Abstract
Background
The type of anaesthesia and choice of anaesthetic drugs may affect the quality of recovery after surgery. Remimazolam is a new benzodiazepine with rapid onset and offset, specifically antagonised by flumazenil. This study aimed to compare remimazolam with propofol on the quality of recovery in patients undergoing ambulatory arthroscopic surgery.
Methods
Patients aged 18–65 yr and scheduled for ambulatory arthroscopic meniscus repair were recruited and randomly assigned to receive either continuous i.v. infusion of remimazolam or plasma target-controlled infusion of propofol. The quality of recovery-15 (QoR-15) scale was administered on postoperative day 1 (POD1) as the primary outcome. Secondary outcomes included the Athens Insomnia Scale (AIS) scores and cardiovascular variables.
Results
In total, 120 patients were randomly assigned to the remimazolam or propofol groups and 114 patients were included in the analysis. The remimazolam group had higher total QoR-15 scores on POD1 (125 [120–127.5] vs 121.5 [119–124], with a median difference of 3 (95% confidence interval: 1–5; P=0.002). Physical independence and psychological support were higher in the remimazolam group (8.5 [8–10] vs 8 [7–9], P=0.043; 17 [13–17] vs 12.5 [12–14], P<0.001). Remimazolam lowered the number of awakenings during the first postoperative night (P=0.042) and the incidence of hypotension (P=0.04).
Conclusions
Remimazolam-based total i.v. anaesthesia was associated with small improvements in the quality of recovery; however, the improvement was less than the minimally clinically important difference.
Clinical trial registration
ChiCTR2100053014.
Keywords: ambulatory, arthroscopic, QoR-15, quality of recovery, remimazolam
Advances in minimally invasive joint surgery have led to increasing numbers of patients to be suitable for ambulatory procedures.1,2 Challenges for day surgery include reducing preoperative anxiety and costs and improving postoperative recovery.3 Propofol, sevoflurane, and desflurane are the three most widely used drugs for day surgery, the effects of which, on the quality of patients' short- and long-term postoperative recovery, continue to be debated.4, 5, 6, 7 Previous studies5,6,8,9 have reported a lower incidence of postoperative nausea and vomiting (PONV), better patient acceptance, better quality of sleep, and postoperative recovery during propofol-based anaesthesia than sevoflurane- or desflurane-based inhalation anaesthesia. However, propofol still has several disadvantages, such as hypotension, injection pain, and higher cost.6,10 Ongoing attempts to find more suitable anaesthetic drugs for day surgery remain the focus of clinical research.
Remimazolam, a new benzodiazepine with rapid onset and offset, is rapidly hydrolysed in vivo by tissue carboxylesterases to inactive metabolites and is specifically antagonised by flumazenil,11 potentially enhancing recovery from anaesthesia and therefore suggesting its utility in day surgery. Clinical trials have demonstrated that remimazolam is associated with a lower incidence of hypotension, hypoxia, and injection pain when compared with propofol,12, 13, 14 and a lower rate of PONV when compared with desflurane after general anaesthesia.15 The effect of remimazolam-based i.v. anaesthesia on the quality of postoperative recovery is controversial. Choi and colleagues16 demonstrated that remimazolam provided a similar quality of recovery for female patients who underwent thyroid surgery, whereas Mao and colleagues17 showed a poorer quality of recovery in urological patients with remimazolam 24 h after surgery.
This study aimed to compare the effect of remimazolam with propofol on the quality of recovery 24 h after ambulatory arthroscopic surgery using the quality of recovery scale (QoR-15) on the first postoperative day (POD1). We hypothesised that remimazolam may lead to a better quality of postoperative recovery.
Methods
This prospective randomised controlled trial followed the CONSORT guidelines and was preregistered in the Chinese Clinical Trial Registry (ChiCTR2100053014, investigator: LT, date of registration: 9 November 2021). The Ethics Committee of the First Affiliated Hospital of Anhui Medical University approved this clinical trial (Ethical Approval no. PJ2021-15-22, on 25 November 2021). All patients signed an informed consent form at the time of trial inclusion.
Patients
From November 2021 to April 2022, the study enrolled patients aged 18–65 yr with ASA 1–2, who underwent arthroscopic meniscus repair under general anaesthesia in the day surgery unit and were hospitalised for one night in the day ward.
The exclusion criteria were as follows: severe respiratory or circulatory system diseases, endocrine system disease, psychosomatic disease, history of chronic analgesic or sedative medication use including benzodiazepines, alcohol abuse, allergy to remimazolam or propofol, history of insomnia, inability to understand the Athens Insomnia Scale (AIS) and QoR-15, and inability to take care of themselves before surgery.
Randomisation and blinding
All the included patients were randomly allocated into the remimazolam group (R group) or the propofol group (P group) at a ratio of 1:1 using computer-generated block randomisation (block size, 4) by an investigator. The allocation numbers were placed in opaque envelopes, which could only be opened by the anaesthetists in the operating room. Follow-up 24 h after the surgery was made by telephone contact with an investigator who did not know the group assignment. Patients, data recorders, and analysis researchers were blinded to the group allocation.
