Abstract
Use of the green fluorescent protein (Gfp) from the jellyfish Aequorea victoria is a powerful method for nondestructive in situ monitoring, since expression of green fluorescence does not require any substrate addition. To expand the use of Gfp as a reporter protein, new variants have been constructed by the addition of short peptide sequences to the C-terminal end of intact Gfp. This rendered the Gfp susceptible to the action of indigenous housekeeping proteases, resulting in protein variants with half-lives ranging from 40 min to a few hours when synthesized in Escherichia coli and Pseudomonas putida. The new Gfp variants should be useful for in situ studies of temporal gene expression.
Studies of specific gene expression in bacteria have been greatly facilitated by the use of reporter genes. The simple and sensitive enzymatic assays for enzymes like β-galactosidase and luciferase have allowed detailed investigations of gene regulation, obtained after construction of the relevant fusions between the promoters of interest and the respective reporter genes. Such investigations have been performed successfully in suspended monocultures of many bacterial species (in vitro experiments), for which the addition and spatial distribution of enzymatic substrates represent no problem. For analysis of heterogeneous and complex populations like surface-bound microbial communities there is a need for gene expression reporters that (i) allow for detection at the single-cell level and (ii) circumvent the problem with introduction and distribution of chemical substrates for the enzymatic reporter assays. A useful option for such cases is the green fluorescent protein (Gfp) obtained from the jellyfish Aequorea victoria. Gfp fluoresces green and requires only the presence of oxygen to maturate; i.e., no external compounds need to be added to organisms expressing Gfp in order to detect green fluorescence (4). The gfp gene may be transferred to and expressed in a wide range of organisms, e.g., mammals (18), fishes (19), insects (28), plants (3), yeasts (20), and a broad variety of bacteria (4, 6). As Gfp normally does not interfere with the growth of the host, it is an excellent choice for nondisruptive studies of bacterial communities or other systems which require live cells to be studied at the single-cell level. The optimal bacterial reporter for studying real-time gene expression in individual cells should possess a species-independent instability permitting monitoring of rates of expression (in the same way as unstable mRNA). A major drawback of Gfp is that once formed it seems to be very stable (24), which in turn renders the protein less valuable for studies of transient (real-time) gene expression.
It was recently shown by Keiler and coworkers (12) that specific C-terminal oligopeptide extensions can render otherwise stable proteins susceptible to degradation by certain intracellular tail-specific proteases. Our strategy has been to exploit this natural protein degradation system, which is based on ssrA-mediated tagging of prematurely terminated polypeptides at the COOH end (12). The ssrA transcript of Escherichia coli is a stable 362-nucleotide RNA molecule (5, 22) that exhibits some tRNA-like properties and can be charged with alanine (15). Genes homologous to ssrA have been identified in both gram-negative (2, 25) and gram-positive (26) bacteria, implying that the ssrA-mediated peptide-tagging system may be a conserved trait in bacteria. In the E. coli model proposed by Keiler et al. (12) the ssrA transcript targets proteins translated from incomplete or damaged mRNAs (e.g., mRNAs lacking a termination codon). Subsequently, a peptide tag with the sequence AANDENYALAA is attached to the carboxyl terminus of the nascent polypeptide chain by cotranslation switching of the ribosome from the damaged mRNA to the ssrA transcript. Finally, the resulting protein, carrying a C-terminal AANDENYALAA peptide tag, is recognized and rapidly degraded by intracellular tail-specific proteases (12). In the periplasm, the proteolytic degradation of AANDENYALAA-tagged proteins has been shown to be executed by the tail-specific Tsp protease, whereas tagged proteins located in the cytoplasm are believed to be broken down by a currently unidentified functional Tsp homolog (12, 23). It is important to be aware of the possibility that a highly expressed protein carrying this type of target tail may compete with naturally occurring products from the translation process, and the consequence could be a reduced growth rate.
Since changes in the last three residues of the AANDENYALAA consensus sequence are known to alter protein stability (13), we reasoned that it should also be possible to obtain Gfp mutants of varying stability by constructing variants carrying C-terminal peptide tags with minor alterations in the Tsp consensus sequence.
MATERIALS AND METHODS
Bacterial strains.
