Abstract
Masticatory function such as chewing is expected to modify human cognitive function, and/or the possibility of improving cognitive function is also predicted. This systematic review investigated whether masticatory function affects cognitive function for older/young adults. Full articles written in English from January 2000 to April 2022 were collected using PubMed and Cochrane Library. Target outcomes were cognitive function test scores, cognitive processing speed (reaction time), and masticatory function. For each research question, two independent reviewers conducted the search and screening, data extraction, quality assessment, and risk of bias assessment. The reviewers resolved any disagreements by discussion. From 226 articles retrieved, 20 were included in this review. Older adults with lower scores on the cognitive function test had lower masticatory performance, lower chewing ability, chewing difficulty, and decreased number of teeth. An increased risk of cognitive impairment was found in older adults with masticatory dysfunction. For young adults, gum chewing significantly reduced the processing speed of cognitive tasks compared to no gum chewing. Although most of the evidence included had a low level of evidence and a high risk of bias because of the research designs, the results still suggest that mastication may be a factor in improving cognitive function.
Keywords: Cognitive function, Cognitive impairment, Cognitive decline, Dementia, Mastication, Chewing
1. Introduction
The increasing prevalence of older people experiencing gradual declines in physical and cognitive function [1], [2] due to aging [2], [3] has emerged as a growing public health concern [1]. Cognitive impairment, commonly observed in older individuals [4], is regarded as an early sign of clinical dementia [5]. Fig. 1 shows a conceptual figure of the relationship between masticatory function and dementia at present.
Fig. 1.
Relationship between cognitive function and masticatory movement Partially based on reference [6].
Dementia is a chronic or progressive syndrome caused by neurodegenerative diseases leading to cognitive decline affecting memory, thinking, behavior, and ability to perform daily activities [1]. The World Health Organization (WHO) estimates an increased prevalence of people with dementia by 139 million by 2050 [1]. In Japan, patients with dementia are expected to reach by 7 million by 2025, with one in every five people over 65 developing dementia [7]. According to WHO, dementia is one of the leading causes of disability and dependency among older people and the seventh leading cause of death among all diseases [1]. Unfortunately, there is no currently available treatment for dementia [1]. Dementia prevention, as well as the maintenance and improvement of cognitive functions, has gained much attention recently [8]. To reduce the risk and progression of dementia, modifiable factors must be identified.
The risk factors for dementia are reported to be multifactorial, including age [9], [10], literacy [9], low educational levels [9], [10], low socioeconomic status [9], head injury [11], obesity [11], smoking [10], [11], high blood pressure [11], diabetes [11], activities of daily living (ADL) [12], [13], nutritional status [14], [15], [6], and oral health, particularly tooth loss [16], [17], [18], [19], [20]. Recent studies suggest that tooth loss, which results in masticatory dysfunction, may be one of the risk factors for dementia [16], [17], [18], [20], [21], [22], [23]. Reports show that masticatory muscle mass and strength decline due to tooth loss, causing chewing difficulty and decreased afferent signals, reducing the brain’s neuroplasticity [24]. Several epidemiological studies have consistently suggested associations between a reduced number of teeth and masticatory dysfunction with cognitive decline and memory deterioration [19], [25], [26], [27], [28], [29]. Emergent evidence on the effects of chewing on increased attention, memory, and cognitive processing shows that the masticatory condition is related to cognitive function. [26], [29], [30], [31], [32], [33], [34], [35].
Several neuroimaging studies, such as Positron Emission Tomography (PET), functional Magnetic Resonance Imaging (fMRI) technologies, and functional Near-Infrared Spectroscopy (fNIRS), have confirmed the association between chewing and increased neural activation of memory centers of the brain, particularly the cortical primary somatosensory area, supplementary motor area, insula, cerebellum, and striatum of basal ganglia were activated during gum chewing [36], [37], [38]. Thus, masticatory function (defined as chewing and eating) [39] is expected to modify human cognitive function, and the possibility of improving cognitive function is also predicted. The relationship between them must first be clarified to confirm the influence of masticatory function on cognitive function. Concrete evidence from systematic reviews investigating the relationship between masticatory function and cognitive function remains limited.
If the relationship between cognitive function and masticatory function is clarified, masticatory functions such as chewing and eating can help maintain or improve cognitive function. This review aimed to clarify whether the literature supports the existence of the relationship between cognitive function and masticatory function. Therefore, this systematic review evaluated the effects of masticatory function on cognitive function.
2. Materials and methods
2.1. Search strategy method and focused question
This systematic review was performed according to the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA 2020) statement [40]. This systematic review was registered with PRISMA before the start of the systematic review (PROSPERO no. CRD42022325708).
We aimed to evaluate the effects of masticatory function on cognitive function. Therefore, the following review questions were formulated using the PICO (participant, intervention, comparison, and outcome) approach [40].
The participants were young and/or older adults. The intervention and control were increased/good masticatory function or decreased/difficulty in masticatory function and at rest or unchanged masticatory function, respectively.
The outcomes were as follows:
① The cognitive status as assessed by the cognitive function test scores on the Mini-Mental State Examination (MMSE) [41], Hasegawa Dementia Scale-Revised (HDSR) [42], [43], Frontal Assessment Battery (FAB) [44], [45], and neuropsychological tests [46] on attention, working memory, and verbal fluency.
② The cognitive function was assessed by the processing speed of cognitive tasks (reaction time) [47].
The following were the research questions (RQ) used:
RQ1
: Is the cognitive status of older adults with dementia associated with masticatory function?
RQ1–1: Is the cognitive status assessed by cognitive function test scores associated with the masticatory function (masticatory performance and chewing ability)?
RQ1–2: Is cognitive impairment associated with decreased number of teeth?
RQ1–3: Is the risk of cognitive impairment associated with masticatory dysfunction? (masticatory dysfunction includes chewing difficulty, decreased chewing ability, and decreased number of present teeth).
RQ2
: Does cognitive function improve with mastication in young adults?
The outcomes for each RQ are shown in Table 1.
Table 1.
List of outcomes for each research question.
| RQ | Outcomes |
|---|---|
| RQ1–1 | cognitive function test scores: MMSE, HDSR, FAB, neuropsychological tests (attention, working memory, verbal fluency) |
| RQ1–2, RQ1–3 | cognitive function test scores: MMSE, HDSR, FAB |
| RQ2 | processing speed of cognitive tasks (reaction time) |
RQ: Research question; MMSE: Mini-Mental State Examination; HDSR: Hasegawa Dementia Scale Revised; FAB: Frontal Assessment Battery.
An electronic search of PubMed and the Cochrane Library database was performed to identify the relevant literature systematically. Articles published between January 1, 2000, to April 11, 2022, were considered. The search string comprised a combination of keywords from the Medical Subject Headings (MeSH) database and free-text terms found in the Title/Abstract. Boolean operators such as “OR” and “AND” were used to link the terms. The search formulas used for each RQ were listed in brain activity Table 2a-d. After the electronic search was completed, the titles and abstracts of the studies were collected and screened for duplicates.
Table 2.
Search formula for each research question. An electronic search of PubMed and the Cochrane Library database was performed. Articles published between January 1, 2000 to April 11, 2022, were considered.
| a: Search formula for RQ1 using PubMed. | |
|---|---|
| #1 | “aged”[MeSH Terms] OR “elderly”[Title/Abstract] OR “aged”[MeSH Terms] OR “older adults”[Title/Abstract] |
| #2 | “dementia”[MeSH Terms] OR dementia[Title/Abstract] |
| #3 | “cognition”[MeSH Terms] OR cognitive function[Title/Abstract] |
| #4 | “cognitive dysfunction”[MeSH Terms] OR cognitive decline[Title/Abstract] OR cognitive impairment[Title/Abstract] |
| #5 | “mastication”[MeSH Terms] OR chewing[Title/Abstract] |
| #6 | #2 OR #3 OR #4 |
| #7 | #1 AND #5 AND #6 |
| B: Search formula for RQ1 using Cochrane Library. | |
| #1 | MeSH descriptor: [Aged] in all MeSH products |
| #2 | MeSH descriptor: [Cognition] explode all trees |
| #3 | MeSH descriptor: [Cognitive Dysfunction] explode all trees |
| #4 | MeSH descriptor: [Dementia] explode all trees |
| #5 | MeSH descriptor: [Mastication] explode all trees |
| #6 | #2 OR #3 OR #5 |
| #7 | #1 AND #5 AND #6 |
| c: Search formula for RQ2 using PubMed. | |
| #1 | "aged"[MeSH Terms] OR "elderly"[Title/Abstract] OR "aged"[MeSH Terms] OR "older adults"[Title/Abstract] |
| #2 | "young adult"[MeSH Terms] OR young adult[Title/Abstract] |
| #3 | "cognition"[MeSH Terms] OR cognitive function[Title/Abstract] |
| #4 | "cognitive dysfunction"[MeSH Terms] OR cognitive decline[Title/Abstract] OR cognitive impairment[Title/Abstract] |
| #5 | "mastication"[MeSH Terms] OR chewing[Title/Abstract] |
| #6 | #3 OR #4 |
| #7 | #1 AND #5 AND #6 |
| #8 | #2 AND #5 AND #6 |
| d: Search formula for RQ2 using Cochrane Library. | |
| #1 | MeSH descriptor: [Aged] in all MeSH products |
| #2 | MeSH descriptor: [Young Adult] explode all trees |
| #3 | MeSH descriptor: [Cognition] explode all trees |
| #4 | MeSH descriptor: [Cognitive Dysfunction] explode all trees |
| #5 | MeSH descriptor: [Mastication] explode all trees |
| #6 | (mastication):ti,ab,kw OR (chewing):ti,ab,kw |
| #7 | #3 OR #4 |
| #8 | #5 OR #6 |
| #9 | #1 AND #7 AND #8 |
| #10 | #2 AND #7 AND #8 |
RQ: Research question.
2.2. Eligibility and inclusion/exclusion Criteria
To conform with the objectives of the review, the following inclusion criteria were applied for the selection of evidence: human experiments on young and/or older adults, articles published on January 2000 to April 2022, and studies that include masticatory functions such as chewing as exposure interests and cognitive function, cognitive impairment, cognitive decline, and dementia as the outcomes of interest, articles such as randomized controlled trials (RCTs), prospective and retrospective studies, cross-sectional studies, clinical studies, with both abstract and full report available and are written in English.
The exclusion criteria applied were articles that were not original studies, studies involving swallowing and eating disorders, meta-analyses, systematic reviews, case reports, in-vitro studies, animal studies, abstracts from conferences, and letters to the editor.
Articles that met at least one exclusion criterion were excluded. The full texts of the relevant articles were then retrieved and analyzed. Additional articles were added by checking the references from the final included articles and manual searching.
