Abstract
Developmental dyslexia is characterized by the pathologically diminished ability to acquire reading and spelling skills. Accurate processing of acoustic information at the phonemic scale is crucial for successful sound-to-letter-mapping which, in turn, is elemental in reading and spelling. Altered activation patterns in the auditory cortex are thought to provide the neurophysiological basis for the inaccurate phonemic perception. Recently, transcranial electrical stimulation has been shown to be an effective method to ameliorate cortical activation patterns in the auditory cortex. In a sample of children and adolescents with dyslexia, we investigated the effect of multi-session transcranial alternating current stimulation delivered concurrently with a phonological training and in combination with a behavioral literacy skills training. Over a 5-week period the participants received 10 training sessions while gamma-tACS was administered over bilateral auditory cortex. We found that gamma-tACS shifted the peak frequency of auditory gamma oscillations reflecting a more fine-grained processing of time-critical acoustic information. This amelioration was accompanied by increased phonemic processing skills. Moreover, individuals who received gamma-tACS showed significant improvements in their spelling skills four months after the intervention. Our results demonstrate that multi-session gamma-tACS enhances the effects of a behavioral intervention and induces long-term improvement on literacy skills in dyslexia.
Keywords: Developmental dyslexia, Phonological processing, Transcranial alternating current stimulation, Individual gamma frequency
Highlights
-
•
Dyslexic children and adolescents received 10 sessions of gamma-tACS concurrently with a phonological and a spelling training.
-
•
Behavioral and electrophysiological effects were assessed immediately as well as four months after the intervention.
-
•
Multi-session gamma-tACS enhanced the effects of a literacy skills training in children and adolescents with dyslexia.
-
•
Effects of the multi-session gamma-tACS remained stable up to four months after the intervention.
1. Introduction
Language is one of the most essential tools for exchanging information between humans, both in the form of speech and written language. However, about 5–10% of the population suffer from a learning disability called developmental dyslexia (DD) which hinders the acquisition of reading and spelling skills (Habib, 2000, Moll et al., 2014, Sanfilippo et al., 2020). DD is a neurodevelopmental disorder characterized by the specific and persistent failure to acquire literacy skills despite normal intelligence, motivation, and adequate schooling (American Psychiatric Association, 2013). In addition to the characteristic slow and error-prone reading and spelling, individuals with DD typically show also substantial deficits in acoustic perception, particularly in the processing of time-critical aspects (Shaywitz et al., 2021). As a consequence, linguistically meaningful information units in the acoustic speech stream such as the voice onset time (VOT), i.e. the temporal difference determining the perception of voiced and voiceless consonants (e.g. b/p, d/t) or between long and short vowels are perceived inaccurately (Maassen et al., 2001, Breier et al., 2001, Ingelghem et al., 2001, Vandermosten et al., 2010, Vandermosten et al., 2011). This imprecise processing of acoustic features also affects the correct mapping from speech sounds to letters (phoneme-to-grapheme correspondence) and vice versa. Individuals with DD not only suffer significant adverse consequences directly associated with impaired literacy competence, such as lower grades in school and less success in future professional or academic careers (Madaus, 2008), but also face a higher prevalence of emotional disorders, e.g. anxiety disorders, depression, or low self-esteem compared to their normal reading and spelling peers (Haft et al., 2019, Hendren et al., 2018, Maughan and Carroll, 2006).
The neurophysiological mechanism underlying successful sound-to-letter-mapping is thought to be reflected by endogenous neural gamma oscillations in the auditory cortex. This rhythmic firing of neurons at about 40 Hz has been claimed as functionally relevant because this oscillatory activity represents the sampling rate at which the auditory cortex parses the acoustic (speech) stream. Phonemes, the smallest linguistically meaningful entities, take place in a time range of about 20 – 60 ms. Since this time range roughly corresponds to the duration of a 40 Hz oscillation, i.e. the endogenous firing rate of neurons in the auditory cortex, it has been claimed that gamma oscillations allow the auditory system to extract linguistically meaningful information units at the phonemic scale from the speech signal (Assaneo et al., 2021, Giraud and Poeppel, 2012, Poeppel, 2003). Altered gamma oscillations are thus hypothesized to either lead to an oversampling - resulting in too many frames to be integrated per time unit - or to an undersampling and, thus, insufficient acoustic details per time unit (Giraud and Poeppel, 2012, Goswami, 2011). Of note, altered gamma oscillations have been found in DD: the individual gamma frequency (IGF), assessed by means of auditory steady state responses (ASSRs) to amplitude-modulated acoustic stimuli, is increased in adults with DD (Lehongre et al., 2011, Lehongre et al., 2013) but decreased in children and adolescents with DD (Rufener and Zaehle, 2021) compared to individuals with typically developed literacy skills. These findings are in line with previously reported age-related differences in the IGF in the typically developing brain. This shift has been suggested to mirror adaptation processes in the auditory cortex due to experience-driven myelination of neuronal projections (Poulsen et al., 2009). Of note, the observed IGF correlated with phonological processing skills in both adolescents and adults diagnosed with DD (Lehongre et al., 2011, Rufener and Zaehle, 2021). These results suggest that endogenous gamma oscillations in individuals diagnosed with DD do not correspond to the time range in that acoustic information at the phonemic scale take place. As a consequence, also sound-to-letter-mapping is affected in individuals diagnosed with DD. Accordingly, it can be assumed that shifting the substantially increased or decreased frequency of gamma oscillations towards the sampling rate observed in typically reading and spelling individuals will result in a more adequate and precise acoustic phoneme perception. Furthermore, since accurate phoneme perception forms the basis for successful reading and spelling acquisition (Blau et al., 2010, Rufener and Zaehle, 2021, Kovelman et al., 2012; but see (Blomert, 2011)), one can further assume that shifting altered gamma oscillations has a positive effect on reading and spelling skills in children and adolescents with DD. Typical interventions in DD focus on the behavioral symptoms while neglecting the neurophysiological basis on that the reduced success in acquiring reading and spelling skills rely on. This might, at least in part, explain why the symptom persists into adulthood in the majority of affected individuals.
Recently, transcranial electrical stimulation (TES) has been demonstrated as a promising adjuvant to behavioral interventions in children and adolescents with learning disorders by positively affecting cortical activation patterns. The method of choice to modulate cortical oscillations is transcranial alternating current stimulation (tACS). By means of tACS, a weak sinusoidal electrical current that rhythmically changes between negative and positive polarity is applied at the subjects' scalp. Via entrainment, that is the alignment of the endogenous oscillatory pattern to the externally applied electrical current, cortical oscillations can be shifted in their frequency or amplitude (Bland and Sale, 2019, Fröhlich and McCormick, 2010). Importantly, there is conclusive evidence confirming the safety of tACS in children, adolescents, and adults. TACS is typically well tolerated comes with only moderate side effects such as itching, tingling, or a heating sensation under the stimulation electrodes (Antal et al., 2017, Krishnan et al., 2015). Recent studies point out that tACS has also potential in modulating language functions (for a review, see: (Turker and Hartwigsen, 2021)). The single application of tACS at 40 Hz over bilateral auditory cortex temporarily increased phoneme categorization acuity in adolescents and adults diagnosed with DD (Rufener et al., 2019). Later, positive effects of gamma-tACS administered over auditory cortex have been reported on reading accuracy and phonological processing skills in a sample of adults diagnosed with dyslexia (Marchesotti et al., 2020). Besides these behavioral improvements gamma-tACS applied over bilateral auditory cortex has also been demonstrated to shift gamma oscillations in the auditory cortex of individuals with DD towards the frequency typically observed in a normally reading and spelling control group (Rufener and Zaehle, 2021). Despite these encouraging findings, there is yet no data available showing the stability of these positive effects. Sustained effects, however, are an essential precondition when considering tACS as a clinical intervention. Studies that applied transcranial direct current stimulation (tDCS) in DD, however, showed that the repetitive application increased neural reactivity as well as related perception and cognition up to four months (Costanzo et al., 2019, Lazzaro et al., 2021).
In the present study we investigated the effects of multi-session gamma-tACS applied during a phonological training and in combination with a behavioral literacy skills training in children and adolescents diagnosed with DD. A modulation of auditory gamma oscillations is thought to affect phonemic processing. However, improvement of reading and spelling abilities also require top-down functions such as blending or orthographic rules. We therefore decided on a combined intervention to increase the probability of training success. The results from the experimental group were compared with those of an active control group that received the identical literacy skills training but combined with sham-tACS. We assessed effects on reading and spelling skills, phonemic awareness, as well as on the IGF. Moreover, in addition to immediate effects, we also investigated long-term effects of the intervention four month after the final training session. We hypothesized that multi-session gamma-tACS (I) causes a shift in the IGF and thereby (II) enhances the positive effect of a literacy skills training as evident in stronger improvements in phonemic awareness, spelling performance, and reading performance as compared to a literacy skills training in combination with sham stimulation. Furthermore, we hypothesized that (III) electrophysiological and behavioral effects of this intervention remain stable over a period of four months after the intervention.
