Abstract
BACKGROUND
Controversy exists as to the optimal treatment approach for ostial left anterior descending (LAD) or ostial left circumflex artery (LCx) lesions. Drug-coated balloons (DCB) may overcome some of the limitations of drug-eluting stents (DES). Therefore, we investigated the security and feasibility of the DCB policy in patients with ostial LAD or ostial LCx lesions, and compared it with the conventional DES-only strategy.
METHODS
We retrospectively enrolled patients with de novo ostial lesions in the LAD or LCx who underwent interventional treatment. They were categorized into two groups based on their treatment approach: the DCB group and the DES group. The treatment strategies in the DCB group involved the use of either DCB-only or hybrid strategies, whereas the DES group utilized crossover or precise stenting techniques. Two-year target lesion revascularization was the primary endpoint, while the rates of major adverse cardiovascular events, cardiac death, target vessel myocardial infarction, and vessel thrombosis were the secondary endpoints. Using propensity score matching, we assembled a cohort with comparable baseline characteristics. To ensure result analysis reliability, we conducted sensitivity analyses, including interaction, and stratified analyses.
RESULTS
Among the 397 eligible patients, 6.25% of patients who were planned to undergo DCB underwent DES. A total of 108 patients in each group had comparable propensity scores and were included in the analysis. Two-year target lesion revascularization occurred in 5 patients (4.90%) and 16 patients (16.33%) in the DCB group and the DES group, respectively (odds ratio = 0.264, 95% CI: 0.093–0.752, P = 0.008). Compared with the DES group, the DCB group demonstrated a lower major adverse cardiovascular events rate (7.84% vs. 19.39%, P = 0.017). However, differences with regard to cardiac death, non-periprocedural target vessel myocardial infarction, and definite or probable vessel thrombosis between the groups were non-significant.
CONCLUSIONS
The utilization of the DCB approach signifies an innovative and discretionary strategy for managing isolated ostial lesions in the LAD or LCx. Nevertheless, a future randomized trial investigating the feasibility and safety of DCB compared to the DES-only strategy specifically for de novo ostial lesions in the LAD or LCx is highly warranted.
Therapeutic management for isolated coronary stenosis involving the left anterior descending (LAD) or left circumflex artery (LCx) ostium is challenging. This is attributed to the unpredictable involvement of the distal left main (LM) coronary artery, as reported in a previous intravascular ultrasound (IVUS) study.[1] Ostial stenting, crossover stenting, and other stenting solutions have been studied. Unfortunately, the results have not always been satisfactory due to the presence of existing struts at the ostium. Although focal stenting is often attempted, it is associated with incomplete lesion coverage or protrusion of the proximal stent margin into the ostium of the adjacent vessel. Moreover, plaque shift, which may compromise adjacent vessels, is a possible impediment.[2,3] Stenting from the main vessel (MV) to the LM, the so-called “crossover approach”, has been reported to achieve favorable outcomes.[4] However, the crossover approach is associated with complications, such as covering of the ostium of the side branch (SB) by metal struts, SB occlusion, or severe stenosis caused by carina and plaque shift. Therefore, the most appropriate approach for ostial LAD or LCx lesion is controversial.
Drug-coated balloons (DCB) deploy antiproliferative drugs without the need to implant permanent struts, and they are effective in the treatment of in-stent restenosis.[5,6] It has been reported that DCBs are effective for the treatment of de novo coronary lesions,[7–10] with major benefits in the context of small vessel disease. Previous studies have suggested that the use of DCBs in bifurcation lesion management may be a potential treatment option.[11,12]
In our previous study,[13] we observed comparable safety and efficacy between the DCB-only strategy and the hybrid strategy combining DCB with drug-eluting stent (DES) for ostial LAD and LCx lesions. This approach shows promise as an effective and technically straightforward method for managing de novo ostial LAD and LCx diseases. In this multicenter study, our objective is to further investigate the reliability and efficacy of the DCB strategies [DCB-only or hybrid (DCB + DES)] in treating ostial LAD and LCx lesions. Additionally, we assessed the two-year outcomes of the DCB strategies compared to the DES alone strategy for the treatment of ostial LAD/LCx stenosis.
METHODS
Study Population
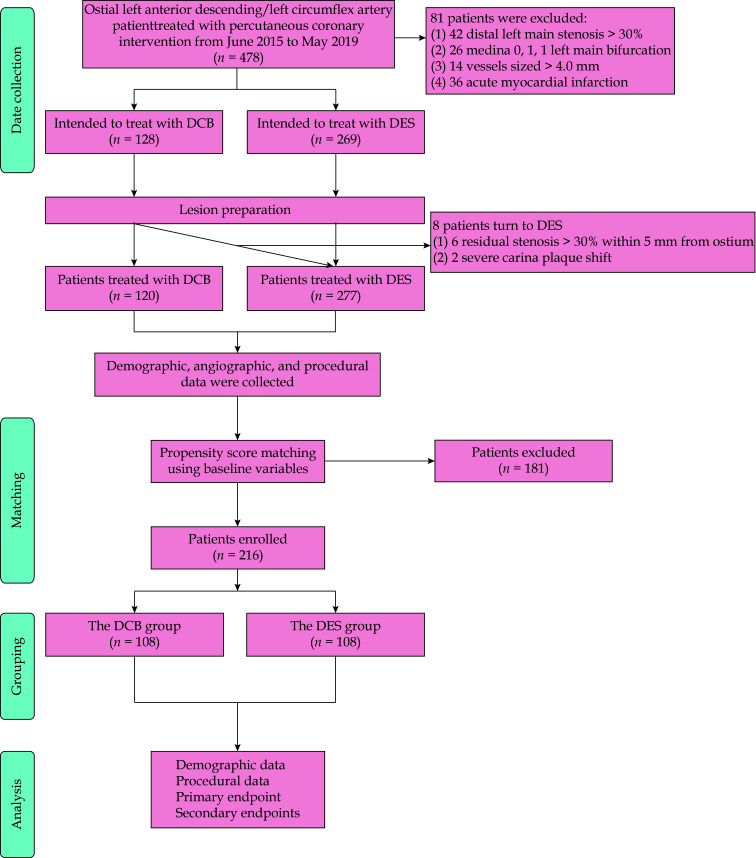
This study was conducted at three large-scale medical centers in China, namely the First Affiliated Hospital of Zhengzhou University, Jincheng People’s Hospital, and the Fifth Affiliated Hospital of Zhengzhou University. Patients were retrospectively enrolled from June 2015 to May 2019. The inclusion criteria were as follows: (1) coronary vessel lesions sized 2.5–4.0 mm; and (2) narrowing (diameter stenosis ≥ 50%) within 3 mm from the LAD/LCx takeoff based on the least foreshortened angiographic projection (scilicet, Medina 0, 1, 0 and 0, 0, 1). The exclusion criteria were as follows: (1) angiographically confirmed concomitant distal LM stenosis > 30%; (2) Medina 0, 1, 1 LM bifurcation; (3) non-adherence to the described procedural optimization steps, as revealed by angiographic films and report reviews; (4) acute myocardial infarction (MI); (5) severe valvular disease or cardiomyopathy; (6) hemodynamic instability or cardiogenic shock; (7) left ventricular ejection fraction (LVEF) ≤ 35%; and (8) severe renal or hepatic dysfunction (Figure 1).
