Abstract
Percutaneous treatments, including thoracic duct embolization (TDE) and thoracic duct disruption (TDD), are reportedly effective and safe alternatives to surgical thoracic duct ligation for refractory chylothorax. When catheterization of the thoracic duct is impossible, TDD can be performed as long as the thoracic duct can be opacified by lymphangiography. However, no report has described percutaneous treatment when the thoracic duct cannot be visualized. In this case, TDE was not feasible because intranodal lymphangiography failed to opacify the thoracic duct: cannulation was not achieved. Therefore, we aimed to disrupt the thoracic duct by puncturing the retrocrural area where it was anatomically suspected to be located. Chylothorax improved thereafter. In cases without lymphangiographic thoracic duct visualization, TDD by puncturing the retrocrural space might improve refractory chylothorax.
Keywords: Chylothorax, Lymphangiography, Thoracic duct, Thoracic duct disruption
Introduction
Chylothorax, primarily caused by damage to lymphatic ducts, is characterized by the presence of lymphatic fluid in the pleural cavity. In recent years, percutaneous treatments including thoracic duct embolization (TDE) and thoracic duct disruption (TDD) have been reported as effective and safe alternatives to surgical thoracic duct ligation for refractory chylothorax [[1], [2]–3]. Nevertheless, these treatments can be challenging in cases where the thoracic duct cannot be visualized. This report describes a case of refractory chylothorax that was improved through puncture of the retrocrural space, where the thoracic duct was anatomically suspected to be located, even though the thoracic duct could not be visualized using lymphangiography.
Case report
