Abstract
Background
To date, all preventive anxiety disorders interventions are one-fit-all and none of them are based on individual level and risk profile. The aim of this project is to design, develop and evaluate an online personalized intervention based on a risk algorithm for the universal prevention of anxiety disorders in the general population.
Methods
A randomized controlled trial (RCT) with two parallel arms (prevANS vs usual care) and 1-year follow-up including 2000 participants without anxiety disorders from Spain and Portugal will be conducted.
The prevANS intervention will be self-guided and can be implemented from the prevANS web or from the participants' Smartphone (through an App). The prevANS intervention will have different intensities depending on the risk level of the population, evaluated from the risk algorithm for anxiety: predictA. Both low and moderate-high risk participants will receive information on their level and profile (risk factors) of anxiety disorders, will have access to stress management tools and psychoeducational information periodically. In addition, participants with a moderate-high risk of anxiety disorders will also have access to cognitive-behavioral training (problem-solving, decision-making, communication skills, and working with thoughts). The control group will not receive any intervention, but they will fill out the same questionnaires as the intervention group.
Assessments will be completed at baseline, 6 and 12-month follow-up. The primary outcome is the cumulative incidence of anxiety disorders. Secondary outcomes include depressive and anxiety symptoms, risk probability of anxiety disorders (predictA algorithm) and depression (predictD algorithm), improvement in physical and mental quality of life, and acceptability and satisfaction with the intervention. In addition, cost-effectiveness and cost-utility analyses will also be carried out from two perspectives, societal and health system, and analyses of mediators and moderators will also be performed.
Discussion
To the best of our knowledge, prevANS study will be the first to evaluate the effectiveness and cost-effectiveness of a personalized online intervention based on a risk predictive algorithm for the universal prevention of anxiety disorders.
Trial registration
Keywords: Anxiety disorders; Prevention; Internet-based interventions, Mobile-based interventions, RCT
1. Introduction
1.1. Anxiety as public health issue
Currently, around 300 million people suffer from anxiety disorders worldwide (GBD, 2020) and in Spain they affect about 2.8 million people (Haro et al., 2006). Recent studies indicate that since the onset of the COVID-19 pandemic, the occurrence of depression and anxiety disorders has multiplied (Luo et al., 2020). During the decade 2007–2017, the anxiety disorders burden (years lived with disability) increased by 12.4 % and 13.6 % for women and men, respectively, ranking eighth (women) and thirteenth (men) in the world (GBD, 2018). In addition to the burden of disease, anxiety disorders are associated with high economic costs. In the year 2010, total costs stemming from anxiety disorders in Europe were estimated at €74,380 million, 62.2 % of which were healthcare costs, 0.2 % were direct non-medical costs, and 37.6 % were indirect costs (sick leave and decrease in productivity work) (Olesen et al., 2012). In Spain anxiety disorders are estimated at €10,365 million annually, of which €4300 million are due to direct healthcare costs, €150 million to direct non-healthcare costs and €5914 to indirect costs (Parés-Badell et al., 2014).
1.2. Need to prevent anxiety disorders
Although there are reasonably effective treatments for anxiety disorders (Cuijpers et al., 2014; Sánchez-Meca et al., 2010), not all affected people receive adequate treatment (Fernández et al., 2007) due to accessibility difficulties, errors in diagnosis, incomplete effectiveness of treatments, and lack of adherence. In developed countries, despite the fact that treatments for anxiety disorders have increased substantially in the last decade, the anxiety prevalence has not decreased (Jorm et al., 2017). This is partly because the incidence of new cases of anxiety disorders is very high compared to the prevalence (preventive gap). Even in the hypothetical case that all existing cases of anxiety disorders would be treated appropriately, new cases could be avoided through primary prevention rather than treatment. Following this line, the European Commission established the prevention of mental disorders as one of its main priorities (Fiorillo et al., 2013).
1.3. International research strategies to prevent anxiety disorders (effectiveness and cost-effectiveness)
A meta-analysis carried out in 2017 showed that psychological and psychoeducational interventions are effective in preventing the onset of new cases of anxiety disorders, although the effect size was small (Moreno-Peral et al., 2017). However, from a public health point of view, even though such effectiveness was small, the generalization of preventive interventions for anxiety disorders to large populations could have a relevant impact on improving health, quality of life and costs. This generalization or scalability would be feasible through information and communication technologies (ICTs). In addition, there is significant room for improvement in the field of psychological interventions to prevent anxiety disorders. This effectiveness of preventive interventions could be optimized through the study of mediators and moderators (Moreno-Peral et al., 2020a; Moreno-Peral et al., 2020b). Regarding the cost-effectiveness of psychological interventions to prevent anxiety disorders, we conducted a systematic review of trial-based economic evaluations, and we concluded that the evidence on the cost-effectiveness of these interventions is very limited, since no firm conclusions can be drawn on the cost-effectiveness of the psychological interventions for the prevention of anxiety disorders (Moreno-Peral et al., in press).
1.4. Predicting future episodes of anxiety: the predictA algorithm
An algorithm for predicting the onset of anxiety disorders at 12 months, called the predictA risk algorithm (Moreno-Peral et al., 2014b) was validated on 3564 primary care patients without anxiety from 6 Spanish regions, and its calibration and discriminant validity were very good (C-index 0.80). The final model of the predictA risk algorithm was composed of 8 risk factors (sex, age, physical and mental quality of life, dissatisfaction with paid and unpaid work, financial difficulties, medication for anxiety, depression or stress). Previously, a risk algorithm to predict anxiety disorders at 6 and 24 months in European population was developed and validated (King et al., 2011), which shared 6 risk factors with the Spanish one. In addition to these algorithms, we had previously developed a Spanish algorithm for predicting the onset of major depressive episodes at 12 months, called predictD (Bellón et al., 2011). The final model of the predictD risk algorithm was composed of 11 risk factors. The two Spanish algorithms for anxiety and depression prediction share 6 risk factors. Intervening on these shared factors could reduce the risk of onset of both disorders, depression and anxiety. This is in line with promising transdiagnostic approaches that increase the potential reach and impact of psychological prevention programs (Barlow et al., 2016).
1.5. Information and communication technologies (ICTs) in health
In Europe, 85 % of citizens used the internet in 2019 (European Commission, 2020). The Internet offers easy and inexpensive access to programs that treat or prevent mental disorders. It is anonymous, treatment can be obtained at any time, by setting the individual's own pace, and its use and adherence can be easily monitored. Furthermore, it enables scaling to large populations, once the interventions are shown to be effective.
M-Health has been defined as the medical and public health practices implemented in mobile devices such as smartphones, patient monitoring devices, personal digital assistants and other wireless devices (WHO, 2011). Smartphones are personal, portable, connected and people tend to carry them with themselves all the time, so they are integrated into daily life. This means that health messages and m-Health programs can be proactively offered to people in an appropriate way over time. In Europe the smartphone penetration is above 85 % and increasing (Ditrendia, 2020). Special mention should be made of the impact of the coronavirus these months on smartphone usage. During the COVID-19 pandemic, the time of use of mobile applications grew by 6 % in Europe. Therefore, health interventions carried out on smartphones have great potential for development, implementation and scalability. However, there is very limited evidence that preventive health programs based on smartphones are effective and ensuring users' adherence is critical (Marcolino et al., 2018; Vodopivec-Jamsek et al., 2012).
Engagement in psychological interventions based on ICTs is generally poor. The rates of attrition are high and data for full completion show a wide range (Eysenbach, 2005; McDonald et al., 2020).
1.6. Internet- and mobile-based interventions (IMIs) for the management of anxiety
The meta-analyses of the internet-based interventions in mental health have been shown to be as effective as the face-to-face treatments and superior to the control groups (Andrews et al., 2010; Barak et al., 2008; Linardon et al., 2019). Regarding the prevention of mental health disorders, several systematic reviews and meta-analyses of internet-based interventions have been published, showing positive results (Deady et al., 2017; Rigabert et al., 2020; Sander et al., 2016; Stratton et al., 2017). However, when we look at preventive health programs based on smartphones there is very limited evidence on their effectiveness (Marcolino et al., 2018; Vodopivec-Jamsek et al., 2012).
Specifically, in relation to anxiety disorders, a meta-analysis concluded that online interventions had a positive, although small, effect on reducing anxiety symptoms in the general population; however, the adherence was low, there were only a few trials to prevent anxiety disorders and most of these preventive trials included population with anxiety disorders at the beginning of the study (Deady et al., 2017). The Beacon 2.0 website of the ‘National Institute for Mental Health Research at the Australian National University’ has registered 40 websites that offer treatment or prevention of anxiety disorders (Beacon 2.0, n.d, Beacon 2.0, n.d, Beacon 2.0, n.d, Beacon 2.0, n.d), among which the interventions based on cognitive-behavioral therapy (CBT) are the most used. In most of these studies, the programs have been evaluated to treat anxiety disorders and very few were evaluated to prevent them. Furthermore, the preventive trials included participants with anxiety disorders at the beginning of the study, making it difficult to separate treatment for prevention. In 2017, a meta-analysis on the prevention of anxiety disorders (Moreno-Peral et al., 2017), only included 4 IMI's with a small effect size and presenting adherence problems.