2. Methods
2.1. Participants
Thirty German speaking children and adolescents in the age range between 8 and 17 years (M = 11.59, SD = 2.4, seven females) with an existing diagnosis for DD participated in this study. We additionally confirmed the diagnosis by administering a standardized behavioral test battery (see 2.3 Behavioral assessment). To exclude subjects who fulfilled the criteria for any psychiatric or neurological disorder (e.g. attention deficit-/hyperactivity disorder, autism spectrum disorder) parents were interviewed with a semi-structured clinical interview (Schedule for Affective Disorders and Schizophrenia for School-Age Children – Present and Lifetime Version (K-SADS-PL), DSM-5 update (Kaufman et al., 2000)). All participants reported normal hearing performance and had normal or corrected-to-normal vision. Prior to the experiment, all participants and their legal representative gave their written informed consent. The procedure was approved by the ethics committee of the Medical Faculty, Otto von Guericke University Magdeburg and was in accordance with the declaration of Helsinki.
Legal representatives of one female participant from the tACS-group withdrew from study participation after the third training session because they faced problems in regularly accompanying their child to and from the University. All other participants completed the full intervention. Thus, all further statistical analyses rely on 29 subjects. Sample size was determined based on previous studies assessing the effects of TES on reading and writing performance in individuals diagnosed with DD (Marchesotti et al., 2020, Costanzo et al., 2019, Lazzaro et al., 2021, Costanzo et al., 2016a, Costanzo et al., 2016b). None of the participants received any further extra-curricular support or training on literacy skills throughout the duration of the study.
2.2. Experimental procedure and study design
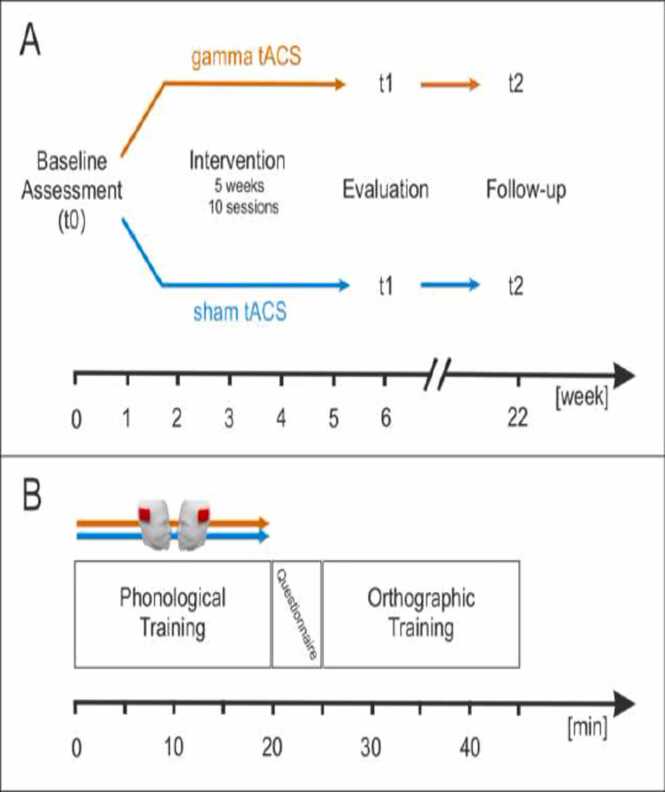
Fig. 1 A shows the study design and the experimental procedure. The participants’ initial literacy skills (see 2.3 Behavioral assessment) and auditory gamma peak frequency (see 2.4 Electrophysiological recording of the auditory gamma oscillations) were assessed at baseline (time point t0). Subsequently, participants were randomly assigned to the treatment group (gamma-tACS) or the sham group. Participants of both groups performed two training sessions per week over a period of five weeks leading to a total of ten training sessions (see 2.5 Intervention). The training sessions were separated by at least 48 h and were performed at the Department of Neurology, Section Neuropsychology, Magdeburg by the first author and an instructed psychologist. Behavioral and EEG-assessment were repeated immediately following the last training session of the multi-session tACS-intervention (Evaluation; time point t1), as well as in a follow up session four month after completing the intervention (time point t2).
2.3. Behavioral assessment
All participants performed a standardized spelling test according to the grade currently attended (Hamburger Schreib-Probe HSP) (May, 2000) and a reading test (Lesegeschwindigkeits- und Verstaendnistest fuer die Klassen 5 – 12; LGVT 5–12 + for participants up from grade 5 (Schneider and Schlagmüller, 2017); Lese- und Rechtschreibtest SLRT II for participants at grade 4 (Moll and Landerl, 2010). Phonemic discrimination and short-term memory skills were assessed using a standardized test (Phonematischer Gedaechtnistest PHOG) (Gruner et al., 2013). In addition, non-verbal intelligence was screened using the CFT 20-R (Weiss, 2019). Developmental dyslexia was defined as a deficit in reading and/or spelling ability resulting in scores at least 1.5 standard deviations (SD) below the individual IQ. Note that IQ was assessed only at time-point t0 and only for diagnostic purposes. We used parallel test versions for the LGVT 5–12 + and the SLRT II (test version A at time point t0 and t2, test version B at time point t1) to reduce retest effects.
Writing performance was defined as T-values in the sub-scales “alphabetic strategies” and “orthographic strategies” of the HSP since these measures best represent the individuals’ competence in phoneme-to-grapheme mapping. Reading performance was quantified as the number of correctly read words (SLRT II) and the number of correct responses in the subscale “text comprehension” (LGVT 5–12 +). In the PHOG, we used the two sub-scales “vowels/syllables” and “words/sentences” (T-values in both sub-scales) instead of the total test score because these subscales best represent the individuals' acuity in phoneme perception. Since we hypothesize that a modulation of auditory gamma oscillations leads to changes in the processing of linguistically meaningful information units at the phonemic scale the effects of gamma-tACS should be most evident in the above mentioned sub-scales of the PHOG.
Due to restrictions of the local authorities in the context of the COVID-19 crisis we had to suspend in house data acquisition from 03.2020 until 05.2020. In order to avoid data loss due to this extraordinary and unforeseeable event the behavioral assessments during this time-period were performed by the participants’ legal representatives at home. To ensure data quality and validity of the results we carefully instructed them individually by phone and in standardized written form. In total, behavioral tests at the evaluation (t1) of four participants (two participants in each group) and of six participants (three participants in each group) at the follow up measurement (t2) were performed by the legal representatives.
2.4. Electrophysiological recording of auditory gamma oscillations
Auditory gamma oscillations as represented by the Individual Gamma Frequency (IGF) were assessed at t0, t1, and t2 using an Auditory Steady-State Response (ASSR) paradigm. Briefly, ASSRs are the auditory cortex’ responses to periodic acoustic signals where the frequency of the ASSR corresponds to the frequency of the input signal. The frequency that evokes the strongest electrophysiological response is termed as IGF. In addition to the individual frequency (in Hz), which represents an electrophysiological estimation of the firing rate and thus the sampling rate of a neuron population in the auditory system, we also assessed the power (in μV^2) of the IGF reflecting the number of neurons in the respective neuron population active at this specific frequency as a measure of the amount of activity in this specific frequency. The stimulus material used to assess the ASSR consisted of 1 kHz pure tone stimuli (10 s duration) that were amplitude modulated (AM) at frequencies from 30 to 70 Hz in steps of 1 Hz (see (Rufener and Zaehle, 2021) for more detailed information on stimulus material and ASSR-paradigm). Each AM-sound was presented three times and in randomized order.
EEG was continuously recorded at 21 sintered Ag/AgCl-electrodes, which were evenly distributed over the scalp and mounted to an elastic cap (Easycap GmbH, Herrsching-Breitbrunn, Germany). Vertical and horizontal eye movements were monitored from two additional electrodes placed lateral and below the right eye. The reference electrode was place on the nose-tip, the ground at AFz. Impedances were kept below 10 kΩ. The EEG signal was digitized at a sampling rate of 1000 Hz using the BrainAmp system and recorded with the BrainVision recorder (BrainProducts, Munich, Germany). Offline, data were down-sampled to 256 Hz and band pass filtered (0.1–80 Hz). Artifacts caused by eye movements and heartbeat were rejected using an independent component analysis (ICA) while muscular artifacts with amplitude > 100 µV were automatically discarded. The pre-processed data were then segmented into epochs of 500–9500 ms after stimulus-onset to eliminate the electrophysiological build-up and ramp-down of the steady state response. The remaining 9000 ms-epochs were segmented in 1000 ms-epochs, resulting in a maximum of 27 epochs for each ASSR-frequency. To determine the IGF, power spectrum analyses were performed on the averaged data for each modulation frequency separately using Fast Fourier Transforms (Hanning window, 1 Hz resolution). To control for the ASSR-typical 1/f characteristic we multiplied the power values by the respective modulation frequency (John et al., 2003, Cone-Wesson et al., 2002). The modulation frequency that elicited the largest ASSR amplitude at electrode Cz was used as estimation for the IGF.
Similar to the behavioral data we had to suspend EEG-data acquisition during this time period. Thus, EEG-data were not acquired from all participants at each time point. Note that there is data from the baseline session as well as at least one further time point from all participants. In total, t1-data of six participants (3 tACS, 3 sham) and t2-data of six different participants (3 tACS, 3 sham) were affected.