E. coli and Pseudomonas putida strains used in this study are listed in Table 1.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strains and plasmids | Relevant genotype and characteristics | Reference |
|---|---|---|
| E. coli | ||
| JM105 | thi rpsL (Strr) endA sbcB15 sbcC hsdR4 (rK− mK−) Δ(lac-proAB) [F′ traD36 lacIq Δ(lacZ)M15 proA+B+] | 29 |
| HB101 | SmrrecA thi pro leu hsdRM+ | 14 |
| MV1190 | Δ(lac-proAB) Δ(srl-recA)306::Tn10 [F′ traD36 proAB lacIq Δ(lacZ)M15] thi supE | 27 |
| MV1190(λ-pir) | As MV1190, lysogenized with λ-pir phage | 11 |
| P. putida | ||
| KT2442 | hsdR Rifr | 11 |
| KT2442 (lacIq) | As KT2442 with a mini-Tn5-lacIq cassette inserted into the chromosome; Rifr | Unpublished |
| JB279 | KT2442 (lacIq) × HB101(RK600) × MV1190(λ-pir)(pJBA29)a; KT2442 (lacIq) with a mini-Tn5-Km-T1-T0-gfpmut3*-PA1/04/03 cassette from pJBA29 randomly inserted into the chromosome; Rifr Kmr | This study |
| JB167 | KT2442 (lacIq) × HB101(RK600) × MV1190(λ-pir)(pJBA115)a; KT2442 (lacIq) with a mini-Tn5-Km-T1-T0-gfp(LAA)-PA1/04/03 cassette from pJBA115 randomly inserted into the chromosome; Rifr Kmr | This study |
| JB391 | KT2442 (lacIq) × HB101(RK600) × MV1190(λ-pir)(pJBA117)a; KT2442 (lacIq) with a mini-Tn5-Km-T1-T0-gfp(LVA)-PA1/04/03 cassette from pJBA117 randomly inserted into the chromosome; Rifr Kmr | This study |
| JB340 | KT2442 (lacIq) × HB101(RK600) × MV1190(λ-pir)(pJBA119)a; KT2442 (lacIq) with a mini-Tn5-Km-T1-T0-gfp(AAV)-PA1/04/03 cassette from pJBA119 randomly inserted into the chromosome; Rifr Kmr | This study |
| JB396 | KT2442 (lacIq) × HB101(RK600) × MV1190(λ-pir)(pJBA121)a; KT2442 (lacIq) with a mini-Tn5-Km-T1-T0-gfp(ASV)-PA1/04/03-cassette from pJBA121 randomly inserted into the chromosome; Rifr Kmr | This study |
| Plasmids | ||
| pUC18Not | Apr; cloning vector | 11 |
| pQE70 | Apr; donor of RBSII-T0-T1 cassette | 21 |
| pUHE24-2 | Apr; donor of PA1/04/03 promoter | 17 |
| pJBA23 | Apr; pUC18Not-RBSII-T0-T1 | This study |
| pJBA24 | Apr; pUC18Not-PA1/04/03-RBSII-T0-T1 | This study |
| pJBA27 | Apr; pUC18Not-PA1/04/03-RBSII-gfpmut3*-T0-T1 | This study |
| pJBA110 | Apr; pUC18Not-PA1/04/03-RBSII-gfp(LAA)-T0-T1 | This study |
| pJBA111 | Apr; pUC18Not-PA1/04/03-RBSII-gfp(LVA)-T0-T1 | This study |
| pJBA112 | Apr; pUC18Not-PA1/04/03-RBSII-gfp(AAV)-T0-T1 | This study |
| pJBA113 | Apr; pUC18Not-PA1/04/03-RBSII-gfp(ASV)-T0-T1 | This study |
| pUT-miniTn5-Km | Apr Kmr; delivery plasmid for mini-Tn5-Km | 9 |
| pJBA28 | Apr; Kmr; delivery plasmid for mini-Tn5-Km-PA1/04/03-RBSII-gfpmut3*-T0-T1 | This study |
| pJBA29 | As pJBA28, but with the PA1/04/03-RBSII-gfpmut3*-T0-T1 cassette of pJBA27 in the opposite orientation | This study |
| pJBA114 | Apr Kmr; delivery plasmid for mini-Tn5-Km-PA1/04/03-RBSII-gfp(LAA)-T0-T1 | This study |
| pJBA115 | As pJBA114, but with the PA1/04/03-RBSII-gfp(LAA)-T0-T1 cassette of pJBA110 in the opposite orientation | This study |
| pJBA116 | Apr Kmr; delivery plasmid for mini-Tn5-Km-PA1/04/03-RBSII-gfp(LVA)-T0-T1 | This study |
| pJBA117 | As pJBA116, but with the PA1/04/03-RBSII-gfp(LVA)-T0-T1 cassette of pJBA111 in the opposite orientation | This study |
| pJBA118 | Apr Kmr; delivery plasmid for mini-Tn5-Km-PA1/04/03-RBSII-gfp(AAV)-T0-T1 | This study |
| pJBA119 | As pJBA118, but with the PA1/04/03-RBSII-gfp(AAV)-T0-T1 cassette of pJBA112 in the opposite orientation | This study |
| pJBA120 | Apr Kmr; delivery plasmid for mini-Tn5-Km-PA1/04/03-RBSII-gfp(ASV)-T0-T1 | This study |
| pJBA121 | As pJBA120, but with the PA1/04/03-RBSII-gfp(ASV)-T0-T1 cassette of pJBA113 in the opposite orientation | This study |
| RK600 | Cmrori ColE1 RK2-Mob+ RK2-Tra+; helper plasmid in triparental matings | 14 |
Three-factor matings were performed as described previously by Kristensen et al. (16).
Media.
The basic medium used was either modified Luria-Bertani (LB) medium (1) containing 4 g of NaCl/liter instead of the normal 10 g of NaCl/liter or ABT minimal medium (AB minimal medium [7] containing 2.5 mg of thiamine/liter).
Plasmids.
