Abstract
Colon cancer (CC) is one of the most common and deadly cancers worldwide. Oncologists are facing challenges such as development of drug resistance and lack of suitable drug options for CC treatment. Flavonoids are a group of natural compounds found in fruits, vegetables, and other plant‐based foods. According to research, they have a potential role in the prevention and treatment of cancer. Apigenin is a flavonoid that is present in many fruits and vegetables. It has been used as a natural antioxidant for a long time and has been considered due to its anticancer effects and low toxicity. The results of this review study show that apigenin has potential anticancer effects on CC cells through various mechanisms. In this comprehensive review, we present the cellular targets and signaling pathways of apigenin indicated to date in in vivo and in vitro CC models. Among the most important modulated pathways, Wnt/β‐catenin, PI3K/AKT/mTOR, MAPK/ERK, JNK, STAT3, Bcl‐xL and Mcl‐1, PKM2, and NF‐kB have been described. Furthermore, apigenin suppresses the cell cycle in G2/M phase in CC cells. In CC cells, apigenin‐induced apoptosis is increased by inhibiting the formation of autophagy. According to the results of this study, apigenin appears to have the potential to be a promising agent for CC therapy, but more research is required in the field of pharmacology and pharmacokinetics to establish the apigenin effects and its dosage for clinical studies.
Keywords: apigenin, apoptosis, colon cancer cells, PI3K/AKT/mTOR, STAT3
Apigenin is a natural flavonoid that has been studied for its potential anticancer effects, including in colon cancer. In summary, research of this study indicates that apigenin may have potential anticancer effects against colon cancer by: Inducing apoptosis and autophagy; Targeting specific signaling pathways such as STAT3 and NF‐B/Snail; Inhibiting EMT, migration, and invasion of colon cancer cells.
1. INTRODUCTION
Colon cancer (CC) ranks among the five most prevalent cancers in men and women (Argilés et al., 2020).
In the year 2020, around 1.9 million CC cases were registered, with the disease expected to increase by approximately 60% (~2.2 million) by the end of 2035 (Xi & Xu, 2021). The incidence of CC is intimately linked to the socioeconomic growth and lifestyle of the population in a given country (Condello & Meschini, 2021).
CC is a cancerous growth that originates from the colon's inner lining and can extend into deeper layers of the intestinal wall. Failure to treat CC can lead to serious health consequences (Jain et al., 2020). The risk factors for CC include age, family history, race, and lifestyle choices. Although some cases of CC are associated with genetic disorders, most are caused by aging and lifestyle habits (Cheng et al., 2022; Mishra et al., 2013). CC is responsible for about 10% of new cancer diagnoses worldwide and 9.4% of mortality associated with cancer, with an estimated 600,000 fatalities annually (Sung et al., 2021). Conventional treatments for CC, including surgery, chemotherapy, and radiation therapy, are frequently used by oncologists to cure this condition (Gündoğdu & Özyurt, 2023).
Chemotherapeutic and radiotherapeutic treatments are frequently used to combat tumors, but tumor cells can either possess or develop a natural tolerance to them (Xu et al., 2020).
This tolerance can result in high recurrence rates following surgery, which is associated with poor 5‐year survival rates.
Patients with stage I or II CC may experience partial or total colon resection surgery alone. In contrast, approximately two‐thirds of those with stage III disease typically receive neoadjuvant chemotherapy and colon resection surgery to mitigate the risk of CC recurrence (Miller et al., 2016).
Nevertheless, the potency of chemotherapeutic drugs is limited due to the development of resistance, which is a significant contributor to the poor clinical outcomes observed in treated patients. (Alfaleh et al., 2022). As a result, it is imperative to find new postoperative chemotherapy drugs that can increase the ability to kill cancer cells at lower doses (Printz, 2021).
Furthermore, traditional chemotherapy, as well as molecular targeted therapy, may cause toxicity in regular tissues. Therefore, it is crucial to develop new treatments that do not cause such problems for patients (Roshani et al., 2022).
There has been a growing interest in using traditional herbal medicines to create new drugs in recent years (Boroushaki et al., 2015; Dehnamaki et al., 2019; Zamanian et al., 2021). Research suggests that regular consumption of natural products can enhance patient well‐being (He et al., 2011; Patel et al., 2010; Shokrzadeh et al., 2018).
Flavonoids, which have antioxidant, antimutagenic, anti‐inflammatory, antiangiogenic, and anticancer properties, are fascinating (Mutha et al., 2021; Slika et al., 2022; Ullah et al., 2020; Zamanian et al., 2021).
They are considered one of the promising chemopreventive agents for cancer (de Sousa Silva et al., 2023; Ferreira et al., 2022).
Apigenin (4′, 5, 7‐trihydroxy flavone, Figure 1) is a biological compound belonging to the flavonoid subclass (Abid et al., 2022; Wang et al., 2019). It is a flavonoid compound that is commonly found in many fruits and vegetables, especially parsley, celery, celeriac, and chamomile tea (Mahbub et al., 2022; Mohammad Nabavi et al., 2015).
FIGURE 1.
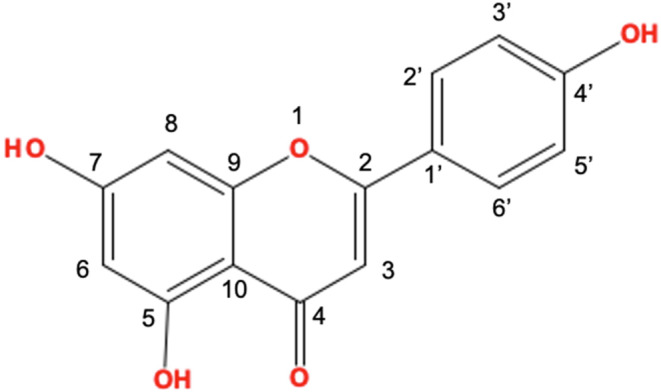
Chemical structure of apigenin.
This compound is not poisonous and is plentiful in plant‐based foods. Apigenin has numerous probable biological activities, including antioxidant, anti‐inflammatory, and anticancer effects, and can concurrently exert multiple anticancer effects by modulating essential molecular targets (Jang et al., 2022; Li et al., 2022; Zafar et al., 2022).
Apigenin displays low cytotoxicity and significantly impacts cancer cells in reference to normal cells (Gupta et al., 2001). According to various studies, apigenin downsizes cell proliferation and causes apoptosis in CC cells (Lee et al., 2014; Turktekin et al., 2011).
Apigenin shows an oncolytic effect through apoptosis induction by modulating the quantity of caspases, BAX, Bcl2, p53, etc (Mabrouk Zayed et al., 2022). Apigenin also regulates cell cycle advancement by stopping the cycle arrest at G2/M or G0/G1 checkpoint (Pandey et al., 2023). In addition, it promotes autophagy, hinders migration and invasion, and deters angiogenesis (Chen et al., 2019). Regarding the molecular anticancer mechanism of apigenin, it controls the PI3K/AKT/mTOR signaling pathway, NF‐κB/MAPK/ERK pathway, and Wnt/JNK pathway (Ahmed et al., 2021).
Thus, the aim of this review is to depict the role of apigenin in the treatment of CC in a cellular and molecular manner.
2. OVERVIEW OF APIGENIN
2.1. Structure and summary of mechanisms
Flavones are in a flavonoid class with a 2‐phenylchromen‐4‐one (2‐phenyl‐1‐benzopyran‐4‐one) backbone (Dajas et al., 2013; Ueda et al., 2004).
Apigenin, a natural flavone, is abundant in diverse plant‐based foods and beverages. It is common in parsley, grapes, red wine, chamomile tea, and apples. Typically, apigenin is conjugated to a glycoside (Jang et al., 2022; Jangdey et al., 2018).
The major source plants of apigenin include Tanacetum, Artemisia, Matricaria genera, and Achillea pertaining to the Artemisia family of plants (Ornano et al., 2016; Sharifi‐Rad et al., 2018; Venditti et al., 2015, 2018).
Certain plants contain apigenin in two forms: as aglycone or as different types of apigenin glycosides, such as apigenin‐7‐O‐glucoside, apigenin‐6‐C‐glucoside (also known as isovitexin), apigenin‐8‐C‐glucoside (also known as vitexin), apigenin‐7‐O‐neohesperidoside (also known as rhoifolin), and apigenin‐6‐C‐glucoside‐8‐C‐arabinoside (Nabavi et al., 2018). A list of various dietary sources of apigenin can be found in Table 1 (Thomas et al., 2023).
TABLE 1.
Various dietary sources of apigenin.
| Sources | Glycoside | Quantity (mg/100 g or mg/100 mL) |
|---|---|---|
| Olive oil, extra virgin | Apigenin aglycone | 1.17 |
| Italian oregano, fresh | Apigenin aglycone | 3.50 |
| Globe artichoke, heads, raw | Apigenin 7‐O‐glucuronide | 7.40 |
| Orange, pure juice | Apigenin 6, 8‐di‐C‐glucoside | 5.53 |
| Kumquats | Apigenin 7‐O‐neohesperidoside | 21.87 |
The synthesis of apigenin takes place on the exterior of the reticular apparatus through a four‐step process involving the synthesis of the intermediate, basic skeleton, precursor, and apigenin structure (Seo et al., 2011). Contemporary data suggest that the chemical structure of apigenin is critical to its bioactivity. By removing molecular subunits linked to specific biological activities, researchers can determine the structure–activity relationship of apigenin. For instance, the blocking of α‐glucosidase and α‐amylase is attributed to the presence of double bonds in the two aromatic rings and hydroxyl groups on C‐7 and C‐4′ (Li et al., 2018). On the other hand, the immune system regulation of apigenin requires a hydroxyl group at C‐4′ in ring B (Kilani‐Jaziri et al., 2016). Finally, OH radicals at positions 5, 7, and 4′ are critical in activating the Liver X receptor (Fouache et al., 2019).
Apigenin has been used as a conventional drug for many years on account of its antioxidant nature (Zafar et al., 2022) and inflammatory properties (Li et al., 2022), and anticancer outcomes (Rahmani et al., 2022; Zhou et al., 2022).
Previous research indicates that it contains broad anticancer properties; for instance, pancreatic, breast, colorectal, and prostate (Feng et al., 2023; Hnit et al., 2022; Hong et al., 2022; Qu et al., 2022). Apigenin works by inhibiting tumor cell proliferation through the induction of cell apoptosis and autophagy and modulating the cell cycle (Liang et al., 2023).
Several studies have investigated the effects of apigenin on metastasis and have found that it can inhibit the process in various types of cancer cells. Apigenin was found to suppress bone metastasis of human breast cancer cells by inducing apoptosis, autophagy, and modulation of the MEK/ERK signaling pathway (Wu et al., 2020). Apigenin inhibited migration and metastasis of human hepatocellular carcinoma (HCC) cells by suppressing the NF‐κB/Snail pathway and reversing increases in epithelial–mesenchymal transition (EMT) marker levels (Qin et al., 2016).
Additionally, it has been shown to control the immune response and the activity of NF‐κB, particularly in the lungs (Cardenas et al., 2016). In the process of tumor inhibition, apigenin modulates a couple of protein kinases and signaling pathways, including MAPK/ERK, PI3K/Akt, JAK/STAT, NF‐κB, and Wnt/β‐catenin (Yan et al., 2017).
