Abstract
Protozoans are gaining recognition as environmental hosts for a variety of waterborne pathogens. We compared the growth of Mycobacterium avium, a human pathogen associated with domestic water supplies, in coculture with the free-living amoeba Acanthamoeba polyphaga with the growth of M. avium when it was separated from amoebae by a 0.1-μm-pore-size polycarbonate membrane (in a parachamber). Although viable mycobacteria were observed within amoebal vacuoles, there was no significant difference between bacterial growth in coculture and bacterial growth in the parachamber. This suggests that M. avium is able to grow saprozoically on products secreted by the amoebae. In contrast, Legionella pneumophila, a well-studied intracellular parasite of amoebae, multiplied only in coculture. A comparison of amoebae infected with L. pneumophila and amoebae infected with M. avium by electron microscopy demonstrated that there were striking differences in the locations of the bacteria within amoebal cysts. While L. pneumophila resided within the cysts, M. avium was found within the outer walls of the double-walled cysts of A. polyphaga. These locations may provide a reservoir for the bacteria when environmental conditions become unfavorable.
The facultative intracellular pathogen Mycobacterium avium is one of the primary health threats to patients with AIDS. It can cause bacteremia and disseminated multiorgan bacterial disease, including pulmonary infections of immunocompetent individuals (10, 15). The interaction of mycobacteria with host phagocytic cells likely is central to mycobacterial pathogenesis. Potential virulence mechanisms against phagocytic cells include prevention of the acidification of phagocytic vesicles and limited fusion of the phagosomes with the endosomal and lysosomal compartments that can lead to bacterial replication within macrophages (12, 13, 18). Within phagocytic cells M. avium bacilli tend to occupy individual vacuoles and to release superoxide dismutase (15) and the cell wall constituent lipoarabinomannan into the cytoplasm and into other lysosomal vesicles (34). Immunoelectron microscopy of macrophages infected with M. avium indicates that the parasitophoric vacuolar membrane possesses the late endosomal marker lysosome-associated membrane protein 1 but lacks the vesicular proton-ATPase (34).
The epidemiology of M. avium has been compared to that of Legionella pneumophila (8) because both organisms are widespread in aquatic environments, including municipal drinking water systems (9, 14, 16, 27, 32). Previous studies have proposed that amoebae might be environmental hosts of certain mycobacteria (4, 24). More recently, it has been shown that M. avium survives intracellularly within Acanthamoeba castellanii and interferes with the fusion of the lysosomal and parasitophoric vacuoles. In addition, the growth of M. avium in environmental amoebae results in increased virulence in the beige mouse model (8). These observations are reminiscent of the intracellular parasitism of L. pneumophila in the trophozoites of a variety of free-living amoebae. Meanwhile, it is well-documented that L. pneumophila multiplies within amoebae and that Acanthamoeba cysts are able to protect legionellae from certain disinfection procedures (17, 22).
Since the biology of mycobacteria and the biology of legionellae have some significant similarities, we compared these two pathogens with regard to their interactions with amoebae. We confirmed that M. avium survives intracellularly and that growth occurs in coculture with amoebae. In contrast to L. pneumophila, M. avium exhibits saprophytic growth on products secreted by Acanthamoeba cells. However, since the mycobacteria are viable in trophozoites and cysts of Acanthamoebae, they might benefit from this interaction under adverse conditions.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The M. avium serotype 4 strain used was a clinical isolate obtained from an AIDS patient (19). The strain was cultured on Middlebrook 7H11 agar (Difco) supplemented with oleic acid, albumin, dextrose, and catalase (BBL) for 10 days at 37°C. For amoeba infection assays, M. avium was grown in Middlebrook 7H9 broth (Difco) supplemented with albumin, dextrose, and catalase (BBL) to the late logarithmic phase with slow shaking (50 rpm) for 8 days at 37°C in the presence of 5% CO2. L. pneumophila Philadelphia I JR32 was grown on buffered charcoal-yeast extract agar. The plates were incubated at 37°C in the presence of 5% CO2 for 3 days. Escherichia coli HB101 was maintained on Luria-Bertani agar at 37°C.
Acanthamoeba polyphaga.
