Abstract
Background and Aims
Eosinophils are present in several solid tumors and have context-dependent function. Our aim is to define the contribution of eosinophils in esophageal squamous cell carcinoma (ESCC), as their role in ESCC is unknown.
Methods
Eosinophils were enumerated in tissues from 2 ESCC cohorts. Mice were treated with 4-NQO for 8 weeks to induce precancer or 16 weeks to induce carcinoma. The eosinophil number was modified by a monoclonal antibody to interleukin-5 (IL5mAb), recombinant IL-5 (rIL-5), or genetically with eosinophil-deficient (ΔdblGATA) mice or mice deficient in eosinophil chemoattractant eotaxin-1 (Ccl11–/–). Esophageal tissue and eosinophil-specific RNA sequencing was performed to understand eosinophil function. Three-dimensional coculturing of eosinophils with precancer or cancer cells was done to ascertain direct effects of eosinophils.
Results
Activated eosinophils are present in higher numbers in early-stage vs late-stage ESCC. Mice treated with 4-NQO exhibit more esophageal eosinophils in precancer vs cancer. Correspondingly, epithelial cell Ccl11 expression is higher in mice with precancer. Eosinophil depletion using 3 mouse models (Ccl11–/– mice, ΔdblGATA mice, IL5mAb treatment) all display exacerbated 4-NQO tumorigenesis. Conversely, treatment with rIL-5 increases esophageal eosinophilia and protects against precancer and carcinoma. Tissue and eosinophil RNA sequencing revealed eosinophils drive oxidative stress in precancer. In vitro coculturing of eosinophils with precancer or cancer cells resulted in increased apoptosis in the presence of a degranulating agent, which is reversed with NAC, a reactive oxygen species scavenger. ΔdblGATA mice exhibited increased CD4 T cell infiltration, IL-17, and enrichment of IL-17 protumorigenic pathways.
Conclusion
Eosinophils likely protect against ESCC through reactive oxygen species release during degranulation and suppression of IL-17.
Keywords: Esophageal Squamous Cell Carcinoma, Eosinophils, Degranulation, Immunotherapy, Oxidative Stress
Summary.
Eosinophils are present and activated in human esophageal squamous cancer, to a greater degree in early stages. In a mouse model of esophageal squamous carcinoma, reducing the eosinophil number exacerbates tumorigenesis and increasing the eosinophil number attenuates tumorigenesis by directly and indirectly modifying the neoplastic microenvironment.
In 2020, there were 604,100 new cases of esophageal cancer, 85% of which were esophageal squamous cell carcinoma (ESCC).1 More importantly, ESCC has an abysmal 5-year survival rate of <20%.2,3 In patients with localized disease, a combination of chemoradiotherapy and surgery has led to a modest increase in median survival in recent years.4,5 However, patients who undergo esophagectomy have decreased quality of life, swallowing difficulties, malnutrition, and poor long-term survival6; thus, new therapies are needed for ESCC.
The tumor microenvironment has a central role in the growth of cancers.7 The advent of immune checkpoint inhibitors to reactivate antitumor immunosurveillance8 and clearance is a new treatment avenue for difficult to manage cancers. While these studies are focused mostly on T cells, myeloid cells are starting to be studied.9,10 Traditionally, studies centered on one of these myeloid cells, eosinophils, have focused on their role in allergic disease or helminth infection. However, recent studies have uncovered varying roles of eosinophils in cancers. Eosinophils are present in several solid tumors, including breast, colon, gastric, ovarian, and lung.11 In colon cancer, they have been shown to be protective,12 but in other cancers, such as cervical,13,14 Hodgkin’s lymphoma,15,16 and ovarian,17 eosinophils have been shown to be protumorigenic. However, relatively little is known about their contribution in esophageal cancer, though early studies suggest that tumor-associated tissue eosinophilia is associated with increased overall survival.18, 19, 20 The role of eosinophils in different cancer types remains a topic of active research, as the interplay between eosinophils, other immune cells, and the tumor microenvironment is still incompletely understood. Understanding the role of eosinophils in cancer is important, as it could lead to new therapeutic strategies. In this study, we show that eosinophils infiltrate human ESCC to a greater extent in early vs late stages. We demonstrate that reduction in eosinophil number by different strategies exacerbates tumorigenesis. Conversely, treatment of mice with recombinant interleukin-5 (rIL-5) reduces esophageal tumor burden. Furthermore, we establish that eosinophils protect from murine ESCC through degranulation and subsequent release of reactive oxygen species (ROS). Therapies targeting increased eosinophil recruitment to the esophagus may be useful in patients with ESCC.
Results
Eosinophils Are Present in Human ESCC and Specific to the Tumor Microenvironment
In order to determine whether eosinophilic presence in ESCC was tumor specific, eosinophil peroxidase (EPX) immunohistochemistry (IHC) was performed on samples for which tumor-adjacent normal tissue was also available. EPX is an enzyme concentrated in granules inside eosinophils, and anti-EPX IHC is used as a histologic marker to identify eosinophils and detect the presence of degranulation.12,21
Within the tumor, the number of EPX+ cells was higher as compared with adjacent normal (Figure 1A). The red arrows highlight the presence of degranulation, and extracellular deposition of eosinophil granule content which was seen in nearly all patient samples in which eosinophils were present. Patient demographic information is reported in Table 2. Given that the esophagus does not normally have resident eosinophils, this suggested that recruitment of eosinophils was tumor-specific.
Figure 1.
Eosinophils are present in greater numbers in patients with early stage ESCC as compared with late stage. (A) There is a significantly greater ratio of EPX+ cells/total cells in human ESCC as compared with adjacent normal (0.0078 [0.0039–0.015] vs 0.00048 [0.00011–0.0011]; P < .0001; n = 28). Red arrows point out degranulating eosinophils. Data are presented as median ± interquartile range. (B) There is a significantly greater ratio of EPX+ cells/total cells in early-stage ≤T2 human ESCC as compared with late-stage ≥T3 ESCC (0.0053 [0.0023–0.0095] vs 0.0026 [0.00068–0.0097]; P = .04; n = 38–67). Data for A and B are represented with violin plots. Red arrows point out degranulating eosinophils. Scale bars in panel A are: top left = 250 μm, top right = 250 μm, bottom left = 50 μm, bottom right = 50 μm. Inserts in bottom left and right = 20 μm. Scale bars in panel B are: top left = 250 μm, top right = 250 μm, bottom left = 50 μm, bottom right = 50 μm. Inserts in bottom left and right = 20 μm. Inserts in bottom left and right = 20 μm. ∗P < .05, ∗∗∗∗P < .0001.
Table 2.
Patient Demographic Information
| Case | Sex | Age (y) | Stage | Surgical Resection, ESD, EMR, or Biopsy | Prior Treatment |
|---|---|---|---|---|---|
| 1 | M | 73 | T1a | Resection | None |
| 2 | M | 77 | T1b | Resection | None |
| 3 | M | 70 | T1b | Resection | None |
| 4 | M | 73 | T3 | Resection | None |
| 5 | F | 79 | T2 | Resection | None |
| 6 | F | 66 | T3 | Resection | None |
| 7 | F | 71 | T1b | Resection | None |
| 8 | M | 61 | T1b | Resection | None |
| 9 | M | 68 | T1b | Resection | None |
| 10 | F | 62 | T2 | Resection | None |
| 11 | M | 63 | T1b | Resection | None |
| 12 | F | 61 | T3 | Resection | None |
| 14 | M | 61 | T3 | Resection | None |
| 15 | M | 60 | T3 | Resection | None |
| 16 | M | 52 | T1b | Resection | None |
| 17 | F | 81 | T1b | Resection | None |
| 19 | M | 73 | T1b | Resection | None |
| 20 | F | 75 | T1b | Resection | None |
| 21 | M | 63 | T1b | Resection | None |
| 22 | M | 74 | T1a | Resection | None |
| 23 | F | 49 | T2 | Resection | None |
| 24 | M | 76 | T3 | Resection | None |
| 25 | F | 67 | T1a | Resection | None |
| 26 | M | 82 | T3 | Resection | None |
| 27 | M | 78 | T1a | Resection | None |
| 28 | M | 60 | T1b | Resection | None |
| 29 | M | 75 | T1b | Resection | None |
| 30 | M | 77 | T3 | Resection | None |
| 31 | M | 77 | T1a | ESD | None |
| 32 | M | 78 | T1a | ESD | None |
| 33 | M | 77 | T1a | ESD | None |
| 34 | F | 62 | T1a | ESD | None |
| 35 | M | 77 | T1a | ESD | None |
| 36 | M | 60 | T1a | ESD | None |
| 37 | M | 77 | T1a | ESD | None |
| 38 | M | 83 | T1a | ESD | None |
| 39 | M | 50 | T1a | ESD | None |
| 40 | M | 70 | T1a | ESD | None |
| 41 | M | 77 | T1a | ESD | None |
| 42 | M | 74 | T1a | ESD | None |
| 43 | M | 78 | T1a | ESD | None |
| 44 | M | 77 | T1b | ESD | None |
| 45 | M | 64 | T1a | ESD | None |
| 46 | M | 70 | T1a | ESD | None |
| 47 | M | 73 | T1a | ESD | None |
| 48 | M | 76 | T1a | ESD | None |
| 49 | F | 65 | T1a | ESD | None |
| 50 | M | 57 | T1a | ESD | None |
| 51 | M | 89 | T1a | ESD | None |
| 52 | M | 60 | T1b | ESD | None |
| 53 | F | 72 | T1a | ESD | None |
| 54 | M | 77 | T1a | ESD | None |
| 55 | M | 70 | T1a | ESD | None |
| 56 | M | 62 | T1b | ESD | None |
| 57 | F | 79 | T1a | ESD | None |
| 58 | M | 53 | T1a | ESD | None |
| 59 | F | 76 | T1a | ESD | None |
| 60 | F | 66 | T1a | ESD | None |
| 61 | M | 71 | T1a | Resection | Chemotherapy and radiation |
| 62 | M | 54 | T3 | Resection | Chemotherapy and radiation |
| 63 | M | 61 | T3 | Biopsy | Chemotherapy and radiation |
| 64 | M | 63 | T1a | Resection | Chemotherapy and radiation |
| 65 | M | 76 | T1b | EMR | Chemotherapy and radiation |
| 66 | M | 89 | Tis | Biopsy | None |
| 67 | M | 48 | T4a | Resection | Chemotherapy and radiation |
| 68 | F | 43 | Tis | Resection | None |
| 69 | M | 51 | T1a | EMR | None |
| 70 | M | 72 | T2 | Resection | Chemotherapy and radiation |
| 71 | M | 44 | T1b | Resection | None |
| 72 | M | 53 | T4a | Resection | Chemotherapy and radiation |
| 73 | M | 74 | T3 | Resection | Chemotherapy and radiation |
| 74 | M | 62 | T3 | Resection | Chemotherapy and radiation |
| 75 | M | 47 | T3 | Biopsy | None |
| 76 | M | 75 | T3 | Biopsy | None |
| 77 | M | 50 | T4 | Biopsy | None |
| 78 | M | 47 | T3 | Biopsy | None |
| 79 | M | 83 | T4 | Biopsy | None |
| 80 | F | 63 | T4 | Biopsy | None |
| 81 | M | 56 | T4 | Biopsy | None |
| 82 | M | 71 | T3 | Biopsy | None |
| 83 | M | 56 | T4 | Biopsy | None |
| 84 | M | 57 | Tis | Biopsy | None |
| 85 | F | 52 | T3 | Biopsy | None |
| 86 | M | 55 | T4 | Biopsy | None |
| 87 | M | 76 | T3 | Biopsy | None |
| 88 | F | 90 | T3 | Biopsy | None |
| 89 | M | 54 | T4 | Biopsy | None |
| 90 | M | 66 | Tis | Biopsy | None |
| 91 | F | 73 | Tis | Biopsy | None |
| 92 | M | 69 | T4 | Biopsy | None |
| 93 | M | 59 | T4 | Biopsy | None |
| 94 | F | 74 | Tis | Biopsy | RFA |
| 95 | F | 70 | T2 | Biopsy | None |
| 96 | F | 65 | T3 | Biopsy | None |
| 97 | M | 59 | T1a | Biopsy | Chemotherapy and radiation |
| 98 | F | 56 | T3 | Biopsy | None |
| 99 | M | 81 | T4 | Biopsy | None |
| 100 | F | 63 | T3 | Biopsy | None |
| 101 | M | 66 | T4 | Biopsy | None |
| 102 | F | 68 | Tis | Biopsy | None |
| 103 | M | 76 | T3 | Biopsy | None |
| 104 | F | 59 | T3 | Biopsy | None |
| 105 | M | 67 | T3 | Biopsy | None |
| 106 | M | 83 | Tis | Biopsy | None |
EMR, endoscopic mucosal resection; ESD, endoscopic submucosal dissection; F, female; M, male; RFA, radiofrequency ablation.
