Abstract
Oxytocin (OT) is a neuropeptide critically involved in social cognition and behavior. Intranasal administration of OT has modulatory effects on both the brain and behavior with potential for therapeutic benefit, especially in individuals with deficits in socioemotional functions. Intranasal OT effects have been well-investigated in younger adults as well as in a variety of clinical populations (e.g., autism, schizophrenia), but there is comparatively less investigation of its function in older adults. To foster more research on OT and aging, the following dataset was made publicly available, which includes data from generally healthy younger (n = 44, age range = 18-31 years [M(SD) = 22.4 (3.0)], 48% female) and older adults (n = 43, age range = 63-81 years [M(SD)= 71.1 (5.3)], 56% female) who self-administered a single dose (24 international units) of either intranasal OT or a placebo (IND 100,860; NCT01823146). The study adopted a randomized, double-blind, between-subject design. The dataset consists of anatomical and functional resting-state neuroimaging scans acquired after nasal spray administration as well as study-specific phenotypic and demographic data. This dataset using both OT administration and neuroimaging is unique in its size and inclusion of both younger and older adults as well as women and men. This data has resulted in published work on OT modulation of cognition, behavior, and neural activation/connectivity. Open access to this data will provide the scientific community with the opportunity to investigate individual differences in the neurocognitive effects of single-dose OT in younger and older adults.
Keywords: Neuroimaging, resting state, fMRI, Cognition, Alzheimer's risk, Neuropeptide, Demographics, ApoE
Specifications Table
| Subject | Psychology |
| Specific subject area | Neuropeptide administration research (Oxytocin (OT) vs. Placebo (PL), Between-subjects) in healthy younger and older women and men; cognitive and neurobiological measures. |
| Data format | Raw |
| Type of data |
|
| Data collection | Data was collected between August 2013 and October 2014. Adults aged 18-31 and 63-81 years were prescreened for study eligibility via phone, during which demographic and cognitive data was collected. Eligible participants then came to the University of Florida (UF) for an in-person screening session during which cognitive data was collected along with blood and saliva samples. Participants returned for an in-person full session during which they self-administered the intranasal spray (OT or PL; randomized, double-blind procedure) and underwent MRI scanning (i.e., T1-weighted structural scan; resting state fMRI scan). Neuroimaging data were collected using a 3T Philips Achieva MRI Scanner. |
| Data source location | Participants were recruited around the Gainesville area in Florida, USA (GPS coordinates: 29.6446° N, 82.3535° W) and attended study sessions at UF. |
| Data accessibility |
Repository name: OpenNeuro Data identification number: doi:10.18112/openneuro.ds004725.v1.0.1 Direct URL to data:https://openneuro.org/datasets/ds004725/versions/1.0.1 Instructions for accessing these data: Data can be accessed via the above link. Please cite this paper and the OpenNeuro repository for any analyses conducted on this data. |
| Related research article | P. Liu, T. Lin, D. Feifel, N.C. Ebner, Intranasal oxytocin modulates the salience network in aging, Neuroimage. 253 (2022) 119045. https://doi.org/10.1016/j.neuroimage.2022.119045. |
1. Value of the Data
-
•
These neuroimaging, phenotypic, and demographic data collected from a single-dose OT intranasal administration trial will promote open investigation on the impact of neuropeptides on the brain and behavior in adulthood and aging. Data can be used to examine cognition and brain regions/networks sensitive to a single-dose, intranasal administration of OT, as well as explore individual differences in the modulatory effects of OT.
-
•
We encourage the use of these data to promote open science, data harmonization, and secondary data analysis. These data also include information on apolipoprotein E (ApoE) biomarkers and cognitive status to address future research questions on OT effects among individuals at increased risk for developing Alzheimer's Disease and Related Dementias (ADRD).
-
•
Sharing these data is an important step toward delineating the role of OT in shaping social cognition in adulthood and aging. These data will be highly beneficial to the broader research community with an interest in the effects of neuropeptides on the brain and cognition and have the unique potential to generate new knowledge on the effects of intranasal OT administration on brain and cognitive function among older adults, a population that is still understudied in this area of research.