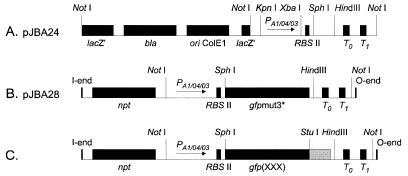
The plasmids used in this study are listed in Table 1. Construction of pJBA24 (Table 1 and Fig. 1A) was done as follows. PCR amplification with the primer set P1 and P2 (Table 2) and with pQE70 (21) as the template produced a 1.05-kb XbaI-StuI fragment containing a synthetic ribosome binding site (RBSII), at an optimal distance from a SphI site, followed by a HindIII site, translational stop codons in all three reading frames, and two strong transcriptional terminators, T0 (derived from phage λ) and T1 (derived from the rrnB operon of E. coli). This fragment was subsequently inserted into the XbaI site and the blunt-ended HindIII site (using the Klenow fragment of DNA polymerase I) of pUC18Not (11). The resulting plasmid, pJBA23, was digested with KpnI and XbaI and ligated to a 0.27-kb KpnI-XbaI fragment carrying the LacI-repressible promoter PA1/04/03 (amplified from pUHE24-2 [17] with the primers P3 and P4 [Table 2]) to create the cloning vector pJBA24. Construction of pJBA28 (Table 1 and Fig. 1B) was done as follows. gfpmut3* was amplified by PCR from gfpmut3b (a kind gift of R. H. Valdivia [8]) with the primers Pgfp(up) and Pgfp(down) (Table 2). From this PCR product, a 0.72-kb SphI-HindIII fragment carrying the gfpmut3* gene was isolated and subsequently ligated to SphI-HindIII-digested pJBA24. The resulting plasmid, pJBA27, was restricted by NotI, and the 2.0-kb NotI fragment containing the PA1/04/03-RBSII-gfpmut3*-T0-T1 cassette was finally inserted into the unique NotI site of the pUT-miniTn5-Km vector (9) to create either pJBA28 or pJBA29, differing only in the orientation of the NotI insert. For the construction of gfp genes encoding variant Gfps with differing terminal amino acids, we used essentially the same procedure as above, substituting primers encoding the entire C-terminal end of the variant Gfps for the down primer (Table 2). The resulting variant genes were transferred to pUT-miniTn5-Km to produce transposon insertion vectors with the gfp gene oriented in either direction (for details, see Tables 1 and 2 and Fig. 1). The novel modified genes were designated gfp(LAA), gfp(LVA), gfp(AAV), and gfp(ASV). Sequencing of the resulting plasmids verified that gfpmut3*, gfp(LAA), gfp(AAV), and gfp(ASV) had no mutations other than those derived from the customized PCR primers, whereas gfp(LVA) had a single (A → G) point mutation in nucleotide 349, resulting in an Asp117 → Gly117 (D117G) amino acid change. This point mutation does not appear to affect the fluorescence spectrum of Gfp(LVA) compared to the other Gfpmut3* variants (not shown).
FIG. 1.
Schematic drawings of cloning and transposon vectors (not to scale). (A) Cloning vector pJBA24. (B) Transposon vector pJBA28. (C) Transposon vectors pJBA114, pJBA116, pJBA118, and pJBA120. In all transposon plasmid vectors only the transposon parts flanked by the O and I ends of mini-Tn5 are shown, and the drawings indicate only the relevant restriction sites. Abbreviations: ori, origin of replication; lacZ′, truncated lacZ gene; bla, ampicillin resistance gene; npt, kanamycin resistance gene; PA1/04/03, LacI-repressible promoter; RBSII, synthetic ribosome binding site; gfpmut3*, gene encoding Gfp(S2R, S65G, S72A) (referred to as Gfpmut3*); T0, transcriptional terminator from phage lambda; T1, transcriptional terminator from the rrnB operon of E. coli; gfp(XXX), gene encoding either Gfp(LAA), Gfp(LVA), Gfp(AAV), or Gfp(ASV) (see text for details).
TABLE 2.
Primers used in this study
| Primer | Sequencea |
|---|---|
| P1 | 5′-GGTCTAGAATTAAAGAGGAGAAATTAAGC-3′ |
| XbaI | |
| P2 | 5′-GAAGGCCTAGCGGCGGATTTGTCCT-3′ |
| StuI | |
| P3 | 5′-CAGGTACCATTTATCAGGGTTATTGTCT-3′ |
| KpnI | |
| P4 | 5′-GGTCTAGATGTGTGAAATTGTTATCCG-3′ |
| XbaI | |
| Pgfp(up) | 5′-ATATAGCATGCGTAAAGGAGAAGAACTTTTCA-3′ |
| SphI | |
| Pgfp(down) | 5′-CTCTCAAGCTTATTTGTATAGTTCATCCATGC-3′ |
| HindIII | |
| Pgfp(down,LAA) | 5′-CTCTCAAGCTTATTAAGCTGCTAAAGCGTAGTTTTCGTCGTTTGCTGCAGGCCTTTTGTATAGTTCATCCATGCCA-3′ |
| HindIII A A L A Y N E D N A A P R ◂— | |
| StuI | |
| Pgfp(down,AAV) | 5′-CTCTCAAGCTTATTAAACTGCTGCAGCGTAGTTTTCGTCGTTTGCTGCAGGCCTTTTGTATAGTTCATCCATGCCA-3′ |
| HindIII V A A A Y N E D N A A P R ◂— | |
| StuI | |
| Pgfp(down,LVA) | 5′-CTCTCAAGCTTATTAAGCTACTAAAGCGTAGTTTTCGTCGTTTGCTGCAGGCCTTTTGTATAGTTCATCCATGCCA-3′ |
| HindIII A V L A Y N E D N A A P R ◂— | |
| StuI | |
| Pgfp(down,ASV) | 5′-CTCTCAAGCTTATTAAACTGATGCAGCGTAGTTTTCGTCGTTTGCTGCAGGCCTTTTGTATAGTTCATCCATGCCA-3′ |
| HindIII V S A A Y N E D N A A P R ◂— | |
| StuI |
Relevant restriction sites are underlined. Regions hybridizing to gfpmut3 (8) are shown in boldface, while regions encoding the C-terminal peptide tags (protease targets) are shown in italics. The arrows indicate the orientation by which the amino acid sequences of the resulting C-terminal peptide tags should be read.
