Abstract
We conducted this research to determine the prevalence rate and presentation patterns with microcystic macular oedema (MMO) in glaucoma patients. The protocol was pre-registered on PROSPERO (CRD42022316367). PubMed, Scopus, Web of Science, EMBASE, ProQuest, EBSCOHost, CENTRAL, clinicaltrials.gov, and Google Scholar were searched for articles reporting MMO in glaucoma patients. The primary outcome was the prevalence of MMO, while secondary outcomes included the comparison between MMO and non-MMO in terms of patients’ characteristics (age, gender), glaucoma stage, and ocular parameters (axial length (AL), intraocular pressure, mean deviation, spherical equivalent). Data are reported as mean difference (MD) or log odds ratio (logOR) along with their corresponding 95% confidence intervals (CI) for continuous and dichotomous outcomes, respectively. The quality of included studies was assessed using the NIH tool, and the certainty of evidence was assessed using GRADE framework. Ten studies (2128 eyes) were included, revealing an overall prevalence rate of MMO of 8% (95%CI: 5–12%). When compared to non-MMO group, MMO was associated with lower age (MD = −5.91; 95%CI: −6.02: −5.20), greater risk of advanced glaucoma stage (LogOR=1.41; 95%CI: 0.72: 2.09), and lower mean deviation of the visual field (MD = −5.00; 95%CI: −7.01: −2.99). No significant difference was noted between both groups in terms of gender, axial length, or spherical equivalent. Three studies had good quality while seven had poor quality. MMO is a prevalent observation in glaucoma patients and is associated with patients’ age and stage of the disease. However, the certainty of evidence remains very low.
Subject terms: Glaucoma, Outcomes research
Abstract
该研究旨在确定青光眼患者中微小黄斑囊性水肿 (microcystic macular oedema, MMO) 的流行率和表现模式。该研究已在PROSPERO进行预注册 (CRD42022316367) 。检索了PubMed, Scopus, Web of Science, EMBASE, ProQuest, EBSCOHost, CENTRAL, clinicaltrials.gov和谷歌学术中报告了青光眼患者MMO的文献。主要结局为MMO的患病率, 次要结局包括MMO和非MMO患者的特征 (年龄, 性别), 青光眼分期和眼部参数 (轴长 (axial length, AL) 、眼压、平均偏差、等效球镜度数) 。对于连续和二分类变量, 数据以平均差 (mean difference, MD) 或对数比值比 (log odds ratio, logOR) 及其95%的置信区间 (confidence intervals, CI) 表示。使用NIH工具评估纳入研究的质量, 使用GRADE框架评估证据的确定性。共纳入了10项研究 (2128只眼), 显示MMO的总患病率为8% (95%CI: 5–12%) 。与非MMO组对比, MMO组与较低的年龄 (MD = −5.91; 95%CI: −6.02: −5.20), 青光眼晚期高风险 (LogOR=1.41; 95%CI: 0.72: 2.09) 以及更低的视野平均偏差 (MD = −5.00; 95%CI: −7.01: −2.99) 相关。两组在性别、轴向长度和等效球镜度数方面无显著差异。纳入的文献中, 有三篇文献的质量较好, 七篇文献质量较差。MMO在青光眼患者中较为常见, 且与患者年龄及青光眼疾病分期密切相关。然而, 目前证据的确定性仍然较低。
Introduction
Glaucoma is a type of optic neuropathy that is distinguished by the progressive loss of retinal ganglion cells (RGCs), which result in a cupping of the optic disc with subsequent visual field (VF) loss [1]. Approximately, 70 million people in the world suffer from glaucoma, making it among the leading causes of irreversible visual loss [2, 3]. The classic glaucomatous disc features include characteristic cupping of the disc, thinning of the neuroretinal rim, diffuse or local loss in the retinal nerve fibre layer (RNFL), parapillary atrophy especially in the Beta zone, and retinal or optic disc haemorrhages [4, 5].
Microcystic macular oedema (MMO), also known as microcystic macular edema (MME), refers to the development of small hypo-reflective round-elliptical cystoid spaces without a confined wall, particularly limited to the INL of the retina. MMO is most commonly seen in the parafoveal area [6, 7]. It is commonly observed with the use of advanced diagnostic technologies, including high resolution or spectral domain optical coherence tomography [6, 8].
MMO was first described in multiple sclerosis (MS) [9]; however, recent studies have shown the occurrence of MMO in optic neuropathy [10, 11] of diverse causes [12–14]. Although the exact pathophysiology of MMO is not well understood yet, many suggested mechanisms have been reported including Müller cell dysfunction, retrograde trans-synaptic degeneration of bipolar cells due to RGC loss, blood-retinal barrier disruption with local inflammation, and vitreous traction from the internal limiting membrane in the setting of inner retinal atrophy [12, 15].
That being said, recent studies highlight the occurrence of MMO in glaucoma patients [16–18], particularly those with primary open-angle glaucoma (POAG) [19, 20], with rates ranging from 1.57% [20] to as high as 17.6% [17]. In addition, MMO has been described to be predictive of severe disease as in MS. Similarly, in 2021, Mahmoudinezhad et al. [21] observed that MMO was associated with glaucoma progression and worse outcomes.
To date, no meta-analysis has been conducted to estimate the prevalence of MMO in glaucoma nor describe the difference between MMO and non-MMO glaucoma patients in terms of patients’ characteristics, glaucoma stage, and ocular parameters. Therefore, we conducted this systematic review and meta-analysis to assess two hypotheses; the first is that MMO is a rare observation (<5%) in glaucoma patients and the second is that demographic characteristics, glaucoma stage, and ocular parameters are not associated with MMO presentation when compared to non-MMO glaucoma patients.
Methods
Protocol registration
This systematic review and meta-analysis were conducted as per the Preferred Reporting for Systematic Review and Meta-Analysis (PRISMA) recommendations [22]. The review protocol was registered on PROSPERO (registration number: CRD42022316367).
Literature search
On March 10, 2022, we systematically searched nine databases: PubMed, Scopus, EMBASE, Web of Science (WOS), ProQuest, EBSCO, Cochrane Central Register of Controlled Trials (CENTRAL), clinicaltrials.gov, and Google Scholar. As per the recent recommendations, only the first 200 records from Google Scholar were screened [23]. The search terms were formulated using the PICO framework [24]: participants were glaucoma patients, no intervention or comparison groups were included, and the primary outcome was the presence of MMO in the INL of the retina. The search included the following keywords (glaucoma OR ‘ocular hypertension’ OR pseudoexfoliation) AND (microcyst). The addition of the keyword ‘pseudoexfoliation’ was to screen all eligible studies where those reporting pseudoexfoliation glaucoma patients (either as the overall population or as a subgroup to patients with pseudoexfoliation syndrome) were included after full-text screening. The search query was adjusted based on the guidelines provided in each searched database respectively (Supplementary Table 1).