1.7. Personalized prevention of anxiety and depression
Most of the time, personalized medicine is related to the identification of genetic and molecular factors to apply individualized treatments. This type of personalized medicine is also applicable to mental illnesses in the case of the choice of antidepressants and antipsychotics. However, in the context of prevention of mental health disorders, specifically anxiety disorders and depression, the clinician's objective is to offer a range of effective intervention options taking into account the different vulnerabilities and risk factors of patients (Cuijpers, 2009). This type of prevention was called personalized prevention (Cuijpers, 2009; Golubnitschaja et al., 2012). Our risk algorithms (Bellón et al., 2011; Moreno-Peral et al., 2014b) include individual information on both risk levels (probability of risk) and the risk factors profile for both anxiety disorders and depression. In the case of depression and based on the depression risk algorithm, we designed an innovative personalized intervention, predictD-CCRT (Bellón et al., 2013), to prevent this disorder in primary care. The intervention consisted of family doctors providing their patients with information about their level and risk profile of suffering depression in the next year. This intervention was tested in a randomized controlled trial (RCT) with 3326 primary care consultants. The predictD-CCRT intervention, compared to usual care, reduced the incidence of depression by 21 % (Bellón et al., 2016) and anxiety disorders by 23 % (Moreno-Peral et al., 2021) and was also cost-effective (Fernández et al., 2018). For anxiety disorders, the evidence in personalized prevention is much more limited. Until now, risk algorithms have not been used to establish different intensities of interventions in relation to the risk level of anxiety disorders of the population. Interventions to prevent anxiety disorders have been designed so that all users work with a standardized intervention (Moreno-Peral et al., 2017).
1.8. The gaps that need to be covered
Taking into account the state of the art described above:
-
1.
There is significant room for improvement in the field of the effectiveness of psychological interventions in general, and IMIs in particular, for the prevention of anxiety disorders.
-
2.
There is very limited evidence on the effectiveness of IMIs, especially based on smartphones, for the prevention of anxiety disorders, given the scarce number of trials.
-
3.
IMIs have adherence problems. Adherence in the real-word is poor and it is still a challenge to improve the adherence of IMIs in mental health.
-
4.
There is very limited evidence on the cost-effectiveness of preventive interventions for anxiety disorders, and this evidence in IMIs is almost non-existent. To incorporate a new health technology, decision-makers need evidence on its cost-effectiveness.
-
5.
Until now, interventions to prevent anxiety disorders have been designed as a one-fits-all solution. None of the interventions developed to prevent anxiety disorders have been personalized according to the anxiety disorders level and risk profile. The potential to produce effective, adherent and transferable-scalable interventions relies on the quality of the design (tailored and personalized, covering people's motivations and supporting users in decision making), the evaluation, and the dissemination process. However, none of the interventions took into account the level of risk to establish different intensities of interventions and the profile of risk for developing personalized interventions in the general population.
-
6.
Little is known about the mediators and moderators of interventions to prevent anxiety disorders, and especially in IMIs. This knowledge would help optimize interventions and increase their effectiveness.
In conclusion, it is necessary to design and evaluate anxiety disorders prevention interventions with higher effectiveness, acceptability, and adherence compared to existing ones. An innovative and promising proposal would include a personalized approach based on the predictA risk algorithm, involving the whole general population, with multiple components according the level of risk, that has evidence of its effectiveness, based on ICTs, and completely self-guided.
1.9. Objectives
The general objective of the prevANS study is to design, develop and evaluate a personalized online intervention to prevent the onset of anxiety disorders, based on an algorithm to calculate the risk of suffering from anxiety disorders in the near future (predictA-algorithm).
The specific aims are:
-
1.
To design and develop a new personalized online intervention (prevANS) based on predictA risk algorithm to prevent anxiety disorders.
-
2.
To evaluate the effectiveness of the prevANS intervention in preventing the onset of anxiety disorders as compared to a usual care group.
-
3.
To evaluate the effectiveness of prevANS intervention in reducing anxiety and depression symptoms, and the risk level of anxiety disorders and depression as compared to a usual care group.
-
4.
To evaluate the effectiveness of prevANS intervention in improving mental and physical quality of life.
-
5.
To evaluate the adherence and usability of prevANS intervention.
-
6.
To evaluate the participants' acceptability and satisfaction on the prevANS intervention.
-
7.
To evaluate the cost-effectiveness and cost-utility of the prevANS to prevent anxiety disorders as compared to a usual care group.
-
8.
To provide evidence on the moderators and mediators of prevANS intervention.
2. Method
2.1. Design
The prevANS study is structured in three phases. In the first phase, the prevANS intervention/tool was designed and developed by clinicians (psychologists, psychiatrists, family doctors) and technical professionals (telecommunications and computer engineers). In the second phase, a qualitative assessment is being conducted to understand the barriers and enablers of the beta version of the prevANS tool. Suggestions for improvement will be collected and implemented before the main trial begins. In the third phase, a randomized-controlled trial (RCT) with two parallel arms and one year follow-up will be conducted. In this trial, the prevANS intervention will be compared with usual care. Primary and secondary outcomes will be assessed at baseline (T0), 6-month (T6) and 12-month (T12) follow-up.
This clinical trial has been approved by Ethics and Research Regional Committee of Malaga and Ethics Committee of Faculty of Psychology and Education Science of the University of Porto (FPCEUP). This study will be reported in accordance with the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT; Chan et al., 2013), the Consolidated Standards of Reporting Trials 2018 Statement for Social and Psychological Interventions (CONSORT-SPI; Montgomery et al., 2018) as well as the guidelines for executing and reporting internet intervention research (Eysenbach, 2013). This study was registered on Clinical Trials (NCT05682365) on January 12, 2023, before the first participant enrolment.
2.2. Participants & procedure
The study will be conducted in subjects of the general population residing in Spain and Portugal. Participants will be recruited using a dissemination campaign on the media (newspapers, the Internet, social networks, Google Adds, etc.), health centers, universities and on the prevANS website itself, which will summon the population to participate. Some of the key messages that the dissemination campaign will use to attract the attention of candidates for inclusion in the study include: ‘How may I prevent anxiety problems? Participate in the prevANS study for free! All you need is access to the Internet’; ‘Have you ever participated in a scientific study? Take part in the prevANS study. It's free! Participate from your mobile phone’ ‘Do you want to prevent anxiety?’ If you are interested in participating, visit the prevANS recruitment website for further information about the study. Prospective participants will be invited to visit the prevANS recruitment website for further information about the study. Those who sign informed consent will be invited to complete the screening questionnaire.
The inclusion criteria will include: 1) being 18 years or older, and 2) having a computer, smartphone, or tablet for personal use connected to the internet during the study year. Participants will be excluded if they: 1) refuse to sign informed consent; 2) reside outside Spain or Portugal; 3) report having a serious mental disorder (psychosis, bipolar, addictions, etc.); 4) have a terminal illness; 5) have cognitive impairment (dementia); 6) have difficulty understanding Spanish or Portuguese; 7) are participating in a research study or involved in a psychological intervention for depression or anxiety; or 8) have experienced clinically relevant anxiety symptoms in the last two weeks according to the General Anxiety Disorder-7 questionnaire (GAD-7; García-Campayo et al., 2010) with a score equal to or higher than 10 points at the beginning of the study. The GAD-7 (score ≤9), for a prevalence of anxiety disorders of 15–20 %, has a Negative Predictive Value (NPV) of 99 % (1 % false negatives) (Spitzer et al., 2006). Prospective participants who obtain a score of ≥10 points will be contacted if they provide their telephone number, and diagnosis of anxiety disorders is confirmed based on CIDI interview, in which case they will be definitively excluded and suggested to visit a health professional.
2.3. Randomized allocation and masking
Those prospective participants who meet all the inclusion criteria without meeting any exclusion criteria will be assigned by the prevANS website automatically and randomly to one of the two groups (prevANS intervention or control) with a 1:1 ratio with a block of random sizes that are not disclosed to ensure concealment. In both groups, participants will create personal passwords that they can use from the personal area of the website or by downloading an app.
The participants will not be blind to the intervention, which is the most common in trials that evaluate psychological and psychosocial interventions (Boutron et al., 2008). However, interviewers (research assistants who assess outcomes) will be blind to the participants´ status (control or intervention group) and those who perform statistical analyses will also be blind to the intervention and control codes.