2. Data Description
Here we introduce publicly available neuroimaging (i.e., anatomical and resting-state functional scans), phenotypic (e.g., plasma oxytocin [OT] and vasopressin [AVP] levels, genotyping), and demographic (e.g., age, sex) data, including study design details from a single-dose OT intranasal administration trial (NCT01823146) conducted by the Social-Cognitive and Affective Development lab (PI: Ebner) at the University of Florida (UF) [1]. Data from this dataset has been used in publications that investigated the modulatory effect of single-dose OT intranasal administration on cognition, behavior, and neural activation/connectivity [2], [3], [4], [5], [6], [7], [8], [9], [10], [11]. By sharing this data, we seek to promote open investigation into the impact of neuropeptides on the brain and behavior in adulthood and aging.
This data has unique potential to generate knowledge on the modulatory, and potential therapeutic, effects of intranasal OT administration in older adults and comprises both women and men. Older women and men are a population understudied regarding the effect of OT on brain and behavioral function despite evidence that OT impacts functions that change with age. For example, previous research has shown that OT modulates trust-related evaluations and decisions [7], dynamic social and emotional information processing [9,12], and functional brain networks relevant to decision-making, including the salience network and the default mode network [2,3]. Making these OT-related data publicly available is an important step toward enhancing scientific knowledge about the role of neuropeptides in shaping social cognition and behavior in adulthood and aging [12]. Additionally, OT research in Alzheimer's Disease and Related Dementias (ADRD) is very limited to date [13] and currently, it is unclear if intranasal OT administration attenuates cognitive impairments in aging and ADRD [14]. This dataset includes information on apolipoprotein E (ApoE) biomarkers (i.e., genotyping for SNP RS429358 and RS7412; ApoE alleles) and cognitive status (e.g., Digit Symbol Substitution Test [DSST] [15]; Rey Auditory Verbal Learning Test [RAVLT] [16], Telephone Interview for Cognitive Status [TICS] [17]) to address future research questions on OT effects among older adults including those with enhanced ADRD risk. Through this public data repository, we will critically expand our lab contributions toward open science, data harmonization, and secondary data analysis.