Anaesthetic methods and interventions
In all patients, sleep during the preceding days was assessed on arrival in the operating room using the AIS.18,19 For this, an investigator read the questions of the questionnaires and recorded the responses of the patients. Patients received standardised monitoring, including noninvasive blood pressure monitoring, electrocardiography, and measurements of oxygen saturation and bispectral index (BIS, Covidien, Inc., Mansfield, MA, USA). No premedication was given to any patients. After upper limb venous access was established, sufentanil 0.4 μg kg−1 was given. Then, the remimazolam group received remimazolam besylate (Rui Ma, Yichang Humanwell Pharmaceutical Co. Ltd., Hubei, China) at a rate of 6 mg kg−1 h−1 i.v. for anaesthesia induction,20,21 whereas the propofol group received propofol (Diprivan, AstraZeneca UK Limited, Macclesfield, U.K.) using plasma target-controlled infusion (SLGO-Diprifusor using the Marsh pharmacokinetic model, Beijing Silugao Medical Technology Co. Ltd., Beijing, China) with a gradual increase in the effect–site concentration (initial concentration 2, up to 3.5 μg mL−1 after 20 s). After the patient lost consciousness, cisatracurium 0.2 mg kg−1 was given, followed by laryngeal mask placement. Anaesthesia was maintained by adjusting the remimazolam dose to 0.4–2 mg kg−1 h−1 or propofol to 1–3 μg ml−1 and remifentanil to 0.1–0.3 μg kg−1 min−1 to achieve a target BIS of 40–60. Sufentanil 0.1 μg kg−1 and cisatracurium 0.05 mg kg−1 were added intermittently as needed for the procedure. Volume-controlled mechanical ventilation was adjusted to provide an end-tidal CO2 (EtCO2) concentration between 4.66 and 5.99 kPa. After the surgery, dexamethasone 5 mg was administered to prevent PONV and ropivacaine 0.5%, 20 ml was injected into the joint cavity. Ringer's lactate solution was administered at a rate of 5–7 ml kg−1 h−1 adjusted according to the heart rate and blood pressure.22 After the resumption of spontaneous breathing, patients were transferred to the post-anaesthesia care unit (PACU). Patients were assessed and findings were recorded by an anaesthesiologist and a nurse who were blinded to the group in the PACU. Flurbiprofen ester 50 mg was administered as a rescue analgesic if the visual analogue scale (VAS) score was >3. Patients were discharged to the ward if they had a Steward score of 6. All anaesthetic procedures were carried out by the same team of experienced anaesthesiologists. All arthroscopic operations were performed by the same team of surgeons.
Perioperative systolic blood pressure and heart rate were maintained within 20% of baseline. Patients with hypotension (decreased by >20% or the mean arterial pressure [MAP] was <60 mm Hg) were immediately treated with ephedrine 6 mg or phenylephrine 20 μg. If the systolic blood pressure increased by >20% above baseline, the patient was given nicardipine 0.3 mg, and atropine 0.5 mg was administered if the patients had bradycardia (HR<50 beats min−1).
Outcome measures
The primary outcome of this trial was the QoR-15 score on POD1 (between 14:00 and 18:00) using the QoR-15 scale,23,24 which is a patient-reported questionnaire and covers 15 items in five dimensions: emotional state (four items), physical comfort (five items), psychological support (two items), physical independence (two items), and pain (two items). The first 10 items are rated on an 11-point Likert scale: from 0 (none of the time, the poorest) to 10 (all the time, the best), whereas the last five items are scored from 10 (none of the time, the best) to 0 (all the time, the poorest). The total QoR-15 score ranges from 0 (poorest recovery) to 150 (excellent recovery).
The secondary outcomes were the time to loss of consciousness (LOC, the time from remimazolam or propofol injection to achieving a modified observer's assessment of alertness/sedation score of 2)25; BIS value of LOC; time of BIS 60 (the time to reach a BIS of 60); recovery time (the time from ceasing all anaesthetics injections to eye opening); extubation time (the time from ceasing all anaesthetics injections to removing the laryngeal mask); orientation recovery time (the time from ceasing all anaesthetic injections for the patient to give their name and location); MAP, HR, BIS, and Ramsay Sedation Score (RSS) at different time points. The doses of the two anaesthetic drugs required for LOC and reaching BIS 60 during anaesthesia induction were also calculated. Sedation levels were assessed using the RSS scores at the time of anaesthesia recovery.
The AIS18 was used to assess sleep problems during the first night after surgery, which consists of eight items: sleep induction, awakenings during the night, early morning awakening, total sleep time and overall quality of sleep, problems with the sense of well-being, overall functioning, and sleepiness during the day. Every AIS item is scored from 0 (no problem at all) to 3 (very serious problem); thus, the total score ranges from 0 (absence of any sleep-related problem) to 24 (the most severe degree of insomnia).18,19 Perioperative adverse reactions were also recorded.
Statistical analysis
According to the results of our preliminary study, the QoR-15 scores on POD1 were 120.3 (9.28). Based on the minimal clinically important difference for the QoR-15 scale of 6 in a previous study,26 we hypothesised that this trial would have 90% power to detect the significance threshold of 0.05 of QoR-15 scores if 51 patients per group were analysed. Considering the loss to follow-up of patients undergoing day surgery, the sample size of each group was increased to 60.
Data were collected and analysed using IBM SPSS Statistics for Windows version 23.0 (IBM Corp., Armonk, NY, USA). All quantitative variables were assessed with the Shapiro–Wilk test and were expressed as mean (standard deviation, sd) or median (inter-quartile range). Normally distributed data were analysed using an independent sample t-test and non-normally distributed data were analysed with the Mann–Whitney U test. All QoR-15 domains and global scores after 24 h in the two groups were compared by the Mann–Whitney U-test, and the median differences with 95% confidence intervals (CI) for each paired difference 24 h after surgery were also calculated using the Hodges–Lehmann test. In both groups, the proportion of patients with QoR-15 ≥118 (good recovery) was recorded.27 Categorical variables were presented as a number (n) and percentage (%) and were compared using the χ2 test or Fisher's exact test. RSS scores, BIS values, and cardiovascular variables at different time points were analysed using repeated-measures analysis of variance. The least significant difference was used for multiple comparisons. For all data analyses, P-values <0.05 were considered statistically significant.