A 40-year-old man, previously diagnosed with left renal cell carcinoma and who underwent a left nephrectomy 5 years prior, was diagnosed with pleural dissemination and para-aortic lymph node metastasis during the first postoperative year. He subsequently received multiple molecular targeted therapies. One year before referral to our institution, CT revealed bilateral pleural effusions. Diagnostic thoracocentesis yielded milky-colored fluid, consistent with a diagnosis of chylothorax. Over time, the dyspnea associated with pleural effusion worsened, necessitating the drainage of approximately 1300 mL per drainage session, twice a week. Despite conservative treatment and 2 sessions of intranodal lymphangiography, the chylothorax did not improve. Neither lymphangiogram depicted the thoracic duct. The patient was subsequently transferred to our institution for additional lymphatic intervention.
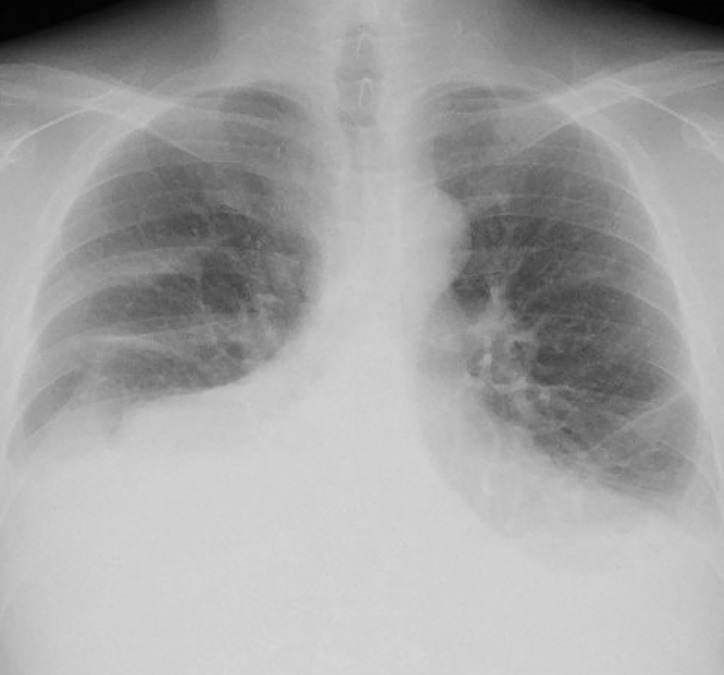
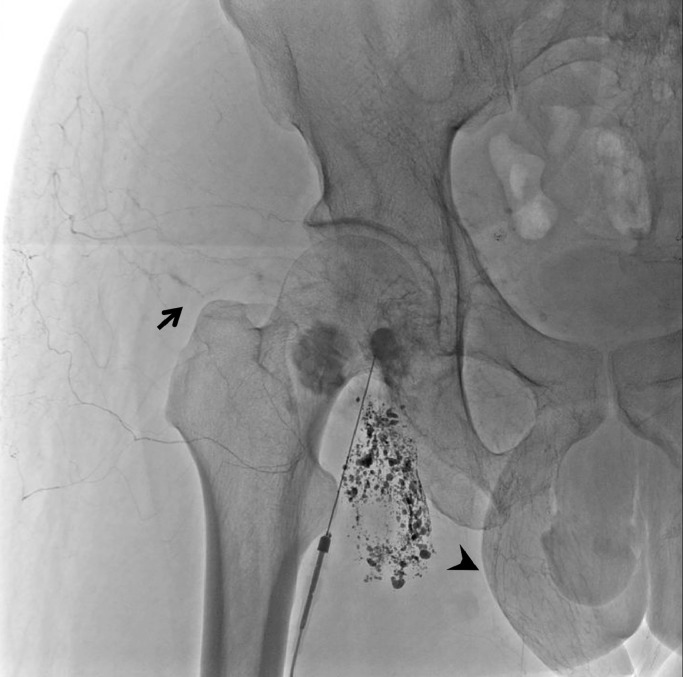
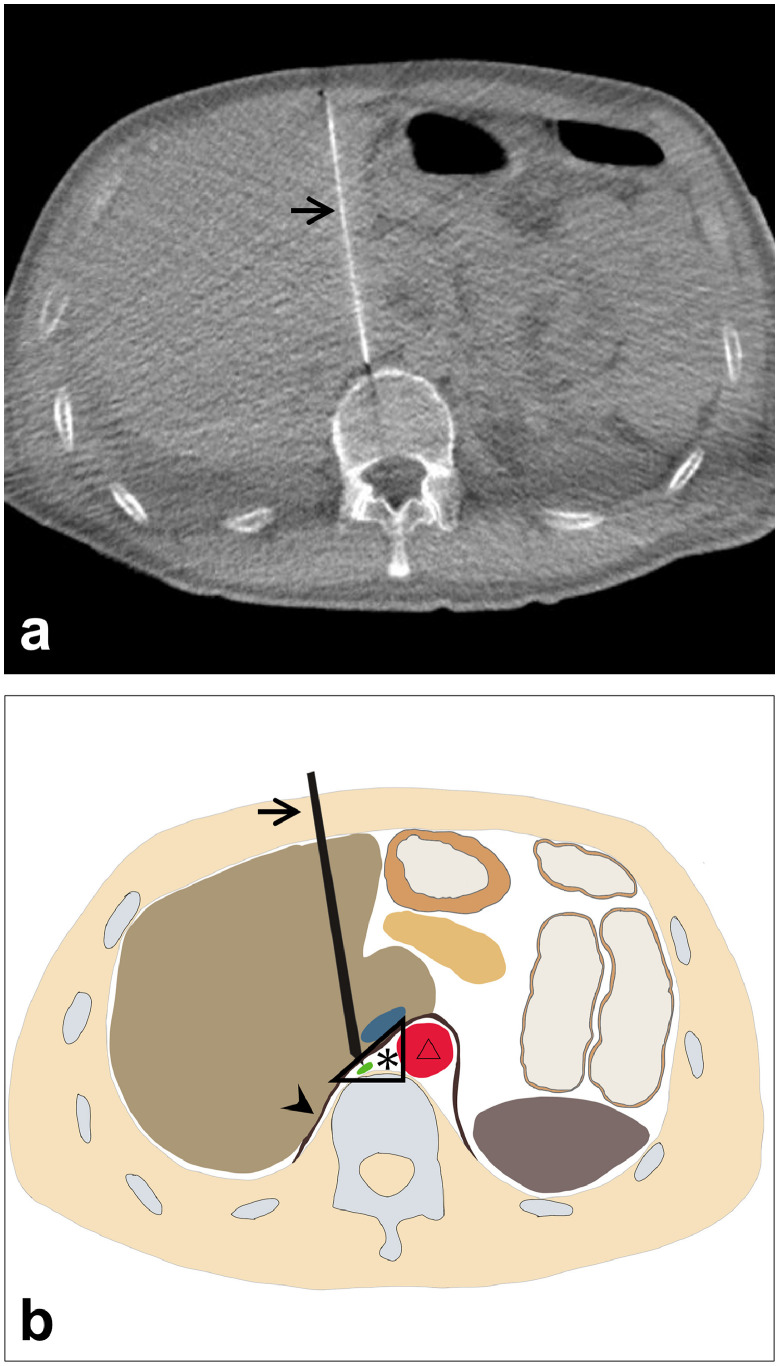
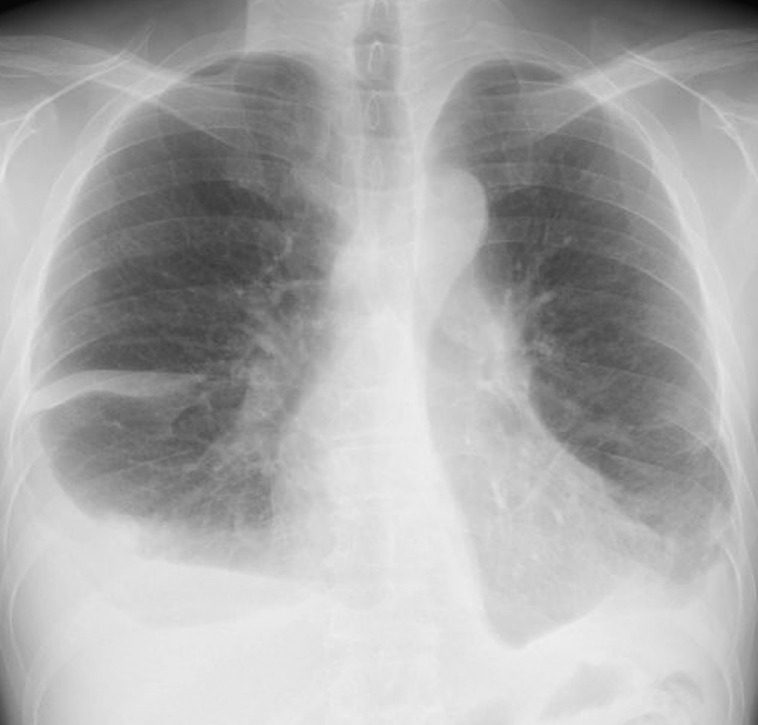
After transfer to our institution, a chest X-ray showed massive bilateral pleural effusion (Fig. 1). A left chest tube was inserted, leading to continuous draining of 900-1500 mL/d of milky fluid, consistent with chylothorax (fluid analysis: 95% lymphocytes; 1.6 mg/dL total protein; 1159 mg/dL triglycerides). Because conservative treatment, including a low-fat diet, failed to reduce chylothorax, and because his serum albumin was as low as 1.6 g/dL, we attempted TDE on the fourth day after admission. Lymphatic tract evaluation by lymphangiography from multiple lymph nodes revealed no identification or location of the thoracic duct. Intranodal lymphangiography only opacified lymphatic vessels toward the pelvis and lateral abdomen and