The relatively uncommon masquerading bundle branch block (MBBB), a concept initially introduced in 1958 by UNGER, et al.[1] and colleagues, refers to a conduction abnormality that consists of the right bundle branch block (RBBB) pattern in the precordial leads, whereas in the limb leads it resembles a left bundle branch block (LBBB). This electrocardiographic pattern signifies an extensive involvement of the left bundle pathway and left ventricle in comparison to a typical bifascicular block,[2] and has been associated with a poorer prognosis, a higher risk of progression to complete heart block, and increased mortality.[2,3] Diagnosing ischemia in patients with MBBB may pose a diagnostic challenge due to the lack of data concerning this specific population. Moreover, right ventricular pacing (RVP) in such patients may lead to additional difficulties when presenting with chest pain. RVP may mask the electrocardiographic identification of ischemia and may prevent interpretation of ST-segment changes.[4] We present a patient with permanent pacemaker, intermittent RVP and MBBB pattern on his baseline electrocardiogram (ECG) who presented with acute coronary syndrome (ACS). We aim to shed light on the unique challenges associated with diagnosing and managing ischemia in these patients, as there is not enough data available on this topic.
An 86-year-old patient with a medical history of diabetes mellitus, hypertension, dyslipidemia, and paroxysmal atrial fibrillation sought medical attention at the Emergency Department. He had no prior history of coronary artery disease. Several years ago, he underwent permanent pacemaker implantation due to combined Mobitz type II atrioventricular block episodes and sinus node dysfunction. Recent echocardiographic study revealed good left ventricular contraction. The patient’s baseline ECG with MBBB is depicted in Figure 1.
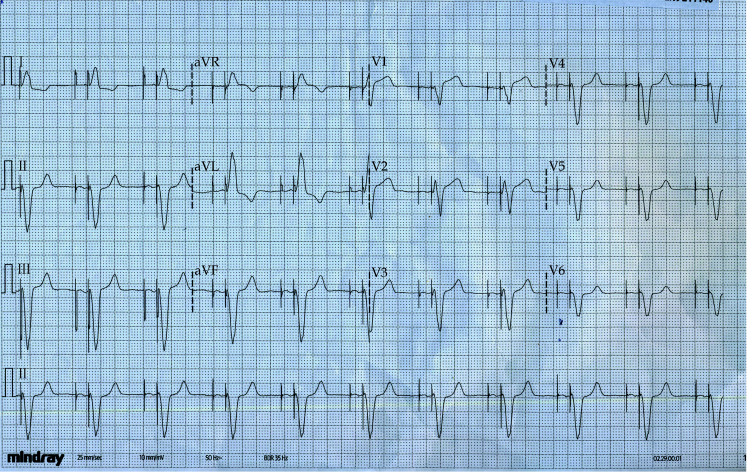
Figure 1.
Baseline electrocardiogram with masquerading bundle branch block pattern.
The patient presented with a chest pain at rest during the last few days, radiating to the left shoulder. Upon admission, he was slightly symptomatic. Physical examination and vital signs were unremarkable. The initial ECG displayed a ventricular paced rhythm with a QRS morphology typical for RVP with LBBB-like pattern (Figure 2). Blood tests revealed elevated troponin T levels (285 ng/L with a normal range < 14 ng/L). Since the presentation took place in the weekend, he was admitted to the Cardiac Care Unit (CCU) awaiting planned coronary angiography. Upon admission to the CCU, another ECG was recorded, interpreted as atrioventricular sequential pacing, as seen in Figure 3. The dynamic changes in the ST-segment in leads V1–V2 were overlooked. The following day after admission, patient’s pacemaker was temporarily adjusted, unveiling the intrinsic rhythm (Figure 4), sinus rhythm with MBBB pattern and various findings suggestive of underlying ischemia (1-mm ST-segment elevation in aVR and V1, a 2-mm ST-segment depression in V4–V6, concordant to QRS axis). These changes were initially masked by ventricular pacing. Echocardiography demonstrated moderately reduced left ventricular function with akinetic apex and an ejection fraction of 40%. Cardiac biomarkers level remained markedly elevated, but fairly stable. After 60 h from his initial presentation, the patient underwent coronary angiography, which revealed a critical lesion (99% narrowing) in the proximal left anterior descending (LAD) artery and a non-obstructive lesion in the ramus intermedius artery (Figure 5A). Percutaneous intervention with a drug-eluting stent was performed on the LAD, resulting in a favorable angiographic outcome (Figure 5B). Following stabilization and a 48-hour monitoring period, the patient was discharged in a stable condition.
Figure 2.
Electrocardiogram of ventricular-paced rhythm with a QRS morphology typical for right ventricular pacing with left bundle branch block-like pattern.