Figure 1.
Flow chart of study population.
*Referred to of the 128 patients intended to be treated with DCB, 8 patients (6.25%) were crossed over to a DES strategy due to unsatisfactory results after lesion preparation. DCB: drug-coated balloons; DES: drug-eluting stents.
Before the intervention, patients who were not already taking long-term aspirin treatment were treated with 300 mg aspirin. A clopidogrel loading dose of 600 mg or a ticagrelor loading dose of 180 mg was administered. Patients who had been subjected to DCB only underwent dual antiplatelet therapy (DAPT) for 1–3 months after the procedure. Patients who underwent concurrent stent implantation underwent DAPT for the guideline-recommended period.[14,15] Patients with known hypersensitivity or contraindications to heparin, DAPT, limus, or paclitaxel; women of childbearing age; and patients with a life expectancy of less than one year were not included in the study. The Ethics Committees of all participating centers in this study have granted approval. Prior to involvement in the study, patients were required to provide written informed consent. The data were obtained using a common electronic case report form.
Study Procedures
In the DES group, ostial LAD/LCx lesions was done using any solutions with DES alone according to European Bifurcation Club guidelines.[16] In cases with favorable anatomical conditions, such as a rectangular LAD-LCx angle, clear visualization of the SB take-off, and absence of disease in the LM, precise ostial stenting can be a viable alternative to crossover stenting. However, in other scenarios, the stent strategy should prioritize the coverage of both the relevant LM and the diseased vessel using a single stent, typically the LAD or, in specific cases, the LCx, through the crossover approach. Subsequently, it is recommended to systematically perform proximal optimization technique (POT). The selected stent should have an appropriate length (8–9 mm) in the LM to accommodate a balloon of the required size for POT. This stent length choice also takes into account the commonly observed diffuse pattern of atherosclerosis in the LM, enabling the coverage of the LM ostium in many instances. If suboptimal results are observed at the SB ostium (indicated by severe carina shift) or if there is a potential future need for downstream percutaneous coronary intervention (PCI), SB rewiring followed by kissing balloon inflation should be considered. Therefore, in younger patients with a significant myocardial mass supplied by the SB or when the LAD is chosen as the secondary branch (stent implanted into LM-LCx), rewiring and kissing techniques may be undertaken.
In the DCB group, the proposed technique has been previously described.[13] Adequate lesion preparations were emphasized prior to DCB angioplasty. Pre-dilatation with a plain balloon, non-compliant balloon, and scoring balloon [including non-slip element (NSE) scoring and cutting balloons] at a balloon/vessel ratio of 0.8–1.0 was compulsory. DCB-only angioplasty was performed with the non-existence of major, flow-limiting dissection (< type C based on the National Heart, Lung, and Blood Institute classification[17]) and where residual stenosis was ≤ 30% based on ≥ 2 perpendicular angiographic views. In cases of residual stenosis ≤ 30% in the proximal 5 mm, regardless of another segment after lesion preparation, the hybrid strategy was used. With the hybrid strategy, DCB angioplasty was performed first, followed by stent deployment. To minimize carina plaque shift, a gap ≥ 3 mm was ensured between the proximal end of the stent and the vessel ostium. The DCB used in this study was coated with a matrix of paclitaxel and iopromide (SeQuent™ Please, B. Braun, Melsungen, Germany). The DCB sizes were fitted to the diameters of the reference vessels using a balloon/vessel ratio of 0.80–1.00. In cases of unsatisfactory DCB results because of severe residual stenosis or dissection, new-generation DESs were implanted.
Follow-up
Clinical follow-up was conducted by telephone or office visit one month after the procedure, and then every three months thereafter for 24 months. All patients were scheduled to undergo angiographic follow-up at 12 months following the index operation (after determining the primary clinical outcome), unless clinical indications necessitated earlier follow-up.
Endpoint Definitions
The primary outcome of this study was two-year target lesion revascularization (TLR). The definition of TLR was either PCI or coronary artery bypass grafting as a result of target lesion restenosis or thrombosis that included distal- and proximal-edge segments, as well as the SB ostium. Several secondary outcomes were evaluated, including the rates of major adverse cardiovascular events (MACEs) [denoted as the combined outcome of cardiac death, TLR, target vessel MI (TVMI), and vessel thrombosis], cardiac death, TVMI, and vessel thrombosis. Periprocedural MI (≤ 48 h) was defined as a cardiac troponin concentration ≥ 5-times the 99th percentile upper reference limit of the assay, plus either (1) new changes in ischemic electrocardiography or development of new pathological Q waves; (2) imaging confirmation of new loss of viable myocardium or new abnormalities in regional wall motion; or (3) angiographically reported grafts, coronary arterial occlusion, or new severe stenosis accompanied by thrombosis. Spontaneous MI (after 48 h) was denoted as a clinical syndrome, consistent with MI with a cardiac troponin concentration more than 1 × upper reference limit and new ST-segment elevation, depression, or other above-mentioned findings.[18] All cases of MI were determined to be TVMI, unless they were attributed to a non-target vessel.[19] Identification of vessel thrombosis was based on the definition of the Academic Research Consortium.[19] In cases of an undeterminable or unknown cause of death, the cause of death was considered to be cardiogenic.
Quantitative Coronary Angiography (QCA) Assessment
The QCA measurements were performed using edge detection techniques and a bifurcation algorithm[20] (QAngio XA 7.3 version; Medis Medical Imaging, Leiden, the Netherlands) with a guiding catheter serving as the calibration reference. Measurements were performed at baseline, after the procedure, and during follow-up angiography. The MV was determined based on the lesion location. The evaluated QCA variables were as follows: (1) lesion length; (2) reference vessel diameter (RVD); (3) percent diameter stenosis; (4) minimal lumen diameter (MLD); (5) percent area stenosis; (6) acute luminal gain (MLD post-intervention minus baseline MLD); and (7) late lumen loss (LLL; MLD instantly after the procedure minus MLD at follow-up). The vessel segment directly after the MV ostial lesion was used as the RVD.