Following the identification of finally eligible studies, a manual search was conducted in order to find potentially missing relevant articles. The search included the following approaches: (1) screening the reference list of finally included articles, (2) screening ‘similar articles’ of finally eligible studies through the ‘similar articles’ option on PubMed [25], and (3) searching for relevant studies through Google with the keywords (glaucoma + microcyst).
Eligibility criteria
We included any original study reporting data regarding microcystic changes of the retinal INL among patients with glaucoma of any type or stage. No limitations were put on language, demographics, study design, or year of publication. The presence of a non-MMO group was not mandatory for inclusion or exclusion.
We excluded studies based on the following criteria: (1) studies reporting MMO in ocular diseases other than glaucoma, (2) the lack of MMO data, (3) studies including glaucoma patients with MMO at baseline, (4) animal or in vitro studies, (5) non-original articles (i.e., reviews, commentaries, guidelines, editorials, correspondence, letters to editors), (6) case reports and case series with <5 cases, (7) studies with unavailable full texts, and (8) duplicated records or records with overlapping datasets [identified by similar country, sample size, and patients’ characteristics].
Screening and study selection
The records found through the primary database search were exported into EndNote software (Version 8) for the removal of duplicates prior to the screening stage. Then, the remaining records were exported to an Excel sheet for formal screening, which was divided into three steps: (1) title screening, (2) abstract screening, and (3) full-text screening. Two groups of two reviewers each helped with the title, abstract, and full-text screening of retrieved records [BEK, HB, RAF, HAS]. If any discrepancies were encountered amongst reviewers, a senior author was consulted to reach a final decision [AA and MMO].
Data extraction
A data extraction sheet was developed following the review of data reported in the finally included studies, which contained three main parts. The first part included the studies’ information (i.e., author name, year of publication, study design, sample size) and patients’ characteristics (glaucoma type, definition, diagnosis of MMO, male, and age). The second part was related to the main outcome of the study, which is the rate of occurrence (prevalence rate) of MMO. The third part was related to secondary outcomes, which included the comparison between MMO and non-MMO glaucoma patients in terms of axial length (AL), spherical equivalent (SE), intraocular pressure (IOP), age, and glaucoma stage (early vs. moderate vs. advanced). Four independent reviewers performed the data extraction [BEK, HB, RAF, HAS], and data were checked for accuracy before the analysis stage by the senior authors [AA and MMO].
Quality assessment
We assessed the quality of the methodology of included case series, cohort, and cross-sectional studies with the National Health Institute (NIH) tool [26]. Two NIH tools were used: one for case series (composed of 9 questions) and one for cohort and cross-sectional studies (14 questions). Each question was given a score of 0, 1, or 2 for not reported, no, or yes. The overall quality was determined as good, fair, or poor based on the overall score. Each study was evaluated by two sets of two reviewers [BEK, HB, RAF, HAS]. Any conflict between the authors was resolved through discussion or with senior author consultation [AA and MMO]. The certainty of reported findings regarding each outcome was assessed using the Grading of Recommendations, Assessment, Development, and Evaluations framework.
Data analysis
All meta-analyses were conducted per eye with the help of STATA Software (Version 17). Important to mention that minor amendments were made to our previous analysis plan (CRD42022316367), mainly due to the unavailability of relevant data regarding secondary outcomes [visual field index and MMO area %] in at least two studies. These parameters were replaced with IOP, AL and SE. We used the metaprop command to pool the overall prevalence of MMO among glaucoma patients [27]. Meanwhile, we used the metan command to pool the difference between MMO and non-MMO groups in terms of secondary outcomes [28]. The choice of random-effects and fixed-effects models was based solely on the presence or absence of heterogeneity, respectively. The presence of heterogeneity was confirmed through the I2 statistic (of >50%) and P value of <0.05.
The prevalence rate of MMO in glaucoma patients was pooled using the random-effects model due to the presence of significant heterogeneity (I2 > 50%), and a subgroup analysis was conducted based on the type of glaucoma.
In terms of secondary outcomes, the mean difference in continuous outcomes (age, IOP, AL, MD, and SE) was calculated along with its corresponding 95% CI through the Inverse Variance (IV) method due to the absence of significant heterogeneity. However, when heterogeneity was encountered (i.e., MD of VF, the restricted maximum likelihood method (REML) was used. Meanwhile, the Mantel Haenszel (MH) method was used to measure the log odds ratio (logOR) of dichotomous outcomes (male gender and stage of glaucoma) between MMO and non-MMO groups. Importantly, the REML method was used to assess the logOR of early glaucoma stage between MMO and non-MMO groups due to the presence of significant heterogeneity. The assessment of publication bias was not feasible due to the inappropriate number of included studies in each analysis (<10 studies). Finally, a leave-one-out sensitivity analysis was performed to assess the impact of removing one study at a time on the pooled effect estimate to determine whether or not the reported estimate was driven by a single study [29].
Results
Database search results
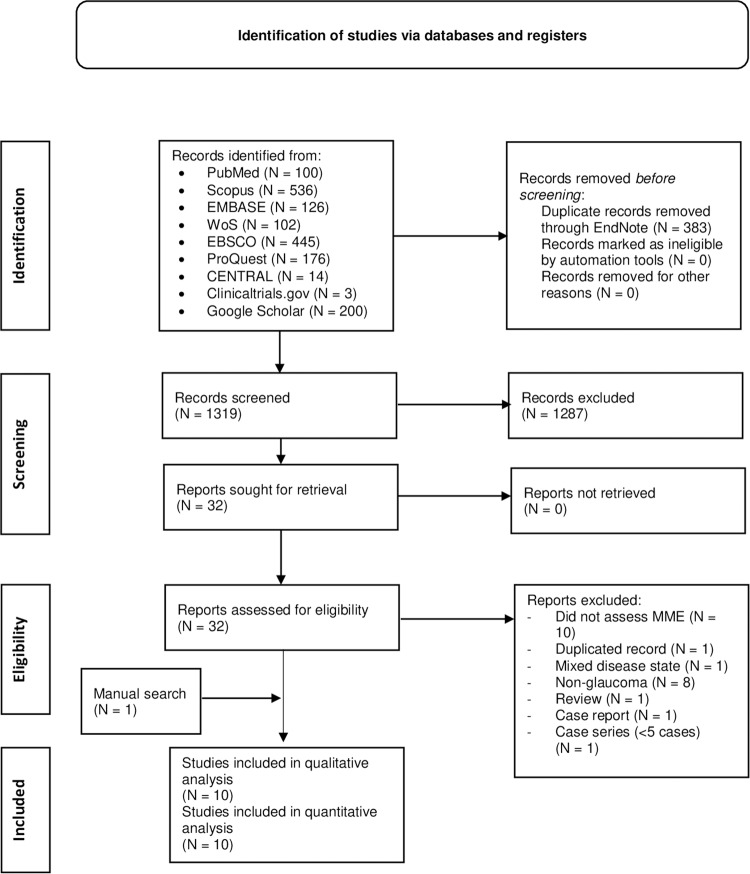
This database search yielded 1702 records, of which 383 were identified as duplicated and were excluded through the use of EndNote (Version 8). The titles and abstracts of 1319 records were then screened, out of which 1287 were found ineligible. The full texts of 32 articles were then retrieved and screened. Eventually, nine articles were found to be consistent with our eligibility criteria and were assessed in this review. Noteworthy, one article was found through manual search [14], resulting in a total number of ten articles that were qualitatively and quantitatively analysed [6, 8, 14, 16, 17, 19–21, 30, 31] [Fig. 1].
