Abstract
The increased occurrence of antibiotic resistance and the harmful use of pesticides are a major problem of modern times. A ban on the use of antibiotics as growth promoters in animal breeding has put a focus on the probiotics market. Probiotic food supplements are versatile and show promising results in animal and human nutrition. Chemical pesticides can be substituted by biopesticides, which are very effective against various pests in plants due to increased research. What these fields have in common is the use of spore‐forming bacteria. The endospore‐forming Bacillus spp. belonging to this group offer an effective solution to the aforementioned problems. Therefore, the biotechnological production of sufficient qualities of such endospores has become an innovative and financially viable field of research. In this review, the production of different Bacillus spp. endospores will be reviewed. For this purpose, the media compositions, cultivation conditions and bioprocess optimization methods of the last 20 years are presented and reflected.
Keywords: Bacillus, bioprocess development, industrial, optimization, spore production
Abbreviations
- ALR
airlift reactor
- CFU
colony forming units
- CSL
corn steep liquor
- DO
dissolved oxygen
- DSM
difco sporulation medium
- LAB
lactic acid bacteria
- OFAT
one‐factor‐at‐a‐time
- rpm
rounds per minute
- RSM
response surface methodology
- SFLAB
spore‐forming lactic acid bacteria
- SmF
submerged fermentation
- SSF
solid‐state fermentation
- STR
stirred tank reactor
1. INTRODUCTION
1.1. Endospores and sporulation
Food spoilage of canned goods or diseases originated from endospore‐forming bacteria of the genus Bacillus spp. [1, 2, 3] are often associated with these metabolically dormant cell forms [4], after their germination in the food source [1, 2]. In contrast to their bad image, endospore‐forming bacteria have proven to be useful tools in the field of biotechnology [5, 6, 7, 8]. These cell types are resistant to environmental stress and are widely distributed in nature [4, 9, 10]. The main trigger for the onset of sporulation is nutrient depletion. Endospores are inhomogenously formed in a subpopulation as a last resort for survival of the population [9]. Sporulation is a cell density‐dependent process initiated by quorum sensing using cell‐cell‐communication [9, 11]. Simplified, it is an eight‐step process [9, 12] involving a complex genetic routine of sigma‐factors and a phosphorelay‐system. Eventually, this results in an asymmetric cell division and autolysis of the so‐called mother cell and the release of a matured endospore [5, 13, 14, 15]. Spore‐forming aerobic bacteria include Bacillus spp., which are Gram‐positive and are thermoresistant with a strain‐dependent thermophilic growth optimum [4, 9]. Commonly used organisms of this genera include Bacillus subtilis as the Gram‐positive model organism, Bacillus licheniformis, Bacillus thuringiensis, Bacillus cereus and Bacillus coagulans [4].
1.2. Applications of endospores in industry
1.2.1. Feed application
The increased appearance of antibiotic resistant bacteria formed a global problem in need of an innovative solution. First, the use of antibiotics was limited resulting in a ban of aforementioned chemicals in livestock nutrition as growth promoters in the EU [5, 16, 17, 18, 19, 20, 21]. Following a vast amount of scientific research, probiotics show a great potential as a substitute for antibiotics with the means as growth promoters in animal nutrition. Especially the poultry industry profits from probiotics [20, 22]. Additionally, probiotics can also improve the health as a nutritional supplement in human nutrition [5, 16, 18, 19, 21, 23, 24]. LAB (lactic acid bacteria) such as Lactobacillus are commonly used as probiotics in human and livestock nutrition [20, 25, 26, 27]. Especially interesting for the industrial use of probiotics are SFLAB (spore‐forming lactic acid bacteria) such as B. coagulans. The organism combines the probiotic properties of LAB with the heat and acid resistance of endospores and thus increases the bacterial survival of gastric acid [25, 28, 29]. Additionally, the shelf life at room temperature of SFLAB probiotics is prolonged due to the resistance of endospores against environmental stress [27, 30, 31]. Most SFLAB are non‐pathogenic, registered GRAS (generally regarded as safe) organisms and thus simplify the use of Bacillus spp. as probiotics [25, 28, 32]. Advantages of SFLAB over LAB include the resistance to environmental stress and their easy cultivation [5, 28]. Moreover, an increasing market for health promoting dietary supplements shows the significance of the industrial production of SFLAB as probiotics [17, 25].
1.2.2. Other applications
In addition to the use of probiotics in nutrition, Bacillus spp. are also used in household chemicals due to their easy formulation and handling. For example, endospore containing sprays are used in hospitals to prevent the colonization of surfaces against Staphylococcus aureus [32].
Furthermore, Bacillus spp. are also heavily used in the market of biopesticides [33]. As a promising alternative for pesticides the endospores and crystals of B. thuringiensis are used [5, 8]. In a futuristic application, endospores are used in self‐healing concrete by precipitation of calcium carbonate and the alkalization of the surrounding [34].
To meet the increasing market demand for endospores, efficient strategies for large‐scale production need to be developed. For this purpose, the field of bioprocess development offers optimal opportunities to produce endospores. For the development of a bioprocess, the understanding of the physiological needs of the organism is paramount. The regulation or specific requirement of nutrients during cell cycles support the process development. These requirements must be specifically defined for each Bacillus organism. For the successful cultivation of endospores, in addition to the strain‐specific selection of the parameter ranges, various conditions must be considered and tested. These include the media type and composition as well as the process mode. Subsequently, various methods for process optimization can be applied during or after the development. An overview of conditions is depicted in Figure 1.
FIGURE 1.
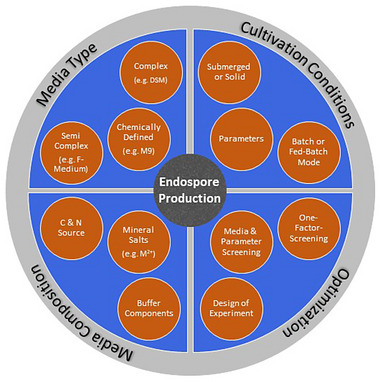
Overview of conditions that should be considered for an endospore producing bioprocess.
1.3. Media composition
Nutrient media are an important basis for process development and play a key role in leading to a successful process [5]. Broadly speaking, the media used can be divided into complex and chemically defined. The former makes it difficult to draw conclusions about the exact composition of the medium and can vary depending on the batch. Additionally, the reproducibility of chemically defined media facilitates the process development. The nutrient needs of the organisms change from growth to sporulation and need to be adjusted accordingly [4]. In addition, the components and the precise composition of the medium influences the induction of sporulation and the successful maturation of the endospore [35, 36, 37]. Besides nutrient components, divalent mineral ions are essential for successful sporulation [5, 38]. Especially Mn2+ shows essential influence on the sporulation of Bacillus spp. [39, 40]. During induction of sporulation Mn2+ is crucial for its continuation [36, 39, 40, 41, 42]. Additionally, Ca2+ is important for the formation of endospores and is chelated with the sporulation biomarker dipicolinic acid (DPA) [43, 44]. Monteiro et al. increased the spore yield by using a Ca2+ concentration of 0.6 gL−1 [45]. The supplementation of 0.1% Ca2+ carbonate helped with the completion of the sporulation [46].
Sporulation can be repressed by high concentrations of glucose [47, 48]. Furthermore, a higher glucose concentration than 15 gL−1 did not improve the sporulation of B. thuringiensis in a partially defined medium [49]. Low glucose concentrations showed high sporulation outcomes [50]. However, a decreased spore number was reported above 5 gL−1 glucose concentrations [51]. The autolysis of vegetative cells, which begins in the stationary phase, can be prevented by the addition of glucose at the end of the exponential growth phase [51, 52, 53]. But the supplementation of an additional carbon source to the medium during the cultivation can delay the sporulation for 24 h [48]. In conclusion, the glucose concentration is a crucial variation point in process development, which requires a closer look. The concentration ranges need to be adjusted strain‐specifically.