Results
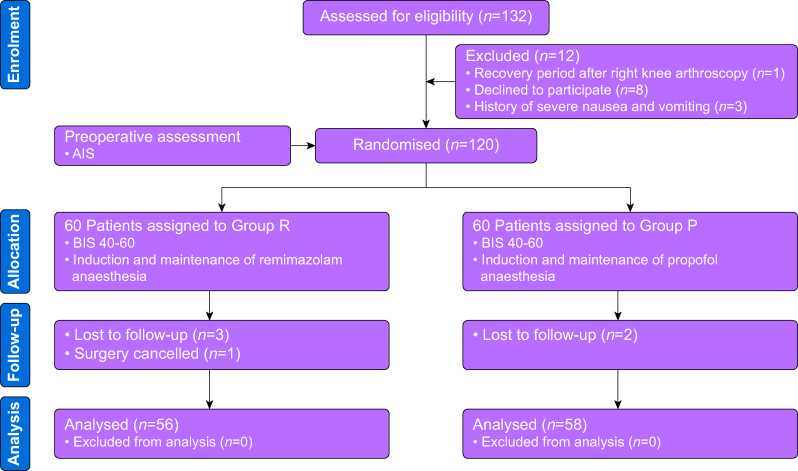
In this study, 132 patients were initially assessed for eligibility and 120 who met the inclusion criteria were randomly allocated to the remimazolam group (R group, n=60) or the propofol group (P group, n=60). Five patients were lost to follow-up, three in the R group, two in the P group, while one operation was cancelled in the R group. Finally, 114 patients were included in the analysis (shown in Figure 1). The baseline data were similar in the two groups (Table 1).
Fig 1.
Consort flowchart. Group R: patients received continuous i.v. infusion of remimazolam for anaesthesia induction and maintenance. Group P: patients received plasma target-controlled infusion of propofol for anaesthesia induction and maintenance. AIS, Athens Insomnia Scale; BIS, bispectral index.
Table 1.
Patient characteristics and surgical variables in the remimazolam (R) and propofol (P) groups. The values are expressed as means (sd), no. (%) or median (inter-quartile range). ASA, American Society of Anesthesiologists; BMI, body mass index. ∗Age is presented as median (minimum–maximum).
| Variables | R Group (n=56) | P Group (n=58) |
|---|---|---|
| Age (yr)∗ | 48.5 (19–62) | 50 (19–64) |
| BMI (kg m−2) | 24.73 (2.93) | 23.95 (2.85) |
| ASA physical status n (%) | ||
| 1 | 3 (5.36) | 4 (6.90) |
| 2 | 53 (94.64) | 54 (93.10) |
| Sex | ||
| Male n (%) | 27 (48.21) | 27 (46.55) |
| Female n (%) | 29 (51.79) | 31 (53.45) |
| Surgical duration (min) | 47.32 (17.88) | 46.64 (16.33) |
| Duration of anaesthesia (min) | 77.91 (19.05) | 76.67 (16.92) |
| Dose of sufentanil (μg) | 35 (30–40) | 35 (30–40) |
| Dose of remifentanil (μg) | 690.75 (590.5–861.5) | 624.00 (535.5–765.5) |
| Crystalloids (ml) | 400 (250–600) | 375 (250–500) |
The QoR-15 total scores were statistically higher in the R group than in the P group (125, [120–127.5] vs 121.5 [119–124], respectively; P=0.002), with a median difference of 3 (95% CI 1–5). Physical independence and psychological support, two of the five QoR-15 domains, were also higher in the R group (8.5 [8–10] vs 8 [7–9], P=0.043; 17 [13–17] vs 12.5 [12–14], P<0.001). In the R group 93% of patients had a QoR-15 ≥118 compared with 90% in the P group (P=0.394) (Table 2). Each item score of the QoR-15 in the two groups is provided in the Supplementary material (Supplementary Table S1). No significant difference in the total AIS scores for the first postoperative night was found between the two groups (5 [3–7] vs 5 [4–8], P=0.242); however, the number of awakenings at night among the eight dimensions of the AIS was lower in the R group (P=0.042) (Table 2).
Table 2.
Comparison of quality of recovery-15 total scores, AIS scores, and domains on POD1 in the remimazolam (R) and propofol (P) groups. The values are expressed as median (inter-quartile range) or number of patients (percentage). The median difference (95% confidence intervals) is the median of every pair-wise difference between the two groups at POD1. AIS, Athens Insomnia Scale; POD1, postoperative 1 day; QoR-15, quality of recovery-15.
