Abstract
Objective
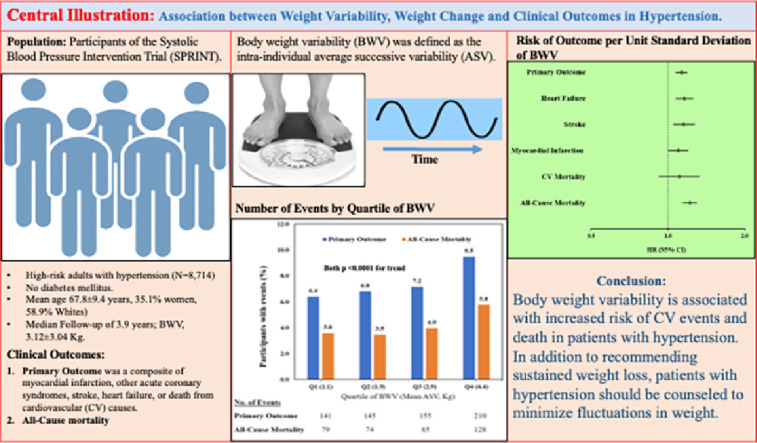
The effect of body weight variability (BWV) and body weight change (BWC) in high-risk individuals with hypertension, but without diabetes mellitus (DM) remains unclear. We examined the effect of BWV and BWC on the primary outcome [the composite of myocardial infarction (MI), other acute coronary syndromes, stroke, acute decompensated heart failure (HF), or cardiovascular (CV) death] and all-cause mortality in the Systolic Blood Pressure Intervention Trial (SPRINT).
Methods
In this post-hoc analysis, we used multivariate Cox regression models to examine the risk associated with BWV and BWC for the primary outcome in SPRINT. BWV was defined as the intra-individual average successive variability (ASV). BWC was defined as baseline weight minus final weight.
Results
A total of 8714 SPRINT participants (mean age 67.8 ± 9.4 years, 35.1 % women, 58.9 % Whites) with available data on body weight were included. The median follow-up was about 3.9 years (IQR, 3.3–4.4). In multivariable-adjusted Cox models, each 1 unit standard deviation (SD) of BWV was significantly associated with a higher risk for the primary outcome, all-cause mortality, HF, MI, and stroke [HR(95 % CI)]: 1.13 (1.07–1.19; p < 0.0001), 1.22 (1.14–1.30; p < 0.0001), 1.16 (1.07–1.26; p < 0.001), 1.10 (1.00–1.20; p = 0.047), and 1.15 (1.05–1.27; p = 0.005), respectively. Similarly, each 1 unit SD of BWC was significantly associated with a higher risk of the primary outcome, all-cause mortality, MI, and HF: 1.11(1.02–1.21; p = 0.017), 1.44 (1.26–1.65; p < 0.0001), 1.16 (1.01–1.32; p = 0.041) and 1.19 (1.02–1.40; p = 0.031) respectively. However, there was no significant association with CV death (for both BWV and BWC) or stroke (BWC).
Conclusion
In high-risk hypertension, BWV and BWC were both associated with higher risk of the primary outcome and all-cause mortality. These results further stress the clinical importance of sustained weight loss and minimizing fluctuations in weight in hypertension.
Keywords: Weight variability, Weight change, Hypertension, Clinical outcomes
Graphical abstract
1. Introduction
Obesity is a major public health problem with an estimated prevalence of over 40 % among U.S. adults [1]. Weight loss is an important component in the management of hypertension among individuals with co-existing obesity or those who are overweight [2]. Unfortunately, attempts to lose weight are often followed by cycles of weight loss and weight gain [3]. Previous studies in various patient populations have linked such intra-individual variability in weight with an increased risk for cardiovascular disease and death, independent of other factors [4,5]. However, it is still unknown whether a similar effect occurs in older high-risk individuals with hypertension but without DM. The U.S population is becoming much older, with nearly one in five U.S. residents aged 65 and older by 2030 [6]. Therefore, it is important to explore factors that may influence outcomes in older patient populations.
In this post-hoc analysis, we used data from the Systolic Blood Pressure Intervention Trial (SPRINT), which enrolled high-risk, older individuals (age ≥ 50 years) with hypertension but without DM, to examine the effect of weight variability and weight change on the trial outcomes [7]. SPRINT compared the benefit of treatment of systolic blood pressure (SBP) to a target of less than 120 mm Hg with treatment to a target systolic SBP of less than 140 mm [7,8]. We hypothesized that higher weight variability and weight change during the study follow-up would be associated with increased rates of cardiovascular events and all-cause mortality.