In addition to the chemical composition of the medium, its physicochemical properties, such as pH, are also important for a successful cultivation. Moreover, the cultivation method may alter the growth requirements of the organism. For example, under anaerobic conditions B. cereus shows higher amino acid and pyruvate needs [54].
In the following an overview of complex and chemically defined media that have been used for the sporulation of Bacillus spp., developed over a period of the last 20 years, is given.
1.3.1. Complex media
This type of media is often used for the sporulation of Bacillus spp. [55]. Besides glucose, carbon sources can for example, include sucrose and xylose for the Bacillus endospore production. Glycerol showed an impaired spore yield compared to another carbon source [48]. Commonly used complex media for sporulation include Difco Sporulation Medium (DSM), F‐medium [56] and Schaeffer Sporulation Medium [57] that support the growth and sporulation of different Bacillus spp. Especially B. subtilis was frequently cultivated in DSM. Tavares et al. yielded a spore concentration of 4 × 108 spores mL−1 after 24 h cultivation time and achieved a sporulation efficiency of 45.5% [56]. Monteiro et al. doubled the concentration of DSM components and supplemented with additional glucose. This modified medium achieved an increased sporulation with an efficiency of 77% instead of 48% [51]. Other complex media for the Bacillus cultivation contained beef and yeast extract [58] or hydrolyzed casein [59].
One media component is being increasingly used for Bacillus endospore production. The by‐product of corn preparation corn steep liquor (CSL) or corn steep powder (CSP) can be used for low cost cultivation and spore production. The high spore counts achieved with this media component can be explained by the more freely available amino acids, carbohydrates and minerals [60]. In addition, CSL is rich in nitrogen and the just mentioned amino acids and nutrients. Moreover, CSL and soybean flour can provide growth factors for bacterial growth and sporulation [61]. Especially thiamine in CSL improved the bacterial sporulation [62, 63]. In media development, the C/N ratio is an interesting and important variation point. The ratio was tested by Pandey et al. for high cell density of B. coagulans in a CSL‐based medium with a C/N ratio of 40:1 [64]. CSL or CSP show promising results when used instead of yeast extract. A shake flask cultivation of B. cereus in a CSL‐based medium improved the sporulation efficiency and spore concentration by a 300‐fold in comparison to a yeast extract‐based medium [60]. Moreover, when replacing yeast extract with CSL and acetate the sporulation of Bacillus sphaericus was increased in a fed‐batch bioprocess by yielding a spore concentration of 1.64×1010 spores mL−1 [63].
Besides CSL, other waste product‐based media are used for the endospore production. Some media are based on corn starch, corn flour or wheat bran [61]. Barley malt powder [34] and rice straw [65] were also used. Soybean flour was tested for B. thuringiensis in a media optimization [49]. Here, soybean powder was used as a nitrogen source. Furthermore, this organism also grew in a medium of tapioca powder, sugar cane and jaggery with additional sucrose and glucose. It was also shown that yeast extract was necessary for growth and sporulation in the used complex media [46]. Khardziani et al. tested lignocellulosic substrates such as banana and mandarin peels. Other tested substrates were corn, soybean, sunflower oil cake and wheat straw. A particularly inexpensive carbon source material, namely, mandarin peels, resulted in a high sporulation of B. subtilis. 40 gL−1 milled mandarin peels with peptone as nitrogen source with cheese whey instead of distilled water were used as a medium. The mandarin peel is rich in cellulose, hemicellulose, pectin, and nitrogen and has water‐soluble sugars [48]. Additionally, an overview of waste and by‐product‐based media can be found in Figure 2.
FIGURE 2.
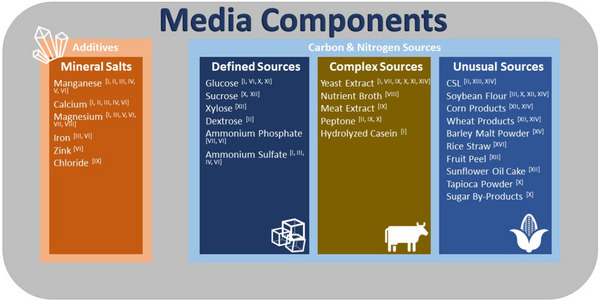
Overview of media components which are commonly used in endospore production. References: [I] Sarrafzadeh et al.[59]; [II] Pandey and Vakil [64]; [III] Elsayed et al. [66]; [IV] Lacerda et al. [67]; [V] Posada‐Uribe et al. [68]; [VI] Monteiro et al. [45]; [VII] Tavares et al. [56]; [VIII] Monteiro et al. [51]; [IX] Murugesan et al. [58]; [X] Gopinathan et al. [46]; [XI] Awad et al. [69]; [XII] Khardziani et al. [48]; [XIII] Lalloo et al. [60]; [XIV] Chen et al. [61]; [XV] Hong et al.[34]; [XVI] Yin et al. [65].
1.3.2. Defined media
In addition to the already widely used and well‐known chemically defined sporulation media such as MOD [70] or CCY (Casein‐Casein‐Yeast) [71], other defined media have been modified or developed over the past 20 years. Based on MOD a chemically defined medium was used for B. cereus to demonstrate anaerobic cultivation conditions in Hungate tubes. The medium contained low concentrations of glucose (10 mM) and amino acids (2.5 gL−1) [72]. Monteiro et al. developed and optimized a chemically defined medium for B. subtilis by varying the concentrations of vitamins, carbon, nitrogen and Ca2+. Furthermore, it was discovered that no additional vitamin mix was necessary if thiamine (10 mgL−1) had been supplemented sufficiently [45]. For cost reduction or growth enhancement complex media components can be added to an otherwise chemically defined medium. Posada‐Uribe et al. developed a defined medium with complex components for the cultivation of B. subtilis and achieved a spore yield of 1.37 × 109 CFU mL−1 and a sporulation efficiency of 93.5%. Especially low concentrations of glucose (1.04 gL−1) were found to be effective [68]. A partially defined medium with yeast extract was optimized for B. thuringiensis and resulted in a spore concentration of 3.29 × 106 spores mL−1 after 36 h in a shake flask cultivation by Awad et al. [69].
1.4. Cultivation and sporulation in bioprocesses of Bacillus spp
For a successful production of endospores in a bioprocess, strain‐specific cultivation and nutrient requirements should be determined [17]. A high cell density is necessary to trigger quorum sensing in Bacillus spp. [68]. Sporulation can be induced at the beginning of the stationary phase by its main trigger nutrient deficiency [9, 73].
1.4.1. Cultivation conditions to produce endospores
Spore formation can only occur in the same parameter ranges which support bacterial growth [5]. Cultivation conditions deviating from the optimum can lead to a prolonged cultivation time and thus entail a further production cost [5, 74, 75, 76, 78, 79, 80]. In this chapter, commonly used cultivation condition ranges from temperature, aeration, agitation as well as pH‐control are summarized.
Particularly different strategies in the production of endospores exist in regard to pH‐control. The views on this vary from a supporting to an inhibiting effect on sporulation. pH‐control can be useful because some Bacillus spp. contribute to acidification of the environment through for example, lactate fermentation and can thus impair growth. Adjusting pH to a fixed value often improved the sporulation of various Bacillus spp. [48, 51, 59, 64, 81]. Another approach is to regulate the pH in a fixed range to support growth and sporulation [57, 61]. Rosenfeld et al. for example, detected an increased growth of B. cereus by using pH‐control [54]. Monteiro et al. conducted experiments with pH‐control and found a minimal pH of 5 necessary for a successful sporulation of B. subtilis. A possible explanation for the benefit of a controlled pH for improved sporulation could be due to a synchronization of the sporulation in the population and thus, increase the sporulation efficiency [51]. In the topic of pH‐control the strain‐specificity was evident again. Uncontrolled pH showed no impact on sporulation in some Bacillus spp. or even increased the spore yield through a natural pH‐shift [68, 69].