Statistical Analysis
The R statistical software 3.6.1 (The R Foundation, Vienna, Austria; http://www.r-project.org) and EmpowerStats software (X&Y Solutions Inc., Boston, MA, USA; http://empowerstats.com) statistical packages were used to analyze the results. Categorical variables are reported as counts (percentages) and continuous variables as mean ± SD. The DCB group and the DES group were compared using the Fisher’s exact probability test for each variable and the non-parametric Wilcoxon–Mann–Whitney test for continuous variables.
Given the differences in the baseline characteristics between eligible participants in the two groups in the observational study, 1:1 propensity score matching was conducted to select patients with comparable baseline data. After evaluating covariates associated clinically and/or statistically with the treatment group and removal of repeatedly defined or collinear variables, including baseline characteristics, risk factors, clinical conditions at admission, and treatments during surgery, thirteen variables (including age, sex, diabetes mellitus, hypertension, hyperlipidemia, renal insufficiency, clinical presentation, family history of coronary artery disease, history of smoking, previous PCI, previous MI, previous coronary artery bypass grafting, and LVEF) were included in the propensity score matching model using greedy nearest neighbor matching without replacement and a caliper of 0.01 (supplemental material, Figure 1S). Analyses of the primary and secondary outcomes were performed in the total population and in the propensity score-matched cohort. The outcomes were compared using the log-rank test, and the results are presented as Kaplan–Meier curves.
Prespecified subgroup analyses were performed according to age (< 60 years vs. ≥ 60 years), sex (male vs. female), diabetes mellitus status (yes vs. no), clinical presentation (stable angina vs. unstable angina), and target vessel (LAD vs. LCx). In the subgroup analyses, to maintain the baseline balance between the DCB group and the DES group, only the corresponding matched pairs in a subgroup were chosen. For example, in the subgroup of patients with diabetes mellitus, only the matched pairs of patients with diabetes mellitus in the DCB group and in the DES group were included in the analysis. Interaction and stratified analyses were performed to assess heterogeneity in the treatment effect among the subgroups. Propensity score-matched cohorts of patients who underwent PCI with DCB versus crossover stenting and precise ostial stenting, respectively, were analyzed in terms of the primary and secondary outcomes. For all analyses, two-sided P-value < 0.05 were considered statistically significant.
RESULTS
Baseline Clinical, Angiographic, and Procedural Characteristics
A total of 397 patients with ostial LAD/LCx lesions treated with PCI satisfied the inclusion criteria. Of these patients, 6.25% of the patients who were planned to undergo DCB crossed underwent DES (Figure 1). Of these patients, 120 patients (40.40%) were treated with DCB (DCB only or hybrid strategy). Table 1 outlines the baseline characteristics of the patients. After matching, 216 patients (108 patients in each group) were selected. Patient matching minimized statistical disparities between the groups in terms of diabetes mellitus and LVEF. During the same procedure, 47 patients (21.76%) underwent interventional therapy for another vessel.
Table 1. Demographic characteristics before and after propensity score matching.
| Variable | All patients | Propensity-matched sample | |||||
| DCB group (n = 120) |
DES group (n = 277) |
P-value | DCB group (n = 108) |
DES group (n = 108) |
P-value | ||
| Data are presented as means ± SD or n (%). DCB: drug-coated balloons; DES: drug-eluting stents. | |||||||
| Age, yrs | 59.75 ± 11.29 | 60.05 ± 10.43 | 0.625 | 58.82 ± 10.38 | 60.62 ± 10.01 | 0.249 | |
| Male | 86 (71.67%) | 194 (70.04%) | 0.743 | 77 (71.30%) | 77 (71.30%) | 1.000 | |
| Comorbidity | |||||||
| Diabetes mellitus | 39 (32.50%) | 123 (44.40%) | 0.027 | 37 (34.26%) | 32 (29.63%) | 0.466 | |
| Hypertension | 59 (49.17%) | 149 (53.79%) | 0.397 | 53 (49.07%) | 57 (52.78%) | 0.586 | |
| Hyperlipidemia | 30 (25.00%) | 90 (32.49%) | 0.136 | 29 (26.85%) | 31 (28.70%) | 0.761 | |
| History of smoking | 45 (37.50%) | 78 (28.16%) | 0.065 | 38 (35.19%) | 35 (32.41%) | 0.666 | |
| Renal insufficiency | 6 (5.00%) | 15 (5.42%) | 0.865 | 4 (3.70%) | 9 (8.33%) | 0.153 | |
| Clinical presentation | 0.952 | 0.580 | |||||
| Stable angina | 45 (37.50%) | 103 (37.18%) | 42 (38.89%) | 46 (42.59%) | |||
| Unstable angina | 75 (62.50%) | 174 (62.82%) | 66 (61.11%) | 62 (57.41%) | |||
| Previous myocardial infarction history | 11 (9.17%) | 44 (15.88%) | 0.075 | 11 (10.19%) | 10 (9.26%) | 0.818 | |
| Previous percutaneous coronary intervention history | 19 (15.83%) | 51 (18.41%) | 0.536 | 16 (14.81%) | 16 (14.81%) | 1.000 | |
| Previous coronary artery bypass grafting history | 4 (3.33%) | 14 (5.05%) | 0.449 | 4 (3.70%) | 4 (3.70%) | 1.000 | |
| Family history of coronary artery disease | 25 (20.83%) | 57 (20.58%) | 0.954 | 24 (22.22%) | 20 (18.52%) | 0.499 | |
| Left ventricular ejection fraction, % | 61.08 ± 4.00 | 59.32 ± 6.75 | 0.043 | 61.00 ± 4.00 | 61.20 ± 4.36 | 0.568 | |
| Other vessel treated during the same procedure | 26 (21.67%) | 67 (24.19%) | 0.586 | 24 (22.22%) | 23 (21.30%) | 0.869 | |
Table 2 displays the procedural and angiographic baseline characteristics. Before matching, there were 397 lesions, of which 120 lesions (30.23%) were treated with the DCB strategy (DCB only vs. hybrid: 58 vs. 62) and 277 lesions (69.77%) were treated with the DES strategy (ostial stenting vs. crossover stenting: 117 vs. 160). The median SYNTAX score for the whole population was 28 (range: 23–36). More than two thirds of the patients had ostial LAD disease after matching, and roughly half of the examined lesions were diffuse. The characteristics of the lesions in both groups, including RVD, stenosis, and lesion length (all P > 0.05), were comparable.