2.4. Intervention
The prevANS is a personalized online intervention for preventing anxiety disorders, based on a validated risk algorithm to predict anxiety disorders (predictA; Moreno-Peral et al., 2014b), which will be used through a mobile phone application (App) as the main user's interface or a user-friendly website. The prevANS intervention will be self-guided and multi-component (Fig. 1), including evidence-based psychological and psychoeducational information and exercises for preventing the emergence of anxiety symptoms (Hofmann and Gómez, 2017; Moreno-Peral et al., 2017; Moreno-Peral et al., 2020a).
Fig. 1.
prevANS intervention.
The first step to be taken by the participants in the intervention group will be to complete a series of questionnaires to assess the level of risk of suffering anxiety disorders in the next year (predictA algorithm). After this, participants will be provided with a simple but detailed report with the results of these questionnaires one by one and information about their probability of suffering anxiety disorders in the next year.
After the participants have received their reports, the tool will classify them as: a) low risk (probability of suffering anxiety disorders ≤ 7 %), b) moderate-high risk (probability of suffering anxiety disorders above 7 %). This classification is based on the data obtained in the study of the predictA algorithm (Moreno-Peral et al., 2014b). The 50th percentile of the scores on the probability of risk of suffering from anxiety disorders has been used as a threshold to rank the participants as low versus moderate-high risk. Depending on this classification, the intervention will be milder (low risk) or more intense (moderate-high risk) in its therapeutic work:
-
a)
Low risk of anxiety disorders: The system will provide information on the level and risk profile to develop anxiety disorders and the detailed report on recommendations for action based on risk factors. The information and recommendations will be directed toward the importance and advantages of having this low probability and the participant will be encouraged to continue with those activities or habits that he/she carries out in his/her life that yield that low risk, identifying and expanding preventive/protective factors. On the other hand, the tool will provide weekly psychoeducational information in the form of videos, audios, quizzes and texts based on cognitive-behavioral bibliotherapy (emotional well-being, stress, self-care, etc.). In addition, it will be offered a series of techniques based on the principles of mindfulness to learn to stop at times that require it, learn to take breaths and learn to relax. These techniques have been shown to be effective in managing anxiety (Hofmann and Gómez, 2017).
-
b)Moderate-high risk of anxiety disorders: Participants classified at this level, in addition to all the therapeutic components included in the low-risk profile, will be provided with a cognitive-behavioral training, which will consist of working on specific aspects that are at the origin of anxiety disorders. This training consists of working on the following modules:
-
•Problem solving module instructs to apply problem-solving strategies. Participants will learn effective strategies for solving problems and turning problems into objectives; then, they will apply them to their own problems. Finally, a review will be performed of the work done.
-
•Communication skills module. Participants will be instructed regarding to the good communication principles addressing components of assertiveness, empathy and active listening.
-
•Decision making module instructs to apply several steps based on decision making strategies. Training involves providing guidance about useful strategies for making decisions and seeking alternatives. We will also help participants make decisions about their own problems. In this module, participants are encouraged to use the strategies learned in a real context.
-
•Working with thoughts module aims to reduce automatic negative thoughts and worries. This training instructs to identify and analyze automatic negative thoughts and turn them into positive thoughts. Concerning to worries, the goal is to analyze the worries ranking them by relevance and priority and reduce them through relaxing exercises, mindfulness, psychoeducation and monitoring.
-
•
Regardless of the level of risk, the prevANS intervention will offer personalized information on the balance between risk and protective factors. Information on the risk level, risk factors, protective factors and recommendations will be transmitted at baseline and at 6 months of follow-up. All inputs will be re-assessed at 6 months. If any participant who was at low risk at baseline were to increase their risk probability to the moderate-high level at the 6-month follow-up, they would be proposed to carry out the components of the most intensive intervention indicated for this level of risk.
2.4.1. The control group
Participants in both groups will continue to receive health care from their usual providers, and the participants at control group will complete the same questionnaires repeatedly over time, also receiving the same notifications to fill them out and in the same agreed sequence. However, they will receive no intervention, no personalization, and no information on the level of risk and risk factors. If prevANS intervention is effective, the participants in the control group will have access to all the contents of prevANS intervention once the study has been completed.
2.5. Potential risk for the participants
Based on our previous studies, the participation of patients in the trial does not increase the risk of harm for the patients (Bellón et al., 2016). If the system detects (during recruitment and/or follow-up) high levels of anxiety symptoms and/or moderate-severe depression or a high risk of suicide, participants from both arms will be informed of this and will receive suggestions for action through messages. Suicidal ideation will be screened using one question from PHQ-9. Participation does not imply making visits to the healthcare system but, during recruitment and follow-up, a trained team member will provide support through email or telephone during office hours for any type of doubts or requests, technical support (e.g., difficulties to download the App) or other types of information (e.g., confidentiality guarantee questions), that the participants may demand during the study. In addition, the appearance of secondary effects and inconveniences derived from the preventive intervention will be evaluated by means of a message sent by the App or website every 3 months with the option of free text response.
2.6. Adherence and strategies for trial retention
A monitoring and support plan for each participant will be established, offering feedback on his/her performance in the intervention and its results through a system of points/awards, with the aim of increasing user adherence and satisfaction to the prevention program.
In order to improve trial retention, the tool will send a reminder to the participants to complete the follow-up questionnaires every 6 months. In addition, a research assistant will contact participants when the following situations happen: 1) participants do not complete questionnaire at baseline, 2) participants have not used the App (which means they have not carried out any activity), and 3) participants have not completed a follow-up questionnaire. The purpose of this call will be to encourage them to complete the questionnaires or use the app and to check that participants do not have any technical problems with the app that prevent/hinder them from using it.
2.7. Power and sample size calculation
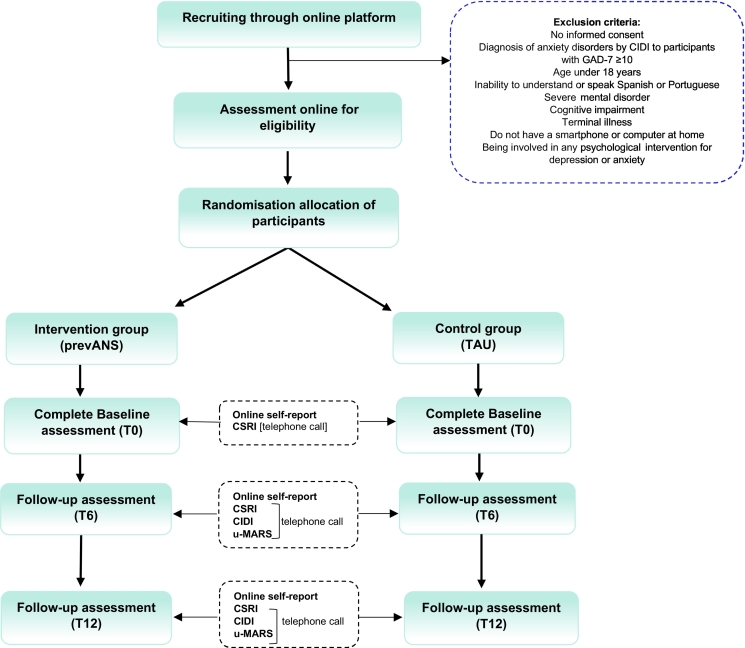
We assume that the participants of this study will have some reason for being interested in the prevention of anxiety disorders, which involves a higher risk of having an anxiety disorder than random subjects of the general population. We expected that the incidence of anxiety disorders in the control group would be similar to that in primary care patients. Therefore, to calculate sample size, the incidence of anxiety disorders in the control group was assumed to be the one reported in the predictA study (Moreno-Peral et al., 2014b), which algorithm was developed based on a cohort of primary care patients. More specifically, we assumed an incidence of anxiety disorders of 14 % for the control group and 8.4 % for the intervention group. Regarding the preventive fraction (difference = 30 %), this was assumed considering previous studies of anxiety prevention for all types of populations (Moreno-Peral et al., 2017; Moreno-Peral et al., 2021). Although the random assignment will be done individually, we cannot rule out the existence of intracluster correlation between participants of the same city; and since we do not have empirical data on its magnitude, in principle we will assume a rho = 0.025. If we assume an alpha error of 5 %, a power of 80 %, and a two-tailed p, we will need 745 individuals for each group, that is, 1.490 individuals in total. Assuming that 30 % of subjects will withdraw from the study, we should increase the sample size to 250 participants per group. Our goal will be to recruit 2000 subjects for the prevANS study (Fig. 2). These calculations assume an average number of participants per city of 50 and that the cluster distribution will be homogeneous (coefficient of variation <0.23) (Eldridge et al., 2006).
Fig. 2.
Flowchart of the prevANS study.
CIDI: Composite International Diagnostic Interview; GAD-7: Generalized Anxiety Disorder-7; CSRI: Client Service Receipt Inventory; u-MARS: User Version of the Mobile Application Rating Scale.