| Variables | R Group (n=56) | P Group (n=58) | Median difference | P |
|---|---|---|---|---|
| QoR-15 value on POD1 | 125 (120–127.5) | 121.5 (119–124) | 3 (1–5) | 0.002 |
| Patients with QoR-15 value on POD1 ≥118 n (%) | 52 (92.86) | 52 (89.66) | — | 0.394 |
| QoR-15 domains on POD1 | ||||
| Pain | 18 (17–18) | 18 (17–18) | 0 (0–0) | 0.855 |
| Physical comfort | 47 (45–48) | 46 (45–47) | 0 (−1 to 1) | 0.531 |
| Physical independence | 8.5 (8–10) | 8 (7–9) | 1 (0–1) | 0.043 |
| Psychological support | 17 (13–17) | 12.5 (12–14) | 1 (1–4) | 0.000 |
| Emotions | 36 (35.5–38) | 37 (36–38) | 0 (−1 to 1) | 0.979 |
| AIS baseline value | 3 (1–5) | 4 (2–6) | 0.383 | |
| AIS scores at postoperative 1st night | 5 (3–7) | 5 (4–8) | 0.242 | |
| AIS domains at postoperative 1st night | ||||
| Sleep induction | 0 (0–1) | 0 (0–1) | 0.585 | |
| Awakenings during the night | 1 (0–1.5) | 1 (1–2) | 0.042 | |
| Early morning awakening | 0 (0–1) | 1 (0–1) | 0.129 | |
| Total sleep time | 1 (0–1) | 1 (0–1) | 1.00 | |
| Overall quality of sleep | 1 (0–1) | 1 (0–1) | 0.445 | |
| Sense of well-being during the day | 0 (0–1) | 0 (0–1) | 0.612 | |
| Functioning (physical and mental) during the day | 1 (0–1) | 1 (0–1) | 0.552 | |
| Sleepiness during the day | 1 (0–1) | 1 (0–1) | 0.103 |
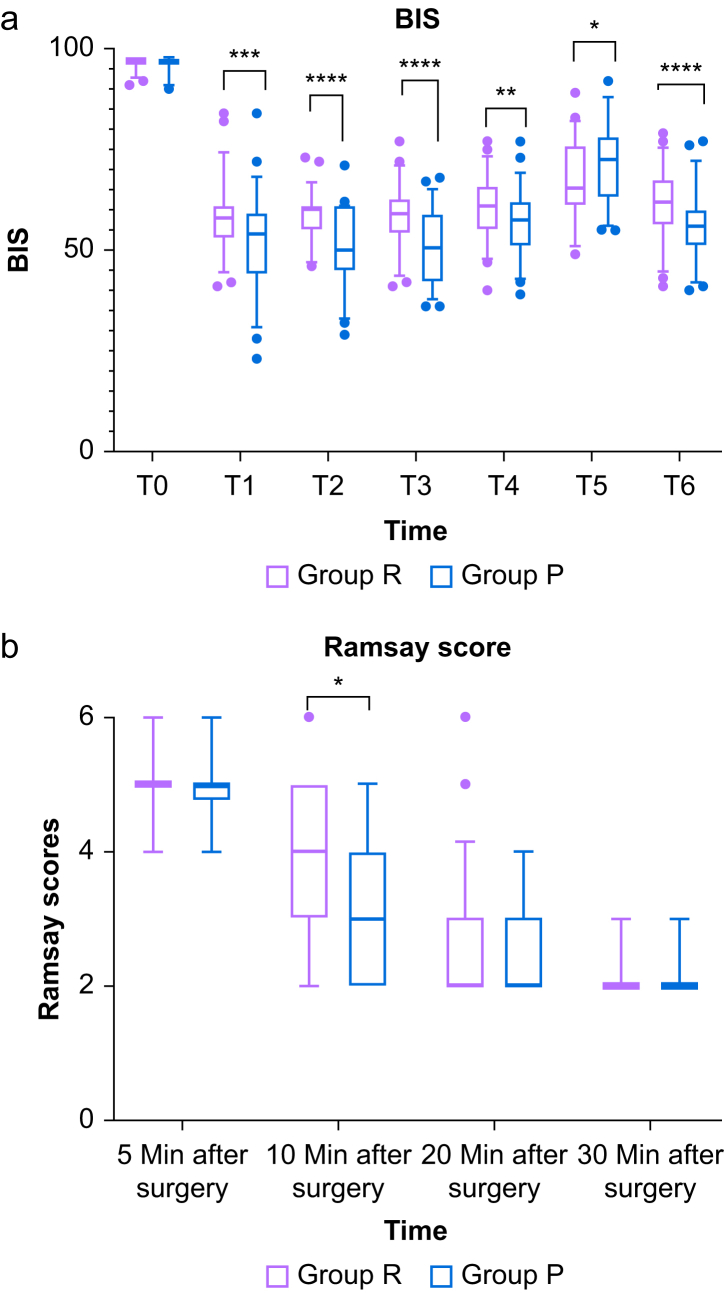
The dose to LOC was 0.14 (0.13–0.16) mg kg−1 remimazolam in the R group and 1.22 (1.08–1.58) mg kg−1 propofol in the P group. The time to LOC was slightly longer in the P group (82 [68–105] vs 79 [65–87.5], P=0.032) (Table 3). The recovery time, the extubation time, orientation recovery time and PACU time were all longer in the R group than in the P group (10 [8–18] vs 8 [7–10], P<0.001; 16 [12–20] vs 13 [11–15], P=0.003; 14.5 (11–20.5) vs 12 [10–15], P=0.001; 35 [33.5–40] vs 33.5 [30–40], P=0.021) (Table 3). Consistent with the results for the time to awakening, the RSS score in the R group was higher 10 min after surgery (Figure 2, P<0.05). The BIS values at different time points were comparable between the two groups (Fig 2). Compared with the baseline, BIS values significantly decreased at all time points after anaesthesia induction in the two groups (P<0.001), and the change in BIS values was greater in the propofol group at T1 (after induction), T2 (after placement of the laryngeal mask), T3 (before tourniquet inflation), T4 (before tourniquet deflation), and T6 (the end of surgery) (P<0.01). Interestingly, BIS values both transiently increased and the value in the propofol group increased faster and higher after tourniquet deflation (P<0.05).
Table 3.