Acanthamoeba polyphaga ATCC 30872 was obtained from the American Type Culture Collection and was maintained axenically at room temperature in PYG 712 broth [2% proteose peptone, 0.1% yeast extract, 0.1 M glucose, 4 mM MgSO4, 0.4 M CaCl2, 0.1% sodium citrate dihydrate, 0.05 mM Fe(NH4)2(SO4)2 · 6H2O, 2.5 mM NaH2PO3, 2.5 mM K2HPO3] as monolayers in 75-cm2 tissue culture flasks. Amoebae were suspended by tapping the flasks. Cell counts were determined with a modified Fuchs-Rosenthal chamber. Amoebae were subcultured at intervals of 10 days.
Infection of A. polyphaga monolayers and parachamber experiments.
L. pneumophila and E. coli were suspended in Acanthamoeba buffer (see below) and centrifuged at the maximum speed in an Eppendorf microcentrifuge, and the pellets were washed twice in Acanthamoeba buffer. Broth cultures of M. avium were likewise centrifuged and washed twice in buffer. After washing, M. avium and L. pneumophila were incubated in Acanthamoeba buffer for 10 days (33°C, 5% CO2) without shaking to deplete the stored nutrients in the bacteria. This 10-day starvation procedure was necessary since the residual growth rate of M. avium in buffer was very high. After this step the bacterial suspensions were vortexed for 1 min and adjusted in Acanthamoeba buffer to a concentration of 108 cells/ml, as determined by optical density at 600 nm.
Axenic cultures of A. polyphaga in the logarithmic phase were centrifuged (200 × g, 15 min), and the pellets were resuspended in Acanthamoeba buffer (PYG 712 medium without proteose peptone, yeast extract, sodium citrate, and glucose). After two repetitions of this washing step, the cells were adjusted to a titer of 105 cells per ml in this buffer. One milliliter of this amoebal suspension was pipetted into each well of a 24-well microtiter plate (Costar, Cambridge, Mass.). Following 2 h of incubation at 33°C, the amoeba monolayer was inoculated with 10 μl of a bacterial suspension (105 bacteria/ml), which resulted in a preparation containing 103 bacteria/ml (multiplicity of infection, 10−2). The numbers of CFU were determined at time zero and after 1, 7, and 14 days. In a control experiment the multiplication of the bacteria in buffer without amoebae was determined. To minimize evaporation, the plates were sealed in plastic bags.
In the parachamber experiment the bacteria were washed as described above, and 1 ml of a suspension containing 103 bacteria/ml in Acanthamoeba buffer was pipetted into each well of a 24-well microtiter plate (Costar). After insertion of a parachamber (diameter, 6.5 mm; transwell pore size, 0.1 μm; Costar) into each well, 200 μl of an amoebal suspension containing 5 × 105 cells/ml in Acanthamoeba buffer was pipetted into each transwell. Colony counts were determined at time zero and after 1, 7, and 14 days. As described above for the Acanthamoeba infection, control experiments in buffer were performed and precautions to prevent evaporation were taken. In addition, the separation of bacteria and amoebae by the 0.1-μm-pore-size polycarbonate membrane filter was confirmed by viable cell counting and by light microscopy of the contents of the lower chamber.
Localization of intracellular bacteria and viability testing of M. avium.
One milliliter of an Acanthamoeba cell suspension (105 cells/ml in Acanthamoeba buffer) was inoculated with a minimal volume containing 107 bacteria (in Acanthamoeba buffer) to yield a multiplicity of infection of 100. At 1, 6, 24, and 48 h the amoeba monolayer was washed twice with 1 ml of phosphate-buffered saline to eliminate noningested bacteria. To localize intracellular bacteria, the cells were processed for acid-fast staining and electron microscopy. For electron microscopy the cells were fixed in 2.5% cacodylate-buffered glutaraldehyde, postfixed with 1% osmium tetroxide in 0.1 M cacodylate buffer (1 h), and embedded in Epon resin. Thin sections were stained with 2% uranyl acetate and lead citrate and examined with a transmission electron microscope (Phillips Electronic Instruments, Mahwah, N.J.) at 40 kV.
To determine the viability of intracellular bacteria, a Baclight Live/Dead kit (Molecular Probes, Junction City, Oreg.) was used as described by the manufacturer. Briefly, after 2 days of incubation at 33°C, infected acanthamoebae (see above) were mounted on glass slides. Saline (100 μl) was placed on the air-dried cells, and 1 μl of SYTO 9 nucleic acid stain and 1 μl of a propidium iodide solution were suspended in the saline. SYTO 9 is a nucleic acid stain that labels bacterial cells with green fluorescence; propidium iodide, a red fluorescent nucleic acid stain, penetrates only bacteria with damaged membranes and effectively competes with SYTO 9 for nucleic acid binding sites. Thus, damaged cells were identified by red fluorescence, while live cells were identified by green fluorescence. The preparation was incubated in the dark for 15 min and then examined by fluorescence microscopy.