Fig. 1. PRISMA flow diagram of the database search and screening processes.
N Number.
Baseline characteristics of included studies
The baseline characteristics of included studies, as well as the definition and diagnostic criteria implemented in each study, are reported in Table 1. Three studies were cross-sectional [16, 19, 30], one was a prospective cohort [17], two were retrospective case series [6, 8], and four were retrospective cohorts in design [14, 20, 21, 31]. Four studies were conducted in the USA [6, 8, 14, 21], two in France [17, 31], three in Japan [16, 19, 20], and one in Switzerland [30]. One study included patients with paediatric glaucoma [8], two studies included patients with mixed glaucoma types [20, 30], and seven studies included patients with primary open-angle glaucoma [6, 14, 16, 17, 19, 21, 31]. The stage of glaucoma was reported in only four studies [6, 16, 20, 21].
Table 1.
Baseline characteristics of included studies (N = 10).
| Author/YOP | Country | Glaucoma | Design | MME | Sample Size | Male | Age | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type | Stage | Definition | Diagnostic Method | Patients | Eyes | N | T | Mean | SD | |||
| Wolff et al. [17] | France | POAG | NR | Prospective Cohort | Hyporeflective perifoveal crescent-shaped lesions [small round hyporeflective microcysts confined to the retinal INL] | ‘En-face’ SD-OCT | NR | 85 | NR | NR | 43.4 | 3.3 |
| Brazerol et al. [30] | Switzerland | POAG - PEXG | NR | Cross-sectional | Vertical vacuoles in the INL of the macula | High-resolution OCT scans | 218 | 282 | NR | NR | 66 | 3.7 |
| El Maftouhi et al. [31] | France | POAG | NR | Retrospective Cohort | Pseudocysts in the INL of the macula | B-scan + ‘en-face’ OCT + central 10–2 VF analysis | 216 | 216 | NR | NR | NR | NR |
| Hasegawa et al. [16] | Japan | POAG | Early, moderate, and advanced | Cross-sectional | Microcystic INL changes [cystic, lacunar areas of hyporeflectivity with clear boundaries, excluding lesions due to speckling artefact, in two or more adjacent B-scans] | Spectralis HRA + OCT | 143 | 258 | NA | 62 | 57.5 | 11.2 |
| Hasegawa et al. [19] | Japan | POAG | NR | Cross-sectional | INL microcystic lesions [coterminous with the dark areas of the cone mosaics on AO-SLO images] | B-scan + SD-OCT + ‘en-face’ SS-OCT | 35 | 35 | 17 | 17 | 60.4 | 10.4 |
| Jiramongkolchai et al. [8] | USA | Paediatric glaucoma | NR | Retrospective Case Series | Isolated microcystic changes [macular INL cysts] | SD-OCT | 227 | 227 | 103 | 227 | 12.16 | 4.18 |
| Mahmoudinezhad et al. [21] | USA | POAG | Mild, moderate, and advanced | Retrospective Cohort | Small hyporeflective round-elliptical cystoid spaces, without cyst walls, located in the INL and not confluent with cystoid spaces in other retinal layers. | Spectralis OCT | 315 | 315 | 146 | 315 | 61.61 | 9.91 |
| Murata et al. [20] | Japan | POAG - NTG - DG - PACG - PEXG - SOAG | Early, moderate, and advanced | Retrospective Cohort | Vacuoles observed in the INL on SD-OCT | ‘En-face’ SS-OCT | 341 | 636 | 183 | 341 | 61.36 | 14.83 |
| Wen et al. [14] | USA | POAG | NR | Retrospective Cohort | Numerous INL microcysts throughout the macula | OCT | 160 | 160 | NR | NR | NR | NR |
| Govetto et al. [6] | USA | POAG | Mild, moderate, and severe | Retrospective Case Series | Multiple, small hyporeflective roundish-elliptical cystoid spaces, without the presence of cyst wall, located in the INL and not confluent with cystoid spaces in other retinal layers | SD-OCT | 51 | 51 | NR | NR | NR | NR |
YOP year of publication, USA United States of America, POAG primary open-angle glaucoma, PEXG pseudoexfoliative glaucoma, NTG normal-tension glaucoma, PACG primary angle-closure glaucoma, SOAG secondary open-angle glaucoma, NR not reported, MME microcystic macular edema, INL inner nuclear layer, OCT optical coherence tomography, SD spectral domain, SS swept-source, VF visual field, HRA.
Quality assessment
The quality of eight studies was assessed using the NIH tool for cross-sectional and cohort studies. Overall, only one study showed good quality [16], while the remaining studies showed fair quality [14, 17, 19–21, 30, 31] (Table 2). Meanwhile, two case series studies were evaluated using the NIH tool for case series, and both showed good overall quality [6, 8] (Table 3).
Table 2.
Quality assessment of included cohort and cross-sectional studies using the NIH quality assessment tool.
| Author, YOP | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Q11 | Q12 | Q13 | Q14 | Overall Quality |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wolff et al. [17] | Y | N | Y | Y | NR | Y | N | N | N | N | Y | NR | NR | N | Fair |
| Brazerol et al. [30] | Y | Y | Y | Y | NR | Y | N | N | N | N | Y | NR | NR | N | Fair |
| El Maftouhi et al. [31] | Y | N | Y | Y | NR | Y | N | N | N | N | Y | NR | Y | N | Fair |
| Hasegawa et al. [16] | Y | Y | Y | Y | NR | Y | Y | Y | N | N | Y | NR | Y | N | Good |
| Hasegawa et al. [19] | N | Y | Y | Y | NR | Y | NR | N | N | N | Y | NR | NR | N | Fair |
| Mahmoudinezhad et al. [21] | Y | Y | N | Y | NR | Y | Y | Y | N | N | Y | NR | NR | Y | Fair |
| Murata et al. [20] | Y | Y | N | Y | NR | Y | NR | Y | N | N | Y | NR | NR | N | Fair |
| Wen et al. [14] | Y | Y | N | N | NR | Y | NR | N | N | N | Y | NR | NR | N | Fair |
Quality was rated as poor (if a study scored 0–4 out of 14 questions), fair (if a study scored 5–10 out of 14 questions), or good (if a study scored 11–14 out of 14 questions).
YOP year of publication, Y yes, N no, NR not reported, NIH National Institute of Health.