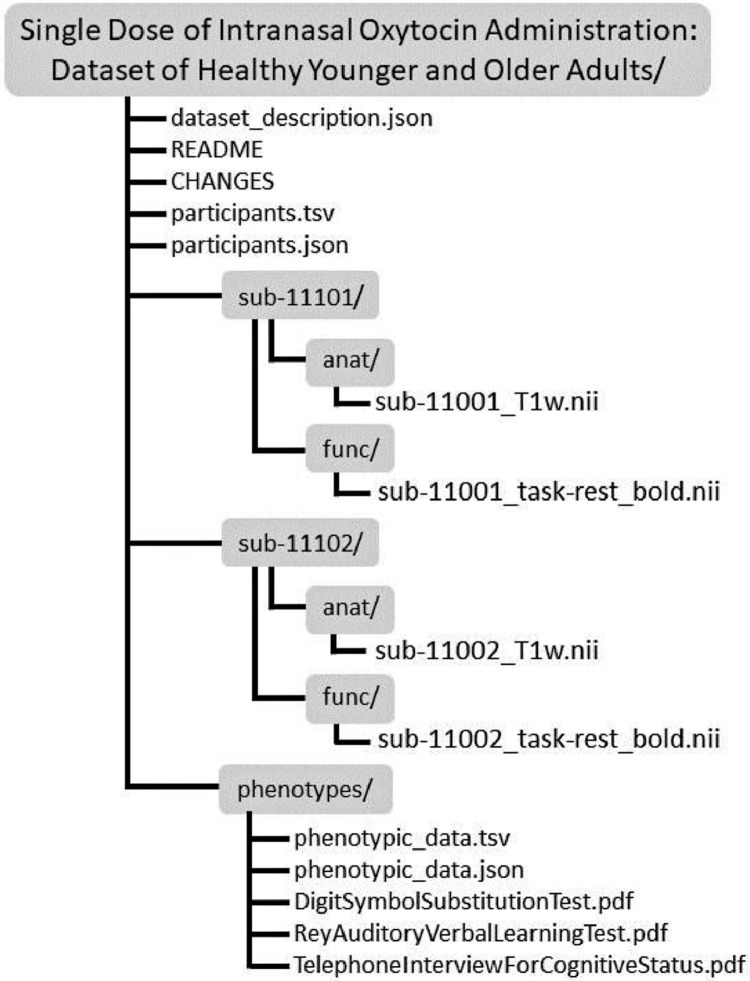
The dataset described in this article can be found on the public neuroimaging repository OpenNeuro under the title “Single Dose Intranasal Oxytocin Administration: Dataset of Healthy Younger and Older Adults” [1]. This uploaded data includes anatomical and resting-state fMRI scans, participant demographics, and participant phenotypic data (e.g., cognitive measures, plasma OT/AVP levels, ApoE genotyping) for a total of 87 younger and older participants (see Participants subsection below). All data is raw and organized in line with the Brain Imaging Data Structure (BIDS) version 1.7.0 specifications [18] (Fig. 1).
Fig. 1.
Directory organization of all folders and files in the dataset.
The root level of the study directory contains the following files:
-
•
dataset_description.json: JSON file containing general dataset description (e.g., authors, funding sources, acknowledgments).
-
•
README.txt: Text file providing a brief overview of the project and data (e.g., participants, study design).
-
•
CHANGES.txt: Text file describing all changes made to the dataset with the date of change.
-
•
participant.tsv: Tab-separated file containing demographics of each participant (i.e., chronological age, sex, handedness, treatment group [OT or PL], years of education, race/ethnicity, Body Mass Index [BMI]).
-
•
participant.json: JSON file accompanying participant.tsv that describes all columns and values.
Subject directories (sub-11001/, sub-11002/, etc.) at the root level lead to neuroimaging anatomical and resting-state functional scans for each participant, where available. In each subject folder is an anat/ and func/ folder, with respective scans in each location (e.g., anat/sub-11001_T1w.nii, func/sub-11001_task-rest_bold.nii). Also, at the root level are JSON files accompanying each scan type (e.g., T1w.json, task-rest_bold.json) to describe scan parameters related to image acquisition. Each participant has the same scan parameters; thus, these files apply to each participant.
The phenotypes/ directory contains the tab-separated file phenotypics_data.tsv as well as the accompanying JSON phenotypics_data.json to describe phenotypic data and values in detail. See Table 1 for an overview of the phenotypic data provided. In addition, this directory contains PDFs of the cognitive measures used (i.e., DigitSymbolSubstitutionTest.pdf, ReyAuditoryVerbalLearningTest.pdf, and TelephoneInterviewForCognitiveStatus.pdf).
Table 1.
Description of phenotypic data.