Synchronous encystment of infected A. polyphaga cells.
The experiment to study infection of A. polyphaga monolayers was set up as described above for the coculture experiment except that the mycobacterial inoculum used was larger (final concentration, 107 bacteria/ml). After incubation for 24 h at 33°C, the supernatant was discarded, and the amoeba monolayer was rinsed with encystment buffer [0.1 M KCl, 0.02 M tris(2-amino-2-hydroxymethyl)-1,3-propandiol, 8 mM MgSO4, 0.4 mM CaCl2, 1 mM NaHCO3] and then incubated in fresh encystment buffer at 33°C. After 3 days, the cell suspension was centrifuged (1,000 × g, 20 min), and the pellet was resuspended in 3% (vol/vol) hydrochloric acid. This acid treatment was sufficient to kill the remaining trophozoites, immature cysts, and extracellular bacteria after 36 h. During the treatment the percentages of viable amoebae and bacteria were determined by Trypan blue exclusion and plating on Middlebrook 7H11 agar, respectively. After the acid treatment the cysts were washed three times with Acanthamoeba buffer. One-half of the sample was processed for electron microscopy (see above), and the other half was incubated in PYG medium at 33°C for 7 days. The excystment of the cysts was examined by light microscopy, and the presence of viable bacteria was determined by viable counting on Middlebrook 7H11 agar plates.
RESULTS
Growth of bacteria in Acanthamoeba buffer.
Despite intensive washing with buffer, unstarved mycobacteria showed significant residual growth in buffer after 10 days of incubation (Table 1). The increases in CFU per milliliter were 72-fold for the unstarved bacilli and 6-fold for the starved mycobacteria. In contrast, the number of CFU of L. pneumophila was almost unaffected by starvation (0.3-fold decrease for unstarved legionellae and 0.7-fold decrease for starved legionellae). The viability of unstarved E. coli decreased 0.049-fold. Although media and growth conditions are known to influence virulence, there was no obvious difference in the initial uptake by amoebae between starved and unstarved bacteria (data not shown).
TABLE 1.
Growth of unstarved and starved bacteria in Acanthamoeba buffer
| Strain | Growth increment in Acanthamoeba buffera
|
|
|---|---|---|
| Unstarved bacteria | Starved bacteriab | |
| M. avium serotype 4 | 72.2 ± 59.6 | 6.1 ± 6.3 |
| L. pneumophila JR32 | 0.31 ± 0.13 | 0.72 ± 0.24 |
| E. coli HB101 | 0.049 ± 0.067 | NDc |
Number of CFU per milliliter after 10 days divided by initial number of CFU per milliliter (mean ± standard deviation from four independent experiments). The preparations were incubated at 33°C.
Starved M. avium and L. pneumophila cells were incubated in nutrient-free buffer for 10 days to deplete the stored nutrients in the bacteria.
ND, not determined.
Direct coculture and parachamber culture.
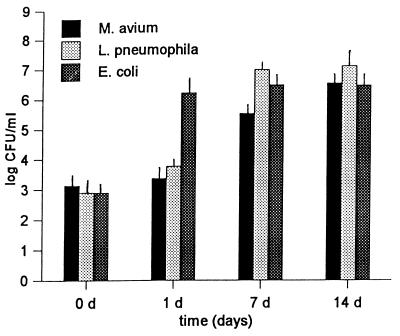
E. coli and starved cells of M. avium and L. pneumophila were tested for the ability to grow in coculture with A. polyphaga. The results of this growth assay are shown in Fig. 1. For M. avium the number of bacteria increased from 1.5 × 103 CFU in the inoculum to 3 × 105 CFU after 7 days and to 4 × 106 CFU after 14 days. In monolayers infected with L. pneumophila the number of bacteria increased from 5 × 102 CFU in the inoculum to 3 × 105 CFU after 2 days to 2 × 107 CFU after 14 days. E. coli also multiplied in coculture; the number of bacteria increased from 866 CFU to 1.6 × 106 CFU within 1 day.
FIG. 1.