The aeration is important for the support of growth in bacteria. A dependency between aeration and spore yield was shown for B. cereus and B. thuringiensis [5, 82, 83, 84]. The agitation is closely tied to the aeration and influences the parameter naturally through technical reasons. The dissolved oxygen (DO) is often used for the monitoring of aeration. The DO can be set so that it acts as a minimum value and is regulated accordingly. The interaction of agitation and aeration was of particular importance for achieving high spore yields of B. subtilis [68]. In other examples, a variable agitation was used to influence the DO [45, 51, 59, 60, 64, 68]. Sarrafzadeh et al. used a periodical adjustment of agitation and aeration to maintain a minimal DO >20% [59]. An increased cell and spore yield was achieved for B. thuringiensis by using a controlled DO of 50%. However, by increasing the aeration during the sporulation phase to a DO 100% or a full limitation of aeration, a decrease of the spore yield was shown in both scenarios. A minimal DO >30% helped with the increase of spore yield but had no significant influence on the growth of B. subtilis [51]. A supplementation of pure oxygen can inhibit the sporulation itself [83] and should thus be tested strain‐specifically.
Temperature ranges are dependent on the growth optimum of the organism and can range for some Bacillus spp. up to thermophilic temperatures [4]. For Bacillus spp. the most commonly used temperature ranges include 30–37°C [45, 48, 51, 56, 57, 58, 59, 60, 61, 67]. A shift in temperature between growth phase and sporulation can also be applied [85]. Additionally, the cultivation temperature influences the heat resistance of mature endospores [74, 76, 80].
1.4.2. Cultivation strategies
Production of endospores in lab scale can be performed in shake flasks or benchtop bioreactors with bioprocess control. The bioprocess needs to be simple enough for the application in an industrial scale.
Submerged cultivations in shake flasks are often the first experiments to be conducted in order to develop a bioprocess. These cultivation vessels provide optimal conditions for getting to know the organism and establishing cultivation parameters. In addition, media compositions and cultivation parameters can be screened quickly and without much effort in these vessels [66]. For example, Tavares et al. compared DSM and F‐medium in shake flask cultivations [56]. The DSM achieved a higher spore concentration of 4 × 108 spores mL−1 after 24 h than the F‐medium (6.5 × 106 spores mL−1). But after 72 h incubation time the spore concentration in the F‐medium reached 1.2 × 109 spores mL−1 and thus being higher than in DSM after 72 h (6.7 × 108 spores mL−1). These findings show that the nutrient media can have a major impact on the process and should therefore be thoroughly investigated.
Upscaling from shake flasks to a bioreactor is an important step in bioprocess development. The process conditions must be adjusted repeatedly during upscaling. Cultivations in batch mode are most commonly used to produce endospores of Bacillus spp. [51, 67, 68, 86]. By applying three different cultivation modes in one batch‐cultivation of B. coagulans Das et al. first supported the growth of the organism. After the growth phase, the parameters were changed after glucose consumption and then again for lipase and spore production until process end [85]. An overview of reviewed cultivation parameters can be found in Table 1.
TABLE 1.
Overview of the bioprocess parameters cultivation mode, pH (controlled or uncontrolled), aeration, agitation and temperature. The listing also includes the cultivated organism and the spore yield produced in the process.
| Publication | Process mode | pH [−] | Aeration | Agitation [rpm] | Temperature [°C] | Organism | Spore yield | |
|---|---|---|---|---|---|---|---|---|
| batch | controlled | 6.7 & 8.0 | 1.0 v/v/min | 300 | 37 | B. subtilis | 6.5 × 1010 spores mL−1 | |
| batch | controlled | 7.5 | 2 L min−1, DO > 30% | 100‐200 | 37 | B. subtilis | 5.6 × 109 spores mL−1 | |
| batch | controlled | 6.5–7.5 | n.n. | 800 | 30 | B. thuringiensis | 1.5 × 109 spores mL−1 | |
| batch | controlled | 6.8–7.2 | 30 L min−1, DO 30%–40% | 400–800 | 30 | B. subtilis | 1.56 × 1010 spores mL−1 | |
| batch | uncontrolled | n.n. | 1 v/v/min | n.n. | n.n. | B. thuringiensis | 3.7 × 106 spores mL−1 | |
| batch | n.n. | n.n. | 1 v/v/min | 700 | 37 | B. subtilis | 1.0 × 108 CFU mL−1 | |
| batch | uncontrolled | 1 v/v/min | 600 | 30 | B. thuringiensis | 1.52 × 109 spores mL−1 | ||
| batch | uncontrolled | 12 L min−1 | 400 | 30 | B. subtilis | 9.33 × 109 CFU mL−1 | ||
| batch | uncontrolled | 106 L h−1 | 158 | 40,95 | B. coagulans | 6.0 × 1012 spores g−1 biomass | ||
| fed‐batch | uncontrolled | 0.5–1.0 v/v/min DO 40%–60% | 250–500 | 37 | B. coagulans | 1.9 × 1011 spores mL−1 | ||
| fed‐batch | controlled | >6.5 | 2 L min−1, DO = 30% | 100–1200 | 37 | B. subtilis | 3.6 × 1010 spores mL−1 | |
| fed‐batch | controlled | 6.8 | DO > 20% | periodical adjustment | 30 | B. thuringiensis | 4.14 × 109 spores mL−1 | |
| fed‐batch | controlled | 7.0 | DO > 30% | 500–1200 | 30 | B. cereus | 1.0 × 1010 CFU mL−1 | |
| fed‐batch | controlled | 7.5 | 2 L min−1, DO > 30% | 100–1000 | 37 | B. subtilis | 7.4 × 109 spores mL−1 | |
| fed‐batch | controlled | 6.5–7.5 | n.n. | 800 | 30 | B. thuringiensis | 3.9 × 1010 spores mL−1 | |
| fed‐batch | controlled | 7.0 | DO > 20% | 700–1000 | 30 | B. sphaericus | 1.64 × 1010 spores mL−1 | |
However, fed‐batch processes can also be used to support sporulation through added supporting components [51]. The feed solution and its components or their ratio are an important control element. Feed solutions often contain glucose or other carbon or nitrogen sources [46, 57, 60, 64, 88] and additionally mineral salts such as Ca2+ [45] or Mn2+ [59]. In this context, fed‐batch bioprocesses often start as batch bioprocesses with a feed starting after or shortly before the induction of sporulation [45, 51, 59, 64]. In a different approach the feed solution was added at variable feed rates [57] or until a desired sporulation efficiency (proportion of spores to the total cell count) has been achieved [60]. The switch between batch and fed‐batch and back to batch mode was applied by Monteiro et al. for B. subtilis [51]. Pandey et al. showed the importance of the C/N ratio (30:1) in the feed solution in a fed‐batch cultivation of B. coagulans which yielded a sporulation efficiency of 81%. Other experiments by Pandey et al. used C/N ratios of 40:1 and 35:1 in batch or fed‐batch mode, resulting in the beforementioned 30:1 C/N ratio. Here, the reduction of the nutrients through the C/N ratio and an additional glucose feed increased the sporulation [64]. Fed‐batch mode can also be used to suppress a catabolic repression through a partial addition of the carbon source [46]. Moreover, the addition of excess glucose can decrease the autolysis in the bioprocess [45].
1.5. Optimization of endospore production
Different methods can be applied to optimize a medium or a bioprocess in general. Also, the use of bioinformatic tools (e.g., genomics) can be used to optimally predict and optimize growth and sporulation. In this way, it is possible to name strain‐specific sporulation requirements and to define the borders of process parameters or media components. High‐throughput tools for analysis and improvement are also particularly useful here [89]. Statistical design of experiments such as Plackett‐Burman design or a one‐factor‐at‐a‐time (OFAT) approach can be applied for the optimization. The prevalent aim is to maximize the spore yield after a successful high cell density cultivation.