2.8. Outcomes
Our primary outcome will be the cumulative incidence of any anxiety disorders according to DSM-V (panic disorder, agoraphobic, generalized anxiety disorder or social anxiety disorder) during 12 months of follow-up assessed through the section of anxiety disorders of the Composite International Diagnostic Interview at 6 and 12 months follow (CIDI; WHO, 1997). It will be considered as an anxiety diagnosis if the participant presents any anxiety disorder during the whole follow-up period. As secondary outcomes, we will use measurements of mental and physical quality of life using the 12-item Short Form (SF-12; Jenkinson et al., 1997), depressive symptomatology (PHQ-9; Kroenke et al., 2001), anxious symptomatology (GAD-7; Spitzer et al., 2006), probability of onset of anxiety disorders and major depression at 12 months (predictA and predictD risk algorithm; Moreno-Peral et al., 2014b; Bellón et al., 2011), social support (DUKE; Bellón et al., 1996), cognitive change as a mediator of the intervention (assessed by the thoughts worked on the program) and subgroup analysis (according to age, sex, education level, anxiety and depression symptoms and level of risk of anxiety disorders and depression). All these variables will be evaluated at baseline, 6- and 12-months follow-up, except the incidence of anxiety disorders, which will be evaluated at 6 and 12 months. In addition, cost-effectiveness and cost-utility estimates will be performed.
2.9. Variables and measurements
Online self-completed questionnaires and questions include:
-
-
The Patient Health Questionnaire (PHQ-9; Kroenke et al., 2001) consists of 9 items assessing the presence of depressive symptoms present in the past 2 weeks. Each item has a severity index ranging from 0 to 3 (0= “not at all”, 1 = “several days”, 2= “more than half of the days” and 3= “almost every day”). The PHQ-9 shows good psychometric properties in Spain and Portugal (Gómez-Gómez et al., 2022; Ferreira et al., 2019).
-
-
The seven-item Generalized Anxiety Disorder questionnaire (GAD-7; Spitzer et al., 2006) which comprises 7 items measuring symptoms and severity of anxiety based on the DSM-V diagnostic criteria for GAD. The GAD-7 is also an effective screener for other related-anxiety disorders (Kroenke et al., 2010). The score ranges from 0 to 21; higher scores indicate greater severity of symptoms. The Spanish and Portuguese version of GAD-7 has shown good metric properties (García-Campayo et al., 2010; Sousa et al., 2015).
-
-The Spanish predictA (Moreno-Peral et al., 2014a) and predictD (Bellón et al., 2011) risk algorithms calculate the individual probability of the onset of anxiety disorders and major depression in the next 12 months. These predictive risk algorithms were previously validated and contain the following predictors:
-
oSpanish province.
-
oAge.
-
oSex.
-
oEducational level (beyond secondary education, secondary education, primary education and incomplete primary education/illiterate).
-
oQuality of life using the 12-item Short Form (SF-12; Jenkinson et al., 1997). This questionnaire consists of two components, one related to physical health and another related to mental health. Scores range from 0 to 100; higher scores indicate better health-related quality of life. The SF-12 has been validated in the Spanish and Portuguese population and has demonstrated adequate levels of reliability and validity.
-
oControls, demands and rewards for unpaid and paid work using an adapted 7-item version of the Job Content Instrument (Karasek and Theorell, 1990). This questionnaire consists of questions about difficulties of unpaid and paid work, the frequency with which help is perceived and satisfaction with the work performed. The result is classified into 3 categories (satisfied, dissatisfied and very dissatisfied) by the sum of the 7 items. The questionnaire has shown good psychometric properties in Spain (Bellón et al., 2013) and Portugal (Boas and Cerqueira, 2017).
-
oEconomic difficulties using a 4-Likert-type response option item ranging from 1 (living comfortably) to 4 (finding it very difficult).
-
oSatisfaction with living together at home using a 5-Likert-type response option item ranging from 1 (very dissatisfied) to 5 (very satisfied).
-
oPresence of serious problems in family members or close persons using 4 different dichotomous response items (yes/no) on serious physical, psychological or substance misuse problems and any serious disability.
-
oChildhood experiences of physical abuse using a 5-Likert-type response item ranging from 1 (never) to 5 (frequently).
-
oLifetime depression using the first 2 questions included in the CIDI (WHO, 1997) on lifetime low mood.
-
oUse of medication for anxiety, depression or stress in the previous 6 months by means of a dichotomous response item (yes/no).
-
o
-
-
Two questions of the Spanish version of the DUKE-UNC-11 instrument to evaluate the perceived functional social support referred to affective social support and confidential social support (Bellón et al., 1996). This questionnaire has been also validated in Portuguese population (Martins et al., 2022).
-
-
Other sociodemographic variables (questions about gender, sexual orientation and identity) will be also collected.
A telephone call will be made based on participant's availability. This interview will be conducted by trained interviewers blind to participant assignment and will include:-
oAnxiety disorders section of the CIDI (WHO, 1997). The CIDI is a structured psychiatric interview that was designed and evaluated by the WHO and has shown excellent evidence of interview validity in different cultures and populations. The purpose of this interview is ascertaining diagnoses based on DSM criteria. This interview will be administered at 6- and 12-months follow-up; also, to the participants with a score of 10 points or more at baseline.
-
oThe use of healthcare resources will be evaluated by means of the Client Service Receipt Inventory (CSRI) that collects information on the use of services, psychotropic drugs, sick leave and loss of productivity (Vazquez-Barquero et al., 1997). This inventory will be administered at baseline, 6- and 12-months follow-up.
-
oQuality Adjusted Life Years (QALYs) will be measured using the five-dimensional EuroQol questionnaire (EQ-5D; Herdman et al., 2001; Badia et al., 1998; Badia et al., 1999; Ferreira et al., 2013). This is a widely used measure of general health and quality of life which includes five domains addressing mobility, personal care, usual activities, pain/discomfort and anxiety/ depression. In addition, it includes a visual analog scale (VAS) graded from 0 (the worst health status) to 100 (the best health status). Spanish and Portuguese tariffs will be used to estimate the utility of health states described by the participants. This questionnaire will be administered at baseline, 6- and 12-months follow-up.
-
oAcceptability and satisfaction with the intervention will be measured using The User Version of the Mobile Application Rating Scale (u-MARS; Martin Payo et al., 2019; Oliveira et al., 2022). The u-MARS scale consists of 26 items assessing app quality, each of which is scored from 1 (‘poor’) to 5 (‘excellent’), except items 13–16, which also include a “not applicable” option, and the last point, which includes an open-ended question. Higher scores are equivalent to higher App quality. This questionnaire will be administered at 6- and 12-months follow-up.
-
o
For an overview of instruments at screening, baseline (T0) and follow-up assessments (T6-T12), see Table 1.
Table 1.
Study variables and assessments.
| Instrumentsa | Assessment area | Data collection method | Time of measurement |
|||
|---|---|---|---|---|---|---|
| Screening | Baseline | T6 | T12 | |||
| Screening instruments | ||||||
| GAD-7 | Anxiety symptoms | Online self-report | x | |||
| CIDI | Diagnosis of anxiety disorders to participants with GAD-7 ≥10 | Telephone call | x | |||
| Primary outcome | ||||||
| CIDI | Diagnosis of anxiety disorders | Telephone call | x | x | ||
| Secondary outcomes | ||||||
| GAD-7 | Anxiety symptoms | Online self-report | x | x | x | |
| PHQ-9 | Depressive symptoms | Online self-report | x | x | x | |
| PredictA | Probability of the onset of anxiety disorders at 12 months | Online self-report | x | x | x | |
| PredictD | Probability of the onset of major depression at 12 months | Online self-report | x | x | x | |
| SF-12 | Health-related quality of life | Online self-report | x | x | x | |
| EuroQol-5D | Quality Adjusted Life Years | Telephone call | x | x | x | |
| CSRI | Healthcare resource use (costs) | Telephone call | x | x | x | |
| Other assessments | ||||||
| Duke-UNC-11 | Perceived functional social support | Online self-report | x | x | x | |
| Other questions | Sexual identity and orientation | Online self-report | x | x | x | |
| u-MARS | Acceptability and satisfaction with the intervention | Telephone call | x | x | ||
PHQ-9: Patient Health Questionnaire-9; CIDI: Composite International Diagnostic Interview; GAD-7: Generalized Anxiety Disorder-7; SF-12: 12-item Short Form; CSRI: Client Service Receipt Inventory; u-MARS: User Version of the Mobile Application Rating Scale.