Table 2. Procedural characteristics before and after propensity score matching.
| Variable | All patients | Propensity-matched sample | |||||
| DCB group | DES group | P-value | DCB group | DES group | P-value | ||
| Data are presented as means ± SD or n (%). DCB: drug-coated balloons; DES: drug-eluting stents. | |||||||
| Number of lesions | 120 | 277 | 108 | 108 | |||
| Vascular access | 0.704 | 1.000 | |||||
| Trans-radial | 115 (95.83%) | 263 (94.95%) | 103 (95.37%) | 104 (96.30%) | |||
| Trans-femoral | 5 (4.17%) | 14 (5.05%) | 5 (4.63%) | 4 (3.70%) | |||
| Treated vessel | 0.093 | 0.530 | |||||
| Left anterior descending artery | 89 (74.17%) | 226 (81.59%) | 79 (73.15%) | 83 (76.85%) | |||
| Left circumflex artery | 31 (25.83%) | 51 (18.41%) | 29 (26.85%) | 25 (23.15%) | |||
| Procedure strategies | |||||||
| DCB-only strategy | 58 (48.33%) | - | 53 (49.07%) | - | |||
| Hybrid strategy | 62 (51.67%) | - | 55 (50.93%) | - | |||
| Ostial stenting | 117 (42.24%) | 48 (44.44%) | |||||
| Crossover stenting | 160 (57.76%) | 60 (55.56%) | |||||
| Feature of lesion | |||||||
| Total occlusion | 9 (7.50%) | 26 (9.39%) | 0.543 | 9 (8.33%) | 12 (11.11%) | 0.491 | |
| Diffuse vessel disease | 58 (48.33%) | 105 (37.91%) | 0.052 | 53 (49.07%) | 44 (40.74%) | 0.218 | |
| Calcified lesions | 9 (7.50%) | 19 (6.86%) | 0.819 | 8 (7.41%) | 8 (7.41%) | 1.000 | |
| Reference vessel diameter | 3.43 ± 0.39 | 3.38 ± 0.38 | 0.132 | 3.43 ± 0.40 | 3.43 ± 0.37 | 0.789 | |
| Diameter stenosis (by quantitative coronary angiography) |
0.70 ± 0.13 | 0.69 ± 0.12 | 0.533 | 0.70 ± 0.13 | 0.71 ± 0.12 | 0.700 | |
| Area stenosis (by quantitative coronary angiography) |
0.89 ± 0.07 | 0.89 ± 0.07 | 0.533 | 0.90 ± 0.07 | 0.90 ± 0.06 | 0.700 | |
| Lesion length, mm | 26.52 ± 14.48 | 24.71 ± 11.75 | 0.901 | 26.46 ± 14.38 | 23.98 ± 10.15 | 0.693 | |
| SYNTAX score | 26.83 ± 5.38 | 27.90 ± 5.75 | 0.081 | 26.96 ± 5.41 | 28.13 ± 5.71 | 0.139 | |
| Lesion preparation | 120 (100%) | 277 (100%) | 108 (100%) | 108 (100%) | |||
| Plain old balloon angioplasty | 94 (78.33%) | 204 (73.65%) | 0.322 | 85 (78.70%) | 85 (78.70%) | 1.000 | |
| Non-slip element scoring balloon | 27 (22.50%) | 45 (16.25%) | 0.137 | 26 (24.07%) | 25 (23.15%) | 0.873 | |
| Cutting balloon | 81 (67.50%) | 94 (33.94%) | < 0.001 | 69 (63.89%) | 39 (36.11%) | < 0.001 | |
| Non-compliant balloon | 22 (18.33%) | 61 (22.02%) | 0.407 | 22 (20.37%) | 21 (19.44%) | 0.865 | |
| Rotational atherectomy | 1 (0.83%) | 3 (1.08%) | 1.000 | 1 (0.93%) | 1 (0.93%) | 1.000 | |
| Maximum pre-dilation balloon diameter, mm | 3.10 ± 0.35 | 2.74 ± 0.37 | < 0.001 | 3.09 ± 0.35 | 2.77 ± 0.37 | < 0.001 | |
| Maximum pre-dilation balloon diameter/reference vessel diameter ratio |
0.91 ± 0.07 | 0.82 ± 0.12 | < 0.001 | 0.91 ± 0.07 | 0.81 ± 0.12 | < 0.001 | |
| DCB/DES use | |||||||
| Number of DCBs/DESs used (per lesion) | 1.56 ± 0.52 | 1.15 ± 0.44 | < 0.001 | 1.05 ± 0.25 | 1.09 ± 0.38 | 0.239 | |
| DCB/DES diameter, mm | 3.14 ± 0.40 | 3.21 ± 0.35 | 0.009 | 3.13 ± 0.41 | 3.25 ± 0.34 | 0.004 | |
| DCB/DES diameter/reference vessel diameter ratio | 0.92 ± 0.08 | 0.95 ± 0.06 | < 0.001 | 0.92 ± 0.08 | 0.95 ± 0.05 | < 0.001 | |
| Length of DCB and/or DES, mm | 34.42 ± 15.03 | 28.57 ± 13.32 | 0.002 | 34.37 ± 14.96 | 27.57 ± 11.36 | 0.006 | |
| Inflation pressure, bar | 8.47 ± 1.50 | 11.00 ± 2.01 | < 0.001 | 8.52 ± 1.56 | 11.05 ± 2.07 | < 0.001 | |
| Coronary dissection after DCB angioplasty | |||||||
| None | 55 (45.84%) | 53 (49.07%) | |||||
| Type A | 25 (20.83%) | 21 (19.44%) | |||||
| Type B | 16 (13.33%) | 13 (12.04%) | |||||
| Type C | 13 (10.83%) | 11 (10.19%) | |||||
| Type D | 10 (8.33%) | 9 (8.33%) | |||||
| Type E-F | 1 (0.83%) | 1 (0.93%) | |||||
| Final kissing inflation | 81 (29.24%) | 33 (30.56%) | |||||
| Proximal optimization technique | 151 (54.51%) | 57 (52.78%) | |||||
| Bailout stenting | 4 (3.33%) | 4 (3.70%) | |||||
All lesions underwent lesion preparation (pre-dilatation with semi-compliant balloons, NSE scoring balloons, cutting balloons, non-compliant balloons, or rotational atherectomy). Interestingly, the use of scoring balloons (NSE scoring or cutting balloons) was significantly greater in the DCB group than in the DES group (90.00% vs. 50.19%, P < 0.001), which is consistent with clinical practice. In addition, the maximum balloon diameter (and balloon/vessel diameter ratio) used for lesion preparation was greater in the DCB group than in the DES group (P < 0.001). It exhibited opposing properties when DCB and DES were used (P < 0.001). The overall length of the DCB and/or DES was significantly longer in the DCB group than in the DES group (34.37 ± 14.96 mm vs. 27.57 ± 11.36 mm, P = 0.006). Compared to the DES group, the DCB and/or DES diameter/RVD ratio was significantly lower in the DCB group (0.92 ± 0.08 vs. 0.95 ± 0.05, P < 0.001). According to the stated procedure, 53 patients underwent DCB only, while 55 patients underwent the hybrid strategy. Therefore, the percentage of bailout stenting was low in the DCB group (3.70%), despite the proportion of type C-F dissection reaching roughly 20% following DCB angioplasty. Only patients who were scheduled to undergo DCB only underwent bailout stenting. In addition, 98 patients (45.37%) underwent IVUS during the procedure.