2.5. Intervention
In each session, participants completed a supervised literacy skills training for 40 min. (cf. Fig. 1B). Each session started with mounting the tACS-electrodes (see 2.6 Transcranial alternating current stimulation). In the first 20 min and simultaneous to tACS the participants performed a phonological training including exercises on phoneme categorization, syllable processing, and rhyme processing, aiming to improve phonemic and phonological skills. In the exercises focusing on phoneme categorization the participants were acoustically presented with stimuli representing a VOT continuum from voiced to unvoiced consonant-vowel stimuli (ba/pa, da/ta; ga/ka). Each of the continua consisted of 11 stimuli which were presented eight times in randomized order (Rufener et al., 2019). After each stimulus, participants had to indicate via button-press whether they perceived the previously presented stimulus as either voiced or unvoiced. In the exercises targeting syllable processing the participants had to segment acoustically presented German words consisting of two to six syllables into their constituent syllables. In the exercises targeting rhyme processing, the participants' task was to identify two objects in a series of objects that build a rhyme pair (e.g. Haus / Maus, Puppe / Suppe). Stimuli consisted of pictures of everyday objects only, on written words only, or a combination. In all tasks, the participants received direct feedback after each response. All three elements of the phonological training (phoneme categorization, syllable processing, rhyme processing) were performed in each training session.
Fig. 1.
Study design and experimental procedure. A) Timeline of the study showing the different time points. Behavioral and EEG variables were assessed at baseline (t0), evaluation (t1) and follow-up (t2). B) Sequence of an exemplary training session. Each session started with exercises targeting phonemic processing while gamma-tACS or sham-tACS was applied. Subsequently, during a short break of about 5 min, tACS-electrodes were removed and the participants completed a short questionnaire on their physical state and potential adverse events due to the electrical stimulation. Immediately after the break, participants performed the orthographic training.
Subsequently, during a short break of about 5 min, the tACS-electrodes were removed and a short questionnaire on potential side effects was completed.
The orthographic training that was performed directly after the break consisted of an adaptive procedure that focused on basic orthographic rules. All exercises and the material were taken from the Marburger Rechtschreibtraining MRT (Schulte-Körne and Mathwig, 2013), an evaluated German spelling training for children and adolescents with reading and spelling deficits (Schulte-Körne et al., 2001). Exercises taken from the MRT focused on vowel duration, since this feature is linguistically meaningful in German and determines the number of consonants following the respective vowel (e.g. Nudel [eng.: noodle]; long vowel followed by one consonant but Suppe [eng.: soup]: short vowel followed by two consonants). In addition, exercises on typical irregularities in German spelling were performed. An individual number of exercises were used for each topic until the participant was able to correctly solve the task. Note that the behavioral intervention including the performed exercises was identical for participants from the tACS group and from the sham group.
2.6. Transcranial alternating current stimulation
TACS was applied by means of a battery-driven stimulator (DC STIMULATOR Plus, NeuroConn, Ilmenau, Germany). A double-blinded design was ensured using the STUDY MODE module provided by the tACS device. Thus, participants, their caregivers as well as any personal involved were naïve regarding the group allocation (verum or sham) until unblinding at the end of the study. Two 5 × 7 cm rubber electrodes in 0.9% saline-soaked sponges were placed horizontally over T7 and T8 (Rufener et al., 2019, Rufener et al., 2016a). Impedance was kept below 15 kOhm. Participants allocated to the gamma-tACS group received tACS with a frequency of 40 Hz-over a period 20 min with a 10 s fade in / out sequence. Participants in the sham group received tACS with a frequency of 40 Hz for 30 s and a fade in/out sequence of 10 s only to allow a successful blinding of the participants (Antal et al., 2017). In all participants (tACS group, sham group) the tACS-intensity was set to 1 mA and the identical electrode montage used was used.
After each stimulation session, participants were asked to fill out a short questionnaire on whether they had felt any sensation due to the electrical stimulation (i.e., headache, nausea, dizziness, loss of concentration, fatigue, skin irritation, itch, prickle on the scalp, heat, burning) by means of a numeric rating scale (0 = no sensation, 4 = strong sensation).
2.7. Statistical analysis
To assess potential pre-existing differences between the two groups (gamma-tACS, sham-tACS) before the intervention, we used separate two-tailed non-parametric independent samples Mann-Whitney U-tests and compared age, IQ, spelling performance, reading performance, phonemic skills, IGF, and power of the IGF between the participants of the tACS group and the sham group. Table 1 shows the demographic data as well as the participants literacy skills and IGF at baseline (t0). Statistical analyses revealed no significant differences in age, IQ or the participants performance in reading, spelling or phonemic skills between the tACS group and the sham group.
Table 1.
Description of the study sample. Demographic data as well as the initial literacy skills of the participants separately depicted for the tACS group and the sham group. Data represent mean values. Standard deviation in parentheses.
| gamma-tACS | Sham-tACS | p-value | |
|---|---|---|---|
| N (gender) | 14 (11 m/3 f) | 15 (11 m/4 f) | |
| Age (in years) | 11.85 (2.51) | 11.29 (2.37) | .505 |
| IQ | 96.07 (10.60) | 100 (11.55) | .252 |
| Phonemic skills (total score)* |
42.64 (9.7) | 42.2 (11.18) | .914 |
| Phonemic skills (subscale vowels /syllables)* |
43.43 (9.67) | 40.27 (10.49) | .591 |
| Phonemic skills (subscale words/sentences)* |
43.29 (10.09) | 47.87 (12.10) | .377 |
| Spelling performance (correct words)* |
32.79 (8.38) | 32.67 (9.05) | > .999 |
| Spelling performance (correct graphemes)* |
32.43 (7.41) | 34.0 (7.93) | .652 |
| Spelling performance (alphabetic strategy)* |
33.86 (10.91) | 39.53 (10.58) | .158 |
| Spelling performance (orthographic strategy)* | 31.86 (7.56) | 36.80 (7.79) | .172 |
| Reading performance (accuracy)* |
38.63 (6.27) | 34.36 (8.24) | .102 |
| Individual gamma frequency (power in µV*2) | 6.14 (3.65) | 8.89 (10.27) | .780 |
| Individual gamma frequency (peak frequency in Hz) | 40.28 (6.22) | 41.07 (6.04) | .683 |
Values represent T-scores. Note that a T-score of 50 represents the norm mean, while scores + /− 10 represent a standard deviation above/below norm, respectively.
To evaluate the effects of the intervention, the data was analyzed using Linear Mixed Models (LMMs) performed with the lmer function from the afex package (Singmann et al., 2022). P-values for the β-estimates were obtained using Satterthwaite’s approximation method. We performed separate LMMs for the dependent variables spelling performance, reading performance, phonemic skills, and IGF. Time (t0, t1, t2), group (tACS, sham), and group x time were considered as fixed factors. Data from the sham group at t0 were used as baseline. Individuals were used as random effects. Age and gender were added as covariates to account for potential group differences. The Akaike Information Criterion (AIC) was used to compare models. A two-sided significance level of α = 0.05 was used. Table 2 shows the β-coefficients of LMMs separately depicted for each variable of interest.
Table 2.
β-coefficients of LMMs.
| Beta | SE | t-value | p-value | |
|---|---|---|---|---|
| Phonemic skills (subscale vowels /syllables) | ||||
| intercept | 40.267 | 2.922 | 13.779 | < .001 |
| time t1 | 3.267 | 1.791 | 1.824 | 0.037 |
| time t2 | 6.693 | 1.837 | 3.643 | < .001 |
| group | 3.162 | 4.206 | 0.752 | 0.229 |
| time t1 * group | -1.481 | 2.578 | 0.574 | 0.284 |
| time t2 * group | -3.564 | 2.688 | 1.326 | 0.095 |
| Phonemic skills (subscale words/sentences) | ||||
| intercept | 47.867 | 2.862 | 16.725 | < .001 |
| time t1 | 2.4 | 1.856 | 1.293 | 0.100 |
| time t2 | 5.625 | 1.904 | 2.955 | 0.002 |
| group | -4.581 | 4.119 | -1.112 | 0.137 |
| time t1 * group | 4.529 | 2.672 | 1.695 | 0.048 |
| time t2 * group | 1.599 | 2.786 | 0.574 | 0.284 |
| Spelling performance (alphabetic strategies) | ||||
| intercept | 39.533 | 2.763 | 14.307 | < .001 |
| time t1 | -0.133 | 2.055 | -0.065 | 0.474 |
| time t2 | 0.133 | 2.107 | 0.063 | 0.475 |
| group | -5.677 | 3.977 | -1.427 | 0.080 |
| time t1 * group | 0.705 | 2.958 | 0.238 | 0.406 |
| time t2 * group | 2.960 | 3.082 | 0.960 | 0.170 |
| Spelling performance (orthographic strategies) | ||||
| intercept | 36.8 | 2.146 | 17.151 | < .001 |
| time t1 | 2.333 | 1.261 | 1.849 | 0.035 |
| time t2 | 2.621 | 1.294 | 2.025 | 0.024 |
| group | -4.943 | 3.088 | -1.601 | 0.059 |
| time t1 * group | -0.691 | 1.816 | -0.380 | 0.353 |
| time t2 * group | 3.503 | 1.894 | 1.850 | 0.035 |
| Individual gamma frequency (power in µV*2) | ||||
| intercept | 8.891 | 2.047 | 4.342 | < .001 |
| time t1 | -1.071 | 1.447 | -0.741 | 0.231 |
| time t2 | 1.651 | 1.447 | 1.141 | 0.13 |
| Group | 2.754 | 2.947 | -0.934 | 0.177 |
| time t1 * group | 4.465 | 2.030 | 2.199 | 0.016 |
| time t2 * group | 2.180 | 2.095 | 1.041 | 0.152 |
| Individual gamma frequency (peak frequency in Hz) | ||||
| intercept | 41.067 | 1.545 | 26.583 | < .001 |
| time t1 | 1.454 | 1.866 | 0.779 | 0.219 |
| time t2 | -1.438 | 1.866 | -0771 | 0.222 |
| Group | -0.781 | 2.223 | -0.351 | 0.363 |
| time t1 * group | 1.591 | 2.621 | 0.607 | 0.273 |
| time t2 * group | 4.883 | 2.696 | 1.811 | 0.038 |
Statistical analysis of the questionnaires on tolerability and side effects after each tACS /sham-application was performed by means of two-tailed non-parametric Mann-Whitney U-tests and for each sensation separately.