2. Methods
2.1. Study design and population
The rationale, protocol, and results of SPRINT have been published [7]. In summary, SPRINT was a randomized, controlled, open-label trial, including 9361 non-diabetic U.S. adults who were at least 50 years of age, at high cardiovascular risk, with hypertension with SBP 130–180 mmHg at enrollment. Increased cardiovascular risk was defined by one or more of the following: clinical or subclinical cardiovascular disease other than stroke; chronic kidney disease, excluding polycystic kidney disease, with an estimated glomerular filtration rate (eGFR) of 20 to less than 60 ml per minute per 1.73 m2 of body surface area, calculated with the use of the four variable Modification of Diet in Renal Disease equation [9]; a 10-year risk of cardiovascular disease of 15 % or greater on the basis of the Framingham risk score [10]; or an age of 75 years or older. One of the exclusion criteria in SPRINT was unintentional weight loss greater than 10 % in the last 6 months. Participants were randomized into intensive treatment arm with goal SBP <120 mmHg or standard treatment arm with goal SBP <140 mmHg. The primary efficacy endpoint SPRINT was defined as the composite of myocardial infarction, other acute coronary syndromes, stroke, acute decompensated heart failure, or death from cardiovascular causes. Secondary efficacy endpoints comprised the individual components of the primary endpoint (stroke, acute decompensated heart failure, and cardiovascular death) and death from any cause. A structured interview was used in both groups every 3 months to obtain self-reported cardiovascular disease outcomes [8].
The primary endpoint of this post-hoc analysis was the SPRINT primary outcome, which was defined as the composite of myocardial infarction, other acute coronary syndromes, stroke, acute decompensated heart failure, or death from cardiovascular causes. All SPRINT participants provided written informed consent for participation in the trial. The trial was approved by the institutional review board at each site and was registered with ClinicalTrials.gov. For the purposes of this analysis, we included 8714 participants on whom data on serial body weight measurements during SPRINT study period was available. The de-identified dataset was obtained from the National Heart, Lung, and Blood Institute's Biologic Specimen and Data Repository Information Coordinating Center after the study was approved by the institutional review board at the Wake Forest School of Medicine.
2.2. Body weight variability and body weight change
Body weight was measured at baseline and annually during the follow-up period. All body weights were measured during the study visits at the participating centers. The predictor variables for this analysis were variability in weight and weight change. We used a prior defined formula for body weight variability [11]. We used average successive variability, defined as the absolute difference between successive values, as the primary body weight variability measurement. Body weight change was computed as baseline weight minus final/close-out weight (kg). We restricted our analysis to participants who had completed at least four weight measurements. We excluded participants (n = 647) who had missing body weight measurements.
2.3. Ascertainment of clinical outcomes
A detailed protocol for ascertainment of outcomes in SPRINT has been previously published [7]. Briefly, a structured interview was used every 3 months to obtain self-reported cardiovascular disease and all-cause mortality outcomes using a standardized protocol with centralized monitoring by the coordinating center. The interviewers were aware of SBP treatment group assignment, to minimize ascertainment bias, they used the same format for interviews. Medical records and electrocardiograms were obtained for documentation of events. Whenever clinical site staff became aware of a death, a standard protocol was used to obtain information on the events.
2.4. Covariates
In the SPRINT study, trained study personnel ascertained baseline sociodemographic data, comorbid conditions, and antihypertensive medications during the screening or randomization visit. Fasting blood and urine samples were collected at that time. Serum and urine creatinine were measured using an enzymatic procedure and an auto-analyzer. Urine albumin was measured using an immuneturbidometric method on an auto-analyzer. All assays were performed in a single SPRINT central laboratory [7].
2.5. Statistical analysis
The SPRINT trial cohort included in the analysis was first classified into low BWV and high BWV using average successive variability (ASV). Low BWV category was defined as those below the median ASV, and high BWV was defined as those equal or greater than median ASV. The baseline demographic, risk factors, and clinical characteristics were assessed according to the two BWV categories using analysis of variance for normally distributed continuous variables or Wilcoxon rank sum analysis for non-normally distributed continuous variables, and chi-square test for categorical variables. Continuous variables were reported as mean and standard deviation (SD) or median and interquartile range (IQR) for skewed variables, and n (%) for categorical variables.
We used Kaplan-Meier analysis to determine the event-free survival of participants with low and high BWV for the primary outcome and all-cause mortality. Next, we used Cox proportional hazards analysis to examine the association between BWV and BWC and our outcomes of interest. BWV and BWC were each introduced into our models as continuous variables to calculate Cox proportional hazard ratios (HR) for outcomes per 1 unit SD. Furthermore, BWC was categorized into stable weight (<5 % change in body weight), weight loss (>5 % weight loss) and weight gain (>5 % weight gain) and performed Cox analysis using stable weight as the reference group.
We also conducted additional analysis using quartiles of BWV and BWC.
Two models were used, with model 1 being unadjusted and model 2 adjusting for age, sex and race/ethnicity and baseline covariates including smoking status, average SBP, average diastolic blood pressure, body mass index (BMI), number of antihypertensive agents, history of cardiovascular disease, urine albumin-creatinine ratio, fasting glucose, total cholesterol, low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), triglycerides, study arm assignment, and time between initial and final body weight measurement. We further stratified analysis by age <75 or ≥75 years at enrolment. This analysis was performed to answer the question of whether BWV is more detrimental in an individual who is ≥75 years of age.