Early-Stage ESCC Is Characterized by Greater Eosinophilic Infiltration Than Late-Stage ESCC
We next compared eosinophil number in early-stage ESCC (≤T2) with later-stage ESCC (≥T3) and found that there were significantly more EPX+ cells in early-stage cancers (Figure 1B). Again, the red arrows highlight the occurrence of degranulation, indicating the eosinophils present were activated. Patient demographic information for ≤T2 group as compared with ≥T3 group is listed in Table 3. While the ≥T3 group was significantly younger, the difference in median age was 9 years and there was considerable overlap in the interquartile ranges. This observation suggests that eosinophils are being recruited to a greater degree early in tumorigenesis.
Table 3.
Comparison of Demographic Information Between Patients With ≤T2 ESCC vs Patients With ≥T3 ESCC
| ≤T2 | ≥T3 | P value | |
|---|---|---|---|
| Age, y | 71 (62–77) | 62.5 (55.8–74.3) | .02 |
| Female | 18/66 (27%) | 9/38 (24%) | .81 |
| Prior therapy | 6/66 (9%) | 6/38 (16%) | .35 |
Values are median (interquartile range) or n/n (%).
ESCC, esophageal squamous cell carcinoma.
Murine ESCC Precancer Is Characterized by Greater Eosinophilic Infiltration as Compared With Carcinoma
The 4-NQO murine model of ESCC mimics the development of ESCC in humans.22 4-NQO contains similar carcinogens to those present in tobacco, a known risk factor for ESCC,2,3,23 and causes DNA damage.24, 25, 26
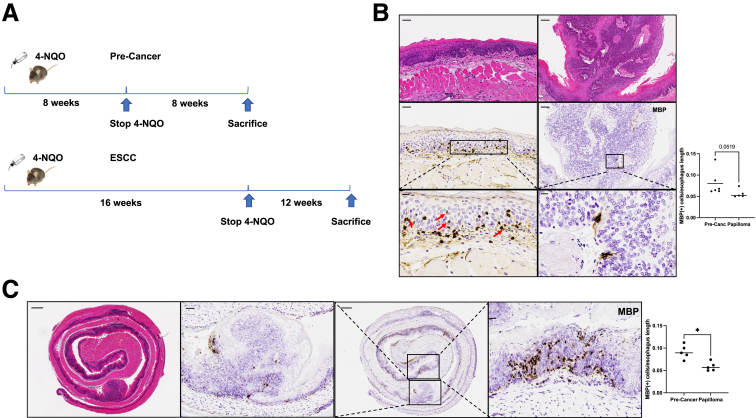
Mice were treated with 4-NQO for 8 weeks followed by 8 weeks of vehicle or with 4-NQO for 16 weeks followed by 12 weeks of vehicle (Figure 2A). Mice treated with 4-NQO for 8 weeks had an increase in major basic protein–positive (MBP+) cells in precancerous areas as compared with papillomas or invasive carcinoma from mice treated with 4-NQO for 16 weeks (Figure 2B). To further investigate this, we also compared areas of papilloma or invasive carcinoma vs precancerous areas in mice treated with 4-NQO for 16 weeks. There were significantly more MBP+ cells in precancer areas as compared with papillomas or invasive carcinoma (Figure 2C). This is easily visualized in the 20× image. This finding provided evidence that murine development of ESCC in the 4-NQO model was similar to what occurs in human ESCC.
Figure 2.
Eosinophils are present in greater numbers in mice with 4-NQO precancer as compared with carcinoma. (A) Timelines for murine 4-NQO induced precancer and carcinoma. In precancer, 4-NQO is given via drinking water for 8 weeks followed by 8 weeks of propylene glycol vehicle. In carcinoma, 4-NQO is given via drinking water for 16 weeks followed by 12 weeks of propylene glycol vehicle. (B) There are significantly more MBP+ cells/length of precancer in μm from mice treated with 4-NQO for 8 weeks as compared with papilloma/length of papilloma) from mice treated with 4-NQO for 16 weeks (0.10 ± 0.03 vs 0.06 ± 0.005; P = .03; n = 5–7). Red arrows highlight degranulating eosinophils. (C) Left = representative H&E of a WT mouse treated with 4-NQO in the carcinoma timeline. Precancerous areas (right panel) show significantly greater MBP+ cells as compared with papillomas (second panel) within the same mice (0.092 ± 0.0069 vs 0.059 ± 0.0044; P = .02; n = 4). MBP quantification was standardized to the length of precancer or papilloma. All comparisons are reported as mean ± SEM and used the Mann-Whitney test. Scale bars in panel B are: top left = 100 μm, middle left = 100 μm, bottom left = 50 μm, top right = 100 μm, middle right = 100 μm, bottom right = 50 μm. Scale bars in panel C are: left = 400 μm, second panel = 100 μm, third panel = 400 μm, and fourth panel = 50 μm. ∗P < .05.
Targeted Eosinophil Depletion Exacerbates Esophageal Precancer
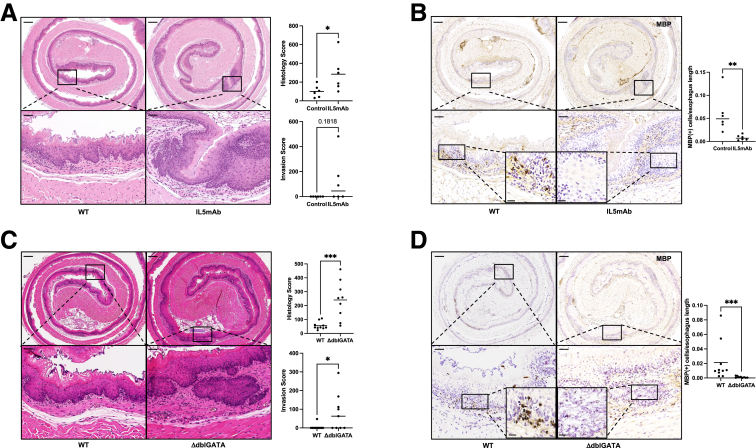
Because we observed significantly greater eosinophilia in mice in precancer vs in papillomas or invasive carcinoma, we hypothesized that eosinophils might protect from ESCC progression. Thus, we treated wild-type (WT) mice with a monoclonal antibody against IL-5 (IL5mAb), which blocks eosinophil differentiation, for the second 8 weeks of the precancer timeline (Figure 1C). The histology score was computed based on the severity of 4 characteristics: rete pegs, nuclear histologic abnormalities, papilloma, and invasive carcinoma.27 Rete pegs and nuclear histologic abnormalities are common precancer model findings, while papilloma and invasive carcinoma are common cancer model findings. Histology score showed significantly worse disease in mice treated with IL5mAb. The hematoxylin and eosin (H&E) highlights, in particular the 20× views, the greater depth of rete pegs and the beginning of invasive disease, indicators of more advanced disease with eosinophil depletion (Figure 3A). While there was not a statistically significant difference in invasion, none of the control mice show invasive disease, while 50% of the mice treated with IL5mAb did. We confirmed eosinophil depletion via MBP IHC in mice treated with IL5mAb (Figure 3B).
Figure 3.
A reduction in eosinophils leads to significantly worse precancer after 8 weeks of 4-NQO. (A) Mice treated with IL5mAb for the second 8 weeks of the 4-NQO precancer protocol had significantly increased histology score as compared with control animals (284.6 ± 77.6 vs 100.8 ± 26.1; P = .04; n = 6). There is a trend toward an increased invasion parameter of the total score (122.7 ± 76.8 vs 0.0 ± 0.0; P = .18; n = 6), and all control animals have a score of 0. (B) Mice treated with IL5mAb had significantly fewer MBP+ cells as compared with control (0.008314 ± 0.002308 vs 0.05944 ± 0.01702; P = .002; n = 6). Inserts on the lower 2 panels are taken at 40× magnification. (C) Eosinophil-deficient (ΔdblGATA) mice have significantly increased histology score as compared with control animals in the precancer protocol (240.7 ± 45.3 vs 55.6 ± 8.8; P < .001; n = 9–10). They demonstrate significantly increased invasion parameter of the total score (82.0 ± 33.4 vs 4.8 ± 4.8; P = .02; n = 9–10) as well. (D) There are significantly fewer MBP+ cells/esophageal length in ΔdblGATA mice as compared with WT control animals (0.0011 ± 0.0004 vs 0.021 ± 0.0086; P < .001; n = 9–10). Inserts on lower 2 panels are taken at 40× magnification. All comparisons are reported as mean ± SEM and used the Mann-Whitney test. Scale bars in panel A are: top left = 250 μm, top right = 250 μm, bottom left = 50 μm, bottom right = 50 μm. Inserts in bottom left and right = 20 μm. Scale bars in panel B are: top left = 250 μm, top right = 250 μm, bottom left = 50 μm, bottom right = 50 μm. Inserts in bottom left and right = 20 μm. Scale bars in panel C are: top left = 250 μm, top right = 250 μm, bottom left = 50 μm, bottom right = 50 μm. Inserts in bottom left and right = 20 μm. Scale bars in panel D are: top left = 250 μm, top right = 250 μm, bottom left = 50 μm, bottom right = 50 μm. Inserts in bottom left and right = 20 μm. ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001.
We next treated genetically eosinophil-deficient (ΔdblGATA) mice with 4-NQO in the precancer timeline. Confirming the phenotype observed with IL5mAb-mediated eosinophil depletion in WT mice, ΔdblGATA mice showed more advanced precancer and invasion, which is highlighted in the 20× view (Figure 3C). In the 4× image, the rete pegs are apparent. MBP IHC confirmed the absence of eosinophils in ΔdblGATA mice (Figure 3D). As such, reducing eosinophil number either with IL5mAb or genetically with ΔdblGATA mice results in more advanced precancer.
Absence of Eosinophils Results in Increased Esophageal Tumorigenesis
Because ΔdblGATA mice displayed more advanced precancer as compared with control animals, we hypothesized that ΔdblGATA mice would also develop more severe tumorigenesis in an ESCC cancer model (Figure 2A). As predicted, ΔdblGATA mice had significantly more esophageal papillomas than WT mice (Figure 4A). Additionally, there was a trend toward decreased survival in ΔdblGATA mice (Figure 4B), and due to this, the experiment was ended 4 weeks early to minimize the number of mice lost in the experiment. 4-NQO can induce oral tumors as well; however, no genotype-specific differences in oral tumors was observed (Figure 4C). Thus, we hypothesized that this survival trend was due to esophageal tumorigenesis, rather than to oral tumorigenesis, as there was no difference in tongue tumor number. Finally, consistent with the survival trend, we observed there was a significantly higher histology score and invasion score in ΔdblGATA mice (Figure 4D). Thus, ΔdblGATA mice demonstrate features of advanced precancer and cancer.
Figure 4.
The absence of eosinophils leads to significantly worse carcinoma in a 4-NQO carcinoma timeline. (A) There are significantly more esophageal papillomas in ΔdblGATA as compared with WT control mice (6.08 ± 0.75 vs 3.36 ± 0.88; P < .01; n = 11–12). (B) There is a trend toward increased mortality for ΔdblGATA as compared with WT mice in the 4-NQO carcinoma model (P = .24; n = 12–16). (C) There is no difference in the number of tumors on the tongue between ΔdblGATA and WT mice. (D) ΔdblGATA have significantly increased histology score as compared with control mice in the 4-NQO carcinoma timeline (1107 ± 227.1 vs 601.7 ± 84.9; P = .03; n = 11–12). They demonstrate significantly increased invasion parameter of the total score (271.7 ± 43.4 vs 150.4 ± 33.9; P = .02; n = 11–12). (E) ΔdblGATA mice have significantly fewer MBP+ cells/esophagus length as compared with WT control mice (0.00062 ± 0.00026 vs 0.029 ± 0.0056; P < .0001; n = 11–12). All comparisons are reported as mean ± SEM and used the Mann-Whitney test. Scale bars in panel D are left = 400 μm, right = 400 μm. Scale bars in panel E are: top left = 400 μm, top right = 400 μm, bottom left = 50 μm, bottom right = 50 μm. Inserts in bottom left and right = 20 μm. ns, not significant. ∗∗P < .01 and ∗∗∗∗P < .0001.
Ccl11 Is Upregulated in Precancer
Because there were significantly more eosinophils in mice that were treated with 4-NQO in precancer (8 weeks) vs cancer (16 weeks), we hypothesized that Ccl11, or Eotaxin-1, an eosinophil chemoattractant, which is expressed in the gastrointestinal tract,28 may also be upregulated in precancer. Thus, we queried a publicly available single-cell RNA sequencing (sc-RNAseq) dataset29 in which transcriptomic profiling was done at several time points throughout 4-NQO treatment. The authors of the study utilized a carcinoma model (16 weeks of 4-NQO, followed by 12 weeks of vehicle) and sacrificed mice at these time points: 0-, 12-, 20-, 22-, 24-, and 26-week time points after initiation of 4-NQO. They defined 0 weeks as normal (NOR), 12 weeks as inflammation (INF), 20 weeks as hyperplasia (HYP), 22 weeks as dysplasia (DYS), 24 weeks as carcinoma in situ (CIS), and 26 weeks as invasive carcinoma (ICA). After reviewing the histologic images, we determined that the 20-week hyperplasia time point was most similar to our precancer experiments in which mice were treated with 4-NQO for 8 weeks followed by 8 weeks of vehicle. Thus, we compared Ccl11 expression at 20 weeks vs the other time points. This analysis focused on epithelial cells and fibroblasts because Ccl11 expression was very low in immune cells. In epithelial cells, Ccl11 was significantly upregulated at 20 weeks as compared with 0, 12, 22, 24, and 26 weeks after the 4-NQO start date (Figure 5A). Additionally, Ccl11 was significantly upregulated at 20 weeks in fibroblasts as compared with 0 and 22 weeks after initiation of 4-NQO but unchanged at 12, 24, or 26 weeks (Figure 5A).