| Phenotypic Data Column | Description |
|---|---|
| participant_id | Subject identification number |
| TICS_SCORE | Telephone Interview for Cognitive Status score |
| DIGIT_TOTAL | Total score for Digit Symbol Substitution Task |
| DIGIT_CORRECT | Total correct items for Digit Symbol Substitution Task |
| RAVLT_TOTAL | Total score for Rey Auditory Verbal Learning Test |
| RAVLT_CORRECT | Total correct items for Rey Auditory Verbal Learning Test |
| CURRENT_MEDICATION_NAME | Names of current medications |
| MEDICATION_STATUS_AT_SCAN | Use of medication within 24 hours of MRI scan |
| CONTRACEPTION_USE_AT_SCAN | Use of contraception at the time of study participation (younger women only) |
| TIMING_BIOLOGICAL_SAMPLING_RELATIVE_TO_TREATMENT | Number of days between blood/saliva sampling and MRI/intranasal administration |
| TIME_OF_DAY_BLOOD_SAMPLING | Time of day (AM) of blood sampling |
| BLOOD_MENSTRUAL_CYCLE_PHASE | Phase of menstrual cycle (younger women only) |
| BLOOD_OXYTOCIN_LEVELS | Level of plasma OT |
| BLOOD_VASOPRESSIN_LEVELS | Level of plasma AVP |
| TIME_OF_DAY_SALIVARY_SAMPLING | Time of day (AM) of saliva sampling |
| SALIVARY_SNP_RS429358 | Genotyping of SNP RS429358 |
| SALIVARY_SNP_RS7412 | Genotyping of SNP RS7417 |
| SALIVARY_APOE_GENOTYPE | ApoE allele based on SNP RS429358 and SNP RS7417 |
| TIME_OF_DAY_RESTING_SCAN | Time of day (AM) for resting-state scan |
2. Experimental Design, Materials, and Methods
Participants. This study examined the effects of a single-dose (24 international units [IU] intranasal OT vs. PL administration on brain, cognitive, and behavioral outcomes in younger (n = 44, age range = 18-31 years [M(SD) = 22.4 (3.0)], 48% female, 55% in OT group) and older (n = 43, age range = 63-81 years [M(SD) = 71.1 (5.3)], 56% female, 49% in OT group) adults. Healthy younger participants were recruited through the UF Psychology Department undergraduate participant pool (i.e., SONA), HealthStreet, handouts, and flyers. Healthy older participants were recruited through HealthStreet and UF participant registries. Both younger and older participants were compensated a total of $65 for study completion plus a bonus depending on task performance.
No participant had neurological or psychiatric disorders, and all participants were able to understand and give informed written consent for this study. All older participants scored ≥ 30 on the Telephone Interview for Cognitive Status [17]. Only white, English-speaking adults were included in this study. All older women included in the study were postmenopausal whereas all younger women were premenopausal. Individuals with contraindications for MRI or intranasal OT spray self-administration were excluded for safety. Individuals with certain metal implants or pacemakers; who were pregnant or breastfeeding; excessively smoked or drank alcohol; and/or had severe or progressive medical illness(es) were not eligible for this study.
Experimental Design and Procedures. The study was conducted in the Department of Psychology, the Institute on Aging, and the McKnight Brain Institute at UF from August 2013 to October 2014. This study utilized a randomized, double-blind, between-group design that comprised 1) an initial phone prescreening to determine study eligibility (∼30 min), 2) an in-person screening session (∼45 min), and 3) an in-person full session (∼3 hrs). The study followed a 2 (age: Younger, Older) X 2 (sex: Female, Male) X 2 (treatment: OT, PL) design. Only the acquisition of measures provided in this dataset is described herein. Additional methods and measures are reported elsewhere [2], [3], [4].
During an initial phone prescreening, older participants underwent the Telephone Interview for Cognitive Status to screen for cognitive decline [17]. All participants completed an MRI Eligibility Form and a study-specific Health Screening and Demographics Form to assess demographic information, present health conditions, and health history. Based on these measures, eligibility for the study was determined. Eligible participants were then scheduled for an in-person screening session and a full session on campus. All participants provided informed written consent before enrollment. All in-person sessions took place at ∼8:00 AM. Participants were also instructed to stay hydrated and abstain from substance use and caffeine for 24 hours and from food, exercise, and sexual activity for at least two hours before the sessions.
During the in-person screening session, participants completed an intake interview and cognitive measures that included the Digit Symbol Substitution Test (DSST), which measures sensorimotor processing speed [15], and the Rey Auditory Verbal Learning task (RAVLT), which measures short-term verbal memory [16] among other questionnaires [2], [3], [4]. For female participants, menstrual cycle phase data was also obtained via self-report.