1.5.1. Medium optimization
Medium optimization is a crucial step in bioprocess development. Additionally, it is often the first step to improve the sporulation and cell density by developing a medium to support both. In a combination of an OFAT factorial design for substrate determination and a two‐level full factorial design, a medium was developed for the industrial application of Bacillus spp. by Hong et al. This approach followed a quadratic model with central composite design to optimize the found substrate concentrations of barley malt powder and mixed grain powder [34].
Regularly the Plackett‐Burman design is used to define the composition and concentration ranges of the medium. Posada‐Uribe et al. used this method for the media optimization to cultivate B. subtilis. Especially Mn2+ was a promising factor as it was having a positive effect on the sporulation. The design matrix was a full factorial design to define the optimization zone and to find the optimum concentrations of the components [68]. In a two‐level Plackett‐Burman design with seven parameters the distillery effluent‐based medium for the growth of B. subtilis was optimized by Shi et al. For the determination of the optimal concentration ranges of the three most promising factors corn flour, Mg2+ and (NH4)2SO4 a Box‐Behnken design was used, yielding a spore concentration of 6.95×108 spores mL−1 [90]. Three media components were screened in a Plackett‐Burman design and a subsequent response surface methodology (RSM) to optimize the medium and bioprocess of B. coagulans. Due to the optimization a spore yield of 5.74 × 1011 CFU mL−1 was achieved in a bioreactor after 48 h [65]. The Plackett‐Burman design was also used by Chen et al. to determine the optimal nitrogen source for B. subtilis. Experiments were conducted with CSL, soybean flour and yeast extract, as the carbon source was optimized beforehand. The improved medium achieved a spore concentration of 1.52 × 1010 spores mL−1 in a shake flask cultivation and 1.56 × 1010 spores mL−1 in a 30 L bioreactor after 40 h of cultivation. Furthermore, Chen et al. supposedly doubled the highest known spore concentration for B. subtilis (by Monteiro et al. [51]) in 2010 with this optimized medium, making CSL a valuable complex media component in spore production [61]. Additionally, this optimization approach showed how these optimization methods can facilitate the process development. Furthermore, a screening experiment followed by an RSM for B. amyloliquefaciens resulted in the determination of an optimized carbon and nitrogen source. The method followed a five‐level four‐factor central composite design to find the optimum of lactose, tapioca, ammonium sulfate and peptone. A significant effect was found between tapioca and peptone, which were determined as the best carbon and nitrogen sources [91].
1.5.2. Parameter optimization
Following the development of a bioprocess and its testing with the organisms, the parameters of the cultivation process are an important regulator to achieve a higher cell and spore density in favorably a shorter cultivation time to minimize production cost. Optimized cultivation parameters can include pH, agitation, aeration, temperature and incubation time.
An enhancement of the growth of B. subtilis and B. coagulans as probiotics was created by optimizing the culture conditions by Murugesan et al. The pH, temperature and incubation time were varied in an OFAT approach. The parameters of pH 7, incubation time of 24 h and a temperature of 30°C for B. subtilis and 37°C for B. coagulans were identified [58]. In a single‐factorial design, Posada‐Uribe et al. optimized a pH‐control at varying values as well as aeration and agitation. The conducted experiments showed a significant effect (p < 0.05) of aeration and agitation on the spore cell density but not the sporulation efficiency [68]. Pandey et al. used a Plackett‐Burman design to choose the culture conditions based on nutrient requirements of a phage‐resistant B. coagulans and applied a strain selection additionally [92]. Furthermore, multivariate response surface modeling of B. coagulans spore production and a genetic algorithm‐based optimization was used to enhance the spore yield in a batch cultivation. This approach focused on cultivation parameters such as temperature, aeration and agitation and their influence on growth and sporulation. The optimized cultivation conditions (158 rpm, 40.95°C, uncontrolled pH and batch mode) resulted in a yield of 6 × 1012 spores g−1 biomass [85]. A different approach was applied by Atehortúa et al. by involving the cell death and a dynamic substrate consumption in a fed‐batch cultivation of B. thuringiensis as a model. The kinetic was based on a sigmoid function and an intermitted fed‐batch cultivation with total cell retention was the result of this optimization [93].
1.6. Cultivation systems for endospore production
1.6.1. Solid‐State fermentation
Submerged cultivation (SmF) is commonly used in Western countries. In contrast, in Asian culture many foods rely on a different production technique called solid‐state fermentation (SSF). Already a commonly used technique for the cultivation of fungus spores [94], SSF appears to be a fitting technique for bacterial spore cultivation as well [17, 20]. In this technique, microorganisms are cultivated on moist, solid substrate and have no aqueous phase [95, 96]. Tray type solid‐state fermenters contain a perforated bottom to enable aeration and to withhold the substrate. Inside the chamber are humid conditions with circulating air [94]. The second type are drum fermenters with drum‐shaped corpuses and a possible control of aeration and agitation. The advantage of this reactor type in contrast to the tray fermenter are more homogenous culture conditions. Column reactors contain a column with lidded endings. A temperature control is possible [94]. Additionally, agitation and aeration can be applied to SSF if needed. Furthermore, batch mode or a continuous operation are possible with this cultivation technique as well [94]. Other advantages of SSF in contrast to SmF cultivation include higher product yield and simplification of product recovery as well as product processing [97] (e.g., no centrifugation steps needed [20]). A disadvantage of SSF can be the heat development during cultivation, which can lead to water loss and limited oxygen supply [95, 98]. A comparison of SmF and SSF based on different properties is shown in Figure 3.
FIGURE 3.
Comparison of SmF and SSF concerning the oxygen supply, shear stress, product yield, product recovery, equipment cost and the industrial implementation [94]. Green implies a positive characteristic and red indicates negative characteristics for common aerobic cultivations of microorganisms. SmF, submerged fermentation; SSF, solid‐state fermentation.
The used substrates can be natural such as cellulose or agricultural by‐products, but due to the construction of the substrate, the basis will be degraded and changes its physicochemical and mechanical properties during cultivation [97]. Raw plant material and waste products are an environmental‐friendly and ecological substrate for SSF [17, 20, 99, 100, 101]. Furthermore, simple equipment and low‐cost educts make SSF a cost‐effective alternative [20]. For an easier product recovery and with the possibility of mass balancing and better process control, inert chemically defined substrates show a different approach. Here, different abiotic materials are saturated with media components. During cultivation, the base material and its mechanical properties stay the same [97].
For natural substrates, a broad range of agricultural products were tested with Bacillus spp. Soybean meal, wheat bran and corn meal were tested and optimized by using RSM to enhance sporulation in SSF of B. amyloliquefaciens. A spore yield of 7.24 × 1010 CFU g−1 wet substrate was achieved [100]. Berikashvili et al. used lignocelluloses and corn cobs for the SSF of B. amyloliquefaciens and yielded 4.7 × 1011 spores g−1 biomass. In the scope of their work, sunflower oil and mushroom were tested as well, but yielded only low sporulation rates. Notably, when distilled water was interchanged with cheese whey and corn cob based medium, an increased spore yield of 1.05 × 1012 spores g−1 biomass was achieved [102]. Soybean was also the substrate in SSF for the cultivation of B. amyloliquefaciens as a feed‐additive for broiler and resulted in a dried spore yield of 9 × 1011 CFU g−1 [103]. Another waste product used as a substrate is rice straw powder with wheat bran for the cultivation of B. licheniformis. This medium containing the beforementioned substrates and glucose, peptone as well as yeast extract achieved a spore dry weight of 1.7 × 1011 spores g−1 [101]. The cultivation and sporulation of B. thuringiensis was compared in SmF and SSF by Lima‐Pérez et al. Interestingly, catabolic repression by glucose occurred in SmF with 25 gL−1, but in SSF at 50 gL−1. In SSF the sporulation started at just 15 h and gained the highest spore yield at 36 h [104]. LB (lysogeny broth) medium supplemented with raw potato flour was used for the endospore production of B. thuringiensis in a mixed cultivation of SmF and SSF. And here as well, the sporulation time was decreased (by 24 h) in comparison to SmF and an enhanced crystal formation occurred [105]. Bacillus atrophaeus production was compared in an SSF column bioreactor, bag reactors and flasks. Here, the ventilation and moisture were varied in the cultivations. An enhanced spore yield of 3.31 × 1010 CFU g−1 dry weight in the column bioreactor with 80% initial humidity and lack of aeration was achieved [106]. For B. subtilis a two‐stage fermentation in SSF was conducted by Zhao et al. with the aim to support cell growth and sporulation. The stages included a temperature shift from 37°C during growth and 47°C for sporulation after 48 h. The parameters Mn2+ concentration and temperature were optimized in a statistical experimental design. This process achieved a spore yield of 1.79 × 1010 spores g−1 dry medium after 72 h cultivation time [107]. In a co‐cultivation of B. subtilis, Bacillus mucilaginosus and Paecilomyces lilacinus on tobacco and its wastewater, sporulation occurred but was decreased compared to single cultivations. By limiting the utilization of P. lilacinus the spore yield of the co‐cultivation was increased [108]. In conclusion, the approach of established SSF methods for endospore production is a promising field. However, due to the more difficult technical implementation possibilities of SSF as an industrial scale process, its application could still be limited in Western countries.