2.10. Statistical analysis
Our primary analysis will be the absolute risk difference in the cumulative incidence of any anxiety disorders at 12 months follow-up, a priori adjusted for risk of anxiety disorders at baseline. Although the random assignment will be done individually, the intracluster correlation coefficient will be calculated (participants nested in provinces). We will use multilevel logistic regression if the correlation between participants nested in provinces is relevant. The “province” variable will be included as a random component. The “group” variable will be used as a fixed component of the model (independent variable), whereas suffering from anxiety disorders during follow up will be used as the dependent variable. The incidence of anxiety disorders will be estimated using STATA command `margins'. In the secondary analyses, multilevel linear regressions including as random components time and province variables, and as fixed components the variables group, time, the interaction group ∗ time and the respective measurements of the dependent variables at baseline will be used. We will calculate standardized mean differences using the margins in STATA. All analysis will be conducted with STATA and will be based on the intention-to-treat principle, analyzing all participants according to their randomized treatment and including all them in the analyses. We will use multiple imputations (MI) for dealing with missing data under a missing-at-random framework. The following sensitivity analyses will be carried out: 1) for the primary and secondary analyses adjusting for the probability of risk of anxiety at the baseline and those other covariates that could have indications of an imbalance between the arms of the trial; 2) performing complete case analysis and adjusting for inverse probability weighting (IPW) (Hernán et al., 2004) to minimize attrition bias during follow-up. We will evaluate some moderators (country, age, sex, educational level, physical and mental quality of life, and anxiety risk level) and a mediator (cognitive change) using multilevel structural equation models to describe the causal chain as suggested by Kraemer (2014).
2.10.1. Economic evaluation
We will calculate the incremental cost-effectiveness ratios (ICERs) for the primary and secondary economic evaluations using GLM models (Glick et al., 2007). In the primary analysis (cost-utility), we will use the difference in quality adjusted life years (QALYs) between the prevANS intervention compared to the control group. In the secondary analysis (cost-effectiveness), we will use the difference in the number of diagnoses of anxiety between the two arms. The mean QALYs per patient will be calculated from the EQ-5D-5L (EQ-5D-5L, 2019), using official tariffs available to estimate participants' utility weights. We will consider two separate cost perspectives, from society and from the health system. Health care costs will be calculated by multiplying the number of health service units (consultations, hospital days, etc.) by their standard cost listed in the official datasets available. Costs of medication will be calculated by multiplying the cost per daily dose (available from national formularies) by the number of prescription days. Indirect costs will include the costs of absenteeism and presenteeism. Costs of productivity loss will be calculated by multiplying the time missed due to absence from work (absenteeism) plus time lost due to reduced productivity while at work (presenteeism) by the minimum wage. Confidence intervals and cost-effectiveness acceptability planes and curves (CEACs) will be generated with bootstrap for each imputed data file, varying the values of the availability to pay from 0 to € 100,000. Sensitivity analyses will be carried out by modifying unit prices, using the average wage instead of the minimum wage.
3. Discussion
The prevANS study will provide new information about the effectiveness and cost-effectiveness of an innovative and personalized online intervention based on a risk prediction algorithm for the universal prevention of anxiety disorders.
This study is characterized by several strengths. Until now, interventions to prevent anxiety disorders have been designed so that all users work on a standardized intervention; prevANS will adapt the therapeutic content to each person. According to the level of risk of the participants, different kinds of interventions will be offered. It is well known that online self-guided interventions have adherence problems, making it difficult for users to complete their programs. By giving participants personalized interventions as prevANS offers, we expect to improve the adherence figures. In addition, the tool will retrofit the system with information on the intervention implementation, with the aim of achieving optimal use's adherence to the prevention program. This personalized approach, in addition to increasing the effectiveness of the interventions conducted until now, could also improve the impact of online preventive interventions through the increase of acceptance and adherence toward these programs. We will recruit a large sample of participants from two European countries and will continue the follow-up for 12 months. We will use a structured interview (CIDI) to assess the cumulative incidence of anxiety disorders during follow-up.
Our study also presents several limitations. First, the selection bias could be relevant, participants who choose to participate may have a different profile to those who do not, this could limit external validity and it should be noted that the prevANS intervention could be less effective when applied as a general program. The Spanish predictA risk algorithm, which provides predictions at 12 months, has not been validated in Portugal. However, the European predictA algorithm, which predicts anxiety at 6 and 24 months and was developed with Portuguese population, is very similar to the Spanish one. Another point to consider is that the inference of this trial will be limited to citizens aged 18 or over with Internet access and a basic knowledge of its functioning. However, the expansion/scalability of prevANS intervention could be higher due to the penetration rate of the mobile phone in European population. A high proportion of dropouts from the RCT are expected. In order to limit this risk, the prevANS tool will send automated reminders to fill in the questionnaires. In addition, the research team will be available to give answers to the different problems that may appear in the follow-up and to give personalized support and advice to those subjects who are slow to respond to automated reminders to continue performing the tasks inherent in the trial. On the other hand, power size calculations took into account that there will be a 30 % of cases lost and MI will be used to deal with missing data. Some degree of contamination could happen due to the coexistence in the same family or friends' groups of participants of both control and intervention arms. This bias is difficult to control; however, this bias would be against the hypothesis of the study. The information bias is also probable but the use of questionnaires of proven validity, reliability and sensitivity to change, standardization of procedures and training of interviewers will minimize this bias. Furthermore, even though a lack of balancing at baseline is unlikely, adjusted analyses will be conducted to address confusion bias. Some post-randomization bias is possible (e.g., the use of anxiolytics or antidepressants during follow-up), which will be addressed by carrying out measurements and analysis of them between intervention and control arms. Another aspect that should be noted is that since we are evaluating a psychological, educational and social intervention, it is very difficult to blind the participants. As in other self-guided health programs based on smartphones or other devices, the adherence can be critical. The prevANS intervention entails personalized prevention plans based on each individual's level and risk profile, which can increase the motivation and adherence in the population. In addition, a qualitative evaluation will be performed for knowing the barriers and facilitators of the beta version of prevANS intervention and tool. Improvement proposals will be collected and implemented before the RCT starts. Also, technological strategies to motivate users and to improve its adherence will be included in the prevANS tool such as gamification or simple and intuitive interface and interactions. Finally, if the prevANS intervention was effective and cost-effective, it would be difficult to determine which of the different components are responsible of the preventive effect, and how each interacts with the others. Although we will conduct a secondary analysis that could provide information about mediators and moderators of the effect, more studies specifically directed toward that goal will be needed.
The prevANS intervention aims to activate and empower people, so that they are the main protagonists of their own health, minimizing the intervention of health services. If the prevANS intervention were effective and cost-effective in preventing anxiety disorders, its overall impact could be relevant due to its scalability potential. The use of an innovative technological tool represents a minimal cost for health services and due to its potential scalability, the reduction of cost for the health system and the society would be relevant. Therefore, even under limited budgets, this economic information could help decision-makers to invest money in this new tool and promote its use.
The prevANS study will provide evidence on novel and innovative intervention for the prevention of anxiety disorders and the management of mental health. This evidence will open a new field of research on personalized preventive interventions.
Ethics statement
This protocol involving human participants was reviewed and approved by the ethic committees: “Ethics and Research Regional Committee of Malaga” and “Ethics Committee of FPCEUP”. Participants are informed that the confidentiality are guaranteed following the Spanish, Portuguese and European data protection laws, and the execution of the program is performed through an encrypted and secure communications system. Participants will be informed that they can withdraw from the study at any time without any negative consequences. Any modifications to the protocol that may impact relevant changes to study procedures (e.g., changes in eligibility criteria, assessments, information on risk/benefit) or administrative routine require a formal amendment to the protocol.
Data management
The management of the study data will be carried out in accordance with Regulation (EU) 2016/679 of the European Parliament and of the Council, of April 27 in 2016, regarding the protection of natural persons with regard to the processing of personal data and the free circulation of these data; and also in accordance with Spanish laws: Organic Law 3/2018, of December 5, on the Protection of Personal Data and guarantee of digital rights, as well as Law 41/2002, of November 14, basic regulating autonomy of the patient and of rights and obligations regarding information and clinical documentation, among others.
All study participants will be informed about the procedures of the study and the use and reuse of the data. They will sign an informed consent to join the study, which, together with the study protocol, have been validated by the competent ethic committees. The informed consent document includes the objectives of the study, a description of the procedure, an explanation about interventions and their randomized allocation process, whom to contact about the research, participants' rights, the potential benefits involved in the study, the costs to the participants (none), and information on anonymized data sharing. After recruitment, the personal data of the participants will be removed from all data and stored in a password-protected encrypted database, each participant will receive a code that will only be linked to the data, except in case of medical emergency.
To ensure data quality, the research coordinator or person designated by her will implement and maintain quality assurance and control procedures with written standard operating procedures to ensure that the study is conducted and that the data is generated, documented, and notify in accordance with the protocol. Quality control procedures will be implemented starting with the data entry system, and data quality control checks will be performed on the database.
The data, which will be collected in the framework of this project, will be destined to the study of anxiety prevention. Access to the data will be through an administrator user in a back-office, or through the export to CSV format. The data will belong to the research group according to the privacy policy and will be located in a database within servers, which will be blocked with encrypted files and passwords. The project research team will be responsible for the curation-purification and conservation of the data. For the preservation of the data, periodic backup copies will be made.