Statistical analyses and production of all plots was performed using R Statistical Software (version 4.2.0, R Core Team, 2022) and JASP software (version 0.16.3, JASP Team, 2022).
3. Results
Table 3 shows the behavioral and electrophysiological data for all three measurement time points separately depicted for the tACS group and the sham group.
Table 3.
Behavioral and electrophysiological data for all three measurement time points separately depicted for the tACS group and the sham group. Data represent mean values. Standard deviation in parentheses. a Behavioral data represent T-scores. Note that T-scores have a mean of 50 and a standard deviation of 10. Asterisks represent significant group-differences as revealed by the LMM analysis.
| gamma-tACS | sham-tACS | |||||
|---|---|---|---|---|---|---|
| t0 | t1 | t2 | t0 | t1 | t2 | |
| Phonemic skills (subscale vowels /syllables)a |
43.43 (9.67) | 45.21 (11.18) | 46.08 (6.82) | 40.27 (10.49) | 43.52 (13.55) | 47.79 (13.18) |
| Phonemic skills (subscale words/sentences)a |
43.29 (10.09) | 50.21 (9.72) * | 50.58 (10.17) | 47.87 (12.10) | 50.27 (12.73) | 54.00 (10.8) |
| Spelling performance (alphabetic strategy)a |
33.86 (10.91) | 34.43 (11.15) | 37.25 (13.15) | 39.53 (10.58) | 39.40 (9.70) | 39.21 (8.98) |
| Spelling performance (orthographic strategy)a |
31.86 (7.56) | 33.50 (10.22) | 38.08 (9.91) * | 36.80 (7.79) | 39.12 (7.91) | 38.63 (6.06) |
| Reading performance (accuracy)a |
38.63 (6.27) | 39.33 (5.72) | 42.19 (7.60) | 34.36 (8.24) | 36.49 (7.40) | 38.22 (10.94) |
| Individual gamma frequency (power in µV*2) | 6.14 (3.65) | 9.65 (4.58) * | 9.85 (9.32) | 8.89 (10.27) | 7.71 (7.75) | 11.50 (11.17) |
| Individual gamma frequency (peak frequency in Hz) | 40.28 (6.22) | 43.54 (6.50) | 43.90 (4.68) * | 41.07 (6.04) | 42.50 (7.37) | 39.92 (4.36) |
3.1. Phonemic awareness
The model to predict performance in the PHOG subscale “vowels/syllables” (VoSy) showed that participants from the sham group numerically increased their performance from t0 to t1 as well as from t1 to t2. However, there was neither a significant effect of group nor an interaction group x time indicating that gamma-tACS had no increasing effect on task performance (cf. Fig. 2a). In the model the fixed and random effects explained approximately 82% of the variance (R2 conditional = 0.819). Including the factor age did not lead to a statistically significant increase in model fit (AIC(lmm VoSy): 590.7; AIC (lmm VoSy + age) = 592.2, p = .482). We found, however, that including the factor sex significantly increased the model fit (AIC(lmm VoSy): 590.7; AIC (lmm VoSy + sex) = 585.49, p = .007). Additionally performed non-parametric Mann-Whitney U-tests showed that female participants in the sham group performed significantly worse compared to male participants from the sham group at time point t0 (U = 34.0; p = .025), time point t1(U = 32.0, p = .052), and time point t2 (U = 30.5, p = .035). No such difference was evident between female and male participants from the tACS group (all time points: p > .340).
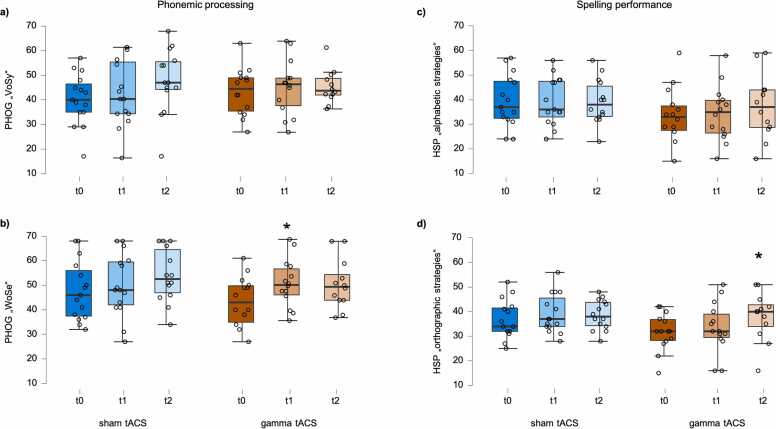
Fig. 2.
Behavioral results of participants that received multi-session gamma-tACS (orange) and the active control group that received sham-tACS (blue). After the intervention (time point t1) participants from the gamma-tACS group showed stronger increases in phonemic processing skills (b). In the follow-up measurement (time point t2) participants from the gamma-tACS group showed stronger increases in their spelling skills compared to the control group (d). Dots represent the individual participants. t0: baseline session; t1: evaluation session, t2: four-month follow-up session. Asterisks represent significant group x time-differences as revealed by the LMM analysis.
Results on the model on the PHOG subscale “words/sentences” (WoSe) revealed that participants from the sham group increased task performance over the course of the experiment. Their initial performance was a T-score of 47.867 and increased by 2.4 T-scores by timepoint t1 [β time t1 = 2.4, 95% CI (−1.17, 5.97), t = 1.203, p=.100] and by 5.625 T-scores by timepoint t2 [β time t2 = 5.625, 95% CI (1.97, 9.29), t = 2.995, p=.002]. Importantly, participants that received gamma-tACS showed a significantly stronger increase by 6.929 T-scores from t0 to t1 as compared to the sham group (β time t1 + β time t1 * group; β time t1 * group = 4.529, 95% CI (−0.60, 9.66), t = 1.695, p = .048; cf. Fig. 2b]. The fixed and random effects explained approximately 81% of the variance (R2 conditional = 0.805). Including the factor age did not lead to a statistically significant increase in the model fit (AIC(lmm WoSe): 592.93; AIC (lmm WoSe + age) = 594.62, p=.577). We found, however, that including the factor sex significantly increased the model fit (AIC(lmm WoSe): 592.93; AIC (lmm WoSe + sex) = 590.86, p=.007). Additionally performed non-parametric Mann-Whitney U-tests revealed that female participants from the sham group performed worse compared to male participants from the sham group at time point t0 (U = 30.0; p=0.097), time point t1(U = 31.5, p=.060), and time point t2 (U = 31.0, p=0.028). No such difference was evident between female and male participants from the tACS group (all time points: p>.310).
3.2. Spelling performance
Regarding the “alphabetic strategies” no effect of group and no effect of time was found. Including the factors sex and age did not lead to a significant increase in model fit (Fig. 2c). The fixed and the random effects explained approximately 74% of the variance (R2 conditional = 0.736).
Results on the model on the “orthographic strategies” (Fig. 2d) showed that participants from the sham group increased task performance over the course of the experiment. Their initial performance was a T-score of 36.8 and increased by 2.33 T-scores by time point t1 [β time t1 = 2.33, 95% CI (−0.09, 4.76), t = 1.849, p=.035] and additional 2.621 T-scores by time point t2 [β time t2 = 2.621, 95% CI (0.13, 5.11), t = 2.025, p=.024]. Importantly, participants who received gamma-tACS showed a significantly stronger increase compared to the sham group: their performance increase by 6.124 T-scores by time point t2 (β time t2 + β time t2 * group; β time t2 * group = 3.503, 95% CI (−0.13, 7.15), t = 1.85, p = .035. In the model the fixed and random effects explained approximately 84% of the variance (R2 conditional = 0.844). The model fit was neither increased by including the factor sex (AIC(lmm OS): 534.55; AIC (lmm OS + sex) = 535.16, p=.239) nor by including the factor age (AIC(lmm OS): 534.55; AIC (lmm OS + age) = 535.30, p=.264).