2.3. Screening Procedures
Two reviewers independently screened each retrieved document for eligibility by examining the titles and abstracts according to the inclusion and exclusion criteria. The selected abstracts were listed and compared. Any discrepancy during the screening and the selection process was resolved by discussion, and a third reviewer was consulted to reach a definitive consensus regarding the inclusion of the articles. The full text of all potentially relevant studies was then obtained for independent assessments by the same reviewers. Only studies with sufficient and specific data available were included for further analysis. Discrepancies and disagreements were resolved through discussion and consensus.
2.4. Data synthesis
We pooled the data into evidence tables and created a descriptive summary to evaluate all data and identify study characteristics and outcome variations. This enabled the identification of similarities and differences between studies and the determination of suitability for additional synthesis or comparison methods.
For pooled data in a statistical meta-analysis, data extraction from either graphs or charts was done with a Review Manager tool (RevMan, Version 5.4, The Cochrane Collaboration, 2020) [48]. Forest plots were presented as standardized mean differences (SMD) with 95% confidence intervals (CI) or odds ratio and 95% CI. Effect sizes were presented as SMD, and 95% CI was calculated for analysis [49]. Heterogeneity was assessed statistically using the standard χ2, Tau2, and I2 tests. Meta-analysis was performed using the random effects model with heterogeneity taken from an inverse variance model to estimate the pooled data effect.
2.5. Quality assessment
The two reviewers independently assessed the quality and risk of bias during the data extraction process, and a discussion was used to resolve disagreements and discrepancies. Inconsistencies and conflicts were resolved through discussion. Quality assessment of included cross-over trials was performed using the Cochrane Collaboration tool, Review Manager (RevMan, Version 5.4, The Cochrane Collaboration, 2020), for assessing the risk of bias and meta-analyses [48], [50].
The Cochrane Collaboration tool assesses the risk of bias from seven domains: selection bias or allocation bias (sequence generation and allocation concealment), performance bias (blinding of participants and personnel), detection bias (blinding of outcome assessors), attrition bias (incomplete outcome data), reporting bias (selective reporting) and an auxiliary domain: “other bias.” The bias judgment for each domain is ‘unclear risk,’ ‘low risk,’ or ‘high risk’ of bias. Since the adopted Newcastle-Ottawa Scale (NOS) for non-randomized studies [51], [52] was not appropriate for our study, a modified version of this scale was used to perform the quality assessment for the studies [53] included in this review.
The modified NOS tool assigns a maximum of 10 stars across three domains: (1) Selection (up to 5 stars), (2) Comparability (up to 2 stars), and (3) Outcome (up to 3 stars). For the Selection domain, a study can be awarded 1 star if the study’s sample is true/somewhat representative of the average target population. Another star can be awarded when the sample size is justified and satisfactory. The study was also awarded another star if the response rate was adequate. Two stars can be granted if the ascertainment of exposure used a validated measurement tool, and one star if non-validated but the tool was available or described. One star could be awarded for the Comparability domain if confounding factors such as age and sex were controlled. Another star was awarded if the study controlled for any additional factor. For the Outcome domain, two stars can be granted if the assessment was blinded and one star if not blinded or self-reported. An additional one star can be awarded if the statistical test used to analyze the data was clearly described and appropriate. Then, the sum of the stars in each domain was calculated to convert the Newcastle-Ottawa Scales to the Agency for Health Research and Quality (AHRQ) standards [54]. The studies evaluated with poor quality have 0 or 1 star in the Selection domain OR 0 star in the Comparability domain OR 0 or 1 star in the Outcome domain, while those with fair quality have two stars in the Selection domain AND 1 or 2 stars in Comparability domain AND 2 or 3 stars in Outcome domain. On the other hand, the studies evaluated with good quality have 3 or 4 stars in the Selection domain, 1 or 2 stars in the Comparability domain, AND 2 or 3 stars in the Outcome domain [54], [55]. Although data from cross-sectional studies are considered low quality compared to other research designs such as RCT, cohort, and longitudinal studies, assessing the relationship between masticatory function and cognitive function remains feasible.
3. Results
3.1. General outcomes
The study selection process is described in Fig. 2 as per the PRISMA flow diagram [56]. The final electronic search of the databases yielded 226 articles. There were 42 articles chosen for the second evaluation based on a review of their titles and abstracts. The second phase included a thorough screening and evaluation of 41 full-text articles. At this point, 23 publications were excluded because they did not meet the inclusion criteria. Two additional articles were added by checking the references from the final included articles and manual searching. The last search date was May 20, 2022. Finally, 20 articles were identified as eligible for this study. A list of corresponding papers is shown in Table 3, Table 4, Table 5, Table 6 for each RQ.
Fig. 2.
Flow chart illustrating the screening process for the selection of articles.
Table 3.
Characteristics of Studies Integrated relating to RQ1–1.
| No | Authors | Study Design | Study Population | Intervention | Comparison | Outcome |
|---|---|---|---|---|---|---|
| 1 * | Matsubara et al., 2021 | RCT | 50 older adults aged 65 and above (MMSE score ≥21 to ≤26) | N = 25 intervention group (mean age: 77.0 years) | N = 25 control group (mean age: 72.8 years) | MMSE, chewing ability with gum chewing, and masticatory performance with test gummy jelly |
| 2 * | Tan et al., 2020 | Pilot quasi-experimental study | 4 completely edentulous patients (mean age: 73.0 ± 1.4years; 2 males, 2 females) | N= 4 implant retained removable denture | N = 4 complete removable denture | 3MS - Modified MMSE, Masticatory performance using color changing gum |
| 3 | Cho et al., 2021 | Case-control | 308 Koreans aged ≥ 65 (mean age: 78.69 6 ± 5.76 years, 68 males, 240 females | N = 89, poor masticatory function; N=109, with cognitive impairment (MMSE score <24) | N = 99, good masticatory function; N= 199, normal (MMSE score ≥ 24) | MMSE, Chewing ability for 5 food items, subjective masticatory ability |
| 4 | Jung et al., 2022 | Case-control | 295 Korean adults aged > 60 years (85 males, 200 females) | N = 100, poor masticatory function | N = 109, good masticatory function | MMSE |
| 5 | Kim et al., 2017 | Case-control | 295 Koreans aged ≥ 70 | N = 60, poor masticatory function; N= 59, with cognitive impairment (MMSE score ≤ 20) | N = 56, good masticatory function; N= 236, normal (MMSE score ≥ 21) | MMSE, Masticatory Performance using color changing gum |
| 6 | Kimura et al., 2013 | Case-control | 269 Japanese aged ≥ 75 (mean age 80.6 ± 4.7 years; 88 males, 181 females) | N = 105, poor masticatory function | N = 164, good masticatory function | MMSE, HDSR, FAB |
| 7 | Shin et al., 2020 | Case-control | 101 Korean women aged ≥ 65 (mean age: 80.64 ± 4.83 years) | N = 29, poor masticatory function; N= 40, with cognitive impairment (MMSE score <24) | N = 36, good masticatory function; N= 61, normal (MMSE score ≥ 24) | MMSE, Masticatory Performance using color-changing gum |
| 8* | Weijenberg et al., 2015 | Case-control | 114 Dutch older persons with dementia aged ≥ 67 | N = 56, poor masticatory function (mean age: 85.2 ± 6.4 years; 4 males, 52 females) | N = 58, good masticatory function (mean age: 85.3 ± 5.4 years; 11 males, 47 females) | MMSE, attention, working memory |
| 9 | Kim et al., 2020 | Case-control | 7029 Korean adults > 45 years | N = 1243, poor masticatory function; N= 2043, with cognitive impairment (MMSE score <24, mean ag:e 75.39 ± 0.21 years; 624 males, 1419 females) | N = 2868, good masticatory function; N= 4986, normal (MMSE score ≥ 24, mean age: 65.38 ± 0.12 years; 2363 males, 2623 females) | MMSE, Chewing ability using self-report questionnaire |
| 10 | Seraj et al., 2017 | Case-control | 50 older adults aged ≥ 60 (25 males, 25 females) | N = 26, poor masticatory function; N= 31, with cognitive impairment (MMSE score <24, mean age: 73.5 ± 11.8 years; 14 males, 17 females) | N = 24,good masticatory function; N= 19, normal (MMSE score ≥ 24, mean age: 67.7 ± 5.4 years; 11 males, 8 females) | MMSE, Index of Chewing ability (questionnaire) |
| 11 | Takehara et al., 2020 | Case-control | 369 Australian men aged ≥ 78 (mean age: 83.8 ± 4.2 years) | N = 101, poor masticatory function; N= 17, with cognitive impairment (MMSE score <24) | N = 268, good masticatory function; N= 352, normal (MMSE score ≥ 24) | MMSE, Chewing ability for 11 food items |
| 12 * | Elsig et al., 2015 | Case-control | 51 older adults aged ≥ 75 | N = 29, with Dementia (mean age: 82.5 ± 6.3 years; 7 males, 22 females) | N = 22, normal (19) or with mild cognitive impairment (3) (mean age: 81.9 ± 6.5 years; 5 males, 17 females) | Masticatory Performance using two-color mixing test |
| 13 * | Miura et al., 2003 | Case-control | 88 Japanese women aged ≥ 65 | N = 44, with cognitive impairment (HDSR score ≤ 20, mean age: 81.1 ± 5.5 years) | N = 44, normal (HDSR score ≥ 21, mean age: 82.3 ± 8.0 years) | HDSR, Chewing ability using food intake questionnaire |
| 14 * | Campos et al., 2017 | Case-control | 32 older adults with removable dentures (11 completely edentulous, 5 partially edentulous in each group) | N = 16, with mild Alzheimer’s Disease (mean age: 76.7 ± 6.3 years; 8 males, 8 females) | N = 16, healthy (mean age: 75.23 ± 4.4 years; 8 males, 8 females) | Masticatory Performance using Optocal artificial test food |
| 15 * | Kugimiya et al., 2019 | Cross-sectional | 1118 Japanese aged ≥ 70 (MMSE ≥24; mean age: 77.0 ± 4.7 years; 445 males, 673 females) | Comparison among gender | Comparison among gender | MMSE |
*Studies not included in the meta-analyses but considered as evidence (see Discussion).
RQ: Research question; RCT: Randomized Controlled Trial; MMSE: Mini-Mental State Examination; HDSR: Hasegawa Dementia Scale-Revised; FAB: Frontal Assessment Battery.
Table 4.