Figure 3.
Electrocardiogram of atrioventricular sequential pacing on admission to the Cardiac Care Unit.
Figure 4.
Electrocardiogram of intrinsic rhythm with sinus rhythm and masquerading bundle branch block pattern.
Figure 5.
Coronary angiography.
Right anterior oblique cranial view before percutaneous coronary intervention (A) and after percutaneous coronary intervention (B).
RVP induces significant QRS and ST-T changes that closely resemble those seen in complete LBBB, making the detection of ischemic changes considerably more challenging.[5] Originally developed in 1996 to assist clinicians to diagnose infarction in the setting of LBBB, Sgarbossa criteria were shown in numerous publications to be applicable to patients with RVP. In 2012, after noting a limitation with one of the criteria, Smith, et al.[6] published the Smith-modified Sgarbossa criteria. Unlike the original criterion referring to excessively discordant ST-segment elevation (STE) > 5 mm, Smith, et al.[6] defined excessively discordant STE relative to the amplitude of the preceding S-wave. The cut-off for ominous discordance was found to be STE/S-wave ratio ≥ 0.25 and was superior to the original criterion. Other validated features of the modified criteria include concordant STE of ≥ 1 mm in ≥ 1 lead, and ≥ 1 mm of concordant ST-segment depression in ≥ 1 lead in V1–V3. Retrospective case-control validation study reported a sensitivity and specificity of 80% and 99%, respectively, when one of the above is positive.[7] The PERFECT study has demonstrated that modified Sgarbossa criteria may be applied to paced patients, presenting with ACS with higher sensitivity of 86%.[8] Nevertheless, ventricular pacing may obscure ischemic changes and lead to delays in urgent revascularization, as happened in our case, underscoring the utmost importance of assessing the underlying ECG for ischemic changes and comparing it to the baseline ECG tracing.
The 2017 European Society of Cardiology (ESC) ST-segment elevation myocardial infarction (STEMI) guidelines supported the use of the abovementioned ECG criteria in patients with myocardial infarction and RVP, though stated they might be less specific than with LBBB. In patients with RVP in the setting of suspected ACS, unless pacemaker-dependent, it is recommended to reprogram the pacemaker in order to enable intrinsic heart rhythm and evaluation of native ST-segment changes.[4] For pacemaker-dependent patients with no underlying rhythm or ambiguous ECG changes, echocardiography can also aid in diagnosing myocardial infarction by revealing segmental wall motion abnormalities consistent with the coronary artery distribution territory.
The ECG tracings presented in our case entail the story in a chronological order. In Figure 1, patient’s baseline ECG can be appreciated. The ECG taken during the initial presentation (Figure 2) shows a typical RVP morphology at a rate of 54 beats/min. The underlying atrial rhythm is probably atrial fibrillation, as no atrial activity is discernible. In response to the atrial tachyarrhythmia, the pacemaker activated automatic mode switch algorithm which allows protection from high-rate RVP.[9] Perhaps the relatively high dose of bisoprolol taken regularly by the patient suppressed the intrinsic ventricular rhythm, resulting in RVP at the lower rate limit. Next, in the CCU, another ECG was obtained (Figure 3), depicting atrioventricular sequential pacing. However, the attending physician disregarded the dynamic changes in the ST-segment in the right precordial leads. The STE/S-wave ratio in lead V1 is 0.3, which fulfills the Smith-modified Sgarbossa criteria.
The most striking tracing, Figure 4 depicts patient’s intrinsic rhythm after the device was reprogrammed, with both atrial and ventricular sensing, at a rate of 75 beats/min.
The presence of ischemic changes (STE in V1, aVR and ST-segment depression in V4–V6) became evident after temporary pacemaker adjustment, despite the MBBB ECG pattern. This provided an opportunity to compare the changes with his baseline tracing, revealing the magnitude of the electrocardiographic changes. While no established ischemic criteria exist for patients with MBBB, the emergence of new ST-segment deviation, concordant to QRS axis, is very suggestive of ongoing ischemia.
Another noteworthy finding is the presence of RBBB in the setting of ACS. The ECG diagnostic criteria of STEMI are not affected by the presence of RBBB, although it may confound the diagnosis.[4] Since the blood supply of the right bundle branch often originates from the septal perforators of the LAD, a proximal occlusion may result in a new RBBB. While the interventricular delay is considered to be reversible with reperfusion therapy, it is associated with an overall larger infarcts and grave prognosis in the setting of acute myocardial infarction.[10] This was also reflected by the ESC STEMI guidelines, where a class IIa indication was given to perform emergent coronary angiography in patients with persistent ischemic symptoms and RBBB. Our subject had a pre-existing QRS morphology resembling RBBB in precordial leads, but as mentioned above, there were new identifiable changes comparing with the previously documented pattern. As RBBB results in baseline repolarization abnormalities with ST-segment depression in the right precordial leads, it is plausible that the magnitude of STE is somewhat obscured by these pre-existing changes. Whether the threshold for STE in anterior STEMI with RBBB should be lowered still remains a subject of debate and requires established prospective data.