Monitoring green fluorescence from single colonies.
The P. putida strains JB279, JB167, and JB340 [i.e., KT2442 (lacIq) carrying a transposon insertion of gfpmut3*, gfp(LAA), or gfp(AAV), respectively, allowing expression of the gfp genes from endogenous P. putida promoters (Table 1)] were streaked onto semisolid agar plates of ABT medium supplemented with 10 mM citrate, 50 μg of kanamycin/ml, 1 mM IPTG (isopropyl-β-d-thiogalactoside), and 20 g of Bacto Agar (Difco)/liter. After 18, 30, 42, 54, 73, 102, and 192 h of incubation at 30°C, the green-fluorescence phenotypes of single colonies were recorded with a charge-coupled device camera mounted on an epifluorescence microscope (Zeiss Axioplan; Zeiss, Oberkochen, Germany) equipped with a 2.5× lens. For fluorescence microscopy we used Zeiss filter set no. 10 (excitation, 470 to 490 nm; emission, 515 to 565 nm; dichroic, 510 nm) and a Zeiss HBO-100 mercury lamp. A CH250 charge-coupled device equipped with a KAF 1400 liquid-cooled chip (Photometrics, Tucson, Ariz.) was used for imaging.
Quantification of green fluorescence from liquid cultures.
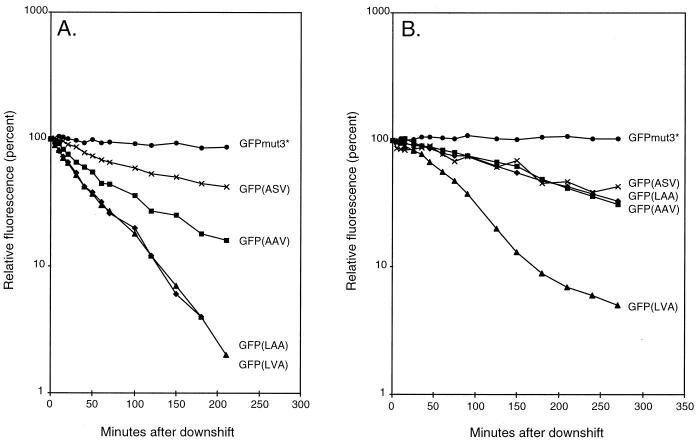
The strains MV1190(λ-pir) harboring either pJBA28 (gfpmut3*), pJBA114 [gfp(LAA)], pJBA116 [gfp(LVA)], pJBA118 [gfp(AAV)], pJBA120 [gfp(ASV)], or pUT-miniTn5-Km (control strain) (Table 1 and Fig. 1) were grown exponentially in LB medium (1) supplemented with 50 μg of kanamycin/ml and 0.2 mM IPTG at 37°C. At an optical density at 450 nm (OD450) of 1.0 the cultures were harvested, washed in ABT minimal medium, shifted to the same volume of preheated (37°C) ABT minimal medium containing 50 μg of kanamycin/ml, and reincubated at 37°C. Following the shift, culture samples were withdrawn at various time intervals and green fluorescence was measured with a fluorometer (model RF-1501; Shimadzu, Tokyo, Japan) set at an excitation wavelength of 475 nm and emission detection at 515 nm. The measured values of green fluorescence per milliliter of culture of strain MV1190(λ-pir) expressing either Gfpmut3* or the C-terminal-modified Gfps were corrected for background green fluorescence by subtracting the corresponding measured values of green fluorescence per milliliter of culture of MV1190(λ-pir) harboring the control plasmid pUT-miniTn5-Km. Finally, the background-corrected values of green fluorescence per milliliter of culture were converted into relative green fluorescence and plotted as a function of postshift time in a semilogarithmic plot (relative green fluorescence was arbitrarily set to 100% in the time zero samples) (see Fig. 3A). Likewise, the P. putida strains based on KT2442 (lacIq) and carrying gfpmut3* (JB279) or the modified gfp genes {JB167 [gfp(LAA)], JB391 [gfp(LVA)], JB340 [gfp(AAV)], and JB396 [gfp(ASV)]} or KT2442 (lacIq) without gfp (Table 1) were grown exponentially in LB medium at 30°C. At an OD450 of 1.0 the cultures were harvested, washed in ABT minimal medium, resuspended in the same volume of preheated ABT minimal medium, and reincubated at 30°C. Following the downshift, 1-ml culture samples were withdrawn after set time intervals, and green fluorescence was measured as described above. The measured values of green fluorescence per milliliter of culture of strain KT2442 (lacIq) expressing either Gfp were corrected for background green fluorescence by subtracting the corresponding measured values of green fluorescence per milliliter of culture of KT2442 (lacIq) and subsequently converted into relative green fluorescence and plotted as a function of postshift time in a semilogarithmic plot (relative green fluorescence was arbitrarily set to 100% in the time zero samples) (see Fig. 3B).
FIG. 3.