Comparison of perioperative time and postoperative outcomes in the remimazolam (R) and propofol (P) groups. The values are expressed as median (inter-quartile range) or number of patients (percentage). BIS, bispectral index; LOC, loss of consciousness; NA, Not Applicable; PONV, postoperative nausea and vomiting; VAS, visual analogue scale.
| Variables | R Group (n=56) | P Group (n=58) | P |
|---|---|---|---|
| Time to LOC (s) | 79 (65–87.5) | 82 (68–105) | 0.032 |
| BIS of LOC | 82 (79–84) | 82 (80–86) | 0.845 |
| Time to BIS 60 (s) | 140.5 (112–182.5) | 135 (106–178) | 0.796 |
| Recovery time (min) | 10 (8–18) | 8 (7–10) | <0.001 |
| Extubation time (min) | 16 (12–20) | 13 (11–15) | 0.003 |
| Orientation recovery time (min) | 14.5 (11–20.5) | 12.0 (10–15) | 0.001 |
| Time in PACU (min) | 35 (33.5–40) | 33.5 (30–40) | 0.021 |
| Dose to LOC (mg kg−1) | 0.14 (0.13–0.16) | 1.22 (1.08–1.58) | NA |
| Dose to BIS 60 (mg kg−1) | 0.21 (0.18–0.28) | 1.49 (0.33) | NA |
| Total dose of anaesthetics (mg) | 84.90 (64.06–103.13) | 636 (532.70–812.70) | NA |
| Hypotension, n (%) | 18 (32.14) | 34 (58.62) | 0.005 |
| Bradycardia, n (%) | 3 (5.36) | 10 (17.24) | 0.089 |
| Injection pain, n (%) | 0 (0) | 3 (5.17) | 0.244 |
| Intraoperative awareness, n (%) | 0 | 0 | NA |
| PONV, n (%) | 6 (10.71) | 5 (8.62) | 0.705 |
| Hypoxia, n (%) | 3 (5.36) | 4 (6.90) | 1.000 |
| VAS>3, n (%) | 1 (1.79) | 5 (8.62) | 0.225 |
| Sore throat, n (%) | 2 (3.57) | 3 (5.17) | 1.000 |
Fig 2.
Percent of the time maintained in the target BIS value range and sedation scores in 30 min after surgery. (a) BIS values; (b) Ramsay Sedation Scores. Group R: patients received continuous i.v. infusion of remimazolam for anaesthesia induction and maintenance. Group P: patients received plasma target-controlled infusion of propofol for anaesthesia induction and maintenance. T0: baseline, T1: after induction, T2: after placement of the laryngeal mask, T3: before tourniquet inflation, T4: before tourniquet deflation, T5: after tourniquet deflation, T6: the end of surgery. ∗P<0.05. ∗∗P<0.01. ∗∗∗P<0.001. ∗∗∗∗P<0.0001.
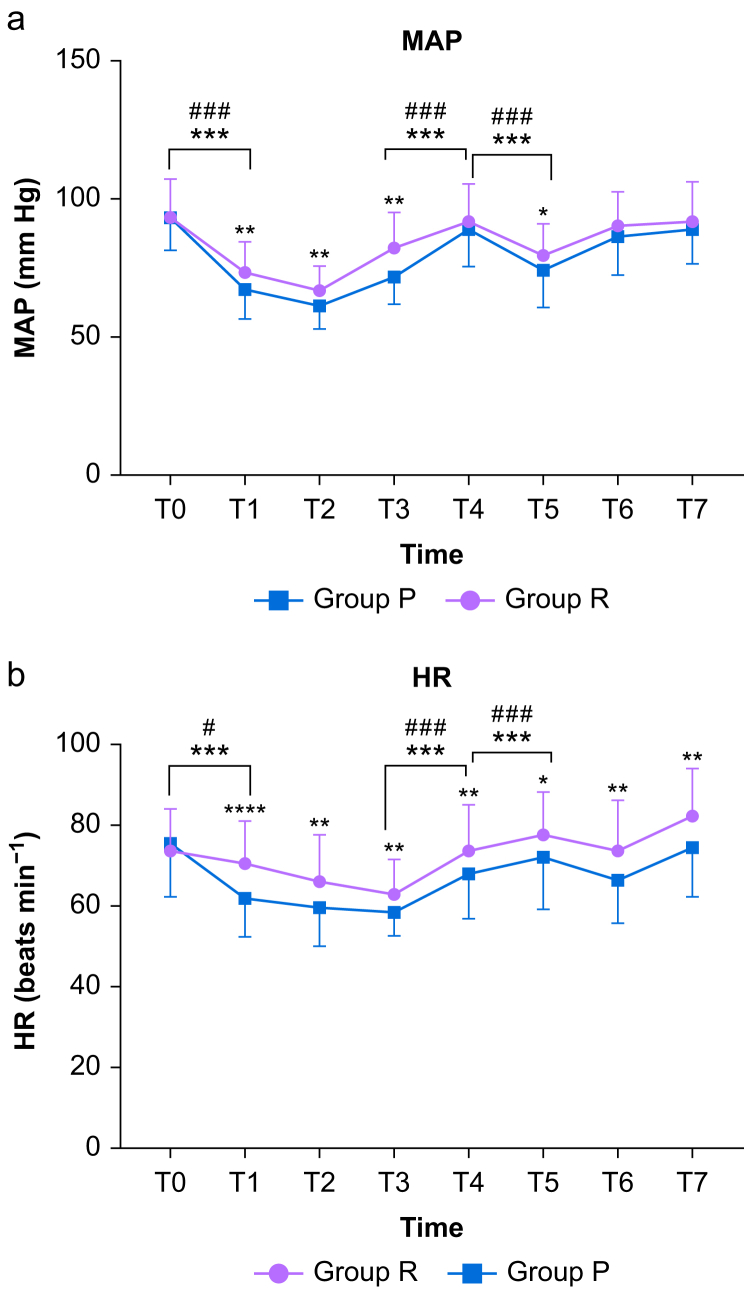
Compared with baseline, the MAP decreased sharply after anaesthesia induction in both groups (P<0.001, P<0.001) (Figure 3). Figure 3 also shows the fluctuations in the MAP and HR during the course of the procedures. The incidence of hypotension was significantly lower in the R group (32.14%) than in the P group (58.62%) (P=0.005, Table 3). No statistically significant difference in the incidence of injection pain was found between the two groups (0% vs 5.17%, P=0.244) (Table 3). No intraoperative awareness or other adverse reactions occurred in either group.
Fig 3.