A two-sided P-value < 0.05 was considered statistically significant, and all statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
3. Results
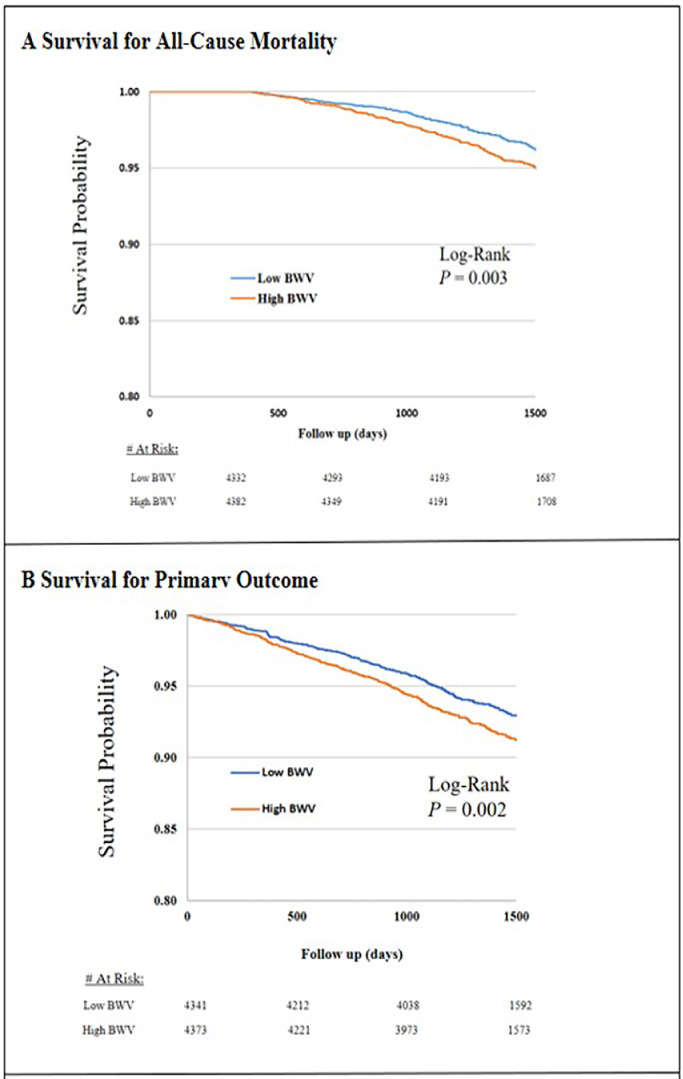
A total of 8714 (mean age ± SD, 67.8 ± 9.4 years, 35.1 % women, 58.9 % Whites) of the SPRINT participants were included in the analysis. The average successive variability (ASV) in this cohort was 2.38 kg (IQR, 1.5–3.7). Compared to participants with low BWV (ASV <2.38 kg), those with high BWV (ASV ≥ 2.38 kg) were more likely to be men, younger, current smokers and have a higher BMI (Table 1). A total of 7703 (88.4 %) participants had their weight measured at least four times during the study period. The change in body weight experienced by participants was 0.73 kg (IQR, −2.49–4.13). The time between initial and close-out weight measurements was 3.8 years (IQR, 3.3–4.4). After a median follow-up of about 3.9 years (IQR, 3.3–4.4), the event rates of the primary outcome, all-cause mortality, heart failure, myocardial infarction, stroke, and cardiovascular mortality were 7.5 %, 4.2 %, 2.3 %, 2.9 %, 1.7 %, and 1.2 %, respectively (Table 1). Compared to participants in the low BWV category, those in the high BWV category had worse survival for the primary composite outcome and all-cause mortality (Fig. 1). In our fully adjusted Cox model, each 1 unit SD of BWV was significantly associated with 13 %, 22 %, 16 % and 15 % higher risk of the primary outcome, all-cause mortality, heart failure and stroke but not with myocardial infarction or cardiovascular mortality (Table 2). Similarly, each 1 unit SD of BWC was associated with 11 %, 44 %, 16 % and 19 % increased risk of the primary outcome, all-cause mortality, myocardial infarction, heart failure but not with cardiovascular mortality or stroke (Table 3).
Table 1.
Demographic and Risk Factor Profile of 8714 Participants included from the SPRINT by Body Weight Variability.
