Abstract
Species interactions can influence species distributions, but mechanisms mitigating competition or facilitating positive interactions between ecologically similar species are often poorly understood. Aardwolves (Proteles cristata) and aardvarks (Orycteropus afer) are nocturnal, insectivorous mammals that co‐occur in eastern and southern Africa, and knowledge of these species is largely limited to their nutritional biology. We used aardwolf and aardvark detections from 105 remote cameras during 2016–2018 to assess their spatial and temporal niche overlap in the grasslands of Serengeti National Park, Tanzania. Using a multispecies occupancy model, we identified a positive interaction between occupancy probabilities for aardwolves and aardvarks. Slope, proportion of grassland and termite mound density did not affect the occupancy probabilities of either species. The probability of aardwolf, but not aardvark, occupancy increased with distance to permanent water sources, which may relate to predation risk avoidance. Diel activity overlap between aardwolves and aardvarks was high during wet and dry seasons, with both species being largely nocturnal. Aardwolves and aardvarks have an important ecological role as termite consumers, and aardvarks are suggested to be ecosystem engineers. Our results contribute to a better understanding of the spatial and temporal niche of insectivores like aardwolves and aardvarks, suggesting high spatial and temporal niche overlap in which commensalism occurs, whereby aardwolves benefit from aardvark presence through increased food accessibility.
Keywords: camera trap, commensalism, occupancy model, Orycteropus afer, Proteles cristata, spatiotemporal niche
We investigated the spatial and temporal niche of two insectivorous mammals, aardwolves (Proteles cristata) and aardvarks (Orycteropus afer) and quantified niche interactions between them in the grasslands of Serengeti National Park, Tanzania. We provide evidence of spatial and temporal niche overlap between aardwolves and aardvarks.
1. INTRODUCTION
Species distributions are determined by environmental conditions and interspecific interactions (Wiens, 2011). Ecological niche overlap between sympatric species can cause competition, resulting in reduced abundance or exclusion of weaker competitors (Creel & Creel, 1996; Miquelle et al., 2005). Competition can be avoided through niche partitioning (Schoener, 1974) which facilitates species diversity (Chesson, 2000; Levine & HilleRisLambers, 2009). Alternatively, sympatric species with high niche overlap may interact non‐competitively. Two species can benefit from a symbiotic interaction through mutualism, while commensalism occurs when one species benefits and the other is unaffected (Mathis & Bronstein, 2020). Understanding species interactions can help conserve ecological communities because they influence species distributions (HilleRisLambers et al., 2012; Wisz et al., 2013) alongside other factors such as anthropogenic landscape change (Broennimann et al., 2012).
Behavioural and morphological similarities between species can cause niche overlap (Brown & Wilson, 1956; Dayan & Simberloff, 2005), with behavioural niche overlap occurring along the axes of space, time and diet (Schoener, 1974). Dietary niche overlap can be mediated through dietary differentiation, which can facilitate coexistence when species specialize in different foods (Emrich et al., 2014; Ferretti et al., 2020; Kartzinel et al., 2015). Symbiotic relationships inherently require spatial niche overlap between species, while competing species can reduce the frequency and intensity of overlap through spatial partitioning (Rodriguez Curras et al., 2022; Sollmann et al., 2012). Similarly, symbiotic relationships may require temporal niche overlap, while competing species may display temporal niche partitioning through differences in foraging time, frequency or effort (Dröge et al., 2017; Kronfeld‐Schor & Dayan, 2003).
Niche overlap can vary spatiotemporally (Wiens, 1989), for example, when dietary niche overlap varies with temporal (Porter et al., 2022) or spatial (Hasui et al., 2009) differences in food availability, or when species display decreased spatial overlap during the reproductive season (McConnell et al., 2008). For example, arctic foxes (Vulpes lagopus) may avoid competition with red foxes (V. vulpes) during the reproductive season by denning at higher elevations (Tannerfeldt et al., 2002). Risk avoidance can also influence the extent of niche overlap between species, such as when avoidance of human disturbance increases spatial and temporal niche overlap between interacting species (Sévêque et al., 2020). Bobcats (Lynx rufus) and pumas (Puma concolor) had increased overlap in activity patterns in areas with more human disturbance (Lewis et al., 2015). Similarly, predator avoidance of sympatric species can influence niche overlap as refuge from predation is shared or partitioned in space and time (Holt, 1984; Sommers & Chesson, 2019).
Aardwolves (Proteles cristata) and aardvarks (Orycteropus afer) are nocturnal mammals co‐occurring in eastern and southern Africa (Kingdon, 2015). Aardvarks have a diverse diet of termites and ants that varies geographically and seasonally (Taylor, 2013), while aardwolves depend on grass‐harvesting termites of the genus Trinervitermes throughout their range (De Vries et al., 2011; Kruuk & Sands, 1972). Different degrees of dietary specialism may limit spatial niche overlap of aardwolves and aardvarks, as Trinervitermes occur only in open habitats such as grasslands (Anderson, 2013; Kruuk & Sands, 1972), while aardvark habitat use is more diverse (Kingdon, 2015; Melton, 1976). Termite activity may be higher on slopes, attracting termite predators, so spatial overlap of aardwolves and aardvarks may increase with slope (Freymann et al., 2010; Sarcinelli et al., 2009). Areas with high large carnivore activity could increase spatial overlap between aardwolves and aardvarks in other areas as they have common predators (Anderson, 2013; Mills, 1984; Taylor, 2013), and aardvarks might avoid areas near water to avoid large carnivores (Epps et al., 2021). A commensal relationship has been suggested whereby aardwolves increase spatial overlap with aardvarks during seasons of lower food availability, to benefit from increased termite availability at locations where aardvarks excavate termite mounds (Taylor & Skinner, 2000). In the Serengeti grasslands, there is lower food availability for aardwolves during the wet season (Kruuk & Sands, 1972), which may coincide with a switch in primary prey of aardvarks from ants to termites (Melton, 1976). Additionally, seasonal variability in termite nocturnality may increase diurnal activity of aardwolves (Richardson, 1987) and aardvarks (Weyer et al., 2020), and there are no reported indications of temporal niche partitioning related to their diel activity.
We used remote camera data to quantify spatial overlap (occupancy) and temporal overlap (diel activity) of aardvarks and aardwolves in southeastern Serengeti National Park, Tanzania. We predicted a positive correlation between the occupancy probabilities of the two species. We predicted that occupancy for both species would be positively associated with termite mound density and that for aardwolves only, this effect would be stronger in the wet season. Furthermore, we predicted occupancy probability for aardvarks and aardwolves would be positively associated with increasing slope and distance to water, and with proportional grass cover (as a proxy for grassland habitat) for aardwolves only. We predicted a positive correlation between the occupancy probabilities of the two species and similar diel activity patterns whereby both species would display strong nocturnality.
2. METHODOLOGY
2.1. Study area
We conducted the study in a 1533 km2 area of southeastern Serengeti National Park, Tanzania (Figure 1), which consisted primarily of grassland (89%), shrubland (7%) and woodland (2%) (Buchorn et al., 2020). Elevations are 1484–1859 m above sea level, with higher elevations in the eastern half of the study area (NASA et al., 2018). The climate is warm with stable temperatures averaging around 21°C (Metzger et al., 2015), and most precipitation occurs during November–May (Norton‐Griffiths et al., 1975). Serengeti National Park has several large carnivores that prey on aardwolves and aardvarks, including spotted hyena (Crocuta crocuta), lion (Panthera leo), leopard (P. pardus) and cheetah (Acinonyx jubatus) (Anderson, 2013; Craft et al., 2015; Taylor, 2013).
FIGURE 1.