(A) Stability of Gfp variants in E. coli following a downshift. •, MV1190(λ-pir)(pJBA28) expressing Gfpmut3*; ×, MV1190(λ-pir)(pJBA120) expressing Gfp(ASV); ▪, MV1190(λ-pir)(pJBA118) expressing Gfp(AAV); ▴, MV1190(λ-pir)(pJBA116) expressing Gfp(LVA); and ⧫, MV1190(λ-pir)(pJBA114) expressing Gfp(LAA). (B) Stability of Gfp variants in P. putida following a downshift. •, KT2442 (lacIq) expressing Gfpmut3*; ×, KT2442 (lacIq) expressing Gfp(ASV); ▪, KT2442 (lacIq) expressing Gfp(AAV); ▴, KT2442 (lacIq) expressing Gfp(LVA); and ⧫, KT2442 (lacIq) expressing Gfp(LAA).
RESULTS AND DISCUSSION
Construction of unstable Gfp variants.
Wild-type Gfp is very stable in its mature, fluorescent form (24). The mutant protein, Gfpmut3, is approximately 20 times more fluorescent than wild-type Gfp when excited at 488 nm, and it is only weakly excited by UV light (8). This unique combination of features may allow efficient spectral separation between Gfpmut3 and other chromophores which are excited at shorter wavelengths, such as the mutant Gfp, Bfp (blue fluorescent protein) (10). We therefore chose Gfpmut3 as the template for construction of new, unstable reporter proteins aimed at applications in bacteria. We genetically modified the gfpmut3 gene in such a way that nucleotide sequences corresponding to variants of the described destabilizing peptide tail consensus sequence were added (separately) to replace the normal stop codon. This was accomplished by the use of PCR in combination with extended primers, the DNA sequences of which would encode new peptide tails of Gfpmut3 (8). The insertion of the upstream primer resulted in a change of amino acid 2 of Gfpmut3 from serine to arginine [in this study referred to as Gfpmut3*(Gfpmut3, S2R)]; we have not registered any phenotypic effects of this replacement. Several versions of the downstream primer were designed in order to introduce homologous but different C-terminal peptide tags of the encoded Gfpmut3* proteins (see Table 2 for details). We have chosen four of the resulting PCR products for further investigation: gfp(LAA), gfp(LVA), gfp(AAV), and gfp(ASV), encoding mutant Gfpmut3* proteins with the C-terminal extension sequences RPAANDENYALAA, RPAANDENYALVA, RPAANDENYAAAV, and RPAANDENYAASV, respectively. As a control we amplified the gfpmut3* gene without any C-terminal modifications in a similar way (see Materials and Methods). The selected fragments were cloned downstream of the strong LacI-repressible promoter PA1/04/03 (17), giving rise to the plasmids pJBA27 (gfpmut3*), pJBA110 [gfp(LAA)], pJBA111 [gfp(LVA)], pJBA112 [gfp(AAV)], and pJBA113 [gfp(ASV)] (Table 1). Next, fragments carrying the expression cassettes from these plasmids were transferred to mini-Tn5 transposon delivery vectors carrying the npt gene conferring resistance to kanamycin (9). Plasmids carrying the expression cassettes in both orientations were isolated (see Materials and Methods).
Expression of gfpmut3*, gfp(LAA), gfp(LVA), gfp(AAV), and gfp(ASV).
The 10 mini-Tn5 plasmid derivatives were transformed to E. coli MV1190(λ-pir) (11), and Gfp phenotypes of single colonies were monitored with an epifluorescence microscope with low magnification (see below). In this strain the lacIq gene is located on an F′ plasmid. The clones were green fluorescent, indicating that the high copy number of the mini-Tn5 plasmids (approximately 26 per genome compared to 1 for the F′ plasmid) may lead to titration of the LacI repressor, resulting in partial induction of the gfp promoter. Subsequent investigations showed that the fluorescence signals from these clones were greatly stimulated after induction with IPTG. Furthermore, our initial screening for fluorescence indicated that the constructs showed the predicted differences in protein stability (see below).
The value of these new Gfp constructs would be significantly increased if the particular instability phenotype was transferable to other bacterial species. Since homologs of the E. coli ssrA gene have been identified in both gram-negative (2, 25) and gram-positive (26) bacteria, and since there is considerable interest in the use of marker genes in in situ experiments with environmental samples, we introduced the mini-Tn5 transposons comprising the five gfp variant genes separately into the chromosome of P. putida KT2442 (lacIq) (Table 1).
