Abstract
Aims
Invariant natural killer T (iNKT) cells, a T cell subset that is CD1d-restricted and expresses a semi-invariant T cell receptor, have been proposed to contribute to dyslipidaemia-driven cardiovascular disease due to their ability to specifically recognize lipid antigens. Studies in mice have attributed pro-atherogenic properties to iNKT cells, but studies in humans investigating associations of iNKT cells with incident coronary events (CE) are lacking.
Methods and results
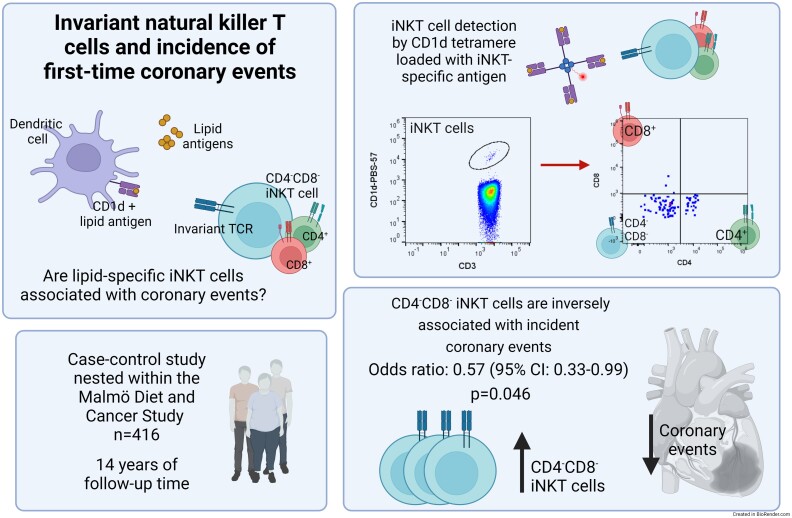
Here, we used flow cytometry to enumerate circulating iNKT cells (CD3+ CD1d-PBS57-Tetramer+) in a case-control cohort nested within the prospective population-based Malmö Diet and Cancer Study (n = 416) to explore associations with incident first-time CE during a median follow-up of 14 years. We found a significant inverse association between CD4− and CD8− double negative (DN) iNKT cells and incident CE, with an odds ratio of 0.62 [95% confidence interval (CI) 0.38–0.99; P = 0.046] comparing the highest vs. the lowest tertile of DN iNKT cells. The association remained significant after adjustment for cardiovascular risk factors with an odds ratio of 0.57 (95% CI 0.33–0.99; P = 0.046). In contrast, total iNKT cells were not significantly associated with incident CE after adjustment, with an odds ratio of 0.74 (95% CI 0.43–1.27; P = 0.276).
Conclusion
Our findings indicate that animal studies suggesting an atherosclerosis-promoting role for iNKT cells may not translate to human cardiovascular disease as our data show an association between high circulating numbers of DN iNKT cells and decreased risk of incident CE.
Keywords: Coronary artery disease, Invariant natural killer T cells, Prospective study, Case-control study, Lymphocytes, Inflammation
Graphical Abstract
Graphical abstract.
Introduction
Atherosclerosis, the main underlying pathology for myocardial infarction and stroke, is a lipid-induced chronic inflammatory disease of the arterial wall. Thus, natural killer T (NKT) cells, immune cells that specifically recognize lipid antigens, are of genuine interest in the pathogenesis of atherosclerosis and are potential treatment targets to ameliorate the burden of cardiovascular disease (CVD). Invariant natural killer T cells (iNKT), also referred to as type I NKT cells, are CD1d-restricted and have a semi-invariant T cell receptor that in humans consists of a Vα24-Jα18 chain coupled with Vβ11.1
Numerous studies in transgenic NKT cell deficient mice (CD1d−, Jα18-deficient mice, or adoptive transfer of CD1d-deficient cells) have led to the conclusion that NKT cells promote atherosclerosis.2–6 Furthermore, several studies in mice treated with the prototypical iNKT cell agonist α-galactosylceramide (α-GalCer) or studies increasing iNKT cell numbers by adoptive transfer have essentially established a pro-atherosclerotic role of iNKT cells in mice, even though atheroprotective effects have been observed in some settings.2–4,7,8 Like conventional T cells, iNKT cells can be stratified based on CD4 and CD8 expression, with most iNKT cells being CD4+ or CD4−CD8− double negative (DN) and rarely CD8+.9 In most atherosclerosis studies in mice the potential different effects of these subsets have not been thoroughly investigated, but To et al. described that CD4+ iNKT cells, but not CD4−CD8− DN cells, exacerbated the development of atherosclerosis.10 It should be noted, however, that the hyperlipidaemic mouse models used in many of these studies may limit the interpretation of the data as both apolipoprotein E and the Low-density lipoprotein (LDL) receptor play an important role in the processing and presentation of lipid antigens by CD1d and absence of either has been shown to limit iNKT cell responses.11,12
Several smaller studies in humans (n = 17–40) have shown that individuals with established atherosclerotic CVD have lower numbers of circulating iNKT cells, mirroring other chronic inflammatory diseases.13–16 A report in systemic lupus erythematosus patients also found lower levels of iNKT cells in patients who had suffered a clinically manifested cardiovascular event, compared to patients with subclinical CVD.17 Importantly, this study found that iNKT cells in patients with subclinical atherosclerosis adopted a more pronounced TH2-like phenotype, characterized by an increased production of IL-4 and increased expression of the TH2-promoting transcription factor GATA3, indicating that iNKT cells with this phenotype may have a protective role in subclinical atherosclerosis. Notably, this potentially protective iNKT cell phenotype was lost in patients with clinically established disease.17
Consequently, the effect of iNKT cells on atherosclerotic CVD in humans might be more complex than anticipated from the studies in mice. Yet, prospective studies in humans investigating circulating iNKT cells and associations with incident cardiovascular events are lacking. We have previously examined interferon-γ expressing NKT-like (CD3+ CD56+) cells and found an inverse association with the incidence of first-time coronary events (CE), although this association was not independent of the LDL/HDL ratio.18
In the current study, we used antigen-loaded CD1d tetramers for the specific detection of iNKT cells to examine associations of circulating iNKT cells and first-time CE in a matched case-control study nested in the Malmö Diet and Cancer (MDC) study.