3.3. Power of the individual gamma frequency (IGF)
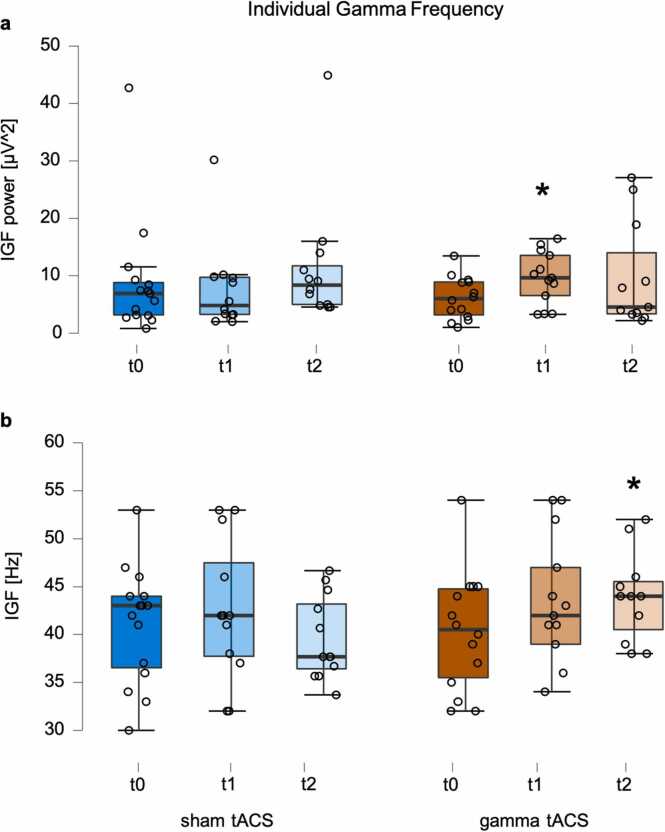
Compared to the sham group, participants that received gamma-tACS show a significantly stronger increase by 3.394 uV^2 at time point t1 (β time t1 + β time t1 * group; β time t1 * group = 4.465, 95% CI (0.58, 8.35), t = 2.030, p = .016, cf. Fig. 3a]. In the model the fixed and random effects explained approximately 60% of the variance (R2 conditional = 0.595). Including the factor sex did not lead to a significant increase in model fit (AIC(lmm pow): 496.08; AIC (lmm pow + sex) = 498.07, p = .981). We found, however, that including the factor age significantly increased the model fit (AIC(lmm pow): 496.08; AIC (lmm pow + age) = 585.49, p = 0.023). The adapted fixed and random effects still explained about 60% of the variance (R2 conditional = 0.605). Further inspection of the data show that one participant in the sham group exposed very strong power values. To ensure that the reported results were not exclusively driven by this subject, we performed the model without this outlier. Still, the stronger power-increase in the tACS group at time point t1 remained statistically significant (β time t1 + β time t1 * group; β time t1 * group = 3.659, 95% CI (0.01, 7.35), t = 1.910, p = .032).
Fig. 3.
Electrophysiological results of participants that received multi-session gamma-tACS (orange) and the active control group that received sham-tACS (blue). After the intervention (time point t1) participants from the gamma-tACS group showed stronger increases in the power of auditory cortex gamma oscillations (a). In the follow-up measurement (time point t2) participants from the gamma-tACS group showed stronger increases in the individual gamma frequency compared to the control participants (b). Dots represent the individual participants. t0: baseline session; t1: evaluation session, t2: four-month follow-up session. Asterisks represent significant group x time-differences as revealed by the LMM analysis.
3.4. Individual gamma frequency (IGF)
Compared to the sham group, participants that received gamma-tACS show a significantly stronger increase by 3.445 Hz at time point t2 (β time t2 + β time t2 * group; β time t2 * group = 4.883, 95% CI (−0.28, 10.05), t = 1.811, p = .038, cf. Fig. 3b]. In the model the fixed and random effects explained approximately 41% of the variance (R2 conditional = 0.409). The model fit was neither increased by including the factor sex (AIC(lmm IGF): 495.14; AIC (lmm IGF + sex) = 497.14, p = .961) nor by including the factor age (AIC(lmm IGF): 534.55; AIC (lmm IGF + age) = 494.81, p = .127).
In sum, the results indicate that the a five weeks literacy skills training combined with gamma-tACS positively affected phonemic processing and the power of individual gamma frequency in individuals with DD. Additionally, we found an ameliorating effect on the IGF as well as on spelling skills in the follow-up session four month after the intervention, indicating a delayed effect of multi-session tACS on the auditory systems’ sampling rate and related behavioral performance. Furthermore, the fact that all participants irrespective of the group allocation increased their reading performance demonstrates the efficacy of our behavioral intervention.
3.5. Tolerability
No participant asked to withdraw from the study or reported significant discomfort at the location of the stimulation electrodes. Subjective reports on potential side effects did not reveal any statistically significant differences between the sham group and the gamma tACS group (all: p > 0.05). None of the participants reported discomfort or adverse effects at any stimulation session or at any post-intervention time-point (t1 and t2).
4. Discussion
In the present study we tested the hypothesis that gamma-tACS can enhance the beneficial effects of a literacy skills training in a sample of children and adolescents with DD. Additionally, we hypothesized that these behavioral improvements would be directly related to neurophysiological parameters such as changes in the auditory cortex’ gamma peak frequency. To this end, we compared behavioral and EEG-data recorded prior and immediately after ten combined tACS-training sessions which were performed twice a week over a period of five weeks. In addition, long-term effects were assessed four month after the end of the intervention. We found that the combined multi-session tACS-intervention positively affected phonemic processing skills, spelling performance, and enhanced the auditory cortex’ sampling rate as indicated by an increase in the IGF. Thereby, we show that the application of gamma-tACS can increase the benefit of a behavioral DD-training and that these fostering effects are already observable after a short-time intervention over five weeks. Our data extend the findings from previous studies that reported positive effects of a single-session of gamma-tACS on phonemic processing in typically reading participants (Rufener et al., 2016a, Rufener et al., 2016b) as well as in individuals with DD (Rufener and Zaehle, 2021, Rufener et al., 2019, Marchesotti et al., 2020). Furthermore, our data complete existing evidence on the ameliorating effect of multi-session transcranial electrical stimulation combined with a behavioral training in children and adolescents with DD (Costanzo et al., 2019, Costanzo et al., 2016b). Finally, we show that multi-session gamma-tACS is feasible and well tolerated in children and adolescents with DD. Our results thus have important implications on future interventions in DD but also on language rehabilitation in general.
Interestingly, we found different trajectories in our variables of interest: While effects on the power of the IGF and on phonemic skills were observable immediately after the intervention the changes on spelling performance and the peak frequency of the IGF were not evident until four months later. We will discuss these findings in the following sections.
4.1. Functional action cascade: from phonemic awareness to written language
One line of argumentation is that auditory gamma oscillations, phonemic processing skills, and spelling performance rely on each other. In other terms, there is a hierarchical structure connecting these functions. There is evidence on phonological processing skills as predictor for future literacy skills already prior to the onset of formal instruction (Puolakanaho et al., 2004, Puolakanaho et al., 2008, Clayton et al., 2020, Bus and van IJzendoorn, 1999, Goswami, 2006; but see Bretherton and Holmes, 2003; Blomert and Wllems, 2010) and that interventions targeting phonological processing can improve reading and spelling performance (for a meta-analysis, see (Melby-Lervåg et al., 2012; Al Otaiba et al., 2009). Furthermore, the direct link between the IGF and phonemic processing has already been demonstrated (Lehongre et al., 2011, Rufener and Zaehle, 2021). The power of the IGF is thought to reflect phase-synchronicity of neuron populations in the auditory cortex to the envelope of a periodic stimulus (Picton et al., 2003). The ability of the auditory system to follow rapidly changing features at about 40 Hz is crucial in phoneme perception (Poulsen et al., 2009, Rojas et al., 2006). The finding of stronger IGF power at time-point t1 might implicate that gamma-tACS increased the auditory systems’ ability to accurately track acoustic information at the phonemic rate. This increase in temporal acuity enabled the participants to perceive and distinguish phonemes more precisely – as evident in increased phonemic processing skills measured at time point t1. The enhanced perception of phonemic information improved speech-to-letter mapping and, finally, the application of simple orthographic rules as evident in our finding of increased spelling performance at time-point t2.
4.2. Multi-session tACS causes long-term plasticity in the auditory system
The finding that multi-session tACS had a fostering effect on oscillatory activity in the auditory cortex both immediately after the intervention but also in the follow-up measurement indicates long-term effects of our intervention. Long-term effects of single-session tACS lasting up to 30 min after tACS application have already been shown on occipital alpha oscillations (Zaehle et al., 2010, Helfrich et al., 2014a, Helfrich et al., 2014b). In the motor-domain, beta-tACS administered over 15 min even lead to ongoing behavioral effects up to 60 min after application (Wischnewski et al., 2019). Furthermore, Ahn et al., 2019 found that alpha-tACS repetitively applied over five days led to a sustained shift in oscillatory activity until one month after the final intervention (although the effect was only numerical and not statistically significant). Our data demonstrate that ten interventions with gamma-tACS over a period of five weeks can cause after-effects even up to four months after the final intervention.