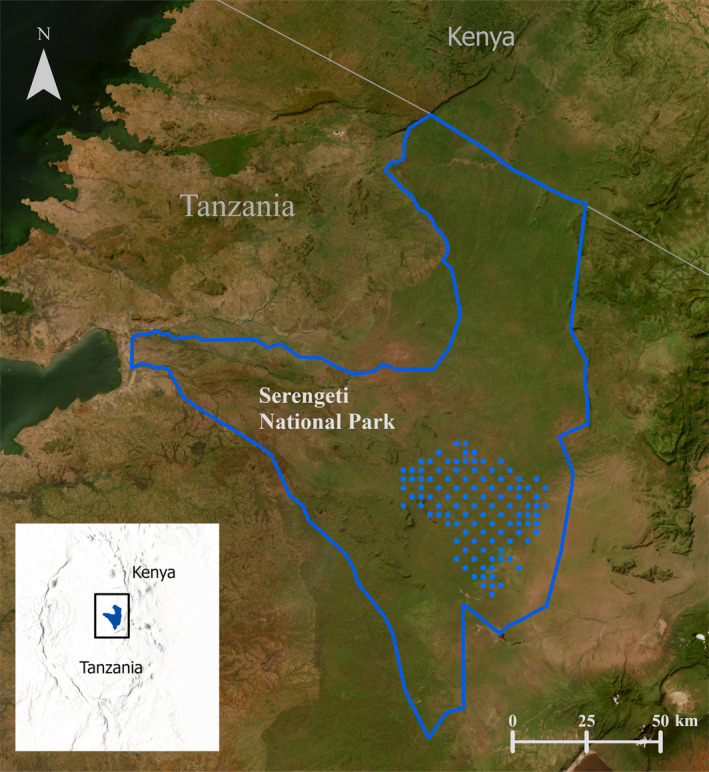
Camera locations (blue circles) to assess occurrences of aardvark (Orycteropus after) and aardwolf (Proteles cristata), Serengeti National Park (blue outline), Tanzania, 8 September 2016–30 June 2018. Map was created using the 2022 World Imagery Basemap in ESRI ArcGIS Pro Version 3.0.
2.2. Data collection and processing
We collected data from August 2016 to June 2018 using 105 remote cameras (Stealth Cam, model N45NG; Irving, Texas, USA). Nearest distance between cameras was 3000 m for 63 cameras and 4225 m for 43 cameras (Figure 1). We attached cameras to metal stakes 50‐cm above ground and cleared vegetation in front of cameras every 6 weeks, with cameras programmed to record 3‐image bursts at each detection with a 30‐s delay. Because of staggered camera installations, we extracted data for a period of 70 consecutive days from each camera (hereafter a ‘camera‐period’) from 26 August 2016 to 1 January 2018, and a second 70‐day camera‐period for 77 cameras from 22 January 2017 to 30 June 2018. Overlap between the two periods of data collection occurred due to staggered camera installation. Most (95%) data were obtained from September 2016 to February 2018. Each 70‐day camera‐period was associated with a season (wet season, November–May; dry season, June–October). For 77 camera‐periods that overlapped two seasons we split the data by season into two separate, shorter camera‐periods each entirely with one season.
2.3. Covariates
We collected environmental covariates in a 50‐m radius around each camera. We used proportional grass cover from the 2017 Copernicus Global Land Cover dataset (range = 0–100, 100‐m resolution; Buchorn et al., 2020) as a proxy for habitat type, with lower proportional grass cover implying higher proportional shrubland or woodland. We derived the slope from the ASTER Global Digital Elevation Model v3 (30‐m resolution; NASA et al., 2018). We derived the distance from each camera to the nearest water feature using the Serengeti GIS and Data Centre (30‐m resolution; Maliti et al., 2008). We used only year‐round water sources for consistency between seasons (Schooler et al., 2022). We counted active termite mounds based on termite presence and signs of recent activity in a 50‐m radius around each camera. We proportionally averaged covariate values where multiple values occurred within a 50‐m radius. We scaled covariates before analysis by subtracting the mean from each value and dividing this by the standard deviation to facilitate comparison of parameter estimates. We used Pearson's correlations to test for multicollinearity (|r| > 0.7), retaining the covariate from pairwise correlations considered most ecologically relevant (Dormann et al., 2013).
2.4. Occupancy models
We used single‐season multispecies occupancy models to estimate the effect of environmental covariates on the occupancy of each species (MacKenzie et al., 2002) and to determine interactions in occupancy probability between the two species (Rota et al., 2016). To allow for covariate effects that vary between wet and dry seasons, we treated camera‐periods (defined above; data from a given camera during a given time period of ≤70 consecutive days, separated by wet and dry season) as separate sites for the purposes of the occupancy model (a ‘stacking’ approach; Kéry & Royle, 2020). Preliminary analysis showed a high proportion of non‐detections led to numerical estimation and optimization problems (Steenweg et al., 2016; Tobler et al., 2015), so we pooled data points into 5‐day detection periods whereby every 5 days of data collection were grouped into one sampling occasion (i.e. ≤ 70 daily sampling occasions became ≤14 5‐day sampling occasions).
Our occupancy models assumed occupancy was spatially and temporally constant for a site at a site throughout a ≤70‐day sampling period (Rota et al., 2009). We considered this reasonable, as aardwolves and aardvarks occupy small territories (1–4 km2) relative to the spacing between cameras (3–4 km) in our study area, with little to no territory overlap between conspecifics (Anderson, 2013; Bothma & Walker, 1999; Taylor, 2013; Van Aarde et al., 1992). Furthermore, aardwolves and aardvarks are considered long‐lived species, based on lifespans in captivity exceeding 20 years (Anderson, 2013; Taylor, 2013).
We defined 10 candidate occupancy models based on our predictions which were implemented in R (v4.2.2, R Core team, 2022) using the unmarked package (Fiske & Chandler, 2011; Kellner et al., 2023). Models included covariates potentially affecting single‐species occupancy probabilities or species interaction: distance to water, proportional grass cover, termite mound density, slope and interactions with seasonality (Table 1). We included the number of trees present at camera sites as a detection covariate in all models. We included no other detection covariates because we cleared vegetation around cameras every 6 weeks, oriented cameras to avoid obstructed views and did not orient cameras toward roads, so we expected no other influences on detectability. A covariate was considered to have a significant effect if the 95% confidence interval did not overlap zero. We ranked models using Akaike's Information Criterion (AIC; Arnold, 2010) and selected a final model from candidate models based on the lowest AIC, or the competing model (ΔAIC <2) with fewer terms (Burnham et al., 2011). We also tested the out‐of‐sample predictive performance of the candidate models with k‐fold cross‐validation using an approach similar to Broms et al. (2016). For each model, we divided the data into k = 10 folds, then re‐fit the candidate models 10 times. For each re‐fit of k, one fold was held out as testing data and the remaining nine were used as training data. We then calculated the total log‐likelihood ll k of the held‐out testing data in fold k. Finally, we calculated the total model deviance as . We assessed goodness‐of‐fit for our top model with a parametric bootstrap (MacKenzie & Bailey, 2004). Using the parboot function in the unmarked package, we simulated 1000 datasets from the model and calculated the sum of squared errors (SSE) for each dataset. If the SSE from the real dataset fell within the distribution of SSEs from simulated datasets, we concluded the model fit the data reasonably well.
TABLE 1.