Saliva (i.e., ApoE status) and blood sampling (i.e., plasma OT and AVP levels) were conducted along with a health review by a clinician. Saliva samples were collected using the OraGene DNA Self Collection Kit OG-500 (http://www.dnagenotek.com/ROW/products/OG500.html); participants salivated approximately 2mL into a collection tube that is part of the kit. Saliva samples were assayed by the Translational Genomics Research Institute (PI: Huentelman) between February and April 2022. Blood plasma was frozen to –70°C directly after collection and only thawed immediately before assay. OT (unextracted) and AVP were measured via Enzyme Immunoassay (EIA), purchased from Enzo Life Sciences, Inc. (Farmingdale, New York); plasma samples were run at the same time with inter- and intra-assay coefficients of variation less than 8%.
Participants eligible for full study participation returned to campus at a later date for the in-person full session. During this session, participants underwent further MRI safety determination and completed another intake interview. Following recommendations for the standardized administration of intranasal OT [19], participants self-administered 24 IU (i.e., one puff per nostril) of OT or PL, which contained the same ingredients as the OT spray except for the synthetic OT (IND 100,860). Compounding, dispensing, and randomization were overseen by the dispensing pharmacy.
Before MRI scanning, participants received instructions about the MRI procedure as well as an overview of the experimental tasks they would complete inside the scanner. Participants were settled into the 3T MRI scanner ∼45 min after self-administration of OT or PL. Participants underwent anatomical image acquisition followed by functional image acquisition across four tasks, described elsewhere [7,9], including an eyes-open resting-state scan [2], [3], [4]. The session concluded with participant debriefing and compensation.
Neuroimaging Data Acquisition. A 3T Philips Achieva MRI Scanner with a 32-channel head coil at the McKnight Brain Institute was used to acquire brain images. Participants were placed in the MRI scanner with their heads comfortably positioned and stabilized with cushions to reduce head motion. Anatomical data was collected in the first 10 min of the MRI scanning for anatomical details. These anatomical scans included a high-resolution three-dimensional T1w scan using an MP-RAGE sequence (sagittal plane, TR/TE/TI = 7/3.2/2750 ms, flip angle = 8°; in-plane FOV = 240 × 240 mm; imaging matrix 240 × 240; 170 contiguous sagittal slices with 1 mm slice thickness, 1 × 1 × 1 mm3 isotropic voxels).
For all functional scans, a single-shot gradient echo, echo-planar imaging sequence sensitized to blood oxygenation level-dependent (BOLD) contrast (TR = 2000 ms, TE = 30 ms, flip angle = 90°, in-plane FOV = 240 × 240 mm, 80 × 80 matrix size, 3 × 3 × 3 mm3 isotropic voxels, 38 interleaved axial slices [ascending 1, 3, 5, etc.], zero inter-slice gap) was used for whole-brain fMRI coverage. Every functional run started with 4 dummy scans (each lasting 1 TR [2000 ms] which is 8 seconds); each run ended with a “fade out” period of 4 dummy scans (each lasting 1 TR [2000 ms] which is 8 seconds). The resting-state scan took place between 70–90 min after spray administration and lasted about 8 min with 240 time points acquired. Participants lay supine and were instructed to relax and look at a white fixation cross on a black screen. Before uploading to OpenNeuro, all data was BIDS formatted, and facial features were removed from anatomical data to de-identify participants [20].
3. Limitations
Some data was not included in this repository due to technical issues, which resulted in missing or corrupted files, as well as study attrition. Several participants did not complete the resting-state functional scan, which was the last scan in the imaging sequence, due to time restrictions (e.g., technical difficulties earlier on in the session; late arrival of participant) and thus are not included in this dataset. Any missing phenotype data are designated with “n/a” (i.e., not applicable) in the dataset. Additionally, there were a variable number of days between in-person sessions for each participant due to scheduling logistics. Days between sessions are listed in the dataset for each participant for use in covariate analyses.
Ethics Statement
Written informed consent was obtained from all participants. All data was de-identified. This research was carried out per the Declaration of Helsinki; was approved by the UF Institutional Review Board (IRB#39–2013); approved by the FDA (IND 100,860), and pre-registered with ClinicalTrials.gov (NCT01823146).