| Variable | Total n = 8714 | Low BWV (ASV < 2.38 Kg) n = 4332 | High BWV n = 4382 | P-value |
|---|---|---|---|---|
| Age, years, mean (SD) | 67.8(9.4) | 69.3(9.2) | 66.5(9.3) | <0.0001 |
| Age ≥75 years, n (%) | 1423(32.9) | 1423(16.3) | 1005(11.5) | <0.001 |
| Female, n (%) | 3054(35.1) | 1634(18.8) | 1420(16.3) | <0.0001 |
| Race or Ethnic group, n (%) | – | – | – | <0.0001 |
| Non-Hispanic black | 2574(29.9) | 1109(12.9) | 1465(17.0) | |
| Hispanic | 923(10.7) | 538(6.3) | 385(4.5) | |
| Non-Hispanic White | 5061(58.9) | 2583(30.0) | 2478(28.8) | |
| Other | 42(0.5) | 32(0.4) | 10(0.1) | |
| BMI, kg/m², mean (SD) | 29.8(5.7) | 28.4(5.0) | 31.3(6.01) | <0.0001 |
| Baseline Weight, Kg (SD) | 86.8(18.7) | 81.3(16.6) | 91.7(19.3) | <0.0001 |
| Current Smoker, n (%) | 1196(13.8) | 458(5.3) | 738(8.5) | <0.0001 |
| Average SBP, mmHg, mean (SD) | 145.1(11.1) | 145.2(11.2) | 145.0(11.1) | 0.361 |
| Average DBP, mmHg mean (SD) | 80.3(11.5) | 79.2(11.4) | 81.1(11.6) | <0.0001 |
| Framingham Risk Score, mean (SD) | 17.4(2.5) | 17.4(2.4) | 17.4(2.5) | 0.388 |
| History of Cardiovascular Disease, n (%) | 1744(20.0) | 864(9.9) | 880(10.1) | 0.873 |
| Chronic Kidney Disease, n (%) | 2434(27.9) | 1230(14.1) | 1204(13.8) | 0.340 |
| Creatinine, (mg/dL), mean (SD) | 1.07(0.34) | 1.06(0.33) | 1.08(0.34) | 0.005 |
| Estimate GFR, (ml/min/1.73 m2) | 71.8(20.5) | 71.1(20.0) | 72.6(20.8) | 0.0008 |
| Urine albumin-Creatinine Ratio, median (IQR) | 2.38 (1.54 - 3.69) | 9.46 (5.66 - 20.19) | 9.45 (5.63 - 22.42) | 0.300 |
| Aspirin use | 4469(51.4) | 2293(26.4) | 2176(25.0) | 0.002 |
| Randomized to intervention group | 4361(50.1) | 2120(24.3) | 2241(25.7) | 0.040 |
| No. of antihypertensive medication classes | 1.80(1.03) | 1.78(1.01) | 1.82(1.04) | 0.042 |
| Fasting Glucose, (mg/dL), mean (SD) | 98.9(13.5) | 98.0(12.3) | 99.7(14.6) | <0.0001 |
| HDL-C, (mg/dL), mean (SD) | 52.8(14.4) | 53.9(14.7) | 51.6(14.0) | <0.0001 |
| LDL-C, (mg/dL), mean (SD) | 112.3(35.1) | 112.6(34.8) | 112.1(35.3) | 0.545 |
| Total Cholesterol (mg/dL), mean (SD) | 190.0(41.1) | 190.8(40.8) | 189.2(41.5) | 0.068 |
| Triglycerides (mg/dL), mean (SD) | 126.2(90.8) | 123.0(79.6) | 129.3(100.6) | 0.001 |
| Study Outcomes, n (%) | – | – | – | |
| Primary Outcome | 651(7.5) | 286(6.6) | 365(8.3) | 0.002 |
| All-Cause Mortality | 366(4.2) | 153(3.5) | 213(4.9) | 0.002 |
| Heart Failure | 198(2.3) | 73(1.7) | 125(2.9) | <0.001 |
| Myocardial Infarction | 254(2.9) | 131(3.0) | 123(2.8) | 0.547 |
| Stroke | 151(1.7) | 59(1.4) | 92(2.1) | 0.008 |
| Cardiovascular Death | 100(1.2) | 44(1.0) | 56(1.8) | 0.250 |
BWV, body weight variability; ASV, successive average variability; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; GFR, glomerular filtration rate; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; SD, standard deviation; IQR, interquartile range. Continuous variables are presented as means and standard deviations or median and IQR. Categorical variables are presented as counts and corresponding percentages.
Fig. 1.
Kaplan Meier Survival Curves for Primary Outcomes and All-Cause Mortality by Degree of Body Weight Variability. Primary outcome was the composite of myocardial infarction, other acute coronary syndromes, stroke, acute decompensated heart failure, or death from cardiovascular causes. BWV denoted body weight variability. Low BWV is defined as average successive variability (ASV) less than median of 2.4 kg and High BWV is defined as ASV of 2.4 kg or higher.
Table 2.
Continuous Body Weight Variability (per Unit SD) and Risk of Outcomes in SPRINT Trial.
| Outcome | Model 1 (Unadjusted) |
Model 2* |
||
|---|---|---|---|---|
| HR (95 % CI) | P-value | HR (95 % CI) | P-value | |
| Primary Outcome | 1.11 (1.05–1.17) | <0.001 | 1.13 (1.07–1.19) | <0.0001 |
| All-Cause Mortality | 1.15 (1.08–1.22) | <0.0001 | 1.22 (1.14–1.30) | <0.0001 |
| Heart failure | 1.13 (1.04–1.24) | 0.006 | 1.16 (1.07–1.26) | <0.001 |
| Myocardial Infarction | 1.06 (0.96–1.18) | 0.269 | 1.10 (1.00–1.20) | 0.047 |
| Stroke | 1.13 (1.02–1.26) | 0.025 | 1.15 (1.05–1.27) | 0.005 |
| Cardiovascular Mortality | 1.02 (0.84–1.23) | 0.848 | 1.11 (0.92–1.33) | 0.297 |
Model 2 adjusted for age, sex and race/ethnicity, smoking status, average systolic blood pressure, average diastolic blood pressure, body mass index (BMI), number of antihypertensive agents, history of cardiovascular disease, fasting glucose, total cholesterol, low-density lipoprotein cholesterol (LDL-C), high density lipoprotein cholesterol (HDL-C), triglycerides, study arm assignment, and urine albumin-creatinine ratio, time between initial and final weight measurement.