Figure 5.
Ccl11–/–mice exhibit significantly worsened ESCC precancer and carcinoma. (A) Analysis of sc-RNAseq (Yao et al, accession number CRA002118)29 shows that epithelial cell expression of Ccl11 was significantly greater in what was termed HYP (20 weeks after initiation of 4-NQO) as compared with 0 weeks (NOR, P = .01), 12 weeks (INF, P < .001), 22 weeks (DYS, P = .001), 24, weeks (CIS, P < .001), and 26 weeks (ICA, P < .001). Fibroblast cell Ccl11 expression was significantly greater in what was termed HYP (20 weeks after initiation of 4-NQO) as compared with 0 weeks (NOR, P < .001) and 22 weeks (DYS, P < .001). There was no difference between 20 weeks and 12 weeks (INF), 24 weeks (CIS), or 26 weeks (ICA). The Wilcoxon rank sum test was used to compare the target gene (ie, Ccl11) expression between HYP and NOR, between HYP and INF, between HYP and DYS, between HYP and CIS and between HYP and ICA. Multiplicity was controlled with the Bonferroni method. (B) Ccl11–/– mice display a significantly increased histology score in the precancer timeline (364.4 ± 95.6 vs 182.1± 63.9; P = .04; n = 9–14). They demonstrate a significantly increased invasion parameter of the total score (121.8 ± 58.3 vs 20.5 ± 13.6; P = .03; n = 9–14). (C) Ccl11–/– mice had significantly fewer MBP+ cells/esophagus length as compared with WT control animals (0.016 ± 0.0017 vs 0.044 ± 0.0076; P = .002; n = 9–14). Scale bars in panel B are: top left = 250 μm, top right = 250 μm, bottom left = 50 μm, bottom right = 50 μm. Scale bars in panel C are: top left = 250 μm, top right = 250 μm, bottom left = 50 μm, bottom right = 50 μm Inserts in bottom left and right = 20 μm. ∗P < .05, ∗∗P <.01, ∗∗∗P < .001, and ∗∗∗∗P < .0001.
Ccl11–/– Mice Exhibit Worse Tumorigenesis
Because Ccl11 was upregulated at a time point when there were more eosinophils and downregulated when there were fewer eosinophils, we tested whether the absence of Ccl11 would lead to worse neoplasia. Ccl11–/– mice showed more advanced disease after 8-week 4-NQO treatment (Figure 5B). Similar to the IL5mAb-treated and ΔdblGATA mice, the H&E highlights the greater degree of invasion in the Ccl11–/– mice. MBP IHC confirms that there were fewer eosinophils in Ccl11–/– mice (Figure 5C). After treatment with 4-NQO for 16 weeks, Ccl11–/– mice displayed a trend toward decreased survival (Figure 6A), and the experiment was concluded 4 weeks early just as in ΔdblGATA mice. However, Ccl11–/– mice also had a significantly greater number of tumors on the tongue (Figure 6B), and thus it is difficult to interpret whether this change in survival was due to esophageal or oral tumorigenesis. Additionally, Ccl11–/– mice displayed significantly more esophageal papillomas and greater histology scores including increased invasion (Figure 6C). MBP IHC revealed significantly fewer eosinophils (Figure 6C). Notably, while there were fewer eosinophils in the Ccl11–/– mice, it was not zero, which could be due to the presence of Ccl24 or other unknown eosinophil chemoattractants.
Figure 6.
Ccl11–/–mice exhibit significantly worsened tumorigenesis in a carcinoma timeline. (A) There is a trend toward worse survival in Ccl11–/– mice as compared with WT control animals (P = .07; n = 9–14). (B) Ccl11–/– mice have significantly more tongue tumors as compared with WT control animals (2.6 ± 0.4 vs 0.9 ± 0.4; P = .009; n = 9–10). (C) Ccl11–/– mice have significantly more esophageal papillomas than WT control animals in a 4-NQO carcinoma timeline (6.1 ± 0.9 vs 2.2 ± 0.5; P < .001; n = 9–10). Ccl11–/– mice demonstrate significantly greater histology score than WT control animals in the same carcinoma timeline (1997 ± 257.8 vs 1104 ± 157.5; P = .04; n = 9–10). Ccl11–/– mice have significantly increased invasion parameter of the total histology score as compared with WT (982.2 ± 185.0 vs 468.2 ± 126.6; P = .04; n = 9–10). Ccl11–/– mice also show significantly fewer MBP+ cells/esophagus length than WT control animals (0.0047 ± 0.00068 vs 0.014 ± 0.0023; P = .02; n = 9–10). The log-rank (Mantel-Cox) test was used for survival analysis. For B and C, all comparisons are reported as mean ± SEM and used the Mann-Whitney test. Scale bars in panel C are: left top and bottom = 2 mm, H&E top and bottom = 100 μm, MBP middle top and bottom = 100 μm, MBP right top and bottom = 20 μm. ∗P < .05 and ∗∗P < .01.
rIL-5 Slows ESCC Tumorigenesis
Because the reduction of eosinophil number exacerbated tumorigenesis, we next hypothesized that enhancing eosinophilic recruitment might reduce ESCC progression. In order to do so, we tested whether rIL-5 treatment, which increases eosinophilia, could slow neoplastic progression. We first treated mice weekly with intranasal rIL-5 in the second 8 weeks of the precancer protocol (Figure 2A). Mice treated with rIL-5 had significantly lower histology scores (Figure 7A) and significantly more MBP+ cells along the length of the esophagus (Figure 7B). The H&E shown highlights the increased smoothness of the epithelium, ie, fewer rete pegs, a feature of precancer. There was no difference in invasion score, but so few control mice develop invasion in this timeline that it is not surprising that there was no difference. The same experiment was conducted in the carcinoma timeline, in which WT mice were treated intranasally with rIL-5 weekly for the last 12 weeks of the experiment (ie, after 4-NQO was stopped) (see Figure 1C). Mice treated with rIL-5 had significantly fewer esophageal papillomas (Figure 7C) and less severe features of carcinoma, including invasion score (Figure 7D). There were significantly more MBP+ cells than controls (Figure 7D), confirming rIL-5 increases recruitment of eosinophils to the esophagus.
Figure 7.
Intranasal rIL-5 attenuates 4-NQO precancer and carcinoma. (A) Mice treated with rIL-5 for the second 8 weeks of the 4-NQO dysplasia timeline have significantly reduced histology score (80.5 ± 9.7 vs 127.8 ± 21.5; P = .04; n = 8–9), but there was no difference in the invasion parameter of the total score. (B) Intranasal rIL-5 results in significantly greater MBP+ cells/esophagus length as compared with WT control animals in the precancer timeline (0.064 ± 0.010 vs 0.034 ± 0.0074; P = .04; n = 8–9). (C) Mice treated with intranasal rIL-5 during the final 12 weeks of the 4-NQO carcinoma timeline have significantly reduced esophageal papillomas (3.3 ± 0.6 vs 6.9 ± 0.8; P < .001; n = 14). (D) Mice treated with intranasal rIL-5 have significantly lower histology score than WT control animals (537.8 ± 90.9 vs 863.9 ± 62.3; P = .03; n = 12–13), a lower invasion parameter of the total score (65.7 ± 11.1 vs 237.3 ± 48.8; P < .001; n = 12–13), and a significantly greater number of MBP+ cells/esophagus length (0.027 ± 0.0068 vs 0.010 ± 0.003; P = .02; n = 12–13). All comparisons are reported as mean ± SEM and used the Mann-Whitney test. Scale bars in panel A are: top left = 250 μm, top right = 250 μm, bottom left = 50 μm, bottom right = 50 μm. Scale bars in panel B are: top left = 250 μm, top right = 250 μm, bottom left = 50 μm, bottom right = 50 μm. Scale bars in panel D are left top/bottom = 250 μm, middle top/bottom = 250 μm, right top/bottom 20 μm. Inserts on right top/bottom = 15μm. ∗P < .05 and ∗∗∗P < .001.
RNA Sequencing Demonstrates Eosinophilic Contribution to Oxidative State in Esophageal Squamous Precancer
Having established that the number of eosinophils modifies tumor progression, we then performed RNA sequencing (RNA-seq) on the whole esophagus of WT and ΔdblGATA mice in the precancer model to understand how the presence of eosinophils affects the precancer esophageal environment. After generating a list of differentially expressed genes between the 2 groups, we input this list into Ingenuity Pathway Analysis (IPA) software to understand which molecular pathways were significantly different. Several of the most dysregulated pathways were related to metabolic alterations, and most of them were downregulated in the absence of eosinophils, including oxidative phosphorylation, glutathione redox reactions, glutathione-mediated detoxification, and endothelial nitric oxide synthase signaling (Figure 8A). The list of all significantly dysregulated pathways is uploaded in the Gene Expression Omnibus (GEO) and as a Supplementary Appendix.
Figure 8.
RNA-seq of tissue and eosinophils reveals a significant eosinophilic contribution to oxidative stress in esophageal squamous precancer. (A) WT and ΔdblGATA mice (n = 6–7) were treated with 4-NQO in the precancer model, and after sacrifice the whole esophagus was collected for RNA. Bulk RNA-seq was performed. The list of differentially expressed genes was analyzed for pathway alterations using IPA, and a heatmap of z scores for altered metabolic pathways is shown. Pathways that were statistically significant (adjusted P value <.10) are shown and pathways of interest are annotated. The majority of oxidant pathways were significantly downregulated in the absence of eosinophils. (B) For eosinophil-specific RNA-seq, eosinophils were retrieved using positive enrichment from the esophageal lamina propria (n = 7) and from the colonic lamina propria from a random subset (n = 4) of the same mice. Bone marrow was also extracted and eosinophils were induced in vitro from the same random subset (n = 4). Eosinophils were subsequently subjected to bulk RNA-seq. Principal component (PC) analysis of gene expression data is shown. (C) Volcano plot highlighting genes of interest in the comparison of esophageal eosinophils vs bone marrow eosinophils. Genes that are above the threshold for statistical significance and fold change are colored red. Many highlighted genes are mitochondria or directly involved in regulating oxidative stress, including Sdha, Sdhd, Bckdk, and Oxa1l. (D) Volcano plot highlighting genes of interest in the comparison of esophageal eosinophils vs colonic eosinophils. Again, many highlighted genes are mitochondrial or regulate oxidative stress, including Sdha, COX3, Oxa1l, Duoxa1, Fmo9, and Bckdk. eNOS, endothelia nitric oxide synthase; TCA, Tricarboxylic acid cycle.
In order to study the transcriptional profiles of eosinophils specifically, we next isolated eosinophils from esophageal tissue of WT mice that had been treated with 4-NQO in the precancer model. Because eosinophils are not present in the esophagus normally, we isolated eosinophils from the colons of a random subset of the same mice. Additionally, we derived eosinophils from the bone marrow of a random subset of the same mice. We then compared the precancer esophageal eosinophils with each of these groups. The principal component analysis showed that the precancer esophageal eosinophils were more similar to the colonic eosinophils than bone marrow–derived eosinophils (Figure 8B). When comparing esophageal precancer eosinophils with bone marrow–derived eosinophils (Figure 8C), we identified several differentially expressed genes that were either mitochondrial or related to the redox state, including Bco1, Cyb5r3, Cyp11a1, Sdha, Bckdk, Sdhd, and Oxa1l. All of these genes were downregulated in the precancer eosinophils. Similarly, when comparing precancer esophageal eosinophils to colonic eosinophils (Figure 8D), we again identified several genes related to redox state which were downregulated: Sdha, Cox3, Oxa1l, Duoxa1, Fmo9, and Bckdk. Notably, in this comparison, we also observed a significant upregulation of Cd74, the receptor of macrophage inhibitory factor (MIF), in the precancer esophageal eosinophils. Additionally, H2-Ab1 (part of major histocompatibility complex II) was upregulated in both comparisons in the precancer esophageal eosinophils, suggesting antigen presentation by esophageal eosinophils may contribute to their function in attenuating esophageal cancer. The full list of dysregulated genes in each comparison is uploaded in the GEO and in the Supplementary Appendix. Altogether, these results suggest that precancer esophageal eosinophils regulate other immune cells as well as metabolism.