Growth of the M. avium serotype 4 strain, L. pneumophila JR32, and E. coli HB101 in cocultures with A. polyphaga. Each coculture experiment was performed three times in Acanthamoeba buffer at 33°C. The values shown are the mean numbers of CFU (± standard deviations) determined at zero time and at 1, 7, and 14 days after coincubation was started.
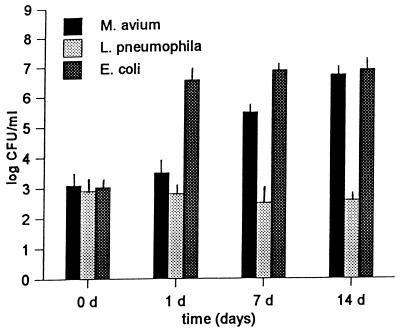
Parachamber experiments were used to determine if the amoeba-associated growth of the bacteria was due to intracellular parasitism of A. polyphaga (Fig. 2). M. avium and E. coli showed approximately the same growth kinetics as those observed in the direct coculture experiment. In contrast, L. pneumophila showed no increase in CFU when it was separated from the amoebae by the 0.1-μm-pore-size polycarbonate membrane. Due to the low-nutrient-content environment the number of amoebal cysts increased during the course of the experiment.
FIG. 2.
Growth of the M. avium serotype 4 strain, L. pneumophila JR32, and E. coli HB101 when they were separated from A. polyphaga by a 0.1-μm-pore-size polycarbonate membrane (parachamber). Each parachamber experiment was performed three times in Acanthamoeba buffer at 33°C. The values shown are the mean numbers of CFU (± standard deviations) determined at zero time and at 1, 7, and 14 days after inoculation.
Intracellular survival.
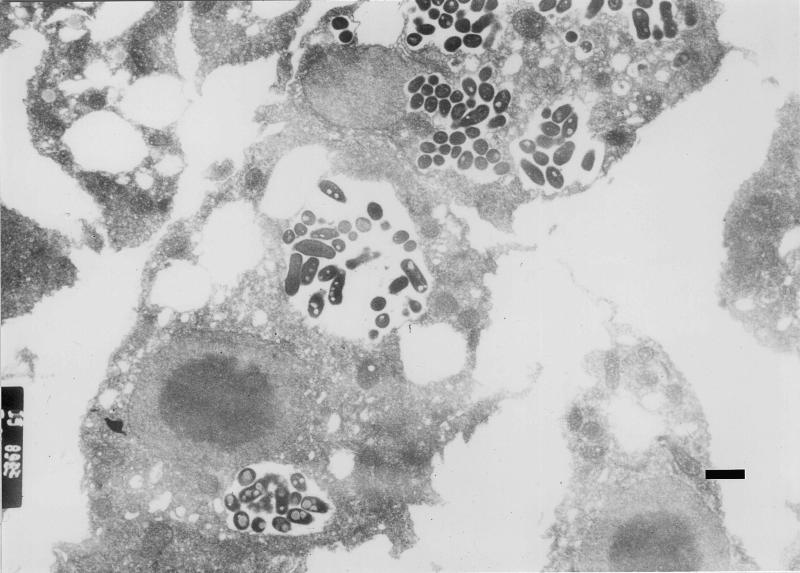
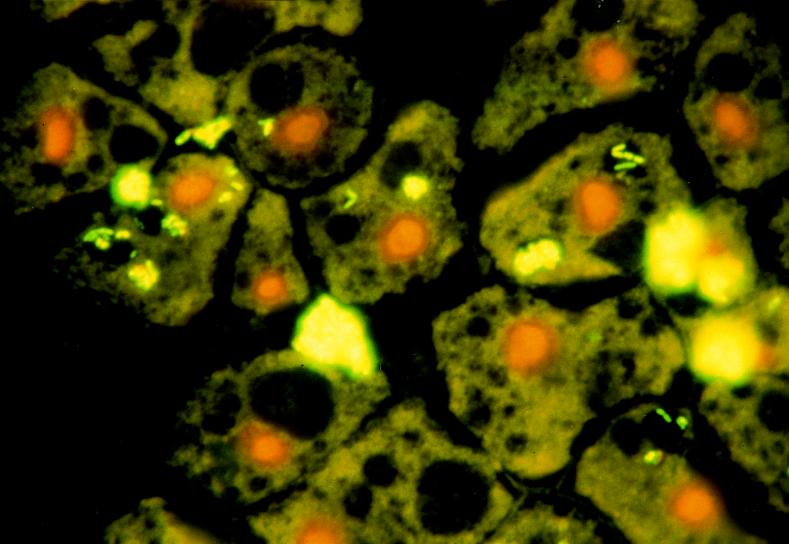
Acid-fast staining, Gimenez staining, and transmission electron microscopy were used to demonstrate bacterial ingestion by A. polyphaga during coculture. These studies confirmed previous observations that great numbers of legionellae are found intracellularly inside a single vacuole. The numbers of legionellae increase over time, and finally the bacteria fill the entire host cell. In contrast, the numbers of intracellular M. avium cells were much lower (1 to 20 bacteria per vacuole), and the bacteria were found in several vacuoles (one to six vacuoles per cell) (Fig. 3). The viabilities of intracellular bacteria could be confirmed by using differential live-dead fluorescence staining (Fig. 4). The percentage of infected amoebae increased over time, while the number of intracellular mycobacteria within a single host cell remained constant after 2 days of cocultivation. This observation suggests that the primary mechanism of mycobacterial growth in coculture is not intracellular. The very few amoebae that contained E. coli cells contained one to three bacteria in a single vacuole.