(a) Mean arterial pressure (MAP) and (b) heart rate (HR) at different time points. Group R: patients received continuous i.v. infusion of remimazolam for anaesthesia induction and maintenance. Group P: patients received plasma target-controlled infusion of propofol for anaesthesia induction and maintenance. T0: baseline, T1: after induction, T2: after intubation, T3: before tourniquet inflation, T4: before tourniquet deflation, T5: after tourniquet deflation, T6: the end of surgery, T7: after extubation. ∗P<0.05. ∗∗P<0.01. ∗∗∗P<0.001. ∗∗∗∗P<0.0001. #P<0.05. ##P<0.01. ###P<0.001.
Discussion
This randomised clinical trial revealed that remimazolam anaesthesia was associated with statistically significant but clinically unimportant increases in QoR-15 scores on 1 day after short-day surgical procedures. The median difference in QoR-15 on POD1 compared with propofol was <6 (the minimal clinically important difference for the QoR-15 scale).26 These findings contrast with those of a previous study comparing remimazolam and propofol in patients undergoing urological surgery. Mao and colleagues17 found that the QoR-15 total scores were significantly reduced on POD1 and POD3 and showed a clinically relevant difference on POD1. This might be related to their inclusion of older patients (up to 84 yr old). Similar results were reported in another study for female patients scheduled for open thyroidectomy. The QoR-15 total scores on POD1 in the remimazolam group were slightly higher than in the propofol group, mainly including emotional status, physical independence, psychological support, and pain.16 However, these data were not significantly different.
Sleep quality is associated with patient comfort, daytime consequences, and recovery from illness or surgery.28,29 We found the number of awakenings on the first postoperative night was lower in the R group. The R group had a longer sleep duration on the first postoperative night, which may contribute to marginally better scores for physical independence and psychological support. Considering a recent systematic review, benzodiazepines extend stage 2 of non-rapid eye movement sleep, which improves sleep quality with no awakenings.30 Remimazolam, as a new ultra-short-acting benzodiazepine drug, could have similar effects and further studies are needed.
Remimazolam-based total i.v. anaesthesia can achieve sufficient anaesthetic depth for induction and maintenance.21,21, 31, 32, 33 In this study, the perioperative BIS values were kept between 50 and 60 in the two groups. Another trial suggested that general anaesthesia with BIS values of 50–59 can diminish propofol consumption and shorten the recovery time compared with maintaining BIS values of 40–49.22 After tourniquet deflation, the BIS values in the two groups transiently increased, which might be associated with a transient increase in the cerebral blood volume34 and a decrease in the blood drug concentration caused by peripheral blood redistribution.
The time to LOC in the R group was shorter than that in the P group and this is similar to other studies,16,20 possibly because of the administration method of propofol with gradual increase in the effect–site concentrations and sufentanil given in advance.32 In the absence of flumazenil antagonism, the time to eye opening, orientation recovery, extubation, and PACU stay were longer in the R group than in the P group. Although flumazenil administration was shown to rapidly reverse sedation after remimazolam infusion, and reduce the median time to 3.5 min,11,35,36 the impact of flumazenil on the quality of postoperative recovery remains unknown. These results indicate that we can taper the dose of remimazolam or discontinue injection earlier as the operation comes to an end.
Similar to previous studies,14,20 remimazolam anaesthesia provided stable haemodynamic conditions and a lower incidence of hypotension. We found no significant differences in the rates of bradycardia and injection pain between the groups. The incidence of PONV, hypoxia in the PACU, and postoperative sore throat in the R group was similar to that in the P group. Inconsistent with previous research in female patients,16 we did not observe a difference in VAS pain scores in PACU between the two groups. The anxiety and pain sensitivity of female patients may contribute to this difference.
However, this clinical trial has several limitations. First, we only recruited patients undergoing ambulatory arthroscopic meniscus repair, which is a relatively short procedure. The implications of remimazolam-based anaesthesia on the quality of recovery for prolonged major surgery are unknown. Second, owing to the rapid discharge of patients after day surgery and the low education level of some patients, the follow-up of QoR-15 questionnaires survey was completed by researchers using telephone calls. Although the researcher was blinded to the group assignment, some biases may occur. Third, sleep quality was evaluated using AIS and more objective tools are needed. Finally, this is a single-centre clinical study, so a multicentre large-sample study is needed to further verify the effect of remimazolam on the quality of recovery during day surgery.
In conclusion, remimazolam-based total i.v. anaesthesia was associated with small, clinically unimportant improvements in the quality of recovery after ambulatory arthroscopic surgery, but reduced adverse effects suggest that remimazolam may be an option for ambulatory arthroscopic surgery.
Authors’ contributions
Study design: LT, YL, XL
Ethics approval and registration: LT, XL
Patient recruitment and randomisation: XH
Data collection: YS, CX, XS, TW, LT
Data analysis: LT, CH, YL, XL
Drafting: LT, YL, XL
Critical revision of paper: all authors
Approved the final version of this manuscript: all authors
Declarations of interest
The authors declare that they have no conflicts of interest.
Funding
The Joint Project of Anesthesiology and Pharmacology of 2021 Peak Discipline Construction Project in the First Affiliated Hospital of Anhui Medical University (9001001813), and this work was partially funded by Bethune Charity Fund project (BCF-RF-WSQZTZJ- 202011-057).
Acknowledgements
The authors thank Meng Ning (Department of Anesthesiology, Hefei, China) for his great assistance during data collection.
Handling editor: Phil Hopkins
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bjao.2023.100237.
Contributor Information
Yao Lu, Email: luyao@ahmu.edu.cn.