CRediT authorship contribution statement
Marilyn Horta: Conceptualization, Validation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing, Supervision, Funding acquisition, Visualization. Rebecca Polk: Data curation, Software, Formal analysis, Investigation, Writing – original draft, Writing – review & editing, Funding acquisition, Conceptualization, Visualization, Validation. Natalie C. Ebner: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Resources, Writing – original draft, Writing – review & editing, Supervision, Project administration, Funding acquisition.
Acknowledgements
This work was supported by the UF Clinical and Translational Science pilot award [NIH/NCATS, UL1 TR000064 to N.C.E.]; the Scientific Research Network on Decision Neuroscience and Aging pilot award [NIH/NIA, R24 AG039350 to N.C.E.]; the NIA Pre-Doctoral Fellowship on Physical, Cognitive and Mental Health in Social Context [T32 AG020499 to M.H. and R.P.]; the Scientific Research Network on Decision Neuroscience and Aging Open Data Award [NIH/NIA, R24-AG054355 to M.H., R.P., and N.C.E.]; an NIH Diversity Supplement [NIA 3R01AG072658-01A1S1 to N.C.E. and M.H.]; an NIH grant [NIA R01AG059809 to N.C.E.]; a U.S. Navy Office of Naval Research grant [N00014-21-1-2201 to N.C.E]; the UF Psychology Department; the Center for Cognitive Aging and Memory; and the Claude D. Pepper Older Americans Independence Center. A portion of this work was performed in the McKnight Brain Institute at the National High Magnetic Field Laboratory's Advanced Magnetic Resonance Imaging and Spectroscopy Facility, which is supported by National Science Foundation Cooperative Agreement No. DMR-1157490 and the State of Florida.
The authors would like to thank Gabriel Maura, Andrew Varan, and the research teams from the Social-Cognitive and Affective Development Lab and the Institute on Aging at UF for assistance in study implementation, data collection, and data management. We would like to thank Tammy Nicholson for their roles in brain imaging; David Feifel for serving as the sponsor for the OT/PL nasal spray; David Feifel, Hakan Fischer, and Kai MacDonald for discussions on central study ideas; Kaat Alaerts for discussions surrounding data sharing.
Declaration of Competing Interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data Availability
References
- 1.Horta M., Polk R., Ebner N.C. 2023. Single Dose Intranasal Oxytocin Administration: Data from Healthy Younger and Older Adults. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu P., Lin T., Feifel D., Ebner N.C. Intranasal oxytocin modulates the salience network in aging. Neuroimage. 2022;253 doi: 10.1016/j.neuroimage.2022.119045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ebner N.C., Chen H., Porges E., Lin T., Fischer H., Feifel D., Cohen R.A. Oxytocin's effect on resting-state functional connectivity varies by age and sex. Psychoneuroendocrinology. 2016;69:50–59. doi: 10.1016/j.psyneuen.2016.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen H., Zhao B., Porges E., Cohen R.A., Ebner N.C. Edgewise and subgraph-level tests for brain networks. Statis. Med. 2016;35:4994–5008. doi: 10.1002/sim.7039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin T., Pehlivanoglu D., Ziaei M., Liu P., Woods A.J., Feifel D., Fischer H., Ebner N.C. Age-related differences in amygdala activation associated with face trustworthiness but no evidence of oxytocin modulation. Front. Psychol. 2022;13 doi: 10.3389/fpsyg.2022.838642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rung J.M., Kidder Q.A., Horta M., Nazarloo H.P., Carter C.S., Berry M.S., Ebner N.C. Associations between alcohol use and peripheral, genetic, and epigenetic markers of oxytocin in a general sample of young and older adults. Brain Behav. 2022;12 doi: 10.1002/brb3.