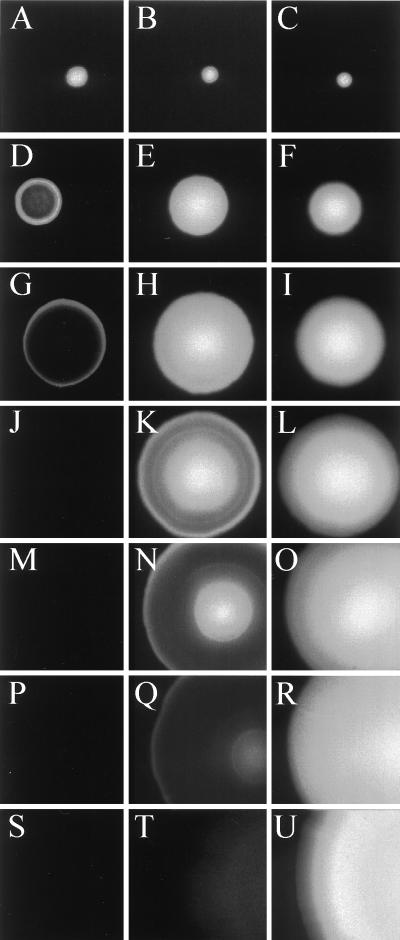
The fluorescence phenotypes of three of the P. putida clones are shown in Fig. 2, and they are very similar to those observed for the corresponding E. coli colonies (not shown). When grown in the presence of the inducer IPTG, single colonies of P. putida KT2442 (lacIq) expressing Gfpmut3* remained brightly green fluorescent even after 192 h of incubation at 30°C, indicating that Gfpmut3* is also extremely stable in the P. putida host. In striking contrast, P. putida KT2442 (lacIq) expressing either Gfp(LAA) or Gfp(AAV) lost the green-fluorescence signal over a period of 54 or 192 h, respectively. P. putida KT2442 (lacIq) cells expressing Gfp(LAA) were initially detected as small homogeneous green-fluorescent colonies. Following 30 to 42 h of incubation they developed into colonies exhibiting green fluorescence exclusively in their border regions, suggesting that Gfp(LAA) is rapidly turned over and therefore only the fastest-growing cells localized in the periphery of the colonies were able to synthesize enough mature Gfp(LAA) to be detected. After 54 h of incubation the colonies were no longer green fluorescent. Since only cells that are sufficiently metabolically active seem to be able to accumulate detectable amounts of green fluorescence, these results indicate that Gfp(LAA) is unstable in the P. putida KT2442 (lacIq) strain. Colonies expressing Gfp(AAV) remained homogeneously green fluorescent for at least 42 h. Following 54, 73, and 102 h of incubation these colonies had developed green-fluorescent cores and green-fluorescent border regions separated by nonfluorescent rings, which expanded in width with time. This temporary persistence of green-fluorescent cores may reflect the fact that significant metabolic activity in the core region is maintained for some time (probably reflecting slow growth based on “cannibalism” in these parts of the colonies). After 102 h P. putida colonies expressing Gfp(AAV) were only weakly green fluorescent in their border and core regions. After 90 additional hours of incubation, green-fluorescent colonies could no longer be detected. Considering that the Gfp derivatives are expressed under analogous conditions, we find it likely that the observed colony phenotypes reflect differences in the stabilities of the Gfp variants. This is in agreement with the experiments performed by Keiler et al. (12), in which the λ repressor carrying a C-terminal AANDENYALAA peptide tag was significantly less stable than the unmodified λ repressor in E. coli. In addition, the suggested stability hierarchy Gfpmut3* > Gfp(AAV) > Gfp(LAA) is similar to that observed in E. coli for unmodified cytochrome b562, cytochrome b562 carrying a WVAAV C-terminal tag, and cytochrome b562 carrying a WVLAA C-terminal tag (13). The remaining two variant gfp genes [gfp(LVA) and gfp(ASV)] appeared by visual inspection to give fluorescence patterns essentially the same as those observed for gfp(LAA) and gfp(AAV), respectively (data not shown). Similar observations of the expression of the unstable Gfps have been made with Serratia liquefaciens MG1 (not shown), and it is therefore likely that the unstable Gfp reporter proteins will show similar behavior in a number of different bacterial species. As far as we know, this is the first demonstration that the design of unstable proteins through the addition of this type of C-terminal tail is possible in bacterial species other than E. coli.
FIG. 2.
Time-resolved epifluorescence images of single colonies of P. putida strains expressing either Gfpmut3* or peptide-tagged variants of Gfpmut3*. (A, D, G, J, M, P, and S) Strain KT2442 (lacIq) expressing Gfp(LAA); (B, E, H, K, N, Q, and T) KT2442 (lacIq) expressing Gfp(AAV); (C, F, I, L, O, R, and U) KT2442 (lacIq) expressing Gfpmut3*. Incubation times at 30°C were 18 h (A, B and C), 30 h (D, E, and F), 42 h (G, H, and I), 54 h (J, K, and L), 73 h (M, N, and O), 102 h (P, Q, and R), and 192 h (S, T, and U).
Stability of Gfpmut3*, Gfp(LAA), Gfp(LVA), Gfp(AAV), and Gfp(ASV) in E. coli and P. putida.
The kinetics of induction of the different forms of the Gfp (the wild type and the four unstable variants) was investigated in an induction experiment in which IPTG was added to exponentially growing cultures of E. coli harboring the plasmids pJBA28, pJBA114, pJBA116, pJBA118, and pJBA120 (Table 1), carrying fusions between the IPTG-inducible promoter and the relevant gfp genes. The times at which fluorescence increased above the background levels for the five strains were determined, and it was found that in all cultures the initial kinetics were the same (data not shown). This strongly suggests that gene expression and maturation of the Gfp follow identical patterns for wild-type and variant genes.