Characteristics of Studies Integrated relating to RQ1–2.
| No | Authors | Study Design | Study Population | Intervention | Comparison | Outcome |
|---|---|---|---|---|---|---|
| 1 | Kimura et al., 2013 | Case-control | 269 Japanese aged ≥ 75 (mean age 80.6 ± 4.7 years; 88 males, 181 females) | N = 105, poor masticatory function | N = 164, good masticatory function | MMSE, HDSR, FAB, number of present teeth |
| 2 | Takehara et al., 2020 | Case-control | 369 Australian men aged ≥ 78 (mean age: 83.8 ± 4.2 years) | N = 101, poor masticatory function; N= 17, with cognitive impairment (MMSE score <24) | N = 268, good masticatory function: N= 352, normal (MMSE score ≥ 24) | MMSE, number of present teeth |
| 3 | Elsig et al., 2015 | Case-control | 29 with dementia (75 years or older); 19 cognitively normal and 3 with mild cognitive impairment | N = 29, with Dementia (mean age: 82.5 ± 6.3 years; 7 males, 22 females) | N = 22, normal (19) or with mild cognitive impairment (3) (mean age: 81.9 ± 6.5 years; 5 males, 17 females) | MMSE, number of present teeth |
| 4 | Miura et al., 2003 | Case-control | 88 Japanese women aged ≥ 65 | N = 44, with cognitive impairment (HDSR score ≤20, mean age: 81.1 ± 5.5 years) | N = 44, normal (HDSR score ≥ 21, mean age: 82.3 ± 8.0 years) | HDSR, number of present teeth |
| 5 | Cho et al., 2021 | Case-control | 308 Koreans aged ≥ 65 (mean age: 78.69 6 ± 5.76 years, 68 males, 240 females | N = 109, with cognitive impairment (MMSE score <24) | N = 199, normal (MMSE score ≥ 24) | MMSE, number of present teeth |
| 6 | Lexomboon et al., 2012 | Case-control | 557 Swedish aged ≥ 77 (mean age: 83.0 ± 4.7 years) | N = 123, with cognitive impairment (MMSE score <24; 41 males, 82 females) | N = 434, normal (MMSE score ≥ 24; 188 males, 246 females) | MMSE, number of present teeth |
| 7 | Seraj et al., 2017 | Case-control | 50 older adults aged ≥ 60 (25 males, 25 females) | N = 31, with cognitive impairment (MMSE score <24, mean age: 73.5 ± 11.8 years; 14 males, 17 females) | N = 19, normal (MMSE score ≥ 24, mean age: 67.7 ± 5.4 years; 11 males, 8 females) | MMSE, number of present teeth |
| 8 * | Shin et al., 2020 | Case-control | 101 Korean women aged ≥ 65 (mean age: 80.64 ± 4.83 years) | N = 29, poor masticatory function; N= 40, with cognitive impairment (MMSE score <24) | N = 36, good masticatory function; N= 61, normal (MMSE score ≥ 24) | MMSE, Number of present teeth |
*Studies not included in the meta-analyses but considered as evidence (see Discussion).
RQ: Research question; MMSE: Mini-Mental State Examination, HDSR: Hasegawa-Dementia Scale-Revised; FAB: Frontal Assessment Battery.
Table 5.
Characteristics of Studies Integrated relating to RQ1–3.
| No | Authors | Study Design | Study Population | Intervention | Comparison | Outcome |
|---|---|---|---|---|---|---|
| 1 | Lexomboon et al., 2012 | Case-control | 557 Swedish aged ≥ 77 (mean age: 83.0 ± 4.7 years) | N = 123, with cognitive impairment (MMSE score <24; 41 males, 82 females) | N = 434, normal (MMSE score ≥ 24; 188 males, 246 females) | MMSE, Chewing difficulty questionnaire |
| 2 | Cho et al., 2021 | Case-control | 308 Koreans aged ≥ 65 (mean age: 78.69 6 ± 5.76 years, 68 males, 240 females | N = 89, poor masticatory function; N=109, with cognitive impairment (MMSE score <24) | N = 99, good masticatory function; N= 199, normal (MMSE score ≥ 24) | MMSE, Chewing ability for 5 food items, subjective masticatory ability |
| 3 | Kim et al., 2017 | Case-control | 295 Koreans aged ≥ 70 | N = 60, poor masticatory function; N= 59, with cognitive impairment (MMSE score ≤ 20) | N = 56, good masticatory function; N= 236, normal (MMSE score ≥ 21) | MMSE, Masticatory Performance using color changing gum |
| 4 | Kim et al., 2020 | Case-control | 7029 Korean adults > 45 years | N = 2043, with cognitive impairment (MMSE score <24, mean ag:e 75.39 ± 0.21 years; 624 males, 1419 females) | N = 4986, normal (MMSE score ≥ 24, mean age: 65.38 ± 0.12 years; 2363 males, 2623 females) | MMSE, Chewing ability questionnaire |
| 5 | Seraj et al., 2017 | Case-control | 50 older adults aged ≥ 60 (25 males, 25 females) | N = 26, poor masticatory function; N= 31, with cognitive impairment (MMSE score <24, mean age: 73.5 ± 11.8 years; 14 males, 17 females) | N = 24、good masticatory function; N= 19, normal (MMSE score ≥ 24, mean age: 67.7 ± 5.4 years; 11 males, 8 females) | MMSE, Index of Chewing ability (questionnaire) |
| 6 | Shin et al., 2020 | Case-control | 101 Korean women aged ≥ 65 (mean age: 80.64 ± 4.83 years) | N = 29, poor masticatory function; N= 40, with cognitive impairment (MMSE score <24) | N = 36, good masticatory function; N= 61, normal (MMSE score ≥ 24) | MMSE, Masticatory Performance using color-changing gum |
| 7 | Takehara et al., 2020 | Case-control | 369 Australian men aged ≥ 78 (mean age: 83.8 ± 4.2 years) | N = 101, poor masticatory function; N= 17, with cognitive impairment (MMSE score <24) | N = 268, good masticatory function; N= 352, normal (MMSE score ≥ 24) | MMSE, Chewing ability for 11 food items |
| 8 * | Scherder et al. 2008 | Case-control | 38 older adults from the Netherlands (MMSE score ≥ 25) | N = 19, with full dentures (mean age: 75.68 ± 3.35 years; 7 males, 12 females) | N = 19, complete natural teeth (mean age 73.21 ± 4.28 years; 10 males, 9 females) | mandibular excursions, bite force, number of occluding pairs, and complaints of the masticatory system |
*Studies not included in the meta-analyses but considered as evidence (see Discussion).
RQ: Research question; MMSE: Mini-Mental State Examination.
Table 6.
Characteristics of Studies Integrated relating to RQ2.
| No. | Authors | Study Design | Study Population | Intervention | Comparison | Outcome |
|---|---|---|---|---|---|---|
| 1 | Sakamoto et al., 2009 | cross-over trial (quasi-RCT) | 11 healthy subjects aged 24–42 years (mean age: 30.9 years; 8 males, 3 females) for Experiment 1. 9 healthy subjects aged 25–43 years (mean age: 30.6 years; 8 males, 1 female) for Experiment 2. | gum chewing | no gum chewing | reaction time |
| 2 | Smith 2010 | cross-over trial (quasi-RCT) | 133 adults aged 19–39 years (mean age: 22.6 ± 4.4 years; 64 males, 69 females) | gum chewing | no gum chewing | reaction time |
| 3 | Tucha & Simpson, 2011 | Randomized cross-over trial (quasi-RCT) | 42 healthy young adults (mean age 22.2 ± 2.4 years; 21 males, 21 females) | gum chewing | no gum chewing | reaction time |
RQ: Research question.
Most included papers were case-control studies rather than randomized controlled clinical trials that considered baseline data for analysis. The included studies evaluated the cognitive status/function of young and older adults and their masticatory function (defined as chewing). Statistical analysis was performed appropriately in all the included studies, except for four articles that failed to report data distribution.
3.2. Association between cognitive function and masticatory function (RQ1-1)
Based on the results of this study, one RCT, one pilot quasi-experimental study, and 13 case-control studies obtained evidence showing that the cognitive status of older adults with dementia is associated with masticatory function.
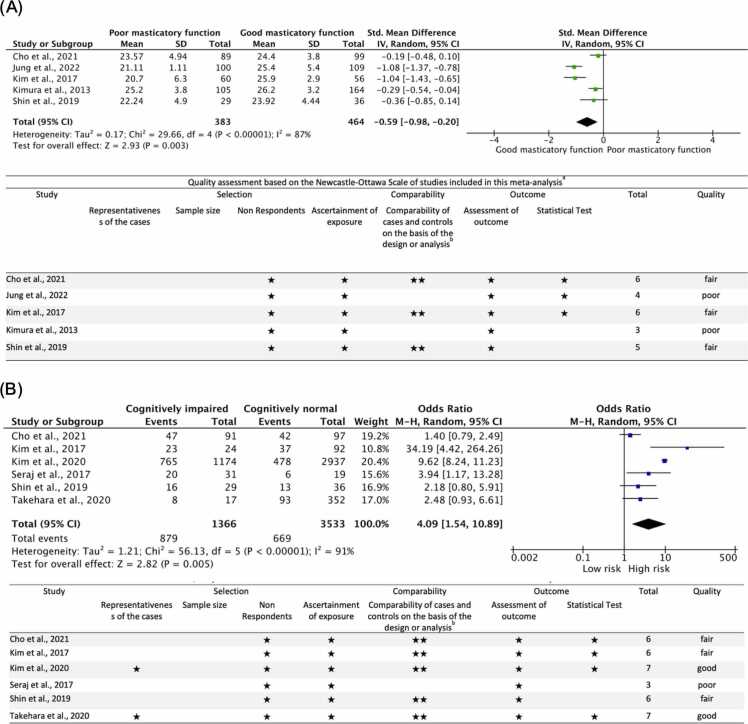
Among these studies, five case-control studies, including 847 subjects, were analyzed to assess the association between the cognitive status of older adults using MMSE and masticatory function. The meta-analysis showed that the MMSE scores of those subjects with poor mastication were lower than those with good masticatory function (Fig. 3a). The standardized mean difference (SMD) was − 0.59 (95% CI −0.98 to −0.20). The heterogeneity by I2 statistics was high at 87%. The test for overall effect (Z) was 2.93 (p = 0.003). The results showed that the cognitive status of older adults with good masticatory functions was better than those with poor ones. This suggests that good masticatory function is considered a factor affecting the cognitive status of dementia patients. However, these results must be interpreted cautiously since only three studies included in this meta-analysis were evaluated for fair quality, and two were assessed as poor. All studies included in this meta-analysis used convenience sampling or selected a specific group, and no study justified the sample size. The response rate in all studies was satisfactory. Although the measurement tools used were non-validated, the tools were available and described. Four studies study controlled for confounding variables such as socio-demographic factors (age, sex, marital status, smoking, and alcohol consumption), health-related factors (hypertension, diabetes, activities of daily living, and nutritional intake), and oral function-related factors (number of teeth, and prostheses). The outcomes assessment in all studies was not blinded; however, sufficient descriptions of the evaluation method were provided. Three studies were considered to have used appropriate and well-described statistical tests; the remaining studies did not describe or provide adequate details.
Fig. 3.