Xuesheng Liu, Email: liuxuesheng@ahmu.edu.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Bailey C.R., Ahuja M., Bartholomew K., et al. Guidelines for day-case surgery 2019: guidelines from the association of anaesthetists and the British association of Day surgery. Anaesthesia. 2019;74:778–792. doi: 10.1111/anae.14639. [DOI] [PubMed] [Google Scholar]
- 2.Gebhardt V., Zawierucha V., Schoffski O., Schwarz A., Weiss C., Schmittner M.D. Spinal anaesthesia with chloroprocaine 1% versus total intravenous anaesthesia for outpatient knee arthroscopy: a randomised controlled trial. Eur J Anaesthesiol. 2018;35:774–781. doi: 10.1097/EJA.0000000000000794. [DOI] [PubMed] [Google Scholar]
- 3.Eger E.I., White P.F., Bogetz M.S. Clinical and economic factors important to anaesthetic choice for day-case surgery. Pharmacoeconomics. 2000;17:262. doi: 10.2165/00019053-200017030-00003. [DOI] [PubMed] [Google Scholar]
- 4.Gupta A., Stierer T., Zuckerman R., Sakima N., Parker S.D., Fleisher L.A. Comparison of recovery profile after ambulatory anesthesia with propofol, isoflurane, sevoflurane and desflurane: a systematic review. Anesth Analg. 2004;98:632–641. doi: 10.1213/01.ane.0000103187.70627.57. [, table of contents] [DOI] [PubMed] [Google Scholar]
- 5.Kumar G., Stendall C., Mistry R., Gurusamy K., Walker D. A comparison of total intravenous anaesthesia using propofol with sevoflurane or desflurane in ambulatory surgery: systematic review and meta-analysis. Anaesthesia. 2014;69:1138–1150. doi: 10.1111/anae.12713. [DOI] [PubMed] [Google Scholar]
- 6.Schraag S., Pradelli L., Alsaleh A.J.O., et al. Propofol vs. inhalational agents to maintain general anaesthesia in ambulatory and in-patient surgery: a systematic review and meta-analysis. BMC Anesthesiol. 2018;18:162. doi: 10.1186/s12871-018-0632-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Besnier E., Perdrix A., Gillibert A., et al. Postoperative hunger after outpatient surgery in patients anesthetized with propofol vs sevoflurane: a randomized-controlled trial. Can J Anaesth. 2020;67:550–559. doi: 10.1007/s12630-020-01584-w. [DOI] [PubMed] [Google Scholar]
- 8.Liu T., Gu Y., Chen K., Shen X. Quality of recovery in patients undergoing endoscopic sinus surgery after general anesthesia: total intravenous anesthesia vs desflurane anesthesia. Int Forum Allergy Rhinol. 2019;9:248–254. doi: 10.1002/alr.22246. [DOI] [PubMed] [Google Scholar]
- 9.Lee W.K., Kim M.S., Kang S.W., Kim S., Lee J.R. Type of anaesthesia and patient quality of recovery: a randomized trial comparing propofol-remifentanil total i.v. anaesthesia with desflurane anaesthesia. Br J Anaesth. 2015;114:663–668. doi: 10.1093/bja/aeu405. [DOI] [PubMed] [Google Scholar]
- 10.Wang J., Duan J., Xie C., Yu Y., Lu Y. Comparison between intravenous nalbuphine and lidocaine in reducing propofol-induced injection pain during gastroscopy: a randomized controlled trial. Pain Ther. 2020;9:563–571. doi: 10.1007/s40122-020-00188-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen X., Sang N., Song K., et al. Psychomotor recovery following remimazolam-induced sedation and the effectiveness of flumazenil as an antidote. Clin Ther. 2020;42:614–624. doi: 10.1016/j.clinthera.2020.02.006. [DOI] [PubMed] [Google Scholar]
- 12.Zhu X., Wang H., Yuan S., et al. Efficacy and safety of remimazolam in endoscopic sedation – a systematic review and meta-analysis. Front Med (Lausanne) 2021;8 doi: 10.3389/fmed.2021.655042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dong S.A., Guo Y., Liu S.S., et al. A randomized, controlled clinical trial comparing remimazolam to propofol when combined with alfentanil for sedation during ERCP procedures. J Clin Anesth. 2023;86 doi: 10.1016/j.jclinane.2023.111077. [DOI] [PubMed] [Google Scholar]
- 14.Liu T., Lai T., Chen J., et al. Effect of remimazolam induction on hemodynamics in patients undergoing valve replacement surgery: a randomized, double-blind, controlled trial. Pharmacol Res Perspect. 2021;9 doi: 10.1002/prp2.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hari Y., Satomi S., Murakami C., et al. Remimazolam decreased the incidence of early postoperative nausea and vomiting compared to desflurane after laparoscopic gynecological surgery. J Anesth. 2022;36:265–269. doi: 10.1007/s00540-022-03041-y. [DOI] [PubMed] [Google Scholar]
- 16.Choi J.Y., Lee H.S., Kim J.Y., et al. Comparison of remimazolam-based and propofol-based total intravenous anesthesia on postoperative quality of recovery: a randomized non-inferiority trial. J Clin Anesth. 2022;82 doi: 10.1016/j.jclinane.2022.110955. [DOI] [PubMed] [Google Scholar]
- 17.Mao Y., Guo J., Yuan J., Zhao E., Yang J. Quality of recovery after general anesthesia with remimazolam in patients undergoing urologic surgery: a randomized controlled trial comparing remimazolam with propofol. Drug Des Devel Ther. 2022;16:1199–1209. doi: 10.2147/DDDT.S359496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Constantin R., Soldatos D.G.D., Thomas J. Paparrigopoulos. Athens Insomnia Scale: validation of an instrument based on ICD-10 criteria. J Psychosom Res. 2000;48:555–560. doi: 10.1016/s0022-3999(00)00095-7. [DOI] [PubMed] [Google Scholar]