Apigenin has been shown to have a significant part in causing apoptosis. This is accomplished by triggering the MAPK signaling pathway and reducing sulfiredoxin expression (Wang et al., 2022). Studies have demonstrated that apigenin increases the cleavage of poly‐(ADP‐ribose) polymerase (PARP) and rapidly enhances caspase‐3 activity, resulting in DNA fragmentation and apoptosis (Ghanbari‐Movahed et al., 2023; Gupta et al., 2002). These effects are attributed to a shift in the Bax/Bcl‐2 ratio in favor of programmed cell death.
According to research, apigenin can hinder the development of lung tumor cells and transcriptional activation of vascular endothelial growth factor (VEGF) in a concentration‐dependent style. The means by which apigenin suppresses VEGF transcription is indicated to be associated with the decrease in hypoxia‐inducible factor 1‐alpha (HIF‐1α; Jin & Ren, 2007).
It has also been indicated that apigenin can enhance autophagy and activate cell death in primary effusion lymphoma while significantly lowering the level of reactive oxygen species (ROS). Additionally, apigenin can activate p53, which improves catalase and inhibits STAT3, as demonstrated by p53 silencing experiments (Granato et al., 2017).
The use of apigenin caused the arrest of lung tumor cells at the G2/M phase of the cell cycle. Treating patients with apigenin was found to increase p53 and p21CIP1/WAF1 (Lee et al., 2014). Apigenin also enhanced cellular cycling and cell suicide by hindering the PI3K/Akt/mTOR pathway (Yang et al., 2018). Scientists have discovered that apigenin has the prospect of preventing cancer by controlling the ERK1/2 MAPK and PI3K/Akt signaling pathways, thus stopping the growth and spread of tumors (Lim et al., 2016). In addition, by inhibiting the Wnt/β‐catenin pathway, apigenin significantly reduced the growth, and spread of malignant cells, their ability to divide, invade surrounding tissues, migrate to new areas, and form organoids (Xu et al., 2016).
2.2. Bioavailability of apigenin
Including natural products derived from plants is recommended as part of prophylactic and treatment strategies for disease management (Chupradit et al., 2022; Zamanian et al., 2021).
Many of the therapeutic effects of therapeutic plants are attributed to different phytochemicals including flavonoids or active compounds (Mutha et al., 2021; Ullah et al., 2020).
Bioavailability is a crucial factor in drug delivery, referring to the amount and rate of the first dose of a prescription that reaches the target site or access the body (Herkenne et al., 2008; Thilakarathna & Rupasinghe, 2013). Hydrophobic compounds generally exhibit poor bioavailability because of limited capability of absorption, resulting in low levels of the drug reaching the target tumor and advanced toxicity to normal tissues (Darakhshan et al., 2015). Bioavailability can also be described as the amount of a compound absorbed, digested, metabolized, and excreted after food is ingested through the mouth (Rein et al., 2013). Typically, when medicine is taken through the mouth, polyphenols are mainly taken up and processed in the small bowel (Kahle et al., 2007), with only a small fraction being absorbed in the stomach (Crespy et al., 2002).
The hydrophilic properties of apigenin range from 0.001 to 1.63 mg per milliliter in nonpolar solvents (Mohammad Nabavi et al., 2015), in contrast, the solubility in a phosphate buffer is 2.16 μg/mL (Zhang et al., 2012). Apigenin is not easily absorbed orally because of its low water solubility, which is only 2.16 g/mL (Salehi et al., 2019), significantly hindering its clinical development. Apigenin is slowly absorbed and eliminated from the body, as evidenced by its half‐life of 91.8 h in the blood, a distribution volume of 259 mL, and a plasmatic clearance of 1.95 mL/h (Gradolatto et al., 2005).
However, a novel mesoporous silica nanoparticle drug carrier has been shown to improve the ability of apigenin to dissolve, be absorbed orally, and become bioavailable in the body (Huang et al., 2019).
2.3. Cytotoxicity of apigenin
Apigenin, a plant‐derived compound, has demonstrated selective anticancer effects and effective cell cytotoxic activity while exhibiting negligible toxicity to ordinary cells. It has also been found to quash cancer stem cells (CSCs; Ketkaew et al., 2017). The purpose of autophagy in the cytotoxic effect caused by apigenin varies depending on the type of cancer cell. Many studies suggest that autophagy induced by apigenin is responsible for developing resistance to cell apoptosis in cancer cells. This has been shown by the fact that combining apigenin with autophagy inhibitors increases cell apoptosis, indicating that autophagy reduces the cytotoxic effects of apigenin on cancer cells. However, in human papillary thyroid carcinoma BCPAP cells, autophagy leads to autophagy‐mediated cell demise (Kim et al., 2015). A study employing the use of 3‐MA, an autophagy suppressor, significantly augmented the level of apoptosis induced by apigenin, indicating that apigenin's autophagy‐mediated tumor protective properties are still present (Cao et al., 2013). Therefore, the function of autophagy in apigenin‐induced cytotoxicity depends on the type of cancer cell, as autophagy helps cancer cells to protect themselves from the cytotoxic effects of apigenin in some cases (Yan et al., 2017).
2.4. Apigenin as an anticancer agent
Anticancer drugs often have numerous harmful side effects that can cause significant harm to the organs of the patients and exacerbate their symptoms. (Schirrmacher, 2019). As such, it is essential to comprehend and be aware of these toxicities and adverse effects, especially with the rising prices of drugs and the reduction in the quantity of medications that are genuinely effective and approved by the FDA. Synthetic small molecule compounds have been found to have acquired drug resistance and negative effects, prompting the search for flavones, which are believed to have significant physiological usefulness as they have low toxicity and are not mutagenic in humans (Slika et al., 2022). Apigenin, a flavone, has gained much traction in developing anticancer agents. It has been demonstrated to have potential as a dietary supplement or a supplementary chemotherapeutic agent for treating cancer (Rahmani et al., 2022). Studies have shown that apigenin displays notable cytotoxicity toward different kinds of cancer cells and suppresses CSCs in an assortment of cancers, making it an attractive candidate for further investigation in cancer treatment. Apigenin has also been found to induce cell cycle arrest, trigger cell apoptosis and autophagy, stimulate an immune reaction, and suppress metastasis and aggression in multiple human cancers in vitro and in vivo via various biological mechanisms (Pandey et al., 2023; Yan et al., 2017; Zhong et al., 2021; Zhou et al., 2022).
2.5. Possible side effects of apigenin
Apigenin is generally considered safe for intentional consumption in higher doses, as the toxicity hazard is low. Apigenin quantity in a person's diet is not likely to get to a level that would cause injury (Shao et al., 2013).
However, intentional consumption of high doses of dietary supplements may result in a slightly increased risk of experiencing adverse effects. Some of the probable adverse reactions include stomachache, muscle relaxation, as well as sleepiness (Abid et al., 2022).
Consuming chamomile extract, which is high in apigenin, may cause stomach discomfort, and it is recommended to discontinue its use if this occurs (Abid et al., 2022). Some people may experience skin irritation when using topical products containing apigenin. It is advisable to consult a healthcare provider before taking apigenin supplements if a person is taking prescription medications due to the risk of drug interactions. The risk is particularly high for those taking cyclosporine, warfarin, or certain types of chemotherapy drugs (Pham et al., 2012).
3. EFFECTS OF APIGENIN ON COLON CANCER
CC is a predominant gastrointestinal cancer with high death rates and increased morbidity in recent years (Zhang et al., 2023). Ineffective treatment interventions and late disease recognition at progressive stages make this cancer a difficult area to manage (Gupta et al., 2019). The use of anticancer drugs for CC treatment is associated with significant side effects that negatively affect patient quality of life (Mao et al., 2011). In this regard, natural plant‐derived molecules have gained significant attention due to their comparatively lower toxicity. Researchers worldwide are focused on identifying and screening natural products against cancer cells to develop efficient drugs that can target colon and other cancers (Yang et al., 2012).
Autophagy and apoptosis are cellular functions that play important roles in maintaining tissue equilibrium and eliminating unwanted or harmful cells, including cancer cells (Amaravadi et al., 2019; Nazio et al., 2019).
The PI3K/AKT/mTOR signal transduction pathway is often anomalously triggered in cancer cells and is involved in their proliferation (Barrett et al., 2012).
In a study on cisplatin‐resistant CC cells, apigenin was found to have significant anticancer effects at doses of 15, 30, and 60 μM. The anticancer action of apigenin was credited to its capacity to cause both autophagy and programmed cell death. Additionally, apigenin was shown to inhibit the PI3K/AKT/mTOR signal transduction pathway in cisplatin‐resistant CC cells (HT‐29 cells). In vivo studies in mice also revealed that apigenin (35 mg/kg dose) hindered the development of xenografted tumors (Chen et al., 2019).
Caspase‐3, an executioner caspase, serves an indispensable function in apoptosis and is a primary target for cancer therapy (Yadav et al., 2021). Caspase‐8 is an apical caspase that initiates programmed cell death in response to the ligation of the death receptor. This essential involvement in apoptosis has sparked tremendous therapeutic interest in controlling caspase‐8 expression and proteolytic activity (Stupack, 2013).
Turktekin et al. observed that a 100 μM concentration of apigenin could force cellular death in CC cells and target multiple molecular pathways involved in the disease's progression. The study also showed that apigenin upregulated caspase‐3 and caspase‐8 while downregulating mTOR and cyclin D1. Interestingly, the researchers found that apigenin induced a p53‐independent caspase cascade, indicating that it can target CC cells that have mutated p53. Finally, the decline in mTOR expression levels suggested that apigenin drives CC cells toward apoptosis rather than autophagy (Turktekin et al., 2011).
In a separate investigation, Lee et al. found that apigenin (25 and 50 μM) impeded the development of HCT116 cells by inducing cell cycle suppression at the G2/M phase. Furthermore, apigenin therapy leads to increased apoptosis as well as autophagy. The use of the autophagy inhibitor, 3‐methyladenine (3‐MA), improved the apoptosis triggered by apigenin by activating pro‐caspases‐8, ‐9, and ‐3, as well as cleaving poly (ADP‐ribose) polymerase (PARP). These findings suggest that suppressing autophagy may be an effective approach to enhance the effectiveness of chemotherapy for CC treatment using anticancer agents (Lee et al., 2014).
Epithelial–mesenchymal transition (EMT) is a crucial process that plays a role in secondary tumor formation and plays a significant role in CC advancement (Bellovin et al., 2005).
Targeting EMT can be helpful for the prediction and treatment of CC (Bates & Mercurio, 2005; Lefort & Blay, 2011). Some regulatory proteins, for instance, the Snail/Slug family of zinc‐finger proteins and Twist Family BHLH Transcription Factor 1 (Twist1), regulate EMT (Yu et al., 2010).
Research indicates that the NF‐κB pathway is involved in controlling EMT in malignant cells by triggering Snail transcription (Chakrabarti et al., 2004; Julien et al., 2007; Liu et al., 1999). The NF‐κB pathway controls Snail expression through transcriptional and posttranslational mechanisms and boosts Snail transcription (Barbera et al., 2004).