Apoptosis of Precancer and Cancer Cells Is Triggered by Release of ROS From Degranulating Eosinophils
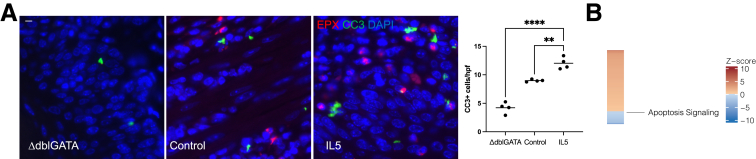
Because precancer eosinophils showed significant downregulation of several mitochondrial genes as compared with colonic and bone marrow–derived eosinophils, we hypothesized that precancer eosinophils may induce apoptosis of surrounding cells through release of ROS during degranulation. In order to test this, we performed cleaved caspase-3 (CC3) and EPX immunofluorescent staining on randomly selected mice (n = 4) treated with rIL-5 and 4-NQO for 16 weeks, control animals treated with 4-NQO for 16 weeks (n = 4), or ΔdblGATA mice treated with 4-NQO for 16 weeks (n = 4). Mice treated with rIL-5 displayed a significantly greater number of CC3+ cells per high-power field as compared with control animals and ΔdblGATA mice (Figure 9A). Consistent with this, bulk RNA-seq from WT and ΔdblGATA mice with precancer showed the apoptosis signaling pathway to be downregulated in the absence of eosinophils (Figure 9B). Moreover, immunofluorescence for EPX revealed that eosinophils were in close proximity to CC3+ cells. Together, these experiments suggested that rIL-5 protects against the development of ESCC by inducing apoptosis of cells adjacent to eosinophils.
Figure 9.
Eosinophils induce apoptosis of surrounding cells. (A) Mice treated with intranasal rIL-5 have significantly more CC3 positive cells/high power field (hpf) as compared with WT control animals (12.0 ± 0.5 vs 9.0 ± 0.1 CC3+ cells/hpf; P = .001; n = 4 mice; 8–12 hpf/mouse) and ΔdblGATA mice (12.0 ± 0.5 vs 4.2 ± 0.49 CC3+ cells/hpf; P < .0001; n = 4 mice; 8–12 hpf/mouse) treated with 4-NQO in the carcinoma timeline. A costain for EPX demonstrates close proximity of EPX+ cells with CC3+ cells in both the rIL-5 and WT conditions. It also confirms the absence of eosinophils in the ΔdblGATA mice. (B) Heatmap of z score of apoptosis pathway retrieved from IPA from tissue bulk RNA-seq from WT and ΔdblGATA mice (n = 6–7), same as in Figure 6A. Scale bars in panel A are 15 μm. ∗∗P < .01 and ∗∗∗∗P < .0001.
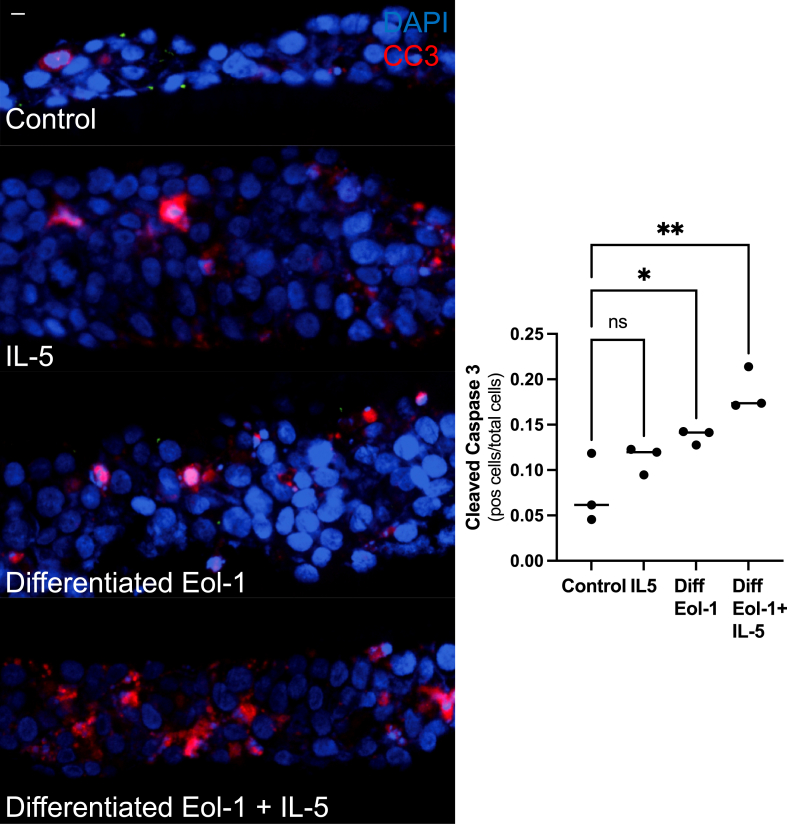
Because rIL-5 treatment of 4-NQO mice demonstrated increased esophageal apoptosis we next sought to define the mechanism by coculturing eosinophils with precancer and cancer cells in vitro. To accomplish this, we cultured murine bone marrow derived eosinophils from WT mice, which were defined as CD45+CD11b+SiglecF+ cells.30 Our culture technique resulted in 95% eosinophils (Figure 10A). These cells were then cocultured with murine precancer organoids which were grown to confluence on a transwell and then made 3-dimensional by removing media from the apical side of the transwell, inducing differentiation. This process is represented in a schematic (Figure 10B). Eosinophils were cultured in the basolateral compartment with or without Lyso-platelet activating factor (Lyso-PAF), which induces eosinophil degranulation. Degranulation was confirmed by performing an Eosinophil Peroxidase assay (Figure 10C), which showed that Lyso-PAF resulted in 40% of degranulation as compared with the positive control CHAPS ((3-((3-cholamidopropyl) dimethylammonio)-1-propanesulfonate), similar to previous studies.30 After 24 hours, the transwells were collected and fixed and immunofluorescence was done for CC3 to measure apoptosis. Without activation, these bone marrow–derived eosinophils did not induce increased apoptosis (Figure 10D, second image).
Figure 10.
Degranulating murine bone marrow–derived eosinophils result in increased apoptosis of precancer esophageal organoids through release of ROS. (A) This is an example of the flow cytometry gating strategy for bone marrow–derived eosinophils. Eosinophils were defined as CD45+CD11b+Siglec-F+. They were further characterized by CCR3 status. (B) Schematic for how coculture experiment is set up. (C) This is an example (1 representative example from 3 different experiments) demonstrating that the addition of Lyso-PAF results in degranulation of bone marrow eosinophils, as measured by an EPX activity assay. EPX activity induced by Lyso-PAF was 40% of EPX activity induced by the detergent CHAPS, a positive control. N-acetylcysteine (NAC) reduced measured EPX activity from 40% to 18%. (D) Coculture of bone marrow derived eosinophils with mouse precancer organoids grown in ALI shows that inducing degranulation of bone marrow eosinophils with Lyso-PAF results in significantly greater CC3+ cells as compared with control cells (6.6 ± 0.4 vs 1.9 ± 0.4; P < .0001; n = 2 independent experiments; 6 replicates), eosinophils alone (6.6 ± 0.4 vs 1.7 ± 0.2; P < .0001; n = 2 independent experiments; 5–6 replicates), or eosinophils plus Lyso-PAF + NAC (6.6 ± 0.4 vs 1.6 ± 0.2; P < .0001; n = 2 independent experiments; 4–6 replicates). Images in panel D were taken using Keyence BZX-810 using a 40× lens. The scale bar in panel D is 20 μm. ns, not significant. ∗∗∗∗P < .0001.
However, when degranulation was triggered with Lyso-PAF, there was significantly increased apoptosis of precancer cells by CC3 immunofluorescence (Figure 10D, third image). Adding both Lyso-PAF and NAC, to buffer ROS, reduced the number of CC3 positive cells (Figure 10D, fourth image). This suggested that eosinophil degranulation was required for inducing apoptosis of surrounding cells and that the release of ROS during degranulation was at least 1 mechanism by which cell death occurred (Figure 10D).
We confirmed these results in a separate model using a human eosinophil cell line (EoL-1 cells) cocultured with a human ESCC cell line (TE-11 cells). In these experiments, TE-11 cells were grown to confluence on transwells and then differentiated using an air-liquid interface (ALI) model, similar to the mouse precancer organoids. EoL-1 cells were differentiated in media with sodium butyrate in order to increase the percentage of mature eosinophils and cultured in the basolateral compartment of the transwell, similar to mouse bone marrow derived eosinophils. Differentiated EoL-1 cells were cocultured with TE-11 cells with or without IL-5, a degranulating agent for human eosinophils. After 24 hours, transwells were fixed and immunofluorescent staining was performed for CC3 to assess apoptosis. When compared with control (ie, coculture with no EoL-1 cells), coculture with differentiated EoL-1 cells minus IL-5 showed a trend toward greater apoptosis of TE-11 cells. However, adding IL-5 to differentiated EoL-1 cells led to significantly greater apoptosis of TE-11 cells as compared with control (Figure 11), confirming our observation in the murine model. Notably, IL-5 alone did not significantly increase apoptosis as compared with control.
Figure 11.
Eol-1 cells cocultured with an ESCC cell line results in apoptosis. Differentiated Eol-1 cells cocultured with TE-11 cells grown in ALI results in a trend toward greater apoptosis as measured by CC3+ cells/total cells as compared with control cells (0.14 ± 0.005 vs 0.075 ± 0.022; P = .05; n = 3 independent experiments). Differentiated Eol-1 cells with IL-5 cocultured with TE-11 cells results in significantly greater apoptosis also measured by CC3+ cells/total cells (0.19 ± 0.014 vs 0.075 ± 0.022; P = .004; n = 3 independent experiments). There is no difference between control and TE-11 cells treated with IL-5. This comparison is reported as mean ± SEM using Kruskall-Wallis test. The scale bar is 15 μm. ns, not significant. ∗P < .05 and ∗∗P <.01.
Taken together, these data support that eosinophil degranulation is a driver of apoptosis of surrounding precancer and cancer cells in our mouse model and that the release of ROS is one mechanism by which this occurs.
Eosinophils Suppress CD4 T Cells in the Precancer Esophageal Squamous Microenvironment
Having defined the direct effect of eosinophils on surrounding cells, we next sought to determine the indirect effect of eosinophils on other immune cells in the precancer microenvironment. We performed IHC for CD4 T cells in WT, ΔdblGATA, and mice treated with rIL-5 in the precancer model. There was a significantly greater number of CD4 T cells in the absence of eosinophils (Figure 12A), but it was significantly fewer in mice treated with rIL-5 (Figure 12B). Given this difference in CD4 T cells, we then performed a Luminex Multiplex Array (Thermo Fisher Scientific, Waltham, MA) on WT and ΔdblGATA mice to measure cytokines associated with T cell subsets. ΔdblGATA showed significantly increased IL-17A and a trend toward an increase in IL-6 and granulocyte-macrophage colony-stimulating factor (Figure 12C). Bulk RNA-seq from WT and ΔdblGATA mice supported these data, as IL-6 signaling and IL-17 signaling pathways were enriched in ΔdblGATA mice in IPA (Figure 12D). Moreover, pathways downstream of IL-17, including Epidermal Growth Factor (EGF) and nuclear factor κB were also enriched in ΔdblGATA mice. Finally, the number of CD8 T cells was not different by IHC in the absence of eosinophils (Figure 12E) or with treatment of rIL-5 (Figure 12F). Thus, our data support that esophageal eosinophils might regulate T helper 17 (Th17) function. The analytes for Th1, Th2, Th9, Th22, and T regulatory cells did not differ (Figure 13).
Figure 12.
The absence of eosinophils alters the precancer immune microenvironment and results in enrichment of protumorigenic pathways. (A) ΔdblGATA have significantly more CD4 T cells/esophagus length as compared with WT mice (0.04 ± 0.005 vs 0.03 ± 0.002; P = .04; n = 9–10). (B) WT mice have significantly more CD4 T cells/esophagus length as compared with WT mice treated with rIL-5 (0.05 ± 0.01 vs 0.02 ± 0.006; P = .02; n = 6). (C) Luminex profiling shows that IL-17a is significantly upregulated (56.6 ± 7.0 vs 38.3 ± 5.0 pg/mg protein; P = .04; n = 14–15) in ΔdblGATA mice. IL-6 (213.2 ± 41.9 vs 133.6 ± 22.5 pg/mg protein; P = .18; n = 14–17) and granulocyte-macrophage colony-stimulating factor (8.2 ± 0.6 vs 7.0 ± 0.9 pg/mg protein; P = .10; n = 14–17) demonstrate a trend toward increase in ΔdblGATA mice. (D) Heatmap of z scores of pathways retrieved from IPA from tissue bulk RNA-seq from WT and ΔdblGATA mice (n = 6–7), same as in Figure 6A. IL-6 signaling, EGF signaling, nuclear factor κB signaling, and IL-17 are all enriched in ΔdblGATA mice. There is no difference in CD8+ cells/esophagus length in WT vs ΔdblGATA mice (E) or in WT vs rIL-5–treated WT mice (F). All comparisons are reported as mean ± SEM and used the Mann-Whitney test. The scale bar in panels A and B in all panels is 50 μm. Inserts in panels A and B are 10 μm. ns, not significant. ∗P < .05.
Figure 13.
Luminex profiling results for cytokines not significantly different between WT and ΔdblGATA mice in the precancer timeline.