1.6.2. Reactor systems
Widespread and established reactor systems such as STRs (stirred tank reactor) are suitable for the cultivation of endospores. New reactor types and high‐throughput‐systems are used for endospore cultivation and bioprocess screening experiments. Such high‐throughput‐systems are for example, already used for E. coli. The developed processes are transferable and thus help with upscaling and lead to reduced experimental effort. So, bioprocess development can be fast‐tracked and discounted [109]. Besides the obvious advantages, high‐throughput screening limits the usage of resources when statistical experimental designs are employed. Disposable STRs are a way to secure lab scale equivalent process development but only in volumes as small as 250 mL [110].
Tzeng et al. used airlift reactors (ALR) for the cultivation of B. amyloliquefaciens and compared it to a STR batch cultivation. By using ALR the spore yield was increased five to eight‐fold compared to STR resulting in 3.82 × 109 spores mL−1. Furthermore, the cultivation time was decreased to 29 h and the minimized shear stress presumably increased the sporulation [111]. B. cereus was cultivated with the purpose of endospore production in a chemically defined medium in an ALR with pH‐control and aeration. The use of the ALR synchronized the sporulation and the airlift was simultaneously used to harvest the endospores [47].
In a two‐stage cultivation system B. thuringiensis was cultivated to produce endospores and bioinsecticide crystals. First, Saccharomyces cerevisiae was cultivated for the purpose of being used as the medium component in a minimal medium. In the same vessel, containing the lysed yeast extract supplemented with 10 gL−1 glycerol B. thuringiensis was cultivated. This system, called pulse fed‐batch one pot (FOP) was inoculated with already heat‐treated endospores instead of vegetative cells. FOP was conducted in lab scale and eventually upscaled to production‐scale bioreactors. This process showed a cost effectiveness and a spore yield of 4 × 109 spores mL−1 in 300 L as well as 3 × 1010 spores mL−1 in 1500 L after 30 h, respectively [112]. But it has to be taken into consideration, the previous cultivation of yeast and the production of endospores on petri dishes for 96 h prolongs and complicates the process distinctly.
2. CONCLUDING REMARKS
This review summarizes and reflects the state‐of‐the‐art in endospore production. The high and constantly increasing demand for endospore‐based products shows the importance of this research field. By using different media, a bioprocess can be fundamentally controlled. Furthermore, the cultivation parameters form an important basis for a successful Bacillus spp. bioprocess. Especially the variety of process parameters and media compositions reflects the diversity of the Bacillus spp. group. Therefore, individual development and adaptation of the bioprocess is necessary on a strain‐specific basis. The cultivation options summarized here are intended to provide a guideline for strain‐specific bioprocess development. Moreover, developed bioprocesses can be further improved by statistical experiment optimizations and so, become industrially attractive.
In addition to SmF, which is very common in Western countries, SSF offers a possible method for the production of endospores. However, despite the emerging use of this method, the application of SSF in large‐scale processes is still difficult. The use of high‐throughput methods can further accelerate and improve the screening of media components and cultivation methods. In the future, these methods could replace the preceding shake flask experiments. This would make bioprocess development significantly more cost‐effective and also shorten the development time.
Especially the emerging new products including endospores put a spotlight on the bioprocess development for these cell types. The established bioprocesses often only focus on high cell density but need to consider the main triggers of sporulation as well.
CONFLICT OF INTEREST STATEMENT
The authors have declared no conflicts of interest.
ACKNOWLEDGMENTS
The authors would like to thank the Open Access fund of Leibniz University Hannover for the funding of the publication of this article. The authors would also like to thank Tessa Habich for her support with the depictions in this review.
Open access funding enabled and organized by Projekt DEAL.
Biermann R, Beutel S. Endospore production of Bacillus spp. for industrial use. Eng Life Sci. 2023;23:e2300013. 10.1002/elsc.202300013
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon request.
REFERENCES
- 1. Bottone EJ. Bacillus cereus, a volatile human pathogen. Clin Microbiol Rev. 2010;23:382‐398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wells‐Bennik MHJ, Eijlander RT, Den Besten HMW, et al. Bacterial spores in food: survival, emergence, and outgrowth. Annu Rev Food Sci Technol. 2016;7:457‐482. [DOI] [PubMed] [Google Scholar]
- 3. Stenfors Arnesen LP, Fagerlund A, Einar Granum P, From soil to gut: Bacillus cereus and its food poisoning toxins. 2008. [DOI] [PubMed]
- 4. Wood BJB, Holzapfel WH. The Genera of Lactic Acid Bacteria. Springer Science and Business Media; 1995. [Google Scholar]
- 5. Bressuire‐Isoard C, Broussolle V, Carlin F. Sporulation environment influences spore properties in Bacillus: evidence and insights on underlying molecular and physiological mechanisms. FEMS Microbiol Rev. 2018;42:614‐626. [DOI] [PubMed] [Google Scholar]
- 6. Wolken WAM, Tramper J, Van Der Werf MJ. What can spores do for us? Trends Biotechnol. 2003;21:338‐345. [DOI] [PubMed] [Google Scholar]
- 7. Cutting SM. Bacillus probiotics. Food Microbiol. 2011;28:214‐220. [DOI] [PubMed] [Google Scholar]
- 8. Pérez‐García A, Romero D, de Vicente A. Plant protection and growth stimulation by microorganisms: biotechnological applications of Bacilli in agriculture. Curr Opin Biotechnol. 2011;22:187‐193. [DOI] [PubMed] [Google Scholar]
- 9. Higgins D, Dworkin J. Recent progress in Bacillus subtilis sporulation. FEMS Microbiol Rev. 2012;36:131‐148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Setlow P. Spores of Bacillus subtilis: their resistance to and killing by radiation, heat and chemicals. J Appl Microbiol. 2006;101:514‐525. [DOI] [PubMed] [Google Scholar]
- 11. Fritsche O. Mikrobiologie. Springer Berlin Heidelberg; 2016. [Google Scholar]
- 12. Riley EP, Schwarz C, Derman AI, Lopez‐Garrido J. Milestones in Bacillus subtilis sporulation research. Microb Cell. 2021;8:1‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Serrano Mó, Neves A, Soares CláM, Moran CP, Henriques AO. Role of the anti‐sigma SpoIIAB in regulation of σG during Bacillus subtilis sporulation. J Bacteriol. 2004:4000‐4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Setlow P. minireview I will survive: protecting and repairing spore DNA. J Bacteriol. 1992;174:2737‐2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tan IS, Ramamurthi KS. Spore formation in Bacillus subtilis . Environ Microbiol Rep. 2014;6:212‐225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brashears MM, Amezquita A, Jaroni D. Lactic acid bacteria and their uses in animal feeding to improve food safety. Adv Food Nutr Res. 2005:1‐31. [DOI] [PubMed] [Google Scholar]