According to Tong et al., apigenin showed curtailing impacts on the metastasis and aggression of colon carcinoma cells. They found that apigenin at loads of 10 and 20 μM suppressed EMT of HCT‐116 and LOVO human CC cells via the NF‐κB/Snail signaling pathway. Furthermore, the effectiveness of apigenin (200 and 300 mg/kg) in treating CC was evaluated by establishing xenografts on Balb/c nude mice. It is worth noting that the use of the PDX (patient‐derived xenografts) model may better reflect the properties of clinical tumor specimens (Tong et al., 2019).
Apoptosis is a regulated cell death process that removes impaired or dangerous cells (Carneiro & El‐Deiry, 2020). The Bcl‐2 proteins regulate apoptosis, including pro‐apoptotic proteins (Bax and Bak) and antiapoptotic proteins (Bcl‐xL and Mcl‐1; García‐Navas et al., 2021; Zamanian et al., 2017). The balance between these proteins can be altered by downregulating antiapoptotic proteins or upregulating proapoptotic proteins, leading to the discharge of cytochrome c and triggering the caspase cascade (Wang & Youle, 2009).
Bcl‐2 is a crucial inhibitor of apoptosis, and its overexpression is associated with malignant transformation and chemotherapy resistance (Burlacu, 2003; Warren et al., 2019). Thus, targeting the Bcl‐2 proteins, especially the antiapoptotic proteins, may represent a promising approach for cancer treatment.
Myeloid cell leukemia 1 (Mcl‐1), a prosurvival Bcl‐2 protein, is commonly overexpressed in cancers that affect humans (Tong et al., 2017).
This protein hinders cell apoptosis by attaching itself to proapoptotic Bcl‐2 proteins, thereby repressing MOMP and caspase activation (Perciavalle & Opferman, 2013). Mcl‐1 has been identified as a critical factor in tumor cell survival and resistance to therapy (Perciavalle & Opferman, 2013).
Recent research shows that apigenin decreases the expression of Mcl‐1 in CC cells (Shao et al., 2013).
Multiple analyses show that STAT proteins, for instance, STAT3, display persistent activity in various human cancer cells and promote the advancement of cancer (Lee et al., 2019; Tolomeo & Cascio, 2021).
In colorectal cancer, sustained activation of STAT3 is linked to increased cellular multiplication and invasion in vitro and tumor growth in vivo, while blocking STAT3 activity can induce apoptosis and reduce cancer cell invasion (Lin et al., 2011; Lu et al., 2016; Wang et al., 2013).
Previous research has suggested that apigenin can suppress tumor growth in various cancers by targeting the STAT3 signaling pathway (Cao et al., 2016; Seo et al., 2015).
Maeda et al. discovered that apigenin (50 μM) causes death in CC cells (DLD‐1 and HCT116 cell lines) by targeting two antiapoptotic proteins, Bcl‐xL as well as Mcl‐1, through STAT3 pathway of cellular signaling. The study showed that IL‐6 stimulation increased p‐STAT3 expression, Bcl‐xL, and Mcl‐1, but administration of apigenin decreased their expression. Furthermore, concurrent inhibition of Bcl‐xL and Mcl‐1 led to increased deaths of CC cells, indicating that these two proteins are essential medicinal targets of CC. These findings suggest that apigenin may be designed as an effective therapeutic agent for CC by concurrently targeting and repressing the expression of Bcl‐xL and Mcl‐1. Figure 2 illustrates the mechanism of action of apigenin on the STAT3 signaling pathway and the Bcl‐xL/Mcl‐1 axis in CC cells (Maeda et al., 2018). Maeda et al., (2018) found that apigenin inhibited the phosphorylation of STAT3 in a dose‐dependent manner, as the levels of p‐STAT3 significantly decreased with increasing concentrations of apigenin. This suggests that apigenin has an inhibitory effect on STAT3 phosphorylation in CC cells.
FIGURE 2.
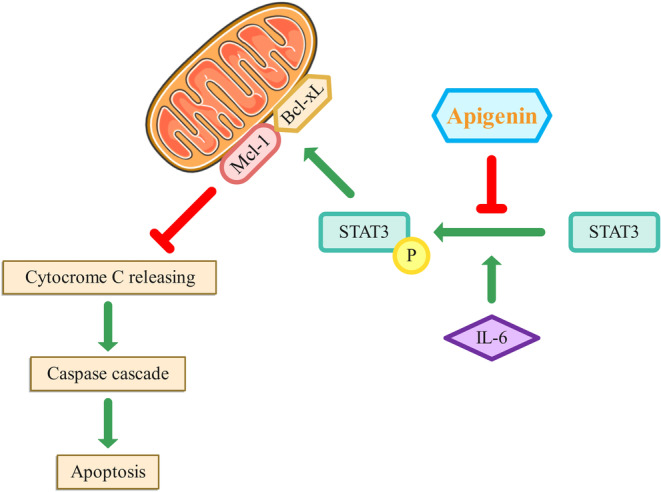
The mechanism of action of apigenin on the STAT3 signaling pathway and the Bcl‐xL/Mcl‐1 axis in CC cells. In CC, the overexpression of Bcl‐xL and Mcl‐1 contributes to the survival and proliferation of cancer cells by inhibiting apoptosis. The upregulation of these proteins helps cancer cells evade cell death signals and promotes tumor growth and resistance to therapy. It was found that treatment with IL‐6 increased the expression of p‐STAT3, Bcl‐xL, and Mcl‐1 in CC cells. Additionally, apigenin treatment resulted in a dose‐dependent decrease in the levels of p‐STAT3 in CC cells. Cytochrome c is a critical mediator of apoptosis. Its release from the mitochondria triggers the activation of caspases and initiates the apoptotic process. The balance between pro‐apoptotic and antiapoptotic Bcl‐2 family members determines whether cytochrome c is released and apoptosis is induced.
NF‐κB is a well‐researched proinflammatory transcription factor, but research indicated its significant inclusion in the development of CC and other types of cancer by promoting neovascularization, metastasis, proliferation, and inhibiting apoptosis (Slattery et al., 2018).
In a pharmacokinetic study, rats administered with 60 mg/kg of apigenin showed a Cmax of 1.33 ± 0.24 μg/mL and an AUC0–t° of 11.76 ± 1.52 μg h/mL (Ding et al., 2014). However, to overcome this limitation, researchers developed apigenin‐loaded lipid–polymer HyNP (LPHyNPs) and evaluated its cytotoxic potential in an in vitro model of CC.
In an analysis conducted by Alfaleh et al. NF‐κB expression level was analyzed following treatment with different formulations. The outcome showed that treatment with LPHyNPs significantly reduced the expression level of NF‐κB compared to blank LPHyNPs in HCT 116 cells. The study also investigated the anticancer properties of LPHyNPs through regulation of the expression of mTOR, JNK, and MDR‐1. Results indicated a noteworthy decrease in mTOR and JNK expression levels with LPHyNPs treatment in comparison with blank LPHyNPs (Alfaleh et al., 2022).
Navitoclax, also known as ABT‐263, is an orally accessible small chemical that inhibits Bcl‐2 selectively and potently (Rudin et al., 2012).
Shao et al. discovered that apigenin (20 μM) enhances the anticancer effects of Navitoclax or ABT‐263, an orally bioavailable small molecule that selectively and potently inhibits Bcl‐2 in various colon cancer cells, including DLD1, HCT116, HCT‐8, HT29, and SW48, by controlling the expression of Mcl‐1 and other pro‐survival effectors. The study then evaluated the antitumor efficacy of ABT‐263 (100 mg/kg) and apigenin (25 mg/kg) in mice. Immunoblotting of tumor tissues revealed that treatment with apigenin or a combination of apigenin and ABT‐263 resulted in decreased expression of Mcl‐1 and reduced phosphorylation of AKT and ERK, suggesting that apigenin can also target these pro‐survival modulators in vivo (Shao et al., 2013).
The Warburg effect, aerobic glycolysis, is noted in neoplastic cells, where glucose is converted into lactate rather than undergoing complete oxidation via the Krebs cycle, even in sufficient oxygen (Ryan et al., 2019).
This shift toward glycolysis is necessary for tumor cell proliferation and growth, as it is the major energy‐generating pathway for these cells (Vander Heiden et al., 2009).
The enzyme pyruvate kinase (PK) facilitates the ultimate rate‐controlling phase of glycolysis (Christofk et al., 2008).
Pyruvate kinase isozymes M1 (PKM1) and PKM2 are ciphered by one PK gene, while another gene encodes PKL and PKR. PKM2 is uniformly depicted in most malignant cells and occupies a key position in cancer progression as a controller of aerobic glycolysis (Chen et al., 2011; Li et al., 2014). Therefore, identifying medications that can hamper PKM2 activity and expression may be an innovative approach to antineoplastic treatment.
Glucose transporter 1 (GLUT1) is a protein that facilitates the transport of glucose across cell membranes and is involved in glucose uptake and metabolism (Pascual et al., 2004). It is expressed in many cell types, including cancer cells, and plays a key role in the Warburg effect and cancer metabolism (Young et al., 2011; Zhang et al., 2015). Apigenin has been found to inhibit GLUT1 activity and glucose uptake in human pancreatic cancer cells (Melstrom et al., 2008). Therefore, it may have the same effect on CC cells.
Polypyrimidine tract binding protein (PTBP1) is an important controller of the splicing of PK genes that selectively promotes the expression of PKM2 (Calabretta et al., 2016).
A study by Minami et al., (2017) showed that c‐Myc, an oncogenic transcription factor, can positively regulate the expression of PTBP1 in malignant cells.
β‐catenin, another protein, is known to be upstream of c‐Myc and can upregulate transcription of PTBP, thereby guaranteeing a high PKM2/PKM1 ratio (David et al., 2010; Noubissi et al., 2006).
According to Shan et al., apigenin (15 and 30 μM) inhibited the activity of PKM2 in CC cells (HCT116, HT29, and DLD1). The study revealed that AP can directly bind to PKM2, leading to a significant inhibition of its activity and expression. This inhibition of PKM2 activity by apigenin ultimately blocks the glycolysis pathway in colon cancer cells. It was also observed that apigenin acts as an allosteric inhibitor of PKM2, as its inhibitory effect on PKM2 was not reversed even in the presence of D‐fructose‐1,6‐diphosphate (FBP), which is a known activator of PKM2. Furthermore, apigenin was found to regulate the PKM2/PKM1 ratio in CC cells by blocking the β‐catenin/c‐Myc/PTBP1 signal pathway. This regulation ensures a low PKM2/PKM1 ratio, which is associated with the suppression of glycolysis and the inhibition of cancer cell proliferation. Overall, the specific targeting and inhibition of PKM2 activity by apigenin in colon cancer cells highlight the potential of apigenin as a novel therapeutic strategy for CC treatment (Shan et al., 2017). Figure 3 illustrates the mechanism of action of apigenin on the PKM2 and PKM1 in CC cells.
FIGURE 3.