Methods
Study design
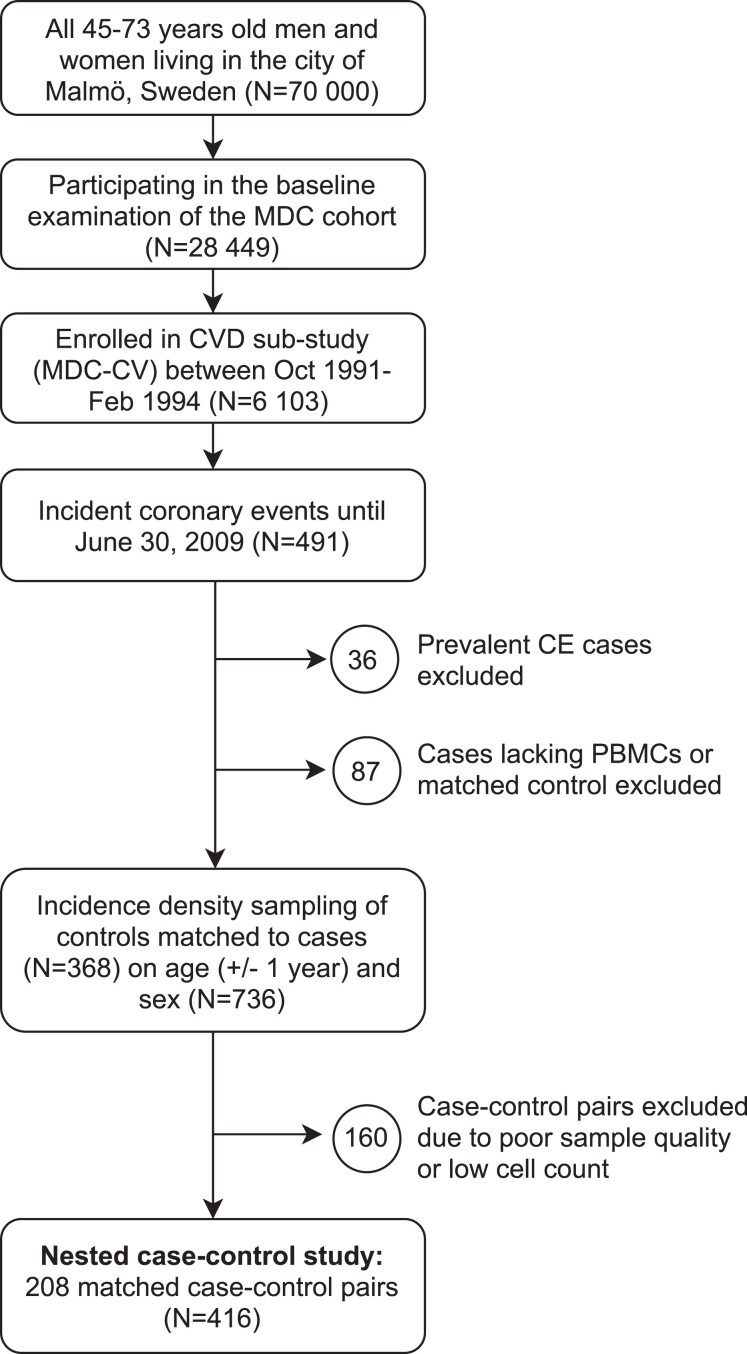
The MDC study is a population-based prospective epidemiological cohort of 28 449 participants enrolled between 1991 and 1996. Between October 1991 and February 1994, every other participant was invited to take part in a sub-study of the epidemiology of CVD (MDC-CV), yielding a cohort of 6103 participants.19 All participants were followed from the baseline examination until the first hospitalization for a CE, death, emigration, or until 30 June 2009. A CE was defined as a nonfatal or fatal myocardial infarction or death due to ischaemic heart disease, based on International Classification of Diseases, Ninth Revision (ICD9) codes 410, 412, and 414 and ICD10 codes I21, I22, I23, and I25. During follow-up, 491 incident cases of patients experiencing a CE (8.0% of the MDC-CV cohort) were retrieved by data linkage to the Swedish Hospital Discharge Register and the Cause of Death Registry of Sweden. CE cases that had suffered a prevalent nonfatal myocardial infarction (n = 36) or lacked a frozen peripheral blood mononuclear cell (PBMC) sample (n = 75) were excluded before an age- and sex-matched case-control study was nested within the prospective population cohort of the MDC study, as described previously.20 Incident CE cases were matched to controls by incidence density sampling of CE-free control participants of the same age (±1 year) and sex. After the exclusion of CE cases where no control could be matched (n = 12), 368 case-control pairs remained. The matching variables were selected because of their nonmodifiable nature. Due to the matched design, the exclusion of samples of poor quality after thawing, samples with too few cells for flow cytometry analysis, samples with less than 25 000 recorded CD3+ events, or non-adherence to the standard operating procedures resulted in a final study cohort of 208 matched case-control pairs. The cohort assembly is described in Figure 1. The study was approved by the regional ethics review board (LU 51-90 and LU 532-2006) and was conducted in accordance with the Declaration of Helsinki. All participants gave written informed consent.
Figure 1.
Malmö Diet and Cancer (MDC) study design flow chart.
Laboratory analyses
Information on anthropometric measures and baseline characteristics was collected from self-administered questionnaires and clinical examinations. Blood pressure (BP) was measured twice in the right arm after a 10-min rest. The average of the two measurements was used. Hypertension was defined as systolic BP ≥140 mmHg or diastolic BP ≥90 mmHg or the use of BP-lowering medication. At a second visit, blood samples were drawn after overnight fasting and fasting venous blood glucose, serum cholesterol, high-density lipoprotein (HDL) cholesterol, C-reactive protein (CRP), white blood cells (WBC), HbA1c, creatinine, and triglycerides were analysed with standard methods at the clinical laboratory of Malmö University Hospital, as described previously.21 LDL was calculated with Friedewald’s formula and the estimated glomerular filtration rate (eGFR) was calculated with the modification of diet in renal disease (MDRD) formula. Interferon γ (IFN-γ), IL-6, IL-18, soluble tumour necrosis factor receptor 1 (sTNF-R1), and sTNF-R2 were analysed in ethylenediaminetetraacetic acid (EDTA) plasma using proximity extension assay. The analysis was performed at the Clinical Biomarkers Facility, Science for Life Laboratory, in Uppsala, Sweden. Diabetes mellitus was defined as fasting whole blood glucose >6.1 mmol/L (corresponding to a threshold of 7.0 mmol/L in fasting plasma glucose), self-reported physician diagnosis of diabetes mellitus, or use of anti-diabetic medication.
Carotid ultrasound
Carotid atherosclerosis was assessed as has been described previously with B-mode ultrasound by specially trained and certified sonographers after completion of an extensive training programme.22 The bifurcation area of the right carotid artery was scanned for plaques, defined as focal thickening of the arterial wall (>1.2 mm) or plaque area >10 mm2, within a pre-defined window encompassing 3 cm of the distal common carotid artery (CCA), the bulb, and 1 cm each of the internal and external carotid arteries.23
Flow cytometry
Peripheral blood mononuclear cell isolation
Blood samples were collected into heparin tubes at baseline. PBMCs were isolated by density gradient centrifugation (Lymphoprep, STEMCELL Technologies; Ficoll-Paque PLUS, GE Healthcare Life Sciences) and resuspended in equal volumes of autologous serum and Roswell Park Memorial Institute (RPMI)-1640 (Thermo Fisher Scientific) containing 20% dimethyl sulfoxide (Sigma-Aldrich). The cells were frozen slowly by placing them in a Styrofoam box at −80°C for at least one hour and then transferred to −140°C for long-term preservation.
Cell processing and reagents
Cryopreserved PBMCs were thawed by holding the vial for 1 min and subsequent stepwise addition of 8 mL of pre-warmed (37°C) RPMI-1640 (Thermo Fisher Scientific) supplemented with 1% human serum (Sigma-Aldrich). PBMCs were centrifuged for 10 min at 330× g and resuspended in 10 mL phosphate-buffered saline (PBS) (HyClone, GE Healthcare Life Sciences). After another wash with PBS, PBMCs were incubated with Zombie Aqua Fixable Viability Dye (Biolegend) for 20 min at room temperature in the dark. After one wash with 3 mL staining buffer (PBS supplemented with 2 mM EDTA), the samples were filtered once and advanced to antibody staining. For this, tubes with the lyophilized antibody-mixes, custom-made by the supplier according to our titrations, were first reconstituted with 50 µL dH2O. Lyophilized antibody-mixes were used as they have been shown to reduce variability.24 The viability dye-stained PBMCs were added to the reconstituted antibody-mixes and incubated for 30 min at room temperature in the dark. PBMCs were washed once with 3 mL staining buffer and resuspended in 200 µL PBS containing formaldehyde (1% final). The cells were acquired within 24 h. The antibodies used in this study were CD27-FITC (clone O323), CCR7-PE (clone G043H7), CD95-PE/Dazzle (clone DX2), CD8α-PerCP (clone RPA-T8), CD45RA-PE/Cy7, (clone HI100), CD4-AF700 (clone RPA-T4), CD3-APC/Cy7 (clone HIT3a), CD28-BV421 (clone CD28.2), (all from Biolegend), and only one lot of each antibody was used throughout the study. APC-labelled PBS-57-loaded CD1d tetramer was obtained from the NIH tetramer core facility. Single-stained compensation beads (BD CompBeads anti-mouse Igκ, Becton, Dickinson and Company Biosciences) were used for calculating compensation.