Table 3.
Continuous Body Weight Change (per Unit SD) and Risk of Outcomes in SPRINT Trial.
| Outcome | Model 1 (Unadjusted) |
Model 2* |
||
|---|---|---|---|---|
| HR (95 % CI) | P-value | HR (95 % CI) | P-value | |
| Primary Outcome | 1.12 (1.05–1.21) | 0.001 | 1.11 (1.02–1.21) | 0.017 |
| All-Cause Mortality | 1.18 (1.09–1.28) | <0.0001 | 1.44 (1.26–1.65) | <0.0001 |
| Heart failure | 1.25 (1.11–1.40) | 0.0003 | 1.19 (1.02–1.40) | 0.031 |
| Myocardial Infarction | 1.17 (1.04–1.31) | 0.007 | 1.16 (1.01–1.32) | 0.041 |
| Stroke | 1.14 (0.99–1.32) | 0.069 | 1.14 (0.95–1.36) | 0.150 |
| Cardiovascular Mortality | 1.05 (0.09–1.24) | 0.519 | 1.20 (0.89–1.60) | 0.232 |
Model 2 adjusted for age, sex and race/ethnicity, smoking status, average systolic blood pressure, average diastolic blood pressure, body mass index (BMI), number of antihypertensive agents, history of cardiovascular disease, fasting glucose, total cholesterol, low-density lipoprotein cholesterol (LDL-C), high density lipoprotein cholesterol (HDL-C), triglycerides, study arm assignment, and urine albumin-creatinine ratio, time between initial and final weight measurement.
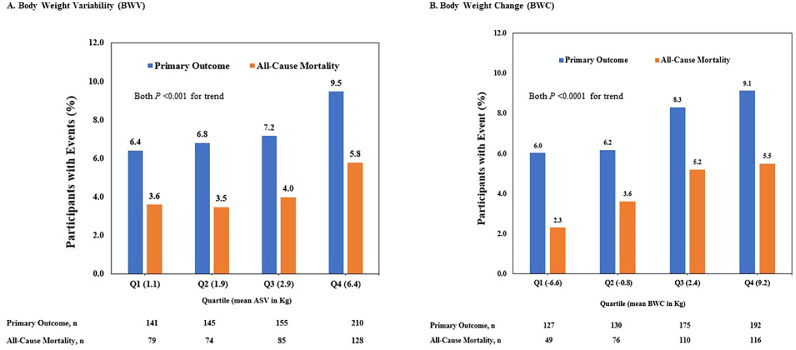
The risk of the primary outcome and all-cause mortality were also higher among participants in the highest compared to lowest quartile of BWV and BWC (Fig. 2).
Fig. 2.
(A) Distribution of the primary outcome and all-cause mortality by quartiles of average successive variability (ASV) which occurred in the SPRINT Trial during the follow-up period. (B) Distribution of the primary outcome and all-cause mortality by quartiles of change in weight which occurred in the SPRINT Trial during the follow-up period. Primary outcome was the composite of myocardial infarction, other acute coronary syndromes, stroke, acute decompensated heart failure, or death from cardiovascular causes.
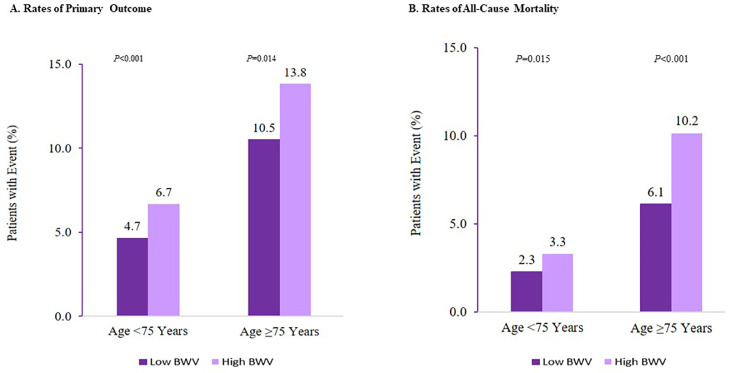
Within both age categories of <75 years and ≥75 years, the event rates of the primary outcome and all-cause mortality were higher among those who experienced high BWV compared to low BWV. BWV and age interaction P-value = 0.011(Fig. 3).
Fig. 3.