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frazier I., Lin T., Liu P., Skarsten S., Feifel D., Ebner N.C. Age and intranasal oxytocin effects on trust-related decisions: behavioral and brain evidence. Psychol. Aging. 2020 doi: 10.1037/pag0000545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ebner N.C., Lin T., Muradoglu M., Weir D.H., Plasencia G.M., Lillard T.S., Pournajafi-Nazarloo H., Cohen R.A., Sue Carter C., Connelly J.J. Associations between oxytocin receptor gene (OXTR) methylation, plasma oxytocin, and attachment across adulthood. Int. J. Psychophysiol. 2019;136:22–32. doi: 10.1016/j.ijpsycho.2018.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horta M., Ziaei M., Lin T., Porges E.C., Fischer H., Feifel D., Spreng R.N., Ebner N.C. Oxytocin alters patterns of brain activity and amygdalar connectivity by age during dynamic facial emotion identification. Neurobiol. Aging. 2019;78:42–51. doi: 10.1016/j.neurobiolaging.2019.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Plasencia G., Luedicke J.M., Nazarloo H.P., Carter C.S., Ebner N.C. Plasma oxytocin and vasopressin levels in young and older men and women: Functional relationships with attachment and cognition. Psychoneuroendocrinology. 2019;110 doi: 10.1016/j.psyneuen.2019.104419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ebner N.C., Horta M., Lin T., Feifel D., Fischer H., Cohen R.A. Oxytocin modulates meta-mood as a function of age and sex. Front. Aging Neurosci. 2015;7 doi: 10.3389/fnagi.2015.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Polk R., Horta M., Lin T., Porges E., Ojeda M., Nazarloo H.P., Carter C.S., Ebner N.C. Evaluating the neuropeptide–social cognition link in ageing: the mediating role of basic cognitive skills. Philos. Trans. R. Soc. B. 2022;377 doi: 10.1098/rstb.2021.0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horta M., Pehlivanoglu D., Ebner N.C. The role of intranasal oxytocin on social cognition: an integrative human lifespan approach. Curr. Behav. Neurosci. Rep. 2020:1–18. doi: 10.1007/s40473-020-00214-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ebner N.C., Kamin H., Diaz V., Cohen R.A., MacDonald K. Hormones as “difference makers” in cognitive and socioemotional aging processes. Front. Psychol. 2015;5:1595. doi: 10.3389/fpsyg.2014.01595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wechsler D. Psychological Corporation; New York: 1981. WAIS-R: Manual : Wechsler Adult Intelligence Scale–Revised. [Google Scholar]
- 16.Rey A. Presses Universitaires de France; 1964. L'examen Clinique en Psychologie. [Google Scholar]
- 17.Brandt J., Spencer M., Folstein M. The telephone interview for cognitive status. Neuropsychiatry Neuropsychol. Behav. Neurol. 1988;1:111–117. [Google Scholar]
- 18.Gorgolewski K.J., Auer T., Calhoun V.D., Craddock R.C., Das S., Duff E.P., Flandin G., Ghosh S.S., Glatard T., Halchenko Y.O., Handwerker D.A., Hanke M., Keator D., Li X., Michael Z., Maumet C., Nichols B.N., Nichols T.E., Pellman J., Poline J.-B., Rokem A., Schaefer G., Sochat V., Triplett W., Turner J.A., Varoquaux G., Poldrack R.A. The brain imaging data structure, a format for organizing and describing outputs of neuroimaging experiments. Sci. Data. 2016;3 doi: 10.1038/sdata.2016.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guastella A.J., Hickie I.B., McGuinness M.M., Otis M., Woods E.A., Disinger H.M., Chan H., Chen T.F., Banati R.B. Recommendations for the standardisation of oxytocin nasal administration and guidelines for its reporting in human research. Psychoneuroendocrinology. 2013;38:612–625. doi: 10.1016/j.psyneuen.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 20.Gulban O.F., Nielson D., Lee John, Poldrack R., Gorgolewski C., Vanessasaurus C.Markiewicz. 2022. poldracklab/pydeface: PyDeface v2.0.2. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.