Google Scholar]
- 101.Palombo E. A. Traditional medicinal plant extracts and natural products with activity against oral bacteria: potential application in the prevention and treatment of oral diseases. Evidence-Based Complementary and Alternative Medicine . 2011;2011:15. doi: 10.1093/ecam/nep067.nep067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Swetha I., Priya V. V., Gayathri R. Benefits of herbal medicine in oral care. Drug Invention Today . 2018;10(7) [Google Scholar]
- 103.Kaur D., Chandrul K. K. Syzygium aromaticum L. (Clove): a vital herbal drug used in periodontal disease. Indian Journal of Pharmaceutical and Biological Research . 2017;5(2):45–51. doi: 10.30750/ijpbr.5.2.9. [DOI] [Google Scholar]
- 104.Voleti V. K., Shaik S. B., Konduru C., et al. Journal of Comprehensive Pharmacy. https://pharmacie-globale.info/
- 105.Ohtani M., Nishimura T. The preventive and therapeutic application of garlic and other plant ingredients in the treatment of periodontal diseases. Experimental and Therapeutic Medicine . 2020;19(2):1507–1510. doi: 10.3892/etm.2019.8382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shetty S., Thomas B., Shetty V., Bhandary R., Shetty R. M. An in-vitro evaluation of the efficacy of garlic extract as an antimicrobial agent on periodontal pathogens: a microbiological study. Ayu . 2013;34(4):p. 445. doi: 10.4103/0974-8520.127732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Karic V., Jaiswal A., Abrahamse H., Thakur A., Ganeshpurkar A. Effectiveness of Allium sativum on bacterial oral infection. Natural Oral Care in Dental Therapy . 2020:345–369. doi: 10.1002/9781119618973.ch22. [DOI] [Google Scholar]
- 108.Zare Javid A., Bazyar H., Gholinezhad H. Rahimlou M., Rashidi H., Salehi P., Haghighi-zadeh M. H. The effects of ginger supplementation on inflammatory, antioxidant, and periodontal parameters in type 2 diabetes mellitus patients with chronic periodontitis under nonsurgical periodontal therapy. A double-blind, placebo-controlled trial. Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy . 2019;12:1751–1761. doi: 10.2147/dmso.s214333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lakshmi T., Krishnan V., Rajendran R., Madhusudhanan N. Azadirachta indica: a herbal panacea in dentistry-an update. Pharmacognosy Reviews . 2015;9(17):p. 41. doi: 10.4103/0973-7847.156337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chatterjee A., Saluja M., Singh N., Kandwal A. To evaluate the antigingivitis and antipalque effect of an Azadirachta indica (neem) mouthrinse on plaque induced gingivitis: a double-blind, randomized, controlled trial. Journal of Indian Society of Periodontology . 2011;15(4):p. 398. doi: 10.4103/0972-124x.92578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Naiktari R. S., Gaonkar P., Gurav A. N., Khiste S. V. A randomized clinical trial to evaluate and compare the efficacy of triphala mouthwash with 0.2% chlorhexidine in hospitalized patients with periodontal diseases. Journal of Periodontal & Implant Science . 2014;44(3):134–140. doi: 10.5051/jpis.2014.44.3.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hamidpour M., Hamidpour R., Hamidpour S., Shahlari M. Chemistry, pharmacology, and medicinal property of sage (Salvia) to prevent and cure illnesses such as obesity, diabetes, depression, dementia, lupus, autism, heart disease, and cancer. Journal of Traditional and Complementary Medicine . 2014;4(2):82–88. doi: 10.4103/2225-4110.130373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Singletary K. Clove: overview of potential health benefits. Nutrition Today . 2014;49(4):207–224. doi: 10.1097/nt.0000000000000036. [DOI] [Google Scholar]
- 114.Shang A., Cao S. Y., Xu X. Y., et al. Bioactive compounds and biological functions of garlic (Allium sativum L.) Foods . 2019;8(7):p. 246. doi: 10.3390/foods8070246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mao Q. Q., Xu X. Y., Cao S. Y., et al. Bioactive compounds and bioactivities of ginger (Zingiber officinale Roscoe) Foods . 2019;8(6):p. 185. doi: 10.3390/foods8060185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Alzohairy M. A. Therapeutics role of Azadirachta indica (Neem) and their active constituents in diseases prevention and treatment. Evidence-Based Complementary and Alternative Medicine . 2016;2016:11. doi: 10.1155/2016/7382506.7382506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ratha K. K., Joshi G. C. Haritaki (Chebulic myrobalan) and its varieties. Ayu . 2013;34(3):p. 331. doi: 10.4103/0974-8520.123139. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are included within the text.