Body-Weight Variability and Rates of All-cause Death and Primary Outcome by Age <75 versus Age ≥75 Years. Primary outcome was the composite of myocardial infarction, other acute coronary syndromes, stroke, acute decompensated heart failure, or death from cardiovascular causes. ASV denotes average successive variability of body weight. Low ASV is defined as less than median (<2.38 kg) ASV, and High ASV is defined as ASV of 2.38 kg or higher.
Furthermore, compared to participants who had stable weight, those who had a weight gain of ≥5 % had a higher risk of the primary outcome (HR 1.21, 95 % CI 1.01–1.46, p = 0.039). In contrast, those with ≥5 % weight loss had a lower risk of all-cause mortality (HR 0.54, 95 % CI 0.35–0.82, p = 0.004) (Supplement Table 1). Supplement Fig. S1 shows outcome rate and number of events by quartiles of BWV. Supplement Fig. S2 shows rate and number of primary and all-cause mortality events by BWC status (i.e., stable weight, weight loss and weight gain). Supplement Tables 2 and 3 show results of model 2 covariates and risk of outcomes for BWV and BWC, respectively.
4. Discussion
The purpose of this post hoc analysis of a randomized controlled trial of non-diabetic participants at high cardiovascular risk was to examine the effect of body weight variability and body weight change on study outcomes. Our results showed that a 1 SD increase in average successive variability (ASV) was associated with a 13 % and a 22 % increased risk of the primary outcome events and all-cause mortality, respectively. Similarly, a 1 SD increase in BWC was associated with 44 % increased risk of all-cause mortality and 11 % increased risk of the primary outcome events in this cohort, respectively. Both BWV and BWC were also associated with a higher risk for heart failure, myocardial infarction, and stroke, but there was no association with cardiovascular mortality.
The negative effect of weight variability on clinical outcomes has been reported in various patient populations [4,5]. Weight variability has several causes but has most frequently been attributed to dieting which has a high risk of rebounding weight gain [3]. In older patients, conditions such as malignancies, heart failure, and the use of diuretics may also contribute to weight changes. Multiple studies among patients with type 2 DM have consistently linked weight variability to increased morbidity and mortality, independent of other risk factors [4]. A similar effect has been observed in prevalent underlying coronary artery disease and in those with varied cardiovascular risk. 11, 12However, prospective studies examining the effect of weight variability on clinical outcomes have yielded inconsistent results. For instance, in the Baltimore Longitudinal Study of Aging, the Stanford Five-City Project, and the Honolulu Heart Program, weight fluctuation was not associated with worse clinical outcomes [[12], [13], [14]]. Yet, earlier data from the Framingham Heart Study and others as well as more recent meta-analyses have all linked weight fluctuation to negative clinical outcomes [4,5,15,16].
The underlying mechanism between weight variability and harm has not been fully elucidated. Future studies are required to support the notion that weight variability leads to impaired glucose tolerance, dyslipidemia, or unfavorable body fat distribution and, thus, rising cardiovascular risk [3]. Weight variability might also lead to increased atherogenesis by accelerating chronic inflammation. Yet, older individuals may be more susceptible to physiologic stress than younger individuals as their adaptive mechanisms are less efficient [17]. Sparse literature that already exists has linked weight variability to incident hypertension independent of other factors [18]. In those with existing hypertension, increased weight variability may also be associated with a higher risk for important outcomes [19].
In our study population, weight variability was associated with a higher risk for poor outcomes particularly among individuals aged ≥75 years. This could indicate that the detrimental effect of body weight variability may be greater in older individuals perhaps due to loss of capacity for physiological compensation with aging. It is well known that weight loss reduces the incidence of hypertension while weight gain has an opposite effect [20,21]. In the elderly, however, the relationship between obesity, weight loss and clinical outcomes is complex [22]. For instance, the aging process is often accompanied by sarcopenic obesity because of changes in body composition [23]. Unintentional weight loss in the elderly is associated with poor outcomes. On the other hand, intentional weight loss in the elderly, especially among those who are overweight or obese, is beneficial [24,25]. In the Systolic Hypertension in the Elderly Program, dynamic measures of weight change were better at predicting mortality compared to static measures of weight such as body mass index [19]. A prior study using data from SPRINT showed that the overall efficacy and safety of intensive blood pressure lowering was not modified by baseline body mass index [26]. However, while BMI is easily measured and widely used in clinical settings for assessing obesity, it has been criticized for being an indirect measurement of total body fat that fails to exclude other tissue types [27]. Other anthropometric measures such as waist circumference and waist-to-hip ratio may be better predictors of weight-related cardiovascular risk [28]. Our results are consistent with the extant literature on weight change in hypertension, but we found that weight gain to be more strongly associated with risk of cardiovascular events while weight loss has a salutary effect on all-cause mortality.
The strength of our study is the use of a large sample size of a uniquely high-risk cohort of patients with hypertension. The data we used was well collected using a standardized process. However, our results must be interpreted in the context of some limitations. First, this is a post hoc analysis of the SPRINT trial. Despite our effort to adjust for confounders, the possibility of residual confounding still exists. Secondly, we were unable to distinguish intentional from unintentional weight loss, a factor with important clinical implications in the elderly population [24]. Additionally, we could not eliminate changes in body weight from causes such as use of diuretics, and fluid retention from kidney failure or heart failure. Finally, the results may not be generalizable to other populations. However, our study does contribute to a growing body of evidence that has linked weight change and weight variability to increased risk for morbidity and mortality.