FIG. 3.
Transmission electron micrograph of M. avium serotype 4 bacilli within cytoplasmic vesicles of an A. polyphaga trophozoite after 2 days of coincubation. Bar = 1 μm.
FIG. 4.
Differential live-dead fluorescence staining of M. avium serotype 4 bacilli within vesicles of A. polyphaga trophozoites after 2 days of coincubation. Green fluorescence indicates live bacterial cells that have an intact membrane.
M. avium within Acanthamoeba cysts.
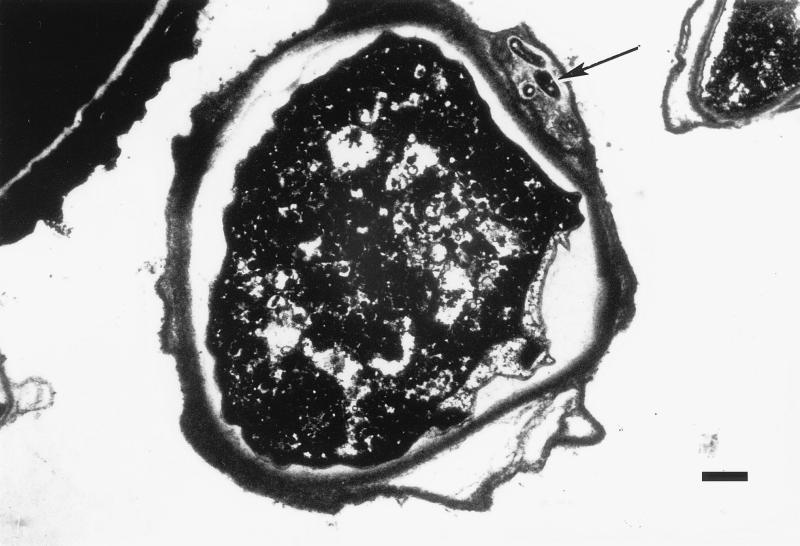
The ability of L. pneumophila to survive within the cysts of A. polyphaga was previously demonstrated by Kilvington and Price (22). To determine whether M. avium and E. coli have similar capabilities, the encystment of amoebae after coculture with these species was induced by incubation in encystment buffer. Six hours after cyst induction the trophozoites rounded up, and encystment of a trophozoite appeared to be complete after 18 h. After 3 days about 92% of the trophozoites produced mature cysts (as determined by the presence of a double wall). The upper temperature limit that permits encystment is 40°C, but optimal numbers of infected cysts were found at 33°C. In order to kill the remaining trophozoites, immature cysts, and extracellular bacteria, a treatment with hydrochloric acid (3%, vol/vol) was performed. Examination of thin sections by transmission electron microscopy revealed double-walled cysts containing one to nine mycobacteria within the amoeba outer cell wall (Fig. 5). E. coli was not found within cysts. Our observations that L. pneumophila was within the cysts but was not between the cell walls of the cysts were consistent with the description given in the previous study. After 7 days in PYG medium the Acanthamoeba cysts were able to excyst and replicate. Legionellae and mycobacteria (at lower numbers), but not E. coli, were culture positive on growth plates after the excystment of the amoebae.
FIG. 5.