Data acquisition
The samples were acquired on a three-laser, 10-color Gallios flow cytometer (Beckman Coulter), which was calibrated and optimized for sensitivity as described in detail previously.20,25 The daily quality assurance routine consisted of running Flow-Check Pro (Beckman Coulter) beads to verify the optical and fluidic function and then set the fluorescent signal in each channel to ±2% of the previously established target median fluorescence intensity (MFI), using single-peak Rainbow beads (Spherotech), which resulted in a high instrument precision with a coefficient of variation of the single-peak MFI of 0.3–0.7%. Sample handling and data acquisition were performed blinded by two trained research engineers.
Data analysis
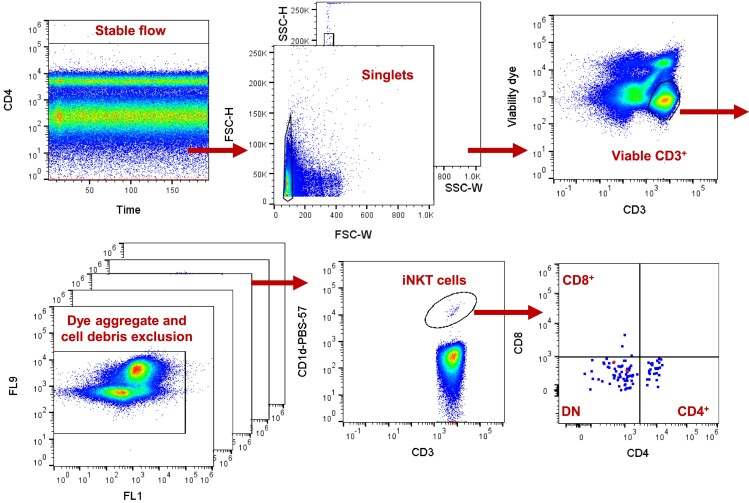
Compensation and gating were performed in FlowJo v10.4 and quality assurance was done in Kaluza Analysis v1.3 (Beckman Coulter). Identification of iNKT cells was done by successive gating on time (to remove any artefacts from unstable flow), forward scatter (FSC) height and width as well as side scatter (SSC) height and width to remove cell doublets, CD3+ live cells on a CD3 vs. viability dye plot, and exclusion of dye aggregates and cell debris, and finally, PBS57-loaded CD1d+ cells (PBS-57 is an analogue of the iNKT agonist α-GalCer). CD4+, CD8+, and CD4−CD8− DN iNKT cells were subsequently gated on a CD4 vs. CD8 plot (Figure 2). Expression of CD45RA, CCR7, CD27, CD28, and CD95 were used to describe the memory phenotype of DN and CD4+ iNKT cell subsets in data aggregated from six individuals(see Supplementary material online, Figure S1). Data analysis was performed blinded.
Figure 2.
Flow cytometry gating of invariant natural killer T (iNKT) cells. Peripheral blood mononuclear cells were first gated on time (to remove any artefacts from unstable flow), and cell duplets were removed by forward scatter height and width as well as side scatter height and width. Live CD3+ cells were gated on a CD3 vs. viability dye plot and dye aggregates and cell debris were excluded from all channels. iNKT cells were gated on a CD1d-PBS-57 vs. CD3 plot (PBS-57 is an analogue of the iNKT cell agonist α-GalCer). CD4+, CD8+, and CD4−CD8− double negative iNKT cells were subsequently gated on a CD4 vs. CD8 plot.
Statistics
Statistical analyses were performed in SPSS Statistics 27 (IBM, Armonk, USA). Figures were made in draw.io diagrams.net 18.0.6 (JGraph Ltd, UK). Continuous baseline data is presented as median and inter-quartile range (IQR) and categorical data is presented with count (n) and percentage of the total. Differences in variables between cases and controls were tested using the Mann–Whitney test or chi-square test. Differences in variables between tertiles of iNKT cells were tested using the Kruskal–Wallis test or chi-square test. Conditional logistic regression conditioned for matched case-control pairs was used to analyse associations between iNKT cells stratified into tertiles and incident CE. Regression models were unadjusted or adjusted for the CVD risk factors (total cholesterol, HDL, diabetes, use of BP-lowering medication, systolic BP, and smoking). In all models, tertile one with the lowest iNKT cell numbers was used as the reference. HDL values were natural logarithm transformed due to their non-normal distribution.
Results
Association of iNKT cells with incident CE
The study population consisted of 416 individuals in 208 age- and sex-matched CE case-control pairs (Figure 1). Invariant natural killer T cells were identified by gating on cells positive for CD3 and a CD1d tetramer loaded with PBS57, an analogue of the canonical iNKT agonist α-GalCer (Figure 2).26 The median percentage of iNKT cells among T cells was 0.017% (IQR 0.008–0.044%), equalling 195 (IQR 89–459) cells/mL, which is in line with previous studies.14,15 Male sex was associated with lower numbers of circulating iNKT cells, while individuals with diabetes had higher numbers of iNKT cells and there was no difference between individuals with or without prevalent cancer (see Supplementary material online, Table S1). Despite the association with cardiovascular risk factors such as diabetes, the number of total iNKT cells was not significantly different between CE cases and controls (see Supplementary material online, Table S1).
Next, we stratified the 208 case-control pairs according to their iNKT cell numbers into tertiles. There were fewer males and current smokers and study participants had lower systolic BP with increasing iNKT tertiles (see Supplementary material online, Table S2). We used conditional logistic regression to examine the associations of iNKT cell tertiles with incident first-time CE during a median of 14 years of follow-up. Elevated numbers of iNKT cells were not associated with incidence of first-time CE when comparing the highest vs. the lowest tertile of iNKT cell numbers (Table 3). Associations remained non-significant after adjustment for CVD risk factors; total cholesterol, HDL, diabetes, use of BP-lowering medication, systolic BP, and smoking with an OR of 0.74 [95% confidence interval (CI) 0.43–1.27], P = 0.276. Since the sex distribution across the tertiles was heterogenous, we analysed men and women separately, but associations with iNKT cells remained non-significant (data not shown).
Table 3.