Multispecies occupancy models for aardwolf (Proteles cristata) and aardvark (Orycteropus after), Serengeti National Park, Tanzania, 26 August 2016–30 June 2018.
| Model | K | AIC | ΔAIC | Deviance |
|---|---|---|---|---|
| Predator avoidance | ||||
| Single species: Water | 9 | 2153.54 | 0 | 2159.53 |
| Species interaction: No covariates | ||||
| Species detection: Trees | ||||
| Predator avoidance (inc. species interaction) | ||||
| Single species: Water | 10 | 2155.30 | 1.76 | 2180.89 |
| Species interaction: Water | ||||
| Species detection: Trees | ||||
| Habitat type | ||||
| Single species: Grass | 9 | 2158.80 | 5.26 | 2172.29 |
| Species interaction: No covariates | ||||
| Species detection: Trees | ||||
| Habitat type (inc. species interaction) | ||||
| Single species: Grass | 10 | 2160.66 | 7.13 | 2163.69 |
| Species interaction: Grass species detection: Trees | ||||
| Termite availability | ||||
| Single species: Termite + Slope | 11 | 2165.96 | 12.43 | 2173.32 |
| Species interaction: No covariates | ||||
| Species detection: Trees | ||||
| Global | ||||
| Single species: Water + Slope + Grass + Termite + (Season: Termite) + (Season: Slope) | 21 | 2167.50 | 13.97 | 2179.02 |
| Species interaction: No covariates | ||||
| Species detection: Trees | ||||
| Null | ||||
| Single species: No covariates | 5 | 2167.52 | 13.99 | 2176.34 |
| Species interaction: No covariates | ||||
| Species detection: No covariates | ||||
| Termite availability (inc. species interaction) | ||||
| Single species: Termite + Slope | 13 | 2168.95 | 15.42 | 2169.19 |
| Species interaction: Termite + Slope | ||||
| Species detection: Trees | ||||
| Seasonal termite availability | ||||
| Single species: (Season + Termite) + (Season + Slope) | 15 | 2173.23 | 19.69 | 2187.49 |
| Species interaction: No covariates | ||||
| Species detection: Trees | ||||
| Seasonal termite availability (inc. species interaction) | ||||
| Single species: (Season + Termite) + (Season + Slope) | 16 | 2174.91 | 21.37 | 2221.92 |
| Species interaction: Season | ||||
| Species detection: Trees | ||||
Note: Models included effects of distance to water (Water), proportional grass cover (Grass), termite mound density (Termite), slope (Slope) and interactions with season (Season), on first‐ and second‐order occupancy. Number of trees (Trees) present was used as a detection covariate.
Abbreviations: AIC, Akaike's Information Criterion; Deviance, total model deviance based on k‐fold cross‐validation; K, number of parameters; ΔAIC, AIC difference from the top model.
2.5. Diel activity
We assessed temporal niche partitioning in aardwolves and aardvarks by estimating overlap in diel activity using the package overlap (v0.3.433, Meredith & Ridout, 2018) in R. We created seasonal models to assess differences in diel activity and overlap between aardwolves and aardvarks, using a coefficient of overlap based on probability density functions. We filtered observations to a maximum of one hourly observation per species for each camera to avoid data clustering (Clauss et al., 2021). We used the estimator Δ4, appropriate when the least‐detected species has >75 observations and calculated 95% confidence intervals of overlap estimates by bootstrapping 1000 generated samples (Meredith & Ridout, 2018). We concluded differences in overlap estimates of diel activity between aardwolves and aardvarks when confidence intervals did not overlap.
3. RESULTS
Using 105 camera locations totaling 12,505 camera days. Aardwolves and aardvarks were detected on 264 and 121 days, respectively. Across the study duration, aardwolves were detected at 71 camera locations (naïve occupancy = 0.68), aardvarks at 35 locations (naïve occupancy = 0.33) and 25 locations had at least one detection of both species. Of a maximum of 14 sampling occasions per camera‐period, the range of sampling occasions across sites was 10–14 (median = 10). Proportional grass cover across locations was 44–80% (median = 63%), distance to water was 25–13,108 m (median = 1842 m), slope was 1.2–27.5% (median = 4.2%) and the number of active termite mounds was 0–12 (median = 1); 65 of 105 camera locations had at least one mound.
3.1. Occupancy models
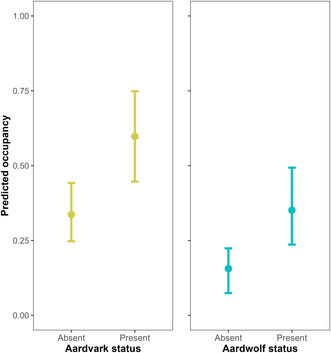
No covariates were omitted from the analysis due to multicollinearity (all |r| ≤ 0.55). Occupancy for aardwolves was positively related to distance to water (CI = 0.222–0.984; Figure 2), but we found no effect of slope, proportional grass cover or termite mound density on aardwolf occupancy probability (Table 2). For aardvarks, no covariates had a significant effect on occupancy probability (Table 1). There were no differences in covariate relationships between seasons for either species. Our top‐ranked model indicated a positive interaction between aardwolf and aardvark occupancy probabilities (CI = 0.227–1.936; Table 2), suggesting a positive relationship between aardwolf occupancy probability and aardvark presence, and between aardvark occupancy probability and aardwolf presence (Figure 3). We found no effect of covariates on the interaction of aardwolf and aardvark occupancy probabilities. The number of trees at camera sites negatively influenced the detection of aardwolves only (CI = −0.868– −0.075, Table 2), though this did not notably affect relationships between our covariates and species occupancy or the interaction between species occupancies. Our top model fits the data reasonably well based on the parametric bootstrap. Deviance estimates based on k‐fold cross‐validation corresponded closely with AIC scores (Table 1).
FIGURE 2.
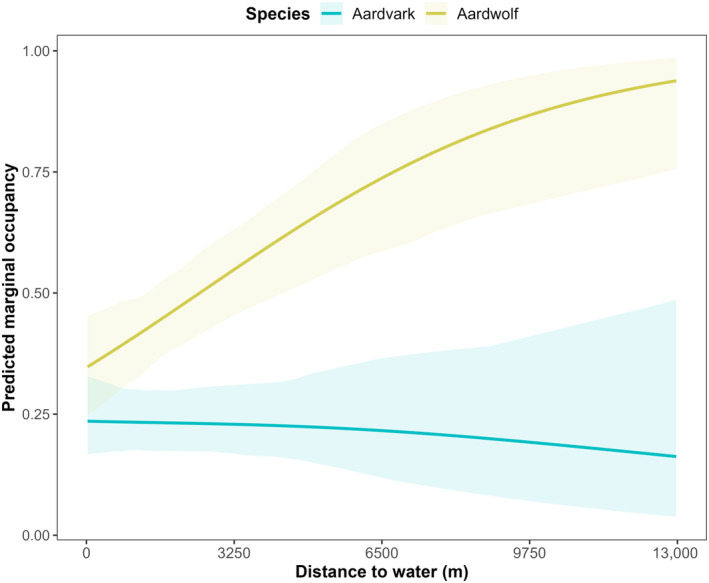
Predicted occupancy probabilities for aardvark (Orycteropus afer) and aardwolf (Proteles cristata) in relation to distance to permanent water, Serengeti National Park, Tanzania, 26 August 2016–30 June 2018. Estimates and 95% confidence intervals were derived from the top‐ranked multispecies occupancy model.
TABLE 2.
Parameter estimates, standard errors and 95% confidence intervals (CI) for the top‐ranked multispecies occupancy model for aardwolf (Proteles cristata) and aardvark (Orycteropus after), Serengeti National Park, Tanzania, 26 August 2016–30 June 2018.
| Parameter | Estimate | SE | CI |
|---|---|---|---|
| Aardwolf occupancy | |||
| Intercept | −0.273 | 0.195 | −0.698 to 0.052 |
| Distance to water | 0.563 | 0.192 | 0.222 to 0.984 |
| Aardvark occupancy | |||
| Intercept | −1.809 | 0.334 | −2.477 to −1.203 |
| Distance to water | −0.168 | 0.180 | −0.506 to 0.197 |
| Aardwolf‐Aardvark occupancy interaction | |||
| Intercept | 1.074 | 0.445 | 0.227 to 1.936 |
| Aardwolf detection | |||
| Intercept | −1.640 | 0.098 | −1.831 to −1.448 |
| Number of trees | −0.471 | 0.202 | −0.868 to −0.075 |
| Aardvark detection | |||
| Intercept | −1.718 | 0.146 | −2.004 to 1.433 |
| Number of trees | −0.394 | 0.273 | −0.929 to 0.142 |
Note: The top‐ranked model included distance to water on first‐order occupancy and a second‐order species interaction term. The intercept for the interaction term had a positive, non‐overlapping confidence interval with zero (0.227–1.936), indicating a significant relationship between aardwolf and aardvark occupancy.
FIGURE 3.