5. Conclusion
Our study shows a strong and independent association of both weight variability and weight change with cardiovascular outcomes and all-cause mortality among high-risk older individuals with hypertension without DM. These results further stress the clinical importance of maintaining a stable weight in patients with hypertension, especially among older individuals.
Author contributions
Conceptualization: R. Kazibwe, J. Yeboah. Formal analysis: R. Kazibwe & J. Yeboah. Visualization: R. Kazibwe. Writing original draft: R. Kazibwe & J. Yeboah. Reviewing and Editing: M.J. Singleton, MI Ahmad, A.D. Kaze, P.A. Chevli, J.H. Namutebi, R.N. Kasozi, D.D. Asiimwe & J. Kazibwe. Critical revision and contribution of important intellectual content: all authors. Supervision: M.D. Shapiro & J. Yeboah.
Data availability
The datasets generated during analysis during the current study are available in the National Heart, Lung, and Blood Institute BioLINCC repository, https://biolincc.nhlbi.nih.gov/studies/sprint/.
Funding
The Systolic Blood Pressure Intervention Trial was funded with Federal funds from the National Institutes of Health (NIH), including the National Heart, Lung, and Blood Institute (NHLBI), the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), the National Institute on Aging (NIA), and the National Institute of Neurological Disorders and Stroke (NINDS), under Contract Numbers HHSN268200900040C, HHSN268200900046C, HHSN268200900047C, HHSN268200900048C, HHSN268200900049C, and Inter-Agency Agreement Number A-HL-13–002–001. This post hoc analysis was unfunded.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors would like to recognize the SPRINT participants, without whom this study would not have been possible. Thank you for your contributions to science. The authors also would like to recognize the SPRINT trial team. A full list of contributors to SPRINT can be found at ClinicalTrials.gov Identifier: NCT01206062.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ajpc.2023.100610.
Appendix. Supplementary materials
References
- 1.Hales C.M.CM., Fryar C.D., Ogden C.L. Prevalence of obesity and severe obesity among adults: united States, 2017–2018. NCHS Data Brief, no 360 Hyattsville, MD: national center for health statistics 2020. 2020. [PubMed]
- 2.Whelton P., Carey R., Aronow W., et al. A guideline for the prevention, detection, evaluation and management of high blood pressure. A report of the American College of Cardiology/American heart association task force on clinical practice guidelines. Hypertension. 2018;71:1269–1324. doi: 10.1161/HYP.0000000000000066. [DOI] [PubMed] [Google Scholar]
- 3.Montani J.P., Viecelli A.K., Prevot A., Dulloo A.G. Weight cycling during growth and beyond as a risk factor for later cardiovascular diseases: the 'repeated overshoot' theory. Int J Obes (Lond) 2006;30(Suppl 4):S58–S66. doi: 10.1038/sj.ijo.0803520. [DOI] [PubMed] [Google Scholar]
- 4.Huang S., Shi K., Ren Y., et al. Association of magnitude of weight loss and weight variability with mortality and major cardiovascular events among individuals with type 2 diabetes mellitus: a systematic review and meta-analysis. Cardiovasc Diabetol. 2022;21(1):1–13. doi: 10.1186/s12933-022-01503-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zou H., Yin P., Liu L., et al. Body-weight fluctuation was associated with increased risk for cardiovascular disease, all-cause and cardiovascular mortality: a systematic review and meta-analysis. Front Endocrinol (Lausanne) 2019;10:728. doi: 10.3389/fendo.2019.00728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vincent G.K., Velkoff V.A. The next four decades, the older population in the United States: 2010 to 2050. Curr Popul Rep. 2010:25. [Google Scholar]
- 7.Ambrosius W.T., Sink K.M., Foy C.G., et al. The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: the Systolic Blood Pressure Intervention Trial (SPRINT) Clin Trials. 2014;11(5):532–546. doi: 10.1177/1740774514537404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wright J.T., Jr., Williamson J.D., Whelton P.K., et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373(22):2103–2116. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levey A.S., Coresh J., Greene T., et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann. Intern. Med. 2006;145(4):247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 10.Wilson P.W., D'Agostino R.B., Levy D., Belanger A.M., Silbershatz H., Kannel W.B. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97(18):1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 11.Bangalore S., Fayyad R., Laskey R., DeMicco D.A., Messerli F.H., Waters D.D. Body-weight fluctuations and outcomes in coronary disease. N Engl J Med. 2017;376(14):1332–1340. doi: 10.1056/NEJMoa1606148. [DOI] [PubMed] [Google Scholar]