Transmission electron micrograph of a mature A. polyphaga cyst containing M. avium serotype 4 bacilli (arrow) within the double cell wall (note that the outer cell wall is divided and surrounds the bacterial cells). Bar = 1 μm.
DISCUSSION
The importance of protozoans in soil and water ecosystems has been recognized for several decades, and the relevance of predatory protozoans in the control of bacterial populations is widely acknowledged (11, 20, 30, 31). However, the potential role of protozoans as reservoirs for human pathogens has only recently received adequate attention (2, 4, 17, 25). The best-studied example of a protozoan-bacterial pathogen interaction is the intracellular parasitism of L. pneumophila. Adaptation of L. pneumophila to parasitism of free-living amoebae might have led to the ability of this environmental bacterium to infect human lung macrophages (4). This observation led to the concepts that selection for resistance to digestion by predatory protozoans is a driving force in the evolution of pathogenic environmental bacteria and that protists are the “missing link between ecology and pathology” (4, 23, 25).
Since M. avium has been isolated from habitats where amoebae normally feed on bacteria (14, 26, 29, 32), we studied the interaction of A. polyphaga with M. avium. Our results show that M. avium does not behave in a manner analogous to L. pneumophila. Since L. pneumophila only grows intracellularly within amoebae, the parasitism of legionellae seems to be much more specific and more highly evolved than the simple growth enhancement of mycobacteria and E. coli by their association with amoebae. In pure buffer (parachamber experiments) M. avium and E. coli were able to grow as free-living saprophytes on products secreted by A. polyphaga. Although we demonstrated that the growth of M. avium in direct cocultures and the growth of M. avium in parachamber experiments are similar, we cannot eliminate the possibility that intracellular multiplication occurs. Consistent with results published recently (8), we found that M. avium is able to survive in the intracellular environment of amoebae, and consequently, these bacteria may receive nutrients and grow within the protozoans. Considering that M. avium is a slowly growing bacterium that lives in a habitat where amoebae feed on bacteria, survival in a hostile intracellular environment may be an important advantage.
Although there are important differences in the interactions of amoebae with legionellae and mycobacteria, intracellular survival provides a possible evolutionary explanation for how a saprozoic-saprophytic or parasitic environmental organism can acquire the ability to survive within human macrophages. M. avium and L. pneumophila are known to inhibit phagolysosomal vacuole fusion in amoebae and macrophages (5, 8, 13, 18, 21). Other defense mechanisms, such as bacterial toxin production, the presence of protective outer membrane structures, and the reduction of vacuole acidification by the bacteria, might also contribute to resistance in phagocytic cells. A comparison of the intracellular activities that occur in protozoans after ingestion of bacteria showed that these activities are very similar to the activities observed in macrophages (1, 3, 5, 7, 8, 25, 33).
It is generally accepted that engulfed bacteria may benefit from the protective coat conferred by protozoans (23). The presence of mycobacteria in domestic water supplies and the results of disinfection studies in which the authors tested various concentration of sodium hypochlorite suggest that chlorination has little effect on mycobacteria (6, 9, 27). Previous studies have demonstrated that cysts of A. polyphaga can contain viable L. pneumophila cells which are protected from disinfection (22). Recovery of M. avium from HCl-treated cysts showed that the exploitation of this amoebal differentiation event is not restricted to L. pneumophila. Therefore, we suggest that the resistance of infected amoebal cysts to biocidal agents may additionally interfere with disinfection of domestic water supplies contaminated with mycobacteria. However, in contrast to L. pneumophila, M. avium is located within the double walls of the cysts, which are known to be composed largely of polysaccharides (one-third is cellulose) (28). The numbers of bacterial cells in the cysts are much lower than the numbers of legionellae, and it is unclear how mycobacteria are distributed to this location.
To control and prevent the dissemination of nontuberculosis mycobacterial infections, it is important to focus on the ecology of the bacterial environment. Although no data are available, protozoans like Hartmannella and Naegleria spp. very likely exhibit interactions with mycobacteria identical to those observed with acanthamoebae. The results of these and other studies in which pathogens were enhanced by associations with protozoans and perhaps biofilms indicate that there is a need for further research on the physiological ecology of these pathogens (16).
ACKNOWLEDGMENT
This work was supported by grant Ste 838/1-1 from the Deutsche Forschungsgemeinschaft.