anastomoses with veins, but not the thoracic duct (Fig. 2). We also considered retrograde venous access to catheterize into a venous angle. Nevertheless, we were unable to cannulate a catheter into the thoracic duct. Consequently, TDE could not be performed because it was not possible to cannulate a microcatheter into the invisible thoracic duct. Therefore, we aimed at disrupting the thoracic duct by puncturing the area in which it was anatomically suspected to be located. Under CT guidance, the dorsal side of the right crus of the diaphragm and the right next to the abdominal aorta was punctured several times using a 21G Chiba needle (Fig. 3). After this procedure, the fluid from the chest tube became reddish and decreased to less than 100 mL/d with no complications. Inferring that TDD had been successful, the chest tube was removed after performing pleurodesis using OK-432 (Picibanil; Chugai Pharmaceutical Co., Ltd., Tokyo, Japan) solution (5KE/ 5 mL). The patient was discharged from the hospital 5 days after treatment. Thereafter, the chylothorax remained reduced without drainage for more than 10 months (Fig. 4).
Fig. 1.
Chest X-ray performed at admission showing a bilateral pleural effusion.
Fig. 2.
Intranodal lymphangiography only opacifies lymphatic vessels toward the scrotum (arrowhead) and buttock (arrow).
Fig. 3.
. (A) The 21G needle (arrow) located at the retrocrural space where the thoracic duct was thought to exist based on anatomical knowledge. (B) Schematic representation of thoracic duct disruption and the retrocrural space (*), which is dorsal to the right crus (arrowhead) of the diaphragm and right next to the abdominal aorta (triangle).
Fig. 4.
Chest X-ray performed 6 months after the intervention showing reduction of bilateral pleural effusion.