Associations between iNKT cell counts and incident first-time coronary events, analysed by conditional logistic regression
| iNKT cell tertile | Unadjusted | Adjusted | |||
|---|---|---|---|---|---|
| OR (95% CI) | P-Value | OR (95% CI) | P-Value | ||
| Total iNKT cells | 1 | 1 (Reference) | 1 (Reference) | ||
| 2 | 0.71 (0.45–1.13) | 0.151 | 0.70 (0.40–1.21) | 0.202 | |
| 3 | 0.81 (0.51–1.29) | 0.373 | 0.74 (0.43–1.27) | 0.276 | |
| CD4+ iNKT cells | 1 | 1 (Reference) | 1 (Reference) | ||
| 2 | 1.12 (0.72–1.75) | 0.610 | 1.17 (0.69–2.00) | 0.556 | |
| 3 | 1.04 (0.65–1.66) | 0.879 | 0.99 (0.581.70) | 0.984 | |
| CD8+ iNKT cells | 1 | 1 (Reference) | 1 (Reference) | ||
| 2 | 1.58 (0.99–2.54) | 0.056 | 1.50 (0.88–2.55) | 0.134 | |
| 3 | 1.10 (0.70–1.74) | 0.670 | 1.03 (0.61–1.74) | 0.913 | |
| CD4−CD8− iNKT cells | 1 | 1 (Reference) | 1 (Reference) | ||
| 2 | 0.53 (0.32–0.87) | 0.012 | 0.55 (0.31–0.98) | 0.043 | |
| 3 | 0.62 (0.38–0.99) | 0.046 | 0.57 (0.33–0.99) | 0.046 | |
n in unadjusted model: 208 pairs. n in adjusted model: 181 pairs. Adjustments were made for total cholesterol, HDL, diabetes, BP-lowering medication, systolic BP and current smoking. Statistically significant P-values are presented in bold.
Associations of iNKT cell subsets and incident CE
Similar to other T cell subsets, iNKT cells can be further stratified based on their expression of CD4 and CD8. iNKT cells are most commonly CD4+ or DN, expressing neither CD4 nor CD8.27 Accordingly, in our cohort the average distribution of iNKT cell subsets was 37% CD4+, 6% CD8+, and 57% DN. CD4+CD8+ double-positive iNKT cells were very rare (on average 0.1% of the total iNKT cell population, but most samples lacked this subset entirely) and were not analysed further. There was no difference in iNKT cell numbers between cases and controls for any iNKT subset, but women had higher numbers of CD4+ iNKT cells than men, P = 0.003, current smokers had fewer DN iNKT cells than non-smokers, P = 0.016, and diabetes patients had more DN iNKT cells than non-diabetics, P = 0.037 (Table 1, Supplementary material online, Table S1).
Table 1.
CD4+ and DN iNKT cell counts separated by baseline characteristics
| n | CD4+ iNKT cells/mL | P | DN iNKT cells/mL | P | ||
|---|---|---|---|---|---|---|
| Coronary event | Yes | 208 | 76 (30–151) | 0.873 | 63 (18–262) | 0.242 |
| No | 208 | 74 (29–157) | 88 (26–268) | |||
| Sex | Female | 152 | 86 (40–228) | 0.003 | 100 (22–442) | 0.106 |
| Male | 264 | 63 (28–118) | 66 (21–227) | |||
| Current smoker | Yes | 117 | 69 (38–110) | 0.578 | 57 (19–173) | 0.016 |
| No | 286 | 77 (27–179) | 85 (25–349) | |||
| Coronary revascularizationa | Yes | 8 | 49 (0–172) | 0.300 | 162 (16–333) | 0.874 |
| No | 408 | 75 (30–156) | 69 (22–262) | |||
| Carotid plaque | Yes | 179 | 69 (27–164) | 0.273 | 72 (22–273) | 0.993 |
| No | 215 | 77 (31–151) | 74 (22–271) | |||
| Diabetes | Yes | 64 | 93 (37–128) | 0.205 | 123 (33–425) | 0.037 |
| No | 352 | 69 (27–158) | 67 (20–245) | |||
| Cancer | Yes | 33 | 60 (30–188) | 0.819 | 61 (18–262) | 0.442 |
| No | 383 | 76 (30–154) | 72 (22–264) | |||
| BP-lowering | Yes | 92 | 65 (36–123) | 0.974 | 78 (22–328) | 0.544 |
| No | 324 | 77 (29–158) | 67 (21–254) | |||
| Beta-blockers | Yes | 56 | 64 (29–130) | 0.705 | 50 (16–422) | 0.643 |
| No | 360 | 76 (30–156) | 73 (22–261) | |||
| Statins | Yes | 7 | 41 (20–89) | 0.154 | 265 (71–581) | 0.116 |
| No | 409 | 76 (30–157) | 67 (22–261) | |||
Values are presented as median (inter-quartile range). Differences in DN and CD4+ INKT cell counts between categorical variables were calculated using chi-square tests and statistically significant P-values are presented in bold.
BP, blood pressure.
aCoronary artery bypass grafting or percutaneous coronary interventions.
In addition, after separating the iNKT cell numbers of each subset into tertiles, we found that the number of current smokers differed across tertiles of both CD4+ and DN iNKT cell numbers, P = 0.005 and P = 0.036, respectively (Table 2, Supplementary material online, Table S2). Also, the number of diabetes patients increased, P = 0.044, whereas WBC, P = 0.025 and systolic BP decreased, P = 0.019, across tertiles of increasing DN iNKT cell numbers (Table 2). Taken together, the different iNKT subsets had partly different associations with CVD risk factors.
Table 2.
Baseline characteristics across tertiles of CD4+ and DN iNKT cells
| CD4+ iNKT cells | DN iNKT cells | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Total n | Tertile 1 | Tertile 2 | Tertile 3 | P | Tertile 1 | Tertile 2 | Tertile 3 | P | |
| Sex. no of females (%) | 416 | 42 (30%) | 47 (34%) | 63 (45%) | 0.026 | 46 (33%) | 48 (35%) | 58 (42%) | 0.291 |
| Age (years) | 416 | 61 (57–64) | 61 (57–63) | 60 (56–63) | 0.470 | 61 (57–64) | 61 (57–64) | 60 (56–63) | 0.189 |
| BMI (kg/m2) | 414 | 25.6 (23.5–28.1) | 25.9 (23.8–29.1) | 25.9 (23.1–29.9) | 0.651 | 25.8 (24.2–28.9) | 25.7 (23.3–28.7) | 26.1 (23.2–29.1) | 0.670 |
| Current smoker | 403 | 34 (25%) | 53 (39%) | 30 (22%) | 0.005 | 42 (32%) | 46 (34%) | 29 (21%) | 0.036 |
| Prevalent disease | |||||||||
| Carotid plaque | 394 | 68 (52%) | 51 (40%) | 60 (45%) | 0.132 | 58 (46%) | 62 (47%) | 59 (44%) | 0.864 |
| Coronary revascularizationa | 416 | 4 (3%) | 2 (1%) | 2 (1%) | 0.594 | 3 (2%) | 1 (1%) | 4 (3%) | 0.410 |
| Stroke | 416 | 2 (1%) | 0 (0%) | 0 (0%) | 0.132 | 0 (0%) | 2 (1%) | 0 (0%) | 0.135 |
| Atrial fibrillation | 416 | 3 (2%) | 1 (1%) | 1 (1%) | 0.440 | 2 (1%) | 3 (2%) | 0 (0%) | 0.243 |
| Diabetes | 416 | 20 (14%) | 20 (14%) | 24 (17%) | 0.753 | 16 (12%) | 18 (13%) | 30 (22%) | 0.044 |
| Cancer | 416 | 14 (10%) | 8 (6%) | 11 (8%) | 0.401 | 14 (10%) | 8 (6%) | 11 (8%) | 0.401 |