There is growing evidence that tACS not only acts immediately via entrainment, i.e. by the alignment of neural oscillations to the externally applied alternating current (Vossen et al., 2015) but that it also causes plasticity and enduring adaptation in the stimulated cortical area (Vosskuhl et al., 2018). The precise neurophysiological mechanism on which this plasticity effect relies on is still a matter of debate (Vossen et al., 2015). Zaehle (Zaehle et al., 2010) suggested spike timing dependent plasticity (STDP) as the driving force that causes long-lasting tACS after-effects on neural oscillations. Later, Vossen (Vossen et al., 2015) confirmed this theoretical assumption in an empirical study by demonstrating that (initial) entrainment gates frequency-specific changes in neural oscillations via long-lasting synaptic plasticity. However, others have argued that changes at the synaptic level such as long-term potentiation LTP and long-term depression LTD might not be sufficient to explain long-term effects and suggested metaplasticity as the crucial underlying mechanism (Costanzo et al., 2019, Korai et al., 2021, Carvalho et al., 2015 Mar). Briefly, metaplasticity refers to the modulation of synaptic plasticity by prior synaptic activity (Abraham and Bear, 1996). Hence, the previous modification in a neuronal network can influence the effects of subsequent interventions to the same network (Carvalho et al., 2015). Depending on the history of the initial synaptic activity the direction and degree of synaptic plasticity is modulated. This can result in either a strengthening or inhibition of future synaptic plasticity, synaptic stabilization or the homeostatic regulation of cellular activity (Müller-Dahlhaus and Ziemann, 2015). However, in order to allow metaplasticity the effects of the previous modification have to be still ongoing when the subsequent intervention occurs (Abraham, 2008, Fricke et al., 2011). In terms of metaplasticity evoked by multi-session tACS, this implicates that the after-effects of the previous tACS session must still be persisting when the second tACS session is performed. Costanzo (Costanzo et al., 2019; 2018) assessed the effect of a six-weeks reading training with concomitant tDCS (three training sessions a week, each lasting about 20 min) and found the strongest behavioral improvements in the two follow-up assessments that took place one month as well as six month after completing the training. Accordingly, and comparable to the results of our present study, the effects of their multi-session tDCS intervention were also delayed. One might therefore cautiously conclude that each 20 min application of gamma-tACS indeed resulted in sufficiently stable after-effects that allowed metaplasticity. Since our study represents, to the best of our knowledge, the first attempt of multi-session gamma-tACS further research is needed to determine the optimal stimulation parameters. In this context, it is of specific importance to shed more light on the ideal timing between the tACS-sessions to achieve strong and long-lasting metaplasticity effects.
Despite the encouraging results of the present work also limiting factors have to be taken into account. Our intervention did not result in positive effects on reading performance. This might be due to the inherent nature of the material and exercises we used in the orthographic training. They focused on basic orthographic rules relying on the perception and discrimination of different vowel lengths. Thus, although the individual exercises required the participant to read single words and short texts the interventions’ focus was mainly on spelling competences. Accordingly, other interventions might have resulted in stronger effects on reading performance. An alternative explanation relies on the test material used to assess the participants literacy skills: while the outcome of the reading tests is represented by the number of correctly read items in a given time period, no time restriction was given in the spelling test. Assessing reading performance without time restriction, e.g., by means of questions assessing text comprehension might be a more sensitive approach to measure the participants’ reading skills.
Second, we had to temporally suspend in-house data acquisition due to regulations of the local authorities during the COVID-19 pandemic. Behavioral assessments during this time-period were performed by the participants’ legal representatives at home. Although we provided the test instructions both in written form as well as in verbal form by phone call we cannot rule out the fact that these environmental factors might have affected the test results. Since data of participants that received gamma-tACS but also of participants from the sham group are affected we belief that our procedure did not systematically affect the reported between-group effects.
Third, because our literacy skills training consisted of a combined phonological and orthographic intervention we cannot disentangle the contribution of each component to the reported outcome. Accordingly, we cannot exclude the possibility that already one component of the intervention would have been sufficient to achieve the presented findings. However, due to the hierarchical structure between auditory gamma oscillations, phonemic processing, and literacy skills we cautiously propose that both parts of the intervention, that is the phonological as well as the orthographic training, are required to eventually increase literacy skills. The application of gamma-tACS combined with the phonological training is thought to specifically affect phonemic processing skills. The implementation of orthographic rules, however, represents a higher-order language skill that is acquired mainly via explicit instruction – as performed by means of the orthographic part of our intervention. Nevertheless, future studies are needed to quantify the impact of the phonological and the orthographic part of the intervention but also their individual susceptibility to gamma-tACS. In the same vein, our study design does not allow to quantify the mere impact of the literacy skills training or the participants’ regular class attendance. Although this information would have been of interest we decided against a waiting group or a group that received gamma-tACS in parallel with a non-linguistic (control) task due to the limited funding period but also due to ethical considerations.
Finally, since our study included of a relatively small sample size consisting of children and adolescents over a broad age range we refrained from performing any subsample analyses on the efficacy of gamma-tACS on individuals of different age groups. Accordingly, our data do not allow to conclude on potential age-related effects of gamma-tACS on the individuals’ reading and spelling performance. Since an age-related trajectory of the IGF with increasing peak frequencies over the course of childhood and adolescence until adulthood has been reported (Poulsen et al., 2009) future studies including larger sample sizes should focus on age-specific susceptibility to gamma-tACS as well as the optimal stimulation parameters depending on the individuals age and IGF.
5. Conclusion
The results of the present study provide first evidence on an ameliorating effect of multi-session gamma-tACS in children and adolescents with DD. Our finding of sustained after-effects of gamma-tACS up to four month together with the high tolerability of the intervention emphasize the need for large-scale replication studies.
Funding
This work was funded by the Deutsche Forschungsgemeinschaft (ZA 626/2–1), the Leibniz Association (SAS-2015-LIN-LWC), and by the federal state of Saxony-Anhalt and the European Regional Development Fund (ERDF) in the Center for Behavioral Brain Sciences (CBBS, ZS/2016/04/78113).
CRediT authorship contribution statement
Katharina S. Rufener: Planned the study, collected the data, analyzed the data, wrote the original draft, Tino Zaehle: Planned the study, wrote the original draft, Kerstin Krauel: Planned the study, supervised the diagnostics. All authors participated in interpreting the results, writing the manuscript and approved the final version.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. The authors declare no competing interests.
Acknowledgments
The authors would like to thank Julia Andreca and Caroline Dietrich for their valuable help in EEG-data acquisition and in performing the literacy skills training. Furthermore, the authors are grateful to Christiane Bier und Magdalena Mischke for performing the diagnostic procedure.
Data statement
Readers seeking access to the data should contact the corresponding authors, Katharina S. Rufener. Requestors must complete a formal data sharing agreement to obtain the data. All data necessary and sufficient information to replicate all data processing steps and analyses will be shared to requestors who meet these requirements.