In order to estimate the half-lives of the mature Gfp variants in vivo, we carried out quantitative fluorometer measurements of green fluorescence expressed by the strains derived from E. coli MV1190(λ-pir) or P. putida KT2442 (lacIq) encoding the variant Gfps (the same strains used for the colony investigations). The strains were grown in rich medium and shifted at mid-log phase to minimal medium without inducer. After the shift there was no increase in OD450 for 4 to 5 h, which means that protein synthesis (including that of Gfp) was negligible during this period. Fluorescence was measured at time intervals after the shift (Fig. 3). The stability of Gfpmut3* and the four tagged versions was estimated by using the relative green fluorescence value as a function of the time after the shift, represented as the slopes of the curves in Fig. 3. In E. coli it was found that the green fluorescence of Gfp(LAA), Gfp(LVA), Gfp(AAV), and Gfp(ASV) in the absence of Gfp synthesis decreased linearly from T = 10 min to T = 80 min after the downshift (Fig. 3A). The slope constants, μ, were determined to be approximately −0.018 min−1 for the strains encoding Gfp(LAA) and Gfp(LVA) and −0.012 and −0.0062 min−1 for the strains encoding Gfp(AAV) and Gfp(ASV), respectively. This corresponds to in vivo half-lives of mature Gfp(LAA) and mature Gfp(LVA) of approximately 40 min (T1/2 = −ln 2/μ), while Gfp(AAV) and Gfp(ASV) appear to have half-lives of approximately 60 and 110 min, respectively. In striking contrast, mature Gfpmut3* appears completely stable within the time of the experiment. This is in accordance with previous observations that both colonies and liquid cultures of E. coli MV1190(λ-pir) expressing Gfpmut3* remain green fluorescent after several weeks of incubation (data not shown). Consequently, we conservatively estimated the in vivo half-life of mature Gfpmut3* to be more than 1 day.
In P. putida KT2442 (lacIq) the green fluorescence derived from Gfpmut3* was similarly unaffected by the shift (Fig. 3B). Because of this, combined with the observation that colonies of this strain continue to be bright green fluorescent for more than a week (Fig. 2), we also consider the in vivo half-life of mature Gfpmut3* in P. putida to be more than 1 day. The strains expressing Gfp(LAA), Gfp(LVA), Gfp(AAV), or Gfp(ASV) exhibited a significant decrease of green fluorescence during the first several hours after the shift (Fig. 3B). During the period in which there was a linear decrease in green fluorescence (T = 30 to 120 min) we determined μ to be approximately −0.0115 min−1 for Gfp(LVA), indicating that the in vivo half-life of mature Gfp(LVA) in P. putida is in the range of 60 min. Interestingly, the other three Gfp variants, Gfp(LAA), Gfp(AAV), and Gfp(ASV), all displayed similar degradation rates of −0.0037 min−1 (corresponding to a half-life of approximately 190 min). It is possible that the increased expression of Gfp, observed as stronger fluorescence, from the chromosomally integrated variant gfp genes in P. putida may affect the degradation rates of the Gfps (e.g., through titration of the protease activity), resulting in apparently more stable proteins. Furthermore, the proteases degrading tagged proteins in P. putida may differ in specificity from the E. coli protease, resulting in altered half-lives.
Consequently, the half-life estimates obtained in the experiments presented are not to be taken as absolute, fixed values. The protease reactions resulting in degradation of Gfp may be dependent on strains, growth conditions, specific features of the surroundings, competing targets in the cell, etc. The important feature, however, is that a protein known to be very stable may be converted to an unstable variant in a semipredictable way through the employment of a natural protease activity, which is apparently found in many different bacteria.
Concluding remarks.
We have constructed new variant gfp genes encoding Gfps with reduced half-lives compared to that of the wild-type protein. The apparent half-lives of these proteins were estimated in liquid cultures, and in all cases the novel proteins displayed half-lives markedly shorter than that of the corresponding unmodified Gfpmut3* protein. We believe that transcriptional fusions between an appropriate gene and the described novel Gfp variants will facilitate studies of real-time gene expression in situ through monitoring of the green-fluorescent phenotype. We are presently assessing the value of these new reporter proteins in connection with physiological studies of bacterial biofilms.
ACKNOWLEDGMENTS
The work was supported by grants to S.M. from the Danish Research Councils under the Biotechnology Program and a contract from EU (BIO4-CT96-0181).
Brendan Cormack, Rafael H. Valdivia, and Stanley Falkow are acknowledged for the gift of the gfpmut3b gene used in this study.
REFERENCES
- 1.Bertani G. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J Bacteriol. 1951;62:293–300. doi: 10.1128/jb.62.3.293-300.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown J W, Hunt D K, Pace N R. Nucleotide sequence of the 10Sa RNA gene of the beta-purple eubacterium Alcaligenes eutrophus. Nucleic Acids Res. 1990;18:2820. doi: 10.1093/nar/18.9.2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Casper S J, Holt C A. Expression of the green fluorescent protein-encoding gene from tobacco mosaic virus-based vector. Gene. 1996;173:69–73. doi: 10.1016/0378-1119(95)00782-2. [DOI] [PubMed] [Google Scholar]