- 17. Elisashvili V, Kachlishvili E, Chikindas ML. Recent advances in the physiology of spore formation for Bacillus probiotic production. Probiotics Antimicrob Proteins. 2019;11:731‐747. [DOI] [PubMed] [Google Scholar]
- 18. Giacchi V, Sciacca P, Betta P. Multistrain probiotics: the present forward the future. Probiotics, Prebiotics, and Synbiotics: Bioactive Foods in Health Promotion. Elsevier; 2016:279‐302. [Google Scholar]
- 19. Krehbiel CR, Rust SR, Zhang G, Gilliland SE. Bacterial direct‐fed microbials in ruminant diets: performance response and mode of action. J Animal Sci. 2003;81:E120‐E132. [Google Scholar]
- 20. Popov IV, Algburi A, Prazdnova EV, et al. A review of the effects and production of spore‐forming probiotics for poultry. Animals. 2021;11:1‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Soccol CR, Vandenberghe LPS, Spier MR. The potential of probiotics. Food Technol Biotechnol. 2010;48:413‐434. [Google Scholar]
- 22. Ruiz Sella SRB, Bueno T, De Oliveira AAB, Karp SG, Soccol CR. Bacillus subtilis natto as a potential probiotic in animal nutrition. Crit Rev Biotechnol. 2021;41:355‐369. [DOI] [PubMed] [Google Scholar]
- 23. Allen HK, Levine UY, Looft T, Bandrick M, Casey TA. Treatment, promotion, commotion: antibiotic alternatives in food‐producing animals. Trends Microbiol. 2013;21:114‐119. [DOI] [PubMed] [Google Scholar]
- 24. Elisashvili V, Kachlishvili E, Chikindas ML. Recent advances in the physiology of spore formation for Bacillus probiotic production. Probiotics Antimicrob Proteins. 2019;11(3):731‐747. [DOI] [PubMed] [Google Scholar]
- 25. Konuray G, Erginkaya Z. Potential use of Bacillus coagulans in the food industry. Foods. 2018;7:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Siezen RJ, Wilson G. Probiotics genomics. Microb Biotechnol. 2010;3:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhou Y, Zeng Z, Xu Y, et al. Application of Bacillus coagulans in animal husbandry and its underlying mechanisms. Animals. 2020;10:454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hyronimus B, Le Marrec C, Sassi AH, Deschamps A. Acid and bile tolerance of spore‐forming lactic acid bacteria. Int J Food Microbiol. 2000;61:193‐197. [DOI] [PubMed] [Google Scholar]
- 29. Jafari M, Alebouyeh M, Mortazavian AM, et al. Influence of heat shock temperatures and fast freezing on viability of probiotic sporeformers and the issue of spore plate count versus true numbers. Food Sci Nutr. 2016;3:35‐42. [Google Scholar]
- 30. Fuller R, Gibson GR. Probiotics and prebiotics: microflora management for improved gut health. Clin Microbiol Infect. 1998;4:477‐480. [Google Scholar]
- 31. Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr. 1995;125:1401‐1412. [DOI] [PubMed] [Google Scholar]
- 32. Jeżewska‐Frąckowiak J, Seroczyńska K, Banaszczyk J, Jedrzejczak G, Żylicz‐Stachula A, Skowron PM. The promises and risks of probiotic Bacillus species. Acta Biochim Pol. 2018;65:509‐519. [DOI] [PubMed] [Google Scholar]
- 33. Nicholson WL. Roles of Bacillus endospores in the environment. Cell Mol Life Sci. 2002;59:410‐416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hong M, Kim W, Park W. Low‐cost cultivation and sporulation of alkaliphilic Bacillus sp. Strain AK13 for self‐healing concrete. J Microbiol Biotechnol. 2019;29:1982‐1992. [DOI] [PubMed] [Google Scholar]
- 35. Sella S, Vandenberghe L, Soccol CR. Life cycle and spore resistance of spore‐forming Bacillus atrophaeus . Microbiol Res. 2014;169:931‐939. [DOI] [PubMed] [Google Scholar]
- 36. Sinnelä M, Park Y, Lee J, et al. Effects of calcium and manganese on sporulation of Bacillus species involved in food poisoning and spoilage. Foods. 2019;8:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Slepecky R, Foster JW. Alterations in metal content of spores of Bacillus megaterium and the effect of some spore properties. J Bacteriol. 1959;78:117‐123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Atrih A, Foster SJ. The role of peptidoglycan structure and structural dynamics during endospore dormancy and germination. Antonie Van Leeuwenhoek. 1999;75:299‐310. [DOI] [PubMed] [Google Scholar]
- 39. Amaha M, Ordal ZJ, Touba A. Sporulation requirements of Bacillus coagulans var. thermoacidurans in complex media. J Bacteriol. 1956;72:34‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Weinberg ED. Manganese requirement for sporulation and other secondary biosynthetic. Appl Microbiol. 1964;12:436‐441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Charney J, Fisher WP, Hegarty CP. Managanese as an essential element for sporulation in the genus Bacillus . J Bacteriol. 1951;62:145‐148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Stöckel S, Meisel S, Böhme R, Elschner M, Rösch P, Popp J. Effect of supplementary manganese on the sporulation of Bacillus endospores analysed by Raman spectroscopy. J Raman Spectrosc. 2009;40:1469‐1477. [Google Scholar]
- 43. Andreoli AJ, Saranto J, Baecker PA, et al. Biochemical Properties of Forespores Isolated from Bacillus Cereus in Spores VI. American Society for Microbiology; 1975. [Google Scholar]
- 44. Errington J. Bacillus subtilis sporulation: regulation of gene expression and control of morphogenesist. Microbiol Rev. 1993;57:1‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Monteiro SMS, Clemente JJ, Carrondo MJT, Cunha AE. Enhanced spore production of Bacillus subtilis grown in a chemically defined medium. Adv Microbiol. 2014;04:444‐454. [Google Scholar]
- 46. Gopinathan C, Chaudhury A, Vivek AT. Novel techniques for cost‐effective production of Bacillus thuringiensis Subsp. israelensis . Int J Mosq Res. 2016;3:17‐29. [Google Scholar]
- 47. De Vries YP, Hornstra LM, De Vos WM, Abee T. Growth and sporulation of Bacillus cereus ATCC 14579 under defined conditions: temporal expression of genes for key sigma factors. Appl Environ Microb. 2004;70:2514‐2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Khardziani T, Kachlishvili E, Sokhadze K, et al. Elucidation of Bacillus subtilis KATMIRA 1933 potential for spore production in submerged fermentation of plant raw materials. Probiotics Antimicrob Proteins. 2017;9:435‐443. [DOI] [PubMed] [Google Scholar]
- 49. Melo LFA, Cabral AM, Melo ACA, et al. Bioprocess development for high cell mass and endospore production by Bacillus thuringiensis var israelensis in semi‐industrial scale. Curr Microbiol. 2014;9:2773‐2783. [Google Scholar]
- 50. Chevanet C, Besson F, Michel G. Effect of various growth conditions on spore formation and bacillomycin L production in Bacillus subtilis . Can J Microbiol. 1986;32:254‐258. [DOI] [PubMed] [Google Scholar]