Discussion
While eosinophils are known effectors in allergic disease, new functions have been discovered in the pathobiology of cancer.31 We investigated the role of eosinophils in ESCC and discovered that activated eosinophils are present in human ESCC, to a greater degree in early-stage vs late-stage cancers. Because tissue-resident eosinophils normally are not present in the esophagus, eosinophils are being specifically recruited during tumorigenesis. Similar to human ESCC, we then showed that there are increased eosinophils in precancer as compared with cancer in the 4-NQO murine model of ESCC, suggesting that this model is relevant for studying eosinophil biology. We demonstrated that expression of Ccl11 by epithelial cells and at some time points fibroblasts increases during the precancer period when there are the most eosinophils, suggesting that CCL11 is one mechanism by which eosinophils are recruited in this model. We then demonstrated that reducing eosinophil number either pharmacologically through IL5mAb treatment or genetically with either eosinophil-deficient mice or Ccl11–/– mice leads to worse precancer and carcinoma, suggesting that eosinophils protect against the development of ESCC. Conversely, increased eosinophilia dramatically reduces the severity of precancer and cancer, notably invasive disease. RNA-seq of whole esophageal tissue and eosinophils revealed that precancer esophageal eosinophils have significantly reduced expression of several genes which regulate oxidative stress. Finally, our in vitro coculture experiments collectively demonstrate that degranulation of eosinophils leads to increased apoptosis of surrounding precancer and cancer cells and that the release of ROS is one mechanism by which eosinophils can induce apoptosis.
To the best of our knowledge, this study is the first to define the contribution of eosinophils in ESCC. Specifically, it is the first to illustrate that eosinophils are activated in tissues from ESCC patients from the United States and Japan using EPX IHC. It is also the first to demonstrate that treatment with intranasal rIL-5 can attenuate the number of tumors in the 4-NQO model, suggesting that this may be a future avenue for therapy in ESCC. Notably, there is one other recently published study that showed that mice treated with 4-NQO and ovalbumin, which induces a Th2 response that leads to increased eosinophilic infiltration, had reduced severity of ESCC as compared with mice treated with 4-NQO alone.32 We were the first to show the direct cytotoxic effect of ROS on precancer cells in a coculture modeling system, though ROS have been previously implicated as an antitumorigenic mechanism.33 It is also notable that in murine colon cancer activated eosinophils displayed an interferon signature,12 but RNA-seq from eosinophils from esophageal precancer showed significantly decreased Irf1 as compared with colonic eosinophils and bone marrow–derived eosinophils, suggesting that this may not be the mechanism of activation in murine esophageal squamous cancer. Additionally, the Luminex array showed that IFN-γ was trending toward being lower in Δdbl-GATA mice in the precancer model. Because esophageal precancer eosinophils had significantly upregulated Cd74, the receptor for MIF, a future direction is to more thoroughly investigate the role of MIF in ESCC development. This is one possible mechanism for how eosinophils are activated in esophageal squamous precancer, as MIF has been shown to activate eosinophils in allergic disease.34 Furthermore, based on the RNA-seq data, we hypothesize that antigen presentation by eosinophils may contribute to reduced disease burden observed in our study.
Finally, while eosinophils have previously been shown to suppress Th17 differentiation in the small intestine,35 we are the first to demonstrate that this may be occurring in the context of esophageal squamous malignancy. This is important because IL-17 has been shown to be protumorigenic in several mouse cancer models, including colon, lung, and skin, and inhibition of IL-17 has been shown to reduce metastasis.36 Additionally, the enrichment of the IL-6, EGF, and nuclear factor κB signaling in eosinophil-deficient mice, all protumorigenic pathways downstream of IL-17,36 is supporting evidence eosinophils suppressing IL-17 and protumorigenic effects. Our lab is currently further investigating the role between eosinophils and IL-17 in the 4-NQO model. In this vein, it is notable that numbers of CD8 T cells were not different in eosinophil-deficient mice or mice treated with rIL-5, as eosinophils have been shown to enhance CD8 T cells in melanoma.37 In summation, for cancers in which eosinophils may be protective, this study provides evidence that strategies should be developed to increase recruitment of eosinophils to the tumor microenvironment.
In addition to the aforementioned new findings, another strength of our study is the use of multiple mouse models to demonstrate that reduction of eosinophil number leads to exacerbated tumorigenesis. We utilized 3-dimensional coculturing methods to show the direct effect of eosinophils on precancer and cancer cells. Eosinophils have been shown to have direct cytotoxic effect in other contexts as well, including in mastocytoma cells (P815 cells),38 hepatocellular carcinoma cells (MH134 cells),39 fibrosarcoma,40 and melanoma (HBL and B16-F10 cells).41
With that being said, there are important limitations to consider as well. Notably, while CCL11 is an important chemoattractant for eosinophil recruitment, it also has been shown to have effects on epithelium.42 Thus, it is possible that the exacerbation of disease in Ccl11–/– mice may be partially related to epithelial changes as well. Additionally, in our coculture studies, murine eosinophils and EoL-1 cells have significant differences from human eosinophils. For example, neither makes all of the granules that human eosinophils do. Another limitation is that intranasal delivery of rIL-5 likely results in eosinophil-mediated lung damage. As such, one of our future directions is to test whether different delivery methods of rIL-5 can also attenuate tumorigenesis.
From a patient-oriented perspective, tumor-associated eosinophilia across all cancers has been associated with increased overall survival (though not disease-free survival),18 suggesting that the presence of eosinophils could be a biomarker for a favorable prognosis. Because we have observed a greater number of EPX positive cells in earlier stage ESCC, this may be the case for this cancer as well. However, many of these studies utilize H&E to quantify eosinophil number instead of IHC for eosinophil granule markers. Future studies should determine whether eosinophil number, perhaps by IHC for an eosinophil granule marker, predicts disease free survival in this cancer. Additionally, it is currently not known whether esophageal eosinophilia has a role as a biomarker or in the development and progression of Barrett’s esophagus to esophageal adenocarcinoma. It has been reported that eosinophils can be present in Barrett’s esophagus43 and that there can be increased esophageal eosinophilia after treatment of Barrett’s with radiofrequency ablation.44 Deconvolution from RNA-seq of Barrett’s and adenocarcinoma has revealed that eosinophils are significantly decreased in adenocarcinoma samples as compared with Barrett’s and dysplasia, a similar pattern to what we have observed in squamous cancer.45 Another study showed that the presence of eosinophils in esophageal adenocarcinoma was associated with less infiltrative disease, again similar to what we observed with squamous cancer.46 However, the function of eosinophils specifically has not been tested in adenocarcinoma modeling. This is also an important future direction which may have impact on patient care.
In conclusion, while eosinophils have context-dependent functions, we discovered that eosinophils are recruited to the early esophageal neoplastic environment at least in part by Ccl11 and protect from progression to ESCC. We determined that treatment with rIL-5, augmenting tissue eosinophilia, reduced tumorigenesis in an ESCC animal model. We showed that the mechanisms by which eosinophils are likely protective is through secretion of ROS and suppression of IL-17. Thus, therapies which either deliver more eosinophils to the esophagus or increase eosinophil recruitment to the esophagus should be further investigated in the treatment of ESCC.
Materials and Methods
ESCC Patient Samples
Our Japanese colleagues (M.N.) in Juntendo University constructed a tissue microarray (TMA) with paraffin embedded sections from 28 ESCC patients from the center of tumor, edge of the tumor, and tumor-adjacent normal tissue. Paraffin-embedded sections from 30 additional patients who underwent endoscopic submucosal dissection as curative therapy were also provided.
At Vanderbilt University Medical Center, a pathology database was queried for ESCC patients from 2012 to 2020. Biopsies and resections were reviewed for availability of blocks. Of these, 14 resections and 32 biopsies were identified. One TMA was constructed from the resections and a second from biopsies. All patients were categorized by AJCC T stage (eighth edition).
Esophageal squamous cancer paraffin blocks from either tissue microarrays or endoscopic resections were cut at 5 μm on positively charged slides. The slides were de-paraffinized in 3 changes of xylene and hydrated to water in a series of graded alcohols. The slides underwent antigen retrieval using a citrate buffer (pH 6.0) solution at 105°C in a pressure cooker for 20 minutes then cooled at room temperature for 10 minutes. The slides were washed in distilled water and placed in Tris-buffered saline with 0.1% Tween wash buffer solution prior to continuation of the staining protocol. Endogenous enzymes were blocked using a 0.03% (H202) peroxidase block solution for 5 minutes, rinsed in wash buffer, and then incubated for 1 hour in the primary antibody (1:1000 primary dilution, EPX Mayo Clinic, clone MM25.82.2.1). The slides were gently rinsed in wash buffer and a peroxidase labelled polymer was applied using Dako EnVision + System-Horseradish Peroxidase labeled polymer (Agilent, Santa Clara, CA) for 30 minutes. After a gentle rinse again in wash buffer the slides were treated with a DAB+ substrate-chromogen for 5 minutes to complete the staining protocol. The slides were washed in distilled water, counterstained in Mayer’s hematoxylin, blued in running tap water, and dehydrated in 3 changes of absolute alcohol and cleared in xylene prior to cover slipping. Slides were digitally scanned by the Vanderbilt Digital Histology Shared Resource core, which uses an Aperio AT2 and Leica SCN400 Slide Scanner for high-resolution brightfield scanning. All slides were uploaded into QuPath47 to analyze whole-slide images.
To calculate the percentage of total cells which were EPX positive, automated cell detection for both total cells and EPX positive cells was utilized in QuPath to account for differences in size of TMA cores and endoscopic submucosal dissection specimens. In all TMAs, each patient had 2–4 cores per patient, and EPX positive cells/total cells were averaged for all cores.
Mice
All experiments were begun with 8- to 12-week-old C57BL/6J mice. These mice were maintained in controlled conditions with a consistent diet and kept in normal light/dark cycle, temperature, and humidity. Ccl11–/– mice were obtained from L.C. and have been previously described.42 CCL11 is knocked out in the whole mouse. The breeding strategy for these mice was Ccl11+/– x Ccl11+/–. ΔdblGATA mice were obtained from the Jackson Laboratory (strain #005653; Bar Harbor, ME). The breeding strategy for these mice was XWTY (WT males) x XMutXWT (heterozygote females) and XMutY (knockout males) crossed with XMutXWT (heterozygote females) to generate WT and knockout mice from the same parents. WT mice were obtained from the Jackson Laboratory.
4-NQO Treatment
Mice were given 4-NQO in 2 different timelines. To model precancer, mice were given 4-NQO for 8 weeks followed by propylene glycol vehicle for 8 weeks.22, 48, 49 For carcinoma modeling, mice were given 4-NQO for 16 weeks and then propylene glycol vehicle for 12 weeks.20, 50, 51
4-NQO (Acros, Antwerp, Belgium; 203792500) was dissolved in propylene glycol (Thermo Fisher Scientific; P355-4) vehicle as a stock solution at a dosage of 50 mg/mL. A new stock solution was prepared weekly and stored at 4°C. The 4-NQO stock solution was then further diluted in drinking water of mice at a dosage of 100 μg/mL, which is the same dose reported in several other publications.48,52,53 Water with 4-NQO was replaced weekly. Mice were continuously allowed access to drinking water.
In the experiment in which mice in the carcinoma protocol were compared with those in the precancer timeline, all 3 experimental groups were coordinated to begin and end at the same time. The 8 week 4-NQO treatment group was given only vehicle for the first 12 weeks of the experiment, then received 4-NQO treatment for 8 weeks, and then given only vehicle for the last 8 weeks of the experiment. The 16-week 4-NQO treatment group was given 4-NQO for 16 weeks and then given only vehicle for the last 12 weeks of the experiment.
For all experiments, each cage contained WT and knockout mice in order to decrease the cage effect. In IL5mAb and rIL-5 experiments, experimental and control treatments were equally distributed among mice in the same cage to decrease the impact of cage effects. For all experiments, the bedding was mixed among all cages with experimental mice monthly. Allocation and conduct of each experiment (ie, weighing mice weekly, changing bedding) in each experiment was done by either Z.A. or A.K., who were blinded to genotype.
After sacrifice, the esophagus was removed from all mice, the inside of the esophagus washed with phosphate-buffered saline (PBS) with a gavage needle and splayed longitudinally. Tumors in the esophagus were counted under a dissecting microscope. Counting was done by an investigator who was blinded to genotype (M.A.B., Z.A., or Y.A.C.). Then, the esophagus was rolled with the distal esophagus in the center and the proximal esophagus on the outside of the roll. Then, the Swiss roll was fixed in 10% buffered formalin for 24 hours, transferred to 70% ethanol overnight, and then embedded in paraffin. 5 μm cross-sections of each sample were cut and stained with H&E for histologic analysis. Paraffin embedding, sectioning, and staining was performed by the Vanderbilt Tissue Pathology Shared Resource (TPSR). For the Ccl11–/– carcinoma experiment, the esophagus was fixed after being splayed longitudinally and sections were cut along the longitudinal axis. Additionally, as 4-NQO can cause oral tumors, the tongue was examined in all experiments and the number of tongue tumors was recorded.