Forest plot and quality assessment of extracted literature on cognitive function and masticatory function in older adults (RQ1–1). Significance level was set at 5%. (A): Forest plot and quality assessment of changes in the masticatory function of older adults as standardized mean differences (SMD) with 95% confidence intervals (95% CI). (B): Forest plot (odds ratio and 95% CI) and quality assessment comparing the risk of cognitive impairment in older adults with poor masticatory function. SD: standard deviation; CI: confidence interval; Std: Standard; MMSE: Mini-mental State Examination; New-Ottawa Scale Quality assessment: a: A study can be awarded a maximum of one star for each item within the Selection and Exposure categories. b: A maximum of two stars can be given for Comparability; Quality: poor: 0 or 1 star in the selection domain, OR 0 stars in the comparability domain OR 0 or 1 Star in the outcome domain; fair: 2 stars in the selection domain AND 1 or 2 stars in comparability domain AND 2 or 3 stars in outcome domain; good: 3 or 4 stars in selection domain AND 1 or 2 stars in compatibility domain AND 2 or 3 stars in outcome domain.
Different measurement tools used to assess cognitive status in the study by Kimura et al. [26] showed evidence that when compared to those with good masticatory function, the subjects with poor masticatory function had lower mean scores in HDSR and FAB. Likewise, Weijenberg et al. [57] also found that subjects with poor masticatory function had lower mean scores in attention, working memory, and verbal fluency.
Furthermore, the results of another meta-analysis, including a total of 4899 subjects, showed that those with poor masticatory function had a higher risk of cognitive impairment than those with good masticatory function (Fig. 3b). The pooled odds ratio (OR) was 4.09 (95% CI 0.69, 0.28). The heterogeneity by I2 statistics was high at 92%. The test for overall effect (Z) was 2.82 (p < 0.005). This suggests that subjects with poor masticatory function are 4.1 times more likely to have cognitive impairment. The quality assessment showed that three studies in this meta-analysis were evaluated as good, two were considered fair, and one had poor quality. Only two studies used a representative population sample; others used convenience sampling or selected a group of subjects. None of the studies justified the sample size, but the response rate in all studies was satisfactory. Although the measurement tools used were non-validated, the tools were available and described. All but one study controlled for confounding variables such as socio-demographic factors, health, and oral function-related factors. The outcomes assessment in all studies was not blinded; however, sufficient descriptions of the evaluation method were provided. Four studies were considered to have used appropriate and well-described statistical tests; the remaining studies did not describe or provide adequate details.
3.3. Association between cognitive impairment and decreased number of teeth (RQ1-2)
Many studies have reported the association of cognitive impairment with the number of present teeth [6], [16], [17], [18], [20]. However, sufficient evidence is still needed to answer RQ1–2. Among the included case-control studies, seven studies reported evidence of the association between the number of present teeth, masticatory function, and cognitive impairment.
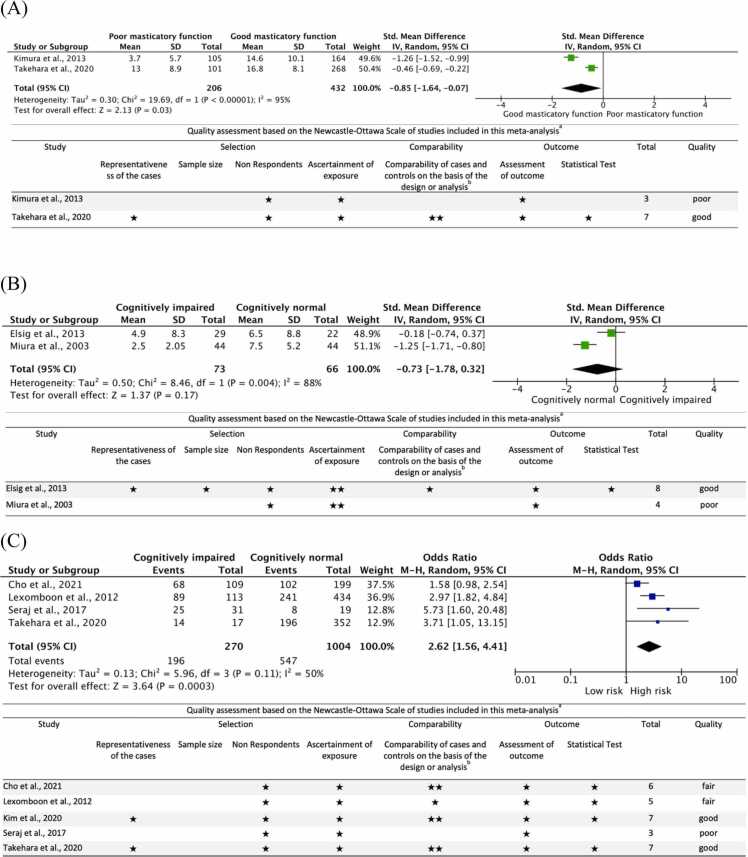
The meta-analysis of two studies, including 638 subjects, showed that older adults with more teeth had good masticatory function than those with poor masticatory function (Fig. 4a). The standardized mean difference was − 0.85 (95% CI −1.64 to −0.07). The heterogeneity by I2 statistics was high at 95%. The test for overall effect (Z) was significant at 2.13 (p = 0.003). This suggests that the number of present teeth is associated with masticatory function. However, the results must be interpreted carefully since there are only two studies in this meta-analysis, and one of the studies was evaluated to have good quality, while the other was considered to be of poor quality. One study used a representative sample, while the other used convenience sampling. Both study did not justify the sample size. The response rate was satisfactory in both studies. Although the measurement tools used were non-validated, the tools were available and described. Only one study controlled for confounding variables, such as oral function-related factors. The outcomes assessment in all studies was not blinded; however, sufficient descriptions of the evaluation method were provided. Only one study was considered to have used appropriate and well-described statistical tests; the other study did not describe or provide adequate details.
Fig. 4.
Forest plot and quality assessment of extracted literature on cognitive function and the number of present teeth in older adults (RQ1–2). Significant if p-value < 0.05. (A): Forest plot and quality assessment of changes in masticatory function based on the number of teeth of older adults as standardized mean differences (SMD) with 95% confidence intervals (95% CI). (B): Forest plot and quality assessment of changes in cognitive function based on the number of teeth of older adults as SMD with 95% confidence intervals (95% CI). (C): Forest plot (odds ratio and 95% CI) and quality assessment comparing the risk of cognitive impairment in older adults with a reduced number of teeth. SD: standard deviation; CI: confidence interval; Std: Standard; MMSE: Mini-mental State Examination; HDSR: Hasegawa Dementia Scale Revised; FAB: Frontal Assessment Battery; New-Ottawa Scale Quality assessment: a: A study can be awarded a maximum of one star for each item within the Selection and Exposure categories. b: A maximum of two stars can be given for Comparability; Quality: poor: 0 or 1 star in the selection domain, OR 0 stars in the comparability domain OR 0 or 1 Star in the outcome domain; fair: 2 stars in the selection domain AND 1 or 2 stars in comparability domain AND 2 or 3 stars in outcome domain; good: 3 or 4 stars in selection domain AND 1 or 2 stars in compatibility domain AND 2 or 3 stars in outcome domain.
Another meta-analysis of two studies, including 139 subjects, showed that the cognitively impaired population had fewer teeth than the cognitively normal population (Fig. 4b). The standardized mean difference was − 0.73 (95% CI −0.32 to −1.78). The heterogeneity by I2 statistics was high at 88%. The test for overall effect (Z) was not significant at 1.37 (p = 0.17). This suggests that the number of present teeth is a factor in improving cognitive function but without certainty. Of the two studies included in this meta-analysis, one was evaluated to have good quality, and the other had poor quality. Both studies used a representative sample, but only one described the sample size calculation. The response rate in all studies was satisfactory. The ascertainment of exposure in both studies was validated. Only one study controlled for confounding variables such as age and sex. Although the outcomes assessment in all studies was not blinded, a sufficient evaluation description was provided. One study was considered to have used appropriate and well-described statistical tests, while the other did not describe or provide enough details.
We confirmed this with another meta-analysis of five studies, including 1274 subjects. The results showed that older adults with fewer teeth are more likely to be cognitively impaired than those with more than 20 teeth (Fig. 4c). The pooled OR was 2.62 (95% CI 1.56–4.41). The heterogeneity by I2 statistics was moderate at 50%. The test for overall effect (Z) was significant at 3.64 (p = 0.0003). This suggests that subjects with less than 20 teeth are 2.6 times more likely to have cognitive impairment. Two of the studies included in this meta-analysis were evaluated to have good quality, two were considered fair, and one had poor quality. Only two studies used a representative population sample; others used convenience sampling or selected a group of subjects. None of the studies justified the sample size, but the response rate in all studies was satisfactory. Although the measurement tools used to assess masticatory function were non-validated, the tools were available and described. One study controlled for socio-demographic factors only; three controlled for confounding variables such as socio-demographic factors, health, and oral function-related factors, while the remaining did not control any. The outcomes assessment in all studies was not blinded; however, sufficient descriptions of the evaluation method were provided. Four studies were considered to have used appropriate and well-described statistical tests, while the remaining study did not describe or provide adequate details.
3.4. Association of the risk of cognitive impairment and masticatory dysfunction (RQ1-3)
The included case-control studies assessed the risk of cognitive impairment based on chewing difficulty [19], poor masticatory function [25], [28], [58], [59], [60], [61], and reduced number of teeth [19], [28], [59], [60], [61].
The meta-analysis of six studies showed that the population with masticatory dysfunction had a higher risk of cognitive impairment (Fig. 5). The pooled OR was 6.82 (95% CI 5.21–8.92). The heterogeneity by I2 statistics was high at 95%. The test for overall effect (Z) was 14.0 (p < 0.00001). This suggests that subjects with masticatory dysfunction are 6.8 times more likely to be cognitively impaired.
Fig. 5.
Forest plot and quality assessment of extracted literature comparing the association between the risk of cognitive impairment and masticatory dysfunction in older adults (RQ1–3). Significant if p-value < 0.05. As odds ratio and 95% CI. SE: standard error; CI: confidence interval; New-Ottawa Scale Quality assessment: a: A study can be awarded a maximum of one star for each item within the Selection and Exposure categories. b: A maximum of two stars can be given for Comparability; Quality: poor: 0 or 1 star in the selection domain, OR 0 stars in the comparability domain OR 0 or 1 Star in the outcome domain; fair: 2 stars in the selection domain AND 1 or 2 stars in comparability domain AND 2 or 3 stars in outcome domain; good: 3 or 4 stars in selection domain AND 1 or 2 stars in compatibility domain AND 2 or 3 stars in outcome domain.
Three studies included in this meta-analysis were evaluated for good quality, three were considered fair, and one had poor quality. Two studies used a representative sample, while the remaining used convenience sampling or selected a specific group. None of the studies described the sample size calculation. The response rate in all studies was satisfactory. All studies used non-validated measurement tools used, except for one. Only one study controlled for confounding variables such as socio-demographic factors, five controlled for health and oral function-related factors, and the remaining did not control for any. The assessment of outcomes in all studies was not blinded. However, a sufficient description of the evaluation was provided. Five studies were considered to have used appropriate and well-described statistical tests; the remaining study did not describe or provide enough details.