- 4.Chalfie M, Tu Y, Euskirchen G, Ward W W, Prasher D C. Green fluorescent protein as a marker for gene expression. Science. 1994;263:802–805. doi: 10.1126/science.8303295. [DOI] [PubMed] [Google Scholar]
- 5.Chauhan A K, Apirion D. The gene for a small stable RNA (10Sa RNA) of Escherichia coli. Mol Microbiol. 1989;3:1481–1485. doi: 10.1111/j.1365-2958.1989.tb00133.x. [DOI] [PubMed] [Google Scholar]
- 6.Christensen B B, Sternberg C, Molin S. Bacterial plasmid conjugation on semi-solid surfaces monitored with the green fluorescent protein (GFP) from Aequorea victoria as a marker. Gene. 1996;173:59–65. doi: 10.1016/0378-1119(95)00707-5. [DOI] [PubMed] [Google Scholar]
- 7.Clark D J, Maaløe O. DNA replication and the division cycle in Escherichia coli. J Mol Biol. 1967;23:99–112. [Google Scholar]
- 8.Cormack B P, Valdivia R H, Falkow S. FACS optimized mutants of the green fluorescent protein (GFP) Gene. 1996;173:33–38. doi: 10.1016/0378-1119(95)00685-0. [DOI] [PubMed] [Google Scholar]
- 9.De Lorenzo V, Herrero M, Jakubzik U, Timmis K N. Mini-Tn5 transposon derivatives for insertion mutagenesis, promoter probing, and chromosomal insertion of cloned DNA in gram-negative eubacteria. J Bacteriol. 1990;172:6568–6572. doi: 10.1128/jb.172.11.6568-6572.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heim R, Prasher D C, Tsien R Y. Wavelength mutations and posttranslational autoxidation of green fluorescent protein. Proc Natl Acad Sci USA. 1994;91:12501–12504. doi: 10.1073/pnas.91.26.12501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herrero M, de Lorenzo V, Timmis K N. Transposon vectors containing non-antibiotic resistance selection markers for cloning and stable chromosomal insertion of foreign genes in gram-negative bacteria. J Bacteriol. 1990;172:6557–6567. doi: 10.1128/jb.172.11.6557-6567.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keiler K C, Waller P R H, Sauer R T. Role of a peptide tagging system in degradation of proteins synthesized from damaged messenger RNA. Science. 1996;271:990–993. doi: 10.1126/science.271.5251.990. [DOI] [PubMed] [Google Scholar]
- 13.Keiler K C, Sauer R T. Sequence determinants of C-terminal substrate recognition by the Tsp protease. J Biol Chem. 1996;271:2589–2593. doi: 10.1074/jbc.271.5.2589. [DOI] [PubMed] [Google Scholar]
- 14.Kessler B, de Lorenzo V, Timmis K N. A general system to integrate lacZ fusions into the chromosomes of gram negative eubacteria: regulation of the Pm promoter in the TOL plasmid studied with all controlling elements in monocopy. Mol Gen Genet. 1992;233:293–301. doi: 10.1007/BF00587591. [DOI] [PubMed] [Google Scholar]
- 15.Komine Y, Kitabatake M, Yokogawa T, Nishikawa K, Inikuchi H. A t-RNA-like structure is present in 10Sa RNA, a small stable RNA from Escherichia coli. Proc Natl Acad Sci USA. 1994;91:9223–9227. doi: 10.1073/pnas.91.20.9223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kristensen C S, Eberl L, Sanchez-Romero J M, Givskov M, Molin S, de Lorenzo V. Site-specific deletions of chromosomally located DNA segments with the multimer resolution system of broad-host-range plasmid RP4. J Bacteriol. 1995;177:52–58. doi: 10.1128/jb.177.1.52-58.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lanzer M, Bujard H. Promoters largely determine the efficiency of repressor action. Proc Natl Acad Sci USA. 1988;85:8973–8977. doi: 10.1073/pnas.85.23.8973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ludin B, Doll T, Meili R, Kaech S, Matus A. Application of novel vectors for GFP-tagging of proteins to study microtubule-associated proteins. Gene. 1996;173:107–111. doi: 10.1016/0378-1119(95)00899-3. [DOI] [PubMed] [Google Scholar]
- 19.Moss J B, Price A L, Raz E, Driever W, Rosenthal N. Green fluorescent protein marks skeletal muscle in murine cell lines and zebrafish. Gene. 1996;173:89–98. doi: 10.1016/0378-1119(95)00729-6. [DOI] [PubMed] [Google Scholar]
- 20.Niedenthal R K, Riles L, Johnston M, Hegemann J H. Green fluorescent protein as a marker for gene expression and subcellular localization in budding yeast. Yeast. 1996;12:773–786. doi: 10.1002/(SICI)1097-0061(19960630)12:8%3C773::AID-YEA972%3E3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 21.Qiagen GmbH. Product guide. Hilden, Germany: Qiagen GmbH; 1997. [Google Scholar]
- 22.Ray B K, Apirion D. Characterization of 10s RNA: a new stable RNA molecule from Escherichia coli. Mol Gen Genet. 1979;174:25–32. doi: 10.1007/BF00433301. [DOI] [PubMed] [Google Scholar]
- 23.Silber K R, Sauer R T. Deletion of the prc (tsp) gene provides evidence for additional tail-specific proteolytic activity in Escherichia coli K-12. Mol Gen Genet. 1994;242:237–240. doi: 10.1007/BF00391018. [DOI] [PubMed] [Google Scholar]
- 24.Tombolini R, Unge A, Davey M E, de Bruijn F J, Jansson J K. Flow cytometric and microscopic analysis of GFP-tagged Pseudomonas fluorescens bacteria. FEMS Microbiol Ecol. 1997;22:17–28. [Google Scholar]
- 25.Tyagi J S, Kinger A K. Identification of the 10Sa RNA structural gene of Mycobacterium tuberculosis. Nucleic Acids Res. 1992;20:138. doi: 10.1093/nar/20.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ushida C, Himeno H, Watanabe T, Muto A. t-RNA-like structures in 10Sa RNAs of Mycoplasma capricolum and Bacillus subtilis. Nucleic Acids Res. 1994;22:3392–3396. doi: 10.1093/nar/22.16.3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vieira J, Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- 28.Wang S, Hazelrigg T. Implications for bcd mRNA localization from spatial distribution of exu protein in Drosophila oogenesis. Nature. 1994;369:400–403. doi: 10.1038/369400a0. [DOI] [PubMed] [Google Scholar]
- 29.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]