For the treatment of the health data of the participants, the provisions of the European Data Protection Regulation will be taken into account, which establishes the following basic principles: transparency and legality, limitation of the purpose, minimization of data, accuracy, limitation of the conservation period, integrity and confidentiality, and proactive responsibility. Following these basic principles, data privacy measures have been applied prior to the launch of the prevANS App, implementing anonymization measures. Technology producers were encouraged to take the right to data protection into account when developing the app and to ensure that the data controllers and managers they are in a position to fulfill their obligations regarding data protection. And finally, the participants are informed about what will happen with the use of the data at the end of the study.
Dissemination policy
Detailed and short reports, leaflets and graphic and video summaries will be produced with the help of experts in communication to be disseminate among the target public and other stakeholders. The results of this study, whether positive, negative, or inconclusive, will be submitted to a peer-reviewed international journal for publication.
Funding
Spanish Ministry of Health, the Institute of Health Carlos III, co-funded by the European Social Fund “Investing in your future” (grant references: CP19/00056), and the Chronicity, Primary Care and Health Promotion Research Network ‘RICAPPS’ (RD21/0016/0012); and Spanish Ministry of Science and Innovation, the State Investigation Agency (PID2020-119652RA-l00). These funding sources had no role in the design of this study and will not have any role during its execution, analyses, interpretation of the data, or decision to submit results.
CRediT authorship contribution statement
PMP and SCC led the scientific specification and conceptualization of the study protocol and JAB, ARM, CGH, HCP, SRM SG, NSA and CMV contributed to it. PMP and SCC drafted the manuscript and JAB, ARM, CGH, HCP, SRM SG, NSA and CMV conducted a critical revision of the manuscript for important intellectual content, provided feedback, discussed and approved the final manuscript.
Declaration of competing interest
None.
Contributor Information
P. Moreno-Peral, Email: patriciamorenoperal@uma.es.
J.A. Bellón, Email: jabellon@uma.es.
Data sharing
The data generated during this study will include detailed information on the protocols and analyses used to allow their reproducibility and future analyses by other groups. We recognize and support the principles of data sharing. The data generated from this research will be made available to affiliated investigators through secure and anonymized databases. Only investigators with specific independent ethics committee approval will have access to any anonymized data. The research coordinator of prevANS study can be contacted for de-identified data requests. Consent for such data sharing will be integral to enrollment in the study, and our participants will be asked about their willingness to have their data shared to advance health research.
References
- Andrews G., Cuijpers P., Craske M.G., McEvoy P., Titov N. Computer therapy for the anxiety and depressive disorders is effective, acceptable and practical health care: a meta-analysis. PLoS One. 2010;5(10) doi: 10.1371/journal.pone.0013196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badia X., Schiaffino A., Alonso J., Herdman M. Using the EuroQoI 5-D in the Catalan general population: feasibility and construct validity. Qual. Life Res. 1998;7(4):311–322. doi: 10.1023/a:1024933913698. [DOI] [PubMed] [Google Scholar]
- Badia X., Roset M., Montserrat S., Herdman M., Segura A. The Spanish versión of EuroQol: a description and its applications. European Quality of Life scale. Med. Clin. (Barc.) 1999;112(Suppl. 1):79–85. (Article in Spanish) [PubMed] [Google Scholar]
- Barak A., Hen L., Boniel-Nissim M., Shapira N. Database of Abstracts of Reviews of Effects (DARE): Quality-assessed Reviews. Centre for Reviews and Dissemination (UK); York (UK): 2008. A comprehensive review and a meta-analysis of the effectiveness of Internet-based psychotherapeutic interventions.https://www.ncbi.nlm.nih.gov/books/NBK76016/ 1995-. Available from: 1995-. Available from: (Internet) [Google Scholar]
- Barlow D.H., Allen L.B., Choate M.L. Toward a unified treatment for emotional disorders - republished article. Behav. Ther. 2016;47(6):838–853. doi: 10.1016/j.beth.2016.11.005. [DOI] [PubMed] [Google Scholar]
- Beacon 2.0 Generalised anxiety disorder. National Institute for Mental Health Research at the Australian National University. Beacon 2.0. https://beacon.anu.edu.au/service/website/browse/2/Generalised_anxiety_disorder (s.f. -a)
- Beacon 2.0 Panic disorder. National Institute for Mental Health Research at the Australian National University. Beacon 2.0. https://beacon.anu.edu.au/service/website/browse/3/Panic_disorder (s.f. -b)
- Beacon 2.0 Social anxiety. National Institute for Mental Health Research at the Australian National University. Beacon 2.0. https://beacon.anu.edu.au/service/website/browse/5/Social_anxiety (s.f. -c)
- Beacon 2.0 Phobias. National Institute for Mental Health Research at the Australian National University. Beacon 2.0. https://beacon.anu.edu.au/service/website/browse/4/Phobias (s.f. -d)
- Bellón J.A., Delgado A., Luna J.D., Lardelli P. Validity and reliability of the Duke-UNC-11 questionnaire of functional social support. Aten. Primaria. 1996;18(4):153–163. 15. [PubMed] [Google Scholar]
- Bellón J.Á., de Dios Luna J., King M., Moreno-Küstner B., Nazareth I., Montón-Franco C., GildeGómez-Barragán M.J., Sánchez-Celaya M., Díaz-Barreiros M.Á., Vicens C., Cervilla J.A., Švab I., Maaroos H.-I., Xavier M., Geerlings M.I., Saldivia S., Gutiérrez B., Motrico E., Martínez-Cañavate M.T., Oliván-Blázquez B., Sánchez-Artiaga M.S., March S., del Mar Muñoz-García M., Vázquez-Medrano A., Moreno-Peral P., Torres-González F. Predicting the onset of major depression in primary care: international validation of a risk prediction algorithm from Spain. Psychol. Med. 2011;41(10):2075–2088. doi: 10.1017/S0033291711000468. [DOI] [PubMed] [Google Scholar]
- Bellón J.Á., Conejo-Cerón S., Moreno-Peral P., King M., Nazareth I., Martín-Pérez C., Fernández-Alonso C., Ballesta-Rodríguez M.I., Fernández A., Aiarzaguena J.M., Montón-Franco C., Ibanez-Casas I., Rodríguez-Sánchez E., Rodríguez-Bayón A., Serrano-Blanco A., Gómez M.C., LaFuente P., del Mar Muñoz-García M., Mínguez-Gonzalo P., Araujo L., Palao D., Espinosa-Cifuentes M., Zubiaga F., Navas-Campaña D., Mendive J., Aranda-Regules J.M., Rodriguez-Morejón A., Salvador-Carulla L., de Dios Luna J. Preventing the onset of major depression based on the level and profile of risk of primary care attendees: protocol of a cluster randomised trial (the predictD-CCRT study) BMC Psychiatry. 2013;13:171. doi: 10.1186/1471-244X-13-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellón J.Á, Conejo-Cerón S., Moreno-Peral P., King M., Nazareth I., Martín-Pérez C., Fernández-Alonso C., Rodríguez-Bayón A., Fernández A., Aiarzaguena J.M., Montón-Franco C., Ibanez-Casas I., Rodríguez-Sánchez E., Ballesta-Rodríguez M.I., Serrano-Blanco A., Gómez M.C., LaFuente P., Muñoz-García M.d.M, Mínguez-Gonzalo P., Araujo L., Palao D., Bully P., Zubiaga F., Navas-Campaña D., Mendive J., Aranda-Regules J.M., Rodriguez-Morejón A., Salvador-Carulla L., de Dios Luna J. Intervention to prevent major depression in primary care: a cluster randomized trial. Ann. Intern. Med. 2016;164(10):656–665. doi: 10.7326/M14-2653. [DOI] [PubMed] [Google Scholar]
- Boas M.V., Cerqueira A. Assessing stress at work: the Portuguese version of the Job Content Questionnaire. Avaliaçao Psicologica: Interamerican Journal of Psychological Assessment. 2017;16(1):70–77. doi: 10.15689/ap.2017.1601.08. [DOI] [Google Scholar]
- Boutron I., Moher D., Altman D.G., Schulz K.F., Ravaud P. Extending the CONSORT statement to randomized trials of nonpharmacologic treatment: explanation and elaboration. Ann. Intern. Med. 2008;148:295–309. doi: 10.7326/0003-4819-148-4-200802190-00008. [DOI] [PubMed] [Google Scholar]