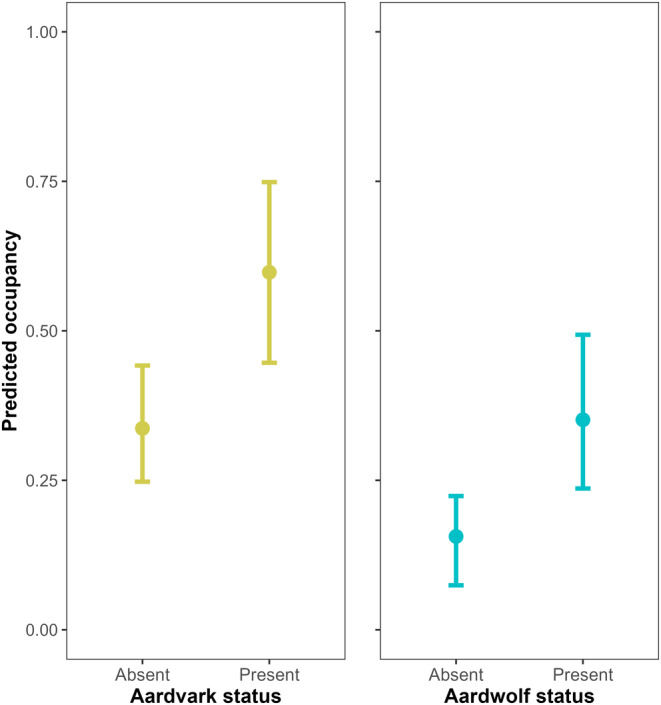
Occupancy probabilities for aardwolf (Proteles cristata) based on aardvark (Orycteropus afer) presence (left panel), and occupancy probabilities for aardvark based on aardwolf presence (right panel), Serengeti National Park, Tanzania, 26 August 2016–30 June 2018. Estimates and 95% confidence intervals were derived from the top‐ranked multispecies occupancy model.
3.2. Diel activity
Overlap in diel activity between aardwolves and aardvarks was high (Δ4 = 0.85, 95% CI = 0.77–0.93), and similar between seasons (wet: Δ4 = 0.87, 95%, CI = 0.80–0.93; dry: Δ4 = 0.83, 95% CI = 0.74–0.93) (Figure 4). Large overlaps in confidence intervals between seasonal models suggested year‐round consistency in diel activity overlap of aardwolves and aardvarks. The greatest overlap in diel activity occurred at night (18:00–6:00), with aardvark activity more frequent than aardwolf activity during 22:00–3:00. There appeared to be limited increases in diurnal activity during the dry season for both species, whereby aardwolves displayed increased morning activity (6:00–12:00), and aardvarks displayed increased afternoon activity (12:00–18:00).
FIGURE 4.
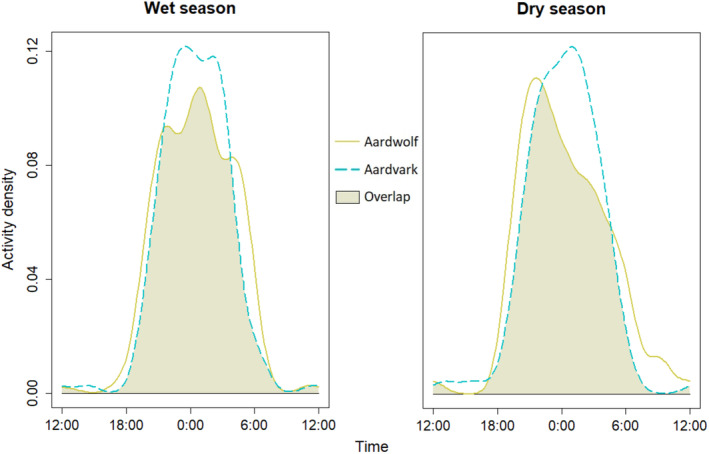
Density estimates of seasonal (wet = June–October, dry = November–May) diel activity of aardwolf (Proteles cristata) and aardvark (Orycteropus after), Serengeti National Park, Tanzania, 26 August 2016–30 June 2018.
4. DISCUSSION
We quantified the spatial and temporal overlap of aardvarks and aardwolves in southeastern Serengeti National Park, Tanzania. Results from the multispecies occupancy model supported our prediction of a positive relationship between the occupancy probabilities of aardwolves and aardvarks. Contrary to our predictions, we did not find evidence of spatial niche segregation through differential habitat use, or spatial niche overlap through similar responses to prey availability or predator avoidance. We found instead a positive relationship between occupancy probability and distance to water, a proxy for predator avoidance, for aardwolves only. We found no relationships between the occupancy probability of aardvarks and any of our covariates. Our prediction of high, year‐round overlap in the diel activity of aardwolves and aardvarks was supported.
Previous observations of aardwolves using burrows dug by aardvarks (Anderson, 2013) and of aardwolves feeding alongside aardvarks when the latter excavates termite mounds (Taylor & Skinner, 2000) corroborate our evidence for spatial niche overlap between these species. Aardwolf occurrence is restricted to open habitats because of their dependence on grass‐harvesting Trinervitermes termites (Anderson, 2013; Kruuk & Sands, 1972), while aardvarks occur in more diverse habitats, presumably due to their wider dietary niche including wood‐harvesting termites such as Macrotermes and Odontotermes (Taylor & Skinner, 2004). In the largely homogenous Serengeti grasslands, these genera of wood‐harvesting termites have adapted to harvest grass (Freymann et al., 2010), potentially increasing dietary niche overlap and by extension, spatial niche overlap between aardwolves and aardvarks.
We found high, year‐round temporal niche overlap in the diel activity of aardwolves and aardvarks, likely due to the nocturnality of their prey and thermoregulatory advantages of being active at night (Anderson, 2004; Weyer et al., 2020). Aardwolves and aardvarks in South Africa increased diurnal activity during winter when nocturnal prey is less active (Richardson, 1987; Taylor & Skinner, 2003), and during droughts (Rey et al., 2017; Weyer et al., 2020). Our analysis suggests limited increases in diurnal activity for both species during the dry season, potentially related to seasonal differences in prey activity (Materu et al., 2013). Year‐round stable temperatures in the Serengeti ecosystem (Metzger et al., 2015) may facilitate consistent foraging opportunities for aardwolves and aardvarks, in contrast to South Africa where aardwolves and aardvarks strongly increase diurnal activity during winter when termite activity was low (Rey et al., 2017; Richardson, 1987; Taylor & Skinner, 2003).
Overall, we found evidence for spatial and temporal niche overlap for aardwolves and aardvarks, congruent with research suggesting co‐occurrence of mammalian insectivores with similar diets is often driven by spatial and temporal activity patterns (Davis et al., 2018). Co‐occurring species that rely on the same, limited food sources may compete for access to these resources in space and time (Hardin, 1960), but aardwolves and aardvarks are myrmecophagous and may benefit from high food availability (Taylor & Skinner, 2004) reducing the potential for competition. High prey abundance could explain the prevalence of inter‐and intraspecific associative feeding, rather than competition for food, between myrmecophagous birds and mammals (e.g. Stenkewitz & Kamler, 2008; Taylor, 2013; Taylor & Skinner, 2000). Alternatively, subtle differences between species may explain coexistence without direct competition (Wiens, 1977). Aardwolves are morphologically adapted to consume termites from the surface (Anderson, 2013; Williams et al., 1997), whereas aardvarks have sharp claws to excavate mounds and an extensible tongue to extract prey from mound tunnels (Taylor et al., 2002). Aardwolves and aardvarks may avoid competition through fine‐scale spatial niche partitioning whereby aardvarks specialize on prey within mounds, and aardwolves on prey surrounding mounds. This idea is supported by reports of commensalism whereby aardwolves consume termites exiting mounds when excavated by aardvarks (Taylor & Skinner, 2003).