REFERENCES
- 1.Allen P G, Dawidowicz E A. Phagocytosis in Acanthamoeba. I. A mannose receptor is responsible for the binding and phagocytosis of yeast. J Cell Physiol. 1990;145:508–513. doi: 10.1002/jcp.1041450317. [DOI] [PubMed] [Google Scholar]
- 2.Amman R, Springer N, Schonhuber W, Ludwig W, Schmid E N, Muller K D, Michel R. Obligate intracellular bacterial parasites of acanthamoebae related to Chlamydia spp. Appl Environ Microbiol. 1997;63:115–121. doi: 10.1128/aem.63.1.115-121.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Avery S V, Harwood J L, Lloyd D. Quantification and characterization of phagocytosis in the soil amoeba Acanthamoeba castellanii by flow cytometry. Appl Environ Microbiol. 1995;61:1124–1132. doi: 10.1128/aem.61.3.1124-1132.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barker J, Brown M R W. Trojan horses of the microbial world: protozoa and the survival of bacterial pathogens in the environment. Microbiology. 1994;140:1253–1259. doi: 10.1099/00221287-140-6-1253. [DOI] [PubMed] [Google Scholar]
- 5.Bozue J A, Johnson W. Interaction of Legionella pneumophila with Acanthamoeba castellanii: uptake by coiling phagocytosis and inhibition of phagosome-lysosome fusion. Infect Immun. 1996;64:668–673. doi: 10.1128/iai.64.2.668-673.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carson L A, Petersen N J, Favero M S, Aguero S M. Growth characteristics of atypical mycobacteria in water and their comparative resistance to disinfectants. Appl Environ Microbiol. 1978;36:839–846. doi: 10.1128/aem.36.6.839-846.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cianciotto N P, Fields B S. Legionella pneumophila mip gene potentiates intracellular infection of protozoa and human macrophages. Proc Natl Acad Sci USA. 1992;89:5188–5191. doi: 10.1073/pnas.89.11.5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cirillo J D, Falkow S, Tompkins L S, Bermudez L E. Interaction of Mycobacterium avium with environmental amoebae enhances virulence. Infect Immun. 1997;65:3759–3767. doi: 10.1128/iai.65.9.3759-3767.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collins C H, Grange J M, Yates M D. A review: mycobacteria in water. J Appl Bacteriol. 1984;57:193–211. doi: 10.1111/j.1365-2672.1984.tb01384.x. [DOI] [PubMed] [Google Scholar]
- 10.Crowle A J, Ross E R, Cohn D L, Gilden J, May M H. Comparison of the abilities of Mycobacterium avium and Mycobacterium intracellulare to infect and multiply in cultured human macrophages from normal and human immunodeficiency virus-infected subjects. Infect Immun. 1992;60:3697–3703. doi: 10.1128/iai.60.9.3697-3703.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Curds C R, Fey G J. The effect of ciliated protozoa on the fate of E. coli in the activated-sludge process. Water Res. 1969;3:853–867. [Google Scholar]
- 12.De Chastellier C, Frehel C, Offredo C, Skamene C. Implication of phagosome-lysosome fusion in restriction of Mycobacterium avium growth in bone marrow macrophages from genetically resistant mice. Infect Immun. 1993;61:3775–3784. doi: 10.1128/iai.61.9.3775-3784.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Chastellier C, Lang T, Thilo L. Phagocytic processing of the macrophage endoparasite Mycobacterium avium in comparison to phagosomes which contain Bacillus or latex beads. Eur J Cell Biol. 1995;68:167–182. [PubMed] [Google Scholar]
- 14.du Moulin G C, Stottmeier K D, Pelletier P A, Tsang A Y, Hedley-Whyte J. Concentration of Mycobacterium avium by hospital hot water systems. JAMA. 1988;260:1599–1601. doi: 10.1001/jama.260.11.1599. [DOI] [PubMed] [Google Scholar]
- 15.Escuyer V, Haddad N, Frehel C, Berche P. Molecular characterization of a surface-exposed superoxide dismutase of Mycobacterium avium. Microb Pathog. 1996;20:41–55. doi: 10.1006/mpat.1996.0004. [DOI] [PubMed] [Google Scholar]
- 16.Falkinham J O. Epidemiology of infection by nontuberculous mycobacteria. Clin Microbiol Rev. 1996;9:177–215. doi: 10.1128/cmr.9.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fields B S. The molecular ecology of legionellae. Trends Microbiol. 1996;4:286–290. doi: 10.1016/0966-842x(96)10041-x. [DOI] [PubMed] [Google Scholar]