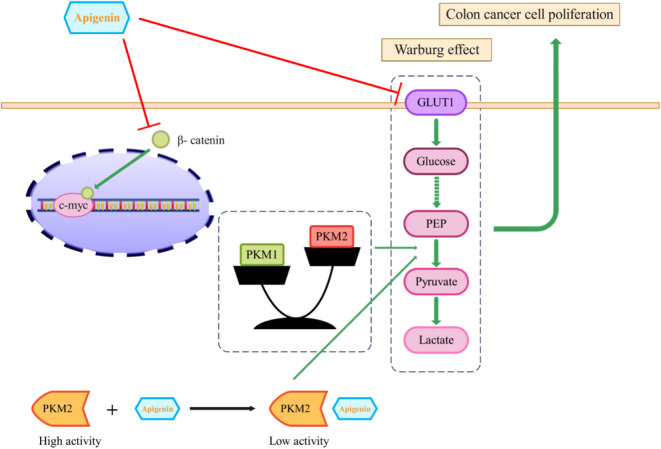
Effects of apigenin on CC cell proliferation via targeted blocking of PKM2‐dependent glycolysis. Apigenin could inhibit the activity and expression of PKM2, which is the last rate‐limiting enzyme in glycolysis. In addition to its effects on PKM2, apigenin also influenced the expression of PKM1. The regulation of PKM2 and PKM1 expression by apigenin was mediated through the β‐catenin/c‐Myc signaling pathway. Apigenin significantly reduced the expression of β‐catenin and c‐Myc, which are downstream target genes of β‐catenin. Overall, these findings suggest that apigenin may play a crucial role in inhibiting CC cell proliferation by targeting the PKM2‐mediated glycolysis pathway and inducing a switch from glycolysis to oxidative phosphorylation.
Adenomatous polyposis coli (APC) is a well‐known antioncogene, and loss of both alleles is required for the initiation of tumorigenesis (Kariv et al., 2020).
Chung et al. demonstrated that apigenin (80 μM) arrested cell cycle progression at the G2/M phase and inhibited cell growth in HT29‐APC and HT29‐GAL cells. In HT29‐APC cells induced with zinc, which expressed wild‐type APC gene, apigenin failed to prompt G2/M the halting of cellular replication, indicating that it may be more efficient in causing G2/M arrest in cells with APC mutations. Interestingly, apigenin significantly increased the expression of APC in HT29‐APC cells induced with zinc, which was linked with boosted noninflammatory CC cell death. These findings imply that apigenin might boost cellular death in CC cells with wild‐type APC and stimulate cell cycle arrest in CC cells with mutant APC (Chung et al., 2007).
Protein kinase CK2 is a serine–threonine kinase known to be disrupted in different human cancers (Trembley et al., 2022).
Colon cancer progression is often accompanied by inflammation, where many inflammatory cytokines are upregulated, including tumor necrosis factor‐alpha (TNF‐α). This cell signaling protein leads to necrosis and inflammation (Lai et al., 2020; Zhao & Zhang, 2018).
Farah et al. performed research demonstrating the effectiveness of two blockers of CK2, 5,6‐dichloro‐ribifuranosylbenzimidazole (DRB), and apigenin (7.5 μM), combined with TNF‐α, leading to a collaborative decrease in cell survival HCT‐116 and HT‐29 cells. Additionally, treatment with either DRB or apigenin decreased MnSOD expression when stimulated with TNF‐α in HCT‐116 CC cells. These findings suggest that these inhibitors may be a viable treatment option for CC (Farah et al., 2003). These findings suggest that apigenin could be a promising therapeutic agent for CC treatment. However, further study is needed to fully understand its mechanisms and efficacy in clinical studies. The results of the studies are summarized in Table 2.
TABLE 2.
Some studies consist of the purpose of the present review.
| Authors | Dosage | Type of study/model | Mechanisms |
|---|---|---|---|
| Chen et al. |
15, 30, and 60 μM 35 mg/kg |
HT29 cells mice | Induces autophagy and programmed cell death inhibits the growth of xenografted tumors |
| Turktekin et al. | 100 μM | HT29 cells | Increases caspase‐3 and caspase‐8 activity |
| Lee et al. | 25 and 50 M | HCT116 cells | Inhibits the G2/M stage of the cell cycle |
| Maeda et al. | 50 μM | DLD‐1 and HCT116 cell lines | Reduces the expression of p‐STAT3, Bcl‐xL, and Mcl‐1 |
| Chung et al. | 80 μM | HT29‐APC and HT29‐GAL cells | Arrests cell cycle progression at the G2/M phase and inhibits cell growth |
| Shao et al. | 25 mg/kg | Severe combined immunodeficient (SCID) Mice | Reduces the expression of Mcl‐1 and the phosphorylation of AKT and ERK in tumor tissues |
| Farah et al. | 7.5 μM | HCT‐116 | Decreases MnSOD expression |
4. CONCLUSION
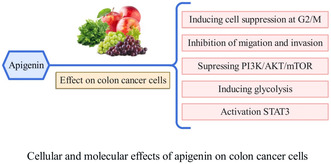
Apigenin as a flavonoid has shown promise in treating various types of cancer, including colon, pancreatic, breast, colorectal, and prostate cancer cells. Studies have demonstrated that combination therapy with apigenin in different types of cancers not only enhances the efficacy of chemotherapy but also reduces the side effects by targeting multiple cell signaling pathways. Apigenin has been reported to suppress CC in vitro and in vivo by multiple biological effects, such as triggering cell apoptosis and autophagy, inducing cell cycle arrest, and suppressing cell migration and invasion. We summarized the effects of apigenin on CC cells in Figure 4. Taken together, these results indicate that apigenin could inhibit the growth of CC cells in vitro and in vivo and may be used for the improvement of therapy for colon cancer.
FIGURE 4.
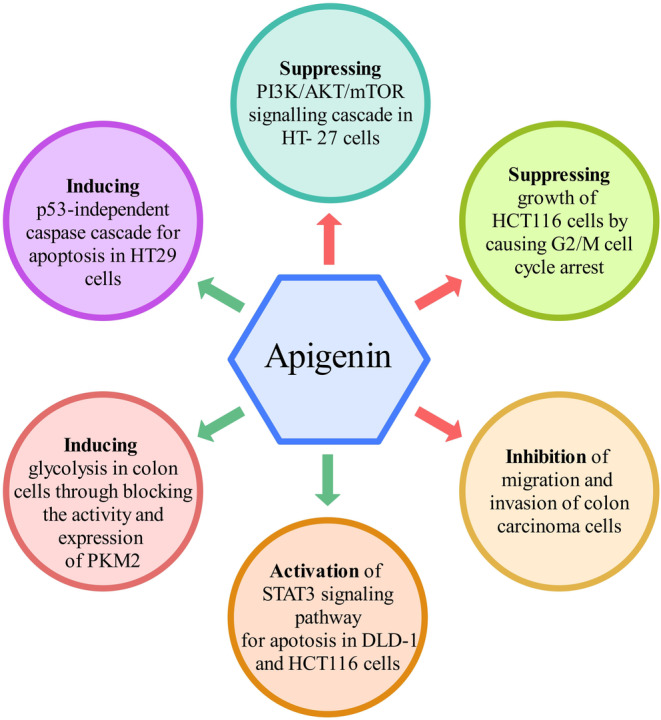
The effects of apigenin on CC cells.
AUTHOR CONTRIBUTIONS
M.Y.Z, M.M, and Gh.B: conception, design, writing, and revising the manuscript. S.D and M.H.: revising and editing the manuscript and graphic drawing. M.G, M.S.I, and Z.K: data gathering and editing the manuscript. S.M.E.P, S.D, and M.H. contributed to data collection, drafting of the manuscript, and table creation. All authors read and approved the final manuscript.
FUNDING INFORMATION
None.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no conflict of interest.
ETHICS STATEMENT
This article does not contain any studies with human participants or animals performed by any of the authors.
ACKNOWLEDGMENTS
Not applicable.
Daneshvar, S. , Zamanian, M. Y. , Ivraghi, M. S. , Golmohammadi, M. , Modanloo, M. , Kamiab, Z. , Pourhosseini, S. M. E. , Heidari, M. , & Bazmandegan, G. (2023). A comprehensive view on the apigenin impact on colorectal cancer: Focusing on cellular and molecular mechanisms. Food Science & Nutrition, 11, 6789–6801. 10.1002/fsn3.3645
Siamak Daneshvar and Mohammad Yasin Zamanian contributed equally to this work.
Contributor Information
Mona Modanloo, Email: m.modanloo@mazums.ac.ir.
Gholamreza Bazmandegan, Email: bkhrbster@gmail.com.
DATA AVAILABILITY STATEMENT
The data relevant to the review article are within the manuscript.