References
- Abraham W.C. Metaplasticity: tuning synapses and networks for plasticity. Nat. Rev. Neurosci. 2008;9(5):387. doi: 10.1038/nrn2356. (May) [DOI] [PubMed] [Google Scholar]
- Abraham W.C., Bear M.F. Metaplasticity: the plasticity of synaptic plasticity. Trends Neurosci. 1996;19(4):160. doi: 10.1016/s0166-2236(96)80018-x. –130. [DOI] [PubMed] [Google Scholar]
- Ahn S., Mellin J.M., Alagapan S., Alexander M.L., Gilmore J.H., Jarskog L.F., et al. Targeting reduced neural oscillations in patients with schizophrenia by transcranial alternating current stimulation. NeuroImage. 2019;186:126–136. doi: 10.1016/j.neuroimage.2018.10.056. (Feb) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Otaiba S., Puranik C.S., Ziolkowski R.A., Montgomery T.M. Effectiveness of early phonological awareness interventions for students with speech or language impairments. J. Spec. Educ. 2009;43(2):107–128. doi: 10.1177/0022466908314869. (Aug) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antal A., Alekseichuk I., Bikson M., Brockmöller J., Brunoni A.R., Chen R., et al. Low intensity transcranial electric stimulation: safety, ethical, legal regulatory and application guidelines. Clin. Neurophysiol. 2017;128(9):1774–1809. doi: 10.1016/j.clinph.2017.06.001. (Sep) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assaneo M.F., Rimmele J.M., Sanz Perl Y., Poeppel D. Speaking rhythmically can shape hearing. Nat. Hum. Behav. 2021;5(1):71–82. doi: 10.1038/s41562-020-00962-0. (Jan) [DOI] [PubMed] [Google Scholar]
- Bland N.S., Sale M.V. Current challenges: the ups and downs of tACS. Exp. Brain Res. 2019;237(12):3071–3088. doi: 10.1007/s00221-019-05666-0. (Dec) [DOI] [PubMed] [Google Scholar]
- Blau V., Reithler J., van Atteveldt N., Seitz J., Gerretsen P., Goebel R., et al. Deviant processing of letters and speech sounds as proximate cause of reading failure: a functional magnetic resonance imaging study of dyslexic children. Brain. 2010;133(3):868–879. doi: 10.1093/brain/awp308. Mar 1. [DOI] [PubMed] [Google Scholar]
- Blomert L. The neural signature of orthographic–phonological binding in successful and failing reading development. NeuroImage. 2011;57(3):695–703. doi: 10.1016/j.neuroimage.2010.11.003. (Aug) [DOI] [PubMed] [Google Scholar]
- Blomert L., Willems G. Is there a causal link from a phonological awareness deficit to reading failure in children at familial risk for dyslexia? Dyslexia. 2010;16(4):300–317. doi: 10.1002/dys.405. (Nov) [DOI] [PubMed] [Google Scholar]
- Breier J.I., Gray L., Fletcher J.M., Diehl R.L., Klaas P., Foorman B.R., et al. Perception of voice and tone onset time continua in children with dyslexia with and without attention deficit/hyperactivity disorder. J. Exp. Child Psychol. 2001;80(3):245–270. doi: 10.1006/jecp.2001.2630. (Nov) [DOI] [PubMed] [Google Scholar]
- Bretherton L., Holmes V.M. The relationship between auditory temporal processing, phonemic awareness, and reading disability. J. Exp. Child Psychol. 2003;84(3):218–243. doi: 10.1016/s0022-0965(03)00023-7. (Mar) [DOI] [PubMed] [Google Scholar]
- Bus A.G., van IJzendoorn M.H. Phonological awareness and early reading: a meta-analysis of experimental training studies. J. Educ. Psychol. 1999;91(3):403–414. [Google Scholar]
- Carvalho S., Boggio P.S., Gonçalves Ó.F., Vigário A.R., Faria M., Silva S., et al. Transcranial direct current stimulation based metaplasticity protocols in working memory. Brain Stimul. 2015;8(2):289–294. doi: 10.1016/j.brs.2014.11.011. [DOI] [PubMed] [Google Scholar]
- Clayton F.J., West G., Sears C., Hulme C., Lervåg A. A longitudinal study of early reading development: letter-sound knowledge, phoneme awareness and RAN, but not letter-sound integration, predict variations in reading development. Sci. Stud. Read. 2020;24(2):91–107. Mar 3. [Google Scholar]
- Cone-Wesson B., Dowell R.C., Tomlin D., Rance G., Ming W.J. The auditory steady-state response: comparisons with the auditory brainstem response. J. Am. Acad. Audio. 2002;13(04):173–187. (Apr) [PubMed] [Google Scholar]
- Costanzo F., Varuzza C., Rossi S., Sdoia S., Varvara P., Oliveri M., et al. Reading changes in children and adolescents with dyslexia after transcranial direct current stimulation. NeuroReport. 2016;27(5):295–300. doi: 10.1097/WNR.0000000000000536. Mar 23. [DOI] [PubMed] [Google Scholar]
- Costanzo F., Varuzza C., Rossi S., Sdoia S., Varvara P., Oliveri M., et al. Evidence for reading improvement following tDCS treatment in children and adolescents with Dyslexia. RNN. 2016;34(2):215–226. doi: 10.3233/RNN-150561. Mar 21. [DOI] [PubMed] [Google Scholar]
- Costanzo F., Rossi S., Varuzza C., Varvara P., Vicari S., Menghini D. Long-lasting improvement following tDCS treatment combined with a training for reading in children and adolescents with dyslexia. Neuropsychologia. 2019;130:38–43. doi: 10.1016/j.neuropsychologia.2018.03.016. (Jul) [DOI] [PubMed] [Google Scholar]
- Fricke K., Seeber A.A., Thirugnanasambandam N., Paulus W., Nitsche M.A., Rothwell J.C. Time course of the induction of homeostatic plasticity generated by repeated transcranial direct current stimulation of the human motor cortex. J. Neurophysiol. 2011;105(3):1141–1149. doi: 10.1152/jn.00608.2009. (Mar) [DOI] [PubMed] [Google Scholar]
- Fröhlich F., McCormick D.A. Endogenous electric fields may guide neocortical network activity. Neuron. 2010;67(1):129–143. doi: 10.1016/j.neuron.2010.06.005. (Jul) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraud A.L., Poeppel D. Cortical oscillations and speech processing: emerging computational principles and operations. Nat. Neurosci. 2012;15(4):511–517. doi: 10.1038/nn.3063. (Apr) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami U. Encyclopedia of Language & Linguistics. Elsevier; 2006. Phonological Awareness and Literacy; pp. 489–497. [Google Scholar]
- Goswami U. A temporal sampling framework for developmental dyslexia. Trends Cogn. Sci. 2011;15(1):3–10. doi: 10.1016/j.tics.2010.10.001. (Jan) [DOI] [PubMed] [Google Scholar]
- Gruner E., Zeller M., Fleck C. Phonematischer Gedächtnistest. Verlag Hans Huber; Bern: 2013. [Google Scholar]
- Habib M. The neurological basis of developmental dyslexia: an overview and working hypothesis. Brain. 2000;123(12):2373–2399. doi: 10.1093/brain/123.12.2373. Dec 1. [DOI] [PubMed] [Google Scholar]
- Haft S.L., Duong P.H., Ho T.C., Hendren R.L., Hoeft F. Anxiety and attentional bias in children with specific learning disorders. J. Abnorm Child Psychol. 2019;47(3):487–497. doi: 10.1007/s10802-018-0458-y. (Mar) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfrich R.F., Schneider T.R., Rach S., Trautmann-Lengsfeld S.A., Engel A.K., Herrmann C.S. Entrainment of brain oscillations by transcranial alternating current stimulation. Curr. Biol. 2014;24(3):333–339. doi: 10.1016/j.cub.2013.12.041. (Feb) [DOI] [PubMed] [Google Scholar]
- Helfrich R.F., Knepper H., Nolte G., Strüber D., Rach S., Herrmann C.S., et al. Vol. 12. 2014. Selective Modulation of Interhemispheric Functional Connectivity by HD-tACS Shapes Perception. (PLoS Biol). Dec 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendren R.L., Haft S.L., Black J.M., White N.C., Hoeft F. Recognizing psychiatric comorbidity with reading disorders. Front Psychiatry. 2018;9 doi: 10.3389/fpsyt.2018.00101. Mar 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingelghem M.V., van Wieringen A., Wouters J., Vandenbussche E., Onghena P., GhesquieÁre P. Psychophysical evidence for a general temporal processing deficit in children with dyslexia. NeuroReport. 2001;12:5. doi: 10.1097/00001756-200111160-00046. (Nov) [DOI] [PubMed] [Google Scholar]
- John M.S., Dimitrijevic A., Picton T.W. Efficient stimuli for evoking auditory steady-state responses. Ear Hear. 2003;24(5):406–423. doi: 10.1097/01.AUD.0000090442.37624.BE. (Oct) [DOI] [PubMed] [Google Scholar]
- Kaufman J., Birmaher B., Brent D.A., Ryan N.D., Rao U. K-SADS-PL. J. Am. Acad. Child Adolesc. Psychiatry. 2000;39(10):1208. doi: 10.1097/00004583-200010000-00002. (Oct) [DOI] [PubMed] [Google Scholar]