Q1: Was the research question or objective in this paper clearly stated? Q2: Was the study population clearly specified and defined? Q3: Was the participation rate of eligible persons at least 50%? Q4: Were all the subjects selected or recruited from the same or similar populations (including the same time period)? Were inclusion and exclusion criteria for being in the study prespecified and applied uniformly to all participants? Q5: Was a sample size justification, power description, or variance and effect estimates provided?’ Q6: For the analyses in this paper, were the exposure(s) of interest measured prior to the outcome(s) being measured? Q7: Was the timeframe sufficient so that one could reasonably expect to see an association between exposure and outcome if it existed? Q8: For exposures that can vary in amount or level, did the study examine different levels of the exposure as related to the outcome (e.g., categories of exposure, or exposure measured as continuous variable)? Q9: Were the exposure measures (independent variables) clearly defined, valid, reliable, and implemented consistently across all study participants? Q10: Was the exposure(s) assessed more than once over time? Q11: Were the outcome measures (dependent variables) clearly defined, valid, reliable, and implemented consistently across all study participants? Q12: Were the outcome assessors blinded to the exposure status of participants? Q13: Was loss to follow-up after baseline 20% or less? Q14: Were key potential confounding variables measured and adjusted statistically for their impact on the relationship between exposure(s) and outcome(s)?
Table 3.
Quality assessment of included cohort and cross-sectional studies using the NIH quality assessment tool.
| Author, YOP | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Overall Quality |
|---|---|---|---|---|---|---|---|---|---|---|
| Jiramongkolchai et al. [8] | Y | Y | Y | Y | Y | Y | Y | Y | Y | Good |
| Govetto et al. [6] | Y | Y | Y | Y | Y | Y | Y | Y | Y | Good |
Quality was rated as poor (if a study scored 0–3 out of 9 questions), fair (if a study scored 4–6 out of 9 questions), or good (if a study scored 7–9 out of 9 questions).
YOP year of publication, Y yes, N no, NR not reported.
Q1: Was the study question or objective clearly stated? Q2: Was the study population clearly and fully described, including a case definition? Q3: Were the cases consecutive? Q4: Were the subjects comparable? Q5: Was the intervention clearly described? Q6: Were the outcome measures clearly defined, valid, reliable, and implemented consistently across all study participants? Q7: Was the length of follow-up adequate? Q8: Were the statistical methods well-described? Q9: Were the results well-described?
The overall prevalence of MMO in glaucoma
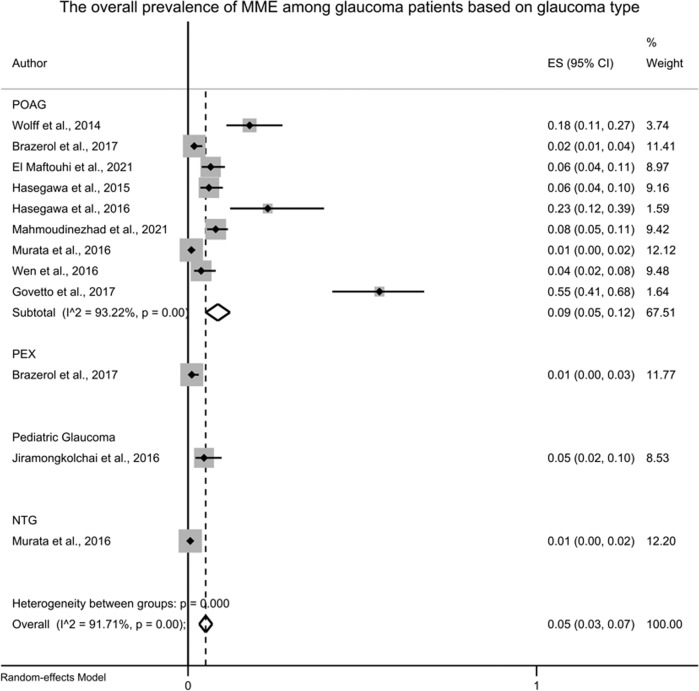
A total of ten studies reported the prevalence rate of MMO among glaucoma patients [6, 8, 14, 16, 17, 19–21, 30, 31], out of which MMO was found in 133 eyes out of 2128 glaucomatous eyes, with a pooled prevalence rate of 8% [95%CI: 5–12%, I2 = 91.28%] which surpasses the rare event assumption (rejects our initial hypothesis). The leave-one-out sensitivity analysis did not reveal any remarkable change from the reported overall effect estimate [Table 4]. The prevalence of MMO was then assessed based on the type of glaucoma [Fig. 2], where MMO was more prevalent in primary open-angle glaucoma [nine studies, ES = 0.09; 95%CI: 0.05–0.12], followed by paediatric glaucoma [one study, ES = 0.05; 95%CI: 0.02: 0.10], pseudoexfoliative glaucoma [one study, ES = 0.01; 95%CI: 0.00: 0.03], and normal-tension glaucoma [one study, ES = 0.01; 95%CI: 0.00: 0.02], respectively. Representative images of MMO in glaucoma and their associated visual fields are presented as Supplementary Fig. 1.
Table 4.
The results of the main meta-analysis and leave-one-out sensitivity analysis of the prevalence of MME among glaucoma patients.
| Model | Model Description | ES (95% CI) | I2 |
|---|---|---|---|
| 1 | Excluding [Wolff et al.] [17] | 0.07 (0.04–0.11) | 91.20% |
| 2 | Excluding [Brazerol et al.] [30] | 0.10 (0.06–0.14) | 92.23% |
| 3 | Excluding [El Maftouhi et al.] [31] | 0.09 (0.05–0.12) | 91.94% |
| 4 | Excluding [Hasegawa et al.] [16] | 0.09 (0.05–0.12) | 92.00% |
| 5 | Excluding [Hasegawa et al.] [19] | 0.08 (0.04–0.11) | 91.62% |
| 6 | Excluding [Jiramongkolchai et al.] [8] | 0.09 (0.05–0.13) | 92.20% |
| 7 | Excluding [Mahmoudinezhad et al.] [21] | 0.08 (0.05–0.12) | 92.40% |
| 8 | Excluding [Murata et al.] [20] | 0.10 (0.06–0.14) | 89.58% |
| 9 | Excluding [Wen et al.] [14] | 0.10 (0.06–0.14) | 89.37% |
| 10 | Excluding [Govetto et al.] [6] | 0.06 (0.04–0.08) | 69.66% |
| Final Model | Inclusive of all studies | 0.08 (0.05–0.12) | 91.28% |
ES effect size (pooled prevalence), I2 measure of heterogeneity, MME microcystic macular edema, CI confidence interval.
Fig. 2. Forest plot showing the overall prevalence of MME based on type of glaucoma.
MME Microcystic Macular Edema, ES Effect Size, POAG Primary Open-Angle Glaucoma, PEX Pseudoexfoliation Glaucoma, NTG Normal-Tension Glaucoma.