REFERENCES
- Abid, R. , Ghazanfar, S. , Farid, A. , Sulaman, S. M. , Idrees, M. , Amen, R. A. , Muzammal, M. , Shahzad, M. K. , Mohamed, M. O. , Khaled, A. A. , Safir, W. , Ghori, I. , Elasbali, A. M. , & Alharbi, B. (2022). Pharmacological properties of 4′, 5, 7‐Trihydroxyflavone (Apigenin) and its impact on cell signaling pathways. Molecules, 27(13), 4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed, S. A. , Parama, D. , Daimari, E. , Girisa, S. , Banik, K. , Harsha, C. , Dutta, U. , & Kunnumakkara, A. B. (2021). Rationalizing the therapeutic potential of apigenin against cancer. Life Sciences, 267, 118814. [DOI] [PubMed] [Google Scholar]
- Alfaleh, M. A. , Hashem, A. M. , Abujamel, T. S. , Alhakamy, N. A. , Kalam, M. A. , Riadi, Y. , & Md, S. (2022). Apigenin loaded lipoid–PLGA–TPGS nanoparticles for colon cancer therapy: Characterization, sustained release, cytotoxicity, and apoptosis pathways. Polymers, 14(17), 3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaravadi, R. K. , Kimmelman, A. C. , & Debnath, J. (2019). Targeting autophagy in cancer: Recent advances and future Directions Targeting autophagy in cancer. Cancer Discovery, 9(9), 1167–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argilés, G. , Tabernero, J. , Labianca, R. , Hochhauser, D. , Salazar, R. , Iveson, T. , Laurent‐Puig, P. , Quirke, P. , Yoshino, T. , Taieb, J. , Martinelli, E. , Arnold, D. , & ESMO Guidelines Committee. Electronic address: clinicalguidelines@esmo.org . (2020). Localised colon cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow‐up. Annals of Oncology, 31(10), 1291–1305. [DOI] [PubMed] [Google Scholar]
- Barbera, M. J. , Puig, I. , Domínguez, D. , Julien‐Grille, S. , Guaita‐Esteruelas, S. , Peiró, S. , Baulida, J. , Francí, C. , Dedhar, S. , Larue, L. , & de Herreros, A. G. (2004). Regulation of snail transcription during epithelial to mesenchymal transition of tumor cells. Oncogene, 23(44), 7345–7354. [DOI] [PubMed] [Google Scholar]
- Barrett, D. , Brown, V. I. , Grupp, S. A. , & Teachey, D. T. (2012). Targeting the PI3K/AKT/mTOR signaling axis in children with hematologic malignancies. Pediatric Drugs, 14, 299–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates, R. C. , & Mercurio, A. (2005). The epithelial‐mesenchymal transition (EMT) and colorectal cancer progression. Cancer Biology & Therapy, 4(4), 371–376. [DOI] [PubMed] [Google Scholar]
- Bellovin, D. I. , Bates, R. C. , Muzikansky, A. , Rimm, D. L. , & Mercurio, A. M. (2005). Altered localization of p120 catenin during epithelial to mesenchymal transition of colon carcinoma is prognostic for aggressive disease. Cancer Research, 65(23), 10938–10945. [DOI] [PubMed] [Google Scholar]
- Boroushaki, M. T. , Rajabian, A. , Farzadnia, M. , Hoseini, A. , Poorlashkari, M. , Taghavi, A. , Dolati, K. , & Bazmandegan, G. (2015). Protective effect of pomegranate seed oil against cisplatin‐induced nephrotoxicity in rat. Renal Failure, 37(8), 1338–1343. [DOI] [PubMed] [Google Scholar]
- Burlacu, A. (2003). Regulation of apoptosis by Bcl‐2 family proteins. Journal of Cellular and Molecular Medicine, 7(3), 249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabretta, S. , Bielli, P. , Passacantilli, I. , Pilozzi, E. , Fendrich, V. , Capurso, G. , Fave, G. D. , & Sette, C. (2016). Modulation of PKM alternative splicing by PTBP1 promotes gemcitabine resistance in pancreatic cancer cells. Oncogene, 35(16), 2031–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, H.‐H. , Chu, J. H. , Kwan, H. Y. , Su, T. , Yu, H. , Cheng, C. Y. , Fu, X. Q. , Guo, H. , Li, T. , Tse, A. K. W. , Chou, G. X. , Mo, H. B. , & Yu, Z. L. (2016). Inhibition of the STAT3 signaling pathway contributes to apigenin‐mediated anti‐metastatic effect in melanoma. Scientific Reports, 6(1), 21731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, X. , Liu, B. , Cao, W. , Zhang, W. , Zhang, F. , Zhao, H. , Meng, R. , Zhang, L. , Niu, R. , Hao, X. , & Zhang, B. (2013). Autophagy inhibition enhances apigenin‐induced apoptosis in human breast cancer cells. Chinese Journal of Cancer Research, 25(2), 212–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas, H. , Arango, D. , Nicholas, C. , Duarte, S. , Nuovo, G. , He, W. , Voss, O. , Gonzalez‐Mejia, M. , Guttridge, D. , Grotewold, E. , & Doseff, A. (2016). Dietary apigenin exerts immune‐regulatory activity in vivo by reducing NF‐κB activity, halting leukocyte infiltration and restoring normal metabolic function. International Journal of Molecular Sciences, 17(3), 323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carneiro, B. A. , & El‐Deiry, W. S. (2020). Targeting apoptosis in cancer therapy. Nature Reviews. Clinical Oncology, 17(7), 395–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti, O. , Veeraraghavalu, K. , Tergaonkar, V. , Liu, Y. , Androphy, E. J. , Stanley, M. A. , & Krishna, S. (2004). Human papillomavirus type 16 E6 amino acid 83 variants enhance E6‐mediated MAPK signaling and differentially regulate tumorigenesis by notch signaling and oncogenic Ras. Journal of Virology, 78(11), 5934–5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, J. , Xie, J. , Jiang, Z. , Wang, B. , Wang, Y. , & Hu, X. (2011). Shikonin and its analogs inhibit cancer cell glycolysis by targeting tumor pyruvate kinase‐M2. Oncogene, 30(42), 4297–4306. [DOI] [PubMed] [Google Scholar]
- Chen, X. , Xu, H. , Yu, X. , Wang, X. , Zhu, X. , & Xu, X. (2019). Apigenin inhibits in vitro and in vivo tumorigenesis in cisplatin‐resistant colon cancer cells by inducing autophagy, programmed cell death and targeting m‐TOR/PI3K/Akt signalling pathway. Journal of BUON, 24(2), 488–493. [PubMed] [Google Scholar]
- Cheng, E. , Ou, F. S. , Ma, C. , Spiegelman, D. , Zhang, S. , Zhou, X. , Bainter, T. M. , Saltz, L. B. , Niedzwiecki, D. , Mayer, R. J. , Whittom, R. , Hantel, A. , Benson, A. , Atienza, D. , Messino, M. , Kindler, H. , Giovannucci, E. L. , van Blarigan, E. L. , Brown, J. C. , … Fuchs, C. S. (2022). Diet‐and lifestyle‐based prediction models to estimate cancer recurrence and death in patients with stage III colon cancer (CALGB 89803/alliance). Journal of Clinical Oncology, 40(7), 740–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christofk, H. R. , Vander Heiden, M. G. , Harris, M. H. , Ramanathan, A. , Gerszten, R. E. , Wei, R. , Fleming, M. D. , Schreiber, S. L. , & Cantley, L. C. (2008). The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature, 452(7184), 230–233. [DOI] [PubMed] [Google Scholar]
- Chung, C. , Jiang, Y. , Cheng, D. , & Birt, D. F. (2007). Impact of adenomatous polyposis coli (APC) tumor supressor gene in human colon cancer cell lines on cell cycle arrest by apigenin. Molecular Carcinogenesis, 46(9), 773–782. [DOI] [PubMed] [Google Scholar]
- Chupradit, S. , Bokov, D. , Zamanian, M. Y. , Heidari, M. , & Hakimizadeh, E. (2022). Hepatoprotective and therapeutic effects of resveratrol: A focus on anti‐inflammatory and antioxidative activities. Fundamental & Clinical Pharmacology, 36(3), 468–485. [DOI] [PubMed] [Google Scholar]
- Condello, M. , & Meschini, S. (2021). Role of natural antioxidant products in colorectal cancer disease: A focus on a natural compound derived from prunus spinosa, trigno ecotype. Cell, 10(12), 3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespy, V. , Morand, C. , Besson, C. , Manach, C. , Demigne, C. , & Remesy, C. (2002). Quercetin, but not its glycosides, is absorbed from the rat stomach. Journal of Agricultural and Food Chemistry, 50(3), 618–621. [DOI] [PubMed] [Google Scholar]
- Dajas, F. , Juan Andres, A. C. , Florencia, A. , Carolina, E. , & Felicia, R. M. (2013). Neuroprotective actions of flavones and flavonols: Mechanisms and relationship to flavonoid structural features. Central Nervous System Agents in Medicinal Chemistry (Formerly Current Medicinal Chemistry‐Central Nervous System Agents), 13(1), 30–35. [DOI] [PubMed] [Google Scholar]
- Darakhshan, S. , Bidmeshki Pour, A. , Hosseinzadeh Colagar, A. , & Sisakhtnezhad, S. (2015). Thymoquinone and its therapeutic potentials. Pharmacological Research, 95, 138–158. [DOI] [PubMed] [Google Scholar]
- David, C. J. , Chen, M. , Assanah, M. , Canoll, P. , & Manley, J. L. (2010). HnRNP proteins controlled by c‐Myc deregulate pyruvate kinase mRNA splicing in cancer. Nature, 463(7279), 364–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Sousa Silva, G. V. , Lopes, A. L. V. F. G. , Viali, I. C. , Lima, L. Z. M. , Bizuti, M. R. , Haag, F. B. , & Tavares de Resende e Silva, D. (2023). Therapeutic properties of flavonoids in treatment of cancer through autophagic modulation: A systematic review. Chinese Journal of Integrative Medicine, 29(3), 268–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehnamaki, F. , Karimi, A. , Pilevarian, A. A. , Fatemi, I. , Hakimizadeh, E. , Kaeidi, A. , Allahtavakoli, M. , Rahmani, M. R. , Khademalhosseini, M. , & Bazmandegan, G. (2019). Treatment with troxerutin protects against cisplatin‐induced kidney injury in mice. Acta Chirurgica Belgica, 119(1), 31–37. [DOI] [PubMed] [Google Scholar]
- Ding, S.‐M. , Zhang, Z.‐H. , Song, J. , Cheng, X.‐D. , Jiang, J. , & Jia, X.‐B. (2014). Enhanced bioavailability of apigenin via preparation of a carbon nanopowder solid dispersion. International Journal of Nanomedicine, 9, 2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farah, M. , Parhar, K. , Moussavi, M. , Eivemark, S. , & Salh, B. (2003). 5, 6‐Dichloro‐ribifuranosylbenzimidazole‐and apigenin‐induced sensitization of colon cancer cells to TNF‐α‐mediated apoptosis. American journal of physiology‐gastrointestinal and liver. Physiology, 285(5), G919–G928. [DOI] [PubMed] [Google Scholar]
- Feng, Y.‐B. , Chen, L. , Chen, F.‐X. , Yang, Y. , Chen, G.‐H. , Zhou, Z.‐H. , & Xu, C.‐F. (2023). Immunopotentiation effects of apigenin on NK cell proliferation and killing pancreatic cancer cells. International Journal of Immunopathology and Pharmacology, 37, 3946320231161174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira, M. , Costa, D. , & Sousa, Â. (2022). Flavonoids‐based delivery systems towards cancer therapies. Bioengineering, 9(5), 197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouache, A. , Zabaiou, N. , de Joussineau, C. , Morel, L. , Silvente‐Poirot, S. , Namsi, A. , Lizard, G. , Poirot, M. , Makishima, M. , Baron, S. , Lobaccaro, J. M. A. , & Trousson, A. (2019). Flavonoids differentially modulate liver X receptors activity—Structure‐function relationship analysis. The Journal of Steroid Biochemistry and Molecular Biology, 190, 173–182. [DOI] [PubMed] [Google Scholar]