- 12.Taylor C.B., Jatulis D.E., Fortmann S.P., Kraemer H.C. Weight variability effects: a prospective analysis from the Stanford five-city project. Am J Epidemiol. 1995;141(5):461–465. doi: 10.1093/oxfordjournals.aje.a117448. [DOI] [PubMed] [Google Scholar]
- 13.Iribarren C., Sharp D.S., Burchfiel C.M., Petrovitch H. Association of weight loss and weight fluctuation with mortality among Japanese American men. New Engl J Med. 1995;333(11):686–692. doi: 10.1056/NEJM199509143331102. [DOI] [PubMed] [Google Scholar]
- 14.Lissner L., Andres R., Muller D.C., Shimokata H. Body weight variability in men: metabolic rate, health and longevity. Int J Obes. 1990;14(4):373–383. [PubMed] [Google Scholar]
- 15.Lissner L., Odell P.M., D'Agostino R.B., et al. Variability of body weight and health outcomes in the Framingham population. N Engl J Med. 1991;324(26):1839–1844. doi: 10.1056/nejm199106273242602. [DOI] [PubMed] [Google Scholar]
- 16.Vergnaud A.-C., Bertrais S., Oppert J.-M., et al. Weight fluctuations and risk for metabolic syndrome in an adult cohort. Int J Obes. 2008;32(2):315–321. doi: 10.1038/sj.ijo.0803739. [DOI] [PubMed] [Google Scholar]
- 17.Rizvi S.I., Maurya P.K. Markers of oxidative stress in erythrocytes during aging in humans. Ann N Y Acad Sci. 2007;1100(1):373–382. doi: 10.1196/annals.1395.041. [DOI] [PubMed] [Google Scholar]
- 18.Schulz M., Liese A.D., Boeing H., Cunningham J.E., Moore C.G., Kroke A. Associations of short-term weight changes and weight cycling with incidence of essential hypertension in the EPIC-Potsdam Study. J Hum Hypertens. 2005;19(1):61–67. doi: 10.1038/sj.jhh.1001776. [DOI] [PubMed] [Google Scholar]
- 19.Somes G.W., Kritchevsky S.B., Shorr R.I., Pahor M., Applegate W.B. Body mass index, weight change, and death in older adults: the systolic hypertension in the elderly program. Am J Epidemiol. 2002;156(2):132–138. doi: 10.1093/aje/kwf019. [DOI] [PubMed] [Google Scholar]
- 20.He J., Whelton P.K., Appel L.J., Charleston J., Klag M.J. Long-term effects of weight loss and dietary sodium reduction on incidence of hypertension. Hypertension. 2000;35(2):544–549. doi: 10.1161/01.hyp.35.2.544. [DOI] [PubMed] [Google Scholar]
- 21.Jayedi A., Rashidy-Pour A., Khorshidi M., Shab-Bidar S. Body mass index, abdominal adiposity, weight gain and risk of developing hypertension: a systematic review and dose–response meta-analysis of more than 2.3 million participants. Obes Rev. 2018;19(5):654–667. doi: 10.1111/obr.12656. [DOI] [PubMed] [Google Scholar]
- 22.Bosello O., Vanzo A. Obesity paradox and aging. Eating and Weight Disorders-Studies on Anorexia. Bulimia Obes. 2021;26(1):27–35. doi: 10.1007/s40519-019-00815-4. [DOI] [PubMed] [Google Scholar]
- 23.Mathus-Vliegen E.M. Obesity and the elderly. J. Clin. Gastroenterol. 2012;46(7):533–544. doi: 10.1097/MCG.0b013e31825692ce. [DOI] [PubMed] [Google Scholar]
- 24.Alharbi T.A., Paudel S., Gasevic D., Ryan J., Freak-Poli R., Owen A.J. The association of weight change and all-cause mortality in older adults: a systematic review and meta-analysis. Age Ageing. 2021;50(3):697–704. doi: 10.1093/ageing/afaa231. [DOI] [PubMed] [Google Scholar]
- 25.Kritchevsky S.B., Beavers K.M., Miller M.E., et al. Intentional weight loss and all-cause mortality: a meta-analysis of randomized clinical trials. PLoS ONE. 2015;10(3) doi: 10.1371/journal.pone.0121993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oxlund C.S., Pareek M., Rasmussen B.S.B., et al. Body mass index, intensive blood pressure management, and cardiovascular events in the SPRINT trial. Am J Med. 2019;132(7):840–846. doi: 10.1016/j.amjmed.2019.01.024. [DOI] [PubMed] [Google Scholar]
- 27.Prentice A.M., Jebb S.A. Beyond body mass index. Obes Rev. 2001;2(3):141–147. doi: 10.1046/j.1467-789x.2001.00031.x. [DOI] [PubMed] [Google Scholar]
- 28.Bener A., Yousafzai M.T., Darwish S., Al-Hamaq A.O., Nasralla E.A., Abdul-Ghani M. Obesity index that better predict metabolic syndrome: body mass index, waist circumference, waist hip ratio, or waist height ratio. J Obes. 2013;2013 doi: 10.1155/2013/269038. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during analysis during the current study are available in the National Heart, Lung, and Blood Institute BioLINCC repository, https://biolincc.nhlbi.nih.gov/studies/sprint/.