| Medications | |||||||||
| BP-lowering | 416 | 32 (23%) | 34 (24%) | 26 (19%) | 0.478 | 32 (23%) | 25 (18%) | 35 (25%) | 0.329 |
| Beta-blockers | 416 | 19 (14%) | 19 (14%) | 18 (13%) | 0.977 | 23 (17%) | 13 (9%) | 20 (14%) | 0.189 |
| Statins | 416 | 4 (3%) | 3 (2%) | 0 (0%) | 0.149 | 1 (1%) | 1 (1%) | 5 (4%) | 0.099 |
| Laboratory parameters | |||||||||
| Systolic BP (mm Hg) | 416 | 149 (138–160) | 150 (130–164) | 142 (130–158) | 0.117 | 150 (138–166) | 142 (130–160) | 144 (130–160) | 0.019 |
| Diastolic BP (mm Hg) | 416 | 90 (85–96) | 90 (80–98) | 90 (80–96) | 0.430 | 90 (84–98) | 90 (80–96) | 90 (80–96) | 0.297 |
| Total cholesterol (mmol/L) | 410 | 6.06 (5.41–6.9) | 6.14 (5.43–7.12) | 6.26 (5.32–6.92) | 0.956 | 6.09 (5.26–6.86) | 6.06 (5.49–6.8) | 6.30 (5.48–7.07) | 0.410 |
| LDL (mmol/L) | 398 | 4.2 (3.5–4.8) | 4.3 (3.6–5.2) | 4.4 (3.6–4.9) | 0.351 | 4.2 (3.4–4.8) | 4.2 (3.6–4.9) | 4.4 (3.6–5.0) | 0.323 |
| HDL (mmol/L | 403 | 1.24 (1.05–1.46) | 1.19 (0.98–1.51) | 1.22 (1.02–1.5) | 0.715 | 1.27 (1.01–1.51) | 1.22 (1.04–1.49) | 1.20 (1.01–1.47) | 0.864 |
| LDL/HDL ratio | 398 | 3.4 (2.6–4.3) | 3.7 (2.8–4.6) | 3.5 (2.6–4.7) | 0.360 | 3.4 (2.6–4.3) | 3.6 (2.6–4.6) | 3.5 (2.8–4.5) | 0.544 |
| Triglycerides (mmol/L) | 410 | 1.38 (1.08–1.79) | 1.23 (0.96–1.63) | 1.22 (0.91–1.79) | 0.208 | 1.23 (0.93–1.66) | 1.32 (0.97–1.84) | 1.26 (0.98–1.80) | 0.396 |
| HbA1c (%) | 409 | 5.0 (4.6–5.3) | 4.8 (4.7–5.1) | 4.9 (4.6–5.3) | 0.566 | 4.9 (4.7–5.2) | 4.9 (4.6–5.2) | 4.8 (4.6–5.2) | 0.939 |
| CRP (mg/L) | 387 | 1.6 (0.9–3.4) | 1.7 (0.9–3.8) | 1.5 (0.8–2.6) | 0.222 | 1.8 (0.9–4.1) | 1.6 (0.8–3.3) | 1.6 (0.8–2.8) | 0.307 |
| WBC (109/L | 416 | 6.1 (5.1–7.1) | 6.3 (5.3–7.4) | 6.1 (5.1–7.0) | 0.740 | 6.5 (5.2–7.7) | 6.1 (5.1–7.0) | 5.9 (5.0–6.9) | 0.025 |
| Lymphocytes (109/L) | 416 | 1.8 (1.5–2.1) | 1.9 (1.6–2.1) | 1.9 (1.6–2.3) | 0.039 | 1.8 (1.5–2.1) | 1.9 (1.6–2.2) | 1.9 (1.6–2.1) | 0.745 |
| eGFR (mL/min/1.73 m2) | 405 | 75.8 (66.1–86.0) | 76.9 (69.2–83.9) | 77.4 (67.6–85.5) | 0.517 | 76.0 (66.7–85.4) | 77.4 (70–85.4) | 77.0 (65.3–84.9) | 0.395 |
Statistically significant P-values are presented in bold.
BMI, body mass index.
aCoronary artery bypass grafting or percutaneous coronary interventions.
Next, conditional logistic regression analysis revealed that the DN iNKT cell subset was significantly inversely associated with incident CE, whereas the CD4+ or CD8+ subsets were not (Table 3). This negative association for DN iNKT cells remained significant after adjustment for CVD risk factors: OR 0.57 (95% CI 0.33–0.99), P = 0.046 (Table 3).
As women had higher numbers of CD4+ iNKT cells than men, we performed separate regression analyses in men and women, but CD4+ iNKT cells were not associated with incident CE in men or women after adjusting for CVD risk factors, with an OR of 1.39 (95% CI 0.68–2.82), P = 0.365 in men and 0.56 (95% CI 0.21–1.49), P = 0.245 in women.
Phenotypic memory markers on DN and CD4+ iNKT cells and correlations with pro-inflammatory cytokines
Functional studies of iNKT cells have mainly focused on which cytokines iNKT cells or iNKT subsets express, but the expression of homing and activation markers could also be of functional importance. We found that most DN and CD4+ iNKT cells were CD45RA−CCR7−, similar to canonical effector memory T cells (see Supplementary material online, Figure S1). Further subdivision of DN and CD4+ iNKT cells based on CD27 and CD28 also showed likeness to canonical effector memory T cells in that the majority of DN and CD4+ iNKT cells were CD27+CD28+ (78%) and CD27−CD28+ (16%) and only few were CD27−CD28− (4%). Notably, while the distribution of DN and CD4+ iNKT cells expressing CD27 and CD95 were similar to canonical effector memory T cells, the CD4+ iNKT subset expressed higher surface levels of CD95 than the DN iNKT subset [CD95 MFI 530 (IQR 310–1110) on DN iNKT cells vs. CD95 MFI 1690 (IQR 560–2490) on CD4+ iNKT cells].
Next, we performed correlation analyses to investigate associations between circulating iNKT cell subsets and pro-inflammatory cytokines. Increased plasma levels of pro-inflammatory cytokines have been associated with CVD and in mice activation of iNKT cells with α-GalCer has been shown to increase serum levels of IFN-γ, TNF-α, and IL-6.3
We found significant negative correlations between DN iNKT cells and plasma levels of the cytokines IL-6, IL-18, and soluble TNF receptor 1 and 2 (considered as surrogate markers for TNF-α),28 whereas no such correlations with CD4+ and CD8+ iNKT cells were found (see Supplementary material online, Table S3). None of the iNKT subsets were correlated with IFN-γ, although IFN-γ measurements were only available in a smaller number of samples (see Supplementary material online, Table S3). Taken together it is noteworthy that only the DN iNKT subset had an independent association with incident CE after risk factor adjustment and displayed inverse correlations with pro-inflammatory cytokines.
Discussion
In this study, we aimed to provide a prospective analysis of the association of circulating iNKT cells with first-time incident CE to explore possible links between iNKT cells and CVD in humans. We found that the DN iNKT cell subset, which lacks expression of both CD4 and CD8, was inversely associated with incident CE independently of CVD risk factors. In contrast, we did not find any significant associations for total iNKT cells, the CD4+ or CD8+ iNKT cell subsets, and incident CE.
The lack of association between total iNKT cells and incident CE indicates that the net effect of iNKT cells might be of minor significance for the risk of suffering a CE.