The difference between MMO and non-MMO glaucoma patients in terms of patients’ characteristic
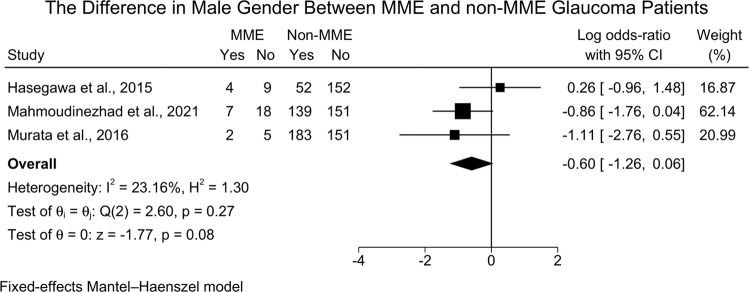
The difference in male gender between MMO and non-MMO glaucoma patients was reported in three studies [873 eyes] [16, 20, 21], and the meta-analysis revealed no significant difference between MMO and non-MMO glaucoma patients [logOR = −0.60; 95%CI: −1.26: 0.06; I2 = 23.16%] [Fig. 3]. However, the leave-one-out sensitivity analysis revealed a statistically significant difference in gender between both groups with the exclusion of the study of Hasegawa et al. [16] [LogOR = −0.92; 95%CI: −1.71: −0.13] [Supplementary Fig. 2].
Fig. 3. Forest plot showing the difference in male gender between MME and non-MME glaucoma patients.
MME Microcystic Macular Edema.
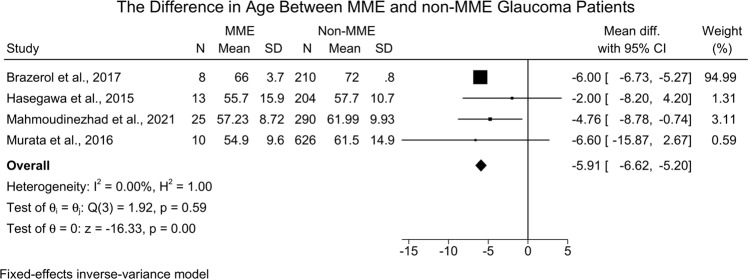
Four studies [1386 eyes] [16, 20, 21, 30] reported the difference in age between MMO and non-MMO groups. The meta-analysis revealed that MMO patients were significantly more likely to present with younger age when compared to the non-MMO group [MD = −5.91; 95%CI: −6.62: −5.20, I2 = 0.00%] (Fig. 4). The leave-one-out sensitivity analysis did not reveal any significant change in the reported effect estimate after ruling out one study at a time [Supplementary Fig. 3].
Fig. 4. Forest plot showing the difference in age between MME and non-MME glaucoma patients.
MME Microcystic Macular Edema, SD Standard Deviation.
The difference between MMO and non-MMO glaucoma patients based on glaucoma stage
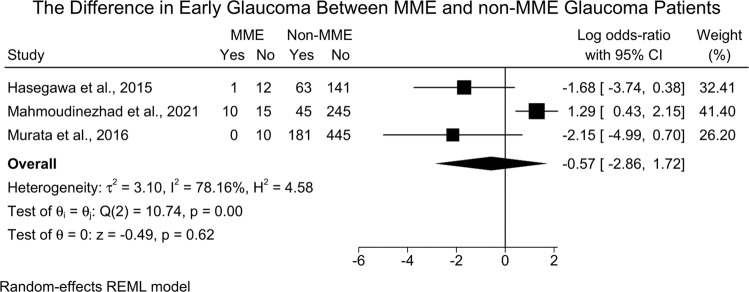
Three studies [16, 20, 21] assessed the difference in MMO and non-MMO glaucoma patients based on stage. No significant difference in the odds of early glaucoma was noted between MMO and non-MMO groups [three studies (1168 eyes), LogOR =−0.57; 95%CI: −2.86: 1.72; I2 = 78.16%] [Fig. 5]. Surprisingly, the leave-one-out sensitivity analysis revealed significantly lower odds of early glaucoma in MMO patients when compared to non-MMO following the exclusion of the study of Mahmoudinezhad et al. [21] [LogOR = −1.84; 95%CI: −3.51: −0.17] [Supplementary Fig. 4].
Fig. 5. Forest plot showing the difference in early glaucoma between MME and non-MME patients.
MME Microcystic Macular Edema.
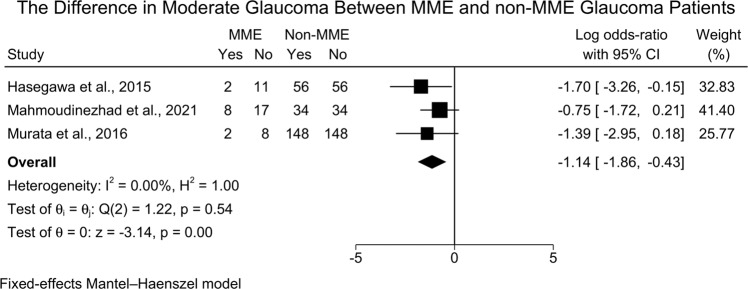
However, MMO patients were less likely to present with moderate glaucoma when compared to non-MMO patients [three studies (524 eyes), LogOR = −1.14; 95%CI: −1.86: −0.43, I2 = 0.00%] [Fig. 6]. The leave-one-out sensitivity analysis did not reveal any significant change in the reported effect estimate after ruling out one study at a time [Supplementary Fig. 5].
Fig. 6. Forest plot showing the difference in moderate glaucoma between MME and non-MME patients.
MME Microcystic Macular Edema.
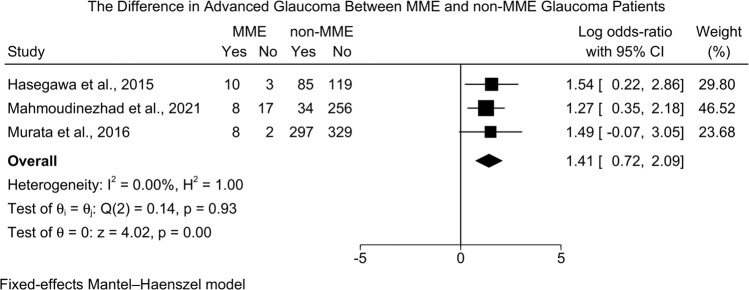
On the other hand, MMO patients were more likely to present with advanced glaucoma as compared to non-MMO patients [three studies (1168 eyes), LogOR=1.41; 95%CI: 0.72: 2.09, I2 = 0.00%] [Fig. 7]. The leave-one-out sensitivity analysis did not reveal any significant change in the reported effect estimate after ruling out one study at a time [Supplementary Fig. 6].
Fig. 7. Forest plot showing the difference in advanced glaucoma between MME and non-MME patients.
MME Microcystic Macular Edema.
The difference between MMO and non-MMO glaucoma patients in terms of ocular parameters
Four ocular parameters were assessed, including AL, IOP, SE, and MD of visual field. No statistically significant difference was noted between MMO and non-MMO groups regarding AL [two studies [532 eyes], MD = −0.04; 95%CI: −0.68: 0.60, I2 = 0.00%]. Similarly, no significant difference was noted between MMO and non-MMO glaucoma patients in terms of IOP [two studies [532 eyes]; MD = −0.04; 95%CI: −1.04: 0.96, I2 = 0.00%] and SE [two studies [532 eyes]; MD = 0.08; 95%CI: −0.93: 1.10, I2 = 0.00%]. The primary analysis revealed no significant change in the MD of visual field [three studies [1168 eyes]; MD = −3.36; 95%CI: −6.91: 0.19, I2 = 0.00%]. However, the leave-one-out sensitivity analysis revealed significant difference with the exclusion of the study of Hasegawa et al. [16] [MD = −5.00; 95%CI: −7.01: −2.99].