- García‐Navas, R. , Gajate, C. , & Mollinedo, F. (2021). Neutrophils drive endoplasmic reticulum stress‐mediated apoptosis in cancer cells through arginase‐1 release. Scientific Reports, 11(1), 12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghanbari‐Movahed, M. , Shafiee, S. , Burcher, J. T. , Lagoa, R. , Farzaei, M. H. , & Bishayee, A. (2023). Anticancer potential of Apigenin and Isovitexin with focus on oncogenic metabolism in cancer stem cells. Metabolites, 13(3), 404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradolatto, A. , Basly, J. P. , Berges, R. , Teyssier, C. , Chagnon, M. C. , Siess, M. H. , & Canivenc‐Lavier, M. C. (2005). Pharmacokinetics and metabolism of apigenin in female and male rats after a single oral administration. Drug Metabolism and Disposition, 33(1), 49–54. [DOI] [PubMed] [Google Scholar]
- Granato, M. , Gilardini Montani, M. S. , Santarelli, R. , D'Orazi, G. , Faggioni, A. , & Cirone, M. (2017). Apigenin, by activating p53 and inhibiting STAT3, modulates the balance between pro‐apoptotic and pro‐survival pathways to induce PEL cell death. Journal of Experimental & Clinical Cancer Research, 36(1), 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gündoğdu, A. Ç. , & Özyurt, R. (2023). Resveratrol downregulates ENaCs through the activation of AMPK in human colon cancer cells. Tissue and Cell, 82, 102071. [DOI] [PubMed] [Google Scholar]
- Gupta, P. , Chiang, S. F. , Sahoo, P. K. , Mohapatra, S. K. , You, J. F. , Onthoni, D. D. , Hung, H. Y. , Chiang, J. M. , Huang, Y. , & Tsai, W. S. (2019). Prediction of colon cancer stages and survival period with machine learning approach. Cancers, 11(12), 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta, S. , Afaq, F. , & Mukhtar, H. (2001). Selective growth‐inhibitory, cell‐cycle deregulatory and apoptotic response of apigenin in normal versus human prostate carcinoma cells. Biochemical and Biophysical Research Communications, 287(4), 914–920. [DOI] [PubMed] [Google Scholar]
- Gupta, S. , Afaq, F. , & Mukhtar, H. (2002). Involvement of nuclear factor‐kappa B, Bax and Bcl‐2 in induction of cell cycle arrest and apoptosis by apigenin in human prostate carcinoma cells. Oncogene, 21(23), 3727–3738. [DOI] [PubMed] [Google Scholar]
- He, Z.‐Y. , Shi, C. B. , Wen, H. , Li, F. L. , Wang, B. L. , & Wang, J. (2011). Upregulation of p53 expression in patients with colorectal cancer by administration of curcumin. Cancer Investigation, 29(3), 208–213. [DOI] [PubMed] [Google Scholar]
- Herkenne, C. , Alberti, I. , Naik, A. , Kalia, Y. N. , Mathy, F. X. , Préat, V. , & Guy, R. H. (2008). In vivo methods for the assessment of topical drug bioavailability. Pharmaceutical Research, 25, 87–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hnit, S. S. T. , Yao, M. , Xie, C. , Bi, L. , Wong, M. , Liu, T. , de Souza, P. , Li, Z. , & Dong, Q. (2022). Apigenin impedes cell cycle progression at G2 phase in prostate cancer cells. Discover Oncology, 13(1), 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong, S. , Dia, V. P. , Baek, S. J. , & Zhong, Q. (2022). Nanoencapsulation of apigenin with whey protein isolate: Physicochemical properties, in vitro activity against colorectal cancer cells, and bioavailability. LWT, 154, 112751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, Y. , Zhao, X. , Zu, Y. , Wang, L. , Deng, Y. , Wu, M. , & Wang, H. (2019). Enhanced solubility and bioavailability of apigenin via preparation of solid dispersions of mesoporous silica nanoparticles. Iranian Journal of Pharmaceutical Research: IJPR, 18(1), 168–182. [PMC free article] [PubMed] [Google Scholar]
- Jain, P. , Patel, K. , Jangid, A. K. , Guleria, A. , Patel, S. , Pooja, D. , & Kulhari, H. (2020). Modulating the delivery of 5‐fluorouracil to human colon cancer cells using multifunctional arginine‐coated manganese oxide nanocuboids with MRI properties. ACS Applied Bio Materials, 3(10), 6852–6864. [DOI] [PubMed] [Google Scholar]
- Jang, J. Y. , Sung, B. , & Kim, N. D. (2022). Role of induced programmed cell death in the chemopreventive potential of apigenin. International Journal of Molecular Sciences, 23(7), 3757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jangdey, M. S. , Gupta, A. , & Sarwa, K. (2018). Apigenin and quercetin: Potential therapeutic challenging effective against in Alzheimer's disease. Pharmaceutical and Biosciences Journal, 6(1), 46–51. [Google Scholar]
- Jin, X. , & Ren, C. (2007). Effect and mechanism of apigenin on VEGF expression in human breast cancer cells. Zhonghua Zhong Liu Za Zhi [Chinese Journal of Oncology], 29(7), 495–499. [PubMed] [Google Scholar]
- Julien, S. , Puig, I. , Caretti, E. , Bonaventure, J. , Nelles, L. , van Roy, F. , Dargemont, C. , de Herreros, A. G. , Bellacosa, A. , & Larue, L. (2007). Activation of NF‐κB by Akt upregulates snail expression and induces epithelium mesenchyme transition. Oncogene, 26(53), 7445–7456. [DOI] [PubMed] [Google Scholar]
- Kahle, K. , Huemmer, W. , Kempf, M. , Scheppach, W. , Erk, T. , & Richling, E. (2007). Polyphenols are intensively metabolized in the human gastrointestinal tract after apple juice consumption. Journal of Agricultural and Food Chemistry, 55(26), 10605–10614. [DOI] [PubMed] [Google Scholar]
- Kariv, R. , Caspi, M. , Fliss‐Isakov, N. , Shorer, Y. , Shor, Y. , Rosner, G. , Brazowski, E. , Beer, G. , Cohen, S. , & Rosin‐Arbesfeld, R. (2020). Resorting the function of the colorectal cancer gatekeeper adenomatous polyposis coli. International Journal of Cancer, 146(4), 1064–1074. [DOI] [PubMed] [Google Scholar]
- Ketkaew, Y. , Osathanon, T. , Pavasant, P. , & Sooampon, S. (2017). Apigenin inhibited hypoxia induced stem cell marker expression in a head and neck squamous cell carcinoma cell line. Archives of Oral Biology, 74, 69–74. [DOI] [PubMed] [Google Scholar]
- Kilani‐Jaziri, S. , Mustapha, N. , Mokdad‐Bzeouich, I. , el Gueder, D. , Ghedira, K. , & Ghedira‐Chekir, L. (2016). Flavones induce immunomodulatory and anti‐inflammatory effects by activating cellular anti‐oxidant activity: A structure‐activity relationship study. Tumor Biology, 37, 6571–6579. [DOI] [PubMed] [Google Scholar]
- Kim, S. H. , Kang, J. G. , Kim, C. S. , Ihm, S.‐H. , Choi, M. G. , Yoo, H. J. , & Lee, S. J. (2015). Suppression of AKT potentiates synergistic cytotoxicity of apigenin with TRAIL in anaplastic thyroid carcinoma cells. Anticancer Research, 35(12), 6529–6537. [PubMed] [Google Scholar]
- Lai, M. , Liu, G. , Li, R. , Bai, H. , Zhao, J. , Xiao, P. , & Mei, J. (2020). Hsa_circ_0079662 induces the resistance mechanism of the chemotherapy drug oxaliplatin through the TNF‐α pathway in human colon cancer. Journal of Cellular and Molecular Medicine, 24(9), 5021–5027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, H. , Jeong, A. J. , & Ye, S.‐K. (2019). Highlighted STAT3 as a potential drug target for cancer therapy. BMB Reports, 52(7), 415–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, Y. , Sung, B. , Kang, Y. J. , Kim, D. H. , Jang, J.‐Y. , Hwang, S. Y. , Kim, M. , Lim, H. S. , Yoon, J.‐H. , Chung, H. Y. , & Kim, N. D. (2014). Apigenin‐induced apoptosis is enhanced by inhibition of autophagy formation in HCT116 human colon cancer cells. International Journal of Oncology, 44(5), 1599–1606. [DOI] [PubMed] [Google Scholar]
- Lefort, É. C. , & Blay, J. (2011). The dietary flavonoid apigenin enhances the activities of the anti‐metastatic protein CD26 on human colon carcinoma cells. Clinical & Experimental Metastasis, 28, 337–349. [DOI] [PubMed] [Google Scholar]
- Li, B. , Hu, Y. , Wu, T. , Feng, Y. , Jiang, C. , du, H. , & Lu, S. (2022). Apigenin‐oxymatrine binary co‐amorphous mixture: Enhanced solubility, bioavailability, and anti‐inflammatory effect. Food Chemistry, 373, 131485. [DOI] [PubMed] [Google Scholar]
- Li, K. , Yao, F. , Xue, Q. , Fan, H. , Yang, L. , Li, X. , Sun, L. , & Liu, Y. (2018). Inhibitory effects against α‐glucosidase and α‐amylase of the flavonoids‐rich extract from Scutellaria baicalensis shoots and interpretation of structure–activity relationship of its eight flavonoids by a refined assign‐score method. Chemistry Central Journal, 12(1), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Z. , Yang, P. , & Li, Z. (2014). The multifaceted regulation and functions of PKM2 in tumor progression. Biochimica et Biophysica Acta (BBA)‐Reviews on Cancer, 1846(2), 285–296. [DOI] [PubMed] [Google Scholar]
- Liang, Y. , Zhong, Q. , Ma, R. , Ni, Z. , Thakur, K. , Zhang, J. , & Wei, Z. (2023). Apigenin, a natural flavonoid, promotes autophagy and ferroptosis in human endometrial carcinoma Ishikawa cells in vitro and in vivo. Food Science and Human Wellness, 12(6), 2242–2251. [Google Scholar]
- Lim, W. , Park, S. , Bazer, F. W. , & Song, G. (2016). Apigenin reduces survival of choriocarcinoma cells by inducing apoptosis via the PI3K/AKT and ERK1/2 MAPK pathways. Journal of Cellular Physiology, 231(12), 2690–2699. [DOI] [PubMed] [Google Scholar]
- Lin, L. , Fuchs, J. , Li, C. , Olson, V. , Bekaii‐Saab, T. , & Lin, J. (2011). STAT3 signaling pathway is necessary for cell survival and tumor sphere forming capacity in ALDH+/CD133+ stem cell‐like human colon cancer cells. Biochemical and Biophysical Research Communications, 416(3–4), 246–251. [DOI] [PubMed] [Google Scholar]
- Liu, Y. , Tergaonkar, V. , Krishna, S. , & Androphy, E. J. (1999). Human papillomavirus type 16 E6‐enhanced susceptibility of L929 cells to tumor necrosis factor α correlates with increased accumulation of reactive oxygen species. Journal of Biological Chemistry, 274(35), 24819–24827. [DOI] [PubMed] [Google Scholar]
- Lu, R. , Wu, S. , Zhang, Y. G. , Xia, Y. , Zhou, Z. , Kato, I. , Dong, H. , Bissonnette, M. , & Sun, J. (2016). Salmonella protein AvrA activates the STAT3 signaling pathway in colon cancer. Neoplasia, 18(5), 307–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabrouk Zayed, M. M. , Sahyon, H. A. , Hanafy, N. A. N. , & el‐Kemary, M. A. (2022). The effect of encapsulated apigenin nanoparticles on HePG‐2 cells through regulation of P53. Pharmaceutics, 14(6), 1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda, Y. , Takahashi, H. , Nakai, N. , Yanagita, T. , Ando, N. , Okubo, T. , Saito, K. , Shiga, K. , Hirokawa, T. , Hara, M. , Ishiguro, H. , Matsuo, Y. , & Takiguchi, S. (2018). Apigenin induces apoptosis by suppressing Bcl‐xl and mcl‐1 simultaneously via signal transducer and activator of transcription 3 signaling in colon cancer. International Journal of Oncology, 52(5), 1661–1673. [DOI] [PubMed] [Google Scholar]