3.5. Cognitive function improves with masticatory movement in young adults (RQ2-1)
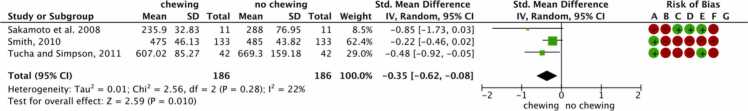
Among the included studies, three focused on cognitive processing speed (reaction time) and chewing. The meta-analysis showed that in healthy young adults who chewed gum, the reaction time was faster than no chewing (Fig. 6).
Fig. 6.
Forest plot and quality assessment of extracted literature comparing the cognitive processing speed (reaction time) in chewing vs. no chewing conditions in young adults (RQ2). Significant if p-value < 0.05. As standardized mean differences (SMD) with 95% confidence intervals (95% CI). ms: millisecond; SD: standard deviation; CI: confidence interval; Std: Standard; CI: confidence interval; Risk of bias legend: (A) Random sequence generation (selection bias); (B) Allocation concealment (selection bias); (C) Blinding of participants and personnel (performance bias); (D) Blinding of outcome assessment (detection bias); (E) Incomplete outcome data (attribution bias); (F) Selective reporting (reporting bias); (G) Other bias.
The standardized mean difference was − 0.35 (95% CI −0.62 to 0.08). The heterogeneity by I2 statistics was at 22%. The test for overall effect (Z) was 2.59 (p < 0.010). This suggests that chewing gum is considered a factor affecting the cognitive processing speed of young adults.
The two studies included in this meta-analysis had a high risk of bias. Although there was randomization, the description of the concealment allocation, blinding of participants, assessors, and outcome assessment were not mentioned. The remaining had a moderate risk of bias since participants, personnel, and the outcomes assessment were blinded, and there was no attrition bias. However, the description of the randomization and allocation concealment were not mentioned.
4. Discussion
Despite the increased attention to dementia prevention and maintenance and improvement of cognitive functions, few studies have explored the relationship between cognitive function and masticatory function. To the best of our knowledge, there is no existing systematic review or meta-analysis on the effects of masticatory function on cognitive function. Therefore, we have conducted this systematic review. We found consistent evidence that masticatory function is directly associated with the cognitive function of older adults. The results show that good masticatory function (with an increased number of teeth, high MP, and chewing ability) is considered a factor in improving the cognitive function of patients with both older and young adults.
The information presented in this systematic review must be interpreted carefully since only one RCT, three quasi-RCT, and 16 case-control studies were included in this review. The methods used to collect data on the masticatory function also lacked homogeneity, limiting the confidence in the level of evidence collected and the overall effect of the meta-analysis.
4.1. Masticatory function affects cognitive function
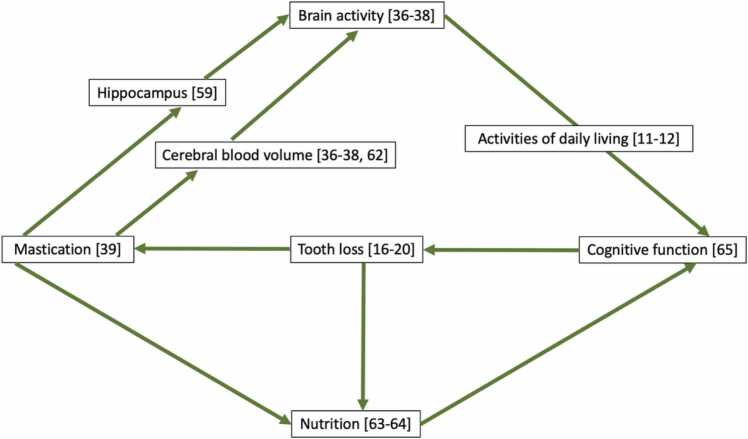
Fig. 7 shows the predictive relationship diagram illustrating several mechanisms of mastication affecting cognitive function based on the literature of this systematic review. Mastication is an intricate process controlled by the central nervous system [62]. Mastication activates brain function. It prevents cognitive impairment by directly stimulating the hippocampus, thereby increasing the neurons responsible for memory and cognitive function [58]. During mastication, cerebral blood volume increases, providing a greater oxygen supply beneficial for promoting brain cell activity and strengthening cognitive function [36], [37], [38], [63]. Mastication also promotes increased nutritional intake [64], particularly vitamin B consumption, which supports brain function [65]. Mastication can also be influenced by numerous factors such as age, gender, dental status, smell, taste, texture, and the hardness of the food [62]. Aging can impair masticatory function, resulting in morphophysiological changes in the body, such as reduced salivary flow, taste impairment, tooth loss [16], [17], [18], [19], [20], and chewing muscle atrophy [24], which can result in decreased masticatory function. Lastly, inflammatory diseases can cause cell damage, which can also induce the loss of brain cells and, consequently, cognitive impairment [66].
Fig. 7.
Predictive relationship diagram illustrating several mechanisms of mastication affecting cognitive function.
Jung et al. [67] reported that MP directly affected cognitive function and indirectly affected how activities of daily living (ADL) and nutritional status assessed by Mini-Nutritional Assessment (MNA) affected cognitive function. Participants with lower MP also had significantly lower ADL [25], [32], [57] and MNA scores (p < 0.0001) [25], [67]. Generally, older adults with poor ADL have difficulty independently brushing and flossing their teeth, consequently deteriorating their oral health and reducing their masticatory function [68].
4.2. Masticatory function assessment
The masticatory function can be evaluated using several methods objectively and subjectively [69], [70], [71], [72]. Although it has been reported that their correlation was significantly weak [70], both still pertain to masticatory function [69]. The included studies used two-color gum [57], [73], color-changing gum [4], [25], [26], [28], [67], gummy jelly [74], [75], and optocal artificial food [21] to measure the objective masticatory performance, while other studies used chewing ability questionnaires [27], [58], [59], [60], [61], [75].
4.3. Cognitive function assessment
We found evidence that masticatory function was associated with the cognitive status of older adults as assessed by MMSE [21], [25], [27], [28], [58], [59], [60], [61], [67], [73], [74], HDSR [26], FAB [26], and cognitive function tests [57] that assessed attention, working memory, and verbal fluency. The HDSR, although not internationally used, is widely known in Japan and is equivalent to MMSE ranging from 0 to 30, with scores of ≥ 21 representing normal cognitive function and ≤ 20 representing a low cognitive function [27], while FAB scores range from 0 to 18 [26].
Evidence shows that subjects with lower MMSE scores and decreased masticatory function have cognitive impairment [25], [27], [28], [58], [59], [61], dementia [73], and Alzheimer’s disease [21]. In patients with Alzheimer’s Disease, the area of the brain corresponding to chewing (cortico-bulbar tract) is affected by neuronal damage and atrophy, which can significantly impact masticatory function [76]. The sense of smell and taste is also affected in patients with AD, possibly impairing sensory feedback needed for mastication [76]. Furthermore, since patients with AD exhibit skeletal muscle atrophy, decreased strength, and physical frailty, it is possible that decreased bite force can also cause their reduced chewing function. It has also been reported that impaired mastication accelerates dementia by reducing the cerebral blood volume essential for brain activity [77].
There were six studies that were not included in the meta-analysis, but they also reported that masticatory function was associated with the cognitive status of older adults ([21], [27], [73], [74], while two other studies reported that cognitive function improves after an oral health intervention program [75] and implant prosthodontic rehabilitation [4].
Matsubara et al. [75] assessed the cognitive function of older adults using MMSE and the Trail Making Test (TMT) parts A (visual attention) and B (working memory). Their study reported a significant improvement after intervention in the MP and TMT scores of older adults with oral health intervention. Their study suggested that TMT scores improved in the intervention group due to the stimulation of the oral cavity resulting in brain stimulation, integration of spatial cognitive function, and improvement of attentional and executive functions.
On the other hand, Cho et al. [60] did not find an association between cognitive function and masticatory function but more on the occlusal balance of individuals. They found that cognitive function in older adults was higher when the relative molar occlusal balance was greater. This result may be due to selection bias since there were fewer male participants in their study, and older adults who could not measure the posterior occlusal balance were excluded.
Although some evidence suggests that masticatory function was associated with cognitive function, the findings should be interpreted with caution because there is variability in the assessment of masticatory function. Some were based on self-reports and subjective questionnaires. The subjects' responses to the questions may be influenced by their cognitive abilities and education level. Other studies might have a possibility of inter-observer variability in the assessment of MP since the evaluators are trained nurses instead of dental experts. It is also unclear whether the human eye can accurately judge the extent of color changes like a machine. It was also possible that there was an attrition bias since the results of the analyses of the first gum samples in the study of Elsig et al. [73] were lost because of too long storage. In addition, possible selection bias may have affected the results of the study of Weijenberg et al. [57], since approximately 50% of the subjects did not participate in the mixing ability test, lowered the cases available for analysis. This is also true in the case of Cho et al. [60] when they excluded subjects for whom they could not measure the posterior occlusal balance. There were studies wherein the subjects were limited to females [27], [28], while Takehara et al. [59] limited it to males. These issues limit the confidence level of the evidence presented.
Nonetheless, the studies mentioned above were consistent with the results of our meta-analysis, which suggests that masticatory function is directly associated with the cognitive function of older adults.
4.4. Association between cognitive impairment and decreased number of teeth (RQ1-2)
We found evidence that reported that the number of teeth was significantly related to masticatory function [26], [59], and cognitive impairment [19], [27], [59], [60], [61]. However, a study reported that the number of teeth was not associated with cognitive impairment (p = 0.553) in patients with dementia [73]. Their findings revealed that MP was lower in patients with dementia than in those with normal cognitive function or mild cognitive impairment, suggesting that MP seems stronger in association with cognitive impairment than the number of teeth. One possible explanation for this result is that their sample size was too small.
The study by Texeira et al. [63] stated that investigations regarding the relationship between tooth loss and cognitive impairment are necessary because the loss of teeth and masticatory function are very closely related. The number of natural teeth also predisposes to substantial changes in the orofacial structures, such as loss of sensory feedback and reduced muscle tone [78], which leads to decreased masticatory function, which could influence dietary preferences, change one’s nutritional status [79], and eventually affect cognitive function [65].
Although the number of teeth and masticatory function are not the same, previous studies have established that number of present teeth is associated with masticatory function [19], [25], [26], [27], [28], [29], [80], [81], [82]. It has been reported that the number of teeth affects the gummy jelly score occlusal force and suggests that the number of present teeth significantly affects the rate of oral hypofunction [74]. Hence, it is necessary to consider the influence of the number of present teeth when conducting research relating to masticatory function.
The small sample size of the included studies limits the confidence of the evidence presented. However, based on the results of the present review, decreased number of teeth can be considered a factor affecting the cognitive function of older adults.