We found a positive relationship between aardwolf occupancy probabilities and distance from water, which could relate to spatial avoidance of large carnivores. Aardwolves and aardvarks largely do not rely on surface water and obtain water through prey consumption (Anderson, 2004; Taylor & Skinner, 2004), so it is unlikely their response to water source proximity is linked to physiological needs. Many herbivores depend on surface water, thus large carnivores preying on herbivores often hunt in close proximity to water (Constant et al., 2015; De Boer et al., 2010; Tagwireyi et al., 2020). Aardvarks in South Africa avoided areas close to water, potentially to avoid predation (Epps et al., 2021). Little is known about aardwolves and aardvarks as prey species, but they have few defences against large carnivores (Anderson, 2013; Taylor, 2013). Aardvarks could benefit from the many burrows they excavate that provide refuge from predators (Melton, 1976; Taylor & Skinner, 2003) while aardwolves may have only one or a few dens to use as refuge (Richardson, 1985). Aardwolves therefore may have fewer escape options from predators (Bothma & Walker, 1999), resulting in stronger spatial avoidance of large carnivores. However, further research is needed to definitively link proximity to water with predation risk for these species.
We note several limitations to our study. The model covariates we selected were based on studies from South Africa and Uganda, but the extent to which the ecological niche of aardwolves and aardvarks differs across their distribution is unknown. Our study area is characterized by limited variability in seasonal temperature differences, slope and proportional grass cover, which may be why no relation between these covariates and occupancy probabilities of aardwolves or aardvarks was found. Additionally, it is unknown whether termite mound density and slope on a 50‐m radius are accurate year‐round estimators of prey availability, particularly for aardvarks which elsewhere rely primarily on ants (Taylor et al., 2002; Willis et al., 1992). Similarly, our distance to water layer was imperfect due to consistent and complete datasets on ephemeral water sources being unavailable (Rich et al., 2017). Finally, our occupancy model assumed an individual present at a site occupied this site throughout each 70‐day period, which may not be the case due to mortality or individuals altering their space use, though we consider this unlikely due to the territoriality and longevity of aardwolves and aardvarks (Anderson, 2013; Taylor, 2013).
Aardwolves and aardvarks have an ecological role as termite consumers (Anderson, 2013), and aardvarks are considered ecosystem engineers through the excavation of burrows which benefit many vertebrate species (Whittington‐Jones et al., 2011). These burrows provide sleeping shelter for various small and medium‐sized mammals, and African wild dogs (Lycaon pictus) might use aardvark burrows to shelter young (Taylor, 2013). Aardvark burrows also contribute to local soil and vegetation diversity (Louw et al., 2017). While aardwolves and aardvarks are currently classified by the IUCN as species of least concern (Green, 2015; Taylor & Lehmann, 2015), a better understanding of their ecological niches can help identify current and future conservation issues including habitat loss (Green, 2015) and climate change (Rey et al., 2017). Finally, species interactions are primarily studied in the form of competitive interactions, but little is known regarding symbiotic relationships, so these results contribute to a better understanding of this understudied species interaction.
AUTHOR CONTRIBUTIONS
Merijn van den Bosch: Conceptualization (lead); data curation (equal); formal analysis (lead); methodology (lead); visualization (lead); writing – original draft (lead); writing – review and editing (equal). Kenneth F. Kellner: Conceptualization (equal); data curation (equal); writing – review and editing (equal). Imani Mkasanga: Data curation (equal); project administration (equal); writing – review and editing (equal). Stanslaus B. Mwampeta: Data curation (equal); project administration (equal); writing – review and editing (equal). Robert Fyumagwa: Conceptualization (supporting); project administration (equal); writing – review and editing (equal). Mariela G. Gantchoff: Conceptualization (equal); writing – review and editing (equal). Brent R. Patterson: Conceptualization (equal); writing – review and editing (equal). Jerrold L. Belant: Conceptualization (equal); funding acquisition (equal); project administration (equal); supervision (lead); writing – review and editing (equal).
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
ACKNOWLEDGEMENTS
Financial support was provided by Safari Club International Foundation. We thank Tanzania National Parks, Tanzania Wildlife Research Institute and the Commission for Science and Technology for permission to conduct this research (permit 2016‐187‐ER‐2016‐125). Project support was provided by C. Comer, M. Eckert, I. Lejora and M. Lewis. P. Kessy, S. Msechu, S. Mwinyimtama, R. Ndaskoi and C. Wilton provided field assistance.
van den Bosch, M. , Kellner, K. F. , Mkasanga, I. , Mwampeta, S. B. , Fyumagwa, R. , Gantchoff, M. G. , Patterson, B. R. , & Belant, J. L. (2023). Spatial and temporal niche overlap of aardwolves and aardvarks in Serengeti National Park, Tanzania. Ecology and Evolution, 13, e10718. 10.1002/ece3.10718
DATA AVAILABILITY STATEMENT
Animal location data and code related to the analysis are available from Dryad through https://doi.org/10.5061/dryad.9kd51c5qb. This link is currently not live but will be after the paper is accepted for publication. Reviewers can access the dataset for peer review purposes through the following link: https://datadryad.org/stash/share/nDrQ5zc37KII8eJ1UNJJ3DDYden0zGKbuTtKMrh9GY0.
REFERENCES
- Anderson, M. D. (2004). Aardwolf adaptations: A review. Transactions of the Royal Society of South Africa, 59(2), 99–104. [Google Scholar]
- Anderson, M. D. (2013). Proteles cristatus: Aardwolf. In Kingdon J. & Hoffmann M. (Eds.), Mammals of Africa: Volume V: Carnivores, pangolins, equids and rhinoceroses (pp. 282–292). Bloomsbury Publishing. [Google Scholar]
- Arnold, T. W. (2010). Uninformative parameters and model selection using Akaike's information criterion. The Journal of Wildlife Management, 74(6), 1175–1178. 10.1111/j.1937-2817.2010.tb01236.x [DOI] [Google Scholar]
- Bothma, J. D. P. , & Walker, C. (1999). The aardwolf. In Bothma J. D. P. & Walker C. (Eds.), Larger carnivores of the African savannas (pp. 236–251). Springer. 10.1007/978-3-662-03766-9_10 [DOI] [Google Scholar]
- Broennimann, O. , Fitzpatrick, M. C. , Pearman, P. B. , Petitpierre, B. , Pellissier, L. , Yoccoz, N. G. , Thuiller, W. , Fortin, M.‐J. , Randin, C. , Zimmermann, N. E. , Graham, C. H. , & Guisan, A. (2012). Measuring ecological niche overlap from occurrence and spatial environmental data. Global Ecology and Biogeography, 21(4), 481–497. 10.1111/j.1466-8238.2011.00698.x [DOI] [Google Scholar]
- Broms, K. M. , Hooten, M. B. , & Fitzpatrick, R. M. (2016). Model selection and assessment for multi‐species occupancy models. Ecology, 97(7), 1759–1770. [DOI] [PubMed] [Google Scholar]