Other outcomes
The assessment of the difference of central corneal thickness (CCT) [21], visual acuity (VA) [21], number of anti-glaucoma medications (AGM) [21], RNFL thickness [30], and the type of glaucoma [30] between MMO and non-MMO glaucoma patients was not feasible due to the lack of appropriate number of studies (<two studies).
GRADE assessment
Since our evidence was based primarily on observational studies, the grading of available evidence in each of the assessed outcomes was reported as very low. A full description of the certainty of evidence regarding each outcome is reported in Table 5.
Table 5.
Summary of findings table including the assessment of the certainty of evidence through the use of the GRADE framework for reported outcomes in our review.
| Outcomes | Category | Outcome Endpoint | Studies (N)/ Eyes (N) | Estimate [95%CI] | Study Limitations | Inconsistency | Indirectness | Imprecision | Publication Bias | Certainty of Evidence (GRADE) |
|---|---|---|---|---|---|---|---|---|---|---|
| Primary Outcome [The overall prevalence of MME in glaucoma] | ||||||||||
| Prevalence of MME | 10/2128 | 0.09 [0.05: 0.12] | Fair quality | Y | — | — | — | ⊕⊝⊝⊝ Very Low | ||
| Secondary Outcomes [MME vs. Non-MME] | ||||||||||
| Patients’ characteristics | ||||||||||
| Age | 4/1386 | MD = −5.91 [−6.62: −5.20] | Fair quality | — | — | — | N/A | ⊕⊝⊝⊝ Very Low | ||
| Gender [Male] | 3/873 | logOR= 0.60 [−1.26: 0.06] | Fair quality | — | — | — | N/A | ⊕⊝⊝⊝ Very Low | ||
| Stage of glaucoma | ||||||||||
| Early | 3/1168 | LogOR = −1.84 [−3.51: −0.17]a | Fair quality | Y | — | Y | N/A | ⊕⊝⊝⊝ Very Low | ||
| Moderate | 3/524 | LogOR = −1.14 [1.86: −0.43] | Fair quality | — | — | — | N/A | ⊕⊝⊝⊝ Very Low | ||
| Advanced | 3/1168 | LogOR= 1.41 [0.72: 2.09] | Fair quality | — | — | — | N/A | ⊕⊝⊝⊝ Very Low | ||
| Ocular parameters | ||||||||||
| Axial Length | 2/532 | MD = −0.04 [−0.68: 0.60] | Fair quality | — | — | — | N/A | ⊕⊝⊝⊝ Very Low | ||
| Intraocular Pressure | 2/532 | MD = −0.04 [−1.04: 0.96] | Fair quality | — | — | — | N/A | ⊕⊝⊝⊝ Very Low | ||
| Mean Deviation of VF | 3/1168 | MD = −5.00 [−7.01: −2.99]b | Fair quality | Y | — | Y | N/A | ⊕⊝⊝⊝ Very Low | ||
| Spherical Equivalent | 2/532 | MD = −0.04 [−0.68: 0.60] | Fair quality | — | — | — | N/A | ⊕⊝⊝⊝ Very Low | ||
N number, N/A not applicable because the meta-analysis was based on only one study, RoB risk of bias, GRADE Grading of Recommendations, Assessment, Development and Evaluation, Y yes, MD mean difference, LogOR log odds ratio, VF visual field, MME microcystic macular edema
aThis effect estimate was reported as per the leave-one-out sensitivity analysis following the exclusion of the study of Mahmoudinezhad et al. [21]
bThis effect estimate was reported as per the leave-one-out sensitivity analysis following the exclusion of the study of Hasegawa et al. [16]
Discussion
This is the first systematic review and meta-analysis to investigate the prevalence rate of MMO among glaucoma patients and to compare MMO to non-MMO glaucoma patients in terms of patients’ characteristics, glaucoma stage, and ocular parameters. Our results reveal that MMO is not a rare observation among glaucoma patients since it exceeds the rare event assumption with an overall rate of 8%, which is slightly higher (9%) in the POAG subgroup. That being said, we propose that the actual rate of MMO in glaucoma could even be higher than ours because MMO is difficult to diagnose, most probably overlooked, and commonly under-reported in the literature. In addition, MMO may be missed using OCTs with a lower number of B-scans, lower resolution, or older generations.
Since MMO is diagnosed based on the presence of hypo-reflective peri-foveal crescentic microcysts (without a cyst wall) confined to the INL of the retina, the use of the ‘en-face’ OCT in this setting would be recommended [17, 20, 31] to properly observe these microcystic changes, which are often used interchangeably with MMO [6, 16, 17]. Our findings reject the initial hypothesis of rare event assumption (<5%); thus, we recommend clinical ophthalmologists to consider MMO investigation in glaucoma diagnostic workup and follow-up. Most of the reported literature discussed the prevalence of MMO among POAG; however, other types of glaucoma, including secondary glaucoma, paediatric glaucoma, or angle closure glaucoma have received little attention. Although our subgroup analysis reveals a greater prevalence of MMO among POAG patients when compared to other types, our findings are not reflective of the actual rates since the other types of glaucoma were reported in only one study. Therefore, future research should be focused on investigating all types of glaucoma to determine whether or not MMO is an observation reflective of the underlying pathology in a certain type of glaucoma.
MME is defined as vertical vacuoles predominantly located in the INL on OCT or B scans [6, 14, 30, 31]. MMO has been first described in multiple sclerosis patients [9]. After that, MMO has been reported in many conditions, including POAG [21]. The pathophysiology of MMO in glaucoma patients is not yet well understood. However, Gelfand et al. [9] suggested that it occurs due to blood-retinal barrier disruption or due to the occurrence of a local inflammatory process. Meanwhile, Abegg et al. [12] explained the occurrence of MMO in optic neuropathy due to retrograde trans-synaptic degeneration of the INL and retinal ganglion cells with secondary formation of cystic spaces. MMO is most commonly located in the parafoveal area [7]. It is thought that a relationship with Müller cell function exists due to MMO’s almost exclusive localisation within the inadequately vascularises perimacular rim [13]. In vitro models of different retinopathies, Müller cells reduced the expression of their major potassium channel Kir4.1, which disrupted the rapid water transport through Müller cell membranes; thus, leading to cellular swelling [32].