4.5. Association of the risk of cognitive impairment and masticatory dysfunction (RQ1-3)
The results of the present review also showed that the risk of cognitive impairment was higher in participants with masticatory dysfunction due to chewing difficulty [19], [83], decreased masticatory performance [25], [28], reduced chewing ability [58], [59], [61], and decreased number of present teeth (RQ1–3) [19], [28], [59], [61].
On the contrary, Shin et al. [28] reported that there was a low risk of cognitive impairment in patients with a low MP (OR=0.95, 95% CI: 0.89–1.01) or a low subjective chewing ability (OR=0.96, 95% CI: 0.91–1.02). They also reported a low risk of cognitive impairment with a reduced number of teeth (OR=0.988, 95% CI: 0.949–1.029). One possible explanation was that their sample size was small and mainly comprised of females, which did not represent the general population.
The number of teeth determines the occlusal surface available for food comminution. It increases the maximum bite force by transferring the occlusal load to the periodontal ligament or acting as an abutment if a removable prosthesis is present [73]. Bite force has been reported to have a positive relationship with masticatory performance. It tends to reduce with aging due to the atrophy of the jaw-closing muscles, even more evident in edentulous than in dentate subjects [84]. Tan et al. [4] reported that a transient decline in the cognitive function of completely edentulous older adults was seen after implant placement and loading in the first week, but improvements to or beyond baseline levels were seen after six weeks and one month. The plausible reason for the cognitive decline was mild post-operative pain and discomfort after implant placement.
Older adults often wear removable dentures, and several studies have examined the relationship between denture-wearing conditions and cognitive function. Scherder et al. [83] found that the relationship between mastication, episodic memory, and executive functions becomes evident when the functional status of the masticatory system decreases in older subjects with complete dentures. The risk of dementia was higher in those chewing with a denture than in those chewing with natural teeth [85], and subjects with fewer teeth who did not wear dentures exhibited a more severe cognitive impairment [64]. Individuals with lesser teeth are at a greater risk of developing nutritional deficiencies, especially vitamin B, which plays a significant role in the pathogenesis of cognitive decline [86], [87], [88], [89]. Because the masticatory function of denture-wearing patients is influenced by the occlusal support provided by the remaining teeth [70], [90], dentures that enable one to chew well are essential in maintaining cognitive function.
Based on these findings, the present review concluded that the risk of cognitive impairment is associated with masticatory dysfunction. However, further research is still needed to examine the risk of cognitive impairment in participants with masticatory dysfunction in a well-designed study and a more significant number of subjects.
4.6. Cognitive function improves with masticatory function in young adults (RQ2)
The effects of masticatory function on cognitive function are not limited to older adults but also the young [91], [92], [93]. Since there have been many studies where young adults were the intervention targets because young people are healthier, and it is easy to keep the subjects in the same state, so we decided to include young adults in the target population. In addition, the evidence suggests that mastication decreases cognitive processing speed (reaction time) during cognitive tasks performed by young adults, confirming the role of masticatory function in improving cognitive function in young adults (RQ2).
Mastication with an object inside the mouth does not give the same effect as rhythmic jaw movement alone regarding cognitive processing. Gum-chewing is a complex behavior involving rhythmic jaw movement, tongue movement, saliva secretion, and tactile sensations of the structures in the oral cavity. Central nervous system is affected by several factors elicited by gum chewing. Mastication speeds up the sequential processing from stimulus onset to the response. In other words, the speed of the evaluation of stimulus in human cognitive processing is influenced by mastication [91]. Studies showed that gum chewing also decreased the subjects’ reaction time (RT) [91], [92], [93] even when distracted [91], reduced attention lapses, increased alertness, and aided concentration [92]. However, gum chewing also affects attention differently. Although attention performance was adversely affected in the early phase of performing the attention task, the subject’s RT was shorter in the gum-chewing condition than in the no-gum condition, in the later stage of the attention task [93]. The included studies used chewing gums with and without taste and flavor as the test food [92], [93]. However, differences in taste and flavor affect cognitive function, so it is not purely an effect of masticatory function alone [94], [95].
Although there were discrepancies with the results of the included studies, we cannot ignore the fact that mastication does not only influence the evaluation of stimulus in human cognitive processing by decreasing cognitive processing speed (reaction time) during cognitive tasks performed by young adults, but also improves attention, alertness, and concentration.
4.7. Limitations
Based on the currently available literature and the limitations of this systematic review, the effects of masticatory function on cognitive function cannot be proven in a scientifically compelling manner because the majority of the studies were case-control with bias in selection, comparability, and outcomes domain. At the same time, the included quasi-experimental studies had unclear allocation concealment processes, blinding of participants, assessors, and outcomes assessment. Because of these limitations, four studies had poor quality, four had fair quality, four had good quality, two had a high risk of bias, and one had a moderate risk of bias. Thus, the studies included in this review were deemed to have a low level of evidence. Furthermore, the assessment of cognitive function between young and older adults differs in.
the studies included in this review. Therefore, comparisons between the effects of masticatory function on the cognitive function of young and older adults cannot be established.
5. Conclusion
This systematic review was conducted to elucidate whether masticatory function affects cognitive status and function for both older and young adults. Based on the findings of this systematic review, the following conclusions were drawn:
-
1.
The cognitive function tests were significantly lower in subjects with lower MP and chewing ability, and a decreased number of teeth.
-
2.
The risk of cognitive impairment is higher in subjects with masticatory dysfunction, such as chewing difficulty, decreased MP, low chewing ability, and a reduced number of teeth.
-
3.
Mastication reduces the cognitive processing speed (reaction time) of young adults when performing cognitive tasks.
Further research with more scientifically robust, well-designed, randomized controlled trials and longitudinal studies with a larger sample size is needed to confirm the effects of masticatory function on cognitive function.
Role of the funding source
No funding was obtained for this study.
CRediT authorship contribution statement
The authors declare that they have contributed significantly to the study's conception and design. Material preparation, data collection, and analysis were performed by Ma. Therese Sta. Maria, Yoko Hasegawa, Aye Mya Mya Khaing, and Simonne Salazar. The first draft of the manuscript was written by Ma. Therese Sta. Maria and Yoko Hasegawa. Takahiro Ono edited the manuscript. All authors critically reviewed and commented on previous versions of the manuscript, and agreed with the content of the final manuscript.
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Organization W.H. World Health Organization; 2021. Dementia. [Google Scholar]
- 2.Organization W.H. Ageing and Health. 2021.
- 3.United Nations Department of Economic and Social Affairs PD. 2020 Highlights: Living arrangements of older persons. New York, 10017, USA: United Nations Publication; 2020.
- 4.Tan D., Foster S., Korgaonkar M.S., Oxenham V., Whittle T., Klineberg I. The role of progressive oral implant rehabilitation in mastication, cognition and oral health‐related quality of life outcomes—A pilot to define the protocol. J Oral Rehabil. 2020;47:1368–1381. doi: 10.1111/joor.13085. [DOI] [PubMed] [Google Scholar]
- 5.Petersen R.C. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 6.Fukai K. Ishiyaku Publishing; Tokyo, Japan: 2019. Oral health and nutrition for healthy longevity (in Japanese) [Google Scholar]
- 7.Ministry of Health LaW. Ministry of Health, Labour and Welfare Service Guide 2020. 2020.
- 8.Ohara T., Hata J., Yoshida D., Mukai N., Nagata M., Iwaki T., et al. Trends in dementia prevalence, incidence, and survival rate in a Japanese community. Neurology. 2017;88:1925–1932. doi: 10.1212/WNL.0000000000003932. [DOI] [PubMed] [Google Scholar]
- 9.Kalaria R.N., Maestre G.E., Arizaga R., Friedland R.P., Galasko D., Hall K., et al. Alzheimer's disease and vascular dementia in developing countries: prevalence, management, and risk factors. Lancet Neurol. 2008;7:812–826. doi: 10.1016/S1474-4422(08)70169-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hugo J., Ganguli M. Dementia and cognitive impairment: epidemiology, diagnosis, and treatment. Clin Geriatr Med. 2014;30:421–442. doi: 10.1016/j.cger.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fillit H., Nash D.T., Rundek T., Zuckerman A. Cardiovascular risk factors and dementia. Am J Geriatr Pharmacother. 2008;6:100–118. doi: 10.1016/j.amjopharm.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 12.Royall D.R., Lauterbach E.C., Kaufer D., Malloy P., Coburn K.L., Black K.J. The cognitive correlates of functional status: a review from the Committee on Research of the American Neuropsychiatric Association. The. J Neuropsychiatry Clin Neurosci. 2007;19:249–265. doi: 10.1176/jnp.2007.19.3.249. [DOI] [PubMed] [Google Scholar]