- Brown, W. L. , & Wilson, E. O. (1956). Character displacement. Systematic Zoology, 5(2), 49–64. 10.2307/2411924 [DOI] [Google Scholar]
- Buchorn, M. , Smets, B. , Bertels, L. , De Roo, B. , Lesiv, M. , Tsendbazar, N. E. , Herold, M. , & Fritz, S. (2020). Copernicus global land service: Land cover 100m: Collection 3, epoch 2017. 10.5281/zenodo.3518036 [DOI]
- Burnham, K. P. , Anderson, D. R. , & Huyvaert, K. P. (2011). AIC model selection and multimodel inference in behavioral ecology: Some background, observations, and comparisons. Behavioral Ecology and Sociobiology, 65(1), 23–35. 10.1007/s00265-010-1029-6 [DOI] [Google Scholar]
- Chesson, P. (2000). Mechanisms of maintenance of species diversity. Annual Review of Ecology and Systematics, 31, 343–366. [Google Scholar]
- Clauss, M. , Scriba, M. , Kioko, J. , Ganzhorn, J. U. , & Kiffner, C. (2021). Camera‐trap data do not indicate scaling of diel activity and cathemerality with body mass in an east African mammal assemblage. Ecology and Evolution, 11(20), 13846–13861. 10.1002/ece3.8090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constant, N. L. , Bell, S. , & Hill, R. A. (2015). The impacts, characterisation and management of human–leopard conflict in a multi‐use land system in South Africa. Biodiversity and Conservation, 24(12), 2967–2989. 10.1007/s10531-015-0989-2 [DOI] [Google Scholar]
- Craft, M. E. , Hampson, K. , Ogutu, J. O. , & Durant, S. M. (2015). Carnivore communities in the greater Serengeti ecosystem. In Sinclair A. R. E., Metzger K. L., Mduma S. A. R., & Fryxell J. M. (Eds.), Serengeti IV: Sustaining biodiversity in a coupled human‐natural system (pp. 419–447). Chicago Scholarship Online. 10.7208/chicago/9780226196336.003.0015 [DOI] [Google Scholar]
- Creel, S. , & Creel, N. M. (1996). Limitation of African wild dogs by competition with larger carnivores. Conservation Biology, 10(2), 526–538. 10.1046/j.1523-1739.1996.10020526.x [DOI] [Google Scholar]
- Davis, C. L. , Rich, L. N. , Farris, Z. J. , Kelly, M. J. , di Bitetti, M. S. , Blanco, Y. D. , Albanesi, S. , Farhadinia, M. S. , Gholikhani, N. , Hamel, S. , Harmsen, B. J. , Wultsch, C. , Kane, M. D. , Martins, Q. , Murphy, A. J. , Steenweg, R. , Sunarto, S. , Taktehrani, A. , Thapa, K. , … Miller, D. A. W. (2018). Ecological correlates of the spatial co‐occurrence of sympatric mammalian carnivores worldwide. Ecology Letters, 21(9), 1401–1412. 10.1111/ele.13124 [DOI] [PubMed] [Google Scholar]
- Dayan, T. , & Simberloff, D. (2005). Ecological and community‐wide character displacement: The next generation. Ecology Letters, 8(8), 875–894. 10.1111/j.1461-0248.2005.00791.x [DOI] [Google Scholar]
- De Boer, W. F. , Vis, M. J. , De Knegt, H. J. , Rowles, C. , Kohi, E. M. , Van Langevelde, F. , Peel, M. , Pretorius, Y. , Skidmore, A. K. , Slotow, R. , van Wieren, S. E. , Prins, H. H. T. , & Prins, H. H. (2010). Spatial distribution of lion kills determined by the water dependency of prey species. Journal of Mammalogy, 91(5), 1280–1286. 10.1644/09-MAMM-A-392.1 [DOI] [Google Scholar]
- De Vries, J. L. , Pirk, C. W. W. , Bateman, P. W. , Cameron, E. Z. , & Dalerum, F. (2011). Extension of the diet of an extreme foraging specialist, the aardwolf (Proteles cristata). African Zoology, 46(1), 194–196. [Google Scholar]
- Dormann, C. F. , Elith, J. , Bacher, S. , Buchmann, C. , Carl, G. , Carré, G. , García Marquéz, J. R. , Gruber, B. , Lafourcade, B. , Leitão, P. J. , Münkemüller, T. , McClean, C. , Osborne, P. E. , Reineking, B. , Schröder, B. , Skidmore, A. K. , Zurell, D. , & Lautenbach, S. (2013). Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography, 36(1), 27–46. 10.1111/j.1600-0587.2012.07348.x [DOI] [Google Scholar]
- Dröge, E. , Creel, S. , Becker, M. S. , & M'soka, J. (2017). Spatial and temporal avoidance of risk within a large carnivore guild. Ecology and Evolution, 7(1), 189–199. 10.1002/ece3.2616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emrich, M. A. , Clare, E. L. , Symondson, W. O. , Koenig, S. E. , & Fenton, M. B. (2014). Resource partitioning by insectivorous bats in Jamaica. Molecular Ecology, 23(15), 3648–3656. 10.1111/mec.12504 [DOI] [PubMed] [Google Scholar]
- Epps, C. W. , Weldy, M. J. , Crowhurst, R. S. , & Spaan, R. S. (2021). Estimating the distribution and habitat suitability for aardvarks (Orycteropus afer) in Kruger National Park, South Africa. African Journal of Ecology, 59(4), 854–865. 10.1111/aje.12916 [DOI] [Google Scholar]
- Ferretti, F. , Lovari, S. , Lucherini, M. , Hayward, M. , & Stephens, P. A. (2020). Only the largest terrestrial carnivores increase their dietary breadth with increasing prey richness. Mammal Review, 50(3), 291–303. 10.1111/mam.12197 [DOI] [Google Scholar]
- Fiske, I. , & Chandler, R. (2011). Unmarked: An R package for fitting hierarchical models of wildlife occurrence and abundance. Journal of Statistical Software, 43, 1–23. 10.18637/jss.v043.i10 [DOI] [Google Scholar]
- Freymann, B. P. , De Visser, S. N. , & Olff, H. (2010). Spatial and temporal hotspots of termite‐driven decomposition in the Serengeti. Ecography, 33(3), 443–450. 10.1111/j.1600-0587.2009.05960.x [DOI] [Google Scholar]
- Green, D. S. (2015). Proteles cristata. The IUCN red list of threatened species 2015: E.T18372A45195681. 10.2305/IUCN.UK.2015-2.RLTS.T18372A45195681.en [DOI]
- Hardin, G. (1960). The competitive exclusion principle. Science, 131, 1292–1297. 10.1126/science.131.3409.1292 [DOI] [PubMed] [Google Scholar]
- Hasui, E. , Gomes, V. S. D. M. , Kiefer, M. C. , Tamashiro, J. , & Silva, W. R. (2009). Spatial and seasonal variation in niche partitioning between blue manakin (Chiroxiphia caudata) and greenish schiffornis (Schiffornis virescens) in southeastern Brazil. Studies on Neotropical Fauna and Environment, 44(3), 149–159. 10.1080/01650520903381729 [DOI] [Google Scholar]
- HilleRisLambers, J. , Adler, P. B. , Harpole, W. S. , Levine, J. M. , & Mayfield, M. M. (2012). Rethinking community assembly through the lens of coexistence theory. Annual Review of Ecology, Evolution, and Systematics, 43, 227–248. 10.1146/annurev-ecolsys-110411-160411 [DOI] [Google Scholar]
- Holt, R. D. (1984). Spatial heterogeneity, indirect interactions, and the coexistence of prey species. The American Naturalist, 124(3), 377–406. 10.1086/284280 [DOI] [PubMed] [Google Scholar]
- Kartzinel, T. R. , Chen, P. A. , Coverdale, T. C. , Erickson, D. L. , Kress, W. J. , Kuzmina, M. L. , Rubenstein, D. I. , Wang, W. , & Pringle, R. M. (2015). DNA metabarcoding illuminates dietary niche partitioning by African large herbivores. Proceedings of the National Academy of Sciences, 112(26), 8019–8024. 10.1073/pnas.1503283112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellner, K. F. , Smith, A. D. , Royle, J. A. , Kéry, M. , Belant, J. L. , & Chandler, R. B. (2023). The unmarked R package: Twelve years of advances in occurrence and abundance modelling in ecology. Methods in Ecology and Evolution, 14(6), 1408–1415. 10.1111/2041-210X.14123 [DOI] [Google Scholar]