Histology Scoring
Histology scoring was performed in accordance with previously published guidelines.27 Slides were digitally scanned by the Vanderbilt Digital Histology Shared Resource core. All slides were uploaded into QuPath, a free open-source software, to analyze whole-slide images. Briefly, 4 parameters which are most commonly observed in mice treated with 4-NQO were used to determine severity of histology score: rete pegs, nuclear histologic abnormalities, papillomas, and invasive carcinoma. Rete pegs and nuclear histologic abnormalities are the most common features in mice treated with 4-NQO for 8 weeks (ie, during precancer), while papillomas and invasive carcinoma are more common in mice treated with 4-NQO for 16 weeks (ie, during cancer).
Nuclear histologic abnormality is defined by the presence of abnormal cells in the epithelium. Rete pegs are epithelial projections that penetrate the connective tissue underneath the epithelium but do not invade beyond the basement membrane. Papillomas are mature acanthotic squamous epithelium arranged along branched fibrovascular cores with an exophytic architectural pattern. Papillomas have increased severity as compared with nuclear histologic abnormality and rete pegs, as they can have invasive disease into the lamina propria within them, though they do not always. Moreover, they are visible grossly and thus can be used as a marker of severity without examining the esophagus microscopically. Invasive disease is marked by neoplastic epithelium that has penetrated the basement membrane and extended into the submucosa.
Severity and degree of each parameter was determined using QuPath. These parameters were scored by an expert gastrointestinal pathologist who was blinded to mouse genotypes (M.K.W.). In the Results, we report both the total histology score and highlight the invasion parameter of the total score (labeled in the graphs as invasion score), as it represents the most severe feature of disease.
MBP IHC
Slides were placed on the Leica Bond Max IHC stainer (Leica, Wetzlar, Germany). All steps besides dehydration, clearing and coverslipping were performed on the Bond Max. Slides were deparaffinized. Enzymatic induced antigen retrieval was performed on the Bond Max using Proteinase K (Dako) for 5 minutes. Slides were incubated with anti-MBP (1:1000; Lee Laboratory, Mayo Clinic, Rochester, MN) for 1 hour and then incubated in a rabbit anti-rat secondary antibody (1:2000, BA-4001; Vector Laboratories, Newark, CA) for 15mins. The Bond Polymer Refine detection system was used for visualization. Slides were then dehydrated, cleared, and coverslipped. This IHC was performed by our Vanderbilt TPSR.
MBP quantification was done in QuPath by highlighting the region of interest and using the positive cell detection feature with a consistent threshold. In the first experiment, in which precancer eosinophilia was compared with eosinophilia in papillomas and invasive carcinoma, the region of interest was defined by presence of precancer features (ie, rete pegs or nuclear histologic abnormality) vs presence of papilloma or invasive carcinoma. This was then standardized to the length of that region of interest, as measured using the polyline tool. For experiments with IL5mAb, ΔdblGATA, Ccl11–/–, or rIL-5, the major purpose of MBP quantification was to illustrate that there were lesser or greater eosinophilic infiltration. Thus, we standardized the MBP quantification to the entire esophagus length, as sections of Swiss rolls can vary slightly.
IL-5mAb Treatment
In the precancer protocol, WT mice were treated with 4-NQO for the first 8 weeks of the experiment. For the second 8 weeks, half the mice were given a 0.1 mg IL5mAb weekly (TRFK5; BioXCell, Lebanon, NH) and the other half given 0.1 mg IgG1 isotype control (BioXCell) weekly by intraperitoneal injection. This dose reduces intestinal eosinophils.12
Recombinant IL-5 Treatment
In the precancer protocol, WT mice were treated with 4-NQO for the first 8 weeks of the experiment. For the second 8 weeks, half the mice were given rIL-5 (R&D Systems, Minneapolis, MN) 100 ng weekly intranasally and the other half given PBS vehicle control. This dose was similar to what has been previously published to induce lung eosinophilia.54 In the carcinoma protocol, the same dose was given for the final 12 weeks of the experiment.
sc-RNAseq Analysis
The sc-RNAseq data analyzed in this study are available from Yao et al29 through the Genome Sequence Archive (Genome Sequence Archive in BIG Data Center, Beijing Institute of Genomics, Chinese Academy of Sciences; http://gsa.big.ac.cn) under the accession number CRA002118. Visualization of target gene expression in each cell type was accomplished with Seurat v4.0.55 A time-ordered single-cell transcriptomic profiling was conducted on 6 esophageal lesions. Mice were sacrificed before (week 0), during (week 12), and after treatment (weeks 20, 22, 24, or 26), and were categorized into 6 stages: NOR, INF, HYP, DYS, CIS, and ICA. These data include CD45+ immune cells (29975 [cells] by 14283 [genes]) and CD45– nonimmune cells (36114 [cells] by 15596 [genes)]. Immune cells were categorized into T cells, B cells, myeloid cells, and natural killer cells, and nonimmune cells were categorized into epithelial cells, fibroblasts, endothelial cells, and myocytes.
The Wilcoxon rank sum test was used to compare the target gene (ie, Ccl11) expression between HYP and NOR, between HYP and INF, between HYP and DYS, between HYP and CIS, and between HYP and ICA in each cell type. Multiplicity was controlled with the Bonferroni method, and adjusted P values ≤.05 were used for statistical significance. All statistical analyses were conducted using R version 4.1 (R Foundation for Statistical Computing, Vienna, Austria).
https://github.com/∼ Script is available upon request.
Eosinophil and Tissue RNA-seq and Analysis
Acquisition of Lamina Propria Cells
Cells from the colonic lamina propria were acquired as previously described56 with the following modifications to enhance viability of eosinophils: (1) 10% fetal bovine serum (FBS) instead of 5% FBS was used in incubation steps that include serum; (2) the digestion media consisted of 10 mL Hank’s Balanced Salt Solution with calcium and magnesium instead of RPMI 1640; (3) the digestion media contained 1.5 mg/mL collagenase and no Liberase; (4) the concentration of DNase was increased to 0.10% DNase I (Sigma-Aldrich, St Louis, MO; D50250); and (5) the maximum centrifugation speed was reduced to 370 g, except for the density gradient (475 g). For cells from the esophageal lamina propria, the esophagus was flushed with PBS, opened longitudinally, and cut into 4 equal-sized pieces. The esophageal tissue was then subjected to the same protocol as the colonic lamina propria with the following modifications to increase eosinophil yield: (1) the initial volume of incubation with DTT was reduced to 5 mL; and (2) the volume of digestion media was reduced to 10 mL and the collagenase concentration was 1.0 mg/mL.
Selection of Lamina Propria Eosinophils
Eosinophils were acquired using positive immunomagnetic selection with the SiglecF+ selection kit (Miltenyi Biotec, Gaithersburg, MD) according to the manufacturers’ instruction with the following modifications: (1) the buffer was supplemented to contain a final concentration of 5 mM EDTA and 0.05% DNase was added; and (2) the working volume was 15 μL buffer with 5 μL of beads. Flow cytometry was used to confirm purity of eosinophils.
RNA Isolation
Extraction of RNA from whole tissue was performed on tissue stored in RNA-later (Sigma-Aldrich) until homogenization using a Tissue-Tearor (Dremel, Racine, WI), followed by phenol/chloroform extraction as described57 and cleanup with the RNeasy Mini Kit (Qiagen, Hilden, Germany) with on-column DNase digestion according to manufacturers’ instruction. For eosinophil RNA-seq, cells were processed using the Ovation RNA-seq System V2 (Tecan, Mannëdorf, Switzerland) by the Vanderbilt Technologies for Advanced Genomics core.
RNA Sequencing
Quality control for RNA was performed by the Vanderbilt Technologies for Advanced Genomics core using RNA 6000 Pico (Agilent). For eosinophil RNA-seq, the complementary DNA library was prepared using a NEB library preparation kit. Paired-end 150-bp sequencing was performed on a NovaSeq 6000 (Illumina, San Diego, CA).
RNA-Seq Analysis
For tissue bulk RNA-seq, analysis was performed as previously described.56 For eosinophil RNA-seq, limma (version 3.50.3) was used for differential expression analysis. The tissue of origin for eosinophils (ie, esophagus, colon, or bone marrow) was used as metadata in the linear model. The cage or which mouse the cells originated from did not measurably affect gene expression as assessed by consensus correlation coefficient (not shown).
Differential Expression Analysis
For differential expression analysis, limma was used on log-CPM transformed counts with prior count set to 3 or DESeq2 (version 1.34.0) was used on non-normalized counts. Unless otherwise indicated, genes with an adjusted P value <.05 (–log10 of 0.05 is 1.3) and log2 fold change >|1| were considered differentially regulated. For heatmaps with gene counts, a z score (ie, the number of standard deviations above or below the mean) of normalized counts was calculated using scale function in R. IPA was used for pathway assessment. Genes with an adjusted P value of <.05 were used as input. Annotation was done with AnnotationDbi (version 1.56.2) using org.Mm.eg.db (version 3.14.0).
Images were generated with pheatmap (version 1.0.12), complexheatmap, RColorBrewer (version 1.1-3), ggplot2 (version 3.3.5), and EnhancedVolcano (version 1.12.0).
Bone Marrow–Derived Eosinophils
Bone marrow eosinophils were isolated and differentiated using a previously published protocol.30 Briefly, bone marrow cells were recovered from the tibias and femurs of WT C57BL/6J mice by spinning individual bones within a cutoff 200 μL pipette tip placed in a 1.5 mL Eppendorf at 10,000 g for 10 minutes at room temperature in fluorescence-activated cell sorting buffer (PBS with 2% FBS and 2 mM EDTA) on day 0. Red blood cell lysis was done twice with ACK lysis buffer (Thermo Fisher Scientific). Cells were plated at 1 × 106 cells/mL in complete RPMI1640 supplemented with 105 ng/mL mouse FLT3 (BioLegend, San Diego, CA) and 100 ng/mL mouse SCF (BioLegend). Cells were washed and re-plated on day 4, 8, 10, and 12 at the original volume in complete RPMI supplemented with 10 ng/mL recombinant mouse IL-5. Only on day 4 were cells were added back to the original plate. On other days, new plates were used. Cells were acquired on day 14 and the percentage of eosinophils was determined by flow cytometry with mouse CD4+ splenocytes used as negative control.
Eosinophil Degranulation EPX Activity Assay
Eosinophil degranulation was assessed in vitro in technical triplicates as previously described58 with modifications. Briefly, eosinophils were mycoplasma free as assessed by a Mycoplasma detection kit (Applied Biological Materials, Vancouver, Canada). Eosinophils were resuspended in eosinophil degranulation media (RPMI 1640 without phenol red) and plated in an untreated flat-bottom tissue culture plate. After resting at 37°C for 1 hour, secretagogues prepared in eosinophil degranulation media were added followed by a 30-minute incubation at 5% CO2 at 37°C to degranulate eosinophils. Then, one volume of freshly prepared OPD buffer (0.5 mg/mL OPD [Thermo Fisher Scientific] in 0.05 M citric acid, 0.05 M sodium phosphate with 0.09% H2O2 [v/v] [Sigma-Aldrich]) was added. Following incubation for 2–8 minutes, the reaction was stopped with 1 volume of 1M H2SO4. The plate was read at 490 nM with a GloMax Discover Microplate Reader (Promega, Madison, WI). EPX activity values were corrected for with a media only control (blank). As a positive control, 100% EPX activity was defined by degranulation after treatment with CHAPS detergent (Thermo Fisher Scientific). EPX activity was measured after treatment of eosinophils with 35 μM platelet activating factor (Lyso-PAF C16; Cayman Chemicals, Ann Arbor, MI; 60906) and 1 mM NAC (Sigma-Aldrich) in combination with Lyso-PAF.
Coculture of Eosinophils With Precancer Organoids
To derive mouse precancer organoids, a WT mouse was treated with 4-NQO for 8 weeks followed by propylene glycol vehicle for 8 weeks. At that time, the mouse was sacrificed and esophagus removed. The esophagus was placed in 1 mg/mL dispase II (Roche) in a thermomixer at 37°C for 10 minutes. Under a dissecting scope, the epithelium was peeled off the submucosa and placed in 1 mL 0.25% trypsin-EDTA. This was incubated again in a thermomixer at 37°C for 10 minutes. The sample was vortexed for 10 seconds. The trypsin-EDTA was then removed and placed in 10 mL of soybean trypsin inhibitor (1 mg/mL, dissolved in Hank’s Balanced Salt Solution; Thermo Fisher Scientific). The remaining tissue was then incubated for a second time in 1 mL fresh 0.25% trypsin-EDTA in a thermomixer at 37°C for 10 minutes. Again, the sample was vortexed for 10 seconds and then transferred to the 10 mL of soybean trypsin inhibitor. The sample (including media and tissue) was then filtered through a 40-μM strainer and rinsed with PBS. The filtered liquid was then centrifuged for 5 minutes at 1000 rpm and resuspended in organoid media. After counting, cells were again centrifuged at 1000 rpm and suspended in Matrigel (Corning, Corning, NY; 356231) at 3000 cells per 20 μL plug. After Matrigel polymerized at 37°C for 20 minutes, organoid media was placed on top of Matrigel plug. Organoid media consisted of Advanced DMEM/F12 (12634010; Gibco, Billings, MT), 1X B-27 Supplement (17504044; Gibco), 1X GlutaMAX (35050061; Gibco), 1X N-2 Supplement (17502048; Gibco), 1 mM HEPES (15630080; Gibco), and 2% (v/v) penicillin/streptomycin (15140122; Gibco) supplemented with 20% (v/v) R-spondin-conditioned media (from R-spondin–expressing cells gifted by Dr Jeff Whitsett, University of Cincinnati, Cincinnati, OH) and 10% (v/v) Noggin-conditioned media (from Noggin-expressing cells gifted by Dr. G.R. van den Brink, Amsterdam, the Netherlands), EGF (500 μg/mL), and NAC (0.5 M). For initial plating and at the time of splitting organoids, Y27632 (10 μM; Thermo Fisher Scientific) was also added.