- 13.Pérès K., Helmer C., Amieva H., Orgogozo J.M., Rouch I., Dartigues J.F., et al. Natural history of decline in instrumental activities of daily living performance over the 10 years preceding the clinical diagnosis of dementia: a prospective population‐based study. J Am Geriatr Soc. 2008;56:37–44. doi: 10.1111/j.1532-5415.2007.01499.x. [DOI] [PubMed] [Google Scholar]
- 14.Morris M.C. Nutritional determinants of cognitive aging and dementia. Proc Nutr Soc. 2012;71:1–13. doi: 10.1017/S0029665111003296. [DOI] [PubMed] [Google Scholar]
- 15.Perez L., Helm L., Sherzai A.D., Jaceldo-Siegl K., Sherzai A. Nutrition and vascular dementia. J Nutr, Health Aging. 2012;16:319–324. doi: 10.1007/s12603-012-0042-z. [DOI] [PubMed] [Google Scholar]
- 16.Tsakos G., Watt R.G., Rouxel P.L., de Oliveira C., Demakakos P. Tooth loss associated with physical and cognitive decline in older adults. J Am Geriatr Soc. 2015;63:91–99. doi: 10.1111/jgs.13190. [DOI] [PubMed] [Google Scholar]
- 17.Gatz M., Mortimer J.A., Fratiglioni L., Johansson B., Berg S., Reynolds C.A., et al. Potentially modifiable risk factors for dementia in identical twins. Alzheimer'S Dement. 2006;2:110–117. doi: 10.1016/j.jalz.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 18.Fernández Martínez M., Castro Flores J., Pérez de las Heras S., Mandaluniz Lekumberri A., Gordejuela Menocal M., Zarranz Imirizaldu J.J. Risk factors for dementia in the epidemiological study of Munguialde County (Basque Country-Spain) BMC Neurol. 2008;8:1–8. doi: 10.1186/1471-2377-8-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lexomboon D., Trulsson M., Wårdh I., Parker M.G. Chewing ability and tooth loss: association with cognitive impairment in an elderly population study. J Am Geriatr Soc. 2012;60:1951–1956. doi: 10.1111/j.1532-5415.2012.04154.x. [DOI] [PubMed] [Google Scholar]
- 20.Xu S., Huang X., Gong Y., Sun J. Association between tooth loss rate and risk of mild cognitive impairment in older adults: a population-based longitudinal study. Aging (Albany NY) 2021;13:21599. doi: 10.18632/aging.203504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Campos C.H., Ribeiro G.R., Costa J.L.R., Rodrigues, Garcia R.C.M. Correlation of cognitive and masticatory function in Alzheimer’s disease. Clin Oral Investig. 2017;21:573–578. doi: 10.1007/s00784-016-1923-z. [DOI] [PubMed] [Google Scholar]
- 22.Cerutti-Kopplin D., Feine J., Padilha D., De Souza R., Ahmadi M., Rompré P., et al. Tooth loss increases the risk of diminished cognitive function: a systematic review and meta-analysis. JDR Clin Transl Res. 2016;1:10–19. doi: 10.1177/2380084416633102. [DOI] [PubMed] [Google Scholar]
- 23.Takeuchi K., Ohara T., Furuta M., Takeshita T., Shibata Y., Hata J., et al. Tooth loss and risk of dementia in the community: the Hisayama study. J Am Geriatr Soc. 2017;65:e95–e100. doi: 10.1111/jgs.14791. [DOI] [PubMed] [Google Scholar]
- 24.Jou Y.-T. Dental deafferentation and brain damage: A review and a hypothesis. Kaohsiung J Med Sci. 2018;34:231–237. doi: 10.1016/j.kjms.2018.01.013. [DOI] [PubMed] [Google Scholar]
- 25.Kim E.-K., Lee S.K., Choi Y.-H., Tanaka M., Hirotsu K., Kim H.C., et al. Relationship between chewing ability and cognitive impairment in the rural elderly. Arch Gerontol Geriatr. 2017;70:209–213. doi: 10.1016/j.archger.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 26.Kimura Y., Ogawa H., Yoshihara A., Yamaga T., Takiguchi T., Wada T., et al. Evaluation of chewing ability and its relationship with activities of daily living, depression, cognitive status and food intake in the community‐dwelling elderly. Geriatr Gerontol Int. 2013;13:718–725. doi: 10.1111/ggi.12006. [DOI] [PubMed] [Google Scholar]
- 27.Miura H., Yamasaki K., Kariyasu M., Miura K., Sumi Y. Relationship between cognitive function and mastication in elderly females. J Oral Rehabil. 2003;30:808–811. doi: 10.1046/j.1365-2842.2003.01124.x. [DOI] [PubMed] [Google Scholar]
- 28.Shin H.E., Cho M.J., Amano A., Song K.B., Choi Y.H. Association between mastication‐related factors and the prevalence of dementia in Korean elderly women visiting senior centres. Gerodontology. 2020;37:177–184. doi: 10.1111/ger.12453. [DOI] [PubMed] [Google Scholar]
- 29.Tada A., Miura H. Association between mastication and cognitive status: a systematic review. Arch Gerontol Geriatr. 2017;70:44–53. doi: 10.1016/j.archger.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 30.Hirano Y., Obata T., Takahashi H., Tachibana A., Kuroiwa D., Takahashi T., et al. Effects of chewing on cognitive processing speed. Brain Cogn. 2013;81:376–381. doi: 10.1016/j.bandc.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 31.Bergdahl M., Habib R., Bergdahl J., Nyberg L., NILSSON L.G. Natural teeth and cognitive function in humans. Scand J Psychol. 2007;48:557–565. doi: 10.1111/j.1467-9450.2007.00610.x. [DOI] [PubMed] [Google Scholar]
- 32.Jung Y.-S., Park T., Kim E.-K., Jeong S.-H., Lee Y.-E., Cho M.-J., et al. Influence of Chewing Ability on Elderly Adults’ Cognitive Functioning: The Mediating Effects of the Ability to Perform Daily Life Activities and Nutritional Status. Int J Environ Res Public Health. 2022;19:1236. doi: 10.3390/ijerph19031236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kondo K., Niino M., Shido K. A case-control study of Alzheimer's disease in Japan–significance of life-styles. Dement Geriatr Cogn Disord. 1994;5:314–326. doi: 10.1159/000106741. [DOI] [PubMed] [Google Scholar]
- 34.Nordenram G., Ryd‐Kjellen E., Johansson G., Nordstrom G., Winblad B. Alzheimer's disease, oral function and nutritional status. Gerodontology. 1996;13:9–16. doi: 10.1111/j.1741-2358.1996.tb00145.x. [DOI] [PubMed] [Google Scholar]
- 35.Wu B., Fillenbaum G.G., Plassman B.L., Guo L. Association between oral health and cognitive status: a systematic review. J Am Geriatr Soc. 2016;64:739–751. doi: 10.1111/jgs.14036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Momose T., Nishikawa J., Watanabe T., Sasaki Y., Senda M., Kubota K., et al. Effect of mastication on regional cerebral blood flow in humans examined by positron-emission tomography with 15O-labelled water and magnetic resonance imaging. Arch Oral Biol. 1997;42:57–61. doi: 10.1016/s0003-9969(96)00081-7. [DOI] [PubMed] [Google Scholar]
- 37.Onozuka M., Fujita M., Watanabe K., Hirano Y., Niwa M., Nishiyama K., et al. Mapping brain region activity during chewing: a functional magnetic resonance imaging study. J Dent Res. 2002;81:743–746. doi: 10.1177/0810743. [DOI] [PubMed] [Google Scholar]
- 38.Narita N., Kamiya K., Yamamura K., Kawasaki S., Matsumoto T., Tanaka N. Chewing-related prefrontal cortex activation while wearing partial denture prosthesis: pilot study. J Prosthodont Res. 2009;53:126–135. doi: 10.1016/j.jpor.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 39.van der Bilt A., Engelen L., Pereira L.J., van der Glas H.W., Abbink J.H. Oral physiology and mastication. Physiol Behav. 2006;89:22–27. doi: 10.1016/j.physbeh.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 40.Minakuchi H., Fujisawa M., Abe Y., Iida T., Oki K., Okura K., et al. Managements of sleep bruxism in adult: A systematic review. Jpn Dent Sci Rev. 2022;58:124–136. doi: 10.1016/j.jdsr.2022.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trivedi D. Cochrane Review Summary: Mini-mental State Examination (MMSE) for the detection of dementia in clinically unevaluated people aged 65 and over in community and primary care populations. Prim Health Care Res Dev. 2017;18:527–528. doi: 10.1017/S1463423617000202. [DOI] [PubMed] [Google Scholar]
- 42.Tsukamoto R., Akisaki T., Kuranaga M., Takata T., Yokono K., Sakurai T. Hasegawa dementia scale - revised, for screening of early Alzheimer's disease in the elderly with type 2 diabetes. Geriatr Gerontol Int. 2009;9:213–215. doi: 10.1111/j.1447-0594.2009.00524.x. [DOI] [PubMed] [Google Scholar]
- 43.Kato K. Revised version of HDR scale. Jpn J Geriat Psychiatry. 1991;2:1339–1347. [Google Scholar]
- 44.Goh W.Y., Chan D., Ali N.B., Chew A.P., Chuo A., Chan M., et al. Frontal assessment battery in early cognitive impairment: psychometric property and factor structure. J Nutr Health Aging. 2019;23:966–972. doi: 10.1007/s12603-019-1248-0. [DOI] [PubMed] [Google Scholar]
- 45.Dubois B., Slachevsky A., Litvan I., Pillon B. The FAB: a frontal assessment battery at bedside. Neurology. 2000;55:1621–1626. doi: 10.1212/wnl.55.11.1621. [DOI] [PubMed] [Google Scholar]
- 46.Zheng H., Onoda K., Nagai A., Yamaguchi S. Reduced dynamic complexity of bold signals differentiates mild cognitive impairment from normal aging. Front Aging Neurosci. 2020;12:90. doi: 10.3389/fnagi.2020.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schmidt S.L., Boechat Y.E.M., Schmidt G.J., Nicaretta D., van Duinkerken E., Schmidt J.J. Clinical utility of a reaction-time attention task in the evaluation of cognitive impairment in elderly with high educational disparity. J Alzheimers Dis. 2021;81:691–697. doi: 10.3233/JAD-210151. [DOI] [PubMed] [Google Scholar]
- 48.Cochrane. The Cochrane Collaboration. 2022.
- 49.Takeshima N., Sozu T., Tajika A., Ogawa Y., Hayasaka Y., Furukawa T.A. Which is more generalizable, powerful and interpretable in meta-analyses, mean difference or standardized mean difference? BMC Med Res Methodol. 2014;14:30. doi: 10.1186/1471-2288-14-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jørgensen L., Paludan-Müller A.S., Laursen D.R.T., Savović J., Boutron I., Sterne J.A.C., et al. Evaluation of the Cochrane tool for assessing risk of bias in randomized clinical trials: overview of published comments and analysis of user practice in Cochrane and non-Cochrane reviews. Syst Rev. 2016;5:80. doi: 10.1186/s13643-016-0259-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Herzog R., Álvarez-Pasquin M.J., Díaz C., Del Barrio J.L., Estrada J.M., Gil Á. Are healthcare workers’ intentions to vaccinate related to their knowledge, beliefs and attitudes? a systematic review. BMC Public Health. 2013;13:154. doi: 10.1186/1471-2458-13-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wells G.A., Wells G., Shea B., Shea B., O'Connell D., Peterson J., et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. 2014.