- Chan A.W., Tetzlaff J.M., Gøtzsche P.C., Altman D.G., Mann H., Berlin J.A., et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ. 2013;346 doi: 10.1136/bmj.e7586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuijpers P. Prevention: an achievable goal in personalized medicine. Dialogues Clin. Neurosci. 2009;11(4):447–454. doi: 10.31887/DCNS.2009.11.4/pcuijpers. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuijpers P., Sijbrandij M., Koole S., Huibers M., Berking M., Andersson G. Psychological treatment of generalized anxiety disorder: a meta-analysis. Clin. Psychol. Rev. 2014;34(2):130–140. doi: 10.1016/j.cpr.2014.01.002. [DOI] [PubMed] [Google Scholar]
- Deady M., Choi I., Calvo R.A., Glozier N., Christensen H., Harvey S.B. eHealth interventions for the prevention of depression and anxiety in the general population: a systematic review and meta-analysis. BMC Psychiatry. 2017;17(1):310. doi: 10.1186/s12888-017-1473-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditrendia Informe Ditrendia: Mobile en España y en el Mundo 2020 + Especial COVID-19. 2020. https://www.amic.media/media/files/file_352_2531.pdf Retrieved from:
- Eldridge S.M., Ashby D., Kerry S. Sample size for cluster randomized trials: effect of coefficient of variation of cluster size and analysis method. Int. J. Epidemiol. 2006;35(5):1292–1300. doi: 10.1093/ije/dyl129. [DOI] [PubMed] [Google Scholar]
- European Commission . 2020. The Digital Economy and Society Index (DESI) 2020. [Google Scholar]
- Eysenbach G. The law of attrition. J. Med. Internet Res. 2005;7(1) doi: 10.2196/jmir.7.1.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eysenbach G. MEDINFO; 2013. CONSORT-EHEALTH: Implementation of a Checklist for Authors and Editors to Improve Reporting of Web-based and Mobile Randomized Controlled Trials. [PubMed] [Google Scholar]
- Fernández A., Haro J.M., Martinez-Alonso M., Demyttenaere K., Brugha T.S., Autonell J., de Girolamo G., Bernert S., Lépine J.P., Alonso J. Treatment adequacy for anxiety and depressive disorders in six European countries. Br. J. Psychiatry J. Ment. Sci. 2007;190:172–173. doi: 10.1192/bjp.bp.106.023507. [DOI] [PubMed] [Google Scholar]
- Fernández Anna, Mendive J.M., Conejo-Cerón S., Moreno-Peral P., King M., Nazareth I., Martín-Pérez C., Fernández-Alonso C., Rodríguez-Bayón A., Aiarzaguena J.M., Montón-Franco C., Serrano-Blanco A., Ibañez-Casas I., Rodríguez-Sánchez E., Salvador-Carulla L., Garay P.B., Ballesta-Rodríguez M.I., LaFuente Pilar, del Mar Muñoz-García M., Mínguez-Gonzalo P., Araujo L., Palao D., Gómez M.C., Zubiaga F., Navas-Campaña D., Aranda-Regules J.M., Rodriguez-Morejón A., de Dios Luna J., Bellón J.Á. A personalized intervention to prevent depression in primary care: cost-effectiveness study nested into a clustered randomized trial. BMC Med. 2018;16(1):28. doi: 10.1186/s12916-018-1005-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira P.L., Ferreira L.N., Pereira L.N. Contributos para a Validação da Versão Portuguesa do EQ-5D [Contribution for the validation of the Portuguese version of EQ-5D] Acta Medica Port. 2013;26(6):664–675. [PubMed] [Google Scholar]
- Ferreira T., Sousa M., Salgado J. Brief assessment of depression: psychometric properties of the Portuguese version of the Patient Health Questionnaire (PHQ-9) Psychol. Pract. Res. J. 2019;1(2):1. doi: 10.33525/pprj.v1i2.36. [DOI] [Google Scholar]
- Fiorillo A., Luciano M., Del Vecchio V., Sampogna G., Obradors-Tarragó C., Maj M., ROAMER Consortium Priorities for mental health research in Europe: a survey among national stakeholders’ associations within the ROAMER project. World Psychiatry. 2013;12(2):165–170. doi: 10.1002/wps.20052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Campayo J., Zamorano E., Ruiz M.A., Pardo A., María Pérez-Páramo M., López-Gómez V., et al. Cultural adaptation into Spanish of the generalized anxiety disorder-7 (GAD-7) scale as a screening tool. Health Qual. Life Outcomes. 2010;8:8. doi: 10.1186/1477-7525-8-8. (Jan 20) [DOI] [PMC free article] [PubMed] [Google Scholar]
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet (London, England) 2018;392(10159):1789–1858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GBD 2019 Diseases and Injuries Collaborators Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet (London, England) 2020;396(10258):1204–1222. doi: 10.1016/S0140-6736(20)30925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick H.A., Doshi J.A., Sonnad S.S., Polsky D. Oxford University Press; Oxford: 2007. Economic Evaluation in Clinical Trials. [Google Scholar]
- Golubnitschaja O., Costigliola V., EPMA General report & recommendations in predictive, preventive and personalised medicine 2012: white paper of the European Association for Predictive, Preventive and Personalised Medicine. EPMA J. 2012;3(1):14. doi: 10.1186/1878-5085-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Gómez I., Benítez I., Bellón J., Moreno-Peral P., Oliván-Blázquez B., Clavería A., Zabaleta-Del-Olmo E., Llobera J., Serrano-Ripoll M.J., Tamayo-Morales O., Motrico E. Utility of PHQ-2, PHQ-8 and PHQ-9 for detecting major depression in primary health care: a validation study in Spain. Psychol. Med. 2022:1–11. doi: 10.1017/S0033291722002835. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haro J.M., Palacín C., Vilagut G., Martínez M., Bernal M., Luque I., Codony M., Dolz M., Alonso J., Grupo ESEMeD-España Prevalencia de los trastornos mentales y factores asociados: resultados del estudio ESEMeD-España [Prevalence of mental disorders and associated factors: results from the ESEMeD-Spain study] Med. Clin. 2006;126(12):445–451. doi: 10.1157/13086324. [DOI] [PubMed] [Google Scholar]
- Herdman M., Badia X., Berra S. EuroQol-5D: a simple alternative for measuring health-related quality of life in primary care. Aten. Primaria. 2001;28:425–429. doi: 10.1016/S0212-6567(01)70406-4. (Article in Spanish) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernán M.A., Hernández-Díaz S., Robins J.M. A structural approach to selection bias. Epidemiology. 2004;15:615–625. doi: 10.1097/01.ede.0000135174.63482.43. [DOI] [PubMed] [Google Scholar]
- Hofmann S.G., Gómez A.F. Mindfulness-based interventions for anxiety and depression. Psychiatr. Clin. N. Am. 2017;40(4):739–749. doi: 10.1016/j.psc.2017.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson C., Layte R., Jenkinson D., Lawrence K., Petersen S., Paice C., Stradling J. A shorter form health survey: can the SF-12 replicate results from the SF-36 in longitudinal studies? J. Public Health Med. 1997;19(2):179–186. doi: 10.1093/oxfordjournals.pubmed.a024606. [DOI] [PubMed] [Google Scholar]
- Jorm A.F., Patten S.B., Brugha T.S., Mojtabai R. Has increased provision of treatment reduced the prevalence of common mental disorders? Review of the evidence from four countries. World Psychiatry. 2017;16(1):90–99. doi: 10.1002/wps.20388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasek R., Theorell T. Basic Books; Nueva York, EE.UU: 1990. Healhty Work Stress, Productivity and the Reconstruction of Working Life. [Google Scholar]
- King M., Bottomley C., Bellón-Saameño J.A., Torres-Gonzalez F., Švab I., Rifel J., Maaroos H.I., Aluoja A., Geerlings M.I., Xavier M., Carraça I., Vicente B., Saldivia S., Nazareth I. An international risk prediction algorithm for the onset of generalized anxiety and panic syndromes in general practice attendees: predictA. Psychol. Med. 2011;41(8):1625–1639. doi: 10.1017/S0033291710002400. [DOI] [PubMed] [Google Scholar]
- Kraemer H.C. A mediator effect size in randomized clinical trials. Int. J. Methods Psychiatr. Res. 2014;23(4):401–410. doi: 10.1002/mpr.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke K., Spitzer R., Williams J. The PHQ-9 validity of a brief depression severity measure. J. Gen. Intern. Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke K., Spitzer Robert L., RL, Williams JBW, Löwe B. The Patient Health Questionnaire Somatic, Anxiety, and Depressive Symptom Scales: a systematic review. Gen. Hosp. Psychiatry. 2010;32:345–359. doi: 10.1016/j.genhosppsych.2010.03.006. [DOI] [PubMed] [Google Scholar]