For coculture with bone marrow–derived eosinophils, precancer organoids were collected on day 3 after plating. Cells were incubated in trypsin-EDTA 0.25% with Y27632 10 μM and DNase (Sigma-Aldrich; 1:200) for 1 hour in order to achieve a single-cell suspension. Then, 150,000 cells were plated on 0.4-μm transwells (Corning; 3413). After 3 days, organoid media was removed from the apical side in order to create an ALI. After another 3 days, 500,000 eosinophils were placed into the basolateral compartment of the transwell with or without Lyso-PAF 35μM or NAC 1 mM. After 24 hours, transwells were collected, fixed in 10% neutral buffered formalin for 24 hours, switched to 70% ethanol, and submitted to Vanderbilt TPSR for processing, embedding, and cutting.
Coculture of Eol-1 Cells With TE-11 Cells
Eol-1 cells (Sigma-Aldrich; 94042252) were maintained as undifferentiated in RPMI media supplemented with 10% FBS and 1% penicillin/streptomycin. Prior to coculture, Eol-1 cells were differentiated in RPMI media with the previous supplements and 500 μM sodium butyrate for 6 days in order to increase the percentage of mature eosinophils.
Meanwhile, TE-11 cells, a generous gift from Anil Rustgi, were also cultured in RPMI supplemented with 10% FBS and 1% penicillin/streptomycin. A total of 75,000 TE-11 cells were plated per transwell for each experiment. Cells were grown to confluence on a transwell for 5 days, with apical and basolateral media changes every other day. Media were then removed from the apical side in order to create ALI and the cells were allowed to differentiate for 5 days, changing the media in the basolateral compartment every other day. After 10 days, 500,000 differentiated Eol-1 cells were placed into basolateral compartment for coculture with TE-11 cells in ALI. Eol-1 cells were cocultured with TE-11 cells with or without 10 ng/mL rIL-5. After 24 hours, the transwell membranes were cut out, fixed in 10% neutral buffered formalin for 24 hours, switched to 70% ethanol, and submitted to Vanderbilt TPSR for processing, embedding, and cutting.
Flow Cytometry
For cell surface staining, cells were incubated in antibody cocktail for 20 minutes at 4°C in the dark. Samples were blocked using 30 μL normal rat serum (StemCell Technologies, Vancouver, Canada). Flow cytometric analysis was performed using a 4-Laser Fortessa or 5-laser LSRII (BD, San Jose, CA) with FACSDiva software (BD). Analyses were performed using FlowJo 10.9.0 (BD). For all flow experiments, a live/dead stain (Thermo Fisher Scientific) was used to only assess live cells. Antibodies used for flow cytometry are listed in Table 1.
Table 1.
List of Fluorescence-Activated Cell Sorting Antibodies
| Antigen Label | Manufacturer | Catalog Number |
|---|---|---|
| CD45-BV785 | BioLegend | 103149 |
| Siglec-F-BV421 | BD | 562681 |
| CD11b-PerCP/Cy5.5 | eBioscience | 45-0112 |
| CCR3-FITC | R&D Systems | FAB729F |
Immunofluorescent Staining
Fluorescent IHC on paraffin-embedded sections was performed as previously described.59 Slides were deparaffinized using CitriSolv (Thermo Fisher Scientific), rehydrated by graded alcohols, and permeabilized using TBS with 0.05% Tween 20. Antigen retrieval consisted of 10-minute boiling in 10 mM pH 6 sodium citrate. After blocking, samples were incubated with the following primary antibodies overnight at 4°C: anti-cleaved caspase 3 (Cell Signaling Technology, Danvers, MA; 1:200), anti-β-catenin (BD; 1610153, 1:500), or anti-EPX Clone MM25.82.2.1 (Mayo Clinic; 1:250). Secondary antibodies were conjugated to either 488 or 594 Alexa Fluor dyes (1:500; Thermo Fisher Scientific) and incubated for 2 hours at room temperature. Nuclear staining was performed using ProLong Gold antifade reagent with DAPI (Thermo Fisher Scientific). Fluorescent-stained slides were visualized on a Keyence BZ-X810 upright microscope. CC3-positive cells were quantified per high-power field using a 40× objective, but images shown in Figure 6 were taken using a 60× oil immersion lens to highlight the important features of the image. A total of 8–12 high-power fields were averaged per mouse, and data are represented as high-power field per mouse.
Luminex Multiplex Array
Luminex Multiplex Array was performed as previously described.59 Esophageal tissues were harvested at the time of sacrifice and homogenized using a handheld pestle-type rotary homogenizer in CelLytic MT lysis extraction reagent from Sigma-Aldrich. The 17 analyte Thermo Fisher Scientific Mouse Cytokine Th1/Th2/Th9/Th17/Th22/T regulatory 17-Plex Mouse ProctaPlex Panel was performed according to the manufacturer’s instructions and analyzed on a FLEXMAP 3D instrument (Luminex, Austin, TX). Tissue protein concentrations, as assessed by the bicinchoninic acid protein assay kit (Pierce, Rockford, IL) were used to standardize the analyte data.
Statistics
Statistical analysis outside of RNA-seq and sc-RNAseq (see previous) was performed in GraphPad Prism (version 9.5.0; GraphPad, San Diego, CA). For 2 groups, the Mann-Whitney nonparametric test was used. For more than 2 groups, Kruskal-Wallis was used. Survival curve analysis was done using the log-rank (Mantel-Cox) test. Data are presented as mean ± SEM, except in analysis of human data, in which they are presented as median with interquartile range. For all experiments, the ROUT (Q = 1%) test was performed to exclude outliers. P < .05 was considered statistically significant.
Study Approval
Human studies were approved by the Vanderbilt University Medical Center Institutional Review Board (192400) and the Hospital Ethics Committee of Juntendo University Hospital (E21-060). All mouse experiments were approved by Vanderbilt University Medical Center Institute for Animal Care and Use Committee (V1900036-01) and Tennessee Valley Health System. All authors had access to the study data and have reviewed and approved the final manuscript.
Acknowledgments
TE-11 cells were kindly provided by Anil Rustgi. The Vanderbilt Flow Cytometry Shared Resource, Translational Pathology Shared Resource, and Division of Animal Care core facilities all supported this work. The authors acknowledge the Tissue Pathology Shared Resource (supported by National Cancer Institute/National Institutes of Health Cancer Center Support Grant 5P30CA68485-19) for assistance with immunohistochemistry (Shared Instrumentation Grant S10 OD023475-01A1). The authors thank the Digital Histology Shared Resource for assistance with data storage and processing. The authors thank Richard M. Peek, Keith T. Wilson, Ariel Munitz, Elizabeth Jacobsen, and Marc Rothenberg for their critical advice in constructing the manuscript. Tissue and eosinophil RNA sequencing from mice have been deposited in Gene Expression Omnibus under the accession numbers GSE233448 and GSE233443. Further details on how to access these files is in the supplement. Supporting analytic code can be made available upon request. A previous version of this article was posted to bioRxiv: https://doi.org/10.1101/2023.06.01.543287.
CRediT Authorship Contributions
Justin Jacobse (Conceptualization: Supporting; Data curation: Equal; Formal analysis: Equal; Methodology: Equal; Writing – original draft: Supporting; Writing – review & editing: Supporting)
Zaryab Aziz (Data curation: Supporting; Formal analysis: Supporting; Writing – review & editing: Supporting)
Lili Sun (Data curation: Supporting; Formal analysis: Supporting; Resources: Supporting; Writing – original draft: Supporting; Writing – review & editing: Supporting)
Jasmine Chaparro (Data curation: Supporting; Formal analysis: Supporting)
Jennifer Pilat (Data curation: Supporting; Formal analysis: Supporting; Writing – review & editing: Supporting)
Aaron Kwag (Data curation: Supporting; Formal analysis: Supporting)
Matthew Buendia (Data curation: Supporting; Writing – review & editing: Supporting)
Mae Wimbiscus (Data curation: Supporting)
Motomi Nasu (Data curation: Equal; Formal analysis: Supporting; Writing – review & editing: Supporting)
Tsuyoshi Saito (Data curation: Supporting)
Shinji Mine (Data curation: Supporting)
Hajime Orita (Data curation: Supporting)
Frank Revetta (Data curation: Supporting; Formal analysis: Supporting; Methodology: Supporting)
Sarah Short (Data curation: Supporting; Formal analysis: Supporting; Methodology: Supporting; Writing – review & editing: Supporting)
Mary Kay Washington (Data curation: Supporting; Formal analysis: Supporting; Supervision: Supporting; Writing – review & editing: Supporting)
Girish Hiremath (Formal analysis: Supporting; Writing – review & editing: Supporting)
Michael Gibson (Data curation: Supporting; Resources: Supporting; Writing – review & editing: Supporting)
Lori Coburn (Data curation: Supporting; Formal analysis: Supporting; Writing – review & editing: Supporting)
Tatsuki Koyama (Formal analysis: Supporting; Methodology: Supporting; Writing – review & editing: Supporting)
Jeremy Goettel (Formal analysis: Supporting; Methodology: Supporting; Writing – review & editing: Supporting)
Christopher Williams (Conceptualization: Supporting; Data curation: Supporting; Formal analysis: Supporting; Methodology: Supporting; Supervision: Supporting; Writing – review & editing: Supporting)
Yash Choksi (Data curation: Lead; Formal analysis: Equal; Funding acquisition: Lead; Investigation: Lead; Supervision: Lead; Writing – original draft: Lead; Writing – review & editing: Lead)
Footnotes
Conflicts of Interest The authors disclose no conflicts.
Funding This work was supported by a VA Career Development Award (IK2BX004648), ACS-Institutional Research Grant (#IRG-19-139-60), DDRC Pilot and Feasibility Grant ApplicationP30 058404, the Vanderbilt Burroughs Welcome Supporting Careers in Research for Interventional Physicians and Surgeons, 5T32DK007673 (to Yash A. Choksi), Veterans Affairs Merit Review grants I01BX004366 (to Lori Coburn) and I01BX001426 (to Christopher S. Williams), the National Institute of Diabetes and Digestive and Kidney Diseases (R03DK123489 to Jeremy A. Goettel, K01DK123495 to Sarah P. Short, and 1K23DK13141 to Girish Hiremath), the Japan Society for the Promotion of Science Grant-in-Aid for Scientific Research (KAKENHI) (22K08878 to Motomi Nasu), and Subsidies for Current Expenditures to Private Institutions of Higher Education from the Promotion and Mutual Aid Corporation for Private Schools of Japan (to Motomi Nasu). Tissue morphology Core Services were performed through Vanderbilt University Medical Center’s Digestive Disease Research Center (P30DK058404 to M. Kay Washington). It was supported by a VICC SPORE in GI cancer grant (P50CA236733 to M. Kay Washington).