Clinical Outcomes
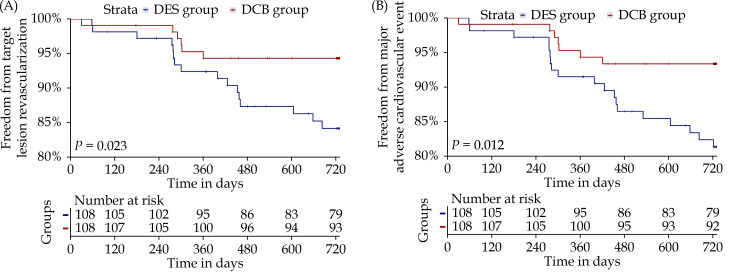
Of the total population, 373 patients (93.95%) were followed up for a mean of 733 days. The incidence rate of TLR was 5.26% and 11.97% in the DCB group and the DES group, respectively [odds ratio (OR) = 0.409, 95% CI: 0.166–1.009, P = 0.046]. After propensity score matching, relative to the DES group, the DCB group exhibited a lower incidence of TLR (4.90% vs. 16.33%, OR = 0.264, 95% CI: 0.093–0.752, P = 0.008; log-rank P = 0.023) (Table 3 & Figure 2A). Subgroup analyses based on certain features yielded findings that were substantially comparable (supplemental material, Table 1S).
Table 3. Risk of primary and secondary outcomes in the propensity score-matched cohort at two-year follow-up.
| Endpoint | All patients | Propensity-matched sample | |||||||
| DCB group | DES group | Odds ratio (95% CI) | P-value | DCB group | DES group | Odds ratio (95% CI) | P-value | ||
| Data are presented as n (%). *Referred to major adverse cardiovascular events defined as the composite outcome of cardiac death, target vessel myocardial infarction, target lesion revascularization, and vessel thrombosis. DCB: drug-coated balloons; DES: drug-eluting stents. | |||||||||
| Patients with clinical follow-up | 114 | 259 | 102 | 98 | |||||
| Target lesion revascularization | 6 (5.26%) | 31 (11.97%) | 0.409 (0.166–1.009) | 0.046 | 5 (4.90%) | 16 (16.33%) | 0.264 (0.093–0.752) | 0.008 | |
| Major adverse cardiovascular events* | 9 (7.89%) | 40 (15.44%) | 0.469 (0.220–1.003) | 0.047 | 8 (7.84%) | 19 (19.39%) | 0.354 (0.147–0.852) | 0.017 | |
| All-cause death | 3 (2.63%) | 14 (5.41%) | 0.473 (0.133–1.679) | 0.237 | 3 (2.94%) | 5 (5.10%) | 0.564 (0.131–2.425) | 0.491 | |
| Cardiac death | 2 (1.75%) | 10 (3.86%) | 0.445 (0.096–2.063) | 0.288 | 2 (1.96%) | 3 (3.06%) | 0.633 (0.104–3.874) | 0.678 | |
| Non-cardiac death | 1 (0.88%) | 4 (1.54%) | 0.564 (0.062–5.104) | 1.000 | 1 (0.98%) | 2 (2.04%) | 0.475 (0.042–5.327) | 0.616 | |
| Target vessel myocardial infarction | 2 (1.75%) | 7 (2.70%) | 0.643 (0.131–3.143) | 0.728 | 2 (1.96%) | 3 (3.06%) | 0.633 (0.104–3.874) | 0.678 | |
| Periprocedural | 0 | 2 (0.77%) | 0 | 0 | |||||
| Non-periprocedural | 2 (1.75%) | 5 (1.93%) | 2 (1.96%) | 3 (3.06%) | |||||
| Vessel thrombosis | 0 | 4 (1.54%) | 0.985 (0.970–1.000) | 0.318 | 0 | 3 (3.06%) | 0.969 (0.936–1.004) | 0.116 | |
| Definite | 0 | 4 (1.54%) | 0 | 3 (3.06%) | |||||
| Probable | 0 | 0 | 0 | 0 | |||||
Figure 2.
Kaplan–Meier analysis for freedom from target lesion revascularization (A) or major adverse cardiovascular event (B) in the propensity score-matched cohort.
DCB: drug-coated balloons; DES: drug-eluting stents.
After matching, the Kaplan–Meier analysis (Table 3 & Figure 2B) revealed that the cumulative rate of MACEs was significantly lower in the DCB group than in the DES group at two years (7.84% vs. 19.39%, OR = 0.354, 95% CI: 0.147–0.866, P = 0.017; log-rank P = 0.012). There were no statistically significant differences in the incidence of cardiac mortality (DCB vs. DES: 1.96% vs. 3.06%, P = 0.678), TVMI (DCB vs. DES: 1.96% vs. 3.06%, P = 0.678), or vessel thrombosis (DCB vs. DES: 0.98% vs. 3.06%, P = 0.361) between the two groups. The subgroup analyses revealed comparable findings (supplemental material, Table 1S).
QCA Measurement
Of the 397 patients, 276 patients (69.5%) underwent angiographic follow-up (Tables 2 & 4). Comparable baseline characteristics were determined in the two groups using the QCA. Due to the features of the DCB/DES selection, the acute lumen gain of the DES group immediately after intervention was greater than that of the DCB group (2.21 ± 0.52 mm vs. 1.63 ± 0.47 mm, P < 0.001). At angiographic follow-up, the MLD and LLL were larger in the DES group than in the DCB group (MLD: 2.94 ± 0.41 mm vs. 2.69 ± 0.63 mm, P = 0.008; LLL: 0.30 ± 0.27 mm vs. −0.02 ± 0.57 mm, P < 0.001). The results were largely similar before and after propensity score matching.