- Mahbub, A. A. , le Maitre, C. L. , Cross, N. A. , & Jordan‐Mahy, N. (2022). The effect of apigenin and chemotherapy combination treatments on apoptosis‐related genes and proteins in acute leukaemia cell lines. Scientific Reports, 12(1), 8858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao, F. , Xiao, B. , Jiang, Z. , Zhao, J. , Huang, X. , & Guo, J. (2011). Anticancer effect of Lycium barbarum polysaccharides on colon cancer cells involves G0/G1 phase arrest. Medical Oncology, 28, 121–126. [DOI] [PubMed] [Google Scholar]
- Melstrom, L. G. , Salabat, M. R. , Ding, X. Z. , Milam, B. M. , Strouch, M. , Pelling, J. C. , & Bentrem, D. J. (2008). Apigenin inhibits the GLUT‐1 glucose transporter and the phosphoinositide 3‐kinase/Akt pathway in human pancreatic cancer cells. Pancreas, 37(4), 426–431. [DOI] [PubMed] [Google Scholar]
- Miller, K. D. , Siegel, R. L. , Lin, C. C. , Mariotto, A. B. , Kramer, J. L. , Rowland, J. H. , Stein, K. D. , Alteri, R. , & Jemal, A. (2016). Cancer treatment and survivorship statistics, 2016. CA: A Cancer Journal for Clinicians, 66(4), 271–289. [DOI] [PubMed] [Google Scholar]
- Minami, K. , Taniguchi, K. , Sugito, N. , Kuranaga, Y. , Inamoto, T. , Takahara, K. , Takai, T. , Yoshikawa, Y. , Kiyama, S. , Akao, Y. , & Azuma, H. (2017). MiR‐145 negatively regulates Warburg effect by silencing KLF4 and PTBP1 in bladder cancer cells. Oncotarget, 8(20), 33064–33077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra, J. , Drummond, J. , Quazi, S. H. , Karanki, S. S. , Shaw, J. J. , Chen, B. , & Kumar, N. (2013). Prospective of colon cancer treatments and scope for combinatorial approach to enhanced cancer cell apoptosis. Critical Reviews in Oncology/Hematology, 86(3), 232–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammad Nabavi, S. , Habtemariam, S. , Daglia, M. , & Nabavi, S. F. (2015). Apigenin and breast cancers: From chemistry to medicine. Anti‐Cancer Agents in Medicinal Chemistry (Formerly Current Medicinal Chemistry‐Anti‐Cancer Agents), 15(6), 728–735. [DOI] [PubMed] [Google Scholar]
- Mutha, R. E. , Tatiya, A. U. , & Surana, S. J. (2021). Flavonoids as natural phenolic compounds and their role in therapeutics: An overview. Future Journal of Pharmaceutical Sciences, 7, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabavi, S. F. , Khan, H. , D'onofrio, G. , Šamec, D. , Shirooie, S. , Dehpour, A. R. , Argüelles, S. , Habtemariam, S. , & Sobarzo‐Sanchez, E. (2018). Apigenin as neuroprotective agent: Of mice and men. Pharmacological Research, 128, 359–365. [DOI] [PubMed] [Google Scholar]
- Nazio, F. , Bordi, M. , Cianfanelli, V. , Locatelli, F. , & Cecconi, F. (2019). Autophagy and cancer stem cells: Molecular mechanisms and therapeutic applications. Cell Death and Differentiation, 26(4), 690–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noubissi, F. K. , Elcheva, I. , Bhatia, N. , Shakoori, A. , Ougolkov, A. , Liu, J. , Minamoto, T. , Ross, J. , Fuchs, S. Y. , & Spiegelman, V. S. (2006). CRD‐BP mediates stabilization of βTrCP1 and c‐myc mRNA in response to β‐catenin signalling. Nature, 441(7095), 898–901. [DOI] [PubMed] [Google Scholar]
- Ornano, L. , Venditti, A. , Donno, Y. , Sanna, C. , Ballero, M. , & Bianco, A. (2016). Phytochemical analysis of non‐volatile fraction ofArtemisia caerulescenssubsp.Densiflora(Viv.) (Asteraceae), an endemic species of La Maddalena archipelago (Sardinia – Italy). Natural Product Research, 30(8), 920–925. [DOI] [PubMed] [Google Scholar]
- Pandey, P. , Khan, F. , & Upadhyay, T. K. (2023). Deciphering the modulatory role of apigenin targeting oncogenic pathways in human cancers. Chemical Biology & Drug Design, 101, 1446–1458. [DOI] [PubMed] [Google Scholar]
- Pascual, J. M. , Wang, D. , Lecumberri, B. , Yang, H. , Mao, X. , Yang, R. , & de Vivo, D. C. (2004). GLUT1 deficiency and other glucose transporter diseases. European Journal of Endocrinology, 150(5), 627–633. [DOI] [PubMed] [Google Scholar]
- Patel, K. R. , Brown, V. A. , Jones, D. J. L. , Britton, R. G. , Hemingway, D. , Miller, A. S. , West, K. P. , Booth, T. D. , Perloff, M. , Crowell, J. A. , Brenner, D. E. , Steward, W. P. , Gescher, A. J. , & Brown, K. (2010). Clinical pharmacology of resveratrol and its metabolites in colorectal cancer patients resveratrol in colorectal cancer patients. Cancer Research, 70(19), 7392–7399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perciavalle, R. M. , & Opferman, J. T. (2013). Delving deeper: MCL‐1's contributions to normal and cancer biology. Trends in Cell Biology, 23(1), 22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham, H. , Chen, M. , Takahashi, H. , King, J. , Reber, H. A. , Hines, O. J. , Pandol, S. , & Eibl, G. (2012). Apigenin inhibits NNK‐induced focal adhesion kinase activation in pancreatic cancer cells. Pancreas, 41(8), 1306–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Printz, C. (2021). Triple chemotherapy combination improves metastatic colorectal cancer outcomes. Cancer, 127(10), 1547. [DOI] [PubMed] [Google Scholar]
- Qin, Y. , Zhao, D. , Zhou, H. G. , Wang, X. H. , Zhong, W. L. , Chen, S. , Gu, W. G. , Wang, W. , Zhang, C. H. , Liu, Y. R. , Liu, H. J. , Zhang, Q. , Guo, Y. Q. , Sun, T. , & Yang, C. (2016). Apigenin inhibits NF‐κB and snail signaling, EMT and metastasis in human hepatocellular carcinoma. Oncotarget, 7(27), 41421–41431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu, W. , Ji, P. , Han, X. , Wang, X. , Li, Y. , & Liu, J. (2022). Highly biocompatible Apigenin‐loaded silk fibroin Nanospheres: Preparation, characterization, and anti‐breast‐cancer activity. Polymers, 15(1), 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmani, A. H. , Alsahli, M. A. , Almatroudi, A. , Almogbel, M. A. , Khan, A. A. , Anwar, S. , & Almatroodi, S. A. (2022). The potential role of apigenin in cancer prevention and treatment. Molecules, 27(18), 6051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rein, M. J. , Renouf, M. , Cruz‐Hernandez, C. , Actis‐Goretta, L. , Thakkar, S. K. , & da Silva Pinto, M. (2013). Bioavailability of bioactive food compounds: A challenging journey to bioefficacy. British Journal of Clinical Pharmacology, 75(3), 588–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roshani, M. , Jafari, A. , Loghman, A. , Sheida, A. H. , Taghavi, T. , Tamehri Zadeh, S. S. , Hamblin, M. R. , Homayounfal, M. , & Mirzaei, H. (2022). Applications of resveratrol in the treatment of gastrointestinal cancer. Biomedicine & Pharmacotherapy, 153, 113274. [DOI] [PubMed] [Google Scholar]
- Rudin, C. M. , Hann, C. L. , Garon, E. B. , Ribeiro de Oliveira, M. , Bonomi, P. D. , Camidge, D. R. , Chu, Q. , Giaccone, G. , Khaira, D. , Ramalingam, S. S. , Ranson, M. R. , Dive, C. , McKeegan, E. M. , Chyla, B. J. , Dowell, B. L. , Chakravartty, A. , Nolan, C. E. , Rudersdorf, N. , Busman, T. A. , … Gandhi, L. (2012). Phase II study of single‐agent Navitoclax (ABT‐263) and biomarker correlates in patients with relapsed small cell lung CancerNavitoclax for recurrent small cell lung cancer. Clinical Cancer Research, 18(11), 3163–3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan, D. G. , Murphy, M. P. , Frezza, C. , Prag, H. A. , Chouchani, E. T. , O'Neill, L. A. , & Mills, E. L. (2019). Coupling Krebs cycle metabolites to signalling in immunity and cancer. Nature Metabolism, 1(1), 16–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehi, B. , Venditti, A. , Sharifi‐Rad, M. , Kręgiel, D. , Sharifi‐Rad, J. , Durazzo, A. , Lucarini, M. , Santini, A. , Souto, E. , Novellino, E. , Antolak, H. , Azzini, E. , Setzer, W. , & Martins, N. (2019). The therapeutic potential of apigenin. International Journal of Molecular Sciences, 20(6), 1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirrmacher, V. (2019). From chemotherapy to biological therapy: A review of novel concepts to reduce the side effects of systemic cancer treatment. International Journal of Oncology, 54(2), 407–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo, H. S. , Ku, J. M. , Choi, H.‐S. , Woo, J.‐K. , Jang, B.‐H. , Go, H. , Shin, Y. C. , & Ko, S.‐G. (2015). Apigenin induces caspase‐dependent apoptosis by inhibiting signal transducer and activator of transcription 3 signaling in HER2‐overexpressing SKBR3 breast cancer cells. Molecular Medicine Reports, 12(2), 2977–2984. [DOI] [PubMed] [Google Scholar]
- Seo, Y. J. , Kim, B. S. , Chun, S. Y. , Park, Y. K. , Kang, K. S. , & Kwon, T. G. (2011). Apoptotic effects of genistein, biochanin‐a and apigenin on LNCaP and PC‐3 cells by p21 through transcriptional inhibition of polo‐like kinase‐1. Journal of Korean Medical Science, 26(11), 1489–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan, S. , Shi, J. , Yang, P. , Jia, B. , Wu, H. , Zhang, X. , & Li, Z. (2017). Apigenin restrains colon cancer cell proliferation via targeted blocking of pyruvate kinase M2‐dependent glycolysis. Journal of Agricultural and Food Chemistry, 65(37), 8136–8144. [DOI] [PubMed] [Google Scholar]
- Shao, H. , Jing, K. , Mahmoud, E. , Huang, H. , Fang, X. , & Yu, C. (2013). Apigenin sensitizes colon cancer cells to antitumor activity of ABT‐263. Molecular Cancer Therapeutics, 12(12), 2640–2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharifi‐Rad, M. , Nazaruk, J. , Polito, L. , Morais‐Braga, M. F. B. , Rocha, J. E. , Coutinho, H. D. M. , Salehi, B. , Tabanelli, G. , Montanari, C. , del Mar Contreras, M. , Yousaf, Z. , Setzer, W. N. , Verma, D. R. , Martorell, M. , Sureda, A. , & Sharifi‐Rad, J. (2018). Matricaria genus as a source of antimicrobial agents: From farm to pharmacy and food applications. Microbiological Research, 215, 76–88. [DOI] [PubMed] [Google Scholar]
- Shokrzadeh, M. , Rajabali, F. , Habibi, E. , & Modanloo, M. (2018). Survey cytotoxicity and genotoxicity of hydroalcoholic extract of Stevia rebaudiana in breast cancer cell line (MCF7) and human fetal lung fibroblasts (MRC‐5). Current Reasearch: Integrative Medicine, 1, 12–17. [Google Scholar]