These results contradict the notion, generated by numerous mouse studies, that iNKT cells are potent drivers of atherosclerotic plaque development.2–6 In contrast to the majority of mouse studies, van Puijvelde et al. have found an atheroprotective effect of iNKT cells in experimental atherosclerosis, which was attributed to increased IL-10 production.7 More importantly, a recent study of human iNKT cells found that these cells can have an atheroprotective TH2-like phenotype in patients with subclinical CVD.17 These two studies highlight an important aspect, namely, that iNKT cells are functionally heterogenous and can be subdivided into TH1-like (NKT1), TH2-like (NKT2), TH17-like (NKT17), and Treg-like (NKT10) cells,29 similar to conventional T cells. This aspect has not been examined in mouse studies of iNKT cells in atherosclerosis so far. The diverse roles of iNKT cells represent a potential explanation for the conflicting results, similar to what has been described for conventional T helper cells, where studies have shown that T helper cells as a whole promote atherosclerosis development, but certain subsets like TH2 can reduce atherosclerosis and clinical events.30,31
In addition, iNKT subsets stratified by expression of CD4 and CD8 are not functionally identical, lending further support to the idea that subset composition of the iNKT pool could be a major determinant of functional outcome.32 The DN and CD4+ iNKT cell subsets have been shown to be distinct lineages with marked differences in their cytokine secretion profile and expression of chemokine receptors, integrins, and NK receptors.33 Several reports have shown that DN iNKT cells have higher expression of inhibitory receptors, such as Ly49, CD94, and NKG2A, compared to CD4+ iNKT cells.33–35 These differences could possibly explain the findings that CD4+, but not DN iNKT cells, can exacerbate atherosclerosis in mice,36 although in humans a shift towards higher CD4+ to DN iNKT cell ratio was associated with increased IL-4 production and a TH2-like phenotype.17 It is tempting to speculate, based on the results of our study in humans, that increased circulating numbers of DN iNKT cells may hamper or delay incident CE, whereas CD4+ iNKT cells do not. However, little is known about the function of DN iNKT cells in humans. iNKT cells have mainly been ascribed functional roles in the liver32making it difficult to infer functional consequences of circulating levels of iNKT cells as it is not clear if increased circulating iNKT cells corresponds to increased or decreased tissue levels of iNKT cells, particularly in tissues relevant in CVD. In addition, although the disparate associations for DN and CD4+ iNKT cells in regard to incident CE and the correlations with pro-inflammatory cytokines observed in our study could be explained by distinct functional properties of the different iNKT cell subsets, more studies are needed to determine if iNKT cells play an active role or whether they are a marker of human CVD. Of note, both the DN and CD4+ iNKT cell populations consisted mainly of effector memory cells, and the DN iNKT cells expressed lower surface levels of the activation marker CD95, which is in line with findings by Montoya et al in healthy adults.37 Although this data is very interesting, it remains to be elucidated whether this difference has any functional consequences in CVD.
iNKT cell numbers as well as cytokine production have been reported to be lower in many autoimmune conditions, such as multiple sclerosis, rheumatoid arthritis, systemic lupus erythematosus and, although there are contradictory results, type 1 diabetes.16,27,38 Notably, we found iNKT cell numbers, specifically of the DN iNKT subset, to be higher in diabetes patients in our study, where the majority of diabetic study participants had type 2 diabetes. Still, a role of iNKT cells in type 2 diabetes remains elusive, but there have been speculations about a role of iNKT cells in obesity-induced inflammation and insulin resistance.39,40 In addition, patients with established CVD have lower numbers of circulating iNKT cells compared to healthy controls,13–15 which, taken together with our data from a cohort of individuals that were CE-free at baseline, suggests that iNKT cells or some iNKT cell subsets may protect against CVD and other conditions characterized by chronic inflammation and autoimmunity.
We found total iNKT cell numbers, particularly the CD4+ iNKT subset, to be higher in females compared to males, even though CD4+ iNKT cells had no association with incident CE when analysed separately in men and women. This observation is in line with previous studies that have also seen elevated iNKT cell numbers in females, although the difference has not been statistically significant in all studies.37,41,42 Increased iNKT cell numbers in females were not observed in the DN or CD8+ iNKT subsets.
There are some limitations of the current study that should be acknowledged. Firstly, it is important to remember that we cannot draw any conclusions about causality from this observational study. We also had to exclude some samples with a low iNKT cell count due to the low frequency of iNKT cells in the volume of blood available, which may have decreased the statistical power or introduced selection biases, especially in the analyses performed in only a subset of participants. Still, the reliability of our iNKT cell counts appears unaffected as our observed counts agree well with what has been reported previously. In addition, our highly standardized state-of-the-art flow cytometry with very low measurement coefficient of variability likely contributed to the validity of our findings.
In conclusion, this study shows that iNKT cells are not associated with an increased incidence of first-time CE in a Swedish cohort of upper-middle aged individuals. This study does, however, show that the CD4−CD8− DN subset of iNKT cells is independently and inversely associated with incident CE, suggesting that iNKT subset diversity may play a role in atherosclerotic CVD which merits further exploration into the detailed function of human iNKT cells.
Supplementary Material
Acknowledgements
The CD1d-PBS57 tetramers were kindly provided by the NIH Tetramer Core Facility.
Contributor Information
Lukas Tomas, Department of Clinical Sciences Malmö, Lund University, Jan Waldenströms gata 35, SE-214 28, Malmö, Sweden.
Pernilla Katra, Department of Clinical Sciences Malmö, Lund University, Jan Waldenströms gata 35, SE-214 28, Malmö, Sweden.
Wiaam Badn, Department of Clinical Sciences Malmö, Lund University, Jan Waldenströms gata 35, SE-214 28, Malmö, Sweden.
Linda Andersson, Department of Clinical Sciences Malmö, Lund University, Jan Waldenströms gata 35, SE-214 28, Malmö, Sweden.
Jan Nilsson, Department of Clinical Sciences Malmö, Lund University, Jan Waldenströms gata 35, SE-214 28, Malmö, Sweden.
Alexandru Schiopu, Department of Translational Medicine, Lund University, Malmö, Sweden; Department of Internal Medicine, Skåne University Hospital, Lund, Sweden.
Daniel Engelbertsen, Department of Clinical Sciences Malmö, Lund University, Jan Waldenströms gata 35, SE-214 28, Malmö, Sweden.
Isabel Gonçalves, Department of Clinical Sciences Malmö, Lund University, Jan Waldenströms gata 35, SE-214 28, Malmö, Sweden; Department of Cardiology, Skåne University Hospital, Malmö, Sweden.
Eva Bengtsson, Department of Clinical Sciences Malmö, Lund University, Jan Waldenströms gata 35, SE-214 28, Malmö, Sweden; Faculty of Health and Society, Malmö University, Malmö, Sweden; Biofilms – Research Center for Biointerfaces, Malmö University, Malmö, Sweden.
Harry Björkbacka, Department of Clinical Sciences Malmö, Lund University, Jan Waldenströms gata 35, SE-214 28, Malmö, Sweden.
Lead author biography
 Lukas Tomas is a Clinician Scientist specializing in Cardiology. After his medical studies in Austria, he successfully pursued a PhD at Lund University in Sweden. Following postdoctoral stays in Sweden and Belgium, he started his Cardiology residency at the LMU University Hospital Munich. His research focuses on immunological mechanisms in cardiovascular disease, with a specific emphasis on the interplay of inflammation and metabolism.
Lukas Tomas is a Clinician Scientist specializing in Cardiology. After his medical studies in Austria, he successfully pursued a PhD at Lund University in Sweden. Following postdoctoral stays in Sweden and Belgium, he started his Cardiology residency at the LMU University Hospital Munich. His research focuses on immunological mechanisms in cardiovascular disease, with a specific emphasis on the interplay of inflammation and metabolism.