MME is thought to be correlated with disease progression and worse clinical outcomes. In the study of Gelfand et al. [9] patients with multiple sclerosis and MMO had a significantly worse disability and higher scores of disease progression than those without MMO. Furthermore, it was observed that the presence of MMO was associated with a poor long-term functional outcome in patients with age-related macular degeneration compared with these patients without MMO [32]. In the same context, we suggest that MMO is associated with glaucoma progression and a worse disease state. Our analysis revealed that MMO patients, when compared to non-MME patients, were at higher risk of advanced glaucoma. This was further supported by the observation that MMO was associated with reduced risk for both early and moderate glaucoma stages. Similarly, Govetto et al. [6] and Abegg et al. [12] also reported that the prevalence of MMO increased significantly at the later stages of glaucoma. Our findings go in line with the observations of Mahmoudinezhad et al. [21] that show that MMO is significantly associated with severe glaucoma and worse visual field outcomes which are reflective of advanced stage and disease progression. That being said, our observations are based mainly on moderate quality research with very low certainty; therefore, we recommend the conduct of more studies, including different types and stages of glaucoma, with enough sample size and power to properly determine if MMO is associated with disease progression and/or poor visual outcomes.
Interestingly, younger age has been reported as one of the main risk factors for the occurrence of MMO in glaucoma patients [21]. Our findings support this observation as MMO was more likely to be diagnosed at a younger age when compared to non-MME. The association between young age and the presence of MMO could be explained by the thicker neural and glial tissues in younger patients, which may make identifying cystic spaces easier [33]. However, this still warrants further investigation.
Our analysis highlights a significant difference in MD of the VF between MMO and non-MME patients, which is in line with the reported literature [21, 31]. Mahmoudinezhad et al. [21] found that patients with MMO were significantly associated with worse baseline superior MD, baseline MD, baseline visual field index (VFI), and baseline VF defects. The authors also reported that MMO location is more frequently seen in the superior hemifield, which is in line with the usual glaucomatous VF loss. Furthermore, El Maftouhi et al. [31] reported a strong correlation between the presence of pseudocysts and paracentral scotoma as measured by central VF analysis. In the study of Murata et al. [20], the authors noted that VF defects detected in 10–2 degrees are more serious than those observed in 24–2 degrees by VF, which is interesting since, in the majority of observed and reported MMO cases, the defects were seen in the parafoveal area. This observation could be reflective of severe disease progression; however, it still warrants further investigation. In this regard, we recommend ophthalmologists to observe MMO-related VF defects in 10–2 degrees as well during glaucoma workup and follow-up assessments.
In our study, we noted no significant difference between MMO and non-MME glaucoma patients in terms of ocular parameters of axial length, spherical equivalent, or IOP. This could be related to the low number of available studies assessing these points. Also, the meta-analysis of the difference between both groups in terms of other parameters such as CCT, VA, number of AGM, and RNFL thickness was not feasible due to the lack of sufficient data. Therefore, further research is still needed in this regard.
Limitations and recommendations for future research
Although our study provides insight into the occurrence rate of MMO as well as associated factors, our study encountered several limitations, and therefore, the findings reported here should be interpreted with caution. First, the reported prevalence rate of MMO among glaucoma patients in our study might not be reflective of the actual rate in clinical practice. For instance, MMO is underdiagnosed and is minimally looked for in diagnostic workup. In addition, the majority of included studies were cohort and/or case series, with minimal studies being of cross-sectional design, which is the most appropriate design for estimating the prevalence of this finding. Second, the reported confidence interval in a number of our analyses was wide, indicating imprecision in the reported findings, and this can be explained by the small number of included studies. Third, we encountered considerable heterogeneity in some analyses, which could not be assessed due to the minimal number of studies. Fourth, the quality of the majority of included studies was fair, and subsequently, the certainty in reported findings was deemed as very low. Finally, we could not meta-analyse the difference between MMO and non-MME glaucoma patients in terms of certain variables due to the unavailability of relevant data (i.e., CCT, VA, AGM, RNFL thickness, and type of glaucoma). Therefore, we encourage the conduct of properly-designed studies for the assessment of MMO prevalence (cross-sectional studies) and for the assessment of differences between MMO and non-MME glaucoma patients (case–control studies) while putting the previously mentioned variables into account.
Conclusion
Microcystic macular oedema surpasses the rare event assumption and is a frequent observation in patients with glaucoma. It is also observed in younger patient populations and is associated with disease progression. Those with the early and moderate disease had a reduced risk of MMO, while those with advanced stages had both statistically and clinically significant increased risk of presenting with MMO. However, the certainty of these findings remains very low. Properly-designed research is still warranted to confirm these findings.
Supplementary information
Description of Additional Supplementary Files
Author contributions
AA: conceptualisation of the research idea, protocol registration, idea validation, data checking, data analysis, manuscript writing, and revision and approval of the final version of the manuscript; MMO: conceptualisation of the research idea, protocol registration, idea validation, data checking, manuscript writing, and revision and approval of the final version of the manuscript; BA: conceptualisation of the research idea, formulating the search query for each database, carrying out the database search, substantial editing and revision of the manuscript, and approval of the final version of the manuscript; BEK, HB, and RAF: screening of retrieved records, extraction of data for analysis, quality assessment, and approval of the final manuscript; HAS: substantial contribution to the writing of the manuscript as well as editing for the improvement of the quality of the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Abdelaziz Abdelaal, Mennatullah Mohamed Eltaras.
Supplementary information
The online version contains supplementary material available at 10.1038/s41433-023-02524-w.
References
- 1.Weinreb RN, Khaw PT. Primary open-angle glaucoma. Lancet. 2004;363:1711–20. doi: 10.1016/S0140-6736(04)16257-0. [DOI] [PubMed] [Google Scholar]
- 2.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262–7. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thylefors B, Negrel A. The global impact of glaucoma. Bull World Health Organ. 1994;72:323. [PMC free article] [PubMed] [Google Scholar]
- 4.Airaksinen P. Clinical evaluation of the optic disc and retinal nerve fiber layer. Glaucomas. 1996.
- 5.Stamper RL, Lieberman MF, Drake MV. Becker-Shaffer’s. Diagnosis and therapy of the glaucomas 7th ed St Louis: Mosby. 1999:15.