- Kéry, M. , & Royle, J. A. (2020). Modeling population dynamics with count data. In Kéry M. & Royle J. A. (Eds.), Applied hierarchical modeling in ecology: Analysis of distribution, abundance and species richness in R and BUGS, Vol. 2 (pp. 65–165). Academic press. 10.1016/B978-0-12-809585-0.00002-8 [DOI] [Google Scholar]
- Kingdon, J. (2015). The Kingdon field guide to African mammals (2nd ed.). Bloomsbury Publishing. [Google Scholar]
- Kronfeld‐Schor, N. , & Dayan, T. (2003). Partitioning of time as an ecological resource. Annual Review of Ecology, Evolution, and Systematics, 34, 153–181. [Google Scholar]
- Kruuk, H. , & Sands, W. A. (1972). The aardwolf (Proteles cristata) as predator of termites. African Journal of Ecology, 10(3), 211–227. 10.1111/j.1365-2028.1972.tb00728.x [DOI] [Google Scholar]
- Levine, J. M. , & HilleRisLambers, J. (2009). The importance of niches for the maintenance of species diversity. Nature, 461(7261), 254–257. 10.1038/nature08251 [DOI] [PubMed] [Google Scholar]
- Lewis, J. S. , Bailey, L. L. , VandeWoude, S. , & Crooks, K. R. (2015). Interspecific interactions between wild felids vary across scales and levels of urbanization. Ecology and Evolution, 5(24), 5946–5961. 10.1002/ece3.1812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louw, M. A. , Le Roux, P. C. , Meyer‐Milne, E. , & Haussmann, N. S. (2017). Mammal burrowing in discrete landscape patches further increases soil and vegetation heterogeneity in an arid environment. Journal of Arid Environments, 141, 68–75. 10.1016/j.jaridenv.2017.02.007 [DOI] [Google Scholar]
- MacKenzie, D. I. , & Bailey, L. L. (2004). Assessing the fit of site‐occupancy models. Journal of Agricultural, Biological, and Environmental Statistics, 9(3), 300–318. 10.1198/108571104X3361 [DOI] [Google Scholar]
- MacKenzie, D. I. , Nichols, J. D. , Lachman, G. B. , Droege, S. , Royle, J. A. , & Langtimm, C. A. (2002). Estimating site occupancy rates when detection probabilities are less than one. Ecology, 83(8), 2248–2255. 10.1890/0012-9658(2002)083[2248:ESORWD]2.0.CO;2 [DOI] [Google Scholar]
- Maliti, H. , von Hagen, C. , Frankfurt Zoological Society, Tanzania National Parks Authority , & Hopcraft, J. G. C. (2008). Serengeti Park rivers. https://serengetidata.weebly.com/rivers‐and‐lakes.html
- Materu, C. , Yarro, J. , Nyundo, B. , Materu, C. L. , & Nyundo, B. A. (2013). Seasonal changes on termite foraging behaviour under different habitats in Rufiji District Tanzania. Journal of Biology, Agriculture and Healthcare, 3(11), 6–12. [Google Scholar]
- Mathis, K. A. , & Bronstein, J. L. (2020). Our current understanding of commensalism. Annual Review of Ecology, Evolution, and Systematics, 51(1), 167–189. 10.1146/annurev-ecolsys-011720-040844 [DOI] [Google Scholar]
- McConnell, S. , O'Connell, T. J. , & Leslie, D. M. (2008). Land cover associations of nesting territories of three sympatric Buteos in shortgrass prairie. The Wilson Journal of Ornithology, 120(4), 708–716. 10.1676/07-048.1 [DOI] [Google Scholar]
- Melton, D. A. (1976). The biology of aardvark (Tubulidentata‐Orycteropodidae). Mammal Review, 6(2), 75–88. 10.1111/j.1365-2907.1976.tb00204.x [DOI] [Google Scholar]
- Meredith, M. , & Ridout, M. (2018). Overview of the overlap package. https://cran.r‐project.org/web/packages/overlap/vignettes/overlap.pdf
- Metzger, K. L. , Sinclair, A. R. , Macfarlane, A. , Coughenour, M. B. , & Ding, J. (2015). Scales of change in the greater Serengeti ecosystem. In Sinclair A. R. E., Metzger K. L., Mduma S. A. R., & Fryxell J. M. (Eds.), Serengeti IV: sustaining biodiversity in a coupled human–natural system (pp. 32–72). Chicago Scholarship Online. [Google Scholar]
- Mills, M. L. (1984). Prey selection and feeding habits of the large carnivores in the southern Kalahari. Koedoe, 27(2), 281–294. [Google Scholar]
- Miquelle, D. G. , Stephens, P. A. , Smirnov, E. N. , Goodrich, J. M. , Zaumyslova, O. Y. , & Myslenkov, A. E. (2005). Tigers and wolves in the Russian Far East: Competitive exclusion, functional redundancy and conservation implications. In Ray J. C., Berger J., Redford K. H., & Steneck R. (Eds.), Large carnivores and the conservation of biodiversity (pp. 179–207). Island Press. [Google Scholar]
- NASA, METI, AIST, Japan Space Systems & US/Japan ASTER Science Team . (2018). ASTER global digital elevation model V003. Distributed by NASA EOSDIS Land Processes DAAC. 10.5067/ASTER/ASTGTM.003 [DOI]
- Norton‐Griffiths, M. , Herlocker, D. , & Pennycuick, L. (1975). The patterns of rainfall in the Serengeti ecosystem, Tanzania. African Journal of Ecology, 13(3–4), 347–374. 10.1111/j.1365-2028.1975.tb00144.x [DOI] [Google Scholar]
- Porter, C. K. , Golcher‐Benavides, J. , & Benkman, C. W. (2022). Seasonal patterns of dietary partitioning in vertebrates. Ecology Letters, 25(11), 2463–2475. 10.1111/ele.14100 [DOI] [PubMed] [Google Scholar]
- R Core Team . (2022). R: A language and environment for statistical computing. R foundation for statistical computing. [Google Scholar]
- Rey, B. , Fuller, A. , Mitchell, D. , Meyer, L. C. , & Hetem, R. S. (2017). Drought‐induced starvation of aardvarks in the Kalahari: An indirect effect of climate change. Biology Letters, 13(7), 20170301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich, L. N. , Davis, C. L. , Farris, Z. J. , Miller, D. A. , Tucker, J. M. , Hamel, S. , Farhadinia, M. S. , Steenweg, R. , Di Bitetti, M. S. , Thapa, K. , Kane, M. D. , Sunarto, S. , Robinson, N. P. , Paviolo, A. , Cruz, P. , Martins, Q. , Gholikhani, N. , Taktehrani, A. , Whittington, J. , … Kelly, M. J. (2017). Assessing global patterns in mammalian carnivore occupancy and richness by integrating local camera trap surveys. Global Ecology and Biogeography, 26(8), 918–929. [Google Scholar]
- Richardson, P. R. K. (1985). The social behaviour and ecology of the aardwolf, Proteles cristatus (Sparrman, 1783) in relation to its food resources. Doctoral dissertation. University of Oxford. [Google Scholar]
- Richardson, P. R. K. (1987). Food consumption and seasonal variation in the diet of the aardwolf Proteles cristatus in southern Africa. Zeitschrift für Säugetierkunde, 52(5), 307–325. [Google Scholar]
- Rodriguez Curras, M. , Donadio, E. , Middleton, A. D. , & Pauli, J. N. (2022). Carnivore niche partitioning in a human landscape. The American Naturalist, 199(4), 496–509. 10.1086/718472 [DOI] [PubMed] [Google Scholar]
- Rota, C. T. , Ferreira, M. A. , Kays, R. W. , Forrester, T. D. , Kalies, E. L. , McShea, W. J. , Parsons, A. W. , & Millspaugh, J. J. (2016). A multispecies occupancy model for two or more interacting species. Methods in Ecology and Evolution, 7(10), 1164–1173. 10.1111/2041-210X.12587 [DOI] [Google Scholar]
- Rota, C. T. , Fletcher, R. J., Jr. , Dorazio, R. M. , & Betts, M. G. (2009). Occupancy estimation and the closure assumption. Journal of Applied Ecology, 46(6), 1173–1181. 10.1111/j.1365-2664.2009.01734.x [DOI] [Google Scholar]
- Sarcinelli, T. S. , Schaefer, C. E. G. , de Souza Lynch, L. , Arato, H. D. , Viana, J. H. M. , de Albuquerque Filho, M. R. , & Gonçalves, T. T. (2009). Chemical, physical and micromorphological properties of termite mounds and adjacent soils along a toposequence in zona da Mata, Minas Gerais state, Brazil. Catena, 76(2), 107–113. 10.1016/j.catena.2008.10.001 [DOI] [Google Scholar]