Author contributions
L.T.: investigation, formal analysis, writing – original draft. P.K.: formal analysis, writing- editing and review, visualization. W.B.: investigation, writing- editing and review. L.A.: investigation, writing- editing and review. J.N.: resources, writing- editing and review. A.S.: data curation, writing- editing and review. D.E.: supervision, writing- editing and review. I.G.: supervision, writing- editing and review. E.B.: supervision, writing- editing and review. H.B.: conceptualization, data curation, writing – Original draft, Supervision.
Data availability
The data that support the findings of this study are available from the authors (P.K. or H.B.) upon reasonable request, but, due to some of the data containing information that could compromise research participant privacy or consent, requests may require ethical review and full compliance to the general data protection regulation.
Supplementary material
Supplementary material is available at European Heart Journal Open online.
Funding
This study was supported by the Swedish Research Council (Strategic Research Area Exodiab Dnr 2009-1039, Linnaeus grant Dnr 349-2006-237, Project Research Grant Dnr 2021-02942, 2015-02523, and 2019-01260), The Swedish Heart Lung Foundation (Project Research Grants 20170617, 20200760, 20220293, 20140097, 20170333, 20200403, and 20140078), The Albert Påhlsson Foundation, Swedish governmental funding of clinical research (ALF) and by Skåne University Hospital funds.
References
- 1. Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol 2007;25:297–336. [DOI] [PubMed] [Google Scholar]
- 2. Nakai Y, Iwabuchi K, Fujii S, Ishimori N, Dashtsoodol N, Watano K, Mishima T, Iwabuchi C, Tanaka S, Bezbradica JS, Nakayama T. Natural killer T cells accelerate atherogenesis in mice. Blood 2004;104:2051–2059. [DOI] [PubMed] [Google Scholar]
- 3. Tupin E, Nicoletti A, Elhage R, Rudling M, Ljunggren H-G, Hansson GK, Berne GP. CD1d-dependent Activation of NKT cells aggravates atherosclerosis. J Exp Med 2004;199:417–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Major AS, Wilson MT, McCaleb JL, Ru Su Y, Stanic AK, Joyce S, Van Kaer L, Fazio S, Linton MF. Quantitative and qualitative differences in proatherogenic NKT cells in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol 2004;24:2351–2357. [DOI] [PubMed] [Google Scholar]
- 5. Aslanian AM, Chapman HA, Charo IF. Transient role for CD1d-restricted natural killer T cells in the formation of atherosclerotic lesions. Arterioscler Thromb Vasc Biol 2005;25:628–632. [DOI] [PubMed] [Google Scholar]
- 6. Rogers L, Burchat S, Gage J, Hasu M, Thabet M, Willcox L, Ramsamy TA, Whitman SC. Deficiency of invariant V alpha 14 natural killer T cells decreases atherosclerosis in LDL receptor null mice. Cardiovasc Res 2008;78:167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. van Puijvelde GHM, van Wanrooij EJA, Hauer AD, de Vos P, van Berkel TJC, Kuiper J. Effect of natural killer T cell activation on the initiation of atherosclerosis. Thromb Haemost 2009;102:223–230. [DOI] [PubMed] [Google Scholar]
- 8. Subramanian S, Goodspeed L, Wang S, Ding Y, O’Brien KD, Getz GS, Chait A, Reardon C. Deficiency of invariant natural killer T cells does not protect against obesity but exacerbates atherosclerosis in ldlr-/- mice. Int J Mol Sci 2018;19:510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brennan PJ, Brigl M, Brenner MB. Invariant natural killer T cells: an innate activation scheme linked to diverse effector functions. Nat Rev Immunol 2013;13:101–117. [DOI] [PubMed] [Google Scholar]
- 10. To K, Agrotis A, Besra G, Bobik A, Toh B-H. NKT Cell subsets mediate differential proatherogenic effects in ApoE-/- mice. Arterioscler Thromb Vasc Biol 2009;29:671–677. [DOI] [PubMed] [Google Scholar]
- 11. van den Elzen P, Garg S, León L, Brigl M, Leadbetter EA, Gumperz JE, Dascher CC, Cheng T-Y, Sacks FM, Illarionov PA, Besra GS, Kent SC, Moody DB, Brenner MB. Apolipoprotein-mediated pathways of lipid antigen presentation. Nature 2005;437:906–910. [DOI] [PubMed] [Google Scholar]
- 12. Allan LL, Hoefl K, Zheng D-J, Chung BK, Kozak FK, Tan R, Tan R, van den Elzen P. Apolipoprotein-mediated lipid antigen presentation in B cells provides a pathway for innate help by NKT cells. Blood 2009;114:2411–2416. [DOI] [PubMed] [Google Scholar]
- 13. Andoh Y, Fujii S, Iwabuchi K, Yokota T, Inoue N, Nakai Y, Mishima T, Yamashita T, Nakagawa T, Kitabatake A, Onoe K, Tsutsui H. Lower prevalence of circulating natural killer T cells in patients with angina: a potential novel marker for coronary artery disease. Coron Artery Dis 2006;17:523–528. [DOI] [PubMed] [Google Scholar]
- 14. Kyriakakis E, Cavallari M, Andert J, Philippova M, Koella C, Bochkov V, Erne P, Wilson SB, Mori L, Biedermann BC, Resink TJ, De Libero G. Invariant natural killer T cells: linking inflammation and neovascularization in human atherosclerosis. Eur J Immunol 2010;40:3268–3279. [DOI] [PubMed] [Google Scholar]
- 15. Liu L-L, Lu J-L, Chao P-L, Lin L-R, Zhang Z-Y, Yang T-C. Lower prevalence of circulating invariant natural killer T (iNKT) cells in patients with acute myocardial infarction undergoing primary coronary stenting. Int Immunopharmacol 2011;11:480–484. [DOI] [PubMed] [Google Scholar]
- 16. van der Vliet HJ, von Blomberg BM, Nishi N, Reijm M, Voskuyl AE, van Bodegraven AA, Polman CH, Rustemeyer T, Lips P, van den Eertwegh AJM, Giaccone G, Scheper RJ, Pinedo HM. Circulating V(alpha24+) Vbeta11+ NKT cell numbers are decreased in a wide variety of diseases that are characterized by autoreactive tissue damage. Clin Immunol 2001;100:144–148. [DOI] [PubMed] [Google Scholar]
- 17. Smith E, Croca S, Waddington KE, Sofat R, Griffin M, Nicolaides A, Isenberg DA, Torra IP, Rahman A, Jury EC. Cross-talk between iNKT cells and monocytes triggers an atheroprotective immune response in SLE patients with asymptomatic plaque. Sci Immunol 2016;1:1–13. [DOI] [PubMed] [Google Scholar]
- 18. Björkbacka H, Berg KE, Manjer J, Engelbertsen D, Wigren M, Ljungcrantz I, Andersson L, Hedblad B, Fredrikson GN, Nilsson J. CD4+ CD56+ natural killer T-like cells secreting interferon-γ are associated with incident coronary events. J Intern Med 2016;279:78–88. [DOI] [PubMed] [Google Scholar]
- 19. Engström G, Berglund G, Göransson M, Hansen O, Hedblad B, Merlo J, Tyden P, Janzon L. Distribution and determinants of ischaemic heart disease in an urban population. A study from the myocardial infarction register in malmö, Sweden. J Intern Med 2000;247:588–596. [DOI] [PubMed] [Google Scholar]