- 6.Govetto A, Su D, Farajzadeh M, Megerdichian A, Platner E, Ducournau Y, et al. Microcystoid macular changes in association with idiopathic epiretinal membranes in eyes with and without glaucoma: clinical insights. Am J Ophthalmol. 2017;181:156–65.. doi: 10.1016/j.ajo.2017.06.023. [DOI] [PubMed] [Google Scholar]
- 7.Kisimbi J, Shalchi Z, Mahroo OA, Mhina C, Sanyiwa AJ, Mabey D, et al. Macular spectral domain optical coherence tomography findings in Tanzanian endemic optic neuropathy. Brain. 2013;136:3418–26. doi: 10.1093/brain/awt221. [DOI] [PubMed] [Google Scholar]
- 8.Jiramongkolchai K, Freedman SF, El-Dairi MA. Retinal changes in pediatric glaucoma and nonglaucomatous optic atrophy. Am J Ophthalmol. 2016;161:188–95.e1. doi: 10.1016/j.ajo.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 9.Gelfand JM, Nolan R, Schwartz DM, Graves J, Green AJ. Microcystic macular oedema in multiple sclerosis is associated with disease severity. Brain. 2012;135:1786–93. doi: 10.1093/brain/aws098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhatti MT, Mansukhani SA, Chen JJ. Microcystic macular edema in optic nerve glioma. Ophthalmology. 2020;127:930. doi: 10.1016/j.ophtha.2020.03.017. [DOI] [PubMed] [Google Scholar]
- 11.Voide N, Borruat FX. Microcystic macular edema in optic nerve atrophy: a case series. Klin Monatsblatter Augenheilkunde. 2015;232:455–8. doi: 10.1055/s-0035-1545797. [DOI] [PubMed] [Google Scholar]
- 12.Abegg M, Dysli M, Wolf S, Kowal J, Dufour P, Zinkernagel M. Microcystic macular edema: retrograde maculopathy caused by optic neuropathy. Ophthalmology. 2014;121:142–9. doi: 10.1016/j.ophtha.2013.08.045. [DOI] [PubMed] [Google Scholar]
- 13.Burggraaff MC, Trieu J, de Vries-Knoppert WA, Balk L, Petzold A. The clinical spectrum of microcystic macular edema. Investig Ophthalmol Visual Sci. 2014;55:952–61. doi: 10.1167/iovs.13-12912. [DOI] [PubMed] [Google Scholar]
- 14.Wen JC, Freedman SF, El-Dairi MA, Asrani S. Microcystic macular changes in primary open-angle glaucoma. J Glaucoma. 2016;25:258–62. doi: 10.1097/IJG.0000000000000129. [DOI] [PubMed] [Google Scholar]
- 15.Reichenbach A, Wurm A, Pannicke T, Iandiev I, Wiedemann P, Bringmann A. Müller cells as players in retinal degeneration and edema. Graefe’s Arch Clin Exp Ophthalmol. 2007;245:627–36. doi: 10.1007/s00417-006-0516-y. [DOI] [PubMed] [Google Scholar]
- 16.Hasegawa T, Akagi T, Yoshikawa M, Suda K, Yamada H, Kimura Y, et al. Microcystic inner nuclear layer changes and retinal nerve fiber layer defects in eyes with glaucoma. PloS One. 2015;10:e0130175. doi: 10.1371/journal.pone.0130175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wolff B, Azar G, Vasseur V, Sahel JA, Vignal C, Mauget-Faÿsse M. Microcystic changes in the retinal internal nuclear layer associated with optic atrophy: a prospective study. J Ophthalmol. 2014;2014:395189. doi: 10.1155/2014/395189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wolff B, Basdekidou C, Vasseur V, Mauget-Faÿsse M, Sahel J-A, Vignal C. Retinal inner nuclear layer microcystic changes in optic nerve atrophy: a novel spectral-domain OCT finding. Retina. 2013;33:2133–8. doi: 10.1097/IAE.0b013e31828e68d0. [DOI] [PubMed] [Google Scholar]
- 19.Hasegawa T, Ooto S, Takayama K, Makiyama Y, Akagi T, Ikeda HO, et al. Cone integrity in glaucoma: an adaptive-optics scanning laser ophthalmoscopy study. Am J Ophthalmol. 2016;171:53–66. doi: 10.1016/j.ajo.2016.08.021. [DOI] [PubMed] [Google Scholar]
- 20.Murata N, Togano T, Miyamoto D, Ochiai S, Fukuchi T. Clinical evaluation of microcystic macular edema in patients with glaucoma. Eye. 2016;30:1502–8. doi: 10.1038/eye.2016.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mahmoudinezhad G, Salazar D, Morales E, Tran P, Lee J, Hubschman JP, et al. Risk factors for microcystic macular oedema in glaucoma. Br J Ophthalmol. 2021. [DOI] [PMC free article] [PubMed]
- 22.Moher D, Altman DG, Liberati A, Tetzlaff J. PRISMA statement. Epidemiology. 2011;22:128. doi: 10.1097/EDE.0b013e3181fe7825. [DOI] [PubMed] [Google Scholar]
- 23.Muka T, Glisic M, Milic J, Verhoog S, Bohlius J, Bramer W, et al. A 24-step guide on how to design, conduct, and successfully publish a systematic review and meta-analysis in medical research. Eur J Epidemiol. 2020;35:49–60. doi: 10.1007/s10654-019-00576-5. [DOI] [PubMed] [Google Scholar]
- 24.Amir-Behghadami M, Janati A. Population, Intervention, Comparison, Outcomes and Study (PICOS) design as a framework to formulate eligibility criteria in systematic reviews. Emerg Med J. 2020;37:387. doi: 10.1136/emermed-2020-209567. [DOI] [PubMed] [Google Scholar]
- 25.Ghozy S, Kacimi SEO, Azzam AY, Farahat RA, Abdelaal A, Kallmes KM, et al. Successful mechanical thrombectomy in acute ischemic stroke: revascularization grade and functional independence. J NeuroInterv Surg. 2022;14:779–82. doi: 10.1136/neurintsurg-2021-018436. [DOI] [PubMed] [Google Scholar]
- 26.National Heart L, Institute B. National Institute of Health, Quality assessment tool for observational cohort and cross-sectional studies. Bethesda: National Heart. Lung, and Blood Institute. 2014.
- 27.Nyaga VN, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Health. 2014;72:1–10.. doi: 10.1186/2049-3258-72-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fisher DJ, Zwahlen M, Egger M, Higgins JP. Meta‐analysis in stata. systematic reviews in health research: meta‐analysis in context. 2022;22:481–509.
- 29.Abdellatif M, Ghozy S, Kamel MG, Elawady SS, Ghorab MME, Attia AW, et al. Association between exposure to macrolides and the development of infantile hypertrophic pyloric stenosis: a systematic review and meta-analysis. Eur J Pediatr. 2019;178:301–14.. doi: 10.1007/s00431-018-3287-7. [DOI] [PubMed] [Google Scholar]
- 30.Brazerol J, Iliev ME, Höhn R, Fränkl S, Grabe H, Abegg M. Retrograde maculopathy in patients with glaucoma. J Glaucoma. 2017;26:423–9. doi: 10.1097/IJG.0000000000000633. [DOI] [PubMed] [Google Scholar]
- 31.El Maftouhi A, Quaranta-El Maftouhi M, Baudouin C, Denoyer A. Cystic maculopathy of the inner nuclear layer in glaucoma patients. J Francais d’Ophtalmol. 2021;44:786–91. doi: 10.1016/j.jfo.2020.11.010. [DOI] [PubMed] [Google Scholar]
- 32.Querques G, Coscas F, Forte R, Massamba N, Sterkers M, Souied EH. Cystoid macular degeneration in exudative age-related macular degeneration. Am J Ophthalmol. 2011;152:100–7. e2. doi: 10.1016/j.ajo.2011.01.027. [DOI] [PubMed] [Google Scholar]
- 33.Leung CK, Ye C, Weinreb RN, Yu M, Lai G, Lam DS. Impact of age-related change of retinal nerve fiber layer and macular thicknesses on evaluation of glaucoma progression. Ophthalmology. 2013;120:2485–92. doi: 10.1016/j.ophtha.2013.07.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files