- Korai S.A., Ranieri F., Di Lazzaro V., Papa M., Cirillo G. Neurobiological after-effects of low intensity transcranial electric stimulation of the human nervous system: from basic mechanisms to metaplasticity. Front Neurol. 2021;12 doi: 10.3389/fneur.2021.587771. Feb 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovelman I., Norton E.S., Christodoulou J.A., Gaab N., Lieberman D.A., Triantafyllou C., et al. Brain basis of phonological awareness for spoken language in children and its disruption in dyslexia. Cereb. Cortex. 2012;22(4):754–764. doi: 10.1093/cercor/bhr094. Apr 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan C., Santos L., Peterson M.D., Ehinger M. Safety of noninvasive brain stimulation in children and adolescents. Brain Stimul. 2015;8(1):76–87. doi: 10.1016/j.brs.2014.10.012. (Jan) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzaro G., Costanzo F., Varuzza C., Rossi S., Vicari S., Menghini D. Effects of a short, intensive, multi-session tDCS treatment in developmental dyslexia: Preliminary results of a sham-controlled randomized clinical trial. Prog. Brain Res. 2021;264:191–210. doi: 10.1016/bs.pbr.2021.01.015. [DOI] [PubMed] [Google Scholar]
- Lehongre K., Ramus F., Villiermet N., Schwartz D., Giraud A.L. Altered low-gamma sampling in auditory cortex accounts for the three main facets of dyslexia. Neuron. 2011;72(6):1080–1090. doi: 10.1016/j.neuron.2011.11.002. (Dec) [DOI] [PubMed] [Google Scholar]
- Lehongre K., Morillon B., Giraud A.L., Ramus F. Impaired auditory sampling in dyslexia: further evidence from combined fMRI and EEG. Front Hum. Neurosci. 2013:7. doi: 10.3389/fnhum.2013.00454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maassen Ben, Groenen Paul, Cru Thom. Identification and discrimination of voicing and place-of-articulation in developmental dyslexia. Clin. Linguist. Phon. 2001;15(4):319–339. (Jan) [Google Scholar]
- Madaus J.W. Employment self-disclosure rates and rationales of university graduates with learning disabilities. J. Learn Disabil. 2008;41(4):291–299. doi: 10.1177/0022219407313805. (Jul) [DOI] [PubMed] [Google Scholar]
- Marchesotti S., Nicolle J., Merlet I., Arnal L.H., Donoghue J.P., Giraud A.L. Vol. 18. 2020. Selective enhancement of low-gamma activity by tACS improves phonemic processing and reading accuracy in dyslexia. (PLoS Biol). Sep 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maughan B., Carroll J. Literacy and mental disorders. Curr. Opin. Psychiatry. 2006;19(4):350–354. doi: 10.1097/01.yco.0000228752.79990.41. (Jul) [DOI] [PubMed] [Google Scholar]
- May P. Hamburger Schreib-Probe (HSP 1–10) Verlag für pädagogische Medien; Stuttgart: 2000. [Google Scholar]
- Melby-Lervåg M., Lyster S.A.H., Hulme C. Phonological skills and their role in learning to read: a meta-analytic review. Psychol. Bull. 2012;138(2):322–352. doi: 10.1037/a0026744. [DOI] [PubMed] [Google Scholar]
- Moll K., Kunze S., Neuhoff N., Bruder J., Schulte-Körne G. Specific learning disorder: prevalence and gender differences. PLoS ONE. 2014;9(7) doi: 10.1371/journal.pone.0103537. Jul 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll K., Landerl K. Lese- und Rechtschreibtest SLRT-II. Verlag Hans Huber; Bern: 2010. [Google Scholar]
- Müller-Dahlhaus F., Ziemann U. Metaplasticity in human cortex. Neuroscientist. 2015;21(2):185–202. doi: 10.1177/1073858414526645. (Apr) [DOI] [PubMed] [Google Scholar]
- Picton T.W., John M.S., Dimitrijevic A., Purcell D. Human auditory steady-state response. Int. J. Audiol. 2003;42(4):177–219. doi: 10.3109/14992020309101316. (Jan) [DOI] [PubMed] [Google Scholar]
- Poeppel D. The analysis of speech in different temporal integration windows: cerebral lateralization as ‘asymmetric sampling in time. Speech Commun. 2003;41(1):245–255. (Aug) [Google Scholar]
- Poulsen C., Picton T.W., Paus T. Age-related changes in transient and oscillatory brain responses to auditory stimulation during early adolescence. Dev. Sci. 2009;12(2):220–235. doi: 10.1111/j.1467-7687.2008.00760.x. (Mar) [DOI] [PubMed] [Google Scholar]
- Puolakanaho A., Poikkeus A.M., Ahonen T., Tolvanen A., Lyytinen H. Emerging phonological awareness differentiates children with and without familial risk for dyslexia after controlling for general language skills. Ann. Dyslexia. 2004;54(2):221–243. doi: 10.1007/s11881-004-0011-2. (Dec) [DOI] [PubMed] [Google Scholar]
- Puolakanaho A., Ahonen T., Aro M., Eklund K., Leppänen P.H.T., Poikkeus A.M., et al. Developmental links of very early phonological and language skills to second grade reading outcomes: strong to accuracy but only minor to fluency. J. Learn Disabil. 2008;41(4):353–370. doi: 10.1177/0022219407311747. (Jul) [DOI] [PubMed] [Google Scholar]
- Rojas D., Maharajh K., Teale P., Kleman M., Benkers T., Carlson J., et al. Development of the 40Hz steady state auditory evoked magnetic field from ages 5 to 52. Clin. Neurophysiol. 2006;117(1):110–117. doi: 10.1016/j.clinph.2005.08.032. (Jan) [DOI] [PubMed] [Google Scholar]
- Rufener K.S., Zaehle T. Dysfunctional auditory gamma oscillations in developmental dyslexia: a potential target for a tACS-based intervention. Prog. Brain Res. 2021;264:211–232. doi: 10.1016/bs.pbr.2021.01.016. [DOI] [PubMed] [Google Scholar]
- Rufener K.S., Zaehle T., Oechslin M.S., Meyer M. 40Hz-Transcranial alternating current stimulation (tACS) selectively modulates speech perception. Int. J. Psychophysiol. 2016;101:18–24. doi: 10.1016/j.ijpsycho.2016.01.002. (Mar) [DOI] [PubMed] [Google Scholar]
- Rufener K.S., Oechslin M.S., Zaehle T., Meyer M. Transcranial alternating current stimulation (tACS) differentially modulates speech perception in young and older adults. Brain Stimul. 2016;9(4):560–565. doi: 10.1016/j.brs.2016.04.002. (Jul) [DOI] [PubMed] [Google Scholar]
- Rufener K.S., Krauel K., Meyer M., Heinze H.J., Zaehle T. Transcranial electrical stimulation improves phoneme processing in developmental dyslexia. Brain Stimul. 2019;12(4):930–937. doi: 10.1016/j.brs.2019.02.007. (Jul) [DOI] [PubMed] [Google Scholar]
- Sanfilippo J., Ness M., Petscher Y., Rappaport L., Zuckerman B., Gaab N. Reintroducing dyslexia: early identification and implications for pediatric practice. Pediatr. 2020;146(1) doi: 10.1542/peds.2019-3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider W., Schlagmüller M. Lesegeschwindigkeits- und Verstaendnistest fuer die Klassen 5 – 12. Hogrefe Verlag; Göttingen: 2017. [Google Scholar]
- Schulte-Körne G., Deimel W., Hülsmann J., Seidler T., Remschmidt H. Das Marburger Rechtschreib-Training - Ergebnisse einer Kurzzeit-Intervention. Z. für Kinder- und Jugend und Psychother. 2001;29(1):7–15. doi: 10.1024//1422-4917.29.1.7. (Feb) [DOI] [PubMed] [Google Scholar]
- Schulte-Körne G., Mathwig F. Das Marburger Rechtschreibtraining. Ein regelgeleitetes Rechtschreibtraining für rechtschreibschwache Kinder. Verlag Dr. Winkler; Bochum: 2013. [Google Scholar]
- Shaywitz S.E., Shaywitz J.E., Shaywitz B.A. Dyslexia in the 21st century. Curr. Opin. Psychiatry. 2021;34(2):80–86. doi: 10.1097/YCO.0000000000000670. (Mar) [DOI] [PubMed] [Google Scholar]
- Turker S., Hartwigsen G. Exploring the neurobiology of reading through non-invasive brain stimulation: a review. Cortex. 2021;141:497–521. doi: 10.1016/j.cortex.2021.05.001. (Aug) [DOI] [PubMed] [Google Scholar]
- Vandermosten M., Boets B., Luts H., Poelmans H., Golestani N., Wouters J., et al. Adults with dyslexia are impaired in categorizing speech and nonspeech sounds on the basis of temporal cues. Proc. Natl. Acad. Sci. 2010;107(23):10389–10394. doi: 10.1073/pnas.0912858107. Jun 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandermosten M., Boets B., Luts H., Poelmans H., Wouters J., Ghesquière P. Impairments in speech and nonspeech sound categorization in children with dyslexia are driven by temporal processing difficulties. Res. Dev. Disabil. 2011;32(2):593–603. doi: 10.1016/j.ridd.2010.12.015. (Mar) [DOI] [PubMed] [Google Scholar]
- Vossen A., Gross J., Thut G. Alpha power increase after transcranial alternating current stimulation at alpha frequency (α-tACS) reflects plastic changes rather than entrainment. Brain Stimul. 2015;8(3):499–508. doi: 10.1016/j.brs.2014.12.004. (May) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosskuhl J., Strüber D., Herrmann C.S. Non-invasive brain stimulation: a paradigm shift in understanding brain oscillations. Front Hum. Neurosci. 2018;12 doi: 10.3389/fnhum.2018.00211. May 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss, R.H. Grundintelligenztest Skala 2 – Revision. Hogrefe; 2019.
- Wischnewski M., Engelhardt M., Salehinejad M.A., Schutter D.J.L.G., Kuo M.F., Nitsche M.A. NMDA receptor-mediated motor cortex plasticity after 20 Hz transcranial alternating current stimulation. Cereb. Cortex. 2019;29(7):2924–2931. doi: 10.1093/cercor/bhy160. Jul 5. [DOI] [PubMed] [Google Scholar]
- Zaehle T., Rach S., Herrmann C.S. Transcranial alternating current stimulation enhances individual alpha activity in human EEG. PLoS ONE. 2010;5(11) doi: 10.1371/journal.pone.0013766. Nov 1. [DOI] [PMC free article] [PubMed] [Google Scholar]