Table 4. Quantitative coronary angiography results before and after propensity score matching.
| Variable | All patients | Propensity-matched sample | |||||
| DCB group | DES group | P-value | DCB group | DES group | P-value | ||
| Data are presented as means ± SD or n. DCB: drug-coated balloons; DES: drug-eluting stents. | |||||||
| Number of lesions | 120 | 277 | 108 | 108 | |||
| Pre-intervention minimal lumen diameter, mm | 1.05 ± 0.48 | 1.03 ± 0.43 | 0.919 | 1.03 ± 0.49 | 0.99 ± 0.44 | 0.522 | |
| Post-intervention minimal lumen diameter, mm | 2.66 ± 0.39 | 3.16 ± 0.37 | < 0.001 | 2.66 ± 0.38 | 3.19 ± 0.35 | < 0.001 | |
| Acute lumen gain, mm | 1.62 ± 0.49 | 2.13 ± 0.50 | < 0.001 | 1.63 ± 0.47 | 2.21 ± 0.52 | < 0.001 | |
| Patients with angiographic follow-up | 87 | 189 | 77 | 75 | |||
| Follow-up minimal lumen diameter, mm | 2.89 ± 0.45 | 2.69 ± 0.63 | 0.005 | 2.69 ± 0.63 | 2.94 ± 0.41 | 0.008 | |
| Late lumen loss, mm | -0.01 ± 0.58 | 0.29 ± 0.27 | < 0.001 | -0.02 ± 0.57 | 0.30 ± 0.27 | < 0.001 | |
Sensitivity Analyses
In the analysis of PCI with DCB versus precise ostial stenting (89 matched pairs), the DCB group was associated with a lower risk of TLR (P = 0.027) and MACEs (P = 0.048), but the risk of other outcomes was similar (supplemental material, Table 2S). In the analysis of PCI with DCB versus crossover stenting (89 matched pairs), DCB was associated with non-significantly lower rates of TLR (3.57% vs. 6.10%, respectively; P = 0.493) and MACEs (5.95% vs. 7.32%, respectively; P = 0.724). However, the risk of the other outcomes was comparable between the groups (supplemental material, Table 3S).
DISCUSSION
We compared the outcomes of DCB with those of DES in the treatment of ostial LCx or LAD lesions at three participating centers. We found that when compared with patients who underwent PCI with the DES strategy, those treated with the DCB strategy had lower rates of TLR and MACEs. The incidences of cardiac death, TVMI, and vessel thrombosis were comparable in the two groups, indicating that both DCB alone and the hybrid strategy were safe and effective for de novo ostial LAD/LCx lesion treatment, and that DCB can be used as an alternative to stenting or even as the first-choice treatment for eligible patients. Our findings are founded on propensity score matching; therefore, our observations are unlikely to be due to negative confounders. In addition, the reliability of the findings was authenticated using sensitivity analysis approaches, including subgroup analyses.
Ostial LAD (LM 0, 1, 0 lesion) has traditionally been regarded as undesirable for PCI because of its technical complexity and potential for severe complications. In this study, 90% of patients had continuous plaques from the LM to the LAD, 66% to the LCx, and 62% to both. Around 9.3% and 17.1% of LAD and LCx vessels, respectively, had plaques in the ostium, without distal LM involvement.[1]
Traditionally, two interventional approaches are used for this type of lesion: precise stent implantation at the LAD ostium level or stenting of the LM toward the LAD.[21] Precise stenting is realistic in cases with large bifurcation angles and IVUS documentation of disease absence in distal LM. Precise LAD ostial stenting entails counter-carina scaffolding, with mild protrusions of the stent covering the ostium of the LCx. For this technique, a proximal stent marker must be positioned just proximal to the angiographic carina. The disadvantages of precise ostial LAD stenting were as follows: (1) potential device protrusion into the LM if positioned too proximally, which may compromise the LCx and make subsequent interventions challenging; and (2) acute recoil and late restenosis in the ostial LAD lesions, leading to incomplete enclosure by the stent.[21] Therefore, optimal stent positioning is vital for the treatment of this type of lesion. Branch ostial disease often involves the distal LM, thereby increasing the risk of incomplete lesion coverage incase stenting is not extended to involve the LM. On this basis, left MV ostial lesions are very comparable to bifurcation disease with similar treatment approaches. Thus, consensus is emerging that ostial lesions in the LCx and LAD should be percutaneously treated by stenting from the LM to the diseased MV with provisional SB stenting. Compared with precise ostial stenting, our strategy, in which the coverage area of the DCB includes part of the LM, ensures that the plaques extending from the target vessel to the LM are completely covered by the DCB without geographic loss. At the same time, the need for accuracy during stent positioning is greatly reduced, thereby simplifying the operation. The technical solution that we have proposed is based on the requirement that the distance between the proximal end of the stent and the ostium of the vessel is ≥ 3 mm. In this way, the lesion can be completely covered with anti-proliferative drugs, while covering the SB ostium and the carina with stent struts is avoided. At the same time, carina plaque shift can be avoided, thereby affecting the SB patency.
Recent research comparing one stent positioned precisely at the LAD ostium with crossover stenting validated the feasibility of crossover stenting and revealed a lower restenosis rate.[4] Thus, based on current knowledge, ostial stenting should be avoided, except if the anatomy is favorable (rectangular angle between the LAD and the LCx, undiseased LM, and perfect visualization of SB takeoff). In all other scenarios, crossover stenting (covering the ostial LCx or LAD and the diseased LM segment) followed by POT and eventual kissing (according to either provisional or “inverted” provisional)[22] is the desirable option. During the crossover procedure, the carina plaque sometimes shifts to the SB, and kissing may be unavoidable. In the present study, nearly one third of the patients underwent final kissing followed by the POT because of SB ostium involvement after crossover. Two patients experienced LCx acute occlusion after LAD–LM crossover. Because of the small diameter of the LCx, the failure to rewire resulted in perioperative MI. It should be noted that precise LAD ostial stenting, crossover stenting, and SB dilation after crossover stenting are all associated with unsatisfactory incidences of long-term clinical events.
Vaquerizo, et al.[23] conducted a retrospective registration and assessed the safety and efficacy of second-generation DCBs in patients with SB ostial lesions (patients with LM bifurcation lesions were excluded). In that study, 49 patients with de novo Medina 0, 0, 1 lesions and related myocardial ischemia were treated using the second-generation DCB-Dior II balloon catheter with an angiographic success rate of 86% [because of coronary dissection of more than type B (n = 2) or acute recoil (n = 5), a bare metal stent was implanted in 14% of patients]. At 12.2 ± 2.2 months, the rate of MACEs was 14.3% (0 cardiac deaths, 7 TLRs, and 1 MI). Occlusion and thrombosis were not observed. At 7.2 ± 1.1 months, binary restenosis was noted in 7 patients (22.5%) with a late loss of 0.32 ± 0.73 mm. However, ostial LAD/LCx lesions were excluded in this study, and RVD was comparatively small (2.18 ± 0.34 mm), which may explain the relatively high TLR rate. Unlike the average RVD, which reached more than 3 mm, the incidence of both MACEs and TLR was much lower.
The feasibility of DCB in the treatment of ostial LAD lesions has not been reported. Through appropriate and adequate lesion preparation, the DCB-only strategy was successfully used in nearly half of the patients. In addition, the number of patients subjected to bailout stenting after DCB angioplasty was markedly low. Importantly, both precision stenting and the crossover approach were associated with considerably reduced rates of TLR or MACEs compared with earlier studies. Moreover, we did not observe any thrombotic events, and it is widely known that thrombotic events of the LAD or the LCx ostium is catastrophic.