It is important to emphasize that along with the growing recognition of the dangers of new RBBB in the context of myocardial infarction, the “presumably new” LBBB, that was traditionally thought to be a STEMI equivalent is no longer considered as such according to the American Heart Association 2013 update.[11]
According to ESC non-STE ACS guidelines, patients with the evidence of troponin elevation and chest pain should undergo revascularization within 24 h. Unfortunately, in our case there was a major delay in coronary angiography, as patient presented in the weekend and ischemic ECG changes were overlooked. Such delays should be avoided, as “time is muscle”, and early revascularization may improve outcomes in these patients.[12]
Diagnosing ischemia in patients with RVP and MBBB presents challenges due to significant ECG alterations. Modified Sgarbossa criteria offer promise for detecting ACS in paced patients, but ventricular pacing may still obscure ischemic changes and evaluation of underlying rhythm is of paramount importance. We found no reports of patients with baseline MBBB presenting with ischemic changes. Timely evaluation and intervention are crucial to improve outcomes for these patients, and further research is needed to optimize clinical decision-making in this population. Lastly, new RBBB in patients with ACS may signify significant ischemia and should be treated promptly.
ACKNOWLEDGMENTS
All authors had no conflicts of interest to disclose.
References
- 1.UNGER PN, LESSER ME, KUGEL VH, et al The concept of masquerading bundle-branch block; an electrocardiographic-pathologic correlation. Circulation. 1958;17:397–409. doi: 10.1161/01.CIR.17.3.397. [DOI] [PubMed] [Google Scholar]
- 2.Kaimoto S, Kawasaki T, Taniguchi T, et al Masquerading bundle branch block as a marker of poor prognosis. J Cardiol Cases. 2013;8:e57–e59. doi: 10.1016/j.jccase.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dhanse S, Kareem H, Devasia T, et al Masquerading bundle branch block: a poor prognostic sign revisited. J Clin Diagn Res. 2016;10:OD01–OD02. doi: 10.7860/JCDR/2016/20572.8472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ibanez B, James S, Agewall S, et al 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC) Eur Heart J. 2018;39:119–177. doi: 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- 5.Diaz CF, Ganim MS, Ellestad MH Electrocardiographic evidence of ischemia during ventricular paced rhythms. Clin Cardiol. 1996;19:520–522. doi: 10.1002/clc.4960190616. [DOI] [PubMed] [Google Scholar]
- 6.Smith SW, Dodd KW, Henry TD, et al Diagnosis of ST-elevation myocardial infarction in the presence of left bundle branch block with the ST-elevation to S-wave ratio in a modified Sgarbossa rule. Ann Emerg Med. 2012;60:766–776. doi: 10.1016/j.annemergmed.2012.07.119. [DOI] [PubMed] [Google Scholar]
- 7.Meyers HP, Limkakeng AT Jr, Jaffa EJ, et al Validation of the modified Sgarbossa criteria for acute coronary occlusion in the setting of left bundle branch block: a retrospective case-control study. Am Heart J. 2015;170:1255–1264. doi: 10.1016/j.ahj.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 8.Dodd KW, Zvosec DL, Hart MA, et al Electrocardiographic diagnosis of acute coronary occlusion myocardial infarction in ventricular paced rhythm using the modified Sgarbossa criteria. Ann Emerg Med. 2021;78:517–529. doi: 10.1016/j.annemergmed.2021.03.036. [DOI] [PubMed] [Google Scholar]
- 9.Stabile G, De Simone A, Romano E Automatic mode switching in atrial fibrillation. Indian Pacing Electrophysiol J . 2005;5:186–196. [PMC free article] [PubMed] [Google Scholar]
- 10.Nikus K, Birnbaum Y, Fiol-Sala M, et al Conduction disorders in the setting of acute STEMI. Curr Cardiol Rev. 2021;17:41–49. doi: 10.2174/1573403X16666200702121937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Gara PT, Kushner FG, Ascheim DD, et al 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127:e362–e425. doi: 10.1161/CIR.0b013e3182742cf6. [DOI] [PubMed] [Google Scholar]
- 12.Collet JP, Thiele H, Barbato E, et al 2020 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2021;42:1289–1367. doi: 10.1093/eurheartj/ehaa575. [DOI] [PubMed] [Google Scholar]