- 53.Modesti P.A., Reboldi G., Cappuccio F.P., Agyemang C., Remuzzi G., Rapi S., et al. Panethnic Differences in Blood Pressure in Europe: A Systematic Review and Meta-Analysis. PLoS One. 2016;11 doi: 10.1371/journal.pone.0147601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shamsrizi P., Gladstone B.P., Carrara E., Luise D., Cona A., Bovo C., et al. Variation of effect estimates in the analysis of mortality and length of hospital stay in patients with infections caused by bacteria-producing extended-spectrum beta-lactamases: a systematic review and meta-analysis. BMJ Open. 2020;10 doi: 10.1136/bmjopen-2019-030266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McLaughlin M., Delaney T., Hall A., Byaruhanga J., Mackie P., Grady A., et al. Associations Between Digital Health Intervention Engagement, Physical Activity, and Sedentary Behavior: Systematic Review and Meta-analysis. J Med Internet Res. 2021;23 doi: 10.2196/23180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int J Surg. 2021;88 doi: 10.1016/j.ijsu.2021.105906. [DOI] [PubMed] [Google Scholar]
- 57.Weijenberg R.A., Lobbezoo F., Visscher C.M., Scherder E.J. Oral mixing ability and cognition in elderly persons with dementia: a cross-sectional study. J Oral Rehabil. 2015;42:481–486. doi: 10.1111/joor.12283. [DOI] [PubMed] [Google Scholar]
- 58.Kim M.S., Oh B., Yoo J.W., Han D.H. The association between mastication and mild cognitive impairment in Korean adults. Med (Baltim) 2020;99 doi: 10.1097/MD.0000000000020653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Takehara S., Wright F.A.C., Waite L.M., Naganathan V., Hirani V., Blyth F.M., et al. Oral health and cognitive status in the Concord Health and Ageing in Men Project: A cross-sectional study in community-dwelling older Australian men. Gerodontology. 2020;37:353–360. doi: 10.1111/ger.12469. [DOI] [PubMed] [Google Scholar]
- 60.Cho M.J., Shin H.E., Amano A., Song K.B., Choi Y.H. Effect of molar occlusal balance on cognitive function in the elderly. Int Dent J. 2022;72:331–337. doi: 10.1016/j.identj.2021.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Seraj Z., Al-Najjar D., Akl M., Aladle N., Altijani Y., Zaki A., et al. The effect of number of teeth and chewing ability on cognitive function of elderly in UAE: a pilot study. Int J Dent. 2017;2017:5732748. doi: 10.1155/2017/5732748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lund J.P. Mastication and its control by the brain stem. Crit Rev Oral Biol Med. 1991;2:33–64. doi: 10.1177/10454411910020010401. [DOI] [PubMed] [Google Scholar]
- 63.Teixeira F.B., Fernandes Ld.M.P., Noronha P.A.T., dos Santos M.A.R., Gomes-Leal W., Maia Cd.S.F., et al. Masticatory deficiency as a risk factor for cognitive dysfunction. Int J Med Sci. 2014;11:209. doi: 10.7150/ijms.6801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim J.M., Stewart R., Prince M., Kim S.W., Yang S.J., Shin I.S., et al. Dental health, nutritional status and recent‐onset dementia in a Korean community population. Int J Geriatr Psychiatry: A J Psychiatry late life Allied Sci. 2007;22:850–855. doi: 10.1002/gps.1750. [DOI] [PubMed] [Google Scholar]
- 65.Listl S. Oral health conditions and cognitive functioning in middle and later adulthood. BMC Oral Health. 2014;14:70. doi: 10.1186/1472-6831-14-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Iwasaki M., Yoshihara A., Kimura Y., Sato M., Wada T., Sakamoto R., et al. Longitudinal relationship of severe periodontitis with cognitive decline in older Japanese. J Periodontal Res. 2016;51:681–688. doi: 10.1111/jre.12348. [DOI] [PubMed] [Google Scholar]
- 67.Jung Y.S., Park T., Kim E.K., Jeong S.H., Lee Y.E., Cho M.J., et al. Influence of chewing ability on elderly adults' cognitive functioning: the mediating effects of the ability to perform daily life activities and nutritional status. Int J Environ Res Public Health. 2022:19. doi: 10.3390/ijerph19031236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Michishige F., Yoshinaga S., Harada E., Hirota K., Miyake Y., Matsuo T., et al. Relationships between activity of daily living, and oral cavity care and the number of oral cavity microorganisms in patients with cerebrovascular diseases. J Med Investig. 1999;46:79–85. [PubMed] [Google Scholar]
- 69.Gonçalves T.M.S.V., Schimmel M., van Der Bilt A., Chen J., van Der Glas H.W., Kohyama K., et al. Consensus on the terminologies and methodologies for masticatory assessment. J Oral Rehabil. 2021;48:745–761. doi: 10.1111/joor.13161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Salazar S., Hasegawa Y., Kikuchi S., Kaneda K., Yoneda H., Nokubi T., et al. The impact of a newly constructed removable denture on the objective and subjective masticatory function. J Prosthodont Res. 2020 doi: 10.2186/jpr.JPR_D_20_00045. [DOI] [PubMed] [Google Scholar]
- 71.Suwanarpa K., Hasegawa Y., Salazar S., Kikuchi S., Yoshimoto T., Paphangkorakit J., et al. Can masticatory performance be predicted by using food acceptance questionnaire in elderly patients with removable dentures? J Oral Rehabil. 2021;48:582–591. doi: 10.1111/joor.13147. [DOI] [PubMed] [Google Scholar]
- 72.Yoshimoto T., Hasegawa Y., Salazar S., Kikuchi S., Hori K., Ono T. Factors affecting masticatory satisfaction in patients with removable partial dentures. Int J Environ Res Public Health. 2021:18. doi: 10.3390/ijerph18126620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Elsig F., Schimmel M., Duvernay E., Giannelli S.V., Graf C.E., Carlier S., et al. Tooth loss, chewing efficiency and cognitive impairment in geriatric patients. Gerodontology. 2015;32:149–156. doi: 10.1111/ger.12079. [DOI] [PubMed] [Google Scholar]
- 74.Kugimiya Y., Watanabe Y., Ueda T., Motokawa K., Shirobe M., Igarashi K., et al. Rate of oral frailty and oral hypofunction in rural community-dwelling older Japanese individuals. Gerodontology. 2020;37:342–352. doi: 10.1111/ger.12468. [DOI] [PubMed] [Google Scholar]
- 75.Matsubara C., Shirobe M., Furuya J., Watanabe Y., Motokawa K., Edahiro A., et al. Effect of oral health intervention on cognitive decline in community-dwelling older adults: a randomized controlled trial. Arch Gerontol Geriatr. 2021;92 doi: 10.1016/j.archger.2020.104267. [DOI] [PubMed] [Google Scholar]
- 76.Friedlander A.H., Norman D.C., Mahler M.E., Norman K.M., Yagiela J.A. Alzheimer's disease: psychopathology, medical management and dental implications. J Am Dent Assoc. 2006;137:1240–1251. doi: 10.14219/jada.archive.2006.0381. [DOI] [PubMed] [Google Scholar]
- 77.Weijenberg R.A., Scherder E.J., Lobbezoo F. Mastication for the mind--the relationship between mastication and cognition in ageing and dementia. Neurosci Biobehav Rev. 2011;35:483–497. doi: 10.1016/j.neubiorev.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 78.Hatch J., Shinkai R., Sakai S., Rugh J., Paunovich E. Determinants of masticatory performance in dentate adults. Arch Oral Biol. 2001;46:641–648. doi: 10.1016/s0003-9969(01)00023-1. [DOI] [PubMed] [Google Scholar]
- 79.Marito P., Hasegawa Y., Tamaki K., Sta, Maria M.T., Yoshimoto T., et al. The association of dietary intake, oral health, and blood pressure in older adults: a cross-sectional observational study. Nutrients. 2022;14:1279. doi: 10.3390/nu14061279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kosaka T., Kida M., Kikui M., Hashimoto S., Fujii K., Yamamoto M., et al. Factors influencing the changes in masticatory performance: the suita study. JDR Clin Trans Res. 2018;3:405–412. doi: 10.1177/2380084418785863. [DOI] [PubMed] [Google Scholar]
- 81.Kosaka T., Ono T., Kida M., Kikui M., Yamamoto M., Yasui S., et al. A multifactorial model of masticatory performance: the suita study. J Oral Rehabil. 2016;43:340–347. doi: 10.1111/joor.12371. [DOI] [PubMed] [Google Scholar]
- 82.Kosaka T., Ono T., Yoshimuta Y., Kida M., Kikui M., Nokubi T., et al. The effect of periodontal status and occlusal support on masticatory performance: the S uita study. J Clin Periodo. 2014;41:497–503. doi: 10.1111/jcpe.12241. [DOI] [PubMed] [Google Scholar]
- 83.Scherder E., Posthuma W., Bakker T., Vuijk P.J., Lobbezoo F. Functional status of masticatory system, executive function and episodic memory in older persons. J Oral Rehabil. 2008;35:324–336. doi: 10.1111/j.1365-2842.2007.01842.x. [DOI] [PubMed] [Google Scholar]
- 84.Ikebe K., Nokubi T., Morii K., Kashiwagi J., Furuya M. Association of bite force with ageing and occlusal support in older adults. J Dent. 2005;33:131–137. doi: 10.1016/j.jdent.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 85.Paganini‐Hill A., White S.C., Atchison K.A. Dentition, Dental Health Habits, and Dementia: The L eisure W orld C ohort S tudy. J Am Geriatr Soc. 2012;60:1556–1563. doi: 10.1111/j.1532-5415.2012.04064.x. [DOI] [PubMed] [Google Scholar]
- 86.Kim H., Kim G., Jang W., Kim S.Y., Chang N. Association between intake of B vitamins and cognitive function in elderly Koreans with cognitive impairment. Nutr J. 2014;13:1–11. doi: 10.1186/1475-2891-13-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.McNeill G., Jia X., Whalley L., Fox H., Corley J., Gow A., et al. Antioxidant and B vitamin intake in relation to cognitive function in later life in the Lothian Birth Cohort 1936. Eur J Clin Nutr. 2011;65:619–626. doi: 10.1038/ejcn.2011.2. [DOI] [PubMed] [Google Scholar]
- 88.Saito Y., Sugawara N., Yasui-Furukori N., Takahashi I., Nakaji S., Kimura H. Cognitive function and number of teeth in a community-dwelling population in Japan. Ann Gen Psychiatry. 2013;12:1–6. doi: 10.1186/1744-859X-12-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tucker K.L., Qiao N., Scott T., Rosenberg I., Spiro A., III High homocysteine and low B vitamins predict cognitive decline in aging men: the Veterans Affairs Normative Aging Study–. Am J Clin Nutr. 2005;82:627–635. doi: 10.1093/ajcn.82.3.627. [DOI] [PubMed] [Google Scholar]
- 90.Kikuchi S., Hasegawa Y., Salazar S.E., Kaneda K., Yoneda H., Hori K., et al. Factors Influencing Changes in Masticatory Performance as a Result of Wearing Removable Partial Dentures in Patients with Partially Edentulous Arches. J Prosthodont. 2021;30:150–156. doi: 10.1111/jopr.13265. [DOI] [PubMed] [Google Scholar]
- 91.Sakamoto K., Nakata H., Kakigi R. The effect of mastication on human cognitive processing: a study using event-related potentials. Clin Neurophysiol. 2009;120:41–50. doi: 10.1016/j.clinph.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 92.Smith A. Effects of chewing gum on cognitive function, mood and physiology in stressed and non-stressed volunteers. Nutr Neurosci. 2010;13:7–16. doi: 10.1179/147683010X12611460763526. [DOI] [PubMed] [Google Scholar]
- 93.Tucha L., Simpson W. The role of time on task performance in modifying the effects of gum chewing on attention. Appetite. 2011;56:299–301. doi: 10.1016/j.appet.2010.12.021. [DOI] [PubMed] [Google Scholar]
- 94.Hasegawa Y., Tachibana Y., Sakagami J., Zhang M., Urade M., Ono T. Flavor-enhanced modulation of cerebral blood flow during gum chewing. PLoS One. 2013;8 doi: 10.1371/journal.pone.0066313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sakuramoto A., Hasegawa Y., Kishimoto H., Ono T. Influence of sourness on higher brain functions. J Nutr Food Sci. 2017;7:2. [Google Scholar]