The efficacy of DCB depends on drug transfer, drug bioavailability, and reduced drug transit times. This is reliant on adequate lesion preparation, as well as meticulous angioplasty using balloons with a diameter of 0.8–1.0 × RVD for > 30 s. Satisfactory lesion preparation is associated with superior acute gain and remodeling, and avoids severe flow-limiting dissection. Tanaka, et al.[24] reported that inadequate angiographic pre-dilatation prior to DCB is an independent predictor of TLR. Ostial lesions exhibit high fibrous tissue and calcium content, with increased elastic recoil. Thus, adjunctive lesion-modifying devices, such as NSE scoring balloons, cutting balloons, and rotational atherectomy, should be used liberally. It should be noted that nearly 90% of the lesions were prepared using scoring balloons in the DCB group in the present study, which is much higher than in previous studies. In our practice, we observed that to achieve the goal of ≤ 30% residual stenosis within 5 mm from the vessel ostium, the average balloon/vessel diameter ratio reached 0.91 and greatly extended the duration of balloon expansion. Therefore, after DCB angioplasty, the proportion of type C-F dissection reached 20%. However, this does not affect our immediate procedural success rate because of the use of the hybrid strategy. Regardless of whether a bailout stent is used in the DCB-only group or a stent is used in the hybrid group, the proximal edge is > 3 mm away from the ostium. A distance of 3–5 mm ensures that the MV does not acutely occlude due to severe dissection or hematoma. Importantly, there are no struts at the ostium after the procedure, allowing numerous possibilities for future intervention. Therefore, the application of this innovative technique achieves a very high success rate while avoiding the shortcomings of the DES-only strategy. It should be noted that because of the lesion location, the entire left coronary artery may often be blocked during DCB angioplasty. Therefore, in patients with extremely poor cardiac function, it is necessary to closely monitor blood pressure when performing DCB expansion.
Our findings indicate significant overlap between DCB and DES during the intervention, which may explain the low TLR and LLL during follow-up.[25] Moreover, the synergy of the two drugs (paclitaxel and -limus) may also play a role. The safety of DCB and DES overlap is consistent with previous findings.[26,27] In this study, we did not observe late acquired stent malapposition or thrombosis.
The suggested method may also be feasible for patients with LM 0, 1, 1 bifurcation who may require a two-stent procedure with DES alone. Moreover, this strategy can be extended to other coronary bifurcation lesions. Ostial SB lesions are particularly important because they can lead to new lesions on the MV. Although not common, isolated SB ostial lesions (0, 0, 1) are very difficult to treat (particularly in narrow, Y-shaped angulations). Thus, surgeons ought to be aware of the “sad story of ostial diagonal lesions” and bear in mind that treating these lesions very aggressively may not be the best tactic as it may cause trauma to the LAD, resulting in new stenosis. Use of the technique proposed in the present study may help to overcome this challenge.
LIMITATIONS
This non-randomized, observational study may include selection and ascertainment bias despite propensity score matching. No a priori sample size was estimated for this exploratory investigation. Due to the lesion complexity, 23.4% of the patients underwent the DES strategy in a different coronary artery during the same procedure [median SYNTAX score: 28 (range: 23–36)], which may have altered the clinical outcomes. The Kaplan–Meier analysis of TLR and MACEs showed that event incidence disparities increased with time. The clinical results of the DCB group may improve with longer-term follow-up. Landmark analyses after longer follow-up periods may lead to different perceptions. Lastly, our study has a limitation in that we did not adequately account for the operator as a confounding factor, which could potentially impact the final results. However, it is worth noting that all operators involved in our study were highly experienced, which to some extent mitigates this concern.
CONCLUSIONS
In this contemporary cohort study of patients with ostial LAD/LCx coronary artery disease, PCI with DCB as compared with any other DES-only strategy was associated with lower long-term risks of TLR and MACEs. It is suggested that use of the DCB strategy alone or use of the hybrid strategy is safe and effective for the treatment of de novo ostial LAD/LCx lesions with a low technical threshold and a high success rate. Certainly, the future implementation of randomized controlled trials is of utmost importance for the validation of our research findings.
SUPPLEMENTARY DATA
Supplementary data to this article can be found online.
ACKNOWLEDGMENTS
This study was supported by the Medical Science and Technique Research Plan of He’nan Province (Provincial and Ministerial Co-construction Project) (SB201901027). All authors had no conflicts of interest to disclose. The authors thank the physicians who helped with this research (especially Guang-Hui LIU, Luo-Sha ZHAO, You-You DU, Zhen-Wen HUANG, Fei-Fei ZHANG, Xu-Le WANG, and Xiao-Lin ZHENG) and the colleagues who participated in data collection (including Peng QIN, Sen GUO, Yan-Jun ZHOU, Wen-Cai ZHANG, Shuai ZHOU, Ran LI, and Qiang-Wei SHI).
Contributor Information
Jian-Zeng DONG, Email: jzdong@zzu.edu.cn.
Chun-Guang QIU, Email: fccqiucg@zzu.edu.cn.