Discussion
Percutaneous treatments, including TDE and TDD, have been reported as effective strategies for managing postoperative chylothorax, serving as an alternative to surgical thoracic duct ligation [1]. In cases where the thoracic duct can be opacified through lymphangiography, postoperative chylothorax was improved with a high success rate through these percutaneous treatments [1,2,4]. However, in cases where the thoracic duct remains invisible through lymphangiography, it can be challenging to improve chylothorax through these percutaneous treatments [4]. A report of an earlier study described malignancy as the main cause of nontraumatic chylothorax [5]. In chylothorax caused by malignancy, a tumor might infiltrate and obstruct the thoracic duct, thereby complicating visualization of the thoracic duct using lymphangiography [5,6]. Our case report demonstrated that TDD might be feasible and effective for managing such chylothorax caused by malignancy, even when the thoracic duct remained invisible to lymphangiography.
Puncturing the area where the thoracic duct is anatomically suspected to be located might lead to successful TDD. Earlier reports have described the main pathway of the thoracic duct as originating from the confluence of the intestinal and lumbar lymphatic trunk at the level of L1–L2. It then ascends through the diaphragmatic aortic hiatus in front of the vertebral column and passes behind the right crus of the diaphragm before entering the thorax [[7], [8]–9]. Therefore, CT-guided puncture of this retrocrural space, which is dorsal to the right crus of the diaphragm and which is right next to the abdominal aorta, is likely to disrupt the thoracic duct, even if lymphangiography is unable to opacify the thoracic duct.
This report has several limitations. First, this method is inapplicable to cases of chylothorax caused by congenital malformations or obstructions of the thoracic duct. Second, we did not evaluate anatomic variations in the thoracic duct using modes such as magnetic resonance lymphangiography.
In conclusion, even if lymphangiography is incapable of visualizing the thoracic duct, TDD by puncturing the retrocrural space, where the thoracic duct is anatomically suspected to be located, might lead to improvement of the refractory chylothorax. Because this report describes a single case, further research must be undertaken to accumulate more cases involving the use of this treatment for lymphatic diseases.
Patient consent
Consent for publication was obtained from the patient.
Footnotes
Competing Interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Cope C, Kaiser LR. Management of unremitting chylothorax by percutaneous embolization and blockage of retroperitoneal lymphatic vessels in 42 patients. JVasc Interv Radiol. 2002;13:1139–1148. doi: 10.1016/s1051-0443(07)61956-3. [DOI] [PubMed] [Google Scholar]
- 2.Itkin M, Kucharczuk JC, Kwak A, Trerotola SO, Kaiser LR. Nonoperative thoracic duct embolization for traumatic thoracic duct leak: experience in 109 patients. J Thorac Cardiovasc Surg. 2010;139:584–589. doi: 10.1016/j.jtcvs.2009.11.025. discussion 9-90. [DOI] [PubMed] [Google Scholar]
- 3.Lyon S, Mott N, Koukounaras J, Shoobridge J, Hudson PV. Role of interventional radiology in the management of chylothorax: a review of the current management of high output chylothorax. Cardiovasc Intervent Radiol. 2013;36:599–607. doi: 10.1007/s00270-013-0605-3. [DOI] [PubMed] [Google Scholar]
- 4.Nadolski GJ, Itkin M. Thoracic duct embolization for nontraumatic chylous effusion: experience in 34 patients. Chest. 2013;143:158–163. doi: 10.1378/chest.12-0526. [DOI] [PubMed] [Google Scholar]
- 5.Valentine VG, Raffin TA. The management of chylothorax. Chest. 1992;102:586–591. doi: 10.1378/chest.102.2.586. [DOI] [PubMed] [Google Scholar]
- 6.Ishii T, Kida K, Jinno S, Nomura K, Yamada K, Katsura H, et al. Two cases of papillary adenocarcinoma of the thyroid gland associated with chylothorax. Nihon Ronen Igakkai Zasshi. 1997;34:421–427. doi: 10.3143/geriatrics.34.421. [DOI] [PubMed] [Google Scholar]
- 7.Rabattu PY, Sole Cruz E, El Housseini N, El Housseini A, Bellier A, Verot PL, et al. Anatomical study of the thoracic duct and its clinical implications in thoracic and pediatric surgery, a 70 cases cadaveric study. Surg Radiol Anat. 2021;43:1481–1489. doi: 10.1007/s00276-021-02764-z. [DOI] [PubMed] [Google Scholar]
- 8.Shieber W The demonstration of thoracic duct abnormalities by lymphangiography. Angiology. 1974;25:73–87. doi: 10.1177/000331977402500111. [DOI] [PubMed] [Google Scholar]
- 9.Hematti H, Mehran RJ. Anatomy of the thoracic duct. Thorac Surg Clin. 2011;21:229–238. doi: 10.1016/j.thorsurg.2011.01.002. ix. [DOI] [PubMed] [Google Scholar]