Supplementary Material
References
- 1.Morgan E., Soerjomataram I., Rumgay H., et al. The global landscape of esophageal squamous cell carcinoma and esophageal adenocarcinoma incidence and mortality in 2020 and projections to 2040: new estimates from GLOBOCAN 2020. Gastroenterology. 2022;163:649–658.e2. doi: 10.1053/j.gastro.2022.05.054. [DOI] [PubMed] [Google Scholar]
- 2.Rustgi A.K., El-Serag H.B. Esophageal carcinoma. N Engl J Med. 2014;371:2499–2509. doi: 10.1056/NEJMra1314530. [DOI] [PubMed] [Google Scholar]
- 3.Siegel R.L., Miller K.D., Jemal A. Cancer statistics. CA Cancer J Clin 2018. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 4.van Hagen P., Hulshof M.C., van Lanschot J.J., et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074–2084. doi: 10.1056/NEJMoa1112088. [DOI] [PubMed] [Google Scholar]
- 5.Shapiro J., van Lanschot JJB, Hulshof M., et al. Neoadjuvant chemoradiotherapy plus surgery vs surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. 2015;16:1090–1098. doi: 10.1016/S1470-2045(15)00040-6. [DOI] [PubMed] [Google Scholar]
- 6.Jacobs M., Macefield R.C., Elbers R.G., et al. Meta-analysis shows clinically relevant and long-lasting deterioration in health-related quality of life after esophageal cancer surgery. Qual Life Res. 2014;23:1155–1176. doi: 10.1007/s11136-013-0576-5. [DOI] [PubMed] [Google Scholar]
- 7.Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 8.Pardoll D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murdoch C., Muthana M., Coffelt S.B., et al. The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer. 2008;8:618–631. doi: 10.1038/nrc2444. [DOI] [PubMed] [Google Scholar]
- 10.Noy R., Pollard J.W. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41:49–61. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grisaru-Tal S., Itan M., Klion A.D., et al. A new dawn for eosinophils in the tumour microenvironment. Nat Rev Cancer. 2020;20:594–607. doi: 10.1038/s41568-020-0283-9. [DOI] [PubMed] [Google Scholar]
- 12.Reichman H., Itan M., Rozenberg P., et al. Activated eosinophils exert antitumorigenic activities in colorectal cancer. Cancer Immunol Res. 2019;7:388–400. doi: 10.1158/2326-6066.CIR-18-0494. [DOI] [PubMed] [Google Scholar]
- 13.Spiegel G.W., Ashraf M., Brooks J.J. Eosinophils as a marker for invasion in cervical squamous neoplastic lesions. Int J Gynecol Pathol. 2002;21:117–124. doi: 10.1097/00004347-200204000-00003. [DOI] [PubMed] [Google Scholar]
- 14.van Driel W.J., Hogendoorn P.C., Jansen F.W., et al. Tumor-associated eosinophilic infiltrate of cervical cancer is indicative for a less effective immune response. Hum Pathol. 1996;27:904–911. doi: 10.1016/s0046-8177(96)90216-6. [DOI] [PubMed] [Google Scholar]
- 15.von Wasielewski R., Seth S., Franklin J., et al. Tissue eosinophilia correlates strongly with poor prognosis in nodular sclerosing Hodgkin's disease, allowing for known prognostic factors. Blood. 2000;95:1207–1213. [PubMed] [Google Scholar]
- 16.Enblad G., Sundstrom C., Glimelius B. Infiltration of eosinophils in Hodgkin's disease involved lymph nodes predicts prognosis. Hematol Oncol. 1993;11:187–193. doi: 10.1002/hon.2900110404. [DOI] [PubMed] [Google Scholar]
- 17.Bishara S., Griffin M., Cargill A., et al. Pre-treatment white blood cell subtypes as prognostic indicators in ovarian cancer. Eur J Obstet Gynecol Reprod Biol. 2008;138:71–75. doi: 10.1016/j.ejogrb.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 18.Hu G., Wang S., Zhong K., et al. Tumor-associated tissue eosinophilia predicts favorable clinical outcome in solid tumors: a meta-analysis. BMC Cancer. 2020;20:454. doi: 10.1186/s12885-020-06966-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ishibashi S., Ohashi Y., Suzuki T., et al. Tumor-associated tissue eosinophilia in human esophageal squamous cell carcinoma. Anticancer Res. 2006;26:1419–1424. [PubMed] [Google Scholar]
- 20.Zhang Y., Ren H., Wang L., et al. Clinical impact of tumor-infiltrating inflammatory cells in primary small cell esophageal carcinoma. Int J Mol Sci. 2014;15:9718–9734. doi: 10.3390/ijms15069718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wright B.L., Doyle A.D., Shim K.P., et al. Image analysis of eosinophil peroxidase immunohistochemistry for diagnosis of eosinophilic esophagitis. Dig Dis Sci. 2021;66:775–783. doi: 10.1007/s10620-020-06230-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang Z., Guan B., Men T., et al. Comparable molecular alterations in 4-nitroquinoline 1-oxide-induced oral and esophageal cancer in mice and in human esophageal cancer, associated with poor prognosis of patients. In Vivo. 2013;27:473–484. [PMC free article] [PubMed] [Google Scholar]
- 23.Panigrahi G.B., Walker I.G. The N2-guanine adduct but not the C8-guanine or N6-adenine adducts formed by 4-nitroquinoline 1-oxide blocks the 3'-5' exonuclease action of T4 DNA polymerase. Biochemistry. 1990;29:2122–2126. doi: 10.1021/bi00460a023. [DOI] [PubMed] [Google Scholar]
- 24.Islami F., Kamangar F., Nasrollahzadeh D., et al. Socio-economic status and oesophageal cancer: results from a population-based case-control study in a high-risk area. Int J Epidemiol. 2009;38:978–988. doi: 10.1093/ije/dyp195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomas D.C., Husain I., Chaney S.G., et al. Sequence effect on incision by (A)BC excinuclease of 4NQO adducts and UV photoproducts. Nucleic Acids Res. 1991;19:365–370. doi: 10.1093/nar/19.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Venkat J.A., Shami S., Davis K., et al. Relative genotoxic activities of pesticides evaluated by a modified SOS microplate assay. Environ Mol Mutagen. 1995;25:67–76. doi: 10.1002/em.2850250110. [DOI] [PubMed] [Google Scholar]
- 27.Aziz Z., Washington M.K., Jacobse J., et al. A method for scoring 4-nitroquinoline 1-oxide-induced murine esophageal squamous neoplasia. Vet Pathol. 2023;60:384–393. doi: 10.1177/03009858231151381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Forbes E., Murase T., Yang M., et al. Immunopathogenesis of experimental ulcerative colitis is mediated by eosinophil peroxidase. J Immunol. 2004;172:5664–5675. doi: 10.4049/jimmunol.172.9.5664. [DOI] [PubMed] [Google Scholar]
- 29.Yao J., Cui Q., Fan W., et al. Single-cell transcriptomic analysis in a mouse model deciphers cell transition states in the multistep development of esophageal cancer. Nat Commun. 2020;11:3715. doi: 10.1038/s41467-020-17492-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dyer K.D., Moser J.M., Czapiga M., et al. Functionally competent eosinophils differentiated ex vivo in high purity from normal mouse bone marrow. J Immunol. 2008;181:4004–4009. doi: 10.4049/jimmunol.181.6.4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosenberg H.F., Dyer K.D., Foster P.S. Eosinophils: changing perspectives in health and disease. Nat Rev Immunol. 2013;13:9–22. doi: 10.1038/nri3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fuller A.D., Karami A.L., Kabir M.F., et al. Eosinophilic esophagitis-associated epithelial remodeling may limit esophageal carcinogenesis. Front Allergy. 2023;4 doi: 10.3389/falgy.2023.1086032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheng J.N., Luo W., Sun C., et al. Radiation-induced eosinophils improve cytotoxic T lymphocyte recruitment and response to immunotherapy. Sci Adv. 2021;7 doi: 10.1126/sciadv.abc7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bozza M.T., Lintomen L., Kitoko J.Z., et al. The role of MIF on eosinophil biology and eosinophilic inflammation. Clin Rev Allergy Immunol. 2020;58:15–24. doi: 10.1007/s12016-019-08726-z. [DOI] [PubMed] [Google Scholar]
- 35.Sugawara R., Lee E.J., Jang M.S., et al. Small intestinal eosinophils regulate Th17 cells by producing IL-1 receptor antagonist. J Exp Med. 2016;213:555–567. doi: 10.1084/jem.20141388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao J., Chen X., Herjan T., Li X. The role of interleukin-17 in tumor development and progression. J Exp Med. 2020;217 doi: 10.1084/jem.20190297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carretero R., Sektioglu I.M., Garbi N., et al. Eosinophils orchestrate cancer rejection by normalizing tumor vessels and enhancing infiltration of CD8(+) T cells. Nat Immunol. 2015;16:609–617. doi: 10.1038/ni.3159. [DOI] [PubMed] [Google Scholar]
- 38.Munitz A., Bachelet I., Fraenkel S., et al. 2B4 (CD244) is expressed and functional on human eosinophils. J Immunol. 2005;174:110–118. doi: 10.4049/jimmunol.174.1.110. [DOI] [PubMed] [Google Scholar]
- 39.Kataoka S., Konishi Y., Nishio Y., et al. Antitumor activity of eosinophils activated by IL-5 and eotaxin against hepatocellular carcinoma. DNA Cell Biol. 2004;23:549–560. doi: 10.1089/dna.2004.23.549. [DOI] [PubMed] [Google Scholar]
- 40.Lucarini V., Ziccheddu G., Macchia I., et al. IL-33 restricts tumor growth and inhibits pulmonary metastasis in melanoma-bearing mice through eosinophils. Oncoimmunology. 2017;6 doi: 10.1080/2162402X.2017.1317420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Simson L., Ellyard J.I., Dent L.A., et al. Regulation of carcinogenesis by IL-5 and CCL11: a potential role for eosinophils in tumor immune surveillance. J Immunol. 2007;178:4222–4229. doi: 10.4049/jimmunol.178.7.4222. [DOI] [PubMed] [Google Scholar]
- 42.Polosukhina D., Singh K., Asim M., et al. CCL11 exacerbates colitis and inflammation-associated colon tumorigenesis. Oncogene. 2021;40:6540–6546. doi: 10.1038/s41388-021-02046-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ravi K., Katzka D.A., Smyrk T.C., et al. Prevalence of esophageal eosinophils in patients with Barrett's esophagus. Am J Gastroenterol. 2011;106:851–857. doi: 10.1038/ajg.2011.7. [DOI] [PubMed] [Google Scholar]
- 44.Villa N., El-Serag H.B., Younes M., et al. Esophageal eosinophilia after radiofrequency ablation for Barrett's esophagus. Dis Esophagus. 2013;26:674–677. doi: 10.1111/dote.12033. [DOI] [PubMed] [Google Scholar]
- 45.Lagisetty K.H., McEwen D.P., Nancarrow D.J., et al. Immune determinants of Barrett's progression to esophageal adenocarcinoma. JCI Insight. 2021;6 doi: 10.1172/jci.insight.143888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dos Santos Cunha A.C., Simon A.G., Zander T., et al. Dissecting the inflammatory tumor microenvironment of esophageal adenocarcinoma: mast cells and natural killer cells are favorable prognostic factors and associated with less extensive disease. J Cancer Res Clin Oncol. 2023;149:6917–6929. doi: 10.1007/s00432-023-04650-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bankhead P., Loughrey M.B., Fernández J.A., et al. QuPath: open source software for digital pathology image analysis. Sci Rep. 2017;7 doi: 10.1038/s41598-017-17204-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hasina R., Mollberg N., Kawada I., et al. Critical role for the receptor tyrosine kinase EPHB4 in esophageal cancers. Cancer Res. 2013;73:184–194. doi: 10.1158/0008-5472.CAN-12-0915. [DOI] [PubMed] [Google Scholar]
- 49.Miyamoto S., Yasui Y., Kim M., et al. A novel rasH2 mouse carcinogenesis model that is highly susceptible to 4-NQO-induced tongue and esophageal carcinogenesis is useful for preclinical chemoprevention studies. Carcinogenesis. 2008;29:418–426. doi: 10.1093/carcin/bgm225. [DOI] [PubMed] [Google Scholar]
- 50.Matrka M.C., Cimperman K.A., Haas S.R., et al. Dek overexpression in murine epithelia increases overt esophageal squamous cell carcinoma incidence. PLoS Genet. 2018;14 doi: 10.1371/journal.pgen.1007227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tang X.H., Knudsen B., Bemis D., et al. Oral cavity and esophageal carcinogenesis modeled in carcinogen-treated mice. Clin Cancer Res. 2004;10:301–313. doi: 10.1158/1078-0432.ccr-0999-3. [DOI] [PubMed] [Google Scholar]
- 52.Li S.H., Lu H.I., Chang A.Y., et al. Angiotensin II type I receptor (AT1R) is an independent prognosticator of esophageal squamous cell carcinoma and promotes cells proliferation via mTOR activation. Oncotarget. 2016;7:67150–67165. doi: 10.18632/oncotarget.11567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lin J., Fu D., Dai Y., et al. Mcl-1 inhibitor suppresses tumor growth of esophageal squamous cell carcinoma in a mouse model. Oncotarget. 2017;8:114457–114462. doi: 10.18632/oncotarget.18772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chiba Y., Srisodsai A., Supavilai P., et al. Interleukin-5 reduces the expression of uteroglobin-related protein (UGRP) 1 gene in allergic airway inflammation. Immunol Lett. 2005;97:123–129. doi: 10.1016/j.imlet.2004.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hao Y., Hao S., Andersen-Nissen E., et al. Integrated analysis of multimodal single-cell data. Cell. 2021;184:3573–3587.e29. doi: 10.1016/j.cell.2021.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jacobse J., Brown R.E., Li J., et al. Interleukin-23 receptor signaling impairs the stability and function of colonic regulatory T cells. Cell Rep. 2023;42 doi: 10.1016/j.celrep.2023.112128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Genzel L., Rossato J.I., Jacobse J., et al. The yin and yang of memory consolidation: hippocampal and neocortical. PLoS Biol. 2017;15 doi: 10.1371/journal.pbio.2000531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ochkur S.I., Jacobsen E.A., Lacy P. Eosinophil shape change and secretion. Methods Mol Biol. 2021;2241:199–219. doi: 10.1007/978-1-0716-1095-4_17. [DOI] [PubMed] [Google Scholar]
- 59.Choksi Y.A., Reddy V.K., Singh K., et al. BVES is required for maintenance of colonic epithelial integrity in experimental colitis by modifying intestinal permeability. Mucosal Immunol. 2018;11:1363–1374. doi: 10.1038/s41385-018-0043-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.