- Slattery, M. L. , Mullany, L. E. , Sakoda, L. , Samowitz, W. S. , Wolff, R. K. , Stevens, J. R. , & Herrick, J. S. (2018). The NF‐κB signalling pathway in colorectal cancer: Associations between dysregulated gene and miRNA expression. Journal of Cancer Research and Clinical Oncology, 144, 269–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slika, H. , Mansour, H. , Wehbe, N. , Nasser, S. A. , Iratni, R. , Nasrallah, G. , Shaito, A. , Ghaddar, T. , Kobeissy, F. , & Eid, A. H. (2022). Therapeutic potential of flavonoids in cancer: ROS‐mediated mechanisms. Biomedicine & Pharmacotherapy, 146, 112442. [DOI] [PubMed] [Google Scholar]
- Stupack, D. G. (2013). Caspase‐8 as a therapeutic target in cancer. Cancer Letters, 332(2), 133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung, H. , Ferlay, J. , Siegel, R. L. , Laversanne, M. , Soerjomataram, I. , Jemal, A. , & Bray, F. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians, 71(3), 209–249. [DOI] [PubMed] [Google Scholar]
- Thilakarathna, S. H. , & Rupasinghe, H. V. (2013). Flavonoid bioavailability and attempts for bioavailability enhancement. Nutrients, 5(9), 3367–3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas, S. D. , Jha, N. K. , Jha, S. K. , Sadek, B. , & Ojha, S. (2023). Pharmacological and molecular insight on the Cardioprotective role of Apigenin. Nutrients, 15(2), 385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolomeo, M. , & Cascio, A. (2021). The multifaced role of STAT3 in cancer and its implication for anticancer therapy. International Journal of Molecular Sciences, 22(2), 603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong, J. , Shen, Y. , Zhang, Z. , Hu, Y. , Zhang, X. , & Han, L. (2019). Apigenin inhibits epithelial‐mesenchymal transition of human colon cancer cells through NF‐κB/snail signaling pathway. Bioscience Reports, 39(5), 39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong, J. , Wang, P. , Tan, S. , Chen, D. , Nikolovska‐Coleska, Z. , Zou, F. , Yu, J. , & Zhang, L. (2017). Mcl‐1 degradation is required for targeted therapeutics to eradicate colon cancer CellsMcl‐1 degradation in targeted therapy. Cancer Research, 77(9), 2512–2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trembley, J. H. , Kren, B. T. , Afzal, M. , Scaria, G. A. , Klein, M. A. , & Ahmed, K. (2022). Protein kinase CK2–diverse roles in cancer cell biology and therapeutic promise. Molecular and Cellular Biochemistry, 478, 1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turktekin, M. , Konac, E. , Onen, H. I. , Alp, E. , Yilmaz, A. , & Menevse, S. (2011). Evaluation of the effects of the flavonoid apigenin on apoptotic pathway gene expression on the colon cancer cell line (HT29). Journal of Medicinal Food, 14(10), 1107–1117. [DOI] [PubMed] [Google Scholar]
- Ueda, H. , Yamazaki, C. , & Yamazaki, M. (2004). A hydroxyl group of flavonoids affects oral anti‐inflammatory activity and inhibition of systemic tumor necrosis factor‐α production. Bioscience, Biotechnology, and Biochemistry, 68(1), 119–125. [DOI] [PubMed] [Google Scholar]
- Ullah, A. , Munir, S. , Badshah, S. L. , Khan, N. , Ghani, L. , Poulson, B. G. , Emwas, A. H. , & Jaremko, M. (2020). Important flavonoids and their role as a therapeutic agent. Molecules, 25(22), 5243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Heiden, M. G. , Cantley, L. C. , & Thompson, C. B. (2009). Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science, 324(5930), 1029–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venditti, A. , Frezza, C. , Sciubba, F. , Serafini, M. , Bianco, A. , Cianfaglione, K. , Lupidi, G. , Quassinti, L. , Bramucci, M. , & Maggi, F. (2018). Volatile components, polar constituents and biological activity of tansy daisy (Tanacetum macrophyllum (Waldst. Et kit.) Schultz Bip.). Industrial Crops and Products, 118, 225–235. [Google Scholar]
- Venditti, A. , Maggi, F. , Vittori, S. , Papa, F. , Serrilli, A. M. , di Cecco, M. , Ciaschetti, G. , Mandrone, M. , Poli, F. , & Bianco, A. (2015). Antioxidant and α‐glucosidase inhibitory activities of Achillea tenorii. Pharmaceutical Biology, 53(10), 1505–1510. [DOI] [PubMed] [Google Scholar]
- Wang, C. , & Youle, R. J. (2009). The role of mitochondria in apoptosis. Annual Review of Genetics, 43, 95–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, M. , Firrman, J. , Liu, L. S. , & Yam, K. (2019). A review on flavonoid apigenin: Dietary intake, ADME, antimicrobial effects, and interactions with human gut microbiota. BioMed Research International, 2019, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, W. , Liu, X. , Zhang, Z. , Yin, M. , Chen, X. , Zhao, S. , & Wu, L. (2022). Apigenin induced apoptosis by downregulating Sulfiredoxin expression in cutaneous squamous cell carcinoma. Oxidative Medicine and Cellular Longevity, 2022, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, W. , Zhao, C. , Jou, D. , Lü, J. , Zhang, C. , Lin, L. , & Lin, J. (2013). Ursolic acid inhibits the growth of colon cancer‐initiating cells by targeting STAT3. Anticancer Research, 33(10), 4279–4284. [PubMed] [Google Scholar]
- Warren, C. F. , Wong‐Brown, M. W. , & Bowden, N. A. (2019). BCL‐2 family isoforms in apoptosis and cancer. Cell Death & Disease, 10(3), 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, Z. , Wang, Z. , Meng, Y. , Tao, Z. , Yan, W. , & du, Y. (2020). Apigenin suppresses proliferation and bone metastasis of human breast cancer cells by inducing apoptosis, autophagy and modulation of the MEK/ERK signalling pathway. Archives of Medical Science, 16(1). 23–34. [Google Scholar]
- Xi, Y. , & Xu, P. (2021). Global colorectal cancer burden in 2020 and projections to 2040. Translational Oncology, 14(10), 101174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, H. , Ma, Y. , Zhang, J. , Gu, J. , Jing, X. , Lu, S. , Fu, S. , & Huo, J. (2020). Identification and verification of core genes in colorectal cancer. BioMed Research International, 2020, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, M. , Wang, S. , Song, Y. U. , Yao, J. , Huang, K. , & Zhu, X. (2016). Apigenin suppresses colorectal cancer cell proliferation, migration and invasion via inhibition of the Wnt/β‐catenin signaling pathway. Oncology Letters, 11(5), 3075–3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav, P. , Yadav, R. , Jain, S. , & Vaidya, A. (2021). Caspase‐3: A primary target for natural and synthetic compounds for cancer therapy. Chemical Biology & Drug Design, 98(1), 144–165. [DOI] [PubMed] [Google Scholar]
- Yan, X. , Qi, M. , Li, P. , Zhan, Y. , & Shao, H. (2017). Apigenin in cancer therapy: Anti‐cancer effects and mechanisms of action. Cell & Bioscience, 7(1), 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, J. , Pi, C. , & Wang, G. (2018). Inhibition of PI3K/Akt/mTOR pathway by apigenin induces apoptosis and autophagy in hepatocellular carcinoma cells. Biomedicine & Pharmacotherapy, 103, 699–707. [DOI] [PubMed] [Google Scholar]
- Yang, P.‐M. , Tseng, H.‐H. , Peng, C.‐W. , Chen, W.‐S. , & Chiu, S.‐J. (2012). Dietary flavonoid fisetin targets caspase‐3‐deficient human breast cancer MCF‐7 cells by induction of caspase‐7‐associated apoptosis and inhibition of autophagy. International Journal of Oncology, 40(2), 469–478. [DOI] [PubMed] [Google Scholar]
- Young, C. D. , Lewis, A. S. , Rudolph, M. C. , Ruehle, M. D. , Jackman, M. R. , Yun, U. J. , Ilkun, O. , Pereira, R. , Abel, E. D. , & Anderson, S. M. (2011). Modulation of glucose transporter 1 (GLUT1) expression levels alters mouse mammary tumor cell growth in vitro and in vivo. PLoS One, 6(8), e23205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, Q. , Zhang, K. , Wang, X. , Liu, X. , & Zhang, Z. (2010). Expression of transcription factors snail, slug, and twist in human bladder carcinoma. Journal of Experimental & Clinical Cancer Research, 29, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zafar, A. , Alruwaili, N. K. , Imam, S. S. , Alsaidan, O. A. , Ahmed, M. M. , Yasir, M. , Warsi, M. H. , Alquraini, A. , Ghoneim, M. M. , & Alshehri, S. (2022). Development and optimization of hybrid polymeric nanoparticles of apigenin: Physicochemical characterization, antioxidant activity and cytotoxicity evaluation. Sensors, 22(4), 1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamanian, M. , Bazmandegan, G. , Sureda, A. , Sobarzo‐Sanchez, E. , Yousefi‐Manesh, H. , & Shirooie, S. (2021). The protective roles and molecular mechanisms of troxerutin (vitamin P4) for the treatment of chronic diseases: A mechanistic review. Current Neuropharmacology, 19(1), 97–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamanian, M. , Shamsizadeh, A. , Esmaeili Nadimi, A. , Hajizadeh, M. , Allahtavakoli, F. , Rahmani, M. , Kaeidi, A. , Safari Khalegh, H. , & Allahtavakoli, M. (2017). Short‐term effects of troxerutin (vitamin P4) on muscle fatigue and gene expression of Bcl‐2 and Bax in the hepatic tissue of rats. Canadian Journal of Physiology and Pharmacology, 95(6), 708–713. [DOI] [PubMed] [Google Scholar]
- Zhang, C. , Cao, Y. , Zuo, Y. , Cheng, H. , Liu, C. , Xia, X. , Ren, B. , Deng, Y. , Wang, M. , & Lu, J. (2023). Bruceine a exerts antitumor effect against colon cancer by accumulating ROS and suppressing PI3K/Akt pathway. Frontiers in Pharmacology, 14, 78–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, J. , Liu, D. , Huang, Y. , Gao, Y. , & Qian, S. (2012). Biopharmaceutics classification and intestinal absorption study of apigenin. International Journal of Pharmaceutics, 436(1–2), 311–317. [DOI] [PubMed] [Google Scholar]
- Zhang, T.‐B. , Zhao, Y. , Tong, Z.‐X. , & Guan, Y.‐F. (2015). Inhibition of glucose‐transporter 1 (GLUT‐1) expression reversed Warburg effect in gastric cancer cell MKN45. International Journal of Clinical and Experimental Medicine, 8(2), 2423. [PMC free article] [PubMed] [Google Scholar]
- Zhao, P. , & Zhang, Z. (2018). TNF‐α promotes colon cancer cell migration and invasion by upregulating TROP‐2. Oncology Letters, 15(3), 3820–3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong, L. , Li, Y. , Xiong, L. , Wang, W. , Wu, M. , Yuan, T. , Yang, W. , Tian, C. , Miao, Z. , Wang, T. , & Yang, S. (2021). Small molecules in targeted cancer therapy: Advances, challenges, and future perspectives. Signal Transduction and Targeted Therapy, 6(1), 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, Y. , Yu, Y. , Lv, H. , Zhang, H. , Liang, T. , Zhou, G. , Huang, L. , Tian, Y. , & Liang, W. (2022). Apigenin in cancer therapy: From mechanism of action to nano‐therapeutic agent. Food and Chemical Toxicology, 168, 113385. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data relevant to the review article are within the manuscript.