References
- 1.Oviedo C, Maehara A, Mintz GS, et al Intravascular ultrasound classification of plaque distribution in left main coronary artery bifurcations: where is the plaque really located? Circ Cardiovasc Interv. 2010;3:105–112. doi: 10.1161/CIRCINTERVENTIONS.109.906016. [DOI] [PubMed] [Google Scholar]
- 2.Park SJ, Lee CW, Hong MK, et al Stent placement for ostial left anterior descending coronary artery stenosis: acute and long-term (2-year) results. Catheter Cardiovasc Interv. 2000;49:267–271. doi: 10.1002/(SICI)1522-726X(200003)49:3<267::AID-CCD9>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 3.Asakaura Y, Takagi S, Ishikawa S, et al Favorable strategy for the ostial lesion of the left anterior descending coronary artery: influence on narrowing of circumflex coronary artery. Cathet Cardiovasc Diagn. 1998;43:95–100. doi: 10.1002/(SICI)1097-0304(199801)43:1<95::AID-CCD28>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 4.Rigatelli G, Zuin M, Baracca E, et al Long-term clinical outcomes of isolated ostial left anterior descending disease treatment: ostial stenting versus left main cross-over stenting. Cardiovasc Revasc Med. 2019;20:1058–1062. doi: 10.1016/j.carrev.2019.01.030. [DOI] [PubMed] [Google Scholar]
- 5.Lu W, Zhu Y, Han Z, et al Drug-coated balloon in combination with bare metal stent strategy for de novo coronary artery disease: a PRISMA-compliant meta-analysis of randomized clinical trials. Medicine (Baltimore) 2017;96:e6397. doi: 10.1097/MD.0000000000006397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang X, Lu W, Wang X, et al Drug-coated balloon angioplasty: predicting outcomes based on different patterns of drug-eluting stent restenosis. Int J Cardiovasc Imaging. 2020;36:171–178. doi: 10.1007/s10554-019-01681-y. [DOI] [PubMed] [Google Scholar]
- 7.Lu W, Zhu Y, Han Z, et al Short-term outcomes from drug-coated balloon for coronary de novo lesions in large vessels. J Cardiol. 2019;73:151–155. doi: 10.1016/j.jjcc.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 8.Jeger RV, Farah A, Ohlow MA, et al Long-term efficacy and safety of drug-coated balloons versus drug-eluting stents for small coronary artery disease (BASKET-SMALL 2): 3-year follow-up of a randomised, non-inferiority trial. Lancet. 2020;396:1504–1510. doi: 10.1016/S0140-6736(20)32173-5. [DOI] [PubMed] [Google Scholar]
- 9.Ali RM, Degenhardt R, Zambahari R, et al Paclitaxel-eluting balloon angioplasty and cobalt-chromium stents versus conventional angioplasty and paclitaxel-eluting stents in the treatment of native coronary artery stenoses in patients with diabetes mellitus. EuroIntervention. 2011;7:K83–K92. doi: 10.4244/EIJV7SKA15. [DOI] [PubMed] [Google Scholar]
- 10.Pan L, Lu W, Han Z, et al Clinical outcomes of drug-coated balloon in coronary lesions: a real-world, all-comers study. Clin Res Cardiol. 2022;111:732–741. doi: 10.1007/s00392-021-01895-y. [DOI] [PubMed] [Google Scholar]
- 11.López Mínguez JR, Nogales Asensio JM, Doncel Vecino LJ, et al A prospective randomised study of the paclitaxel-coated balloon catheter in bifurcated coronary lesions (BABILON trial): 24-month clinical and angiographic results. EuroIntervention. 2014;10:50–57. doi: 10.4244/EIJV10I1A10. [DOI] [PubMed] [Google Scholar]
- 12.Worthley S, Hendriks R, Worthley M, et al Paclitaxel-eluting balloon and everolimus-eluting stent for provisional stenting of coronary bifurcations: 12-month results of the multicenter BIOLUX-I study. Cardiovasc Revasc Med. 2015;16:413–417. doi: 10.1016/j.carrev.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 13.Lu WJ, Pan L, Han ZY, et al Stentless at ostium: a novel approach for treating ostial left anterior descending or left circumflex coronary artery lesions with drug-coated balloons. Am J Transl Res . 2022;14:6256–6267. [PMC free article] [PubMed] [Google Scholar]
- 14.Valgimigli M, Bueno H, Byrne RA, et al 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: the Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS) Eur Heart J. 2018;39:213–260. doi: 10.1093/eurheartj/ehx419. [DOI] [PubMed] [Google Scholar]
- 15.Neumann FJ, Sousa-Uva M, Ahlsson A, et al 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. 2019;40:87–165. doi: 10.1093/eurheartj/ehy394. [DOI] [PubMed] [Google Scholar]
- 16.Burzotta F, Lassen JF, Banning AP, et al Percutaneous coronary intervention in left main coronary artery disease: the 13th consensus document from the European Bifurcation Club. EuroIntervention. 2018;14:112–120. doi: 10.4244/EIJ-D-18-00357. [DOI] [PubMed] [Google Scholar]
- 17.Huber MS, Mooney JF, Madison J, et al Use of a morphologic classification to predict clinical outcome after dissection from coronary angioplasty. Am J Cardiol. 1991;68:467–471. doi: 10.1016/0002-9149(91)90780-O. [DOI] [PubMed] [Google Scholar]
- 18.Thygesen K, Alpert JS, Jaffe AS, et al Fourth universal definition of myocardial infarction (2018) J Am Coll Cardiol. 2018;72:2231–2264. doi: 10.1016/j.jacc.2018.08.1038. [DOI] [PubMed] [Google Scholar]
- 19.Mauri L, Hsieh WH, Massaro JM, et al Stent thrombosis in randomized clinical trials of drug-eluting stents. N Engl J Med. 2007;356:1020–1029. doi: 10.1056/NEJMoa067731. [DOI] [PubMed] [Google Scholar]
- 20.Collet C, Onuma Y, Cavalcante R, et al Quantitative angiography methods for bifurcation lesions: a consensus statement update from the European Bifurcation Club. EuroIntervention. 2017;13:115–123. doi: 10.4244/EIJ-D-16-00932. [DOI] [PubMed] [Google Scholar]
- 21.Burzotta F, Lassen JF, Lefèvre T, et al Percutaneous coronary intervention for bifurcation coronary lesions: the 15th consensus document from the European Bifurcation Club. EuroIntervention. 2021;16:1307–1317. doi: 10.4244/EIJ-D-20-00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burzotta F, Lassen JF, Louvard Y, et al European Bifurcation Club white paper on stenting techniques for patients with bifurcated coronary artery lesions. Catheter Cardiovasc Interv. 2020;96:1067–1079. doi: 10.1002/ccd.29071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vaquerizo B, Fernández-Nofreiras E, Oategui I, et al Second-generation drug-eluting balloon for ostial side branch lesions (001-bifurcations): mid-term clinical and angiographic results. J Interv Cardiol. 2016;29:285–292. doi: 10.1111/joic.12292. [DOI] [PubMed] [Google Scholar]
- 24.Tanaka A, Latib A, Jabbour RJ, et al Impact of angiographic result after predilatation on outcome after drug-coated balloon treatment of in-stent coronary restenosis. Am J Cardiol. 2016;118:1460–1465. doi: 10.1016/j.amjcard.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 25.Ielasi A, Buono A, Pellicano M, et al A hybrid approach evaluating a drug-coated balloon in combination with a new-generation drug-eluting stent in the treatment of de novo diffuse coronary artery disease: the HYPER Pilot Study. Cardiovasc Revasc Med. 2021;28:14–19. doi: 10.1016/j.carrev.2020.07.036. [DOI] [PubMed] [Google Scholar]
- 26.Cortese B, Silva Orrego P, Agostoni P, et al Effect of drug-coated balloons in native coronary artery disease left with a dissection. JACC Cardiovasc Interv. 2015;8:2003–2009. doi: 10.1016/j.jcin.2015.08.029. [DOI] [PubMed] [Google Scholar]
- 27.Mitomo S, Jabbour RJ, Mangieri A, et al Mid-term clinical outcomes after bailout drug-eluting stenting for suboptimal drug-coated balloon results: insights from a Milan registry. Int J Cardiol. 2018;263:17–23. doi: 10.1016/j.ijcard.2018.04.050. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data to this article can be found online.