- 51. Monteiro SM, Clemente JJ, Henriques AO, Gomes RJ, Carrondo MJ, Cunha AE. A procedure for high‐yield spore production by Bacillus subtilis . Biotechnol Prog. 2005;21:1026‐1031. [DOI] [PubMed] [Google Scholar]
- 52. Hu P, Leighton T, Ishkhanova G, Kustu S. Sensing of nitrogen limitation by Bacillus subtilis: comparison to enteric bacteria. J Bacteriol. 1999;181:5042‐5050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Jolliffe LK, Doyle RJ, Streips UN, The energized membrane and cellular autolysis in Bacillus subtilis . 1981. [DOI] [PubMed]
- 54. Rosenfeld E, Duport C, Zigha A, Schmitt P. Characterization of aerobic and anaerobic vegetative growth of the food‐borne pathogen Bacillus cereus F4430/73 strain. Can J Microbiol. 2005;51:149‐158. [DOI] [PubMed] [Google Scholar]
- 55. Chen Y, Dong F, Wang Y. Systematic development and optimization of chemically defined medium supporting high cell density growth of Bacillus coagulans . Appl Microbiol Biotechnol. 2016;100:8121‐8134. [DOI] [PubMed] [Google Scholar]
- 56. Tavares MB, Souza RD, Luiz WB, et al. Bacillus subtilis endospores at high purity and recovery yields: optimization of growth conditions and purification method. Curr Microbiol. 2013;66:279‐285. [DOI] [PubMed] [Google Scholar]
- 57. Bihari V, Tripathi CKM, Sur B, et al. Mass cultivation of Bacillus thuringiensis var. kurstaki in fed‐batch culture for high spore count and improved insecticidal activity. Indian J Biotechnol. 2002;1:205‐208. [Google Scholar]
- 58. Murugesan GS, Tharani J, Vivek I, Karthick PJ. Bioprocess optimization and evaluation of probiotics efficacy on growth performance of Broilers and Country Chicks. Int J Curr Microbiol App Sci. 2014;3:575‐583. [Google Scholar]
- 59. Sarrafzadeh MH, Guiraud JP, Lagneau C, Gaven B, Carron A, Navarro J‐M. Growth, sporulation, δ‐endotoxins synthesis, and toxicity during culture of Bacillus thuringiensis H14. Curr Microbiol. 2005;51:75‐81. [DOI] [PubMed] [Google Scholar]
- 60. Lalloo R, Maharajh D, Görgens J, Gardiner N. High‐density spore production of a B. cereus aquaculture biological agent by nutrient supplementation. Appl Microbiol Biotechnol. 2009;83:59‐66. [DOI] [PubMed] [Google Scholar]
- 61. Chen ZM, Li Q, Liu HM, et al. Greater enhancement of Bacillus subtilis spore yields in submerged cultures by optimization of medium composition through statistical experimental designs. Appl Microbiol Biotechnol. 2010;85:1353‐1360. [DOI] [PubMed] [Google Scholar]
- 62. Hageman JH, Shankweiler GW, Wall PR, et al. Single, chemically defined sporulation medium for Bacillus subtilis: growth, sporulation, and extracellular protease production. J Bacteriol. 1984;160:438‐441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sasaki K, Jiaviriyaboonya S, Rogers PL. Enhancement of sporulation and crystal toxin production by cornsteep liquor feeding during intermittent fed‐batch culture of Bacillus sphaericus 2362. Biotechnol Lett. 1998;20:165‐168. [Google Scholar]
- 64. Pandey KR, Vakil BV. Development of bioprocess for high density cultivation yield of the probiotic Bacillus coagulans and its spores. J Biosci Bioeng. 2016;5:173‐181. [Google Scholar]
- 65. Yin L, Chen MX, Zeng TH, Liu XM, Zhu F, Huang RQ. Improving probiotic spore yield using rice straw hydrolysate. Lett Appl Microbiol. 2021;72:149‐156. [DOI] [PubMed] [Google Scholar]
- 66. Elsayed EA, Othman NZ, Malek R, et al. Bioprocess development for high cell mass and endospore production by Bacillus thuringiensis var israelensis in semi‐industrial scale. J Pure Appl Microbiol. 2014;8:2773‐2783. [Google Scholar]
- 67. Lacerda A, De Carvalho U, Henrique F, et al. Growth, sporulation and production of bioactive compounds by Bacillus subtilis R14. Arch Biol Technol. 2010;53(3):643‐652. [Google Scholar]
- 68. Posada‐Uribe LF, Romero‐Tabarez M, Villegas‐Escobar V. Effect of medium components and culture conditions in Bacillus subtilis EA‐CB0575 spore production. Bioprocess Biosyst Eng. 2015;38:1879‐1888. [DOI] [PubMed] [Google Scholar]
- 69. Awad H, Abd Malek R, Othman NZ, et al. Bioprocess development for enhanced spore production in shake flask and pilot scale bioreactors of Bacillus thuringiensis var. israelensis in submerged culture. IOSR J Pharm Biol Sci. 2015;10:103‐108. [Google Scholar]
- 70. Glatzl BA, Goepfert AM. Production of Bacillus cereus enterotoxin in defined media in fermenter‐grown cultures. J Food Prot. 1977;40:472‐474. [DOI] [PubMed] [Google Scholar]
- 71. Stewart GS, Johnstone K, Hagelberg E, Ellar DJ. Commitment of bacterial spores to germinate. A measure of the trigger reaction. Biochem J. 1981;198:101‐106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Abbas AA, Planchon S, Jobin M, Schmitt P. A new chemically defined medium for the growth and sporulation of Bacillus cereus strains in anaerobiosis. J Microbiol Methods. 2014;105:54‐58. [DOI] [PubMed] [Google Scholar]
- 73. Molière N, Turgay K. General and regulatory proteolysis in Bacillus subtilis . In: Dougan DA, ed. Bacillus Subtilis: Subcellular Biochemistry. Springer Science and Business Media; 2013:73‐103. [DOI] [PubMed] [Google Scholar]
- 74. Baril E, Coroller L, Couvert O, et al. Modeling heat resistance of Bacillus weihenstephanensis and Bacillus licheniformis spores as function of sporulation temperature and pH. Food Microbiol. 2012;30:29‐36. [DOI] [PubMed] [Google Scholar]
- 75. Baweja RB, Zaman MS, Mattoo AR, et al. Properties of Bacillus anthracis spores prepared under various environmental conditions. Arch Microbiol. 2008;189:71‐79. [DOI] [PubMed] [Google Scholar]
- 76. Garcia D, van der Voort M, Abee T. Comparative analysis of Bacillus weihenstephanensis KBAB4 spores obtained at different temperatures. Int J Food Microbiol. 2010;140:146‐153. [DOI] [PubMed] [Google Scholar]
- 77. Mazas M, López M, González I, Bernardo A, Martín R. Effects of sporulation pH on the heat resistance and the sporulation of Bacillus cereus . Lett Appl Microbiol. 1997;25:331‐334. [DOI] [PubMed] [Google Scholar]
- 78. Nguyen Thi Minh H, Perrier‐Cornet JM, Gervais P. Effect of the osmotic conditions during sporulation on the subsequent resistance of bacterial spores. Appl Microbiol Biotechnol. 2008;80:107‐114. [DOI] [PubMed] [Google Scholar]
- 79. Nguyen Thi Minh H, Durand A, Loison P, Perrier‐Cornet J‐M, Gervais P. Effect of sporulation conditions on the resistance of Bacillus subtilis spores to heat and high pressure. Appl Microbiol Biotechnol. 2011;90:1409‐1417. [DOI] [PubMed] [Google Scholar]
- 80. Planchon S, Dargaignaratz C, Levy C, Ginies C, Broussolle V, Carlin F. Spores of Bacillus cereus strain KBAB4 produced at 10°C and 30°C display variations in their properties. Food Microbiol. 2011;28:291‐297. [DOI] [PubMed] [Google Scholar]
- 81. Pandav PV, Ahire RS, Patil SR, Pawar SB. Process development for high density cultivation yield for Bacillus subtilis . International Journal of Recent Advances in Multidisciplinary Topics. 2021;2:335‐339. [Google Scholar]