- Linardon J., Cuijpers P., Carlbring P., Messer M., Fuller-Tyszkiewicz M. The efficacy of app-supported smartphone interventions for mental health problems: a meta-analysis of randomized controlled trials. World Psychiatry. 2019;18(3):325–336. doi: 10.1002/wps.20673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo M., Guo L., Yu M., Jiang W., Wang H. The psychological and mental impact of coronavirus disease 2019 (COVID-19) on medical staff and general public - a systematic review and meta-analysis. Psychiatry Res. 2020;291 doi: 10.1016/j.psychres.2020.113190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcolino M.S., Oliveira J., D’Agostino M., Ribeiro A.L., Alkmim M., Novillo-Ortiz D. The impact of mHealth interventions: systematic review of systematic reviews. JMIR mHealth uHealth. 2018;6(1) doi: 10.2196/mhealth.8873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin Payo R., Fernandez Álvarez M.M., Blanco Díaz M., Cuesta Izquierdo M., Stoyanov S.R., Llaneza Suárez E. Spanish adaptation and validation of the Mobile Application Rating Scale questionnaire. Int. J. Med. Inform. 2019;129:95–99. doi: 10.1016/j.ijmedinf.2019.06.005. (Sep, Epub 2019 Jun 5. PMID: 31445295) [DOI] [PubMed] [Google Scholar]
- Martins S., Martins C., Almeida A., Ayala-Nunes L., Gonçalves A., Nunes C. The adapted DUKE-UNC functional social support questionnaire in a community sample of Portuguese parents. Res. Soc. Work. Pract. 2022;32(5):596–606. doi: 10.1177/10497315221076039. [DOI] [Google Scholar]
- McDonald A., Eccles J., Fallahkhair S., Critchley H. Online psychotherapy: trailblazing digital healthcare. BJPsych. Bull. 2020;44(2):60–66. doi: 10.1192/bjb.2019.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery P., Grant S., Mayo-Wilson E., Macdonald G., Michie S., Hopewell S., et al. Reporting randomised trials of social and psychological interventions: the CONSORT-SPI 2018 Extension. Trials. 2018;19:407. doi: 10.1186/s13063-018-2733-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Peral P., Conejo-Cerón S., Motrico E., Rodríguez-Morejón A., Fernández A., García-Campayo J., Roca M., Serrano-Blanco A., Rubio-Valera M., Bellón J.Á. Risk factors for the onset of panic and generalised anxiety disorders in the general adult population: a systematic review of cohort studies. J. Affect. Disord. 2014;168:337–348. doi: 10.1016/j.jad.2014.06.021. [DOI] [PubMed] [Google Scholar]
- Moreno-Peral P., Luna J., Marston L., King M., Nazareth I., Motrico E., GildeGómez-Barragán M.J., Torres-González F., Montón-Franco C., Sánchez-Celaya M., Díaz-Barreiros M.Á., Vicens C., Muñoz-Bravo C., Bellón J.Á. Predicting the onset of anxiety syndromes at 12 months in primary care attendees. The predictA-Spain study. PLoS One. 2014;9(9) doi: 10.1371/journal.pone.0106370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Peral P., Conejo-Cerón S., Rubio-Valera M., Fernández A., Navas-Campaña D., Rodríguez-Morejón A., Motrico E., Rigabert A., Luna J.D., Martín-Pérez C., Rodríguez-Bayón A., Ballesta-Rodríguez M.I., Luciano J.V., Bellón J.Á. Effectiveness of psychological and/or educational interventions in the prevention of anxiety: a systematic review, meta-analysis, and meta-regression. JAMA Psychiatry. 2017;74(10):1021–1029. doi: 10.1001/jamapsychiatry.2017.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Peral P., Bellón J.Á., Huibers M., Mestre J.M., García-López L.J., Taubner S., Rodríguez-Morejón A., Bolinski F., Sales C., Conejo-Cerón S. Mediators in psychological and psychoeducational interventions for the prevention of depression and anxiety. A systematic review. Clin. Psychol. Rev. 2020;76 doi: 10.1016/j.cpr.2020.101813. [DOI] [PubMed] [Google Scholar]
- Moreno-Peral P., Bellón J.Á., Motrico E., Campos-Paíno H., Martín-Gómez C., Ebert D.D., Buntrock C., Roca M., Conejo-Cerón S. Moderators of psychological and psychoeducational interventions for the prevention of anxiety: a systematic review. J. Anxiety Disord. 2020;76 doi: 10.1016/j.janxdis.2020.102317. [DOI] [PubMed] [Google Scholar]
- Moreno-Peral P., Conejo-Cerón S., de Dios Luna J., King M., Nazareth I., Martín-Pérez C., Fernández-Alonso C., Ballesta-Rodríguez M.I., Fernández A., Aiarzaguena J.M., Montón-Franco C., Bellón J.Á. Use of a personalised depression intervention in primary care to prevent anxiety: a secondary study of a cluster randomised trial. Br. J. Gen. Practice. 2021;71(703):e95–e104. doi: 10.3399/bjgp20X714041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Peral P., Conejo-Cerón S., Wijnen B., Lokkerbol J., Fernández A., Smit F., Bellón J.A. Health-economic evaluation of psychological interventions for anxiety prevention: a systematic review. Psychiatr. Serv. 2023 doi: 10.1176/appi.ps.20230101. (in press) [DOI] [PubMed] [Google Scholar]
- Olesen J., Gustavsson A., Svensson M., Wittchen H.U., Jönsson B., CDBE2010 study group, European Brain Council The economic cost of brain disorders in Europe. Eur. J. Neurol. 2012;19(1):155–162. doi: 10.1111/j.1468-1331.2011.03590.x. [DOI] [PubMed] [Google Scholar]
- Oliveira C., Pereira A., Sousa Maria, Vagos P., Stoyanov S. 2022. Translation and Validation of the User Version of the Mobile Application Rating Scale (uMars) to the Portuguese Population. (in press) [Google Scholar]
- Parés-Badell O., Barbaglia G., Jerinic P., Gustavsson A., Salvador-Carulla L., Alonso J. Cost of disorders of the brain in Spain. PLoS One. 2014;9(8) doi: 10.1371/journal.pone.0105471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigabert A., Motrico E., Moreno-Peral P., Resurrección D.M., Conejo-Cerón S., Cuijpers P., Martín-Gómez C., López-Del-Hoyo Y., Bellón J.Á. Effectiveness of online psychological and psychoeducational interventions to prevent depression: systematic review and meta-analysis of randomized controlled trials. Clin. Psychol. Rev. 2020;82 doi: 10.1016/j.cpr.2020.101931. [DOI] [PubMed] [Google Scholar]
- Sánchez-Meca J., Rosa-Alcázar A.I., Marín-Martínez F., Gómez-Conesa A. Psychological treatment of panic disorder with or without agoraphobia: a meta-analysis. Clin. Psychol. Rev. 2010;30(1):37–50. doi: 10.1016/j.cpr.2009.08.011. [DOI] [PubMed] [Google Scholar]
- Sander L., Rausch L., Baumeister H. Effectiveness of internet-based interventions for the prevention of mental disorders: a systematic review and meta-analysis. JMIR Ment. Health. 2016;3(3) doi: 10.2196/mental.6061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa T.V., Viveiros V., Chai M.V., Vicente F.L., Jesus G., Carnot M.J., Gordo A.C., Ferreira P.L. Reliability and validity of the Portuguese version of the Generalized Anxiety Disorder (GAD-7) scale. Health Qual. Life Outcomes. 2015;13:50. doi: 10.1186/s12955-015-0244-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer R.L., Kroenke K., Williams J.W., Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch. Intern. Med. 2006;166(10):1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- Stratton E., Lampit A., Choi I., Calvo R.A., Harvey S.B., Glozier N. Effectiveness of eHealth interventions for reducing mental health conditions in employees: a systematic review and meta-analysis. PLoS One. 2017;12(12) doi: 10.1371/journal.pone.0189904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez-Barquero J.L., Gaite L., Cuesta M.J., Carcia-Usieto E., Knapp M., Beecham J. Spanish version of the CSRI: a mental health cost evaluation interview. Arch. Neurobiol. (Madr.) 1997;60:171–184. [Google Scholar]
- Vodopivec-Jamsek V., de Jongh T., Gurol-Urganci I., Atun R., Car J. Mobile phone messaging for preventive health care. Cochrane Database Syst. Rev. 2012;12(12):CD007457. doi: 10.1002/14651858.CD007457.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . vol. 3. 2011. mHealth: new horizons for health through mobile technologies. (Global Observatory for eHealth Series). (Geneva, Switzerland) [Google Scholar]
- World Health Organization (WHO) World Health Organization; Geneva: 1997. Composite International Diagnostic Instrument (CIDI). Version 2.1. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data generated during this study will include detailed information on the protocols and analyses used to allow their reproducibility and future analyses by other groups. We recognize and support the principles of data sharing. The data generated from this research will be made available to affiliated investigators through secure and anonymized databases. Only investigators with specific independent ethics committee approval will have access to any anonymized data. The research coordinator of prevANS study can be contacted for de-identified data requests. Consent for such data sharing will be integral to enrollment in the study, and our participants will be asked about their willingness to have their data shared to advance health research.