- 20. Tomas L, Bengtsson E, Andersson L, Badn W, Tengryd C, Persson A, Edsfeldt A, Nilsson PM, Schiopu A, Nilsson J, Gonçalves I, Björkbacka H. Low levels of CD4+ CD28null T cells at baseline are associated with first-time coronary events in a prospective population-based case-control cohort. Arterioscler Thromb Vasc Biol 2020;40:426–436. [DOI] [PubMed] [Google Scholar]
- 21. Hedblad B, Nilsson P, Janzon L, Berglund G. Relation between insulin resistance and carotid intima-media thickness and stenosis in non-diabetic subjects. Results from a cross-sectional study in malmö, Sweden. Diabet Med 2000;17:299–307. [DOI] [PubMed] [Google Scholar]
- 22. Berglund GL, Riley WA, Barnes RW, Furberg CD. Quality control in ultrasound studies on atherosclerosis. J Intern Med 1994;236:581–586. [DOI] [PubMed] [Google Scholar]
- 23. Hedblad B, Wikstrand J, Janzon L, Wedel H, Berglund G. Low-dose metoprolol CR/XL and fluvastatin slow progression of carotid intima-media thickness: main results from the Beta-blocker cholesterol-lowering asymptomatic plaque study (BCAPS). Circulation 2001;103:1721–1726. [DOI] [PubMed] [Google Scholar]
- 24. Finak G, Langweiler M, Jaimes M, Malek M, Taghiyar J, Korin Y, Raddassi K, Devine L, Obermoser G, Pekalski ML, Pontikos N, Diaz A, Heck S, Villanova F, Terrazzini N, Kern F, Qian Y, Stanton R, Wang K, Brandes A, Ramey J, Aghaeepour N, Mosmann T, Scheuermann RH, Reed E, Palucka K, Pascual V, Blomberg BB, Nestle F, Nussenblatt RB, Brinkman RR, Gottardo R, Maecker H, McCoy JP. Standardizing flow cytometry immunophenotyping analysis from the human ImmunoPhenotyping consortium. Sci Rep 2016;6:20686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Perfetto SP, Ambrozak D, Nguyen R, Chattopadhyay PK, Roederer M. Quality assurance for polychromatic flow cytometry using a suite of calibration beads. Nat Protoc 2012;7:2067–2079. [DOI] [PubMed] [Google Scholar]
- 26. Liu Y, Goff RD, Zhou D, Mattner J, Sullivan BA, Khurana A, Cantu C, Ravkov EV, Ibegbu CC, Altman JD, Teyton L, Bendelac A, Savage PB. A modified alpha-galactosyl ceramide for staining and stimulating natural killer T cells. J Immunol Methods 2006;312:34–39. [DOI] [PubMed] [Google Scholar]
- 27. Berzins SP, Smyth MJ, Baxter AG. Presumed guilty: natural killer T cell defects and human disease. Nat Rev Immunol 2011;11:131–142. [DOI] [PubMed] [Google Scholar]
- 28. Paccalet A, Crola Da Silva C, Mechtouff L, Amaz C, Varillon Y, de Bourguignon C, Cartier R, Prieur C, Tomasevic D, Genot N, Leboube S, Derimay F, Rioufol G, Bonnefoy-Cudraz E, Mewton N, Ovize M, Bidaux G, Bochaton T. Serum soluble tumor necrosis factor receptors 1 and 2 are early prognosis markers after ST-segment elevation myocardial infarction. Front Pharmacol 2021;12:656928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Getz GS, Reardon CA. Natural killer T cells in atherosclerosis. Nat Rev Cardiol 2017;14:304–314. [DOI] [PubMed] [Google Scholar]
- 30. Zhou X, Robertson A-KL, Rudling M, Parini P, Hansson GK. Lesion development and response to immunization reveal a complex role for CD4 in atherosclerosis. Circ Res 2005;96:427–434. [DOI] [PubMed] [Google Scholar]
- 31. Engelbertsen D, Andersson L, Ljungcrantz I, Wigren M, Hedblad B, Nilsson J, Björkbacka H. T-helper 2 immunity is associated with reduced risk of myocardial infarction and stroke. Arterioscler Thromb Vasc Biol 2013;33:637–644. [DOI] [PubMed] [Google Scholar]
- 32. Crosby CM, Kronenberg M. Tissue-specific functions of invariant natural killer T cells. Nat Rev Immunol 2018;18:559–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lee PT, Benlagha K, Teyton L, Bendelac A. Distinct functional lineages of human V(alpha)24 natural killer T cells. J Exp Med 2002;195:637–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sköld M, Cardell S. Differential regulation of Ly49 expression on CD4+ and CD4-CD8- (double negative) NK1.1+ T cells. Eur J Immunol 2000;30:2488–2496. [DOI] [PubMed] [Google Scholar]
- 35. Maeda M, Lohwasser S, Yamamura T, Takei F. Regulation of NKT cells by Ly49: analysis of primary NKT cells and generation of NKT cell line. J Immunol 2001;167:4180–4186. [DOI] [PubMed] [Google Scholar]
- 36. Li Y, To K, Kanellakis P, Hosseini H, Deswaerte V, Tipping P, Smyth MJ, Toh B-H, Bobik A, Kyaw T. CD4+ natural Killer T cells potently augment aortic root atherosclerosis by perforin- and granzyme B-dependent cytotoxicity. Circ Res 2015;116:245–254. [DOI] [PubMed] [Google Scholar]
- 37. Montoya CJ, Pollard D, Martinson J, Kumari K, Wasserfall C, Mulder CB, Rugeles MT, Atkinson MA, Landay AL, Wilson SB. Characterization of human invariant natural killer T subsets in health and disease using a novel invariant natural killer T cell-clonotypic monoclonal antibody, 6B11. Immunology 2007;122:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bagchi S, Genardi S, Wang C-R. Linking CD1-restricted T cells with autoimmunity and dyslipidemia: lipid levels matter. Front Immunol 2018;9:1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lynch L, O’Shea D, Winter DC, Geoghegan J, Doherty DG, O’Farrelly C. Invariant NKT cells and CD1d(+) cells amass in human omentum and are depleted in patients with cancer and obesity. Eur J Immunol 2009;39:1893–1901. [DOI] [PubMed] [Google Scholar]
- 40. Lynch L, Nowak M, Varghese B, Clark J, Hogan AE, Toxavidis V, Balk SP, O’Shea D, O’Farrelly C, Exley MA. Adipose tissue invariant NKT cells protect against diet-induced obesity and metabolic disorder through regulatory cytokine production. Immunity 2012;37:574–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sandberg JK, Bhardwaj N, Nixon DF. Dominant effector memory characteristics, capacity for dynamic adaptive expansion, and sex bias in the innate Valpha24 NKT cell compartment. Eur J Immunol 2003;33:588–596. [DOI] [PubMed] [Google Scholar]
- 42. Kee S-J, Park Y-W, Cho Y-N, Jin H-M, Kim M-J, Lee S-J, Kim T-J, Lee S-S, Kwon Y-S, Jang H-C, Kim N, Shin M-G, Shin J-H, Suh S-P, Ryang D-W. Age- and gender-related differences in circulating natural killer T cells and their subset levels in healthy Korean adults. Hum Immunol 2012;73:1011–1016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the authors (P.K. or H.B.) upon reasonable request, but, due to some of the data containing information that could compromise research participant privacy or consent, requests may require ethical review and full compliance to the general data protection regulation.