- 18.Frehel C, De Chastellier C, Lang T, Rastogi N. Evidence for inhibition of fusion of lysosomal and prelysosomal compartments with phagosomes in macrophages infected with pathogenic Mycobacterium avium. Infect Immun. 1986;52:252–262. doi: 10.1128/iai.52.1.252-262.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gan H, Newman G W, Remold H G. Human macrophages acquire a hyporesponsive state of tumor necrosis factor alpha production in response to successive Mycobacterium avium serovar 4 stimulation. Infect Immun. 1995;63:1921–1926. doi: 10.1128/iai.63.5.1921-1926.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Habte M, Alexander M. Further evidence for the regulation of bacterial populations in soil by protozoa. Arch Microbiol. 1977;113:181–183. doi: 10.1007/BF00492022. [DOI] [PubMed] [Google Scholar]
- 21.Horwitz M A. The Legionnaires’ disease bacterium (Legionella pneumophila) inhibits phagosome-lysosome fusion in human monocytes. J Exp Med. 1983;158:2108–2126. doi: 10.1084/jem.158.6.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kilvington S, Price J. Survival of L. pneumophila within cysts of Acanthamoeba polyphaga following chlorine exposure. J Appl Bacteriol. 1990;68:519–525. doi: 10.1111/j.1365-2672.1990.tb02904.x. [DOI] [PubMed] [Google Scholar]
- 23.King C H, Shotts E B, Wooley R E, Porter K G. Survival of coliforms and bacterial pathogens within protozoa during chlorination. Appl Environ Microbiol. 1988;54:3023–3033. doi: 10.1128/aem.54.12.3023-3033.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krishna-Prasad B, Gupta S K. Preliminary report on engulfment and retention of mycobacteria by trophozoites of axenically grown Acanthamoeba castellanii Douglas. Curr Sci. 1978;47:245–247. [Google Scholar]
- 25.Ly T M C, Müller H E. Ingested Listeria monocytogenes survive and multiply in protozoa. J Med Microbiol. 1990;33:51–54. doi: 10.1099/00222615-33-1-51. [DOI] [PubMed] [Google Scholar]
- 26.Ma P, Visvesvara G S, Martinez A J, Theodore F H, Daggett P M, Sawyer T K. Naegleria and Acanthamoeba infections: review. Rev Infect Dis. 1990;12:490–513. doi: 10.1093/clinids/12.3.490. [DOI] [PubMed] [Google Scholar]
- 27.Mollohan C S, Romer M S. Public health significance of swimming pool granuloma. Am J Public Health. 1961;51:883–891. doi: 10.2105/ajph.51.6.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neff R J, Ray S A, Benton W F, Wilborn M. Induction of synchronous encystment (differentiation) in Acanthamoeba sp. In: Prescott D M, editor. Methods in cell physiology. Vol. 1. London, United Kingdom: Academic Press; 1964. pp. 55–83. [Google Scholar]
- 29.Schulze-Robbecke R. Mykobakterien in der Umwelt. Immun Infekt. 1993;21:126–131. [PubMed] [Google Scholar]
- 30.Singh B N. Selectivity in bacterial food by soil amoebae in pure and mixed culture in sterilized soil. Ann Appl Biol. 1941;28:52–65. [Google Scholar]
- 31.Stout J D. The relationship between protozoan populations and biological activity in soils. Am Zool. 1973;13:191–201. [Google Scholar]
- 32.von Reyn C F, Maslow J N, Barber T W, Falkinham III J O, Arbeit R D. Persistent colonisation of potable water as a source of Mycobacterium avium infection in AIDS. Lancet. 1994;343:1137–1141. doi: 10.1016/s0140-6736(94)90239-9. [DOI] [PubMed] [Google Scholar]
- 33.Wintermeyer E, Ludwig B, Steinert M, Schmitt B, Fischer G, Hacker J. Influence of site-specific altered Mip proteins on intracellular survival of Legionella pneumophila in eukaryotic cells. Infect Immun. 1995;63:4576–4583. doi: 10.1128/iai.63.12.4576-4583.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu S, Cooper A, Sturgill-Koszycki S, van Heyningen T, Chatterjee D, Orme L, Allen P, Russel D G. Intracellular trafficking in Mycobacterium tuberculosis and Mycobacterium avium-infected macrophages. J Immunol. 1994;153:2568. [PubMed] [Google Scholar]