- 19.Soldatos C.R., Dikeos D.G., Paparrigopoulos T.J. The diagnostic validity of the Athens insomnia scale. J Psychosom Res. 2003;55:263–267. doi: 10.1016/s0022-3999(02)00604-9. [DOI] [PubMed] [Google Scholar]
- 20.Doi M., Hirata N., Suzuki T., Morisaki H., Morimatsu H., Sakamoto A. Safety and efficacy of remimazolam in induction and maintenance of general anesthesia in high-risk surgical patients (ASA Class III): results of a multicenter, randomized, double-blind, parallel-group comparative trial. J Anesth. 2020;34:491–501. doi: 10.1007/s00540-020-02776-w. [DOI] [PubMed] [Google Scholar]
- 21.Doi M., Morita K., Takeda J., Sakamoto A., Yamakage M., Suzuki T. Efficacy and safety of remimazolam versus propofol for general anesthesia: a multicenter, single-blind, randomized, parallel-group, phase IIb/III trial. J Anesth. 2020;34:543–553. doi: 10.1007/s00540-020-02788-6. [DOI] [PubMed] [Google Scholar]
- 22.Ning M., Sun Y., Zhang H., et al. Effects of different anesthetic depth during propofol anesthesia on postoperative recovery 24 h after arthroscopic day surgery: a randomized clinical trial. Front Pharmacol. 2022;13 doi: 10.3389/fphar.2022.972793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stark P.A., Myles P.S., Burke J.A. Development and psychometric evaluation of a postoperative quality of recovery score the QoR-15. Anesthesiology. 2013;118:8. doi: 10.1097/ALN.0b013e318289b84b. [DOI] [PubMed] [Google Scholar]
- 24.Bu X.S., Zhang J., Zuo Y.X. Validation of the Chinese version of the quality of recovery-15 score and its comparison with the post-operative quality recovery scale. Patient. 2016;9:251–259. doi: 10.1007/s40271-015-0148-6. [DOI] [PubMed] [Google Scholar]
- 25.Schuttler J., Eisenried A., Lerch M., Fechner J., Jeleazcov C., Ihmsen H. Pharmacokinetics and pharmacodynamics of remimazolam (CNS 7056) after continuous infusion in healthy male volunteers: Part I. pharmacokinetics and clinical pharmacodynamics. Anesthesiology. 2020;132:636–651. doi: 10.1097/ALN.0000000000003103. [DOI] [PubMed] [Google Scholar]
- 26.Myles P.S., Myles D.B. An updated minimal clinically important difference for the QoR-15 scale. Anesthesiology. 2021;135:934–935. doi: 10.1097/ALN.0000000000003977. [DOI] [PubMed] [Google Scholar]
- 27.Barrington M.J., Seah G.J., Gotmaker R., Lim D., Byrne K. Quality of recovery after breast surgery: a multicenter randomized clinical trial comparing pectoral nerves interfascial plane (pectoral nerves ii) block with surgical infiltration. Anesth Analg. 2020;130:1559–1567. doi: 10.1213/ANE.0000000000004371. [DOI] [PubMed] [Google Scholar]
- 28.Hillman D.R. Sleep loss in the hospitalized patient and its influence on recovery from illness and operation. Anesth Analg. 2021;132:1314–1320. doi: 10.1213/ANE.0000000000005323. [DOI] [PubMed] [Google Scholar]
- 29.Sattler S., Seddig D., Zerbini G. Assessing sleep problems and daytime functioning: a translation, adaption, and validation of the Athens Insomnia Scale for non-clinical application (AIS-NCA) Psychol Health. 2023;38:1006–1031. doi: 10.1080/08870446.2021.1998498. [DOI] [PubMed] [Google Scholar]
- 30.de Mendonca F.M.R., de Mendonca G., Souza L.C., et al. Benzodiazepines and sleep architecture: a systematic review. CNS Neurol Disord Drug Targets. 2023;22:172–179. doi: 10.2174/1871527320666210618103344. [DOI] [PubMed] [Google Scholar]
- 31.Chae D., Kim H.C., Song Y., Choi Y.S., Han D.W. Pharmacodynamic analysis of intravenous bolus remimazolam for loss of consciousness in patients undergoing general anaesthesia: a randomised, prospective, double-blind study. Br J Anaesth. 2022;129:49–57. doi: 10.1016/j.bja.2022.02.040. [DOI] [PubMed] [Google Scholar]
- 32.Lohmer L.L., Schippers F., Petersen K.U., Stoehr T., Schmith V.D. Time-to-event modeling for remimazolam for the indication of induction and maintenance of general anesthesia. J Clin Pharmacol. 2020;60:505–514. doi: 10.1002/jcph.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dai G., Pei L., Duan F., et al. Safety and efficacy of remimazolam compared with propofol in induction of general anesthesia. Minerva Anestesiol. 2021;87:1073–1079. doi: 10.23736/S0375-9393.21.15517-8. [DOI] [PubMed] [Google Scholar]
- 34.Khandelwal A., Srivastava A., Nayak S.S., Prabhakar S., Sinha S. Transient neurological dysfunction and intracranial hypertension after tourniquet deflation in a patient with a head injury: a case report. A A Pract. 2021;15 doi: 10.1213/XAA.0000000000001486. [DOI] [PubMed] [Google Scholar]
- 35.Murata H., Yokoyama A., Hara T. Remimazolam and low-dose flumazenil for awake craniotomy. J Anesth. 2022;36:789–790. doi: 10.1007/s00540-022-03103-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoshida A., Kurata S., Kida K., Tsubokawa T. Anesthetic management for the sleep-awake-sleep technique of awake craniotomy using a novel benzodiazepine remimazolam and its antagonist flumazenil. JA Clin Rep. 2021;7:14. doi: 10.1186/s40981-021-00417-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.