- Schoener, T. W. (1974). Resource partitioning in ecological communities: Research on how similar species divide resources helps reveal the natural regulation of species diversity. Science, 185(4145), 27–39. 10.1126/science.185.4145.2 [DOI] [PubMed] [Google Scholar]
- Schooler, S. L. , Finnegan, S. P. , Fowler, N. L. , Kellner, K. F. , Lutto, A. L. , Parchizadeh, J. , van den Bosch, M. , Zubiria Perez, A. , Masinde, L. M. , Mwampeta, S. B. , Boone, H. M. , Gantchoff, M. G. , Hill, J. E. , Kautz, T. M. , Wehr, N. H. , Fyumagwa, R. , & Belant, J. L. (2022). Factors influencing lion movements and habitat use in the western Serengeti ecosystem, Tanzania. Scientific Reports, 12(1), 1–11. 10.1038/s41598-022-22053-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sévêque, A. , Gentle, L. K. , López‐Bao, J. V. , Yarnell, R. W. , & Uzal, A. (2020). Human disturbance has contrasting effects on niche partitioning within carnivore communities. Biological Reviews, 95(6), 1689–1705. 10.1111/brv.12635 [DOI] [PubMed] [Google Scholar]
- Sollmann, R. , Furtado, M. M. , Hofer, H. , Jácomo, A. T. , Tôrres, N. M. , & Silveira, L. (2012). Using occupancy models to investigate space partitioning between two sympatric large predators, the jaguar and puma in Central Brazil. Mammalian Biology, 77(1), 41–46. 10.1016/j.mambio.2011.06.011 [DOI] [Google Scholar]
- Sommers, P. , & Chesson, P. (2019). Effects of predator avoidance behavior on the coexistence of competing prey. The American Naturalist, 193(5), 132–148. 10.1086/701780 [DOI] [PubMed] [Google Scholar]
- Steenweg, R. , Whittington, J. , Hebblewhite, M. , Forshner, A. , Johnston, B. , Petersen, D. , Shepherd, B. , & Lukacs, P. M. (2016). Camera‐based occupancy monitoring at large scales: Power to detect trends in grizzly bears across the Canadian Rockies. Biological Conservation, 201, 192–200. 10.1016/j.biocon.2016.06.020 [DOI] [Google Scholar]
- Stenkewitz, U. , & Kamler, J. F. (2008). Birds feeding in association with bat‐eared foxes on Benfontein game farm, South Africa. Ostrich‐Journal of African Ornithology, 79(2), 235–237. 10.2989/OSTRICH.2008.79.2.16.590 [DOI] [Google Scholar]
- Tagwireyi, P. , Wenga, T. , Ndaimani, H. , & Mpakairi, K. S. (2020). Environmental correlates of cheetah (Acinonyx jubatus) space‐use in a savanna landscape. African Journal of Wildlife Research, 50(1), 157–166. [Google Scholar]
- Tannerfeldt, M. , Elmhagen, B. , & Angerbjörn, A. (2002). Exclusion by interference competition? The relationship between red and arctic foxes. Oecologia, 132(2), 213–220. 10.1007/s00442-002-0967-8 [DOI] [PubMed] [Google Scholar]
- Taylor, A. (2013). Orycteropus afer: Aardvark. In Kingdon J., Happold D., Hoffmann M., Butynski T., Happold M., & Kalina J. (Eds.), Mammals of Africa: Volume I: Introductory chapters and Afrotheria (pp. 290–295). Bloomsbury Publishing. 10.5040/9781472926913 [DOI] [Google Scholar]
- Taylor, A. , & Lehmann, T. (2015). Orycteropus afer. The IUCN red list of threatened species 2015: E.T41504A21286437. 10.2305/IUCN.UK.2015-2.RLTS.T41504A21286437.en [DOI]
- Taylor, W. A. , Lindsey, P. A. , & Skinner, J. D. (2002). The feeding ecology of the aardvark Orycteropus afer . Journal of Arid Environments, 50(1), 135–152. 10.1006/jare.2001.0854 [DOI] [Google Scholar]
- Taylor, W. A. , & Skinner, J. D. (2000). Associative feeding between aardwolves (Proteles cristatus) and aardvarks (Orycteropus afer). Mammal Review, 30(2), 141–143. 10.1046/j.1365-2907.2000.00062.x [DOI] [Google Scholar]
- Taylor, W. A. , & Skinner, J. D. (2003). Activity patterns, home ranges and burrow use of aardvarks (Orycteropus afer) in the Karoo. Journal of Zoology, 261(3), 291–297. 10.1017/S0952836903004217 [DOI] [Google Scholar]
- Taylor, W. A. , & Skinner, J. D. (2004). Adaptations of the aardvark for survival in the Karoo: A review. Transactions of the Royal Society of South Africa, 59(2), 105–108. 10.1080/00359190409519169 [DOI] [Google Scholar]
- Tobler, M. W. , Zúñiga Hartley, A. , Carrillo‐Percastegui, S. E. , & Powell, G. V. (2015). Spatiotemporal hierarchical modelling of species richness and occupancy using camera trap data. Journal of Applied Ecology, 52(2), 413–421. 10.1111/1365-2664.12399 [DOI] [Google Scholar]
- Van Aarde, R. J. , Willis, C. K. , Skinner, J. D. , & Haupt, M. A. (1992). Range utilization by the aardvark, Orycteropus afer (Pallas, 1766) in the Karoo, South Africa. Journal of Arid Environments, 22(4), 387–394. 10.1016/S0140-1963(18)30581-0 [DOI] [Google Scholar]
- Weyer, N. M. , Fuller, A. , Haw, A. J. , Meyer, L. C. R. , Mitchell, D. , Picker, M. , Rey, B. , & Hetem, R. S. (2020). Increased diurnal activity is indicative of energy deficit in a nocturnal mammal, the aardvark. Frontiers in Physiology, 11. 10.3389/fphys.2020.00637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittington‐Jones, G. M. , Bernard, R. T. , & Parker, D. M. (2011). Aardvark burrows: A potential resource for animals in arid and semi‐arid environments. African Zoology, 46(2), 362–370. 10.1080/15627020.2011.11407509 [DOI] [Google Scholar]
- Wiens, J. A. (1977). On competition and variable environments: Populations may experience "ecological crunches" in variable climates, nullifying the assumptions of competition theory and limiting the usefulness of short‐term studies of population patterns. American Scientist, 65(5), 590–597. [Google Scholar]
- Wiens, J. A. (1989). Spatial scaling in ecology. Functional Ecology, 3(4), 385–397. 10.2307/2389612 [DOI] [Google Scholar]
- Wiens, J. J. (2011). The niche, biogeography and species interactions. Philosophical Transactions of the Royal Society B: Biological Sciences, 366(1576), 2336–2350. 10.1098/rstb.2011.0059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, J. B. , Anderson, M. D. , & Richardson, P. R. K. (1997). Seasonal differences in field metabolism, water requirements, and foraging behavior of free‐living aardwolves. Ecology, 78(8), 2588–2602. 10.1890/0012-9658(1997)078[2588:SDIFMW]2.0.CO;2 [DOI] [Google Scholar]
- Willis, C. K. , Skinner, J. D. , & Robertson, H. G. (1992). Abundance of ants and termites in the false Karoo and their importance in the diet of the aardvark Orycteropus afer. African Journal of Ecology, 30(4), 322–334. 10.1111/j.1365-2028.1992.tb00509.x [DOI] [Google Scholar]
- Wisz, M. S. , Pottier, J. , Kissling, W. D. , Pellissier, L. , Lenoir, J. , Damgaard, C. F. , Dormann, C. F. , Forchhammer, M. C. , Grytnes, J. A. , Guisan, A. , Heikkinen, R. K. , Høye, T. T. , Kühn, I. , Luoto, M. , Maiorano, L. , Nilsson, M. C. , Normand, S. , Öckinger, E. , Schmidt, N. M. , … Svenning, J. C. (2013). The role of biotic interactions in shaping distributions and realised assemblages of species: Implications for species distribution modelling. Biological Reviews, 88(1), 15–30. 10.1111/j.1469-185X.2012.00235.x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Animal location data and code related to the analysis are available from Dryad through https://doi.org/10.5061/dryad.9kd51c5qb. This link is currently not live but will be after the paper is accepted for publication. Reviewers can access the dataset for peer review purposes through the following link: https://datadryad.org/stash/share/nDrQ5zc37KII8eJ1UNJJ3DDYden0zGKbuTtKMrh9GY0.