- 82. Avignone‐Rossa C, Arcas J, Mignone C. Bacillus thuringiensis growth, sporulation and δ‐endotoxin production in oxygen limited and non‐limited cultures. World J Microbiol Biotechnol. 1992;8:301‐304. [DOI] [PubMed] [Google Scholar]
- 83. Boniolo FS, Rodrigues RC, Prata AMR, et al. Oxygen supply in Bacillus thuringiensis fermentations: bringing new insights on their impact on sporulation and δ‐endotoxin production. Appl Microbiol Biotechnol. 2012;94:625‐636. [DOI] [PubMed] [Google Scholar]
- 84. Abbas AA, Planchon S, Jobin M, Schmitt P. Absence of oxygen affects the capacity to sporulate and the spore properties of Bacillus cereus. Food Microbiol. 2014;42:122‐131. [DOI] [PubMed] [Google Scholar]
- 85. Das S, Kharkwal S, Pandey SK, Sen R. Multi‐objective process optimization and integration for the sequential and increased production of biomass, lipase and endospores of a probiotic bacterium. Biochem Eng J. 2010;50:77‐81. [Google Scholar]
- 86. Elsayed EA, Othman N, Malek R, et al. Bioprocess development for high cell mass and endospore production by Bacillus thuringiensis var israelensis in semi‐industrial scale. J Pure Appl Microbiol. 2014;8:2773‐2783. [Google Scholar]
- 87. Sasaki K, Idachaba MA, Rogers PL. An improved method for cell and spore counts during aerobic cultivation of crystal toxin producing Bacillus sphaericus . Biotechnol Tech. 1995;9:321‐326. [Google Scholar]
- 88. Ahmed AA, Dirar HA. Effect of aeration and method of addition of glucose sugar to culture medium on growth and sporulation of some Bacillus thuringiensis isolates from Sudan soils. Gezira J Agric Sci. 2011;9. [Google Scholar]
- 89. Deckwer WD, Jahn D, Hempel D, Zeng AP. Systems biology approaches to bioprocess development. Eng Life Sci. 2006;6:455‐469. [Google Scholar]
- 90. Shi F, Zhu Y. Application of statistically‐based experimental designs in medium optimization for spore production of Bacillus subtilis from distillery effluent. BioControl. 2007;52:845‐853. [Google Scholar]
- 91. Rao YK, Tsay KJ, Wu WS, Tzeng YM. Medium optimization of carbon and nitrogen sources for the production of spores from Bacillus amyloliquefaciens B128 using response surface methodology. Process Biochem. 2007;42:535‐541. [Google Scholar]
- 92. Pandey KR, Joshi C, Vakil BV. Statistical optimization for enhanced yields of probiotic Bacillus coagulans and its phage resistant mutants followed by kinetic modelling of the process. Springerplus. 2016;5;1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Atehortúa P, Álvarez H, Orduz S. Modeling of growth and sporulation of Bacillus thuringiensis in an intermittent fed batch culture with total cell retention. Bioprocess Biosyst Eng. 2007;30:447‐456. [DOI] [PubMed] [Google Scholar]
- 94. Pandey A. Aspects of fermenter design for solid‐state fermentations. Process Biochem. 1991;26:355‐361. [Google Scholar]
- 95. Hölker U, Höfer M, Lenz J. Biotechnological advantages of laboratory‐scale solid‐state fermentation with fungi. Appl Microbiol Biotechnol. 2004;64:175‐186. [DOI] [PubMed] [Google Scholar]
- 96. Hölker U, Lenz J. Solid‐state fermentation—Are there any biotechnological advantages? Curr Opin Microbiol. 2005;8:301‐306. [DOI] [PubMed] [Google Scholar]
- 97. Ooijkaas LP, Weber FJ, Buitelaar RM, Tramper J, Rinzema A. Defined media and inert supports: their potential as solid‐state fermentation production systems. Trends Biotechnol. 2000;18:356‐360. [DOI] [PubMed] [Google Scholar]
- 98. Gangadharan D, Sivaramakrishnan S, Nampoothiri KM, Pandey A. Solid culturing of Bacillus amyloliquefaciens for alpha amylase production. Food Technol Biotechnol. 2006;44:269‐274. [Google Scholar]
- 99. Mahoney R, Weeks R, Zheng T, et al. Evaluation of an industrial soybean byproduct for the potential development of a probiotic animal feed additive with Bacillus species. Probiotics Antimicrob Proteins. 2020;12:1173‐1178. [DOI] [PubMed] [Google Scholar]
- 100. Su Y, Liu C, Long Z, et al. Poultry‐beneficial solid‐state Bacillus amyloliquefaciens B‐1895 fermented soybean formulation. Probiotics Antimicrob Proteins. 2019;10:593‐599. [Google Scholar]
- 101. Zhao S, Hu N, Huang J, Liang Y, Zhao B. High‐yield spore production from Bacillus licheniformis by solid state fermentation. Biotechnol Lett. 2008;30:295‐297. [DOI] [PubMed] [Google Scholar]
- 102. Berikashvili V, Sokhadze K, Kachlishvili E, Elisashvili V, Chikindas ML. Bacillus amyloliquefaciens spore production under solid‐state fermentation of lignocellulosic residues. Probiotics Antimicrob Proteins. 2018;10:755‐761. [DOI] [PubMed] [Google Scholar]
- 103. Chistyakov V, Mlnikov V, Chikindas ML, et al. Poultry‐beneficial solid‐state Bacillus amyloliquefaciens B‐1895 fermented soybean formulation. Biosci Microbiota Food Health. 2015;34:25‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Lima‐Pérez J, López‐Pérez M, Viniegra‐González G, Loera O. Solid‐state fermentation of Bacillus thuringiensis var kurstaki HD‐73 maintains higher biomass and spore yields as compared to submerged fermentation using the same media. Bioprocess Biosyst Eng. 2019;42:1527‐1535. [DOI] [PubMed] [Google Scholar]
- 105. Smitha RB, Jisha VN, Pradeep S, Josh MS, Benjamin S. Potato flour mediated solid‐state fermentation for the enhanced production of Bacillus thuringiensis‐toxin. J Biosci Bioeng. 2013;116:595‐601. [DOI] [PubMed] [Google Scholar]
- 106. Regina Barroso Ruiz Sella S, Palácio Guizelini B, Porto de Souza Vandenberghe L, Medeiros ABP, Soccol CR. Lab‐scale production of Bacillus atrophaeus’ spores by solid state fermentation in different types of bioreactors. Arch Biol Technol. 2009;52:159‐170. [Google Scholar]
- 107. Zhao ZM, Xi JT, Xu JF, Ma L‐T, Zhao J. Enhancement of Bacillus subtilis growth and sporulation by two‐stage solid‐state fermentation strategy. Processes. 2019;7:644. [Google Scholar]
- 108. Dai JY, Yang Y, Dong YS, Xiu ZL. Solid‐state co‐cultivation of Bacillus subtilis, Bacillus mucilaginosus, and Paecilomyces lilacinus using tobacco waste residue. Appl Biochem Biotechnol. 2020;190:1092‐1105. [DOI] [PubMed] [Google Scholar]
- 109. Fink M, Cserjan‐Puschmann M, Reinisch D, Striedner G. High‐throughput microbioreactor provides a capable tool for early stage bioprocess development. Sci Rep. 2021;11:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Bareither R, Bargh N, Oakeshott R, Watts K, Pollard D. Automated disposable small scale reactor for high throughput bioprocess development: a proof of concept study. Biotechnol Bioeng. 2013;110:3126‐3138. [DOI] [PubMed] [Google Scholar]
- 111. Tzeng YM, Rao YK, Tsay KJ, Wu WS. Effect of cultivation conditions on spore production from Bacillus amyloliquefaciens B128 and its antagonism to Botrytis elliptica . J Appl Microbiol. 2008;104:1275‐1282. [DOI] [PubMed] [Google Scholar]
- 112. Rojas NL, Lewkowicz ES, Nobile ML. Alternative low‐cost process for large‐scale production of Bacillus thuringiensis in a simple and novel culture system. J Environ Sci Health B. 2018;53:719‐728. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon request.


